Abstract
The sphingolipid family of lipids modulate several cellular processes, including proliferation, cell cycle regulation, inflammatory signaling pathways, and cell death. Several members of the sphingolipid pathway have opposing functions and thus imbalances in sphingolipid metabolism result in deregulated cellular processes, which cause or contribute to diseases and disorders in humans. A key cellular process regulated by sphingolipids is apoptosis, or programmed cell death. Sphingolipids play an important role in both extrinsic and intrinsic apoptotic pathways depending on the stimuli, cell type and cellular response to the stress. During mitochondrial-mediated apoptosis, multiple pathways converge on mitochondria and induce mitochondrial outer membrane permeabilization (MOMP). MOMP results in the release of intermembrane space proteins such as cytochrome c and Apaf1 into the cytosol where they activate the caspases and DNases that execute cell death. The precise molecular components of the pore(s) responsible for MOMP are unknown, but sphingolipids are thought to play a role. Here, we review evidence for a role of sphingolipids in the induction of mitochondrial-mediated apoptosis with a focus on potential underlying molecular mechanisms by which altered sphingolipid metabolism indirectly or directly induce MOMP. Data available on these mechanisms is reviewed, and the focus and limitations of previous and current studies are discussed to present important unanswered questions and potential future directions.
Keywords: Sphingolipid, mitochondria, apoptosis, ceramide, cancer, Bcl-2 proteins
Sphingolipids, sterols and glycerolipids are three classes of lipids that constitute the majority of eukaryotic cell membranes. Sphingolipids were initially discovered in brain extract in 1870 and their nomenclature was established by J.L.W. Thudichum in 1884 (Blaschko 1975; Hawthorne 1975; McIlwain 1975). Their structure, metabolism and cellular functions have been extensively investigated and reported. Sphingolipids provide structural integrity to cell membranes, and modulate numerous important cellular processes including apoptosis, proliferation, endocytosis, transport, migration, senescence, and inflammation through signal transduction and gene regulation (Patwardhan and Liu 2011). The function of sphingolipids depends largely on their level, the cell type, and the subcellular location in which they are generated. Imbalances in sphingolipid levels result in aberrant cellular processes, which contribute to the onset and progression of various diseases such as COPD, Gaucher disease, Alzheimer’s disease, inflammatory bowel disease, autoimmune diseases, chronic heart failure, asthma, diabetes, sepsis, and cancer (Baranowski and Gorski 2011; Kolter 2011; Russo et al. 2013; van Echten-Deckert and Walter 2012; Yang and Uhlig 2011). Such disease states may be triggered by deviant intracellular apoptotic signaling regulated by alterations in the levels of individual sphingolipids.
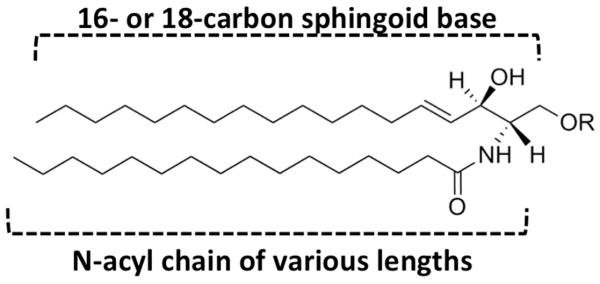
Sphingolipid family members generally contain a common sphingoid base backbone. Structural and functional diversity of sphingolipids is introduced through alterations of the backbone, including the introduction of a double bond(s), differing length (sphingoid base backbones of 16 and 18 carbons are possible), addition of a N-linked fatty acyl chain, and additions to the terminal hydroxyl group. The length of the N-linked fatty acyl chain varies as well as its degree of saturation and hydroxylation. Groups that can be added to the C1-hydroxyl group include a phosphate, carbohydrates, and phosphorylcholine moieties, giving rise to thousands of different sphingolipid species ((Hannun and Obeid 2008); Fig. 1).
Figure 1.
Basic structure of a sphingolipid. Sphingolipid family members generally contain a common sphingoid base backbone, which can be altered in a number of ways including the introduction of a double bond(s), differing length (sphingoid base backbones of 16 and 18 carbons are possible), addition of a N-linked fatty acyl chain, and additions to the terminal hydroxyl group. The length of the N-linked fatty acyl chain varies as well as its degree of saturation and hydroxylation. Groups that can be added to the C1- hydroxyl group of the sphingoid base backbone include a phosphate, carbohydrates, and phosphorylcholine moieties, giving rise to thousands of different sphingolipid species.
Sphingolipid Metabolism
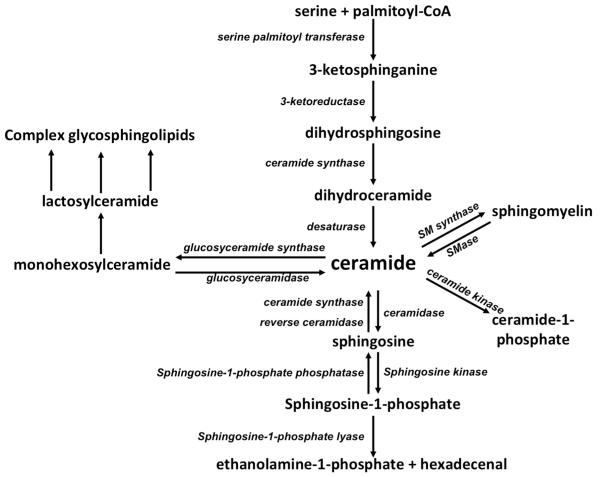
Sphingolipid metabolism (Fig. 2) centers on ceramide, which can be generated in cells by sphingomyelin (SM) hydrolysis, de novo synthesis, the salvage pathway, or from breakdown of more complex sphingolipids (Fig 2). Sphingomyelinases (SMases) catalyze the hydrolysis of SM to form ceramide and phosphocholine and are classified by their pH optima. The acid and Mg+2-dependent neutral sphingomyelinases have been implicated in stress-induced ceramide generation. There is also a secretory form of acid sphingomyelinase that has been implicated in stress responses (Jenkins et al. 2009). De novo ceramide synthesis occurs in the ER. The first step in de novo synthesis is catalyzed by serine palmitoyl transferase (SPT) and involves the condensation of serine and palmitoyl-CoA to form 3-ketosphinganine, which is then reduced to form sphinganine. Ceramide synthases catalyze the N-acylation of sphinganine to form dihydroceramide (DH). A dihydroceramide desaturase catalyzes the conversion of dihydroceramide to ceramide through the insertion of a trans double bond at the 4-5 position of the sphingoid base backbone.
Figure 2.
Schematic of sphingolipid metabolism. Ceramide is the center of sphingolipid metabolism and can be synthesized de novo from serine and palmitoyl CoA, the hydrolysis of sphingomyelin, breakdown of glycosphingolipids, or via salvaging free sphingosine. Once synthesized, ceramides can be utilized to form glycosphingolipids, ceramide-1-phosphate, or sphingomyelin. Alternatively, the N-linked fatty acyl chain can be cleaved off of ceramide to generate sphingosine that can be utilized to form sphingosine-1-phosphate. Sphingosine-1-phosphate can be turned back into sphingosine or can broken down to form hexadecenal and phosphoethanolamine (ethanolamine-1-phosphate).
Ceramide synthases catalyze the formation of ceramide in two distinct pathways: de novo synthesis and the recycling/ salvage pathway. In mammals, there are 6 ceramide synthases (CerS1-6) that have preferential activity for adding fatty acyl CoAs of particular chain lengths. The different CerS are expressed in a cell-type specific manner and their expression changes during development (Mullen et al. 2012). CerS activity is regulated at least in part by homo- and heterodimerization between the different CerS family members (Laviad et al. 2012). For instance, catalytic activity of CerS2 depends on dimer formation between CerS2 and CerS5 (Laviad et al. 2012). In vitro data shows that CerS2 activity is lower by itself compared to other CerS (ı5 μM V/S ı2 μM Km for sphinganine), but increases significantly when co-expressed with other CerS or expressed as a constitutive dimer (Laviad et al. 2012). Data indicates that in addition to homo- and heterodimerization, the specific CerS are regulated at the post-translational level by other mechanisms. For example, a variety of cellular stresses induce proteosomal processing of CerS1 thereby reducing its activity and inducing its translocation from the ER to the Golgi (Sridevi et al. 2010; Sridevi et al. 2009). This may be one mechanism by which cells have evolved to survive particular cellular stresses. Recent data implicates the involvement of proteins in the Bcl-2 family in regulating the activity of specific CerS at the post-translational level during cell death (Beverly et al. 2013; Jensen et al. 2014; Siskind et al. 2010). Thus, CerS activity in cells is determined by many factors such as availability of other CerS for dimerization, allosteric binding of substrate to monomer or dimer, differential regulation by the different transcript variants, proteolytic processing, translocation, and post-translational regulation by proteins in the Bcl-2 family. Ascribing distinct cellular functions to the individual CerS is at present not well established (Park et al. 2014) at least in part because of a lack of specific inhibitors against each of the individual CerS, but data suggest that individual CerS have distinct roles in regulating cellular processes. Studies utilizing CerS knockout mice (single and double knockout mice) are beginning to become available and are uncovering specific pathologies associated with the deletion of specific CerS (Ebel et al. 2014; Ebel et al. 2013; Ginkel et al. 2012; Imgrund et al. 2009; Jennemann et al. 2012; Park et al. 2013b; Petrache et al. 2013; Pewzner-Jung et al. 2010a; Pewzner-Jung et al. 2010b; Zigdon et al. 2013). The interpretation of data from knockout mice requires one to keep in mind the potential compensatory metabolic pathways that may result from perturbing one arm of sphingolipid metabolism (Park et al. 2013a; Park et al. 2013b; Petrache et al. 2013; Pewzner-Jung et al. 2010a; Pewzner-Jung et al. 2010b; Silva et al. 2012). For example, data in cell culture studies indicate that siRNA mediated knockdown of a single CerS, induces a compensatory increase in the expression of other CerS-family members (Mullen et al. 2011). In addition, some CerS may be coupled to specific downstream metabolic arms of ceramide metabolism whereas other CerS may not be coupled to these same processes.
Glycosphingolipids are a very heterogeneous class of lipids, varying in both the hydrophilic and hydrophobic region of the molecule. The hydrophilic portion of glycosphingolipids consists of a sugar headgroup and can contain neutral or charged groups. The hydrophobic portion can vary in the length of the N-linked fatty acyl chain and sphingoid base as well as the degree of saturation, hydroxylation, and branching. In general, glycosphingolipids are divided into two main classes based on the first sugar moiety linked to the ceramide backbone, namely galactose or glucose to form either galactosylceramide (GalCer) or glucosylceramide (GluCer), respectively (collectively known as hexosylceramides). GalCer is formed in the ER via the enzyme UDP-Gal:ceramide galactosyltransferase or GalCer synthase (CGalT) via the transfer of galactose from UDP-galactose to ceramide. GalCer is then transported to the Golgi where sulfatide or galactose groups can be added.
Glucosylceramides are generated from ceramide synthesized in the ER that are transported to the Golgi where UDP-Glc:ceramide glucosyltransferase (glucosylceramide synthase) catalyzes the addition of glucose onto ceramide from an activated nucleotide precursor (UDP-glucose). In mammals, a galactose group is then added from UDP-galactose to generate lactosylceramide, a reaction catalyzed by lactosylceramide synthases. Several sequential modifications to lactosylceramides can then occur to give rise to the more complex gangliosides, lactosylsulfatides, globotriosylceramides, and fucosylactosylceramides. Thus, LacCer is essential to the formation of complex glycosphingolipids. Gangliosides are a class of complex glycosphingolipids that arise from the addition of sialic acid residues to lactosylceramide and are highly abundant in the kidney. Degradation of complex glycosphingolipids leads to the accumulation of “simple” glycosphingolipids such as lactosylceramide and glucosylceramide. This degradation occurs mainly in the lysosome, but is known to occur at the plasma membrane and mitochondrion as well.
Ceramide synthesized in the ER can be transported to the Golgi for synthesis of sphingomyelin (SM). The ceramide transport protein CERT is located in the cytosol and interacts with both the ER and Golgi to extract ceramide from the ER and deliver it to the Golgi for synthesis of SM (Hanada 2006; Hanada et al. 2007; Kawano et al. 2006). CERT function is essential for embryogenesis, specifically for proper heart development (Wang et al. 2009). Interestingly, deletion of CERT in primary mouse embryonic fibroblasts does not results in the accumulation of ceramide, but rather hexosylceramides in the ER and mitochondria, resulting in decreased retrograde transport of plasma membrane proteins to the Golgi, increased levels of ER stress, mitochondrial dysfunction, increased levels of mitophagy, and eventual cell senescence (Rao et al. 2014). These data indicate the key role that CERT plays in maintaining balanced sphingolipid metabolism as well as the toxic impact when it becomes unbalanced.
Ceramidases catalyze the breakdown of ceramide via the removal of the N-linked fatty acyl chain to form sphingosine. Three different types of mammalian ceramidases have been identified. Acid ceramidases are lysosomal (Ferlinz et al. 2001; Park and Schuchman 2006), neutral ceramidases (Hwang et al. 2005; Ito et al. 2014; Tani et al. 2005) are localized to the plasma membrane, mitochondria, and nuclear membranes, and alkaline ceramidases reside in the Golgi and ER (Mao et al. 2001; Mao et al. 2003; Sun et al. 2010). Neutral/alkaline ceramidase has also been shown to catalyze the reverse reaction to generate ceramide from sphingosine and fatty acids (El Bawab et al. 2001; Novgorodov et al. 2011; Tani et al. 2000). Mitochondria have been shown to be capable of generating ceramide via the action of reverse ceramidase activity (Bionda et al. 2004; El Bawab et al. 2000; Novgorodov et al. 2011) and mitochondria isolated from the livers of neutral ceramidase deficient mice have significantly decreased generation of ceramide from sphingosine and palmitoyl-CoA or palmitate; a mitochondrial thioesterase has been shown to hydrolyze palmitoyl-CoA to palmitate for reverse synthesis of ceramide within the mitochondria by a neutral ceramidase (Novgorodov et al. 2011).
Sphingosine generation, via ceramidase catalyzed hydrolysis of ceramide, can also be used to generate sphingosine-1-phosphase (S1P). Phosphorylation of sphingosine is catalyzed by sphingosine kinase (SK) (Heffernan-Stroud and Obeid 2013; Lima and Spiegel 2013; Pitson 2011; Pyne et al. 2012; Siow et al. 2011; Snider 2013; Wattenberg 2010). There are two sphingosine kinases, namely SK1 and SK2. SK1 is thought to be cytosolic and can translocate to the plasma membrane, where it generates S1P that is secreted outside the cell where it can act as a ligand for certain G-coupled protein S1P receptors (Pitson 2011; Pitson et al. 2005; Wattenberg 2010). The subcellular location of SK2 is highly debated, but data suggests that it could be localized to the ER, mitochondria, and nuclei (Antoon et al. 2011; Don and Rosen 2009; Hait et al. 2007; Igarashi et al. 2003; Liu et al. 2000; Liu et al. 2003; Weigert et al. 2010). S1P has been shown to be protective/ anti-apoptotic, although data suggests that S1P may also exert a pro-apoptotic role under certain circumstances (Gandy and Obeid 2013; Heffernan-Stroud and Obeid 2013; Pitson 2011; Zhang et al. 2014). The subcellular localization in which S1P is generated and the ratio of S1P to ceramide/ sphingosine most likely dictate whether S1P exerts a pro- or anti-apoptotic function. S1P is further irreversibly metabolized to hexadecenal and phosphoethanolamine by S1P lyase (S1PL). S1PL is localized in ER and controls S1P signaling (Ikeda et al. 2004). S1P can also be dephosphorylated to regenerate sphingosine by lipid phosphate phosphatases or S1P phosphatase (SPP). SPPs are lipid phosphohydrolases that are predominantly localized in the ER; two SPPs, SPP1 and SPP2 have been identified that demonstrated high sphingolipid species specificity. Both S1PL and SPPs are implicated in cancer underscoring their role in cell death.
Mitochondrial Apoptosis
There are two main pathways for apoptosis, namely the extrinsic receptor-mediated pathway and an intrinsic mitochondrial pathway. The extrinsic pathway of apoptosis involves the binding of a death ligand (for example tumor necrosis factor-α, TNFα) to its receptor (for example, TNF receptor-1) on the external surface of the plasma membrane, which leads to the formation of a death-inducing signaling complex (DISC). The DISC recruits multiple effectors and these are responsible for the execution of the cell. Sphingolipids have been implicated as being key regulators in the extrinsic pathway of apoptosis (Bollinger et al. 2005; Grassme et al. 2003; Grassme et al. 2001a; Grassme et al. 2001b; Gulbins and Kolesnick 2003; Heinrich et al. 2000; Schneider-Brachert et al. 2004). This data will not be discussed here as the current review focuses on the role of sphingolipids specifically in the intrinsic pathway of apoptosis that is mediated by mitochondria.
Mitochondria play a major role in the intrinsic apoptotic pathway whereby stress signals converge on mitochondria to induce mitochondrial outer membrane permeabilization (MOMP). MOMP allows the release of proteins from the intermembrane space of mitochondria to the cytosol where they activate the caspase cascade. Upon activation of caspases, cells display several features of apoptosis, including cleavage of PARP, externalization of plasma membrane phosphatidylserine, chromatin condensation and rapid cell death within minutes. Thus, MOMP is thought to be an irreversible event that is tightly regulated (Galluzzi et al. 2012). MOMP is controlled largely by pro and anti-apoptotic members of Bcl-2 family (Chipuk et al. 2006; Chipuk and Green 2008; Chipuk et al. 2010). Both pro- and anti-apoptotic members in the Bcl-2 family share homology at one or more specific domains referred to as Bcl-2 homology domains (BH domains)(Chipuk et al. 2010). The anti-apoptotic proteins include Bcl-2, Bcl-xL, Mcl-1, Bfl1, Bcl-b and Bcl-w and their over-expression often rescues cells from cell death induced by several stimuli (Chipuk et al. 2010). The pro-apoptotic proteins are subdivided into two classes: the multi-domain proteins such as Bax and Bak, and BH3-only proteins like Bid, Bim, Bad, Puma, Noxa, and Bik (Chipuk et al. 2010). The Bcl-2 family can be regulated by p53, which transcriptionally activates proapoptotic proteins (Bax, PUMA) or directly binds and inhibits activity of anti-apoptotic proteins (Bcl-2) (Chipuk and Green 2004; Chipuk and Green 2006; Chipuk et al. 2004; Chipuk et al. 2003; Follis et al. 2013; Perfettini et al. 2004). The proteins in the Bcl-2 family are present in various cellular compartments, including mitochondria, ER, cytosol, and nuclei (Annis et al. 2001; Malina and Hess 2004; Scorrano et al. 2003; Zong et al. 2003). Some Bcl-2 family members change their intracellular localization in response to apoptotic stimuli. For example, Bax is present as a soluble cytosolic protein and following an apoptotic signal it changes conformation, translocates to mitochondria, and forms a proteolipidic channel in the MOM to induce MOMP (Nomura et al. 1999; Wolter et al. 1997). Bak on the other hand is an integral membrane protein localized to the ER and the MOM. Bax and Bak form homo and hetero-oligomers with other Bcl-2 proteins to regulate MOMP. The interaction of Bax and/ or Bak and with anti-apoptotic Bcl-2 proteins inhibits their ability to induce MOMP, while interactions with particular pro-apoptotic BH3-only members promotes MOMP (Chipuk and Green 2008; Chipuk et al. 2010). Cells deficient in both Bax and Bak are extremely resistant to mitochondrial-mediated apoptosis and thus one of these proteins is thought to be necessary for MOMP to be induced (Cartron et al. 2003; Lindsten et al. 2000; Rathmell et al. 2002; Wei et al. 2001). The exact nature of the interactions between the various members of the Bcl-2 family and the manner in which these interactions regulate MOMP are still under intense investigation. It is clear, however, that MOMP is regulated by more than just Bcl-2 proteins. Data indicate that lipids, including sphingolipids, derived from ER and mitochondrial-associated membranes are also required for Bax and Bak activation and channel formation in the MOM (discussed below). Sphingolipids have numerous other effects on ER and mitochondria that may lead to MOMP induction. These potential mechanisms by which sphingolipids alter mitochondrial function and induce MOMP are discussed below.
Sphingolipids and mitochondrial apoptosis
Obeid et al. in their pioneering 1993 paper in Science illustrated for the first time that ceramide could induce apoptosis when present endogenously in tissues or when exogenously added to cells (Obeid et al. 1993). Countless studies have since validated this initial discovery and indicate that cellular ceramide levels increase early in apoptosis in a variety of cell types and in response to a multitude of apoptotic stimuli including TNFα (Garcia-Ruiz et al. 1997; Geilen et al. 1997), UV-irradiation (Charruyer et al. 2007; Chatterjee and Wu 2001; Deng et al. 2002; Siskind et al. 2010; Uchida et al. 2010; Yao et al. 2013), IL3 withdrawal (Siskind et al. 2010), ionizing radiation (Alphonse et al. 2013; Chmura et al. 1997; Deng et al. 2008; Haimovitz-Friedman et al. 1994; Lu and Wong 2004; Mesicek et al. 2010; Moeller et al. 2009; Vit and Rosselli 2003), serum withdrawal (Caricchio et al. 2002; Fernandez-Ayala et al. 2000), etoposide (Chen et al. 2006; Lin et al. 2005; Perry et al. 2000; Sawada et al. 2000), staurosporine (Wiesner and Dawson 1996), daunorubicin (Bettaieb et al. 1999; Bose et al. 1995; Chen et al. 2000; Come et al. 1999; Jaffrezou et al. 1996; Mansat et al. 1997), taxol (Charles et al. 2001), and cisplatin (Min et al. 2007; Noda et al. 2001; Siskind et al. 2010). Thus, ceramide generation appears to be a common cellular response to apoptosis-inducing agents. Increases in cellular ceramide levels have been shown to occur prior to the mitochondrial phase of apoptosis (Kroesen et al. 2001; Rodriguez-Lafrasse et al. 2001; Thomas et al. 1999) and to be accompanied by the release of proteins from the mitochondrial intermembrane space, increased mitochondrial reactive oxygen species (ROS) generation, and collapse in the mitochondrial inner membrane potential (Fig. 3) (Andrieu-Abadie et al. 2001; Gentil et al. 2003; Hearps et al. 2002; Lin et al. 2004). Interventions that suppress mitochondrial dysfunction also suppress ceramide-induced apoptosis, including bongkrekic acid (Lin et al. 2004; Stoica et al. 2003) cyclosporine A (Pacher and Hajnoczky 2001), Bcl-2 (Karasavvas et al. 1996; Zhang et al. 1996), and Bcl-xL (Wiesner et al. 1997). Thus, ceramide has been established as a bona fide pro-death molecule.
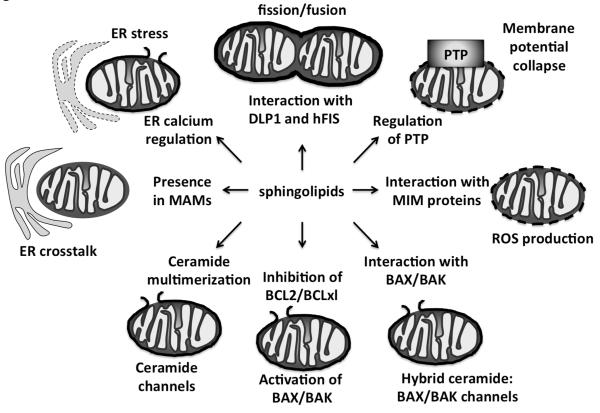
Figure 3.
Diagram illustrating the proposed mechanisms by which sphingolipids alter mitochondrial structure and function to induce mitochondrial outer membrane permeabilization (MOMP) and apoptosis.
Metabolism of ceramide is one mechanism by which cells evade the apoptotic process. The enzyme glucosylceramide synthase (GCS) converts ceramide to glucosylceramide, which is utilized to generate downstream complex glycosphingolipids, including gangliosides. GCS not only enables cells to escape ceramide-induced apoptosis but also renders cells resistant to many of the aforementioned apoptotic stimuli. For example, Liu et al. showed that MCF-7 breast cancer cells with upregulated GCS are resistant to ceramide induced apoptosis when exposed to TNF-α and doxorubicin (Liu et al. 2008). Complex glycosphingolipids derived from ceramides (for example gangliosides) have been reported to increase cell growth. For example, GD1a increases EGFR dimerization and phosphorylation and increases activation of downstream EGFR signaling pathways, which results in cell proliferation (Li et al. 2001; Liu et al. 2004). Metabolites of ceramide such as S1P are thought to help cells evade ceramide-induced apoptosis through activation of ERK and other anti-apoptotic signaling pathways (Shida et al. 2008; Stevenson et al. 2011; Takabe and Spiegel 2014). However, S1P and its breakdown product hexadecanal have also been proposed to exert a pro-apoptotic role when generated in mitochondrial-associated membranes (MAMs) (Chipuk et al. 2012). S1P and hexadecanal were shown in vitro to activate the pro-apoptotic Bcl-2 proteins Bak and Bax, respectively (Chipuk et al. 2012). Thus, levels of a particular sphingolipid, changes in enzymes involved in sphingolipid metabolism, and behavior of these sphingolipids according to site of generation/metabolism (e.g. lipid rafts) determines whether cells will undergo apoptosis or proliferation.
There are several reports implicating ceramide in mitochondrial-induced apoptosis. Ceramide either directly participates in apoptosis by having direct effects on mitochondria to induce MOMP or activates proteins that regulate MOMP. In cells, elevated ceramide levels have been shown to modulate the function of a number of proteins involved in the apoptotic process, including ceramide-activated protein kinase (CAPK), ceramide-activated protein phosphatases PP1 and PP2A, protein kinase C-ζ (PKCζ), and the lysosomal protease cathepsin D (Hannun and Obeid 2008). Besides these indirect mechanisms by which sphingolipds could contribute to mitochondrial apoptosis, a plethora of data suggests that sphingolipids such as ceramide may directly alter mitochondrial function to induce apoptosis. Generation of ceramide within mitochondria via mitochondrial targeting of bacterial sphingomyelinase results in MOMP and apoptosis (Birbes et al. 2001; Birbes et al. 2002; Birbes et al. 2005). There are pools of ER and mitochondrial sphingolipids such as ceramide, sphingomyelin, and glycosphingolipids (see below) and data suggests and that when the levels of these sphingolipids are altered, mitochondrial function declines and mitochondrial-mediated apoptosis is induced. There are several ways by which sphingolipids contribute to apoptosis. Here, we will focus only on their impact on mitochondrial-mediated events.
Sphingolipid metabolism, mitochondria, ER, and Mitochondrial-associated Membranes
Mitochondria are highly dynamic and organized organelles that are closely associated with the ER through mitochondrial-associated membranes (MAMs) that tether the ER and mitochondria. It is estimated that 5-20% of the mitochondrial surface is in close contact with ER networks (Rizzuto et al. 1998) and the distance between ER and mitochondria at the MAM is estimated to be only 10-25 nm (Csordas et al. 2006). MAMs contain enzymes for lipid synthesis and proteins for lipid transfer (Csordas et al. 2006) and ER-mitochondrial cross talk is crucial for the regulation of apoptosis (Fig. 3) (Iwasawa et al. 2011; Pinton et al. 2011). The ER is a major site for the synthesis of sphingolipids (see above) and data indicates that sphingolipids generated in the ER can transfer to mitochondria (Stiban et al. 2008), possibly by way of vesicles or MAMs. In addition, sphingolipids are present in both MAMs and mitochondria (Bionda et al. 2004). Several enzymes in the sphingolipid metabolic pathway have been localized to mitochondria, including ceramide synthase, ceramidase, sphingosine kinases, and neuraminidase 4 (Bigi et al. 2010; Bionda et al. 2004; Novgorodov and Gudz 2009; Novgorodov et al. 2014; Novgorodov et al. 2011; Strub et al. 2011; Yamaguchi et al. 2005). Bionda et al., confirmed the presence of ceramide synthase in ultrapure MAM-free mitochondria and showed that both mitochondrial outer and inner membranes are capable of ceramide synthesis (Bionda et al. 2004). Data indicate that the levels of MAM and possibly mitochondrial outer or inner membrane sphingolipids are altered during apoptosis (Babiychuk et al. 2011; Kujjo et al. 2013; Lee et al. 2011; Novgorodov and Gudz 2011; Novgorodov et al. 2011; Rego et al. 2012; Siskind 2005). Thus, sphingolipid metabolism can occur in and is altered during apoptosis in ER, MAMs, and/ or mitochondria during apoptosis. Functionally, sphingolipids have been documented to have numerous effects on mitochondrial function, permeabilization of the outer membrane and release of intermembrane space proteins. Further, sphingolipids can alter mitochondrial morphology, mitophagy, and mitochondrial respiration via impacting individual components of the mitochondrial electron transport chain. These data are discussed below (Fig. 3).
Alterations in ER function by sphingolipids that could play a role in mitochondrial-mediated apoptosis: De novo ceramide synthesis occurs in the ER (see above). Sphingolipids are also known to play a key role in ER Ca+2 homeostasis and ER stress responses, which also can play a role in mitochondrial-mediated apoptosis. For example, inhibition of neutral sphingomyelinase protected retinal epithelial cells from ER stress-induced apoptosis (Kucuksayan et al. 2014). In addition, knockdown of CERT in mouse embryonic fibroblasts induced ER stress that was linked to an accumulation of hexosylceramide, rather than ceramide (Rao et al. 2014). Interestingly, decreased CerS6 expression actually induced ER stress via induction of calcium release from the ER and activation of transcription factor 6 (ATF6) (Senkal et al. 2010; Senkal et al. 2011). Dihydroceramides have also been implicated in ER stress; treatment of cells with a dihydroceramide desaturase inhibitor induced ER stress as determined by induction of eIFalpha and Xbp1 (Gagliostro et al. 2012). These data suggest that dysregulated sphingolipid metabolism plays a role in ER stress responses. Since it is known that ER stress can induce MOMP and apoptosis via altering ER Ca+2 homeostasis, sphingolipids could indirectly induce MOMP via their effects on the ER (Fig. 3). Alternatively, sphingolipids generated in the ER could directly regulate mitochondrial events via transfer to mitochondrial membranes via MAMs or an unidentified transfer protein. Stiban et al. showed that ceramide generated in the ER and MAMs are rapidly transferred to the mitochondrial outer membrane in sufficient amounts to allow for the induction of MOMP (Stiban et al. 2008). Mesicek et al. showed that ionizing radiation of HeLa cells induces CerS activity in MAM, elevating ceramide levels eventually in MOM and inducing MOMP; these effects were inhibited by ceramide synthase inhibitor fumonisin B1 (FB1)(Mesicek et al. 2010). Further, data indicate that CerS5 and CerS6 are indispensable for IR-induced CerS activity and apoptosis. Interestingly, Mesicek et al., proposed that IR-induced CerS5 mediated C16-ceramide is pro-apoptotic whereas CerS2 mediated C24:1- and C24:0-ceramides may be pro-survival (Mesicek et al. 2010). The ER-resident sphingomyelin synthase-related protein (SMSr, also known as sterile a-motif domain-containing protein; SAMD8) synthesizes the sphingomyelin analogue ceramide phosphoethanolamine (CPE); SMSr appears to act as a suppressor of ceramide-mediated cell death as its downregulation increases ceramides in the ER and in turn in mitochondria, triggering MOMP (Tafesse et al. 2014). Thus, dysregulated sphingolipid metabolism may contribute to MOMP and apoptosis indirectly via effects on the ER or directly via transfer to mitochondria or generation directly within mitochondria.
Impacts of sphingolipids on the mitochondrial outer membrane (MOM) that to could contribute to apoptosis: As discussed above mitochondrial outer membrane permeabilization (MOMP) plays a pivotal role in the induction of mitochondrial-mediated apoptosis. Sphingolipids have been proposed to play a key role in the regulation and/ or induction of MOMP. Data suggests that if sufficient quantities of ceramides accumulate within the MOM, then MOMP and apoptosis would result. There are two main mechanisms by which sphingolipids have been implicated to alter the MOM to induce MOMP, namely ceramide channel formation and the interaction with Bcl-2 proteins.
Ceramide channels: In 2000, Siskind and Colombini first reported that ceramides form channels in membranes as determined by their ability to induce vertical increments in current under voltage clamp conditions (Siskind and Colombini 2000). Since their initial discovery, ceramide channels have been extensively characterized (see reviews (Colombini 2010; Colombini 2013) for further information). The size of the ceramide channels was estimated to be large enough to allow the passage of proteins (Samanta et al. 2011; Siskind et al. 2003; Siskind et al. 2002). A typical ceramide channel is reported to have pore diameter approximately of 10 nm based on the molecular weight of proteins released from mitochondria, conductance of single channels and visualization of pores by electron microscopy (Samanta et al. 2011; Siskind et al. 2003; Siskind et al. 2002; Siskind et al. 2006). Ceramide channels are directly inhibited by anti-apoptotic Bcl-2 proteins (Siskind et al. 2008) and ceramides can form hybrid channels with the pro-apoptotic Bcl-2 protein Bax (Ganesan and Colombini 2010). While ceramides are capable of forming a channel that could allow for MOMP to occur, the evidence for their formation in vivo is still lacking. In an attempt to determine whether ceramides could induce MOMP in vivo, we utilized cells deficient in Bax and Bak as one of these proteins is required for the induction of MOMP in vivo. Our data indicated a much more complicated situation in that cells deficient in Bax and Bak were incapable of generating ceramides in response to apoptotic stimuli (Siskind et al. 2010). Data indicate that the protein Bak regulates ceramide synthases and thus is required for ceramide generation in mitochondrial-mediated apoptosis (Siskind et al. 2010). Activated Bak is more efficient at activating CerS (Beverly et al. 2013). In fact, ABT263, which directly activates Bax and/ or Bak by facilitating their release from anti-apoptotic Bcl-2 proteins such as Bcl-2 and Bcl-xL, induces CerS activation, ceramide generation, and apoptosis of cells, which is enhanced if the downstream metabolism of ceramide to glucosylceramide or S1P is inhibited (Beverly et al. 2013; Casson et al. 2013). Thus, data indicate a complicated system of cross-talk between sphingolipids and Bcl-2 proteins which is further discussed below.
Interaction with Bcl-2 proteins: Interaction of Bcl-2 proteins with sphingolipids is intriguing, given the redundancy and involvement of Bcl-2 family members in various stages and pathways of apoptosis. In general, in vitro data from studies utilizing isolated mitochondria or planar phospholipid membranes indicate that anti-apoptotic Bcl-2 family proteins disassemble ceramide channels whereas pro-apoptotic Bcl-2 family members directly enhance them (Ganesan and Colombini 2010; Ganesan et al. 2010; Perera et al. 2012). Ganeshan et al. demonstrated that activated Bax and ceramide act synergistically to permeabilize MOM and the amount of Bax required for MOMP was inversely proportional to amount of ceramide available (Ganesan et al. 2010). In fact, very small amounts of Bax facilitate the growth of ceramide channels, but once the optimal channel size is achieved, additional Bax ceases to affect the channel (Ganesan et al. 2010). Synergistic channel formation by ceramides and Bax is very exciting given that in cells, ceramide generation has been shown to be required for Bax activation in response to particular apoptotic stimuli (Belaud-Rotureau et al. 2000; Jin et al. 2008; Kim et al. 2001; Kim et al. 2008; Lee et al. 2011; Martinez-Abundis et al. 2009; von Haefen et al. 2002). Anti-apoptotic proteins in the Bcl-2 family inhibit both Bax and ceramide induced apoptosis. It is possible that anti-apoptotic Bcl-2 proteins bind to ceramide and Bax/ Bak to prevent their interaction and synergistic channel formation in the MOM (Fig. 3).
Interestingly, other sphingolipids can activate Bcl-2 family mediated MOMP, possibly independent of ceramide. Phillips et al. reported that sphingosine activates Bax causing its translocation to MOM promoting release of mitochondrial cytochrome c and Smac/Diablo, but not other mitochondrial proteins (Phillips et al. 2007). Whilst cytochrome c and Smac/Diablo were released by sphingosine and Bax, there was no decrease in mitochondrial inner membrane potential or enhanced production of reactive oxygen species (Phillips et al. 2007). Chipuk et al., presented a possibility that S1P and its metabolite hexadecenal activate Bak and Bax, respectively, depending on the cell death stimulus; data suggest that these sphingolipid products specifically cooperate with pro-apoptotic BH3-only proteins to coordinate MOMP and cytochrome c release (Chipuk et al. 2012). The levels of total cellular levels of S1P and hexadecenal generally exceedingly low, but local concentrations within or near mitochondria may be higher. Thus, future studies will need to determine if sufficient levels of mitochondrial S1P and hexadecanal are achieved in mitochondria during the early stages of apoptosis to induce Bak and Bax activation.
Impacts of sphingolipids on the mitochondrial inner membrane (MIM) that could contribute to apoptosis: The mitochondrial inner membrane contains two small electron carriers (cytochrome c and ubiquinol) and five multimeric protein complexes (complexes I-V) that produce an electrochemical proton gradient (ΔΨm) across the MIM necessary for complex V (F1F0-ATP synthase) to generate ATP in a process commonly referred to as oxidative phosphorylation. ATP is required for the activation of caspases and the execution phase of apoptosis. Thus, it is still highly debated whether the collapse in ΔΨm is a cause or a consequence of MOMP and apoptosis. Disruption in the functioning of the complexes of the MIM electron transport chain can lead to the generation of free radicals and reactive oxygen species (ROS) as well as changes in ΔΨm. Generation of ROS is an important factor in mitochondrial-mediated apoptosis. Numerous data implicate sphingolipids as playing a role in changes in ΔΨm or mitochondrial ROS generation. Early in vitro data utilizing isolated mitochondria or whole cells treated with sphingolipids shed light on potential in vivo effects of sphingolipids. Gudz et al. showed that ceramide addition to isolated mitochondria inhibits complex III (Gudz et al. 1997). Garcia-Ruiz et al. illustrated that TNF treatment of cells increased mitochondrial ceramide levels, which was upstream of induced mitochondrial ROS production (Garcia-Ruiz et al. 1997). The involvement of ceramide in mitochondrial apoptosis through ROS is bolstered by studies by Quillet et al. that demonstrated that in human myeloid leukemia U937 cells, addition of the short chain ceramide analogue C6–ceramide (but not dihydroceramide) rapidly induced H2O2 at the ubiquinone step of the mitochondrial respiratory chain, resulting in apoptosis (Quillet-Mary et al. 1997). Park et al. showed that in mesenchymal stem cells derived from human adipose tissue (hASCs), ceramide generates ROS and disrupts mitochondrial potential (ΔΨm), and the effect was inhibited by addition of antioxidant NAC (Park et al. 2011). This is an important finding as mesenchymal stem cells (MSCs) are being examined as a therapy for regenerative medicines and tissue engineering. Although MSCs have shown some promise in pre-clinical and early clinical trials, apoptosis of MSCs may hamper the repair of the tissue. Other sphingolipids have been implicated in ROS production. For example, sphingosine induced a similar effect as ceramide, although much higher concentrations were required. Further, there are reports that sphingosine induces mitochondrial dysfunction via inhibition of complex IV (cytochrome c oxidase) of the electron transport chain (Abrahan et al. 2010; Novgorodov et al. 2014). Gorria et al. demonstrated that exogenously added monosialoganglioside GM1 protects rat hepatic F258 epithelial cells from benzo[a]pyrene (B[a]P) induced apoptosis by preventing the collapse in ΔΨm and mitochondrial ROS production . Interestingly, GM1 does not affect activation of the p53 pathway by B[a]P, indicating that it prevents apoptosis at the mitochondrial level (Gorria et al. 2006). In vitro effects of sphingolipids on the function of the MIM are bolstered by several in vivo studies. First, Fabry disease, which is characterized by altered function of components of the electron transport chain, is cause by a defect in α-galactosidase leading to an accumulation of specific species of glycosphingolipids (Lucke et al. 2004). Second, mice deficient in ceramide synthase 2 have an altered sphingolipid profile in liver mitochondria, concomitant with reduced complex IV activity and progressive hepatopathy (Pewzner-Jung et al. ; Pewzner-Jung et al. ; Zigdon et al. 2013). A third example comes from mice deficient for the ceramide-ER transport protein (CERT) where hexosylceramide accumulates in mitochondria that have defects in oxygen consumption, resulting in degeneration of mitochondria, and embryonic lethality (Kujjo et al. 2013). In vitro data from studies utilizing isolated mitochondria and whole cells grown in culture when combined with in vivo studies in humans and mice provide powerful evidence for a role of sphingolipids in altered functions of the MIM. These changes in the structure and function of the MIM may play a pivotal role in the ability of sphingolipids to induce apoptosis. For additional details of the effects of sphingolipids on the mitochondrial electron transport chain and ROS generation see an extensive review written by Kogot-Levin and Saada (Kogot-Levin and Saada 2014).
Mitochondrial lipid rafts and mitochondrial morphology: Sphingolipids are thought to be a major component of lipid rafts. In plasma membranes, lipid rafts play a role in receptor clustering to facilitate signaling cascades. Lipids rafts containing glycosphingolipid enriched microdomains (GEMs), have been found in the mitochondrial outer membrane. In normal rat hepatocytes, GD3 is primarily present in plasma membrane. In response to TNF-α exposure, GD3 undergoes rapid redistribution depleting its levels in the plasma membrane and instead accumulating in mitochondrial domains through actin dependent vesicle trafficking (Garcia-Ruiz et al. 2002). Similar results were seen in human colon carcinoma cells HT-29 (Garcia-Ruiz et al. 2002). GD3 accumulation selectively in mitochondrial domains is derived by decrease in Glutathione (GSH) levels in mitochondria; low levels of GSH themselves does not lead to decrease in mitochondrial apoptosis, but attracts GD3 to mitochondria making it a two way process (Garcia-Ruiz et al. 2002). These domains are also home to mitochondrial fission proteins (hFis1, DLP1) and pro-apoptotic Bcl-2 family proteins (Bid, Bax). Assembly of these proteins together in lipid microdomains may promote mitochondrial fission and apoptosis. Ciarlo et al. observed that upon anti CD95/Fas stimulation of HeLa cells there was significant co-localization of fission protein DLP1 in GD3 enriched microdomains in mitochondria undergoing fragmentation, as compared to control cells (Ciarlo et al. 2010). Similar results were seen for hFis1 and GD3 in MOM, emphasizing the fact that fission proteins may selectively be recruited to GD3 enriched domains (Ciarlo et al. 2010). Disruption of these rafts by fumonisin-B1 and PDMP led to reduced recruitment of fission proteins to microdomains, decreased mitochondrial fission, and inhibition of apoptosis (Ciarlo et al. 2010; Garofalo et al. 2007; Martinez-Abundis et al. 2009). Gangliosides such as GD3 are broken down by the action of sialidases (neuraminidases, Neu) that catalyze the removal of the sialic acid residues. Interestingly, neuraminidase 4 is localized to mitochondria (Bigi et al. 2010; Yamaguchi et al. 2005). Thus, a system exists to decrease ganglioside levels directly in mitochondria and potentially regulate mitochondrial morphology and apoptosis.
In addition to gangliosides, ceramides have been reported to alter mitochondrial morphology, which may play a role in their ability to induce MOMP. Ceramides may either directly or indirectly manipulate mitochondrial morphology by altering expression of mitochondrial fusion and fission proteins. C2-ceramide (a short-chain cell permeable ceramide analogue) treatment in neonatal rat cardiomyocytes led to increased mitochondrial fission proteins Drp-1 and Fis-1 in mitochondrial fractions, mitochondrial fragmentation and apoptosis (Parra et al. 2008). Recently, Smith et al. demonstrated that C2-ceramide increased expression of Drp-1 and induced ROS generation in C2C12 cells (Smith et al. 2013). Galectin-1 (Gal-1) induces fission via increased expression of Drp-1 and Fis-1; data indicate that Gal-1 regulates induction of mitochondrial fission requires generation of ceramide (Matarrese et al. 2005). Alternatively, data indicate that ceramide may also regulate mitochondrial fusion through influencing expression of mitochondrial fusion protein Mfn2 (Rojas-Charry et al. 2014). Lastly, sphingolipids such as ceramides are known to alter membrane curvature, which plays a role in fission and fusion events (Holopainen et al. 2000; Rogasevskaia and Coorssen 2006; Silva et al. 2012; Sonnino et al. 2007; Sot et al. 2005). Thus, sphingolipids alter mitochondrial morphology via direct biophysical effects on mitochondrial outer and inner membranes, in addition to direct regulation of known mitochondrial fission and fusion proteins.
Future directions/challenges
Apoptosis is a vital process in normal development and homeostasis. Manipulating or targeting apoptotic pathways is a cornerstone for prospective therapy of many diseases. Sphingolipids form an essential and integral part of cell system influencing apoptosis, potentially through induction of MOMP. There are still several unanswered questions regarding mechanisms of sphingolipids mediated mitochondrial apoptosis. The first and foremost question pertains to the formation of ceramide channels in vivo. While it is clear that ceramides do indeed form channels (Colombini 2010), there is lack of solid evidence to show ceramide channel formation in vivo, due to a lack of adequate analytical methods. In vitro work with isolated mitochondria and electrophysiological studies of ceramide channels in planar phospholipid membranes indicate that these channels have the required characteristics to facilitate MOMP (reviewed (Colombini 2010)). However, the immediate ceramide metabolites dihydroceramide and sphingosine as well as anti-apoptotic Bcl-2 proteins inhibit ceramide channels (Elrick et al. 2006; Ganesan and Colombini 2010). Thus, cells appear to have evolved a variety of mechanisms to ensure that channel formation by ceramides is prevented.
The mechanism of transport between the ER and mitochondria is unknown. Ceramide is transported from the ER to the Golgi and plasma membrane by vesicular transport and via transport proteins. It is not known if such a system exists for transport to mitochondria. In the context of mitochondrial ceramide levels, the location (ER or mitochondria) in which the specific ceramides are generated may not be as important as we think if a mechanism for rapid transport of ceramide between ER and mitochondria exists. Recently, a mitochondrial associated neutral sphingomyelinase (manSMase) was identified (Wu et al. 2010). In some cell types, this enzyme showed localization at the periphery of mitochondria (consistent with MAMs), whereas in other cell types it co-localized with mitochondria (Wu et al. 2010). A role for this enzyme in apoptosis is unknown and requires further study.
The presence of sphingolipid metabolism enzymes in the vicinity of mitochondria certainly hints at in situ generation of ceramide within mitochondria, and raises the question of regulation of these enzymes. It would be interesting to determine if enzymes like manSMase and nCDase are localized to specific mitochondrial subcompartments that may define their mechanism of apoptosis. This would be very interesting as elevated ceramide levels specifically in the MOM could lead to formation of pathways for release of intermembrane space proteins, whereas increases in ceramide in the MIM would result in reduction of mitochondrial inner membrane potential and generation of ROS. Data indicate that ceramides do not form channels in the MIM, nor do they from channels in the plasma membrane (Siskind et al. 2002). Determining the factors that influence the propensity for ceramide to form channels within specific membrane environments will be important for understanding the physiological significance of ceramide channel formation with regards to apoptosis. Thus, the submitochondrial location of ceramide generation/accumulation is an important determinant of the impact of ceramides on mitochondrial function.
Of particular importance for future studies is elucidation of the complex system of cross talk between sphingolipids and proteins within the Bcl-2 family. Bcl-2 and Bcl-xL involvement in ceramide-induced MOMP has been well deduced by several studies (Geley et al. 1997; Wiesner et al. 1997; Zhang et al. 1996). For instance, Chen et al. in 1995, showed that IR exposure in HL-60 and U-937 human leukemia cells suppresses Bcl-2 levels and induces ceramide mediated apoptosis (Chen et al. 1995). Siskind et al. demonstrated that Bcl-2 actually dissembles ceramide channels in isolated rat mitochondria and mitochondria isolated from yeast lacking Bcl-2 proteins (Siskind et al. 2008). Ganeshan et al. showed that ceramide and Bax synergistically lead to MOMP (Ganesan and Colombini 2010; Ganesan et al. 2010). It is possible that Bax-ceramide hybrid channels are capable of inducing MOMP at levels far below that required for either factor alone. Data suggests that the individual Bcl-2 family members may have distinct mechanisms in regulating ceramide-induced apoptosis. Recently, data suggests that members of the Bcl-2 family also regulate enzymes involved in ceramide generation during apoptosis. The Bcl-2 family protein Bcl-2L13 inhibits CerS activity and prevents ceramide-induced apoptosis (Jensen et al. 2014). In glioblastoma cells and other solid tumors, Bcl-2L13 is over-expressed and contributes to drug resistance. Suppressing Bcl-2L13 expression or developing drugs to interfere with its inhibition of CerS2 and CerS6 could help sensitize glioblastomas to conventional therapies. The pro-apoptotic Bcl-2 family member Bak activates CerS during apoptosis (Beverly et al. 2013; Siskind et al. 2010). Again, understanding the specific mechanism for this activation will facilitate the development of drugs that can interfere with or mimic the activation of CerS by Bak for regulation of apoptosis. Once elevated, ceramide may itself form channels in the MOM or interact with Bax to form synergistic channels. Data also indicate that downstream ceramide metabolites such as S1P and hexadecanal may be important for activation of Bak and Bax and induction of MOMP (Chipuk et al. 2012). These precise sphingolipid/ Bcl-2 protein interactions need further elucidation as they represent novel drug targets.
For cancer treatment, sphingolipid mediated mitochondrial apoptosis is a highly exploited area in in vitro and in vivo studies. Many chemotherapeutic agents have been shown to induce apoptosis through sphingolipids and in the case of drug resistance, altering levels of sphingolipids has resulted in encouraging outcomes. Several studies indicate that prevention of ceramide metabolism to either glucosylceramides or S1P via inhibition of glucosylceramide synthase or sphingosine kinase sensitizes cancer cells to standard of care therapeutics (for example (Casson et al. 2013)). Ceramide analogues are also being exploited for potential use in combinatorial therapies. For example, a cationic analogue of ceramide LCL124 that specifically accumulates within mitochondria induces apoptosis in gemcitabine and 5-FU resistant pancreatic cancer cells, whilst sparing normal cells (Beckham et al. 2013). Thus LCL124 has promise for treatment of rare and difficult cancers like pancreatic cancer with potentially minimal side effects (Beckham et al. 2013). Liu et al. developed C6-ceramide nanoliposomal formulations for specific delivery to NK-LGL leukemia cells resulting in the induction of disease remission in animal models (Liu et al. 2010). Thus, novel methods for delivery of ceramides or drugs that regulate ceramide metabolism specifically to tumor cells could prove to highly fruitful for the development of targeted therapies utilized alone or in combination with standard of care therapeutics.
Conclusion
Sphingolipids are metabolized in the vicinity of mitochondria and regulating mitochondrial structure and function. It is clear from the many studies cited within this review (and those that the authors inadvertently failed to cite) that deregulated sphingolipid metabolism contributes to mitochondrial dysfunction and disease. However, the exact molecular mechanisms by which specific sphingolipids alter mitochondrial biology are not fully understood. Determining the specific protein-lipid interactions that occur in vivo is challenging but is required for more adequately targeting this pathway for the development of novel therapeutics for treatment of a variety of diseases.
References
- Abrahan CE, Miranda GE, Agnolazza DL, Politi LE, Rotstein NP. Synthesis of sphingosine is essential for oxidative stress-induced apoptosis of photoreceptors. Investigative ophthalmology & visual science. 2010;51:1171–1180. doi: 10.1167/iovs.09-3909. doi:10.1167/iovs.09-3909. [DOI] [PubMed] [Google Scholar]
- Alphonse G, et al. p53-independent early and late apoptosis is mediated by ceramide after exposure of tumor cells to photon or carbon ion irradiation. BMC cancer. 2013;13:151. doi: 10.1186/1471-2407-13-151. doi:10.1186/1471-2407-13-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrieu-Abadie N, Gouaze V, Salvayre R, Levade T. Ceramide in apoptosis signaling: relationship with oxidative stress. Free radical biology & medicine. 2001;31:717–728. doi: 10.1016/s0891-5849(01)00655-4. [DOI] [PubMed] [Google Scholar]
- Annis MG, Zamzami N, Zhu W, Penn LZ, Kroemer G, Leber B, Andrews DW. Endoplasmic reticulum localized Bcl-2 prevents apoptosis when redistribution of cytochrome c is a late event. Oncogene. 2001;20:1939–1952. doi: 10.1038/sj.onc.1204288. [DOI] [PubMed] [Google Scholar]
- Antoon JW, et al. Targeting NFkB mediated breast cancer chemoresistance through selective inhibition of sphingosine kinase-2. Cancer biology & therapy. 2011;11:678–689. doi: 10.4161/cbt.11.7.14903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiychuk EB, Atanassoff AP, Monastyrskaya K, Brandenberger C, Studer D, Allemann C, Draeger A. The targeting of plasmalemmal ceramide to mitochondria during apoptosis. PloS one. 2011;6:e23706. doi: 10.1371/journal.pone.0023706. doi:10.1371/journal.pone.0023706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranowski M, Gorski J. Heart sphingolipids in health and disease. Advances in experimental medicine and biology. 2011;721:41–56. doi: 10.1007/978-1-4614-0650-1_3. doi:10.1007/978-1-4614-0650-1_3. [DOI] [PubMed] [Google Scholar]
- Beckham TH, et al. LCL124, a cationic analog of ceramide, selectively induces pancreatic cancer cell death by accumulating in mitochondria. The Journal of pharmacology and experimental therapeutics. 2013;344:167–178. doi: 10.1124/jpet.112.199216. doi:10.1124/jpet.112.199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaud-Rotureau MA, et al. Early transitory rise in intracellular pH leads to Bax conformation change during ceramide-induced apoptosis. Apoptosis. 2000;5:551–560. doi: 10.1023/a:1009693630664. [DOI] [PubMed] [Google Scholar]
- Bettaieb A, Plo I, Mansat-De Mas V, Quillet-Mary A, Levade T, Laurent G, Jaffrezou JP. Daunorubicin- and mitoxantrone-triggered phosphatidylcholine hydrolysis: implication in drug-induced ceramide generation and apoptosis. Molecular pharmacology. 1999;55:118–125. doi: 10.1124/mol.55.1.118. [DOI] [PubMed] [Google Scholar]
- Beverly LJ, Howell LA, Hernandez-Corbacho M, Casson L, Chipuk JE, Siskind LJ. BAK activation is necessary and sufficient to drive ceramide synthase-dependent ceramide accumulation following inhibition of BCL2-like proteins. The Biochemical journal. 2013;452:111–119. doi: 10.1042/BJ20130147. doi:10.1042/BJ20130147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigi A, et al. Human sialidase NEU4 long and short are extrinsic proteins bound to outer mitochondrial membrane and the endoplasmic reticulum, respectively. Glycobiology. 2010;20:148–157. doi: 10.1093/glycob/cwp156. doi:10.1093/glycob/cwp156. [DOI] [PubMed] [Google Scholar]
- Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D. Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? The Biochemical journal. 2004;382:527–533. doi: 10.1042/BJ20031819. doi:10.1042/BJ20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbes H, El Bawab S, Hannun YA, Obeid LM. Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15:2669–2679. doi: 10.1096/fj.01-0539com. [DOI] [PubMed] [Google Scholar]
- Birbes H, El Bawab S, Obeid LM, Hannun YA. Mitochondria and ceramide: intertwined roles in regulation of apoptosis. Advances in enzyme regulation. 2002;42:113–129. doi: 10.1016/s0065-2571(01)00026-7. [DOI] [PubMed] [Google Scholar]
- Birbes H, Luberto C, Hsu YT, El Bawab S, Hannun YA, Obeid LM. A mitochondrial pool of sphingomyelin is involved in TNFalpha-induced Bax translocation to mitochondria. The Biochemical journal. 2005;386:445–451. doi: 10.1042/BJ20041627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschko H. The first Thudichum lecture, 15 January 1974: Biochemical specificity in neuromal function. Society transactions. 1975;3:27–37. doi: 10.1042/bst0030027. [DOI] [PubMed] [Google Scholar]
- Bollinger CR, Teichgraber V, Gulbins E. Ceramide-enriched membrane domains. Biochim Biophys Acta. 2005;1746:284–294. doi: 10.1016/j.bbamcr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z, Kolesnick R. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 1995;82:405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- Caricchio R, D’Adamio L, Cohen PL. Fas, ceramide and serum withdrawal induce apoptosis via a common pathway in a type II Jurkat cell line. Cell death and differentiation. 2002;9:574–580. doi: 10.1038/sj.cdd.4400996. doi:10.1038/sj/cdd/4400996. [DOI] [PubMed] [Google Scholar]
- Cartron PF, Juin P, Oliver L, Martin S, Meflah K, Vallette FM. Nonredundant role of Bax and Bak in Bid-mediated apoptosis. Molecular and cellular biology. 2003;23:4701–4712. doi: 10.1128/MCB.23.13.4701-4712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casson L, et al. Inhibition of ceramide metabolism sensitizes human leukemia cells to inhibition of BCL2-like proteins. PloS one. 2013;8:e54525. doi: 10.1371/journal.pone.0054525. doi:10.1371/journal.pone.0054525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles AG, Han TY, Liu YY, Hansen N, Giuliano AE, Cabot MC. Taxol-induced ceramide generation and apoptosis in human breast cancer cells. Cancer chemotherapy and pharmacology. 2001;47:444–450. doi: 10.1007/s002800000265. [DOI] [PubMed] [Google Scholar]
- Charruyer A, Jean C, Colomba A, Jaffrezou JP, Quillet-Mary A, Laurent G, Bezombes C. PKCzeta protects against UV-C-induced apoptosis by inhibiting acid sphingomyelinase-dependent ceramide production. The Biochemical journal. 2007;405:77–83. doi: 10.1042/BJ20061528. doi:10.1042/BJ20061528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Wu S. Cell line dependent involvement of ceramide in ultraviolet light-induced apoptosis. Molecular and cellular biochemistry. 2001;219:21–27. doi: 10.1023/a:1011083818452. [DOI] [PubMed] [Google Scholar]
- Chen CL, Lin CF, Chiang CW, Jan MS, Lin YS. Lithium inhibits ceramide- and etoposide-induced protein phosphatase 2A methylation, Bcl-2 dephosphorylation, caspase-2 activation, and apoptosis. Molecular pharmacology. 2006;70:510–517. doi: 10.1124/mol.106.024059. doi:10.1124/mol.106.024059. [DOI] [PubMed] [Google Scholar]
- Chen JS, Chai MQ, Chen HH, Zhao S, Song JG. Regulation of phospholipase D activity and ceramide production in daunorubicin-induced apoptosis in A-431 cells. Biochim Biophys Acta. 2000;1488:219–232. doi: 10.1016/s1388-1981(00)00125-6. [DOI] [PubMed] [Google Scholar]
- Chen M, Quintans J, Fuks Z, Thompson C, Kufe DW, Weichselbaum RR. Suppression of Bcl-2 messenger RNA production may mediate apoptosis after ionizing radiation, tumor necrosis factor alpha, and ceramide. Cancer research. 1995;55:991–994. [PubMed] [Google Scholar]
- Chipuk JE, Bouchier-Hayes L, Green DR. Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell death and differentiation. 2006;13:1396–1402. doi: 10.1038/sj.cdd.4401963. doi:10.1038/sj.cdd.4401963. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Green DR. Cytoplasmic p53: bax and forward. Cell Cycle. 2004;3:429–431. [PubMed] [Google Scholar]
- Chipuk JE, Green DR. Dissecting p53-dependent apoptosis. Cell death and differentiation. 2006;13:994–1002. doi: 10.1038/sj.cdd.4401908. doi:10.1038/sj.cdd.4401908. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends in cell biology. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. doi:10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. doi:10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Maurer U, Green DR, Schuler M. Pharmacologic activation of p53 elicits Bax-dependent apoptosis in the absence of transcription. Cancer cell. 2003;4:371–381. doi: 10.1016/s1535-6108(03)00272-1. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, et al. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell. 2012;148:988–1000. doi: 10.1016/j.cell.2012.01.038. doi:10.1016/j.cell.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Molecular cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. doi:10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmura SJ, Nodzenski E, Beckett MA, Kufe DW, Quintans J, Weichselbaum RR. Loss of ceramide production confers resistance to radiation-induced apoptosis. Cancer research. 1997;57:1270–1275. [PubMed] [Google Scholar]
- Ciarlo L, et al. Association of fission proteins with mitochondrial raft-like domains. Cell death and differentiation. 2010;17:1047–1058. doi: 10.1038/cdd.2009.208. doi:10.1038/cdd.2009.208. [DOI] [PubMed] [Google Scholar]
- Colombini M. Ceramide channels and their role in mitochondria-mediated apoptosis. Biochim Biophys Acta. 2010;1797:1239–1244. doi: 10.1016/j.bbabio.2010.01.021. doi:10.1016/j.bbabio.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Colombini M. Membrane channels formed by ceramide. Handbook of experimental pharmacology. 2013:109–126. doi: 10.1007/978-3-7091-1368-4_6. doi:10.1007/978-3-7091-1368-4_6. [DOI] [PubMed] [Google Scholar]
- Come MG, Bettaieb A, Skladanowski A, Larsen AK, Laurent G. Alteration of the daunorubicin-triggered sphingomyelin-ceramide pathway and apoptosis in MDR cells: influence of drug transport abnormalities. International journal of cancer Journal international du cancer. 1999;81:580–587. doi: 10.1002/(sici)1097-0215(19990517)81:4<580::aid-ijc13>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Csordas G, et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. The Journal of cell biology. 2006;174:915–921. doi: 10.1083/jcb.200604016. doi:10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Zhang H, Kloosterboer F, Liao Y, Klostergaard J, Levitt ML, Hung MC. Ceramide does not act as a general second messenger for ultraviolet-induced apoptosis. Oncogene. 2002;21:44–52. doi: 10.1038/sj.onc.1204900. doi:10.1038/sj.onc.1204900. [DOI] [PubMed] [Google Scholar]
- Deng X, et al. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science. 2008;322:110–115. doi: 10.1126/science.1158111. doi:10.1126/science.1158111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don AS, Rosen H. A lipid binding domain in sphingosine kinase 2. Biochemical and biophysical research communications. 2009;380:87–92. doi: 10.1016/j.bbrc.2009.01.075. doi:10.1016/j.bbrc.2009.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel P, et al. Ceramide synthase 4 deficiency in mice causes lipid alterations in sebum and results in alopecia. The Biochemical journal. 2014;461:147–158. doi: 10.1042/BJ20131242. doi:10.1042/BJ20131242. [DOI] [PubMed] [Google Scholar]
- Ebel P, et al. Inactivation of ceramide synthase 6 in mice results in an altered sphingolipid metabolism and behavioral abnormalities. The Journal of biological chemistry. 2013;288:21433–21447. doi: 10.1074/jbc.M113.479907. doi:10.1074/jbc.M113.479907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bawab S, Birbes H, Roddy P, Szulc ZM, Bielawska A, Hannun YA. Biochemical characterization of the reverse activity of rat brain ceramidase. A CoA-independent and fumonisin B1-insensitive ceramide synthase. The Journal of biological chemistry. 2001;276:16758–16766. doi: 10.1074/jbc.M009331200. doi:10.1074/jbc.M009331200. [DOI] [PubMed] [Google Scholar]
- El Bawab S, Roddy P, Qian T, Bielawska A, Lemasters JJ, Hannun YA. Molecular cloning and characterization of a human mitochondrial ceramidase. The Journal of biological chemistry. 2000;275:21508–21513. doi: 10.1074/jbc.M002522200. doi:10.1074/jbc.M002522200. [DOI] [PubMed] [Google Scholar]
- Elrick MJ, Fluss S, Colombini M. Sphingosine, a product of ceramide hydrolysis, influences the formation of ceramide channels. Biophysical journal. 2006;91:1749–1756. doi: 10.1529/biophysj.106.088443. doi:10.1529/biophysj.106.088443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlinz K, et al. Human acid ceramidase: processing, glycosylation, and lysosomal targeting. The Journal of biological chemistry. 2001;276:35352–35360. doi: 10.1074/jbc.M103066200. doi:10.1074/jbc.M103066200. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ayala DJ, et al. Coenzyme Q protects cells against serum withdrawal-induced apoptosis by inhibition of ceramide release and caspase-3 activation. Antioxidants & redox signaling. 2000;2:263–275. doi: 10.1089/ars.2000.2.2-263. [DOI] [PubMed] [Google Scholar]
- Follis AV, et al. PUMA binding induces partial unfolding within BCL-xL to disrupt p53 binding and promote apoptosis. Nature chemical biology. 2013;9:163–168. doi: 10.1038/nchembio.1166. doi:10.1038/nchembio.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliostro V, et al. Dihydroceramide delays cell cycle G1/S transition via activation of ER stress and induction of autophagy. The international journal of biochemistry & cell biology. 2012;44:2135–2143. doi: 10.1016/j.biocel.2012.08.025. doi:10.1016/j.biocel.2012.08.025. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nature reviews. 2012;13:780–788. doi: 10.1038/nrm3479. doi:10.1038/nrm3479. [DOI] [PubMed] [Google Scholar]
- Gandy KA, Obeid LM. Regulation of the sphingosine kinase/sphingosine 1-phosphate pathway. Handbook of experimental pharmacology. 2013:275–303. doi: 10.1007/978-3-7091-1511-4_14. doi:10.1007/978-3-7091-1511-4_14. [DOI] [PubMed] [Google Scholar]
- Ganesan V, Colombini M. Regulation of ceramide channels by Bcl-2 family proteins. FEBS letters. 2010;584:2128–2134. doi: 10.1016/j.febslet.2010.02.032. doi:10.1016/j.febslet.2010.02.032. [DOI] [PubMed] [Google Scholar]
- Ganesan V, Perera MN, Colombini D, Datskovskiy D, Chadha K, Colombini M. Ceramide and activated Bax act synergistically to permeabilize the mitochondrial outer membrane. Apoptosis. 2010;15:553–562. doi: 10.1007/s10495-009-0449-0. doi:10.1007/s10495-009-0449-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. The Journal of biological chemistry. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- Garcia-Ruiz C, Colell A, Morales A, Calvo M, Enrich C, Fernandez-Checa JC. Trafficking of ganglioside GD3 to mitochondria by tumor necrosis factor-alpha. The Journal of biological chemistry. 2002;277:36443–36448. doi: 10.1074/jbc.M206021200. doi:10.1074/jbc.M206021200. [DOI] [PubMed] [Google Scholar]
- Garofalo T, et al. Do mitochondria act as “cargo boats” in the journey of GD3 to the nucleus during apoptosis? FEBS letters. 2007;581:3899–3903. doi: 10.1016/j.febslet.2007.07.020. doi:10.1016/j.febslet.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Geilen CC, Bektas M, Wieder T, Kodelja V, Goerdt S, Orfanos CE. 1alpha,25-dihydroxyvitamin D3 induces sphingomyelin hydrolysis in HaCaT cells via tumor necrosis factor alpha. The Journal of biological chemistry. 1997;272:8997–9001. doi: 10.1074/jbc.272.14.8997. [DOI] [PubMed] [Google Scholar]
- Geley S, Hartmann BL, Kofler R. Ceramides induce a form of apoptosis in human acute lymphoblastic leukemia cells that is inhibited by Bcl-2, but not by CrmA. FEBS letters. 1997;400:15–18. doi: 10.1016/s0014-5793(96)01284-7. [DOI] [PubMed] [Google Scholar]
- Gentil B, Grimot F, Riva C. Commitment to apoptosis by ceramides depends on mitochondrial respiratory function, cytochrome c release and caspase-3 activation in Hep-G2 cells. Molecular and cellular biochemistry. 2003;254:203–210. doi: 10.1023/a:1027359832177. [DOI] [PubMed] [Google Scholar]
- Ginkel C, et al. Ablation of neuronal ceramide synthase 1 in mice decreases ganglioside levels and expression of myelin-associated glycoprotein in oligodendrocytes. The Journal of biological chemistry. 2012;287:41888–41902. doi: 10.1074/jbc.M112.413500. doi:10.1074/jbc.M112.413500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorria M, Huc L, Sergent O, Rebillard A, Gaboriau F, Dimanche-Boitrel MT, Lagadic-Gossmann D. Protective effect of monosialoganglioside GM1 against chemically induced apoptosis through targeting of mitochondrial function and iron transport. Biochemical pharmacology. 2006;72:1343–1353. doi: 10.1016/j.bcp.2006.07.014. doi:10.1016/j.bcp.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Grassme H, Cremesti A, Kolesnick R, Gulbins E. Ceramide-mediated clustering is required for CD95-DISC formation. Oncogene. 2003;22:5457–5470. doi: 10.1038/sj.onc.1206540. doi:10.1038/sj.onc.1206540. [DOI] [PubMed] [Google Scholar]
- Grassme H, et al. CD95 signaling via ceramide-rich membrane rafts. The Journal of biological chemistry. 2001a;276:20589–20596. doi: 10.1074/jbc.M101207200. doi:10.1074/jbc.M101207200. [DOI] [PubMed] [Google Scholar]
- Grassme H, Schwarz H, Gulbins E. Molecular mechanisms of ceramide-mediated CD95 clustering. Biochemical and biophysical research communications. 2001b;284:1016–1030. doi: 10.1006/bbrc.2001.5045. doi:10.1006/bbrc.2001.5045 S0006-291X(01)95045-4 [pii] [DOI] [PubMed] [Google Scholar]
- Gudz TI, Tserng KY, Hoppel CL. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. The Journal of biological chemistry. 1997;272:24154–24158. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. doi: 10.1038/sj.onc.1207146. doi:10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- Haimovitz-Friedman A, Kan CC, Ehleiter D, Persaud RS, McLoughlin M, Fuks Z, Kolesnick RN. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. The Journal of experimental medicine. 1994;180:525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Bellamy A, Milstien S, Kordula T, Spiegel S. Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. The Journal of biological chemistry. 2007;282:12058–12065. doi: 10.1074/jbc.M609559200. doi:10.1074/jbc.M609559200. [DOI] [PubMed] [Google Scholar]
- Hanada K. Discovery of the molecular machinery CERT for endoplasmic reticulum-to-Golgi trafficking of ceramide. Molecular and cellular biochemistry. 2006;286:23–31. doi: 10.1007/s11010-005-9044-z. doi:10.1007/s11010-005-9044-z. [DOI] [PubMed] [Google Scholar]
- Hanada K, Kumagai K, Tomishige N, Kawano M. CERT and intracellular trafficking of ceramide. Biochim Biophys Acta. 2007;1771:644–653. doi: 10.1016/j.bbalip.2007.01.009. doi:10.1016/j.bbalip.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nature reviews. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Hawthorne JN. A note on the life of J.L.W. Thudichum (1829-1901) Biochemical Society transactions. 1975;3:591. doi: 10.1042/bst0030591. [DOI] [PubMed] [Google Scholar]
- Hearps AC, Burrows J, Connor CE, Woods GM, Lowenthal RM, Ragg SJ. Mitochondrial cytochrome c release precedes transmembrane depolarisation and caspase-3 activation during ceramide-induced apoptosis of Jurkat T cells. Apoptosis. 2002;7:387–394. doi: 10.1023/a:1020034906200. [DOI] [PubMed] [Google Scholar]
- Heffernan-Stroud LA, Obeid LM. Sphingosine kinase 1 in cancer. Advances in cancer research. 2013;117:201–235. doi: 10.1016/B978-0-12-394274-6.00007-8. doi:10.1016/B978-0-12-394274-6.00007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich M, et al. Ceramide as an activator lipid of cathepsin D. Advances in experimental medicine and biology. 2000;477:305–315. doi: 10.1007/0-306-46826-3_33. [DOI] [PubMed] [Google Scholar]
- Holopainen JM, Angelova MI, Kinnunen PK. Vectorial budding of vesicles by asymmetrical enzymatic formation of ceramide in giant liposomes. Biophysical journal. 2000;78:830–838. doi: 10.1016/S0006-3495(00)76640-9. doi:10.1016/S0006-3495(00)76640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang YH, Tani M, Nakagawa T, Okino N, Ito M. Subcellular localization of human neutral ceramidase expressed in HEK293 cells. Biochemical and biophysical research communications. 2005;331:37–42. doi: 10.1016/j.bbrc.2005.03.134. doi:10.1016/j.bbrc.2005.03.134. [DOI] [PubMed] [Google Scholar]
- Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura S. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. The Journal of biological chemistry. 2003;278:46832–46839. doi: 10.1074/jbc.M306577200. doi:10.1074/jbc.M306577200. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Kihara A, Igarashi Y. Sphingosine-1-phosphate lyase SPL is an endoplasmic reticulum-resident, integral membrane protein with the pyridoxal 5′-phosphate binding domain exposed to the cytosol. Biochemical and biophysical research communications. 2004;325:338–343. doi: 10.1016/j.bbrc.2004.10.036. doi:10.1016/j.bbrc.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Imgrund S, et al. Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. The Journal of biological chemistry. 2009;284:33549–33560. doi: 10.1074/jbc.M109.031971. doi:M109.031971 [pii] 10.1074/jbc.M109.031971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Okino N, Tani M. New insight into the structure, reaction mechanism, and biological functions of neutral ceramidase. Biochim Biophys Acta. 2014;1841:682–691. doi: 10.1016/j.bbalip.2013.09.008. doi:10.1016/j.bbalip.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Iwasawa R, Mahul-Mellier AL, Datler C, Pazarentzos E, Grimm S. Fis1 and Bap31 bridge the mitochondria-ER interface to establish a platform for apoptosis induction. The EMBO journal. 2011;30:556–568. doi: 10.1038/emboj.2010.346. doi:10.1038/emboj.2010.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffrezou JP, et al. Daunorubicin-induced apoptosis: triggering of ceramide generation through sphingomyelin hydrolysis. The EMBO journal. 1996;15:2417–2424. [PMC free article] [PubMed] [Google Scholar]
- Jenkins RW, Canals D, Hannun YA. Roles and regulation of secretory and lysosomal acid sphingomyelinase. Cell Signal. 2009;21:836–846. doi: 10.1016/j.cellsig.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennemann R, et al. Loss of ceramide synthase 3 causes lethal skin barrier disruption. Human molecular genetics. 2012;21:586–608. doi: 10.1093/hmg/ddr494. doi:10.1093/hmg/ddr494. [DOI] [PubMed] [Google Scholar]
- Jensen SA, et al. Bcl2L13 is a ceramide synthase inhibitor in glioblastoma. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:5682–5687. doi: 10.1073/pnas.1316700111. doi:10.1073/pnas.1316700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, et al. Ceramide generated by sphingomyelin hydrolysis and the salvage pathway is involved in hypoxia/reoxygenation-induced Bax redistribution to mitochondria in NT-2 cells. The Journal of biological chemistry. 2008;283:26509–26517. doi: 10.1074/jbc.M801597200. doi:10.1074/jbc.M801597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasavvas N, Erukulla RK, Bittman R, Lockshin R, Hockenbery D, Zakeri Z. BCL-2 suppresses ceramide-induced cell killing. Cell death and differentiation. 1996;3:149–151. [PubMed] [Google Scholar]
- Kawano M, Kumagai K, Nishijima M, Hanada K. Efficient trafficking of ceramide from the endoplasmic reticulum to the Golgi apparatus requires a VAMP-associated protein-interacting FFAT motif of CERT. The Journal of biological chemistry. 2006;281:30279–30288. doi: 10.1074/jbc.M605032200. doi:10.1074/jbc.M605032200. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Mun JY, Chun YJ, Choi KH, Kim MY. Bax-dependent apoptosis induced by ceramide in HL-60 cells. FEBS letters. 2001;505:264–268. doi: 10.1016/s0014-5793(01)02836-8. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Oh JE, Kim SW, Chun YJ, Kim MY. Ceramide induces p38 MAPK-dependent apoptosis and Bax translocation via inhibition of Akt in HL-60 cells. Cancer letters. 2008;260:88–95. doi: 10.1016/j.canlet.2007.10.030. doi:10.1016/j.canlet.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Kogot-Levin A, Saada A. Ceramide and the mitochondrial respiratory chain. Biochimie. 2014;100:88–94. doi: 10.1016/j.biochi.2013.07.027. doi:10.1016/j.biochi.2013.07.027. [DOI] [PubMed] [Google Scholar]
- Kolter T. A view on sphingolipids and disease. Chemistry and physics of lipids. 2011;164:590–606. doi: 10.1016/j.chemphyslip.2011.04.013. doi:10.1016/j.chemphyslip.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Kroesen BJ, Pettus B, Luberto C, Busman M, Sietsma H, de Leij L, Hannun YA. Induction of apoptosis through B-cell receptor cross-linking occurs via de novo generated C16-ceramide and involves mitochondria. The Journal of biological chemistry. 2001;276:13606–13614. doi: 10.1074/jbc.M009517200. [DOI] [PubMed] [Google Scholar]
- Kucuksayan E, Konuk EK, Demir N, Mutus B, Aslan M. Neutral sphingomyelinase inhibition decreases ER stress-mediated apoptosis and inducible nitric oxide synthase in retinal pigment epithelial cells. Free radical biology & medicine. 2014;72:113–123. doi: 10.1016/j.freeradbiomed.2014.04.013. doi:10.1016/j.freeradbiomed.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Kujjo LL, et al. Ceramide and its transport protein (CERT) contribute to deterioration of mitochondrial structure and function in aging oocytes. Mechanisms of ageing and development. 2013;134:43–52. doi: 10.1016/j.mad.2012.12.001. doi:10.1016/j.mad.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Laviad EL, Kelly S, Merrill AH, Jr., Futerman AH. Modulation of ceramide synthase activity via dimerization. The Journal of biological chemistry. 2012;287:21025–21033. doi: 10.1074/jbc.M112.363580. doi:10.1074/jbc.M112.363580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, et al. Mitochondrial ceramide-rich macrodomains functionalize Bax upon irradiation. PloS one. 2011;6:e19783. doi: 10.1371/journal.pone.0019783. doi:10.1371/journal.pone.0019783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Liu Y, Ladisch S. Enhancement of epidermal growth factor signaling and activation of SRC kinase by gangliosides. The Journal of biological chemistry. 2001;276:42782–42792. doi: 10.1074/jbc.M101481200. doi:10.1074/jbc.M101481200. [DOI] [PubMed] [Google Scholar]
- Lima S, Spiegel S. Sphingosine kinase: a closer look at last. Structure. 2013;21:690–692. doi: 10.1016/j.str.2013.04.006. doi:10.1016/j.str.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CF, et al. Bcl-2 rescues ceramide- and etoposide-induced mitochondrial apoptosis through blockage of caspase-2 activation. The Journal of biological chemistry. 2005;280:23758–23765. doi: 10.1074/jbc.M412292200. doi:10.1074/jbc.M412292200. [DOI] [PubMed] [Google Scholar]
- Lin CF, et al. Sequential caspase-2 and caspase-8 activation upstream of mitochondria during ceramideand etoposide-induced apoptosis. The Journal of biological chemistry. 2004;279:40755–40761. doi: 10.1074/jbc.M404726200. [DOI] [PubMed] [Google Scholar]
- Lindsten T, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Molecular cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, et al. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. The Journal of biological chemistry. 2000;275:19513–19520. doi: 10.1074/jbc.M002759200. doi:10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- Liu H, et al. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. The Journal of biological chemistry. 2003;278:40330–40336. doi: 10.1074/jbc.M304455200. doi:10.1074/jbc.M304455200. [DOI] [PubMed] [Google Scholar]
- Liu X, et al. Targeting of survivin by nanoliposomal ceramide induces complete remission in a rat model of NK-LGL leukemia. Blood. 2010;116:4192–4201. doi: 10.1182/blood-2010-02-271080. doi:10.1182/blood-2010-02-271080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li R, Ladisch S. Exogenous ganglioside GD1a enhances epidermal growth factor receptor binding and dimerization. The Journal of biological chemistry. 2004;279:36481–36489. doi: 10.1074/jbc.M402880200. doi:10.1074/jbc.M402880200. [DOI] [PubMed] [Google Scholar]
- Liu YY, et al. A role for ceramide in driving cancer cell resistance to doxorubicin. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:2541–2551. doi: 10.1096/fj.07-092981. doi:10.1096/fj.07-092981. [DOI] [PubMed] [Google Scholar]
- Lu FG, Wong CS. Radiation-induced apoptosis of oligodendrocytes and its association with increased ceramide and down-regulated protein kinase B/Akt activity. International journal of radiation biology. 2004;80:39–51. doi: 10.1080/09553000310001642876. doi:10.1080/09553000310001642876. [DOI] [PubMed] [Google Scholar]
- Lucke T, Hoppner W, Schmidt E, Illsinger S, Das AM. Fabry disease: reduced activities of respiratory chain enzymes with decreased levels of energy-rich phosphates in fibroblasts. Molecular genetics and metabolism. 2004;82:93–97. doi: 10.1016/j.ymgme.2004.01.011. doi:10.1016/j.ymgme.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Malina HZ, Hess OM. Xanthurenic acid translocates proapoptotic Bcl-2 family proteins into mitochondria and impairs mitochondrial function. BMC cell biology. 2004;5:14. doi: 10.1186/1471-2121-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansat V, Bettaieb A, Levade T, Laurent G, Jaffrezou JP. Serine protease inhibitors block neutral sphingomyelinase activation, ceramide generation, and apoptosis triggered by daunorubicin. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1997;11:695–702. doi: 10.1096/fasebj.11.8.9240970. [DOI] [PubMed] [Google Scholar]
- Mao C, Xu R, Szulc ZM, Bielawska A, Galadari SH, Obeid LM. Cloning and characterization of a novel human alkaline ceramidase. A mammalian enzyme that hydrolyzes phytoceramide. The Journal of biological chemistry. 2001;276:26577–26588. doi: 10.1074/jbc.M102818200. doi:10.1074/jbc.M102818200. [DOI] [PubMed] [Google Scholar]
- Mao C, et al. Cloning and characterization of a mouse endoplasmic reticulum alkaline ceramidase: an enzyme that preferentially regulates metabolism of very long chain ceramides. The Journal of biological chemistry. 2003;278:31184–31191. doi: 10.1074/jbc.M303875200. doi:10.1074/jbc.M303875200. [DOI] [PubMed] [Google Scholar]
- Martinez-Abundis E, Correa F, Pavon N, Zazueta C. Bax distribution into mitochondrial detergent-resistant microdomains is related to ceramide and cholesterol content in postischemic hearts. The FEBS journal. 2009;276:5579–5588. doi: 10.1111/j.1742-4658.2009.07239.x. doi:10.1111/j.1742-4658.2009.07239.x. [DOI] [PubMed] [Google Scholar]
- Matarrese P, et al. Galectin-1 sensitizes resting human T lymphocytes to Fas (CD95)-mediated cell death via mitochondrial hyperpolarization, budding, and fission. The Journal of biological chemistry. 2005;280:6969–6985. doi: 10.1074/jbc.M409752200. doi:10.1074/jbc.M409752200. [DOI] [PubMed] [Google Scholar]
- McIlwain H. The Second Thudichum Lecture. Cerebral isolates and neurochemical discovery. Biochemical Society transactions. 1975;3:579–590. doi: 10.1042/bst0030579. [DOI] [PubMed] [Google Scholar]
- Mesicek J, et al. Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell Signal. 2010;22:1300–1307. doi: 10.1016/j.cellsig.2010.04.006. doi:10.1016/j.cellsig.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J, Mesika A, Sivaguru M, Van Veldhoven PP, Alexander H, Futerman AH, Alexander S. (Dihydro)ceramide synthase 1 regulated sensitivity to cisplatin is associated with the activation of p38 mitogen-activated protein kinase and is abrogated by sphingosine kinase 1. Molecular cancer research : MCR. 2007;5:801–812. doi: 10.1158/1541-7786.MCR-07-0100. doi:10.1158/1541-7786.MCR-07-0100. [DOI] [PubMed] [Google Scholar]
- Moeller BJ, Pasqualini R, Arap W. Ceramide-mediated apoptosis following ionizing radiation in human prostate cancer cells: PKCalpha joins the fray. Cancer biology & therapy. 2009;8:64–65. doi: 10.4161/cbt.8.1.7447. [DOI] [PubMed] [Google Scholar]
- Mullen TD, Hannun YA, Obeid LM. Ceramide synthases at the centre of sphingolipid metabolism and biology. The Biochemical journal. 2012;441:789–802. doi: 10.1042/BJ20111626. doi:10.1042/BJ20111626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen TD, Spassieva S, Jenkins RW, Kitatani K, Bielawski J, Hannun YA, Obeid LM. Selective knockdown of ceramide synthases reveals complex interregulation of sphingolipid metabolism. Journal of lipid research. 2011;52:68–77. doi: 10.1194/jlr.M009142. doi:10.1194/jlr.M009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda S, Yoshimura S, Sawada M, Naganawa T, Iwama T, Nakashima S, Sakai N. Role of ceramide during cisplatin-induced apoptosis in C6 glioma cells. Journal of neuro-oncology. 2001;52:11–21. doi: 10.1023/a:1010624823158. [DOI] [PubMed] [Google Scholar]
- Nomura M, Shimizu S, Ito T, Narita M, Matsuda H, Tsujimoto Y. Apoptotic cytosol facilitates Bax translocation to mitochondria that involves cytosolic factor regulated by Bcl-2. Cancer research. 1999;59:5542–5548. [PubMed] [Google Scholar]
- Novgorodov SA, Gudz TI. Ceramide and mitochondria in ischemia/reperfusion. Journal of cardiovascular pharmacology. 2009;53:198–208. doi: 10.1097/FJC.0b013e31819b52d5. doi:10.1097/FJC.0b013e31819b52d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novgorodov SA, Gudz TI. Ceramide and mitochondria in ischemic brain injury. International journal of biochemistry and molecular biology. 2011;2:347–361. [PMC free article] [PubMed] [Google Scholar]
- Novgorodov SA, et al. Essential roles of neutral ceramidase and sphingosine in mitochondrial dysfunction due to traumatic brain injury. The Journal of biological chemistry. 2014;289:13142–13154. doi: 10.1074/jbc.M113.530311. doi:10.1074/jbc.M113.530311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novgorodov SA, Wu BX, Gudz TI, Bielawski J, Ovchinnikova TV, Hannun YA, Obeid LM. Novel pathway of ceramide production in mitochondria: thioesterase and neutral ceramidase produce ceramide from sphingosine and acyl-CoA. The Journal of biological chemistry. 2011;286:25352–25362. doi: 10.1074/jbc.M110.214866. doi:10.1074/jbc.M110.214866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Pacher P, Hajnoczky G. Propagation of the apoptotic signal by mitochondrial waves. The EMBO journal. 2001;20:4107–4121. doi: 10.1093/emboj/20.15.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Schuchman EH. Acid ceramidase and human disease. Biochim Biophys Acta. 2006;1758:2133–2138. doi: 10.1016/j.bbamem.2006.08.019. doi:10.1016/j.bbamem.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Park JW, Park WJ, Futerman AH. Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim Biophys Acta. 2014;1841:671–681. doi: 10.1016/j.bbalip.2013.08.019. doi:10.1016/j.bbalip.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Park JW, Park WJ, Kuperman Y, Boura-Halfon S, Pewzner-Jung Y, Futerman AH. Ablation of very long acyl chain sphingolipids causes hepatic insulin resistance in mice due to altered detergent-resistant membranes. Hepatology. 2013a;57:525–532. doi: 10.1002/hep.26015. doi:10.1002/hep.26015. [DOI] [PubMed] [Google Scholar]
- Park JY, Kim MJ, Kim YK, Woo JS. Ceramide induces apoptosis via caspase-dependent and caspase-independent pathways in mesenchymal stem cells derived from human adipose tissue. Archives of toxicology. 2011;85:1057–1065. doi: 10.1007/s00204-011-0645-x. doi:10.1007/s00204-011-0645-x. [DOI] [PubMed] [Google Scholar]
- Park WJ, et al. Protection of a ceramide synthase 2 null mouse from drug-induced liver injury: role of gap junction dysfunction and connexin 32 mislocalization. The Journal of biological chemistry. 2013b;288:30904–30916. doi: 10.1074/jbc.M112.448852. doi:10.1074/jbc.M112.448852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra V, et al. Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovascular research. 2008;77:387–397. doi: 10.1093/cvr/cvm029. [DOI] [PubMed] [Google Scholar]
- Patwardhan GA, Liu YY. Sphingolipids and expression regulation of genes in cancer. Progress in lipid research. 2011;50:104–114. doi: 10.1016/j.plipres.2010.10.003. doi:10.1016/j.plipres.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera MN, Lin SH, Peterson YK, Bielawska A, Szulc ZM, Bittman R, Colombini M. Bax and Bcl-xL exert their regulation on different sites of the ceramide channel. The Biochemical journal. 2012;445:81–91. doi: 10.1042/BJ20112103. doi:10.1042/BJ20112103. [DOI] [PubMed] [Google Scholar]
- Perfettini JL, Kroemer RT, Kroemer G. Fatal liaisons of p53 with Bax and Bak. Nature cell biology. 2004;6:386–388. doi: 10.1038/ncb0504-386. [DOI] [PubMed] [Google Scholar]
- Perry DK, Carton J, Shah AK, Meredith F, Uhlinger DJ, Hannun YA. Serine palmitoyltransferase regulates de novo ceramide generation during etoposide-induced apoptosis. The Journal of biological chemistry. 2000;275:9078–9084. doi: 10.1074/jbc.275.12.9078. [DOI] [PubMed] [Google Scholar]
- Petrache I, et al. Ceramide synthases expression and role of ceramide synthase-2 in the lung: insight from human lung cells and mouse models. PloS one. 2013;8:e62968. doi: 10.1371/journal.pone.0062968. doi:10.1371/journal.pone.0062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pewzner-Jung Y, et al. A critical role for ceramide synthase 2 in liver homeostasis: II. insights into molecular changes leading to hepatopathy. The Journal of biological chemistry. 285:10911–10923. doi: 10.1074/jbc.M109.077610. doi:M109.077610 [pii] 10.1074/jbc.M109.077610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pewzner-Jung Y, et al. A critical role for ceramide synthase 2 in liver homeostasis: II. insights into molecular changes leading to hepatopathy. The Journal of biological chemistry. 2010a;285:10911–10923. doi: 10.1074/jbc.M109.077610. doi:10.1074/jbc.M109.077610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pewzner-Jung Y, et al. A critical role for ceramide synthase 2 in liver homeostasis: I. alterations in lipid metabolic pathways. The Journal of biological chemistry. 285:10902–10910. doi: 10.1074/jbc.M109.077594. doi:M109.077594 [pii] 10.1074/jbc.M109.077594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pewzner-Jung Y, et al. A critical role for ceramide synthase 2 in liver homeostasis: I. alterations in lipid metabolic pathways. The Journal of biological chemistry. 2010b;285:10902–10910. doi: 10.1074/jbc.M109.077594. doi:10.1074/jbc.M109.077594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DC, Martin S, Doyle BT, Houghton JA. Sphingosine-induced apoptosis in rhabdomyosarcoma cell lines is dependent on pre-mitochondrial Bax activation and post-mitochondrial caspases. Cancer research. 2007;67:756–764. doi: 10.1158/0008-5472.CAN-06-2374. doi:10.1158/0008-5472.CAN-06-2374. [DOI] [PubMed] [Google Scholar]
- Pinton P, Giorgi C, Pandolfi PP. The role of PML in the control of apoptotic cell fate: a new key player at ER-mitochondria sites. Cell death and differentiation. 2011;18:1450–1456. doi: 10.1038/cdd.2011.31. doi:10.1038/cdd.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitson SM. Regulation of sphingosine kinase and sphingolipid signaling. Trends in biochemical sciences. 2011;36:97–107. doi: 10.1016/j.tibs.2010.08.001. doi:10.1016/j.tibs.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Pitson SM, et al. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. The Journal of experimental medicine. 2005;201:49–54. doi: 10.1084/jem.20040559. doi:10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne NJ, Tonelli F, Lim KG, Long J, Edwards J, Pyne S. Targeting sphingosine kinase 1 in cancer. Advances in biological regulation. 2012;52:31–38. doi: 10.1016/j.advenzreg.2011.07.001. doi:10.1016/j.advenzreg.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Quillet-Mary A, Jaffrezou JP, Mansat V, Bordier C, Naval J, Laurent G. Implication of mitochondrial hydrogen peroxide generation in ceramide-induced apoptosis. The Journal of biological chemistry. 1997;272:21388–21395. doi: 10.1074/jbc.272.34.21388. [DOI] [PubMed] [Google Scholar]
- Rao RP, et al. Ceramide transfer protein deficiency compromises organelle function and leads to senescence in primary cells. PloS one. 2014;9:e92142. doi: 10.1371/journal.pone.0092142. doi:10.1371/journal.pone.0092142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell JC, Lindsten T, Zong WX, Cinalli RM, Thompson CB. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nature immunology. 2002;3:932–939. doi: 10.1038/ni834. doi:10.1038/ni834. [DOI] [PubMed] [Google Scholar]
- Rego A, et al. Modulation of mitochondrial outer membrane permeabilization and apoptosis by ceramide metabolism. PloS one. 2012;7:e48571. doi: 10.1371/journal.pone.0048571. doi:10.1371/journal.pone.0048571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lafrasse C, Alphonse G, Broquet P, Aloy MT, Louisot P, Rousson R. Temporal relationships between ceramide production, caspase activation and mitochondrial dysfunction in cell lines with varying sensitivity to anti-Fas-induced apoptosis. The Biochemical journal. 2001;357:407–416. doi: 10.1042/0264-6021:3570407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogasevskaia T, Coorssen JR. Sphingomyelin-enriched microdomains define the efficiency of native Ca(2+)-triggered membrane fusion. Journal of cell science. 2006;119:2688–2694. doi: 10.1242/jcs.03007. doi:10.1242/jcs.03007. [DOI] [PubMed] [Google Scholar]
- Rojas-Charry L, Cookson MR, Nino A, Arboleda H, Arboleda G. Downregulation of Pink1 influences mitochondrial fusion-fission machinery and sensitizes to neurotoxins in dopaminergic cells. Neurotoxicology. 2014;44C:140–148. doi: 10.1016/j.neuro.2014.04.007. doi:10.1016/j.neuro.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SB, Ross JS, Cowart LA. Sphingolipids in obesity, type 2 diabetes, and metabolic disease. Handbook of experimental pharmacology. 2013:373–401. doi: 10.1007/978-3-7091-1511-4_19. doi:10.1007/978-3-7091-1511-4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta S, Stiban J, Maugel TK, Colombini M. Visualization of ceramide channels by transmission electron microscopy. Biochim Biophys Acta. 2011;1808:1196–1201. doi: 10.1016/j.bbamem.2011.01.007. doi:10.1016/j.bbamem.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, et al. Ordering of ceramide formation, caspase activation, and Bax/Bcl-2 expression during etoposide-induced apoptosis in C6 glioma cells. Cell death and differentiation. 2000;7:761–772. doi: 10.1038/sj.cdd.4400711. doi:10.1038/sj.cdd.4400711. [DOI] [PubMed] [Google Scholar]
- Schneider-Brachert W, et al. Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity. 2004;21:415–428. doi: 10.1016/j.immuni.2004.08.017. doi:10.1016/j.immuni.2004.08.017 S1074761304002341 [pii] [DOI] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- Senkal CE, Ponnusamy S, Bielawski J, Hannun YA, Ogretmen B. Antiapoptotic roles of ceramidesynthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:296–308. doi: 10.1096/fj.09-135087. doi:10.1096/fj.09-135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkal CE, et al. Alteration of ceramide synthase 6/C16-ceramide induces activating transcription factor 6-mediated endoplasmic reticulum (ER) stress and apoptosis via perturbation of cellular Ca2+ and ER/Golgi membrane network. The Journal of biological chemistry. 2011;286:42446–42458. doi: 10.1074/jbc.M111.287383. doi:10.1074/jbc.M111.287383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida D, Takabe K, Kapitonov D, Milstien S, Spiegel S. Targeting SphK1 as a new strategy against cancer. Current drug targets. 2008;9:662–673. doi: 10.2174/138945008785132402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva LC, et al. Ablation of ceramide synthase 2 strongly affects biophysical properties of membranes. Journal of lipid research. 2012;53:430–436. doi: 10.1194/jlr.M022715. doi:10.1194/jlr.M022715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siow DL, Anderson CD, Berdyshev EV, Skobeleva A, Natarajan V, Pitson SM, Wattenberg BW. Sphingosine kinase localization in the control of sphingolipid metabolism. Advances in enzyme regulation. 2011;51:229–244. doi: 10.1016/j.advenzreg.2010.09.004. doi:10.1016/j.advenzreg.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ. Mitochondrial ceramide and the induction of apoptosis. Journal of bioenergetics and biomembranes. 2005;37:143–153. doi: 10.1007/s10863-005-6567-7. doi:10.1007/s10863-005-6567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, Colombini M. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. The Journal of biological chemistry. 2000;275:38640–38644. doi: 10.1074/jbc.C000587200. doi:10.1074/jbc.C000587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, Davoody A, Lewin N, Marshall S, Colombini M. Enlargement and contracture of C2-ceramide channels. Biophysical journal. 2003;85:1560–1575. doi: 10.1016/S0006-3495(03)74588-3. doi:10.1016/S0006-3495(03)74588-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, et al. Anti-apoptotic Bcl-2 Family Proteins Disassemble Ceramide Channels. The Journal of biological chemistry. 2008;283:6622–6630. doi: 10.1074/jbc.M706115200. doi:10.1074/jbc.M706115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, Kolesnick RN, Colombini M. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. The Journal of biological chemistry. 2002;277:26796–26803. doi: 10.1074/jbc.M200754200. doi:10.1074/jbc.M200754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, Kolesnick RN, Colombini M. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion. 2006;6:118–125. doi: 10.1016/j.mito.2006.03.002. doi:10.1016/j.mito.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, Mullen TD, Romero Rosales K, Clarke CJ, Hernandez-Corbacho MJ, Edinger AL, Obeid LM. The BCL-2 protein BAK is required for long-chain ceramide generation during apoptosis. The Journal of biological chemistry. 2010;285:11818–11826. doi: 10.1074/jbc.M109.078121. doi:10.1074/jbc.M109.078121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME, et al. Mitochondrial fission mediates ceramide-induced metabolic disruption in skeletal muscle. The Biochemical journal. 2013;456:427–439. doi: 10.1042/BJ20130807. doi:10.1042/BJ20130807. [DOI] [PubMed] [Google Scholar]
- Snider AJ. Sphingosine kinase and sphingosine-1-phosphate: regulators in autoimmune and inflammatory disease. International journal of clinical rheumatology. 2013;8 doi: 10.2217/ijr.13.40. doi:10.2217/ijr.13.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnino S, Mauri L, Chigorno V, Prinetti A. Gangliosides as components of lipid membrane domains. Glycobiology. 2007;17:1R–13R. doi: 10.1093/glycob/cwl052. doi:10.1093/glycob/cwl052. [DOI] [PubMed] [Google Scholar]
- Sot J, Aranda FJ, Collado MI, Goni FM, Alonso A. Different effects of long- and short-chain ceramides on the gel-fluid and lamellar-hexagonal transitions of phospholipids: a calorimetric, NMR, and x-ray diffraction study. Biophysical journal. 2005;88:3368–3380. doi: 10.1529/biophysj.104.057851. doi:10.1529/biophysj.104.057851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridevi P, et al. Stress-induced ER to Golgi translocation of ceramide synthase 1 is dependent on proteasomal processing. Experimental cell research. 2010;316:78–91. doi: 10.1016/j.yexcr.2009.09.027. doi:10.1016/j.yexcr.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridevi P, Alexander H, Laviad EL, Pewzner-Jung Y, Hannink M, Futerman AH, Alexander S. Ceramide synthase 1 is regulated by proteasomal mediated turnover. Biochim Biophys Acta. 2009;1793:1218–1227. doi: 10.1016/j.bbamcr.2009.04.006. doi:S0167-4889(09)00102-5 [pii] 10.1016/j.bbamcr.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson CE, Takabe K, Nagahashi M, Milstien S, Spiegel S. Targeting sphingosine-1-phosphate in hematologic malignancies. Anti-cancer agents in medicinal chemistry. 2011;11:794–798. doi: 10.2174/187152011797655122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiban J, Caputo L, Colombini M. Ceramide synthesis in the endoplasmic reticulum can permeabilize mitochondria to proapoptotic proteins. Journal of lipid research. 2008;49:625–634. doi: 10.1194/jlr.M700480-JLR200. doi:10.1194/jlr.M700480-JLR200. [DOI] [PubMed] [Google Scholar]
- Stoica BA, Movsesyan VA, Lea PMt, Faden AI. Ceramide-induced neuronal apoptosis is associated with dephosphorylation of Akt, BAD, FKHR, GSK-3beta, and induction of the mitochondrial-dependent intrinsic caspase pathway. Molecular and cellular neurosciences. 2003;22:365–382. doi: 10.1016/s1044-7431(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Strub GM, et al. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:600–612. doi: 10.1096/fj.10-167502. doi:10.1096/fj.10-167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, et al. Substrate specificity, membrane topology, and activity regulation of human alkaline ceramidase 2 (ACER2) The Journal of biological chemistry. 2010;285:8995–9007. doi: 10.1074/jbc.M109.069203. doi:10.1074/jbc.M109.069203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafesse FG, et al. Sphingomyelin synthase-related protein SMSr is a suppressor of ceramide-induced mitochondrial apoptosis. Journal of cell science. 2014;127:445–454. doi: 10.1242/jcs.138933. doi:10.1242/jcs.138933. [DOI] [PubMed] [Google Scholar]
- Takabe K, Spiegel S. Export of sphingosine-1-phosphate and cancer progression. Journal of lipid research. 2014;55:1839–1846. doi: 10.1194/jlr.R046656. doi:10.1194/jlr.R046656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani M, Igarashi Y, Ito M. Involvement of neutral ceramidase in ceramide metabolism at the plasma membrane and in extracellular milieu. The Journal of biological chemistry. 2005;280:36592–36600. doi: 10.1074/jbc.M506827200. doi:10.1074/jbc.M506827200. [DOI] [PubMed] [Google Scholar]
- Tani M, Okino N, Mitsutake S, Tanigawa T, Izu H, Ito M. Purification and characterization of a neutral ceramidase from mouse liver. A single protein catalyzes the reversible reaction in which ceramide is both hydrolyzed and synthesized. The Journal of biological chemistry. 2000;275:3462–3468. doi: 10.1074/jbc.275.5.3462. [DOI] [PubMed] [Google Scholar]
- Thomas RL, Jr., Matsko CM, Lotze MT, Amoscato AA. Mass spectrometric identification of increased C16 ceramide levels during apoptosis. The Journal of biological chemistry. 1999;274:30580–30588. doi: 10.1074/jbc.274.43.30580. [DOI] [PubMed] [Google Scholar]
- Uchida Y, et al. Hydrolytic pathway protects against ceramide-induced apoptosis in keratinocytes exposed to UVB. The Journal of investigative dermatology. 2010;130:2472–2480. doi: 10.1038/jid.2010.153. doi:10.1038/jid.2010.153. [DOI] [PubMed] [Google Scholar]
- van Echten-Deckert G, Walter J. Sphingolipids: critical players in Alzheimer’s disease. Progress in lipid research. 2012;51:378–393. doi: 10.1016/j.plipres.2012.07.001. doi:10.1016/j.plipres.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Vit JP, Rosselli F. Role of the ceramide-signaling pathways in ionizing radiation-induced apoptosis. Oncogene. 2003;22:8645–8652. doi: 10.1038/sj.onc.1207087. doi:10.1038/sj.onc.1207087. [DOI] [PubMed] [Google Scholar]
- von Haefen C, Wieder T, Gillissen B, Starck L, Graupner V, Dorken B, Daniel PT. Ceramide induces mitochondrial activation and apoptosis via a Bax-dependent pathway in human carcinoma cells. Oncogene. 2002;21:4009–4019. doi: 10.1038/sj.onc.1205497. doi:10.1038/sj.onc.1205497. [DOI] [PubMed] [Google Scholar]
- Wang X, et al. Mitochondrial degeneration and not apoptosis is the primary cause of embryonic lethality in ceramide transfer protein mutant mice. The Journal of cell biology. 2009;184:143–158. doi: 10.1083/jcb.200807176. doi:10.1083/jcb.200807176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattenberg BW. Role of sphingosine kinase localization in sphingolipid signaling. World journal of biological chemistry. 2010;1:362–368. doi: 10.4331/wjbc.v1.i12.362. doi:10.4331/wjbc.v1.i12.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei MC, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert A, Cremer S, Schmidt MV, von Knethen A, Angioni C, Geisslinger G, Brune B. Cleavage of sphingosine kinase 2 by caspase-1 provokes its release from apoptotic cells. Blood. 2010;115:3531–3540. doi: 10.1182/blood-2009-10-243444. doi:10.1182/blood-2009-10-243444. [DOI] [PubMed] [Google Scholar]
- Wiesner DA, Dawson G. Staurosporine induces programmed cell death in embryonic neurons and activation of the ceramide pathway. Journal of neurochemistry. 1996;66:1418–1425. doi: 10.1046/j.1471-4159.1996.66041418.x. [DOI] [PubMed] [Google Scholar]
- Wiesner DA, Kilkus JP, Gottschalk AR, Quintans J, Dawson G. Anti-immunoglobulin-induced apoptosis in WEHI 231 cells involves the slow formation of ceramide from sphingomyelin and is blocked by bcl-XL. The Journal of biological chemistry. 1997;272:9868–9876. doi: 10.1074/jbc.272.15.9868. [DOI] [PubMed] [Google Scholar]
- Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. The Journal of cell biology. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu BX, Rajagopalan V, Roddy PL, Clarke CJ, Hannun YA. Identification and characterization of murine mitochondria-associated neutral sphingomyelinase (MA-nSMase), the mammalian sphingomyelin phosphodiesterase 5. The Journal of biological chemistry. 2010;285:17993–18002. doi: 10.1074/jbc.M110.102988. doi:10.1074/jbc.M110.102988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, et al. Evidence for mitochondrial localization of a novel human sialidase (NEU4) The Biochemical journal. 2005;390:85–93. doi: 10.1042/BJ20050017. doi:10.1042/BJ20050017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Uhlig S. The role of sphingolipids in respiratory disease. Therapeutic advances in respiratory disease. 2011;5:325–344. doi: 10.1177/1753465811406772. doi:10.1177/1753465811406772. [DOI] [PubMed] [Google Scholar]
- Yao J, et al. Ultraviolet (UV) and Hydrogen Peroxide Activate Ceramide-ER Stress-AMPK Signaling Axis to Promote Retinal Pigment Epithelium (RPE) Cell Apoptosis International journal of molecular sciences. 2013;14:10355–10368. doi: 10.3390/ijms140510355. doi:10.3390/ijms140510355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Alter N, Reed JC, Borner C, Obeid LM, Hannun YA. Bcl-2 interrupts the ceramide-mediated pathway of cell death. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5325–5328. doi: 10.1073/pnas.93.11.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang Y, Wan Z, Liu S, Cao Y, Zeng Z. Sphingosine kinase 1 and cancer: a systematic review and meta-analysis. PloS one. 2014;9:e90362. doi: 10.1371/journal.pone.0090362. doi:10.1371/journal.pone.0090362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigdon H, et al. Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. The Journal of biological chemistry. 2013;288:4947–4956. doi: 10.1074/jbc.M112.402719. doi:10.1074/jbc.M112.402719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong WX, Li C, Hatzivassiliou G, Lindsten T, Yu QC, Yuan J, Thompson CB. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. The Journal of cell biology. 2003;162:59–69. doi: 10.1083/jcb.200302084. [DOI] [PMC free article] [PubMed] [Google Scholar]