Abstract
Erythropoietin (EPO) is the cytokine that regulates red blood cell production. Less understood is the nonerythroid action of EPO, including metabolic regulation of fat accumulation and glucose homeostasis. Although EPO treatment increased hematocrit and improved glucose tolerance in male and female mice, we observed a gender difference in EPO effects in weight control. EPO treatment reduced diet-induced weight gain from 9.6 ± 1.5 to 4.2 ± 1.4 g in male mice (P < 0.001), while the weight gain in female mice was similar (4.7 ± 2.0 g with PBS treatment and 3.3 ± 2.1 g with EPO treatment). EPO treatment also reduced weight gain in ovariectomized female mice, while the effect was abrogated with estradiol supplementation, suggesting that the sex-differential response to EPO was associated with estrogen. Furthermore, mice with targeted deletion of EPO receptor in white adipose tissue exhibited sex-differential phenotype in weight control and glucose sensitivity, and EPO receptor gene expression was reduced in wild-type female mice, suggesting that white adipose tissue plays an integral role in mediating the metabolic effects of EPO. Our data provide evidence for a sex-differential response to EPO in weight control in mice and underscore the potential for gender specific EPO action beyond erythropoiesis.—Zhang, Y., Rogers, H. M., Zhang, X., Noguchi, C. T. Sex difference in mouse metabolic response to erythropoietin.
Keywords: adipose tissue, estrogen, nonhematopoietic, weight loss
The cytokine erythropoietin (EPO) is required for the survival, proliferation, and differentiation of erythroid progenitor cells to produce red blood cells (erythropoiesis). In adults, EPO is primarily produced in the kidney, is secreted into the circulation, and targets erythroid progenitor cells in the bone marrow to stimulate red blood cell production. EPO is used clinically to treat anemia due to low EPO production associated with chronic kidney disease, antiretroviral therapy in HIV, and chemotherapy in patients with cancer. Beyond its indispensable role in erythropoiesis, EPO also functions in nonerythroid tissues, such as endothelium, brain, and adipose tissue (1). For example, in the regulation of oxygen delivery beyond increasing red blood cell production, in endothelial cells EPO can induce the production of nitric oxide, a regulator of vascular tone, as an acute response to increase oxygen delivery (2, 3). EPO also plays a protective role in cardiac ischemia/reperfusion injury and neural ischemic stroke (4–8). Furthermore, EPO can also directly and/or indirectly regulate inflammatory response in animal models of ischemic and traumatic injury, including reducing immune cell response and inhibiting proinflammatory cytokine production (9–12).
Studies in animal models and patients suggest that EPO is involved in metabolic regulation. EPO treatment reduces body mass and improves glycemic control in rodents (13–16), while transgenic mice overexpressing human EPO exhibit higher hematocrit (Hct) accompanied with lower body weight and fat mass, reduced blood glucose and serum insulin levels, and improved glucose tolerance and insulin sensitivity (13). EPO protection in metabolism in rodents is suggested to be mediated via multiple mechanisms, such as improving the pancreatic β cell activities (17, 18), altering hypothalamus proopiomelanocortin production (19), inducing browning of white adipose tissue (WAT) (20), and reducing inflammatory response (21, 22). In Pima Indians with high prevalence of obesity and type 2 diabetes, endogenous EPO levels are associated with percentage weight change per year in a gender-specific manner (23). Early studies of EPO treatment in hemodialysis patients, or repeated phlebotomies in patients with diabetes accompanied by increased endogenous EPO levels, suggested that elevated EPO improves glycemic control and insulin sensitivity (24–26). Healthy young men treated with a single dose of EPO exhibit an acute increase in resting energy expenditure (27), providing further evidence that EPO administration may affect metabolic response.
Previously we discovered that EPO action in nonerythroid tissues stimulated a metabolic response in nonerythroid tissues. While mice with deletion of EPO or EPO receptor (EpoR) died in utero of severe anemia, mice with EpoR expression only in erythroid tissue (ΔEpoRE mice) survived and exhibited normal erythropoiesis (28). However, ΔEpoRE mice also developed obesity and insulin resistance (19). Interestingly, obesity developed earlier and to a greater extent in female ΔEpoRE mice than male ΔEpoRE mice. Female ΔEpoRE mice became noticeably obese and insulin resistant at the age of 4 mo compared to female wild-type (WT) mice, while male ΔEpoRE mice became obese and insulin resistant at 6 mo of age compared to male WT mice (19). These results suggested that there are sex-related differences in the metabolic response due to the loss of EPO signaling in nonerythroid tissues. Gender-specific metabolic effects of EPO have not been previously noted, although EPO exhibited gender-specific ventilatory response to hypoxia (29).
Here we examined the hematopoietic and metabolic response to EPO treatment, including EPO-induced changes in food intake, body weight, body composition, and glucose homeostasis. We found that EPO induced significant weight loss in WT male mice, which was not observed in WT female mice. Of note, EPO-stimulated red blood cell production and improvement in glucose homeostasis were not different between male and female mice. We linked the EPO-associated sex differential metabolic response to estrogen production in female mice using ovariectomy surgery and estradiol pellet implantation. The weight loss effect of EPO in female mice was demonstrated after ovariectomy surgery, while estradiol supplementation abrogated this EPO response in ovariectomized (OVA) female mice. The sex-specific EPO metabolic response was also observed in transgenic EpoR (TgEpoR) mice that lacked EpoR expression in adipose tissue and was explained in part by estrogen suppression of EpoR expression in the adipose tissue. Our results revealed a sex difference in the metabolic function of EPO that is associated with EpoR expression in nonhematopoietic tissues, and this nonerythroid metabolic EPO response is abrogated by the presence of estrogen.
MATERIALS AND METHODS
For animal studies, 16 wk old C57BL/6 mice were maintained under a 12 h light/dark cycle and were fed on either regular chow NIH07 diet (Zeigler Brothers, Gardners, PA, USA) (RCD) or high-fat diet (HFD) with 60% calories from fat (Research Diets, New Brunswick, NJ, USA). Food intake was determined by monitoring food consumption every 2 d for up to 3 wk. Recombinant human epoetin α (Amgen, Thousand Oaks, CA, USA) was administered s.c. at a dose of 3000 U/kg body weight, 3 times a week, for 3 wk. PBS was injected into the control mice. Generation of TgEpoR mice with targeted deletion of EpoR in adipose tissue, using aP2-cre (The Jackson Laboratory, Bar Harbor, ME, USA), and generation of EpoRfloxp/floxp mice were previously described (20). HFD feeding and EPO or PBS treatment were initiated at 6 mo for 3 wk. All animal protocols were conducted under the U.S. National Institutes of Health (NIH) guidelines, approved by the National Institute of Diabetes and Digestive and Kidney Diseases Animal Care and Use Committee, and carried out at the NIH.
For ovariectomy and estradiol pellet implantation, bilateral ovaries were removed from female mice aged 14 wk. OVA mice were allowed 14 d for recovery and the depletion of endogenous estrogen, and then placed on HFD feeding and treated with EPO or PBS. For the pellet implantation, a small incision was made on the lateral side of the neck of the OVA mouse. Then a pocket about 2 cm beyond the incision site was made with forceps, and the placebo or estradiol pellet (Innovative Research of America, Sarasota, FL, USA) was put into the pocket. The estradiol pellet released 5 µg 17β-estradiol per day, lasting for 60 d. This dose was previously demonstrated to establish physiologic estradiol levels in OVA mice (30, 31). Placebo pellets were implanted in another group of OVA mice as control. The day after pellet implantation, mice were fed a HFD and injected with PBS or EPO.
Hct was determined from blood collected from the tail vein of the mice using heparin-coated capillary tubes. The capillary tubes were centrifuged using a micro-Hct centrifuge (Unico, Dayton, NJ, USA), and Hct was measured with a VIN micro-Hct capillary tube reader (Veterinary Information Network Bookstore, Davis, CA, USA).
For metabolic assessment, body composition was measured using the EchoMRI 3-in-1 device (Echo Medical Systems, Houston, TX, USA) by the same person. For the glucose tolerance test, mice were fasted overnight and injected with glucose (2 g/kg body weight, i.p.). Blood glucose levels were measured before glucose injection, then at 15, 30, 60, 90, and 120 min after glucose injection with the AlphaTRAK Glucometer (Abbott Animal Health, Abbott Park, IL, USA). Glucose in serum was determined by conversion to hydrogen peroxide. In brief, mutarotase was used for complete conversion to β-d-glucose, glucose oxidase to produce hydrogen peroxide, ascorbate oxidase to eliminate ascorbic acid interference, and peroxidase and 4-aminoantipyrine to form a quinoneimine dye for quantification at 505 nm according to the manufacturer’s instructions (Autokit Glucose Assay; Wako Diagnostics, Richmond, VA, USA). Serum insulin levels were determined by ELISA using antibody specific for mouse insulin and anti-insulin horseradish peroxidase (HRP) conjugate according to the manufacturer’s instructions (Crystal Chem, Downers Grove, IL, USA). In brief, serum was added to an anti-insulin antibody–coated 96-well plate, anti-insulin HRP conjugate was added, and the amount of bound anti-insulin HRP conjugate was detected using tetramethylbenzidine substrate and absorbance difference between 450 and 630 nm.
For gene expression analysis, in brief, total RNA was extracted from adipose tissue using QIAzol Lysis Reagent, chloroform was added for aqueous phase extraction, total RNA was isolated using an RNeasy spin column following the manufacturer’s instructions (RNeasy Lipid Tissue Mini procedure; Qiagen, Germantown, MD, USA), and cDNA was synthesized using MultiScribe Reverse Transcriptase (Thermo Fisher Scientific, Waltham, MA, USA). Gene expression was quantified by real-time quantitative PCR (qPCR) using SYBR Green dye and qPCR SuperMix containing hot-start Taq DNA polymerase (Roche Applied Science, Indianapolis, IN, USA) and gene-specific primers (Table 1), and an ABI 7900HT Real-Time PCR System (Thermo Fisher Scientific). Results were analyzed by the ΔΔCt method and presented as fold changes compared to controls after normalizing to internal control RPL13a.
TABLE 1.
qPCR primers used for quantification of gene expression
| Primer, 5′–3′ |
||||
|---|---|---|---|---|
| GenBank ID | Gene | Name | Forward | Reverse |
| NM_009438 | RPL13a | Ribosomal protein L13A | AGCCTACCAGAAAGTTTGCTTAC | GCTTCTTCTTCCGATAGTGCATC |
| NM_010149.3 | Epor | EPO receptor (EPOR) | GGGCTCCGAAGAACTTCTGTG | ATGACTTTCGTGACTCACCCT |
| NM_016774 | Atp5b | ATP synthase, H+ transporting mitochondrial F1 complex, β subunit | GGTTCATCCTGCCAGAGACTA | AATCCCTCATCGAACTGGACG |
| NM_009941 | Cox4i1 | Cytochrome c oxidase subunit IV isoform 1 | ATTGGCAAGAGAGCCATTTCTAC | CACGCCGATCAGCGTAAGT |
| NM_007751.3 | Cox8b | Cytochrome c oxidase, subunit VIIIb | TGTGGGGATCTCAGCCATAGT | AGTGGGCTAAGACCCATCCTG |
| NM_029573 | Idh3a | Isocitrate dehydrogenase 3 (NAD+) α (nuclear encoding mito gene; rate-limiting step of TCA) | ACAGGTGACAAGAGGTTTTGC | CTCCCACTGAATAGGTGCTTTG |
| NM_008904.2 | Ppargc1a | PGC1a | TATGGAGTGACATAGAGTGTGCT | CCACTTCAATCCACCCAGAAAG |
For statistical analysis, the data were reported as means ± sd with 95% confidence intervals. Comparisons among multiple groups were performed with ANOVA by XLSTAT (Addinsoft, New York, NY, USA) and SPSS (IBM SPSS, Chicago, IL, USA) software. Comparisons between 2 groups were performed by Student’s t test. Values of P < 0.05 were considered to be statistically significant.
RESULTS
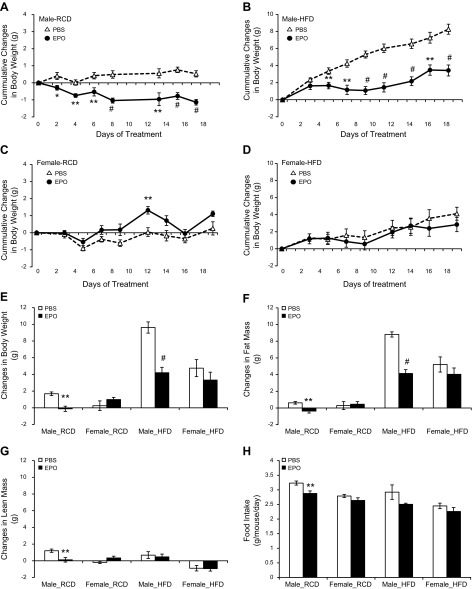
The effect of EPO treatment on body weight and body composition of male and female mice was monitored to determine the gender-specific metabolic EPO response. We treated age-matched male and female mice at 16 wk of age with PBS or EPO (3000 U/kg body weight), 3 times a week for 3 wk, and monitored the changes in body weight (Supplemental Table 1) and body composition (Supplemental Table 2). EPO-treated male mice exhibited a reduction in body weight on regular chow diet (RCD) and a slower accumulation of body mass on HFD (60% kcal fat) compared to PBS treatment (Fig. 1A, B). The body weight of EPO-treated female mice was transiently higher than PBS-treated female mice on RCD (Fig. 1C). Under RCD feeding, PBS-treated male mice gained 1.68 ± 0.49 g, while body weight decreased in EPO-treated male mice by −0.12 ± 0.70 g. In female mice, EPO treatment did not significantly affect weight gain compared to PBS treatment on HFD (4.75 ± 2.04 g with PBS vs. 3.32 ± 2.12 g with EPO) (Fig. 1D, E) or on RCD (0.25 ± 1.42 g with PBS vs. 0.98 ± 0.64 g with EPO) (Fig. 1C, E). Under HFD feeding for 3 wk, PBS-treated male mice gained 9.62 ± 1.50 g, while EPO treatment reduced weight gain in male mice to 4.20 ± 1.45 g (P < 0.001) (Fig. 1E).
Figure 1.
Male and female mice aged 16 wk were treated with PBS (open symbol) or 3000 U/kg EPO (solid symbol) 3 times a week for 3 wk. A, B) Cumulative weight changes in male mice on RCD (A) and HFD (B). C, D) Cumulative weight changes in female mice on RCD (C) and HFD (D). E–G) Summary of changes in body weight (E), fat mass (F), and lean mass (G) before and after PBS or EPO treatment. H) Average food intake in mice treated with PBS or EPO (n = 6 in each group). *P < 0.05; **P < 0.01; #P < 0.001.
Reduction in body weight of EPO-treated male mice was concomitant with a decrease in fat mass (Supplemental Table 2). Under RCD feeding, EPO treatment in male mice decreased fat mass by −0.37 ± 0.54 g, while fat mass in PBS-treated male mice increased by 0.62 ± 0.35 g (P < 0.01). Under HFD feeding, EPO treatment in male mice showed a fat mass increase of 4.13 ± 1.02 g, while PBS-treated male mice increased fat mass by 8.81 ± 0.70 g (P < 0.001) (Fig. 1F). However, the changes in fat mass in female mice were not significantly different between PBS and EPO treatment. Under RCD feeding, EPO-treated female mice gained 0.45 ± 0.75 g fat mass, and PBS-treated female mice gained 0.29 ± 1.16 g fat mass. Under HFD feeding, EPO-treated female mice gained 4.04 ± 1.68 g fat mass, while PBS-treated female mice gained 5.22 ± 1.81 g fat mass (Fig. 1F). The changes in lean mass in response to EPO treatment were only different in male mice on RCD (1.20 ± 0.45 g with PBS vs. 0.12 ± 0.61 g with EPO, P < 0.01), and lean mass was comparable in male mice on HFD treated with PBS (0.68 ± 0.93 g) and EPO (0.47 ± 0.75 g), in female mice on RCD treated with PBS (−0.19 ± 0.33 g) and EPO (0.34 ± 0.52 g), and in female mice on HFD treated with PBS (−0.89 ± 0.66 g) and EPO (−0.94 ± 0.66 g) (Fig. 1G).
Food intake was monitored with EPO treatment on RCD and HFD (Supplemental Table 1). EPO treatment in male mice on RCD reduced the average food intake to 2.85 ± 0.22 g/d per mouse compared to PBS treatment (3.27 ± 0.17 g/d per mouse, P < 0.01). The food intake in female mice was not significantly changed on RCD between PBS treatment (2.79 ± 0.09 g/d per mouse) and EPO treatment (2.64 ± 0.16 g/d per mouse). On HFD feeding, the average food intake in EPO-treated male mice on HFD (2.51 ± 0.33 g/d per mouse) was comparable to the food intake of PBS-treated male mice (2.92 ± 0.29 g/d per mouse). The food intake in EPO-treated female mice (2.26 ± 0.23 g/d per mouse) was also similar to PBS-treated female mice (2.45 ± 0.16 g/d per mouse) on HFD (Fig. 1H).
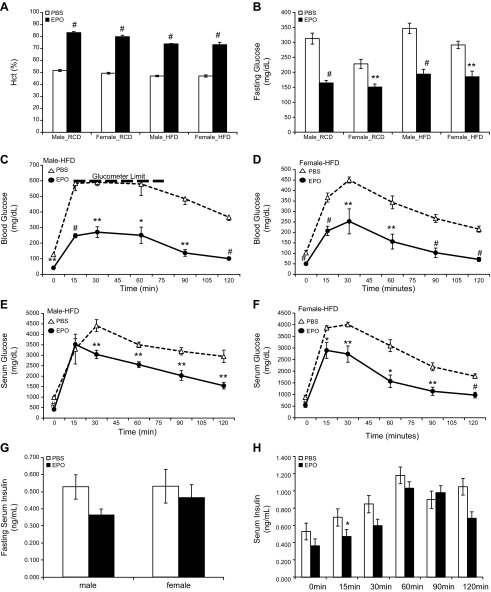
EPO treatment affected Hct and blood glucose level in mice of both genders. EPO-stimulated increase in erythropoiesis was observed in male and female mice on RCD and HFD (Fig. 2A and Supplemental Table 3). On RCD, EPO treatment in male mice increased Hct from 51.6 ± 2.1 to 83.2 ± 1.9% (P < 0.001) and in female mice from 49.3 ± 1.7 to 79.8 ± 3.0% (P < 0.001). On HFD, EPO treatment in male mice increase Hct from 47.0 ± 1.4 to 73.8 ± 0.8% (P < 0.001) and in female mice from 47.0 ± 1.8 to 73.2 ± 4.4% (P < 0.001). The effect of EPO treatment on glycemic control was also monitored (Fig. 2B and Supplemental Table 3). On RCD, EPO treatment in male mice on decreased fasting serum glucose level from 312 ± 41 to 165 ± 19 mg/dl (P < 0.001) and in female mice from 228 ± 37 to 151 ± 24 mg/dl (P < 0.005). On HFD, EPO treatment in male mice decreased fasting serum glucose level from 346 ± 39 to 194 ± 36 mg/dl (P < 0.001) and in female mice from 291 ± 25 to 185 ± 42 mg/dl (P < 0.005).
Figure 2.
Glycemic control in male and female mice treated with PBS or EPO. A) Hct in mice treated with PBS (open bar) or EPO (solid bar). B) Fasting serum glucose levels in male and female mice after PBS (open bar) or EPO (solid bar) treatment (n = 6 in each group). C–F) Blood glucose levels (C, D) and serum glucose levels (E, F) during glucose tolerance test in male (C, E) and female (D, F) mice on HFD with PBS (open symbol) or EPO (solid symbol) treatment (n = 4–5 in each group). Heavy dashed line (C) indicates maximum limit of glucometer reading. G) Fasting serum insulin levels in male and female mice after PBS (open bar) or EPO (solid bar) treatment. H) Fasting serum insulin levels in male mice with PBS (open symbol) or EPO (solid symbol) treatment during glucose tolerance test. *P < 0.05; **P < 0.01; #P < 0.001.
The glucose tolerance test was used to explicitly assess the effect of EPO treatment in HFD-induced glucose intolerance (Fig. 2C–F). EPO treatment improved HFD-induced glucose intolerance in both male and female mice, indicated by the changes in blood glucose levels during the glucose tolerance test (Fig. 2C, D). Because the higher Hct associated with EPO treatment influenced blood glucose levels, we also measured serum glucose levels as an indication of the changes in glucose metabolism. Changes in fasting serum glucose levels also showed that EPO-treated mice exhibited an improved glucose tolerance compared to PBS-treated mice for both males and females (Fig. 2. E, F). The fasting serum insulin levels were not significantly changed by EPO treatment in either male mice or female mice on HFD (Fig. 2G). The fasting insulin levels in male mice were 0.53 ± 0.14 ng/ml with PBS treatment and 0.36 ± 0.07 ng/ml with EPO treatment. In female mice, the fasting serum insulin levels were 0.53 ± 0.24 ng/ml with PBS treatment and 0.46 ± 0.16 ng/ml with EPO treatment.
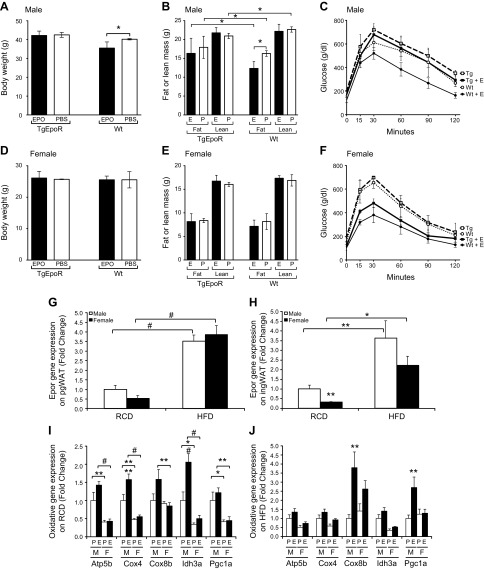
To determine the contribution of adipose tissue response to the metabolic effect of EPO, TgEpoR mice at 6 mo were placed on HFD and treated with EPO or saline for 3 wk (Fig. 3). Male TgEpoR mice were more susceptible to diet-induced obesity, and after 3 wk on HFD (saline treated), the percentage fat mass was 47 ± 3% compared to 41 ± 2% for WT mice (P < 0.05). No difference was observed in percentage fat mass in TgEpoR female mice on HFD (saline treated) at 33 ± 2% compared to percentage fat mass in WT female mice (31 ± 4%). EPO treatment increased Hct similarly in both WT and TgEpoR male and female mice (Supplemental Fig. 1). In male WT mice, EPO administration reduced body weight by about 10% from 40.2 ± 0.4 g with PBS treatment to 35.5 ± 3.3 g (P < 0.05) with EPO treatment (Fig. 3A). Fat mass was also decreased with EPO treatment, dropping from 16.3 ± 0.7 g to 12.3 ± 1.4 g (P < 0.05) with EPO treatment (Fig. 3B). Lean mass was not changed with EPO treatment. EPO treatment also improved glucose tolerance, as indicated by the glucose tolerance test (Fig. 3C). Of note, EPO treatment in male TgEpoR mice did not significantly affect body weight or body composition (Fig. 3. A, B). TgEpoR male mice were more glucose intolerant than WT male mice, and minimal improvement in glucose tolerance was observed with EPO treatment in TgEpoR male mice (Fig. 3C). In female WT mice, body weight did not significantly change with EPO administration (25.6 ± 1.2 g with EPO treatment vs. 25.9 ± 2.6 g with PBS treatment), with no changes in fat mass or lean mass (Fig. 3D, E). Similarly, female TgEpoR mice treated with EPO exhibited no significant change in body weight, fat mass, or lean mass (Fig. 3D, E). Glucose tolerance was not significantly different between WT and TgEpoR female mice, and both WT and TgEpoR female mice showed significant improvement with EPO treatment in glucose tolerance (Fig. 3F).
Figure 3.
Contribution of WAT to EPO metabolic response. A–F) Male (A–C) and female (D–F) WT mice and TgEpoR mice at 6 mo of age were fed HFD and treated with EPO (solid bar: A, B, D, E; solid line: C, F; bold for TgEpoR) or PBS (open bar: A, B, D, E; dashed line: TgEpoR; dotted line: WT) for 3 wk, and body weight (A, D), fat and lean mass (B, E), and glucose tolerance test (C and F) were determined. G–J) EpoR and oxidative gene expression in adipose tissue isolated from male and female mice treated with PBS or EPO was quantified. EpoR gene expression in pgWAT (G) and ingWAT (H) isolated from male (open bar) and female (solid bar) mice fed on RCD and HFD was determined. Oxidative gene expression in ingWAT isolated from male (M) and female (F) mice fed RCD (I) and HFD (J) treated with PBS (P; open bar) or EPO (E; solid bar) was assessed (n = 4–6 in each group). *P < 0.05; **P < 0.01; #P < 0.001. For glucose tolerance (C, F), P values are indicated for comparison between PBS and EPO for WT mice; P values in brackets indicate comparison in TgEpoR mice.
To determine the potential gender difference in EpoR in adipose tissue, EpoR expression in WT mice was determined in the perigonadal WAT (pgWAT; visceral fat) and inguinal WAT (ingWAT; subcutaneous fat) isolated from male and female mice on RCD and HFD. The difference in EpoR expression in the pgWAT was not significant between female and male mice (Fig. 3G). In ingWAT, female mice on RCD expressed 67% less (0.33 ± 0.09–fold) EpoR normalized to age-matched male mice on RCD, and EpoR expression on HFD was 40% lower in female mice (2.22 ± 1.36–fold) compared to male mice (3.64 ± 2.50–fold) (Fig. 3H). Expression of mitochondrial oxidative genes in the WAT, such as Cox4, Ih3a, and Pgc1a, were up-regulated by EPO treatment in the ingWAT of male mice on RCD (up to 2-fold) and on HFD (up to 4-fold), but the increases were not evident in the ingWAT of female mice on RCD or HFD (Fig. 3I, J). The effects of EPO treatment on mitochondrial oxidative gene expression in pgWAT were less apparent (Supplemental Fig. 2A, B).
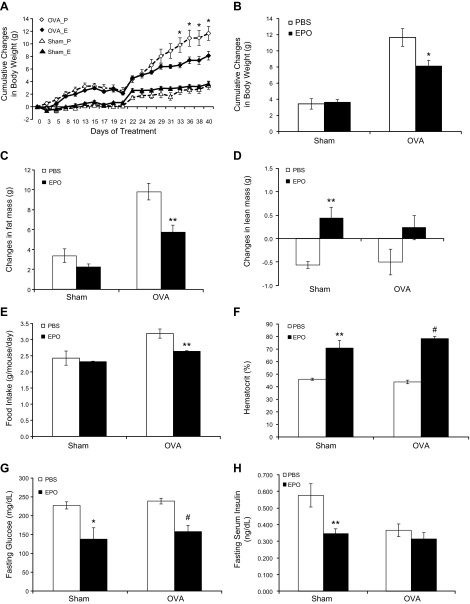
The influence of female hormones in EPO metabolic response was assessed using OVA surgery to eliminate production of ovarian hormones at 14 wk of age. During the first 3 wk after OVA surgery and on RCD feeding, PBS-treated OVA mice gained more body weight than PBS-treated sham-surgery control mice, but the body weight was not significantly different comparing the PBS-treated OVA mice and EPO-treated OVA mice (Fig. 4A and Supplemental Table 4). After 3 wk of RCD, mice were put on HFD feeding. After an additional 3 wk on HFD, EPO-treated OVA mice exhibited a reduction in body weight gain of 8.12 ± 1.69 g compared to weight gain in PBS-treated OVA mice of 11.66 ± 2.70 g (P < 0.01) (Fig. 4A, B). In contrast, there was no significant difference in body weight gain between EPO-treated sham-surgery mice (3.64 ± 0.79 g) and PBS-treated sham-surgery mice (3.42 ± 1.45 g). The reduced body weight in EPO-treated OVA mice was mainly attributed to the reduction in fat mass (Supplemental Table 5), and the changes of fat mass in EPO-treated OVA mice (5.74 ± 1.73 g) was lower than that in PBS-treated OVA mice (9.80 ± 2.06 g) (P < 0.01) (Fig. 4C). However, the changes of lean mass were not significantly different in EPO-treated OVA mice (0.23 ± 0.63 g) from that in PBS-treated OVA mice (−0.50 ± 0.67 g) (Fig. 4D). The average food intake in PBS-treated OVA mice at 3.19 ± 0.35 g/d per mouse was reduced by EPO treatment to 2.64 ± 0.05 g/d per mouse (P < 0.005) (Supplemental Table 4). Nonetheless, the food intake was not significantly different between PBS-treated sham-surgery mice (2.43 ± 0.54 g/d per mouse) and EPO-treated sham-surgery mice (2.32 ± 0.04 g/d per mouse) (Fig. 4E).
Figure 4.
Weight-loss effect of EPO demonstrated in OVA mice. A) Body weight of sham-surgery (triangle) and OVA (diamond) mice with PBS (P; open symbol) or EPO (E; solid symbol) treatment. B–H) Summary of changes during 6 wk PBS (open bar) or EPO (solid bar) treatment in sham-surgery control and OVA mice for cumulative change in body weight (B), cumulative change in fat mass (C), lean mass (D) on HFD, average food intake (E), Hct (F), fasting serum glucose levels (G), and fasting serum insulin levels (H) (n = 6 in each group). *P < 0.05; **P < 0.01; #P < 0.001.
As observed for EPO treatment in male and female mice (Fig. 2), EPO significantly increased Hct in both sham-surgery mice and OVA mice (Fig. 4F and Supplemental Table 6). EPO treatment in sham-surgery mice increased Hct from 46.0 ± 1.9 to 70.8 ± 14.9% (P < 0.01), and EPO treatment in OVA mice increased Hct from 43.8 ± 3.5 to 78.4 ± 4.2% (P < 0.001). EPO treatment also significantly reduced fasting serum glucose levels in sham-surgery mice (from 227.33 ± 23.14 to 137.67 ± 74.07 mg/dl; P < 0.05), as well as in OVA mice (from 238.83 ± 18.59 to 157.83 ± 40.92 mg/dl; P < 0.001) (Fig. 4G). Fasting serum insulin levels were reduced by EPO treatment in sham-surgery mice (from 0.58 ± 0.17 to 0.35 ± 0.07 ng/ml; P < 0.01), but were not significantly altered by EPO treatment in OVA mice (from 0.37 ± 0.09 ng/ml with PBS treatment and 0.31 ± 0.09 ng/ml with EPO treatment; Fig. 4H).
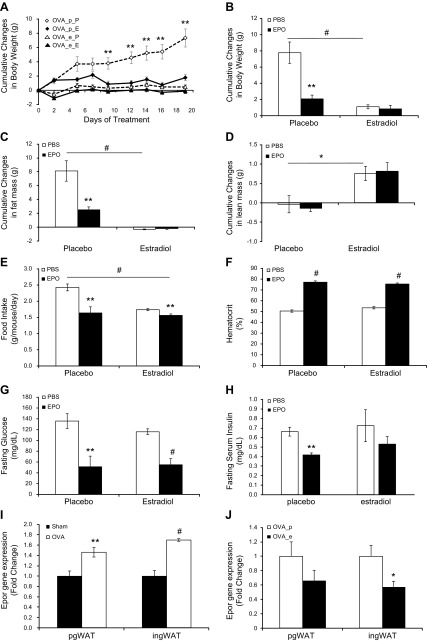
To confirm the potential for estradiol to affect metabolic EPO response, OVA mice were supplemented with estradiol and treated with EPO. Estradiol supplementation and treatment of OVA mice with either estradiol or EPO markedly reduced body weight gain on HFD (Supplemental Table 7). The cumulative weight change in PBS-treated OVA mice with implanted 17β-estradiol pellets after 3 wk HFD feeding at 1.10 ± 0.61 g was lower than that in PBS-treated OVA mice with placebo pellets at 7.77 ± 3.23 g (P < 0.001) (Fig. 5A, B). OVA mice with placebo pellets receiving EPO treatment also had a reduction in weight gain of 2.08 ± 1.13 g compared to PBS-treated OVA mice with placebo pellets (P < 0.01) (Fig. 5A, B). However, the combination of estradiol pellets and EPO treatment did not induce further weight loss in OVA mice compared to estradiol pellets alone, as the weight changes were not significantly different between PBS-treated OVA mice with estradiol pellets and EPO-treated OVA mice with estradiol pellets at 0.84 ± 1.04 g (Fig. 5B).
Figure 5.
Estradiol supplementation abrogated EPO effect in weight change in OVA mice. A) Cumulative changes in body weight of placebo (p) or estradiol (e) pellet-supplemented OVA mice treated with PBS (P) or EPO (E), respectively (n = 6 in each group). B–H) Summary of changes in placebo or estradiol pellet-supplemented OVA mice with PBS (open bar) or EPO (solid bar) treatment on HFD for cumulative change in body weight (B), cumulative change in fat mass (C), lean mass (D), average food intake (E), Hct (F), fasting serum glucose levels (G), and fasting serum insulin levels (H) (n = 6 in each group). I, J) EpoR expression in pgWAT and ingWAT isolated from (I) sham-surgery mice (solid bar) and OVA mice (open bar) fed on HFD and from (J) OVA mice supplemented with placebo pellets (OVA_p; open bar) or estradiol pellets (OVA_e; solid bar) (n = 4–6 in each group). *P < 0.05; **P < 0.01; #P < 0.001.
The cumulative changes in fat mass were consistent with changes in body weight (Fig. 5C and Supplemental Table 8). Compared to PBS-treated OVA mice with placebo pellets, which gained 8.11 ± 3.65 g fat mass, EPO treatment reduced the increase in fat mass to 2.48 ± 1.01 g (P < 0.01). Similarly, estradiol supplementation also reduced the increase in fat mass in OVA mice, and the cumulative change in fat mass in PBS-treated OVA mice with estradiol pellets was −0.33 ± 0.15 g (P < 0.001). Interestingly, EPO treatment in OVA mice implanted with placebo pellets did not alter the cumulative change in lean mass, which was −0.14 ± 0.22 g with EPO treatment compared to −0.04 ± 0.53 g with PBS treatment (Fig. 5D). In contrast, estradiol supplementation increased lean mass in OVA mice to 0.76 ± 0.45 g (P < 0.05) (Fig. 5D). Food intake in PBS-treated OVA mice with implanted placebo pellets at 2.43 ± 0.26 g/d per mouse was reduced by EPO treatment to 1.64 ± 0.46 g/d per mouse (P < 0.01) and was reduced by estradiol supplementation to 1.74 ± 0.08 g/d per mouse (P < 0.001) (Fig. 5E and Supplemental Table 7).
Erythropoietic EPO response was similar in OVA mice implanted with either estradiol or placebo pellets (Fig. 5F and Supplemental Table 9). EPO treatment increased Hct in OVA mice with placebo pellets to 77.3 ± 2.2% compared to PBS-treated OVA mice with placebo pellets with Hct of 50.5 ± 2.6%. EPO treatment in OVA mice with estradiol supplementation increased Hct to 75.5 ± 2.3% compared to PBS-treated OVA mice with implanted estradiol pellets and Hct of 53.5 ± 2.6%. Moreover, EPO treatment reduced fasting serum glucose levels in the OVA mice with placebo pellets to 51.25 ± 39.24 mg/dl compared to serum glucose with PBS treatment of 135.83 ± 33.80 mg/dl (P < 0.01). EPO treatment also reduced fasting serum glucose in the OVA mice with estradiol pellets to 55.00 ± 22.66 mg/dl compared to PBS-treatment level of 116.00 ± 13.33 mg/dl (P < 0.001) (Fig. 5G). In OVA mice implanted with placebo pellets, fasting serum insulin levels were reduced from 0.66 ± 0.11 ng/ml in PBS-treated OVA mice to 0.42 ± 0.04 ng/ml (P < 0.01) in EPO-treated OVA mice, while fasting serum insulin levels were not significantly altered by EPO treatment in OVA mice with estradiol pellets, at 0.53 ± 0.20 ng/ml with EPO treatment and 0.72 ± 0.41 ng/ml with PBS treatment (Fig. 5H).
Of note, EpoR gene expression was higher in the WAT of OVA mice than sham-surgery control mice, increasing 1.46 ± 0.32–fold in pgWAT (P < 0.01) and 1.70 ± 0.05–fold in ingWAT (P < 0.001) (Fig. 5I). When OVA mice were supplemented with estradiol pellets, EpoR expression was decreased to 0.57 ± 0.26–fold with estradiol pellets compared to PBS pellets in the ingWAT (P < 0.05), and in pgWAT EpoR expression tended to be 0.66 ± 0.42–fold with estradiol pellets compared to PBS pellets (Fig. 5J).
DISCUSSION
While recombinant human EPO has been used in the clinic for over 2 decades, research demonstrating the sex difference in response to EPO is limited. The increased rate in weight gain in female ΔEpoRE mice compared to male ΔEpoRE mice (19) led us to examine the metabolic response to exogenous EPO in age-matched WT male mice and WT female mice. We found that EPO stimulation of red blood cell production and the resultant increase in Hct were comparable in mice fed on RCD or HFD, and that the increase in Hct with EPO treatment was not significantly different between male and female mice, exemplifying the gender-independent hematopoietic effect of EPO. The EPO effect in improving glucose tolerance was also observed in both male and female mice. However, EPO treatment for 3 wk in male mice significantly reduced body weight and fat mass, which was not seen in female mice. Additionally, with EPO treatment in male mice, food intake was reduced on RCD and tended to be lower on HFD, while in female mice, little difference was apparent. In contrast to WT control female mice, OVA animals lacking ovary-derived female hormones exhibited the weight-loss effect of EPO, demonstrating the negative influence of female hormones on this EPO activity. The effect of estrogen on the metabolic response to EPO in female mice was shown by estradiol supplementation in OVA mice. While estradiol supplementation or EPO treatment alone decreased body weight in OVA mice, these effects were not additive: EPO treatment did not further enhance the weight-loss effect seen with estradiol alone in OVA mice, analogous to the absence of weight loss with EPO treatment in WT female mice.
In contrast to EPO doses used to promote erythropoiesis, here we used high-dose EPO to stimulate EPO action in nonhematopoietic tissue. Previously, as in the current study, EPO treatment at 3000 U/kg 3 times a week was used to demonstrate loss of body weight in WT male mice (19). High-dose EPO was also used other in mouse studies of EPO action in diabetes such as 7500 U/kg EPO 3 times a week in a diabetic mouse model for EPO protection of pancreatic β cells (17) and 180 U 3 times a week in a mouse model of diabetes/obesity to reduce body mass (13). As with the EPO effect on Hct, reduction of body weight gain and fat mass by EPO is dose dependent beginning at 150 U/kg 3 times a week administered subcutaneously in mice for 5 wk (14).
Although previous animal studies have reported EPO effects in weight loss and reducing fat mass, the experiments were carried out predominantly in male rodents, and a gender-associated differential metabolic response was not observed (14, 15, 19). To our knowledge, our data are the first to demonstrate the sex difference in the metabolic response to EPO treatment. Gender-specific EPO response was previously observed in the ventilatory response in rodents. EPO enhanced the ventilatory response to hypoxia in a sex-dependent manner, with female mice coping better with reduced oxygenation while male mice exhibited a larger ventilatory response to intracisternal injections of soluble EpoR (29, 32–34). The protective effect of EPO against cisplatin-induced nephrotoxicity was stronger in male rats than female rats (35), and the neuroprotective effect of EPO provided after neonatal stroke seemed to be more effective in female rats than male rats (36). In addition, EPO reduced postischemic structural damage while preserving kidney function, particularly in male rats (37). While a detailed mechanism for these differential gender responses to EPO was not elucidated, estrogen was proposed to be involved in meditating EPO activity on respiration and ventilation in the brain (32, 33). The data presented here illustrate the gender-independent effect of EPO stimulated increase in Hct that is also not affected by RCD or HFD, by OVA in female mice, or by estrogen supplementation in OVA mice. EPO was previously shown to improve glycemic control by reducing fasting blood glucose levels, particularly enhancing glucose tolerance in HFD-fed male mice (17, 19). We also observed that EPO treatment improved glucose tolerance in WT mice in a gender-independent manner. These results suggest that the gender-differential effect of EPO on weight control is not related directly to the effect of EPO on glucose metabolism.
On the basis of our observation that EPO treatment significantly reduced body weight and fat mass in male mice, but not in female mice, we proposed that estrogen might be responsible for the gender difference in EPO metabolic response and may interfere or compete with the metabolic action of EPO. We determined the involvement of female hormones in EPO response using ovariectomy surgery, which was shown to eliminate the protective effect of ovarian hormones on susceptibility to obesity in female mice (31, 38). Furthermore, in order to specifically demonstrate the role of estrogen in modifying EPO response, we also implanted 17β-estradiol pellets into OVA mice 2 wk after the ovariectomy surgery, when endogenous estrogen was depleted. The 17β-estradiol pellet released 5 µg 17β-estradiol per day, which was shown to reestablish physiologic estradiol levels in OVA mice (30, 31). We found that estrogen can account for the sex difference in the metabolic response to EPO, as EPO treatment stimulated weight loss in OVA mice but did not induce further weight loss in estradiol pellet–supplemented OVA mice. Several reports suggest that the overall production of EPO is suppressed by estrogen in rodents under normoxia or hypoxia conditions (39, 40), although EPO production in female reproductive organs is stimulated by estrogen for the regulation of the estrus cycles (41, 42). However, suppression of EPO production is not likely to be the major reason for abrogated EPO response in female mice because the dose of exogenous EPO treatment used here is considerably higher than the endogenous EPO levels, which minimizes the influence of changes in endogenous EPO production.
As for other mechanisms by which estrogen alters the EPO response, we propose 2 possibilities. The first is that estrogen does not directly interact with EPO activity but, as shown in OVA mice, exerts similar, albeit stronger, effect in weight control compared to EPO, which masks the effect of EPO when present in combination. The second possibility is that estrogen directly interferes with EPO action to suppress the metabolic effect of EPO. To determine whether estrogen can directly interfere with EPO action, we examined EpoR expression. EPO acts by binding to its cell surface receptor EpoR, and the extent of EpoR expression determines EPO response. EpoR is expressed at the highest levels on erythroid progenitor/precursor cells, and EpoR in WAT is up to half that observed in erythroid tissues and about an order of magnitude higher than other nonerythroid tissues such as kidney, heart, muscle, and brown fat (19). We found that EpoR expression in WAT is lower in female mice than in male mice. Furthermore, ovariectomy resulted in an increased EpoR expression in WAT, while estradiol supplementation in OVA mice reduced EpoR expression in WAT. These results demonstrate that estradiol suppresses EpoR expression in the adipose tissue in vivo in female mice and may explain, in part, the absence of EPO regulation of fat mass accumulation in female mice. Interestingly, TgEpoR mice exhibit increased sensitivity to diet-induced obesity and glucose intolerance, suggesting that adipose tissue response contributes importantly to the metabolic activity of EPO (20). Our results show that increased sensitivity to diet-induced obesity in TgEpoR mice is observed only in male mice and not female mice, suggesting that gender-associated expression pattern of EpoR in the WAT contributes to the gender difference in response to EPO treatment.
Our findings may have potential clinical relevance in understanding the metabolic function of EPO. Recently we reported on the possible association between endogenous EPO levels and body weight changes in full-heritage Southwestern Native Americans, an ethnic population known to exhibit increased susceptibility to type 2 diabetes (23). In a substudy of 109 individuals, the association between plasma EPO levels and weight changes per year exhibited opposing behaviors in men and women. Higher plasma EPO was negatively associated with weight change per year in men but was positively associated with weight change per year in women. These findings suggest that sex hormones may modulate nonhematopoietic EPO response, and they raised the possibility that EPO metabolic activity in males is protective against obesity, while in females estrogen-regulated EPO production in reproductive organs (41, 42) and blood loss with regular menses may provide a signal to maintain or increase energy stores in preparation for reproduction (23). This is consistent with our animal studies, which suggest that the association of EPO treatment with weight loss is clearly evident in male mice but not in female mice. The studies reported here of EPO treatment in mice reveal a sex-differential response in the metabolic effect of EPO on weight control, and they identify estrogen as the major regulator of the gender-specific EPO response. Studies of the interaction between estrogen and EPO signaling might further elucidate the direct and indirect contribution of sex hormone to gender-specific action of EPO treatment, particularly in nonhematopoietic tissues. For example, preliminary data from primary adipocytes isolated from male and female mice suggest that EPO and estradiol similarly stimulate p38 MAPK activation in a gender-specific manner. Further studies to identify signaling pathways with potential gender-specific response to EPO and estradiol are warranted.
CONCLUSIONS
EPO treatment significantly reduced HFD-induced weight gain and accumulation of fat in male mice but not in female mice, which may be associated with the gender-differential expression of EpoR and the effect of the female sex hormone estrogen. The role of adipose tissue in this gender-associated EPO metabolic response was further demonstrated in TgEpoR mice. Furthermore, EPO protection against HFD-induced weight gain was recovered in female mice after ovariectomy surgery to eliminate ovarian hormones and was abrogated in female OVA mice with implantation of estradiol pellets. This sex-specific response to EPO of weight regulation is in contrast to the gender-independent EPO stimulated erythropoiesis. Our data emphasized the sex difference in the metabolic response to EPO treatment, calling for the consideration of potential gender-specific EPO action beyond erythropoiesis.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases and the U.S. National Institutes of Health.
Glossary
- ΔEpoRE
erythropoietin receptor expression only in erythroid tissue
- EPO
erythropoietin
- EpoR
erythropoietin receptor
- Hct
hematocrit
- HFD
high-fat diet
- HRP
horseradish peroxidase
- ingWAT
inguinal white adipose tissue
- OVA
ovariectomized
- pgWAT
perigonadal white adipose tissue
- qPCR
quantitative PCR
- RCD
regular chow diet
- TgEpoR
transgenic erythropoietin receptor
- WAT
white adipose tissue
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Y. Zhang designed and performed the experiments, analyzed the data, and wrote the article; H. M. Rogers performed experiments and analyzed the data; X. Zhang performed the ovariectomy surgery; and C. T. Noguchi developed the concept, supervised the study, and wrote the article.
REFERENCES
- 1. Zhang Y., Wang L., Dey S., Alnaeeli M., Suresh S., Rogers H., Teng R., Noguchi C. T. (2014) Erythropoietin action in stress response, tissue maintenance and metabolism. Int. J. Mol. Sci. 15, 10296–10333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beleslin-Cokic B. B., Cokic V. P., Yu X., Weksler B. B., Schechter A. N., Noguchi C. T. (2004) Erythropoietin and hypoxia stimulate erythropoietin receptor and nitric oxide production by endothelial cells. Blood 104, 2073–2080 [DOI] [PubMed] [Google Scholar]
- 3.Beleslin-Čokić B. B., Cokić V. P., Wang L., Piknova B., Teng R., Schechter A. N., Noguchi C. T. (2011) Erythropoietin and hypoxia increase erythropoietin receptor and nitric oxide levels in lung microvascular endothelial cells. Cytokine 54, 129–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parsa C. J., Matsumoto A., Kim J., Riel R. U., Pascal L. S., Walton G. B., Thompson R. B., Petrofski J. A., Annex B. H., Stamler J. S., Koch W. J. (2003) A novel protective effect of erythropoietin in the infarcted heart. J. Clin. Invest. 112, 999–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright G. L., Hanlon P., Amin K., Steenbergen C., Murphy E., Arcasoy M. O. (2004) Erythropoietin receptor expression in adult rat cardiomyocytes is associated with an acute cardioprotective effect for recombinant erythropoietin during ischemia–reperfusion injury. FASEB J. 18, 1031–1033 [DOI] [PubMed] [Google Scholar]
- 6.Teng R., Calvert J. W., Sibmooh N., Piknova B., Suzuki N., Sun J., Martinez K., Yamamoto M., Schechter A. N., Lefer D. J., Noguchi C. T. (2011) Acute erythropoietin cardioprotection is mediated by endothelial response. Basic Res. Cardiol. 106, 343–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrenreich H., Kästner A., Weissenborn K., Streeter J., Sperling S., Wang K. K., Worthmann H., Hayes R. L., von Ahsen N., Kastrup A., Jeromin A., Herrmann M. (2011) Circulating damage marker profiles support a neuroprotective effect of erythropoietin in ischemic stroke patients. Mol. Med. 17, 1306–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrenreich H., Weissenborn K., Prange H., Schneider D., Weimar C., Wartenberg K., Schellinger P. D., Bohn M., Becker H., Wegrzyn M., Jähnig P., Herrmann M., Knauth M., Bähr M., Heide W., Wagner A., Schwab S., Reichmann H., Schwendemann G., Dengler R., Kastrup A., Bartels C., Group E. P. O. S. T.; EPO Stroke Trial Group (2009) Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke 40, e647–e656 [DOI] [PubMed] [Google Scholar]
- 9.Villa P., Bigini P., Mennini T., Agnello D., Laragione T., Cagnotto A., Viviani B., Marinovich M., Cerami A., Coleman T. R., Brines M., Ghezzi P. (2003) Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J. Exp. Med. 198, 971–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rui T., Feng Q., Lei M., Peng T., Zhang J., Xu M., Abel E. D., Xenocostas A., Kvietys P. R. (2005) Erythropoietin prevents the acute myocardial inflammatory response induced by ischemia/reperfusion via induction of AP-1. Cardiovasc. Res. 65, 719–727 [DOI] [PubMed] [Google Scholar]
- 11.Nairz M., Schroll A., Moschen A. R., Sonnweber T., Theurl M., Theurl I., Taub N., Jamnig C., Neurauter D., Huber L. A., Tilg H., Moser P. L., Weiss G. (2011) Erythropoietin contrastingly affects bacterial infection and experimental colitis by inhibiting nuclear factor-κB-inducible immune pathways. Immunity 34, 61–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng R., Zhu D., Bi Y., Yang D., Wang Y. (2013) Erythropoietin inhibits gluconeogenesis and inflammation in the liver and improves glucose intolerance in high-fat diet–fed mice. PLoS One 8, e53557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz O., Stuible M., Golishevski N., Lifshitz L., Tremblay M. L., Gassmann M., Mittelman M., Neumann D. (2010) Erythropoietin treatment leads to reduced blood glucose levels and body mass: insights from murine models. J. Endocrinol. 205, 87–95 [DOI] [PubMed] [Google Scholar]
- 14.Foskett A., Alnaeeli M., Wang L., Teng R., Noguchi C. T. (2011) The effects of erythropoietin dose titration during high-fat diet–induced obesity. J. Biomed. Biotechnol. 2011, 373781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikolás E., Cseh J., Pap M., Szijárto I. A., Balogh A., Laczy B., Bekő V., Fisi V., Molnár G. A., Mérei A., Szeberényi J., Wittmann I. (2012) Effects of erythropoietin on glucose metabolism. Horm. Metab. Res. 44, 279–285 [DOI] [PubMed] [Google Scholar]
- 16.Caillaud C., Mechta M., Ainge H., Madsen A. N., Ruell P., Mas E., Bisbal C., Mercier J., Twigg S., Mori T. A., Simar D., Barrès R. (2015) Chronic erythropoietin treatment improves diet-induced glucose intolerance in rats. J. Endocrinol. 225, 77–88 [DOI] [PubMed] [Google Scholar]
- 17.Choi D., Schroer S. A., Lu S. Y., Wang L., Wu X., Liu Y., Zhang Y., Gaisano H. Y., Wagner K. U., Wu H., Retnakaran R., Woo M. (2010) Erythropoietin protects against diabetes through direct effects on pancreatic beta cells. J. Exp. Med. 207, 2831–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L. N., Sun Q., Liu S. Q., Hu H., Lv J., Ji W. J., Wang M., Chen M. X., Zhou J. (2015) Erythropoietin improves glucose metabolism and pancreatic β-cell damage in experimental diabetic rats. Mol. Med. Rep. 12, 5391–5398 [DOI] [PubMed] [Google Scholar]
- 19.Teng R., Gavrilova O., Suzuki N., Chanturiya T., Schimel D., Hugendubler L., Mammen S., Yver D. R., Cushman S. W., Mueller E., Yamamoto M., Hsu L. L., Noguchi C. T. (2011) Disrupted erythropoietin signalling promotes obesity and alters hypothalamus proopiomelanocortin production. Nat. Commun. 2, 520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L., Teng R., Di L., Rogers H., Wu H., Kopp J. B., Noguchi C. T. (2013) PPARα and Sirt1 mediate erythropoietin action in increasing metabolic activity and browning of white adipocytes to protect against obesity and metabolic disorders. Diabetes 62, 4122–4131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lifshitz L., Tabak G., Gassmann M., Mittelman M., Neumann D. (2010) Macrophages as novel target cells for erythropoietin. Haematologica 95, 1823–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alnaeeli M., Noguchi C. T. (2015) Erythropoietin and obesity-induced white adipose tissue inflammation: redefining the boundaries of the immunometabolism territory. Adipocyte 4, 153–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reinhardt M., Dey S., Tom Noguchi C., Zhang Y., Krakoff J., Thearle M. S. (2016) Non-hematopoietic effects of endogenous erythropoietin on lean mass and body weight regulation. Obesity (Silver Spring) 24, 1530–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bofill C., Joven J., Bages J., Vilella E., Sans T., Cavallé P., Miralles R., Llobet J., Camps J. (1994) Response to repeated phlebotomies in patients with non-insulin-dependent diabetes mellitus. Metabolism 43, 614–620 [DOI] [PubMed] [Google Scholar]
- 25.Mak R. H. (1996) Effect of recombinant human erythropoietin on insulin, amino acid, and lipid metabolism in uremia. J. Pediatr. 129, 97–104 [DOI] [PubMed] [Google Scholar]
- 26.Mak R. H. (1998) Metabolic effects of erythropoietin in patients on peritoneal dialysis. Pediatr. Nephrol. 12, 660–665 [DOI] [PubMed] [Google Scholar]
- 27.Christensen B., Vendelbo M. H., Krusenstjerna-Hafstrom T., Madsen M., Pedersen S. B., Jessen N., Moller N., Jorgensen J. O. (2012) Erythropoietin administration acutely stimulates resting energy expenditure in healthy young men. J. Appl. Physiol. 112, 1114–1121 [DOI] [PubMed] [Google Scholar]
- 28.Suzuki N., Ohneda O., Takahashi S., Higuchi M., Mukai H. Y., Nakahata T., Imagawa S., Yamamoto M. (2002) Erythroid-specific expression of the erythropoietin receptor rescued its null mutant mice from lethality. Blood 100, 2279–2288 [DOI] [PubMed] [Google Scholar]
- 29.Soliz J., Thomsen J. J., Soulage C., Lundby C., Gassmann M. (2009) Sex-dependent regulation of hypoxic ventilation in mice and humans is mediated by erythropoietin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1837–R1846 [DOI] [PubMed] [Google Scholar]
- 30.Karas R. H., Schulten H., Pare G., Aronovitz M. J., Ohlsson C., Gustafsson J. A., Mendelsohn M. E. (2001) Effects of estrogen on the vascular injury response in estrogen receptor alpha, beta (double) knockout mice. Circ. Res. 89, 534–539 [DOI] [PubMed] [Google Scholar]
- 31.Stubbins R. E., Holcomb V. B., Hong J., Núñez N. P. (2012) Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur. J. Nutr. 51, 861–870 [DOI] [PubMed] [Google Scholar]
- 32.Gassmann M., Pfistner C., Doan V. D., Vogel J., Soliz J. (2010) Impaired ventilatory acclimatization to hypoxia in female mice overexpressing erythropoietin: unexpected deleterious effect of estradiol in carotid bodies. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R1511–R1520 [DOI] [PubMed] [Google Scholar]
- 33.Gassmann M., Tissot van Patot M., Soliz J. (2009) The neuronal control of hypoxic ventilation: erythropoietin and sexual dimorphism. Ann. N. Y. Acad. Sci. 1177, 151–161 [DOI] [PubMed] [Google Scholar]
- 34.Ballot O., Joseph V., Soliz J. (2015) Endogenous brain erythropoietin is a potent sex-specific respiratory stimulant in adult and newborn mice. J. Appl. Physiol. 118, 1386–1395 [DOI] [PubMed] [Google Scholar]
- 35.Eshraghi-Jazi F., Nematbakhsh M., Pezeshki Z., Nasri H., Talebi A., Safari T., Mansouri A., Mazaheri S., Ashrafi F. (2013) Sex differences in protective effect of recombinant human erythropoietin against cisplatin-induced nephrotoxicity in rats. Iran. J. Kidney Dis. 7, 383–389 [PubMed] [Google Scholar]
- 36.Wen T. C., Rogido M., Peng H., Genetta T., Moore J., Sola A. (2006) Gender differences in long-term beneficial effects of erythropoietin given after neonatal stroke in postnatal day-7 rats. Neuroscience 139, 803–811 [DOI] [PubMed] [Google Scholar]
- 37.Prókai A., Fekete A., Bánki N. F., Müller V., Vér A., Degrell P., Rusai K., Wagner L., Vannay A., Rosta M., Heemann U., Langer R. M., Tulassay T., Reusz G., Szabó A. J. (2011) Renoprotective effect of erythropoietin in rats subjected to ischemia/reperfusion injury: gender differences. Surgery 150, 39–47 [DOI] [PubMed] [Google Scholar]
- 38.Hong J., Stubbins R. E., Smith R. R., Harvey A. E., Núñez N. P. (2009) Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr. J. 8, 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang R. Y., Tsai S. C., Lu C. C., Tung Y. F., Wang S. W., Wang P. S. (1998) Effects of aging on erythropoietin secretion in female rats. Mech. Ageing Dev. 103, 81–90 [DOI] [PubMed] [Google Scholar]
- 40.Mukundan H., Resta T. C., Kanagy N. L. (2002) 17Beta-estradiol decreases hypoxic induction of erythropoietin gene expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R496–R504 [DOI] [PubMed] [Google Scholar]
- 41.Yasuda Y., Masuda S., Chikuma M., Inoue K., Nagao M., Sasaki R. (1998) Estrogen-dependent production of erythropoietin in uterus and its implication in uterine angiogenesis. J. Biol. Chem. 273, 25381–25387 [DOI] [PubMed] [Google Scholar]
- 42.Chikuma M., Masuda S., Kobayashi T., Nagao M., Sasaki R. (2000) Tissue-specific regulation of erythropoietin production in the murine kidney, brain, and uterus. Am. J. Physiol. Endocrinol. Metab. 279, E1242–E1248 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.