Abstract
Cell surface glycosylation is thought to be involved in barrier function against microbes at mucosal surfaces. Previously we showed that the epithelium of healthy mouse corneas becomes vulnerable to Pseudomonas aeruginosa adhesion if it lacks the innate defense protein MyD88 (myeloid differentiation primary response gene 88), or after superficial injury by blotting with tissue paper. Here we explored their effect on corneal surface glycosylation using a metabolic label, tetra-acetylated N-azidoacetylgalactosamine (Ac4GalNAz). Ac4GalNAz treatment labeled the surface of healthy mouse corneas, leaving most cells viable, and bacteria preferentially associated with GalNAz-labeled regions. Surprisingly, corneas from MyD88−/− mice displayed similar GalNAz labeling to wild-type corneas, but labeling was reduced and patchy on IL-1 receptor (IL-1R)-knockout mouse corneas (P < 0.05, ANOVA). Tissue paper blotting removed GalNAz-labeled surface cells, causing DAPI labeling (permeabilization) of underlying cells. MS of material collected on the tissue paper blots revealed 67 GalNAz-labeled proteins, including intracellular proteins. These data show that the normal distribution of surface glycosylation requires IL-1R, but not MyD88, and is not sufficient to prevent bacterial binding. They also suggest increased P. aeruginosa adhesion to MyD88−/− and blotted corneas is not due to reduction in total surface glycosylation, and for tissue paper blotting is likely due to cell permeabilization.—Jolly, A. L., Agarwal, P., Metruccio, M. M. E., Spiciarich, D. R., Evans, D. J., Bertozzi, C. R., Fleiszig, S. M. J. Corneal surface glycosylation is modulated by IL-1R and Pseudomonas aeruginosa challenge but is insufficient for inhibiting bacterial binding.
Keywords: GalNAz metabolic label, imaging, mass spectrometry, Muc4, MyD88
Pseudomonas aeruginosa is a leading cause of corneal infection associated with injury or contact lens wear (1). Yet healthy mouse corneas readily resist P. aeruginosa adhesion and subsequent infection (2). Even in the face of extremely large inocula, bacteria are rapidly cleared from healthy mouse eyes (2, 3). This capacity to resist infection is perhaps not surprising given the critical role corneal transparency plays in vision and thus survival.
Multiple mechanisms participate in ocular surface defense against adhesion of P. aeruginosa and other bacteria, including tear fluid effects on the epithelium (4), epithelial cell polarity (5), secretory IgA (6), mucin glycoproteins (7–10), surfactant proteins (2, 11, 12), and antimicrobials (e.g., defensins, cathelicidins, RNase7, and keratin-derived antimicrobial peptides) (3, 4, 13–15).
Our previous studies showed that mouse corneas could be rendered more vulnerable to P. aeruginosa adhesion by gently blotting the cornea with tissue paper (Kimwipe; Kimberly-Clark, Irving, TX, USA) before bacterial exposure (16). This suggested that factors removed by blotting otherwise are responsible for inhibiting adhesion, or else that bacterial adhesion follows cell damage. Suggesting the former, corneas of MyD88 (myeloid differentiation primary response gene 88) gene-knockout mice (not blotted) were more susceptible to P. aeruginosa adhesion in the absence of any loss of barrier function to the small molecule fluorescein (17). MyD88, which is a critical adaptor protein for TLR- and IL-1 receptor (IL-1R)-mediated signaling, can modulate expression of various factors including mucins, cytokines, and antimicrobial peptides. Indeed, we have demonstrated that corneal lysates from MyD88−/− mice exhibited reduced antimicrobial activity (18). The targets modulated by MyD88 to inhibit P. aeruginosa binding to the mouse cornea, as well as how those targets relate to enhanced P. aeruginosa adhesion after tissue paper blotting, remain unknown.
Like other mucosal epithelia, ocular surface epithelial cells express an apical surface glycocalyx consisting of transmembrane mucin glycoproteins, predominantly Muc1, Muc4, and Muc16 (19, 20), although expression of a novel transmembrane protein Muc20 has also been demonstrated (21). This glycocalyx forms an interface between the ocular surface cells and tear film; it is thought to serve multiple functions, including conferring wettability, barrier function, and formation of an antiadhesive surface toward other cells and microbes (7–9, 19, 22, 23). Many glycocalyx functions are attributable to O-glycosylation of the transmembrane mucins (19, 22), and in particular are associated with Muc16 (7–9). For example, knockdown of Muc16 reduced multiple aspects of epithelial barrier function and increased epithelial susceptibility to Staphylococcus aureus binding and host cell invasion (8). Secretion of a metalloproteinase by Streptococcus pneumoniae that causes shedding of the Muc16 ectodomain compromised epithelial barrier function and increased bacterial adhesion (9).
We tested the hypothesis that tissue paper blotting and MyD88 deficiency each alter O-glycosylation at the ocular surface, and that P. aeruginosa binds preferentially to areas of reduced O-glycosylation. To test this, we used the metabolic label tetra-acetylated N-azidoacetylgalactosamine (Ac4GalNAz) ex vivo to label O-glycosylated mucin glycoproteins at the surface of intact mouse corneas. This reagent has been used previously to study cultured cells (24), mucin turnover in the murine gastric mucosa before and after Helicobacter pylori infection (25), and glycan biosynthesis in developing zebrafish (26). Images collected from healthy mouse corneas illustrated the normal pattern of Ac4GalNAz (mucin) expression, showing homogenous expression across individual epithelial cells but with cell-to-cell variation in turnover of O-glycosylated mucins, also associated with natural exfoliation of cells from the tissue surface. Surprisingly however, bacteria preferentially bound to areas with greater (not reduced) GalNAz labeling and caused no obvious change in the distribution of GalNAz labeling. Also surprisingly, GalNAz labeling appeared normal on MyD88−/− mouse corneas but was disrupted on corneas of IL-1R−/− mice. Tissue paper blotting removed GalNAz-labeled superficial cells, and a subsequent exposure to bacteria induced a reemergence of surface GalNAz labeling. Thus, the normal distribution of surface glycosylation requires IL-1R, but not MyD88, and is insufficient to prevent bacterial binding.
MATERIALS AND METHODS
Bacteria
P. aeruginosa strain PAO1 containing a plasmid encoding dTomato (p67T1) (27) was used in this study. Bacteria were cultured at 37°C overnight on tryptic soy agar (BD Biosciences, San Jose, CA, USA) containing carbenicillin (300 μg/ml; Sigma-Aldrich, St. Louis, MO, USA). Bacterial inocula were prepared by resuspension in warm minimum essential medium (MEM; without antibiotics) to an optical density of 1.0 at 650 nm (Spectronic 21D; Spectronic, Milton Roy, PA, USA) corresponding to ∼1 × 109 colony-forming units (CFU)/ml. Inoculum sizes were confirmed by viable count.
Mice
Eyes used in experiments were obtained from C57BL/6 wild-type (WT) mice or from C57BL/6 gene-knockout mice (MyD88−/− or IL-1R−/−). Female and male mice aged 6 to 32 wk were used. Animal care and use procedures were approved by the animal care and use committee of the University of California, Berkeley, an Association for Assessment and Accreditation of Laboratory Animal Care International–accredited institution.
Labeling with Ac4GalNAz
Freshly enucleated eyes were washed once in PBS then gently inserted face up into a 48-well plate containing Ac4GalNAz agar stands prepared as follows. A 1% agar solution was prepared in MEM (500 µl per well) with 5% fetal bovine serum (FBS), 2.5 mg/L amphotericin B, 400 µg/ml carbenicillin, and 5 mM Ac4GalNAz. After the agar stands solidified, medium containing antibiotics and Ac4GalNAz, but without agar or FBS (200 µl), was added to each well. The edge wells of the 48-well plate were then filled with water to prevent evaporation. The eyes were submerged in the agar so that a meniscus formed over the cornea and roughly half of the globe was embedded in agar. The plate was incubated at 37°C for 16 to 24 h to allow incorporation of the Ac4GalNAz. Just before imaging, new agar stands were made in 48-well plates to perform washes. The 1.5% agarose stands (approximately 0.5 ml per well) were made up in DMEM with 5% FBS, amphotericin B, and carbenicillin. (Antimicrobial agents were added after cooling the solution.) Eyes were washed briefly in a dish of PBS, then lodged in new agar stands using the optic nerve to anchor the eyeball. Then 500 µl of medium (phenol-free DMEM without FBS and with carbenicillin and amphotericin) containing dibenzo-aza-cyclooctyne (DIBAC)-biotin (100 µM) was added on top of the eye for 30 min. Eyes were washed 5 times in PBS, then incubated in media containing streptavidin–Alexa Fluor 488 for an additional 30 min on top of a new agar stand. For live/dead assays, eyes were incubated in 37°C for 30 min in DAPI and SYTO Green 16 dyes, then washed in PBS before mounting and imaging. For experiments involving bacteria, 500 µl of P. aeruginosa suspension in warm MEM (without antibiotics) containing 5 × 108 CFU was added to eyes for 1 h before DIBAC-biotin and streptavidin–Alexa Fluor 488 steps.
Imaging
Imaging of eyes was performed after mounting globes to a glass coverslip, using glue to adhere the region surrounding the optic nerve. The center of each eye was imaged with a ×20 lens in phenol-free DMEM (no FBS), giving a maximal Nyquist resolution of 650 nm in the xy plane and 3.7 µm in the z plane. The center of each eye was located using the reflectance image (exciting and collecting at 650 nm) and focusing on the basement membrane just underneath the corneal epithelium.
Image processing
CellProfiler
Labeled corneal epithelial cells were differentiated from unlabeled cells using a combination of image processing steps including application of a threshold, separation of foreground and background, size and shape of the fluorescent object, and segmentation algorithms. The pipeline used can be found online (http://www.cellprofiler.com).
Imaris
Surfaces of green cells were made in Imaris software (Bitplane; Oxford Instruments, Abingdon, United Kingdom) using the surface generation function. Then a distance transformation to the surface was calculated using a MatLab extension (https://www.mathworks.com/). Spot detection was used to identify bacteria and colocalize the bacteria with the surfaces.
Mass spectrometry
Preparation of tissue paper blots of murine corneas for MS analysis
Four sets of murine corneas were blotted with tissue paper after incubation in Ac4GalNAz (as previously described) and DIBAC-biotin. The tissue paper was cut into squares of ∼4 cm2 and added to cell lysis buffer [4 ml of lysis buffer containing 1% Triton X-100, 20 mM Tris pH 7.4, 300 mM NaCl, and protease inhibitors (inhibitor cocktail III from Calbiochem, San Diego, CA, USA)], and cooled in ice. To free cell surface glycoproteins, RIPA buffer was added to make a final concentration of 150 mM sodium chloride, 1.0% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0, and rotated in the dark overnight at 4°C. The slurry was strained using a 40 μm cell strainer (BD Falcon; 352340) and washed (2 × 1.5 ml RIPA buffer).
Immunoblotting for MS
After preparation and normalization of protein concentration by bicinchoninic acid assay, samples were separated by SDS-PAGE (Criterion system; Bio-Rad, Hercules, CA, USA), transferred onto nitrocellulose, blocked in 5% bovine serum albumin (Sigma-Aldrich) in Tris-buffered saline with 0.1% Tween 20, and analyzed using enhanced chemiluminescence immunoblotting (Pierce, Rockford, IL, USA). Monoclonal mouse anti-biotin horseradish peroxidase reagent (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) was diluted 1:100,000 to 1:50,000 in blocking buffer.
Cell surface glycoprotein capture and MS analysis
Samples were filtered through a disposable PD-10 desalting column (GE Healthcare Life Sciences, Little Chalfont, United Kingdom) and eluted with PBS. SDS stock (10%) was added for a final concentration of 0.5% SDS, and samples were boiled at 90°C for 7 min. Samples were cooled on ice for 5 min; then 8 ml of PBS and 100 ml of avidin slurry (A9207; Sigma-Aldrich) were added and rotated at room temperature for 1 h. Beads were concentrated by centrifugation at 1200 g for 3 min at 4°C. The supernatant was removed, and the beads were transferred to a washed biospin column (Bio-Rad) on a vacuum manifold. Beads were washed with 1 ml of 0.2% SDS in PBS, 1 ml of freshly made 6 M urea (Spectrum Chemical, New Brunswick, NJ, USA), 100 ml of 100 mM ProteaseMax surfactant (Promega, Madison, WI, USA), and PBS (2 × 1 ml). Beads were resuspended in 200 µl PBS, 20 µl of 100 mM Tris(2-carboxyethyl)phosphine was delivered, and the sample was rotated for 30 min at room temperature. Freshly prepared iodoacetamide (20 µl, 200 mM) was delivered, and the sample was wrapped in foil and rotated for 30 min at room temperature. Beads were then washed 3 times with 500 µl of PBS and transferred to an RNase-free microcentrifuge tube with 2 washes of 300 µl PBS. Beads were concentrated by centrifugation at 3800 g for 3 min, and supernatant was removed. Beads were resuspended in freshly made 2 M urea in PBS (200 µl). Enzymatic digestion proceeded by resuspending trypsin (V5111; Promega) formed into aliquots (20 μg) in buffer (40.0 µl). A total of 4 µl was delivered to each sample, and samples were rotated overnight at 37°C. The next morning, the beads were pelleted at 3800 g for 3 min. The supernatant was collected, and the beads were washed with an additional 100 µl of PBS. Formic acid (16 µl) was added, and samples were stored at −80°C.
MS analysis
Recovered glycopeptides were analyzed using a Dionex UltiMate3000 RSLCnano liquid chromatograph (Thermo Fisher Scientific, Waltham, MA, USA) that was connected in line with an LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific) equipped with a nanoelectrospray ionization (nanoESI) source. Full-scan mass spectra were acquired in the positive-ion mode over the range m/z = 400–1800 using the Orbitrap mass analyzer, in profile format, with a mass resolution setting of 60,000. The 3 most intense ions exceeding an intensity threshold of 50,000 counts were selected from each full-scan mass spectrum for tandem MS (MS/MS) analysis using collision-induced dissociation. A fully automated chromatographic run was performed on each sample using a 2-mobile-phase system consisting of buffer A (100% water, 0.1% formic acid) and buffer B (100% ACN, 0.1% formic acid). The cycle consisted of a 140 min linear gradient from 5 to 100% buffer B, followed by 20 min of 100% buffer B followed by 20 min of 5% buffer A. MS/MS spectra were acquired using the linear ion trap or the Orbitrap analyzer (in the latter case, with a resolution setting of 7500 at m/z = 400, full width at half maximum), in centroid format, with the following parameters: isolation width, 4 m/z units; normalized collision energy, 28%; default charge state, 3+; activation Q, 0.25; and activation time, 30 ms.
Data analysis
Raw data MS/MS fragmentation spectra were searched with the Sequest HT algorithm within the Proteome Discoverer software framework (version 1.4; Thermo Fisher Scientific) against a mouse SwissProt database (April 2015; http://www.uniprot.org). Search parameters included the following: 2 maximum missed trypsin cleavage sites, 10 ppm precursor mass tolerance, 0.8 Da fragment mass tolerance, carbamidomethyl-Cys as a static modification, and oxidation of Met and phosphorylation of Ser/Thr/Tyr as dynamic modifications. Significance scoring of identified peptides was done with the Percolator node of Proteome Discoverer, which uses a support vector machine model trained on actual and decoy search results. We filtered the data set for a peptide-match false discovery rate <0.01. Proteins were collapsed to groups using the protein grouping algorithm of Proteome Discoverer.
Statistical analysis
Data were expressed as means ± sd, and the significance of differences between 2 groups was determined by Student’s t test. For multiple comparisons, a 1-way ANOVA was performed with Dunnett’s multiple comparison test. A value of P < 0.05 was considered significant.
RESULTS
Ex vivo metabolic labeling of murine ocular surface glycoproteins
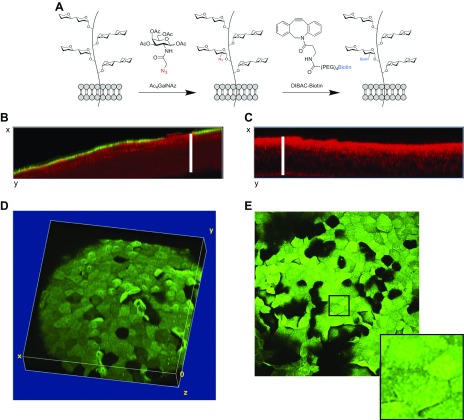
Enucleated eyes of C57BL/6 mice were incubated ex vivo for 24 h with Ac4GalNAz, an azido-containing analog of N-acetylgalactosamine. On the basis of previous work using various types of host cell (24), it was expected that Ac4GalNAz would be taken up by metabolically active corneal epithelial cells and incorporated into O-linked mucin glycoproteins on the cell surface. Once inside the cell, the O-acetyl groups of Ac4GalNAz are quickly cleaved off by esterases to reveal free GalNAz. While N-acetylgalactosamine is a major constituent of O-linked glycans, there is a possibility that some degree of N-linked glycosylation can be visualized as well via further metabolism of GalNAz (28). However, the ocular glycocalyx is composed of O-linked glycoproteins (mucins), making this analog a good tool for imaging the glycocalyx on the surface of living ocular epithelial cells. GalNAz incorporation was visualized by incubation of eyes with DIBAC-biotin (15 min) to allow a click-chemistry reaction to covalently link incorporated GalNAz with biotin, then incubation with streptavidin–Alexa Fluor 488 (30 min) to allow fluorescence imaging (green) of GalNAz-labeled mucin glycoproteins on the ocular surface (Fig. 1A). Confocal imaging revealed successful labeling of most corneal epithelial surface cells, with labeling limited to the surface layer (Fig. 1B). No fluorescent labeling was observed in control eyes without Ac4GalNAz (i.e., vehicle incubation followed by treatment with DIBAC-biotin and streptavidin–Alexa Fluor 488) (Fig. 1C). Images were taken of the entire thickness of the epithelium using tissue reflectance at 635 nm (red) to locate the central corneal region. Three-dimensional images (Figs. 1D, E) revealed that fluorescence intensity varied from cell to cell, with some cells much brighter than others, and some lacking a detectable signal. Similar fluorescence intensity was observed across the surface of individual cells, although numerous punctae were evident in each cell in maximum intensity projections (Fig. 1E).
Figure 1.
Incorporation and labeling of GalNAz in murine cornea of ex vivo cultured eyes. A) Schematic of labeling process. Incubation of corneal cells in Ac4GalNAz overnight (24 h) was followed by DIBAC-biotin to label first sugar moiety bound to serine or threonine of corneal mucins and other O-linked glycoproteins. Biotin was then labeled with streptavidin–Alexa Fluor 488 for imaging. B) Orthogonal view of GalNAz (mucin) fluorescence (green) and tissue reflectance (red) showing that GalNAz labeling was restricted to epithelial surface cells. White bar indicates corneal epithelial thickness determined using reflectance image. C) Orthogonal view of red and green channels in labeled control eye (DMSO instead of Ac4GalNAz). D) Volumetric reconstruction of confocal z stack in green channel (original magnification, ×20) of GalNAz-labeled eye. E) Maximum intensity projection of GalNAz-labeled eye with inlay indicating punctate mucin labeling. Representative images are shown, but similar results were seen in 8 WT eyes from 8 different mice (1 central corneal scan was captured per cornea; see Materials and Methods).
Ocular surface health after GalNAz labeling
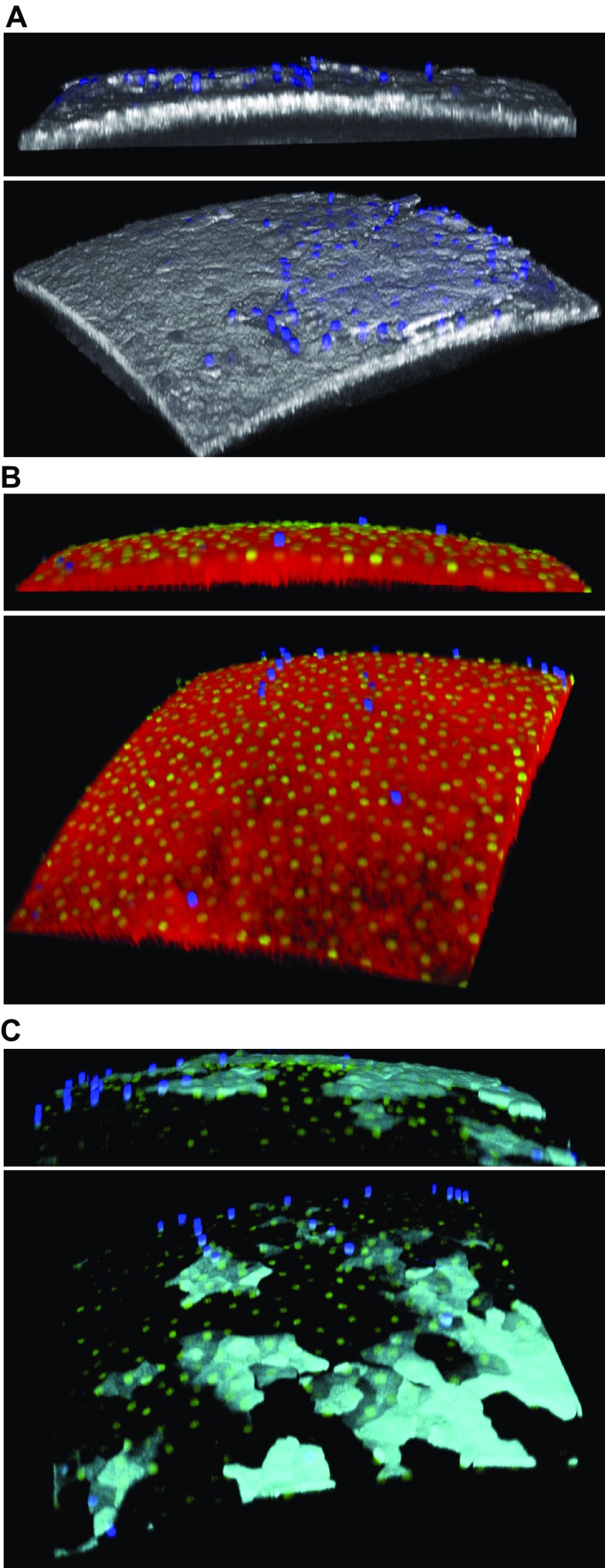
It was next determined whether 24 h ex vivo culturing of murine eyes with Ac4GalNAz followed by the DIBAC-biotin and streptavidin–Alexa Fluor 488 labeling described above damaged the eye or otherwise affected individual cell health. DAPI staining of Ac4GalNAz-labeled eyes, combined with reflectance imaging at 635 nm, revealed the presence of exfoliating dead cells (blue), as might be expected in a viable cornea (Fig. 2A). DAPI and SYTO Green 16 dyes were then used to label dead and living cells, respectively. The majority of cells on the ocular surface were viable (green) after GalNAz labeling, and a few dead cells (blue) were exfoliating (Fig. 2B). We also observed GalNAz labeling of both live and dead cells, suggesting that some cells had lost viability after incorporation of GalNAz, which was expected considering the time frame of these experiments (Fig. 2C).
Figure 2.
Murine corneal epithelial cell health after overnight (24 h) Ac4GalNAz incubation ex vivo followed by DIBAC-biotin and streptavidin–Alexa Fluor 488 labeling. A) Reflectance image (white) after additional DAPI labeling shows several exfoliating dead cells (blue). B) Live/dead stain of epithelial surface (reflectance in red). DAPI was added to incubation medium to show dead cells (blue), and SYTO Green 16 dye was added to show nuclei of live cells (green). C) GalNAz labeling (cyan) merged with DAPI and SYTO Green 16 images revealed live and dead cells colocalizing with sugar label. In total, 3 eyes from 3 WT mice were analyzed in 2 separate assays using live/dead dyes after GalNAz labeling; representative images are shown.
P. aeruginosa interactions with GalNAz-labeled corneas
Exposure of GalNAz-labeled eyes to P. aeruginosa–dTomato (for 1 h) after streptavidin treatment resulted in complete loss of the fluorescent label (data not shown). However, if exposure to bacteria occurred before DIBAC-biotin and streptavidin–Alexa Fluor 488 labeling steps, surface glycosylation labeling was similar to uninoculated corneas (Fig. 3), suggesting that the bacteria do not remove molecules that label with GalNAz, nor do they prevent DIBAC-biotin and streptavidin–Alexa Fluor 488 labeling. This modified method was used for the remaining experiments utilizing bacteria. In an example experiment, the percentage of bacteria colocalizing with green epithelial cells in 3 corneas was 81, 94, and 97%, suggesting that P. aeruginosa was capable of binding to cells expressing surface glycoproteins. Whether this association involves P. aeruginosa binding to previously identified epithelial surface targets, such as galectin-3 (29), N-glycans, or heparan sulfate proteoglycans (30), remains to be determined.
Figure 3.
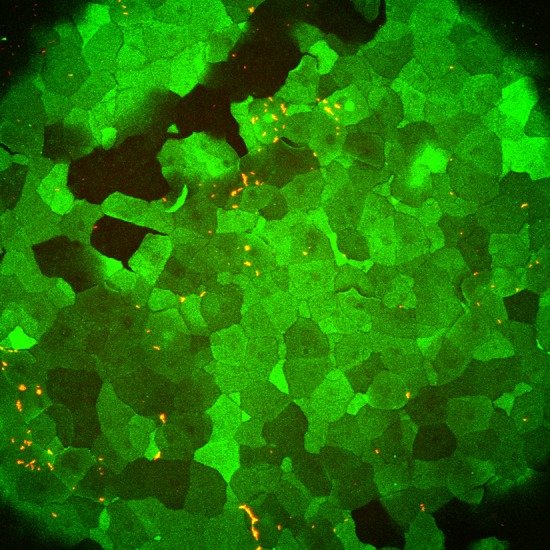
P. aeruginosa colocalization with GalNAz-labeled cells on murine cornea ex vivo. Approximately 5 × 108 CFU of P. aeruginosa (dTomato, red) was added for 1 h to cornea that was previously incubated with Ac4GalNAz overnight before washing the eye and incubating with DIBAC-biotin and streptavidin–Alexa Fluor 488. Representative image is shown. Three WT mouse eyes were quantified to determine degree of association between bacteria and GalNAz-labeled cells (see Results). Surfaces were generated in Imaris software to determine bacterial colocalization in 3 dimensions.
Effects of tissue paper blotting on GalNAz-labeled corneal epithelium
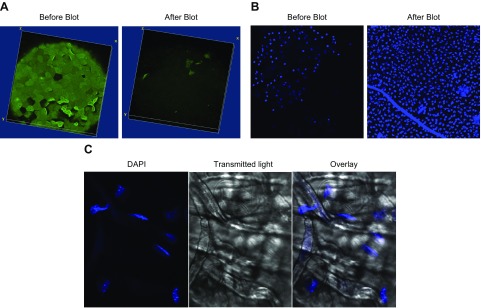
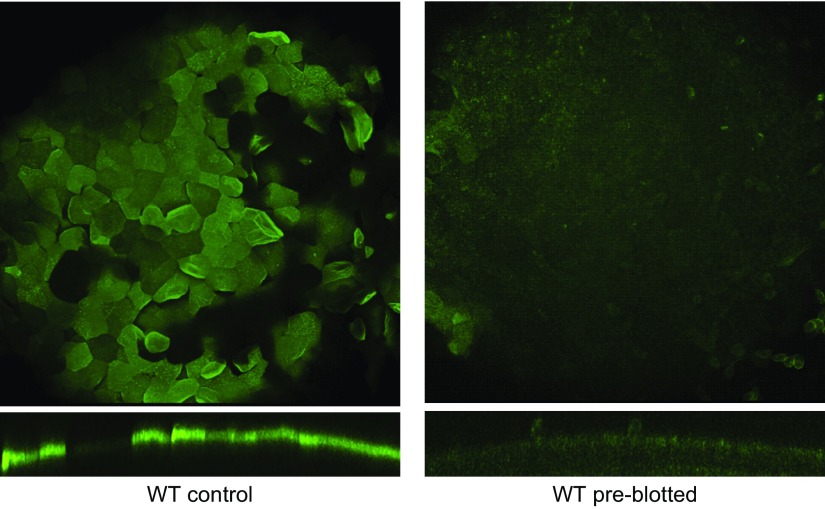
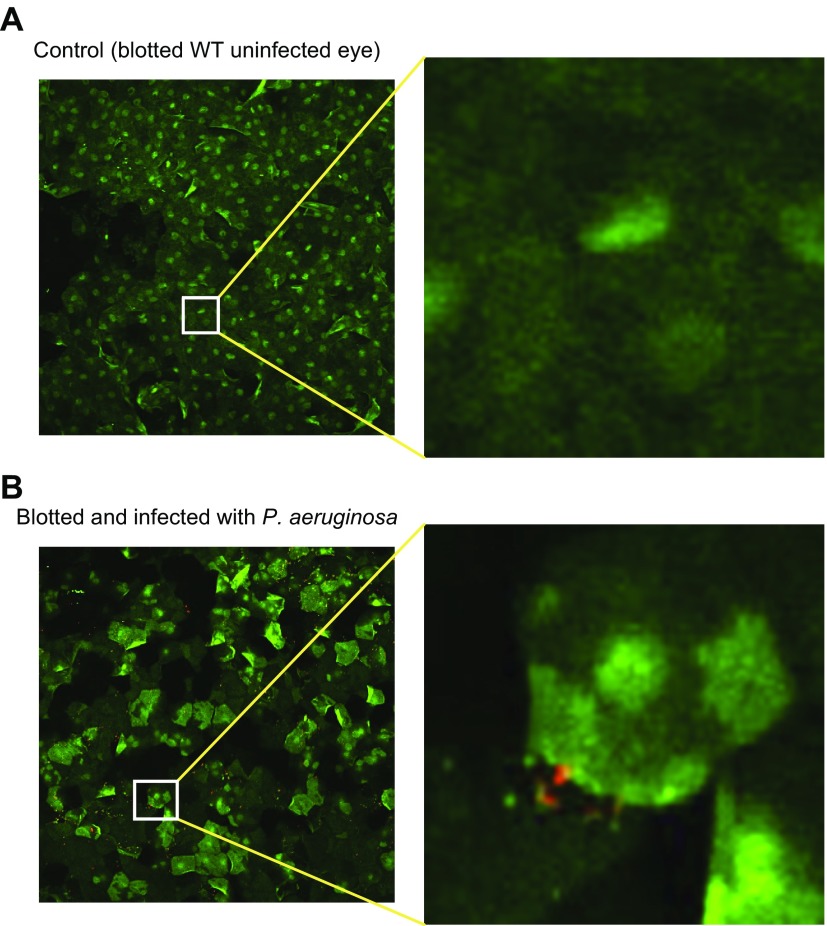
Blotting the GalNAz-labeled ocular surface with tissue paper removed nearly all the GalNAz-labeled surface glycoproteins compared to a nonblotted eye (Fig. 4A). Interestingly, underlying epithelial cells showed extensive DAPI labeling after blotting compared to nonblotted eyes, suggesting enhanced permeability and loss of cell viability (Fig. 4B). Imaging of the tissue paper used for blotting revealed what appeared to be multiple nuclei of epithelial cells, many of which were labeled with DAPI (Fig. 4C). Blotting of the cornea greatly reduced uptake of Ac4GalNAz added after blotting (Fig. 5), further supporting the idea that underlying cells were permeabilized and had lost viability (Fig. 5).
Figure 4.
Tissue paper blotting of GalNAz-labeled WT murine cornea ex vivo removes GalNAz-expressing surface epithelial cells. A) Representative image of murine cornea before (left) and 10 min after (right) blotting with tissue paper, showing almost complete removal of GalNAz-labeled epithelial cells. B) DAPI staining of GalNAz-labeled cornea before (left) and 10 min after (right) blotting, showing extensive labeling of cells remaining after blotting, indicating permeabilization and cell death. C) DAPI stain (blue) and phase-contrast image (right) of tissue paper used to blot murine cornea revealed extensive numbers of cell nuclei on tissue paper with multiple permeabilized (dead) cells (blue). Images are representative of 4 eyes analyzed in 2 separate assays.
Figure 5.
Murine corneas blotted before Ac4GalNAz incubation do not incorporate GalNAz. WT mouse eyes were untreated (control; left) or blotted immediately after enucleation and before 24 h incubation in Ac4GalNAz (preblotted; right), then labeled with DIBAC-biotin and streptavidin–Alexa Fluor 488. Maximum intensity projections (top) and orthogonal views of volumes (bottom) revealed greatly reduced GalNAz incorporation and expression in blotted eyes compared to controls. Images are representative of 4 eyes per condition.
MS from corneal surface tissue blots
To examine which glycosylated proteins were removed by blotting, we next performed MS on the material collected from the ocular surface during the procedure. WT mouse eyes were first treated with Ac4GalNAz (or DMSO control) and incubated in DIBAC-biotin, but not streptavidin–Alexa Fluor 488. After tissue paper blotting for MS (see Materials and Methods), extracts from the paper samples were passed through an avidin column to enrich for biotinylated proteins. Digestion of the captured glycoproteins with trypsin yielded peptides that were subsequently analyzed by MS/MS. Table 1 provides a partial list of proteins only found in blots from GalNAz-labeled corneas. The complete list of GalNAz-specific proteins identified by MS analysis is shown in Supplemental Table S1. There were 67 proteins unique to blots from Ac4GalNAz-treated eyes, not found in the DMSO control. In addition, 14 proteins were unique to the DMSO control, and 69 were found in both (not shown). Murine Muc4, a transmembrane mucin and a major constituent of the glycocalyx, was heavily enriched by the GalNAz label. Blots were also enriched for other transmembrane, cytoplasmic, and nuclear proteins (e.g., vimentin, desmosome proteins periplakin and envoplakin, myosin-14, glutathione-S-transferase B1, and annexin A1), supporting the idea that surface epithelial cells were removed by blotting, in addition to constituents of the glycocalyx.
TABLE 1.
MS of tissue paper blots from GalNAz-labeled murine corneas
| Name | Description | Peptide (n) | PSM (n) | Score | Coverage (%) | aa (n) | MW (kDa) | Calc pI | Accession no. |
|---|---|---|---|---|---|---|---|---|---|
| Ppl | Periplakin | 19 | 19 | 64.54 | 12.71 | 1755 | 203.9 | 5.54 | Q9R269 |
| Evpl | Envoplakin | 16 | 16 | 57.45 | 11.11 | 2035 | 231.9 | 6.6 | Q9D952 |
| Muc4a | Mucin-4 | 6 | 7 | 27.08 | 2.90 | 3443 | 364.9 | 7.43 | Q8JZM8 |
| Myh14 | Myosin-14 | 7 | 7 | 23.76 | 4.30 | 2000 | 228.4 | 5.55 | Q6URW6 |
| Gstp1 | Glutathione S–transferase P 1 | 4 | 4 | 15.3 | 26.67 | 210 | 23.6 | 7.87 | P19157 |
| Anxa1 | Annexin A1 | 4 | 4 | 14.91 | 16.47 | 346 | 38.7 | 7.37 | P10107 |
| Pkm | Pyruvate kinase PKM | 3 | 3 | 13.09 | 10.73 | 531 | 57.8 | 7.47 | P52480 |
| Plet1b | Placenta-expressed transcript 1 protein | 2 | 2 | 7.54 | 10.97 | 237 | 25 | 5.86 | Q8VEN2 |
| Sptan1 | Spectrin α chain, non–erythrocytic 1 | 2 | 2 | 7.39 | 1.29 | 2472 | 284.4 | 5.33 | P16546 |
| Ckb | Creatine kinase B type | 2 | 2 | 7.05 | 6.30 | 381 | 42.7 | 5.67 | Q04447 |
| Calb2 | Calretinin | 2 | 2 | 6.76 | 9.96 | 271 | 31.4 | 5.02 | Q08331 |
| Eef2 | Elongation factor 2 | 2 | 2 | 6.46 | 3.15 | 858 | 95.3 | 6.83 | P58252 |
| Gprc5ab | Retinoic acid–induced protein 3 | 2 | 2 | 6.43 | 7.58 | 356 | 40.1 | 7.62 | Q8BHL4 |
| Krt80 | Keratin, type II cytoskeletal 80 | 2 | 2 | 6.18 | 5.97 | 452 | 50.6 | 6.27 | Q0VBK2 |
| Slc6a14b | Na/Cl-dependent aa transporter B | 2 | 2 | 5.31 | 3.61 | 638 | 71.4 | 7.24 | Q9JMA9 |
| Vima | Vimentin | 2 | 2 | 5.24 | 4.08 | 466 | 53.7 | 5.12 | P20152 |
| Dpysl2 | Dihydropyrimidinase-related protein 2 | 2 | 2 | 5.13 | 5.94 | 572 | 62.2 | 6.38 | O08553 |
| Plec | Plectin | 2 | 2 | 4.91 | 0.43 | 4691 | 533.9 | 5.96 | Q9QXS1 |
| Lgals1 | Galectin-1 | 1 | 2 | 4.27 | 11.85 | 135 | 14.9 | 5.49 | P16045 |
Known O-linked glycosylated protein. bKnown N-linked glycosylated protein.
Bacterial interactions with blotted corneas
Previously we showed that blotting mouse corneas with tissue paper (Kimwipe) rendered the ocular surface more vulnerable to P. aeruginosa adhesion (16, 17). To study how bacteria interact with glycosylated proteins after blotting, corneas were labeled with Ac4GalNAz overnight, then blotted, before exposure to control medium or P. aeruginosa for 1 h. As in our previous studies, blotted eyes were more susceptible to bacterial adhesion: mean ± sd bacterial count per field was 869.5 ± 484.4 (blotted) vs. 90.3 ± 74.8 (nonblotted) (P = 0.043, Student’s t test). As shown in Fig. 6, bacteria (red) on blotted corneas interacted with cells expressing the GalNAz label (green). Blotted corneas displayed what appeared to be a nuclear GalNAz staining in the absence of bacteria (Fig. 6A). However, bacterial exposure for 1 h caused the GalNAz label to concentrate in patches of the plasma membrane in many cells (Fig. 6B). Because only metabolically active cells are capable of presenting the label on the plasma membrane, these brightly stained cells may have been partially buried beneath cells now dead or exfoliated. However, this could not be verified by microscopy because of resolution limitations of the microscope and because of the close and sometimes overlapping association of the first and second layers of corneal epithelial cells.
Figure 6.
P. aeruginosa enhances GalNAz labeling after blotting. WT mouse eyes were incubated in Ac4GalNAz overnight, blotted, and then after 10 min incubated in control MEM (A) or ∼5 × 108 CFU P. aeruginosa (B) for 1 h before addition of DIBAC-biotin and streptavidin–Alexa Fluor 488. GalNAz-labeled mucins (green) are shown as maximum intensity projection; they appear upregulated and redistributed in blotted corneas incubated with bacteria compared to medium-only control (original magnification, ×20 for both corneas). Surfaces were generated in Imaris software to determine bacterial colocalization in 3 dimensions. Images are representative of 3 mouse corneas (A) and 5 mouse corneas (B) analyzed in 2 separate assays.
GalNAz labeling in corneas of MyD88−/− and IL-1R−/− mice and blotted WT mice
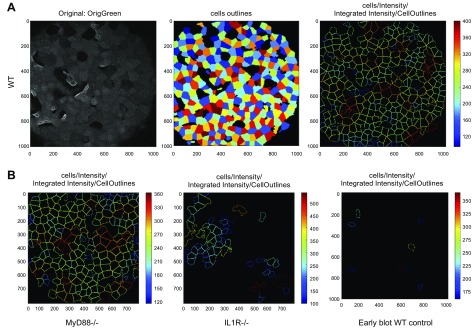
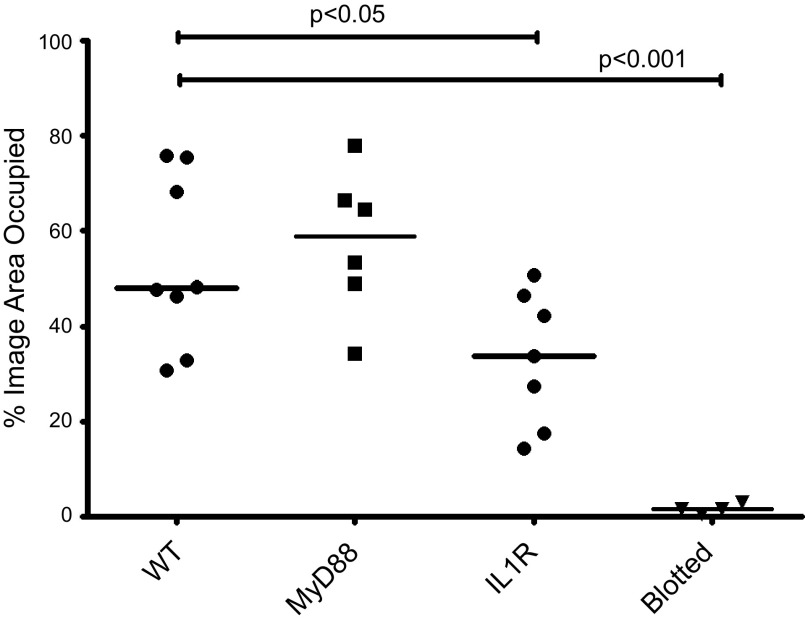
To evaluate the degree of GalNAz labeling across the corneal surface, we developed an image analysis method using the open-source software CellProfiler (31). This software allowed us to identify cells on the corneal surface that had incorporated GalNAz and labeled with DIBAC-biotin and streptavidin–Alexa Fluor 488. Using this image analysis method, we quantified the degree to which MyD88−/− and IL-1R−/− mice incorporated GalNAz compared to WT corneas (Fig. 7). MyD88−/− mice appeared similar to WT, but IL-1R−/− corneas displayed perturbed GalNAz incorporation (Fig. 7A, third panel, compared to Fig. 7B). Labeling of IL-1R−/− corneas also appeared less homogenous across the surface of individual cells compared to WT and MyD88−/− corneas (data not shown). Quantification of CellProfiler data showed significantly reduced GalNAz labeling in the IL-1R−/− corneas and in corneas blotted before Ac4GalNAz exposure (Fig. 8).
Figure 7.
Corneas of IL-1R−/− mice show reduced GalNAz labeling compared to WT and MyD88−/− mice. A) WT mouse corneas were labeled with GalNAz as previously described (left), then analyzed using combination of fluorescence intensity, object shape, and segmentation with CellProfiler software to generate pattern of GalNAz-labeled cells (center) and distinguish those cells independently of cell-to-cell heterogeneity in fluorescence intensity (right). B) Eyes from MyD88−/− mice (left) looked similar to WT, whereas IL-1R−/− mouse corneas showed disrupted (patchy) mucin labeling (center). WT corneas blotted before Ac4GalNAz exposure showed little or no labeling (right). Representative processed images with cell outlines are shown. In total, 8 WT, 6 MyD88−/−, 7 IL-1R−/−, and 4 blotted corneas were analyzed (Fig. 8).
Figure 8.
Significant differences in GalNAz labeling were found between nonblotted WT corneas and IL-1R−/− corneas (P < 0.05, ANOVA), but not between WT and MyD88−/− corneas. WT corneas blotted before Ac4GalNAz exposure showed significantly reduced labeling compared to nonblotted WT (P < 0.001, ANOVA). Each data point represents 1 eye. Bars represent median values.
DISCUSSION
Previously, we showed that corneal epithelial defenses against bacterial adhesion can be compromised by blotting the cornea with tissue paper or by using knockout mice that lack MyD88, an important adaptor protein for TLR and IL-1R-mediated innate defenses. Here, to begin to explore the mechanism or mechanisms for these compromising effects, we tested the hypothesis that both manipulations alter glycosylation. For this purpose, we used a metabolic label (Ac4GalNAz) to image glycosylated proteins at the corneal surface. Ac4GalNAz incubation revealed effective, comprehensive GalNAz labeling of surface corneal epithelial cells in WT and MyD88-knockout mice, with no obvious difference between them. IL-1R-deficient mice, however, showed uneven and disrupted labeling, while tissue paper blotting removed cells from the ocular surface and permeabilized the underlying cell layer. In blotted eyes, the bacteria induced expression of GalNAz-labeled factors within the first hour of bacterial inoculation.
More than 20 yr ago, we reported that mucins rinsed from the rat ocular surface can bind P. aeruginosa and prevent its adherence to the corneal surface (10). Protective effects of ocular mucins against epithelial cell adherence and invasion by nontypable S. pneumoniae have also been shown (9, 32). Muc16 contributes to ocular epithelial barrier function and protects epithelial cells against adherence and invasion of Staphylococcus aureus (8). Given the protective role of mucins against bacterial adhesion, it was expected that the mechanism by which blotting enabled adhesion in this study related to the loss of mucins after this procedure. However, tissue paper blotting also damaged or killed underlying cells, as shown by their uniform permeabilization to DAPI. Indeed, MS analysis revealed many intracellular factors present on the paper used to blot the corneas. Because P. aeruginosa can target exposed basolateral cell surfaces and injured cells (5, 30, 33–35), increased adherence after tissue paper blotting likely follows cell damage, not just removal of any protective glycosylated molecules.
Addition of P. aeruginosa to blotted corneas increased GalNAz labeling at the corneal surface after 1 h and caused a change in GalNAz labeling from the nucleus to the plasma membrane in some cells. New labeling even appeared in areas of the corneal epithelium that were free of bacteria. Because GalNAz is only incorporated into newly glycosylated proteins in metabolically active host cells, this raised the possibility that bacteria, or their secreted factors (e.g., flagellin, LPS), up-regulated mucin expression on epithelial cells. However, another possible explanation for the results is that the bacteria damaged the surface epithelial cells, induced their exfoliation to expose underlying cells, or both. Cell damage could expose intracellular targets to azidosugar incorporation (e.g., nuclear or cytoplasmic proteins) (28, 36), and the exposure of underlying cells could stimulate glycocalyx expression. For example, Gipson and colleagues showed that subapical ocular epithelial cells gained a mucin coat as the superficial layer of cells was shed (37, 38). The same research group also showed that Muc1 mRNA was present in all layers of the corneal epithelium, but the protein only expressed in the apical exposed layer (38). Labeled nuclear and cytoplasmic proteins in blotted murine corneas could also have resulted from GalNAz metabolism to N-azidoacetylglucosamine (GlcNAz). Permeabilization accompanying blotting would have facilitated access of the DIBAC-biotin and streptavidin–Alexa Fluor 488 to those intracellular GlcNAz-labeled proteins. Thus, bacterial-induced cell shedding or blotting-induced injury could each explain the subsequent appearance of GalNAz labeling after bacterial addition. However, we cannot rule out the interesting possibility that bacteria were directly responsible for increased expression of the ocular glycocalyx.
MS analysis of tissue paper blots revealed extensive amounts of GalNAz-labeled murine Muc4, which shares a high degree of homology with human Muc4 (39). Human Muc4 is a membrane-bound glycosylated mucin known to be expressed by ocular surface epithelial cells (20, 40, 41) and known to be localized primarily to the superficial side of surface cells, where it complexes with the growth factor receptor Erb2 (42, 43). It is not clear why other well-known ocular surface membrane-bound mucins (Muc1 and Muc16) or associated proteins (galectin-3) (44) were not identified on the tissue paper blots, GalNAz-labeled or otherwise.
MyD88−/− corneas showed similar GalNAz labeling to WT corneas, suggesting surface glycosylation was not affected by loss of MyD88, at least in total quantity and distribution. While this suggests that susceptibility of MyD88-deficient corneas to P. aeruginosa binding and epithelial traversal does not involve changes in distribution and amount of glycosylation, we cannot rule out the possibility that there are differences in the profile of proteins glycosylated at the tissue surface. However, we have shown that MyD88-deficient corneal lysates exhibit reduced antimicrobial activity against P. aeruginosa, suggesting that other mechanisms are also worthy of exploration (18). Indeed, targeted deletion of MyD88 in intestinal epithelial cells in vivo while compromising expression of Muc2 and antimicrobial peptide expression reduced other epithelial defenses, including the polymeric IgA receptor (45). Mucins have been shown important at mucosal surfaces for sequestering antimicrobial peptides at or near the cell surface (46). Thus, it is feasible that the importance of corneal surface bound mucins in barrier function relates to antimicrobial peptide sequestration rather than a direct role of mucins in preventing adhesion.
IL-1R deficiency did affect the pattern of GalNAz labeling on mouse corneas, suggesting a novel role for this receptor in glycosylation of corneal mucins or other epithelial surface factors. Whatever the mechanism for this regulatory role of IL-1R, it appears to be MyD88 independent, given the normal glycosylation pattern of MyD88-deficient corneas. Although many IL-1R-associated functions depend on MyD88 (47, 48), PI3K association with IL-1R has been demonstrated to activate NF-κB and AP-1 transcription factors via Akt, leading to multiple intracellular signaling events including proinflammatory cytokine expression (49–51). Thus, PI3K/Akt may provide a signaling pathway for IL-1R regulation of ocular surface glycosylation. Alternatively, presence of the IL-1R may influence surface glycosylation independent of intracellular signaling. Although IL-1R signaling promotes ocular surface inflammation and mucin acidification in the context of dry eye diseases (52), we could not find prior evidence for a role in homeostasis of surface glycosylation at the ocular surface or elsewhere. Given the potential use of IL-1R antagonists in the management of dry eye and other ocular surface inflammatory diseases (52–54), the notion that IL-1R affects ocular surface homeostasis warrants further investigation. Considering the blotting procedure removes more than just the cell glycocalyx, other methods will need to be used to study how IL-1R regulates corneal surface glycosylation at the molecular level.
In summary, this study provides insights into the relationship between bacterial binding and glycosylation of the corneal surface. The data suggest that bacterial binding is not dictated purely by the level of glycosylation. Further work must be done to examine the relationship between bacterial adhesion and the expression of glycoproteins at the ocular surface. The methods we used examined the distribution of glycosylation, and it remains possible that blotting and bacterial interactions may change the composition of the specific glycosylated proteins. For example, there might be some glycosylated factors expressed that actually do inhibit bacterial binding, but their effect may have been masked by the presence of other glycosylated proteins that have no effect on bacterial binding, or that actually promote it.
ACKNOWLEDGMENTS
This work was supported, in part, by the National Institutes of Health (National Eye Institute Grants EY011221 and EY024060 to S.M.J.F.; National Cancer Institute Grant CA200423 to C.R.B.; and National Institute of General Medical Sciences Grant GM058867 to C.R.B.). D.R.S. was supported by a predoctoral fellowship from the National Science Foundation. The authors thank J. Singer (University of Maine, Orono, ME, USA) for providing the plasmid construct p67T1 (dTomato), G. Barton (University of California, Berkeley) for providing gene-knockout mice, and M. Gadjeva (Harvard Medical School, Boston, MA, USA) for helpful discussions.
Glossary
- Ac4GalNAz
tetra-acetylated N-azidoacetylgalactosamine
- CFU
colony-forming unit
- DIBAC
dibenzo-aza-cyclooctyne
- FBS
fetal bovine serum
- GalNAz
N-azidoacetylgalactosamine
- GlcNAz
N-azidoacetylglucosamine
- IL-1R
IL-1 receptor
- MEM
minimum essential medium
- MS/MS
tandem MS
- MyD88
myeloid differentiation primary response gene 88
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
A. Jolly, P. Agarwal, M. Metruccio, and D. Spiciarich conducted the experiments; and all authors contributed to the data analysis, writing the article, and research design.
REFERENCES
- 1.Ng A. L., To K. K., Choi C. C., Yuen L. H., Yim S. M., Chan K. S., Lai J. S., Wong I. Y. (2015) Predisposing factors, microbial characteristics, and clinical outcome of microbial keratitis in a tertiary centre in Hong Kong: a 10-year experience. J. Ophthalmol. 2015, 769436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mun J. J., Tam C., Kowbel D., Hawgood S., Barnett M. J., Evans D. J., Fleiszig S. M. (2009) Clearance of Pseudomonas aeruginosa from a healthy ocular surface involves surfactant protein D and is compromised by bacterial elastase in a murine null-infection model. Infect. Immun. 77, 2392–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augustin D. K., Heimer S. R., Tam C., Li W. Y., Le Due J. M., Evans D. J., Fleiszig S. M. (2011) Role of defensins in corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Infect. Immun. 79, 595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mun J. J., Tam C., Evans D. J., Fleiszig S. M. (2011) Modulation of epithelial immunity by mucosal fluid. Sci. Rep. 1, 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleiszig S. M., Evans D. J., Do N., Vallas V., Shin S., Mostov K. E. (1997) Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect. Immun. 65, 2861–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masinick S. A., Montgomery C. P., Montgomery P. C., Hazlett L. D. (1997) Secretory IgA inhibits Pseudomonas aeruginosa binding to cornea and protects against keratitis. Invest. Ophthalmol. Vis. Sci. 38, 910–918 [PubMed] [Google Scholar]
- 7.Ricciuto J., Heimer S. R., Gilmore M. S., Argüeso P. (2008) Cell surface O-glycans limit Staphylococcus aureus adherence to corneal epithelial cells. Infect. Immun. 76, 5215–5220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gipson I. K., Spurr-Michaud S., Tisdale A., Menon B. B. (2014) Comparison of the transmembrane mucins MUC1 and MUC16 in epithelial barrier function. PLoS One 9, e100393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Govindarajan B., Menon B. B., Spurr-Michaud S., Rastogi K., Gilmore M. S., Argüeso P., Gipson I. K. (2012) A metalloproteinase secreted by Streptococcus pneumoniae removes membrane mucin MUC16 from the epithelial glycocalyx barrier. PLoS One 7, e32418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleiszig S. M., Zaidi T. S., Ramphal R., Pier G. B. (1994) Modulation of Pseudomonas aeruginosa adherence to the corneal surface by mucus. Infect. Immun. 62, 1799–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni M., Evans D. J., Hawgood S., Anders E. M., Sack R. A., Fleiszig S. M. (2005) Surfactant protein D is present in human tear fluid and the cornea and inhibits epithelial cell invasion by Pseudomonas aeruginosa. Infect. Immun. 73, 2147–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bräuer L., Kindler C., Jäger K., Sel S., Nölle B., Pleyer U., Ochs M., Paulsen F. P. (2007) Detection of surfactant proteins A and D in human tear fluid and the human lacrimal system. Invest. Ophthalmol. Vis. Sci. 48, 3945–3953 [DOI] [PubMed] [Google Scholar]
- 13.Redfern R. L., Reins R. Y., McDermott A. M. (2011) Toll-like receptor activation modulates antimicrobial peptide expression by ocular surface cells. Exp. Eye Res. 92, 209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDermott A. M., Redfern R. L., Zhang B., Pei Y., Huang L., Proske R. J. (2003) Defensin expression by the cornea: multiple signalling pathways mediate IL-1beta stimulation of hBD-2 expression by human corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 44, 1859–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tam C., Mun J. J., Evans D. J., Fleiszig S. M. (2012) Cytokeratins mediate epithelial innate defense through their antimicrobial properties. J. Clin. Invest. 122, 3665–3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alarcon I., Tam C., Mun J. J., LeDue J., Evans D. J., Fleiszig S. M. (2011) Factors impacting corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Invest. Ophthalmol. Vis. Sci. 52, 1368–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tam C., LeDue J., Mun J. J., Herzmark P., Robey E. A., Evans D. J., Fleiszig S. M. (2011) 3D quantitative imaging of unprocessed live tissue reveals epithelial defense against bacterial adhesion and subsequent traversal requires MyD88. PLoS One 6, e24008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan A. B., Tam K. P., Metruccio M. M., Evans D. J., Fleiszig S. M. (2015) The importance of the Pseudomonas aeruginosa type III secretion system in epithelium traversal depends upon conditions of host susceptibility. Infect. Immun. 83, 1629–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzman-Aranguez A., Argüeso P. (2010) Structure and biological roles of mucin-type O-glycans at the ocular surface. Ocul. Surf. 8, 8–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gipson I. K. (2004) Distribution of mucins at the ocular surface. Exp. Eye Res. 78, 379–388 [DOI] [PubMed] [Google Scholar]
- 21.Woodward A. M., Argüeso P. (2014) Expression analysis of the transmembrane mucin MUC20 in human corneal and conjunctival epithelia. Invest. Ophthalmol. Vis. Sci. 55, 6132–6138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ablamowicz A. F., Nichols J. J. (2016) Ocular surface membrane–associated mucins. Ocul. Surf. 14, 331–341 [DOI] [PubMed] [Google Scholar]
- 23.Sumiyoshi M., Ricciuto J., Tisdale A., Gipson I. K., Mantelli F., Argüeso P. (2008) Antiadhesive character of mucin O-glycans at the apical surface of corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 49, 197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hang H. C., Yu C., Kato D. L., Bertozzi C. R. (2003) A metabolic labeling approach toward proteomic analysis of mucin-type O-linked glycosylation. Proc. Natl. Acad. Sci. USA 100, 14846–14851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navabi N., Johansson M. E., Raghavan S., Lindén S. K. (2013) Helicobacter pylori infection impairs the mucin production rate and turnover in the murine gastric mucosa. Infect. Immun. 81, 829–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laughlin S. T., Baskin J. M., Amacher S. L., Bertozzi C. R. (2008) In vivo imaging of membrane-associated glycans in developing zebrafish. Science 320, 664–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singer J. T., Phennicie R. T., Sullivan M. J., Porter L. A., Shaffer V. J., Kim C. H. (2010) Broad-host-range plasmids for red fluorescent protein labeling of gram-negative bacteria for use in the zebrafish model system. Appl. Environ. Microbiol. 76, 3467–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyce M., Carrico I. S., Ganguli A. S., Yu S. H., Hangauer M. J., Hubbard S. C., Kohler J. J., Bertozzi C. R. (2011) Metabolic cross-talk allows labeling of O-linked beta-N-acetylglucosamine-modified proteins via the N-acetylgalactosamine salvage pathway. Proc. Natl. Acad. Sci. USA 108, 3141–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta S. K., Masinick S., Garrett M., Hazlett L. D. (1997) Pseudomonas aeruginosa lipopolysaccharide binds galectin-3 and other human corneal epithelial proteins. Infect. Immun. 65, 2747–2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bucior I., Mostov K., Engel J. N. (2010) Pseudomonas aeruginosa–mediated damage requires distinct receptors at the apical and basolateral surfaces of the polarized epithelium. Infect. Immun. 78, 939–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamprecht M. R., Sabatini D. M., Carpenter A. E. (2007) CellProfiler: free, versatile software for automated biological image analysis. Biotechniques 42, 71–75 [DOI] [PubMed] [Google Scholar]
- 32.Williamson Y. M., Gowrisankar R., Longo D. L., Facklam R., Gipson I. K., Ades E. P., Carlone G. M., Sampson J. S. (2008) Adherence of nontypeable Streptococcus pneumoniae to human conjunctival epithelial cells. Microb. Pathog. 44, 175–185 [DOI] [PubMed] [Google Scholar]
- 33.Roger P., Puchelle E., Bajolet-Laudinat O., Tournier J. M., Debordeaux C., Plotkowski M. C., Cohen J. H., Sheppard D., de Bentzmann S. (1999) Fibronectin and alpha5beta1 integrin mediate binding of Pseudomonas aeruginosa to repairing airway epithelium. Eur. Respir. J. 13, 1301–1309 [PubMed] [Google Scholar]
- 34.Tsang K. W., Rutman A., Tanaka E., Lund V., Dewar A., Cole P. J., Wilson R. (1994) Interaction of Pseudomonas aeruginosa with human respiratory mucosa in vitro. Eur. Respir. J. 7, 1746–1753 [DOI] [PubMed] [Google Scholar]
- 35.Plotkowski M. C., Tournier J. M., Puchelle E. (1996) Pseudomonas aeruginosa strains possess specific adhesins for laminin. Infect. Immun. 64, 600–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peter-Katalinić J. (2005) Methods in enzymology: O-glycosylation of proteins. Methods Enzymol. 405, 139–171 [DOI] [PubMed] [Google Scholar]
- 37.Gipson I. K., Yankauckas M., Spurr-Michaud S. J., Tisdale A. S., Rinehart W. (1992) Characteristics of a glycoprotein in the ocular surface glycocalyx. Invest. Ophthalmol. Vis. Sci. 33, 218–227 [PubMed] [Google Scholar]
- 38.Inatomi T., Spurr-Michaud S., Tisdale A. S., Gipson I. K. (1995) Human corneal and conjunctival epithelia express MUC1 mucin. Invest. Ophthalmol. Vis. Sci. 36, 1818–1827 [PubMed] [Google Scholar]
- 39.Desseyn J. L., Clavereau I., Laine A. (2002) Cloning, chromosomal localization and characterization of the murine mucin gene orthologous to human MUC4. Eur. J. Biochem. 269, 3150–3159 [DOI] [PubMed] [Google Scholar]
- 40.Hori Y., Spurr-Michaud S., Russo C. L., Argüeso P., Gipson I. K. (2004) Differential regulation of membrane-associated mucins in the human ocular surface epithelium. Invest. Ophthalmol. Vis. Sci. 45, 114–122 [DOI] [PubMed] [Google Scholar]
- 41.Pflugfelder S. C., Liu Z., Monroy D., Li D. Q., Carvajal M. E., Price-Schiavi S. A., Idris N., Solomon A., Perez A., Carraway K. L. (2000) Detection of sialomucin complex (MUC4) in human ocular surface epithelium and tear fluid. Invest. Ophthalmol. Vis. Sci. 41, 1316–1326 [PubMed] [Google Scholar]
- 42.Lomako J., Lomako W. M., Carothers Carraway C. A., Carraway K. L. (2010) Regulation of the membrane mucin Muc4 in corneal epithelial cells by proteosomal degradation and TGF-beta. J. Cell. Physiol. 223, 209–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swan J. S., Arango M. E., Carothers Carraway C. A., Carraway K. L. (2002) An ErbB2-Muc4 complex in rat ocular surface epithelia. Curr. Eye Res. 24, 397–402 [DOI] [PubMed] [Google Scholar]
- 44.Argüeso P., Guzman-Aranguez A., Mantelli F., Cao Z., Ricciuto J., Panjwani N. (2009) Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J. Biol. Chem. 284, 23037–23045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frantz A. L., Rogier E. W., Weber C. R., Shen L., Cohen D. A., Fenton L. A., Bruno M. E., Kaetzel C. S. (2012) Targeted deletion of MyD88 in intestinal epithelial cells results in compromised antibacterial immunity associated with downregulation of polymeric immunoglobulin receptor, mucin-2, and antibacterial peptides. Mucosal Immunol. 5, 501–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thornton D. J., Rousseau K., McGuckin M. A. (2008) Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 70, 459–486 [DOI] [PubMed] [Google Scholar]
- 47.Takeuchi O., Akira S. (2002) MyD88 as a bottle neck in Toll/IL-1 signaling. Curr. Top. Microbiol. Immunol. 270, 155–167 [DOI] [PubMed] [Google Scholar]
- 48.Janssens S., Beyaert R. (2002) A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem. Sci. 27, 474–482 [DOI] [PubMed] [Google Scholar]
- 49.Reddy S. A., Huang J. H., Liao W. S. (1997) Phosphatidylinositol 3-kinase in interleukin 1 signaling. Physical interaction with the interleukin 1 receptor and requirement in NFkappaB and AP-1 activation. J. Biol. Chem. 272, 29167–29173 [DOI] [PubMed] [Google Scholar]
- 50.Reddy S. A., Lin Y. F., Huang H. J., Samanta A. K., Liao W. S. (2004) The IL-1 receptor accessory protein is essential for PI 3-kinase recruitment and activation. Biochem. Biophys. Res. Commun. 316, 1022–1028 [DOI] [PubMed] [Google Scholar]
- 51.Cahill C. M., Rogers J. T. (2008) Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J. Biol. Chem. 283, 25900–25912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vijmasi T., Chen F. Y., Chen Y. T., Gallup M., McNamara N. (2013) Topical administration of interleukin-1 receptor antagonist as a therapy for aqueous-deficient dry eye in autoimmune disease. Mol. Vis. 19, 1957–1965 [PMC free article] [PubMed] [Google Scholar]
- 53.Hou J., Townson S. A., Kovalchin J. T., Masci A., Kiner O., Shu Y., King B. M., Schirmer E., Golden K., Thomas C., Garcia K. C., Zarbis-Papastoitsis G., Furfine E. S., Barnes T. M. (2013) Design of a superior cytokine antagonist for topical ophthalmic use. Proc. Natl. Acad. Sci. USA 110, 3913–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bielory B. P., Shah S. P., O’Brien T. P., Perez V. L., Bielory L. (2016) Emerging therapeutics for ocular surface disease. Curr. Opin. Allergy Clin. Immunol. 16, 477–486 [DOI] [PubMed] [Google Scholar]