Abstract
Brain atrophy is a common feature of numerous neurologic diseases in which the role of neuroinflammation remains ill-defined. In this study, we evaluated the contribution of major histocompatibility complex class I molecules to brain atrophy in Theiler’s murine encephalomyelitis virus (TMEV)–infected transgenic FVB mice that express the Db class I molecule. FVB/Db and wild-type FVB mice were evaluated for changes in neuroinflammation, virus clearance, neuropathology, and development of brain atrophy via T2-weighted MRI and subsequent 3-dimensional volumetric analysis. Significant brain atrophy and hippocampal neuronal loss were observed in TMEV-infected FVB/Db mice, but not in wild-type FVB mice. Brain atrophy was observed at 1 mo postinfection and persisted through the 4-mo observation period. Of importance, virus-infected FVB/Db mice elicited a strong CD8 T-cell response toward the immunodominant Db-restricted TMEV-derived peptide, VP2121-130, and cleared TMEV from the CNS. In addition, immunofluorescence revealed CD8 T cells near virus-infected neurons; therefore, we hypothesize that class I restricted CD8 T-cell responses promote development of brain atrophy. This model provides an opportunity to analyze the contribution of immune cells to brain atrophy in a system where persistent virus infection and demyelination are not factors in long-term neuropathology.—Huseby Kelcher, A. M., Atanga, P. A., Gamez, J. D., Cumba Garcia, L. M., Teclaw, S. J., Pavelko, K. D., Macura, S. I., Johnson. A. J. Brain atrophy in picornavirus-infected FVB mice is dependent on the H-2Db class I molecule.
Keywords: neurodegeneration, MHC class I, viral clearance, TMEV, CD8 T cells
Brain atrophy is a common feature among a broad range of debilitating neurologic diseases, including Alzheimer’s disease, multiple sclerosis, epilepsy, and encephalitis (1–4). The cellular and molecular mechanisms that initiate brain atrophy are not fully understood; however, neuroinflammation is associated with all of these diseases (5–7). In addition, the role that specific immune cell subtypes play in brain atrophy is unknown; however, a reduction of brain atrophy development in patients with multiple sclerosis who are treated with immune-modulating therapies has been reported in multiple clinical studies (8–11). Of importance, brain atrophy occurs in both demyelinating and nondemyelinating neurologic disease. This implies that brain atrophy could be associated with an underlying neuropathology that is more universal than loss of myelin alone. Our understanding of the etiology of brain atrophy is hindered by a lack of animal models available to assess CNS neurodegeneration; therefore, the continued development of model systems designed to study brain atrophy are greatly needed.
Theiler’s murine encephalomyelitis virus (TMEV) infection of SJL mice results in chronic infection, persistent demyelination, inflammation, and motor deficits (12, 13). This TMEV-induced demyelinating disease (TMEV-IDD) serves as an established model of primary progressive multiple sclerosis (14). Previous studies by our team demonstrated the development of highly significant brain and spinal cord atrophy in TMEV-infected SJL mice, which correlated with functional disability (15, 16); therefore, TMEV-IDD in SJL mice serves as a classic model by which primary progressive multiple sclerosis can be studied. In contrast, mouse strains that exhibit resistance to TMEV-IDD have specific major histocompatibility complex (MHC) class I haplotypes that differ from SJL mice (17, 18). In mice with H-2b haplotype, the Db class I molecule confers resistance to chronic TMEV infection and demyelinating syndrome, as demonstrated by studies that used the C57BL/6, congenic C57BL/10, and FVB mouse strains (18–23). Further analysis revealed that resistance to TMEV-IDD coincides with, and is dependent on, development of a CD8 T-cell response restricted against the immunodominant TMEV antigen VP2121-130 presented in the context of the H-2Db class I molecule (22, 24).
Whereas resistance to TMEV-IDD is linked to antiviral CD8 T-cell responses, the contribution of immune cells to brain atrophy remains poorly understood. We previously demonstrated development of brain atrophy in TMEV-infected SJL mice (H-2s haplotype); however, this strain has limited reagents and transgenic mice available to dissect brain inflammation as a pathomechanism of neurologic disease. For this reason, we assessed the development of atrophy in TMEV-IDD–susceptible FVB mice. We also developed transgenic FVB mice that express the Db class I gene. Introduction of the Db transgene into FVB mice confers resistance to TMEV-IDD in this otherwise susceptible mouse strain (25). TMEV-IDD resistance is conferred by the Db molecule’s contribution to the development of a robust antiviral CD8 T-cell response that is specific for the immunodominant Db:VP2121-130 virus epitope (22). Analysis of wild-type FVB and Db transgenic FVB mouse genotypes therefore provided an opportunity to analyze development of brain atrophy in both TMEV-IDD–resistant and –susceptible mice on the same genetic background.
In this study, we employed FVB and FVB/Db mice to evaluate the contribution of an MHC class I molecule to brain atrophy after TMEV infection. Our hypothesis was that the development of brain atrophy would be altered by expression of the Db class I molecule, which enhances the CD8 T-cell immune response against TMEV infection. We further hypothesized that enhanced CD8 T-cell responses in the CNS have the capacity to contribute to atrophy development via direct cytotoxic effects exerted upon CNS cell types that present TMEV antigen in the context of the Db class I molecule. Transgenic expression of the H-2Db MHC class I molecule enables us to assess the onset of atrophy in chronic TMEV-infected wild-type FVB mice as well as in virus-clearing FVB/Db mice; therefore, this work sets the stage for an analysis of how the MHC class I molecule influences neurodegeneration in a murine model system of neuroinflammation.
MATERIALS AND METHODS
Animals
FVB/Db LoxP (FVB/Db) mice were bred in-house and generated as previously described (23). FVB/NJ (FVB) mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). All experiments were conducted in accordance with the Institutional Animal Care and Use Committee at Mayo Clinic. FVB/Db and FVB mice aged 6–10 wk were used in experiments.
Induction of viral infection
TMEV infection is initiated by intracranial virus injection of 6-wk-old mice anesthetized with inhalational isoflurane (2% in O2). With a 27-gauge needle attached to a Hamilton syringe, 10 μl volume that contained 2 × 106 PFU purified Daniel’s strain of TMEV was injected intracerebrally, which resulted in transient acute meningoencephalomyelitis in all strains and chronic demyelination in susceptible strains (26).
Analysis of viral load
CNS viral control was assessed with RT-PCR of viral genome present in the brain tissue. RT-PCR was performed as previously described (27). In brief, whole-brain homogenate was mixed with TRIzol LS Reagent (Thermo Fisher Scientific, Waltham, MA, USA) and chloroform. After incubation, samples were spun down, and the aqueous phase was precipitated with isopropanol. RNA was washed with 75% cold ethanol and resuspended in RNase-free water. RNA concentration and quality were determined by using a NanoVue Spectrophotometer (GE Healthcare Life Sciences, Pittsburgh, PA, USA). TMEV RNA was quantified by using the One Step QuantiTect SYBR Green RT-PCR Kit (Qiagen, Gaithersburg, MD, USA) for the VP2 gene of TMEV (forward primer: 5′-TGGTCGACTCTGTGGTTACG; reverse primer: 5′−GCCGGTCTTGGAAAGATAGT). All sample data were normalized to mouse β-actin (forward primer: 5′-CTGGCACCACACCTTCTACAATGAGCTG; reverse primer: 5′-GCACAGCTTCTCTTTGATGTCACGCACGATTTC). RT-PCR was performed on a StepOnePlus Real Time PCR System (Applied Biosystems, Foster City, CA, USA) under the following conditions: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 55°C for 1 min. Computed tomography was set just before amplification plateau. All samples were normalized to sample-matched β-actin levels. Relative quantities were calculated by using the 2−ΔΔCt method and reported as an increase over background (28).
MRI acquisition
A Bruker Avance 300-MHz (7-T) vertical bore small-animal MRI imaging system (Bruker Biospin, Billerica, MA, USA) was used. Inhalational isoflurane anesthesia (1.5–2% in O2) was delivered via nose cone. Electrocardiogram, respiratory rate, and temperature were monitored by using the SAII MRI-compatible monitoring and gating system (SA Instruments, Stony Brook, NY, USA). A respiratory gated T2-weighted volume acquisition rapid acquisition with refocused echoes (RARE) sequence was used to visualize the brain in vivo (repetition time: 1500 ms, echo time: 70 ms, RARE factor: 16, FOV: 3.20 × 1.92 × 1.92 cm, matrix: 256 × 128 × 128).
Image analysis
To quantify brain atrophy, 3-dimensional volumetric studies of the lateral ventricles were performed by using Analyze 11, as previously reported (15, 29). In brief, T2-weighted brain images were segmented by using semiautomated thresholding and seed growing–based algorithms to generate object maps, which define subvolumes of the brain. In this case, the subvolume of interest represented the 2 lateral ventricles. After object map generation, the Region of Interest Scan Tool was used to calculate volumes. Investigators were blinded and trained on test data sets. Intra- and interrater reliability between investigators was >95%.
Flow cytometry
A minimum of 4 FVB and 4 FVB/Db mouse brains were harvested at 1 and 6 wk post-TMEV infection. Harvested brains were subsequently strained through a nylon mesh 100-mm filter into RPMI 1640, and 700 mg collagenase type IV (Worthington Biochemical, Lakewood, NJ, USA) was added to each 5-ml tissue suspension. Suspensions were then incubated in a 42°C water bath for 45 min. Brain-infiltrating lymphocytes were isolated via collagenase digestion and a Percoll gradient as previously described (30, 31). As an alternative, brains were harvested and homogenized with a Dounce homogenizer and 7-ml Tenbroeck tissue grinder, and the resulting suspension was then added to a Percoll gradient. Isolated brain lymphocytes were washed twice with fluorescence-activated cell sorting (FACS) buffer and incubated with the following peptide-MHC tetramer conjugated to APC for 40 min: Db:VP2121-130 (FHAGSLLVFM) followed by a 20-min incubation with Abs against CD8α (PE-Cy7; 561097), CD4 (PerCP for 1 wk postinfection analysis and BV650 for 6-wk postinfection analysis; BioLegend, San Diego, CA, USA), and CD45 (PE for 1 wk postinfection analysis and PerCP for 6 wk postinfection analysis; BD Biosciences, San Jose, CA, USA). The H-2Db MHC class I tetramer Db:VP2121-130 was constructed as previously described (32, 33). Cells were then washed twice with FACS buffer and fixed in 4% paraformaldehyde (PFA) and 1× PBS. Samples were analyzed on a Becton Dickinson LSRII flow cytometer (BD Biosciences) using BD FACS Diva software (BD Biosciences) and FlowJo analysis (FlowJo, Ashland, OR, USA). Gating strategy was as follows: populations of cells that were previously defined as lymphocytes were gated from plots depicting forward and side scatter (FSC-A vs SSC-A), similar to previously published gating strategies (24). From this, a second plot was generated and cells that were highly expressed CD45 were gated to exclude microglia and to enrich for blood-derived cells. Data were analyzed as shown. Percentage of CD8+ T-cell populations were calculated from CD4+ by CD8+ data plots, and percentage of CD8+ Db:VP2121-130+ T-cells populations were calculated from CD8+ by Db:VP2121-130+ data plots. Data are representative of a minimum of 4 mice per group and 4 independent experiments for acute infection and 2 independent experiments for chronic infection analysis.
Histology
At 4 mo postinfection, FVB and FVB/Db mice were anesthetized with 10 mg of pentobarbital and perfused via intracardiac puncture with 50 ml of 3% PFA. Three brains from FVB and FVB/Db mice were removed, postfixed with 3% PFA, cut into coronal sections, and embedded in paraffin (12, 13). We applied standard hematoxylin and eosin stain to assess overall tissue integrity as described by our team earlier (34). In brief, tissue sections were deparaffinized with xylene and rehydrated with alcohol and distilled water. Sections were then stained in Gill’s hematoxylin, differentiated with acid alcohol, blued with ammonia water, and counterstained with eosin-phloxine. Sections were then dehydrated with alcohol and washed in xylene before a coverslip was placed on the slide. The resulting sections were blindly analyzed. The presence of a discontinuous neuronal staining in the CA1 region of the hippocampus was considered neuronal loss. Comparison of neuronal loss in FVB and FVB/Db mice was analyzed by χ2 analysis.
Immunofluorescence
Fresh frozen brains were harvested from 7-d TMEV-infected FVB and FVB/Db mice. Frozen brains were embedded in optimum cutting temperature (OCT) compound (Sakura Finetek USA, Torrance, CA, USA) and cut into 20-μm-thick sections by using a cryostat. Sections were air dried for 30 min, then washed with PBS and fixed with PFA for 15 min. Primary Abs were added at a 1:100 dilution for 2 h at room temperature, and secondary Abs were applied for 1 h at 1:250 dilution. Secondary only controls were also stained. Reagents used were as follows: primary rat, anti-mouse CD8α mAb (Bio-Rad, Hercules, CA, USA), mouse, anti-NeuN mAb (EMD Millipore, Billerica, MA, USA), rabbit, anti-TMEV polyclonal Ab was generously provided by Moses Rodriguez (Mayo Clinic), and nuclei were stained with Vectashield with DAPI (Vector Laboratories, Burlingame, CA, USA). Secondary Abs used were goat, anti-mouse Alexa Fluor 635, anti-rat Alexa Fluor 555, anti-rabbit Alexa Fluor 488, and anti-rabbit Alexa Fluor 532 (all from Thermo Fisher Scientific).
Experimental design
Viral load
Eight FVB and 8 FVB/Db mouse brains were harvested at 4 mo post-TMEV infection, as well as 2 FVB uninfected controls.
Flow cytometry
A minimum of 4 FVB and 4 FVB/Db mouse brains were harvested at 1 wk and 6 wk post-TMEV infection. Data shown are representative of 4 independent experiments at 1 wk postinfection and 2 independent experiments conducted during the chronic phase of infection (>30 d post-TMEV infection).
MRI and histology
Seven FVB and 13 FVB/Db mice had a preinfection MRI scan, followed by monthly MRI acquisitions out to 4 mo. Animals were euthanized and brains were removed at 4 mo postinfection. Persons who conducted MRI image analysis and histology analysis were blinded to the experimental groups.
Immunofluorescence
FVB and FVB/Db mouse brains were harvested at 1 wk post-TMEV infection and frozen fresh on dry ice for subsequent tissue analysis.
Statistical analysis
We used SigmaPlot 11.0 (Systat Software, San Jose, CA, USA) and GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA) for all statistical analysis. All data are presented as means ± sem. For comparisons of 2 groups, significance was determined by using Student’s t test or Mann-Whitney rank sum test if the data did not pass normality. Hippocampal neuronal loss was assessed by χ2 analysis on histologic brain sections.
RESULTS
Expression of the Db class I molecule confers resistance to chronic TMEV infection in FVB mice
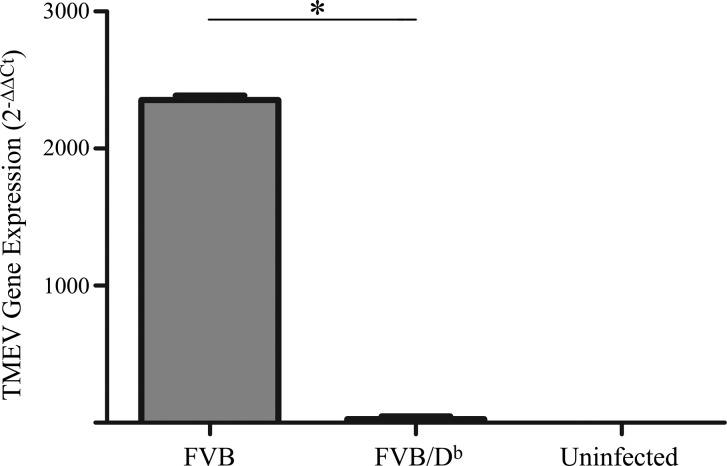
Transgenic introduction of the Db class I molecule into FVB mice resulted in a strong antiviral CD8 T-cell response and viral clearance, which led to resistance to TMEV-IDD (1, 18, 21, 22); therefore, we assessed the extent to which transgenic expression of the Db class I molecule protected mice from persistent TMEV infection in the current study designed to analyze brain atrophy. Wild-type FVB and transgenic FVB/Db mice were infected with TMEV. At 4 mo postinfection, brains were harvested from both groups of mice. RT-PCR analysis was conducted to assess viral load in each individual mouse brain. This experiment revealed virus persistence in wild-type FVB mice and only residual viral transcript in transgenic FVB/Db mice (Fig. 1). This finding is consistent with previous analyses that demonstrated that expression of the Db class I molecule confers antiviral resistance in the TMEV model (22, 25); therefore, in all subsequent experiments in this study, we are confident that the FVB/Db mice used were resistant to TMEV infection. In addition, wild-type FVB mice are susceptible to chronic TMEV infection. Regardless of susceptibility to chronic TMEV infection, all animals exhibited evidence of having acute infection. This is demonstrated by generation of an anti-viral CD8 T-cell response (Fig. 2). Furthermore, in a parallel experiment, all FVB and transgenic FVB/Db mice had measurable anti-TMEV IgG Abs (data not shown). These findings are consistent with previous reports in which 100% of FVB and FVB/Db mice that were infected with TMEV generate anti-TMEV IgG responses (35).
Figure 1.
Transgenic introduction of the Db class I molecule confers resistance to chronic TMEV infection in FVB mice. FVB and transgenic FVB/Db mice were intracranially infected with TMEV. At 4 mo post-TMEV infection, FVB/Db mice, and not wild-type FVB mice, had cleared TMEV infection. RT-PCR analysis of virus load was determined via amplification of the VP2 gene. Wild-type FVB and FVB/Db mice were infected with TMEV. Brain homogenate was collected for RT-PCR analysis. Viral VP2 gene and β-actin mRNA concentrations in each sample were calculated, and relative gene expression was assessed by using the Ct method. The bar graph represents means and sem of 8 replicates in FVB and FVB/Db mice and 2 replicates in FVB uninfected controls. *P < 0.05, Student’s t test.
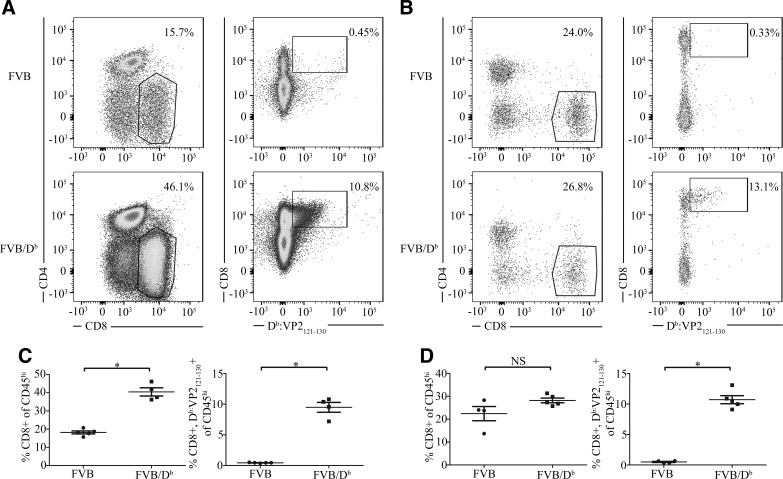
Figure 2.
Transgenic FVB/Db mice present with an enhanced CD8 T-cell response during TMEV infection. A) Flow cytometric analysis of brain infiltrating lymphocytes at 1 wk post-TMEV infection FVB/Db mice (n = 4) shows an increased percentage of brain-infiltrating CD8 T cells compared with FVB mice (n = 5) as well as a significant increase in antiviral Db:VP2121-130 epitope-specific CD8 T cells. B) At 6 wk post-TMEV infection, the proportion of overall CD8+ T cells entering the brain is not significantly different between FVB mice (n = 4) and FVB/Db mice (n = 5). C) CD8+, antigen-specific, and CD8+ Db:VP2121-130+ T cells were quantified as a percentage of CD45high lymphocytes and demonstrate a significant increase in FVB/Db mice compared with wild-type FVB mice. In addition, the percentage of antiviral Db:VP2121-130 epitope-specific CD8 T cells is consistent with acute infection of FVB/Db mice. D) The frequency of CD8+ T cells and antigen-specific CD8+ Db:VP2121-130+ T cells from 1 experiment are quantified as a percentage of CD45high lymphocytes, demonstrating a significant increase in antigen-specific CD8+ T cells in FVB/Db mice. Data are shown as means with error bars denoting sem. *P < 0.05, Mann-Whitney U test.
FVB/Db transgenic mice have an augmented CD8 T-cell response during TMEV infection
TMEV-infected wild-type FVB mice (H-2q haplotype) have persistent infection and demyelination. In addition, transgenic expression of the Db class I molecule in FVB mice conferred resistance to TMEV-IDD. Resistance to TMEV-IDD has been shown to be mediated by antiviral CD8 T cells that are specific for the TMEV peptide, VP2121-130, presented in the context of the Db class I molecule (22). Db:VP2121-130 MHC tetramers have been used extensively to study CD8 T-cell responses against TMEV (24, 30, 31, 36, 37). To confirm this finding in our studies analyzing brain atrophy, wild-type FVB and FVB/Db mice were intracranially infected with TMEV, and brain tissue was harvested for analysis at 1 wk and 6 wk post-TMEV infection. In Fig. 2A, B, we determined the percentage of brain-infiltrating CD8 T cells, as well as the proportion of Db:VP2121-130 epitope specific CD8 T cells in wild-type FVB and transgenic FVB/Db mice during acute and chronic stages of infection, respectively. During acute TMEV infection, FVB/Db mice generated a significant increase in the overall percentage of both CD8 T cells and Db:VP2121-130 epitope-specific CD8 T cells (Fig. 2A, C). At 6 wk postinfection, a greatly reduced, but significant frequency of Db:VP2121-130 epitope-specific CD8 T cells persisted (Fig. 2D); however, an overall difference in the percentage of infiltrating CD8 T cells was not observed (Fig. 2D). Of importance, at 6 wk postinfection, FVB mice had chronic TMEV infection (20). Moreover, FVB/Db mice will have cleared virus as shown by Fig. 1. These findings demonstrate that introduction of the Db class I molecule into the FVB strain enabled the generation of a Db:VP2121-130 epitope-specific CD8 T-cell response and clearance of virus, which is consistent with previous reports (22, 38).
Transgenic FVB/Db mice develop significant brain atrophy during TMEV infection
We next assessed the extent to which expression of the Db class I molecule in FVB mice contributed to brain atrophy during TMEV infection. We conducted baseline MRI of wild-type FVB and FVB/Db transgenic mice. These 2 groups were then infected with TMEV and monitored monthly by MRI. Significant brain atrophy developed in FVB/Db mice, but not in wild-type FVB mice (Fig. 3A, B). The difference between FVB and FVB/Db mean ventricle volume at 1 mo was significant (P = 0.0018), with ventricular volume being >3-fold larger in FVB/Db mice than in wild-type FVB mice (Fig. 3C). The volume increase between baseline and 1 mo post-TMEV infection in FVB/Db mice was also significant (P = 0.00047), again exhibiting a >3-fold increase in mean ventricle volume between these 2 time points (Fig. 3C). Brain atrophy in FVB/Db mice reached its peak at 1 mo and persisted throughout the observation period; there was no significant difference in ventricle volume between the 1- and 4-mo time points (P = 0.39). In the same 4-mo timeframe post-TMEV infection, FVB mice did not develop detectable brain atrophy (P = 0.54). Development of sustained brain atrophy was therefore exclusive to TMEV-infected FVB/Db mice after TMEV infection.
Figure 3.
Development of brain atrophy in TMEV-infected transgenic FVB/Db mice, but not wild-type FVB mice. Shown is ventricle size obtained in TMEV-infected FVB and FVB/Db mice at baseline and once per month for 4 mo using 3-dimensional volumetric analysis of T2 MRI-acquired images. A, B) Representative axial and coronal images are shown in FVB mice (A) and FVB/Db mice (B; n = 7 and 13, respectively). Ventricle size is denoted by red enhancement. C) Significant increased ventricular volume was observed in mo 1–4 in FVB/Db mice compared with wild-type FVB mice. Data are shown as means with error bars denoting sem. *P < 0.05, Mann-Whitney U test.
CD8 T cells are found in tight approximation to virally infected neurons
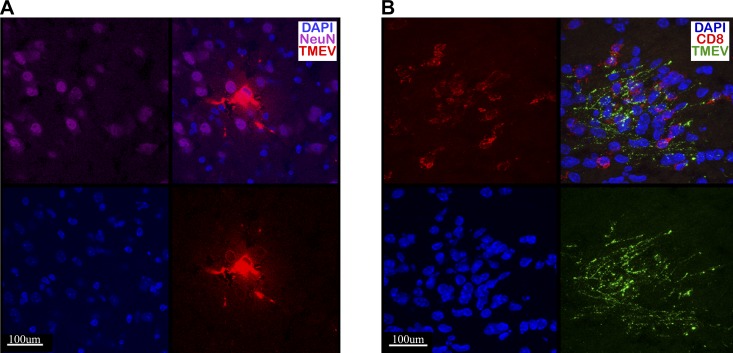
CD8 T cells elicit a strong response to the Db:VP2121-130 epitope concurrently with the development of brain atrophy in FVB/Db mice (Figs. 2D and 3B); therefore, we next assessed the extent to which CD8 T cells directly interacted with TMEV-infected neurons in vivo. We have previously shown that CD8 T cells engage virus-infected neurons during acute TMEV infection in the C57BL/6 mouse strain (31). FVB/Db mice were infected with TMEV. At 7 d postinfection, brains were harvested, sliced, and immunostained for neuronal marker NeuN, TMEV antigen, and CD8α for T cells (Fig. 4A, B). Consistent with previous findings, we observed that virus was localized to neurons and neuronal processes during acute TMEV infection (Fig. 4A). In addition, CD8 T cells were found in close proximity with and tightly opposed to TMEV-infected neurons (Fig. 4B).
Figure 4.
CD8 T cells are found in tight opposition to TMEV-infected neurons during acute infection. FVB/Db mice were intracranially infected with TMEV, and brains were harvested at d 7 postinfection. A) Frozen tissue sections were stained for TMEV (polyclonal Ab, red), nuclei (DAPI, blue), and NeuN (monoclonal anti-mouse NeuN, purple), demonstrating that TMEV infects neurons and neuronal processes during acute TMEV infection. B) Frozen tissue was stained for TMEV (polyclonal Ab, green), nuclei (DAPI, blue), and CD8 (monoclonal anti-mouse CD8α, red). CD8 T cells infiltrate the brain and are found in tight proximity to TMEV-infected neuronal processes. Shown is stratum lucidum of the hippocampal region of a FVB/Db mouse.
Neuronal loss in transgenic FVB/Db mice
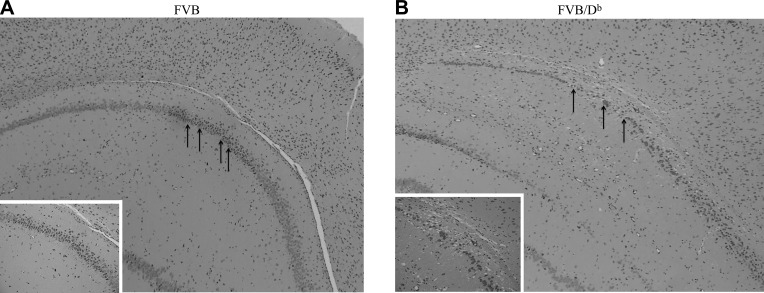
Brain atrophy is defined as cell loss (neurons, oligodendrocytes, astrocytes, and microglia), degradation of extracellular matrix, and/or loss of extracellular proteins, including myelin basic protein (39–41); therefore, we examined the extent to which neuropathology occurred in the brains of 4-mo TMEV-infected FVB and FVB/Db mice. Brain atrophy was assessed as hippocampal neuronal loss and measured via blinded analysis of the hippocampal CA1 region in coronal slices. Any incident of neuronal loss, as shown through discontinuous neuronal cell bodies in the CA1 region, was recorded as an animal with incidence of neuropathology. FVB mice had overall normal histology despite having chronic TMEV infection (Fig. 5A). In contrast, FVB/Db mice exhibited a loss of hippocampal neurons (Fig. 5B). Neuronal loss presented as nuclear disintegration and disrupted architecture of neuronal cell bodies in the CA1 region of the hippocampus and cortical regions (Fig. 5B). A χ2 analysis was performed and revealed that hippocampal neuronal loss occurred in animals with coinciding brain atrophy; therefore, transgenic expression of Db in FVB mice results in long term hippocampal neuronal loss, which is observable after TMEV infection is cleared.
Figure 5.
Hippocampal neuronal loss in FVB mice that transgenically express the Db class I molecule. Hematoxylin and eosin staining of paraffin-embedded brain tissue from wild-type FVB mice (A) and FVB/Db mice (B). Loss of hippocampal neurons is seen in Db-expressing mice, indicated by black arrows in panel B. Brain slices were obtained from 4-mo TMEV-infected animals. Slices are representative of 3 animals in each group [0 of 3 FVB mice and 3 of 3 FVB/Db mice had hippocampal neuronal loss (P < 0.05, χ2 analysis)].
DISCUSSION
In this study, we demonstrate the importance of MHC class I molecule Db in influencing development of brain atrophy in the TMEV model of neuroinflammation. Of importance, brain atrophy occurred in TMEV-infected FVB/Db transgenic mice, a mouse genotype that clears virus and does not undergo TMEV-IDD. These results demonstrate that atrophy can occur independently of the demyelinating syndrome associated with chronic TMEV infection. Furthermore, atrophy occurred concurrently with development of a strong Db:VP2121-130 epitope-restricted CD8 T-cell response and hippocampal neuronal loss. These findings strongly imply that inflammation—and potentially the virus-specific CD8 T-cell response that occurs in FVB/Db transgenic mice during acute infection—contributes to hippocampal neuronal loss and brain atrophy.
TMEV infection has been widely used as a model of primary progressive multiple sclerosis, as persistent infection of the CNS results in extensive demyelination (26). The concept of epitope spreading, in which the immune response shifts from the recognition of virus antigens to myelin proteins, has been a major hypothesis of how TMEV-IDD occurs (42); however, neuropathology and demyelination has primarily been studied in the context of chronic virus infection. For this reason, in TMEV-infected mouse strains susceptible to TMEV-IDD, the contribution of virus-specific or immune-mediated pathology cannot be separated. In this study, we demonstrate development of brain atrophy in the absence of chronic TMEV infection. FVB/Db mice clear TMEV infection and do not develop demyelination (23). This demonstrates that the onset of brain atrophy in FVB/Db mice is not the result of chronic TMEV infection or demyelination.
The increased CD8 T-cell response observed in TMEV-infected FVB/Db mice occurred concurrently with hippocampal neuronal loss and brain atrophy. This raises the question of how these lymphocytes potentially contribute to neuropathology. We observed considerable expansion of Db:VP2121-130 epitope-specific CD8 T cells in TMEV-infected FVB/Db mice but not in wild-type FVB mice (Fig. 2). In addition to this augmented CD8 T-cell response, we observed that the neuron is the CNS cell type that is predominantly infected with TMEV (Fig. 4A). Furthermore, CD8 T cells were observed tightly opposed to TMEV-infected neurons (Fig. 4B). Finally, neuronal death in FVB/Db mice occurred as a result of TMEV infection. All of the above observations we made in this study using FVB/Db mice are consistent with earlier reports that analyzed the C57BL/6 mouse strain (31). In addition, our MRI analysis demonstrated that significant brain atrophy occurred in brain regions near this neuronal loss in the hippocampal region (Fig. 3A); therefore, it is possible that the Db:VP2121-130 epitope-specific CD8 T-cell response that develops early in TMEV-infected FVB/Db mice contributes to the development of brain atrophy via neuronal loss.
Altogether, the above observations support a hypothesis that brain atrophy is the result of a class I–restricted CD8 T-cell response against TMEV-infected neurons which results in neuropathology. This hypothesis, if ultimately supported, puts forward neuroinflammation—namely, CD8 T cells—as a putative mechanism of brain atrophy in neurologic disease. Of importance, these studies highlight that brain atrophy occurs during acute stages of TMEV infection. This implies that brain atrophy coincides with the encephalitic phase of disease. Moreover, at later time points, sustained antiviral CD8 T-cell responses were generated, and only residual virus transcripts were observed in the brains of FVB/Db mice. In these later phases, the level of brain atrophy remained constant. Future work will entail understanding the extent to which antiviral CD8 T-cell response initiates and sustains brain atrophy in both acute and chronic phases of disease, respectively.
Although our working model is that the Db class I molecule potentiates brain atrophy via promotion of the development of a new CD8 T-cell response during neuroinflammation, it remains important to consider alternative mechanisms by which an MHC class I molecule could work via a nonclassical role in this process. MHC class I molecules have been evaluated in brain development and neuronal plasticity (43–47). In these studies, a potential role for MHC class I in pruning and expanding neuronal connections within the CNS was observed (43, 44). Although interesting and of potential significance to our studies, we did not observe a developmental effect of transgenic expression of the Db class I molecule in our FVB mice. The effect of the Db class I molecule on brain atrophy that was observed in our studies is only observed after TMEV infection and the onset of neuroinflammation; therefore, we did not observe neurologic abnormalities in uninfected FVB/Db mice. Nevertheless, a potential, nonclassical role for MHC class I molecules in brain atrophy development remains a topic of interest and will be the object of subsequent studies. Given the FVB/Db transgenic mouse strain used in this analysis enables a Cre-Lox conditional gene silencing approach, future work will entail identifying the critical H-2Db MHC class I–bearing cell type that promotes development of brain atrophy. Experiments are currently underway to dissect both inflammatory and developmental roles of the Db class I molecule in brain atrophy onset.
In summary, increased brain atrophy was observed during neuroinflammation in FVB mice that expressed the Db class I molecule. From these findings, we put forward a working model in which viral clearance is mediated by cytotoxic CD8 T cells that are specific for the Db:VP2121-130 virus epitope. Virus clearance achieved by the CD8 T-cell response against the Db:VP2121-130 epitope was achieved, consequently, with the loss of hippocampal neurons and the onset of brain atrophy. Of importance, the FVB/Db transgenic model presented in this paper allows the analysis of immune-mediated pathology without the consequences of chronic viral-mediated pathology. This final observation provides insight into neurologic disease via implication that a putative mechanism of neurodegeneration associated with brain atrophy is the consequence of neuroinflammation, namely, CD8 T cells.
ACKNOWLEDGMENTS
The authors thank and acknowledge the contributions of their former collaborator, Dr. Istvan Pirko (Department of Neurology, Mayo Clinic), who they will miss. The authors also thank Dr. Yong Guo (Department of Neurology, Mayo Clinic), for help with the histology aspects of this study. This work was supported by U.S. National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke Grant R21 NS094765 and intramural funds, as well as funding from NIH Ph.D. Training Grant in Basic Immunology (T32 AI07425-20) and the Mayo Graduate School. The authors declare no conflicts of interest.
Glossary
- FACS
fluorescence-activated cell sorting
- MHC
major histocompatibility complex
- PFA
paraformaldehyde
- TMEV
Theiler’s murine encephalomyelitis virus
- TMEV-IDD
Theiler’s murine encephalomyelitis virus–induced demyelinating disease
AUTHOR CONTRIBUTIONS
A. M. Huseby Kelcher, P. A. Atanga, J. D. Gamez, L. M. Cumba Garcia, S. J. Teclaw, and K. D. Pavelko performed research and analyzed data; A. M. Huseby Kelcher, P. A. Atanga, J. D. Gamez, K. D. Pavelko, and S.I. Macura designed research; A. M. Huseby Kelcher, P. A. Atanga, and A. J. Johnson wrote the manuscript; and A. J. Johnson conceived of and oversaw the study.
REFERENCES
- 1.Johnson A. J., Suidan G. L., McDole J., Pirko I. (2007) The CD8 T cell in multiple sclerosis: suppressor cell or mediator of neuropathology? Int. Rev. Neurobiol. 79, 73–97 [DOI] [PubMed] [Google Scholar]
- 2.Petito C. K., Torres-Muñoz J. E., Zielger F., McCarthy M. (2006) Brain CD8+ and cytotoxic T lymphocytes are associated with, and may be specific for, human immunodeficiency virus type 1 encephalitis in patients with acquired immunodeficiency syndrome. J. Neurovirol. 12, 272–283 [DOI] [PubMed] [Google Scholar]
- 3.Likeman M., Anderson V. M., Stevens J. M., Waldman A. D., Godbolt A. K., Frost C., Rossor M. N., Fox N. C. (2005) Visual assessment of atrophy on magnetic resonance imaging in the diagnosis of pathologically confirmed young-onset dementias. Arch. Neurol. 62, 1410–1415 [DOI] [PubMed] [Google Scholar]
- 4.Farid N., Girard H. M., Kemmotsu N., Smith M. E., Magda S. W., Lim W. Y., Lee R. R., McDonald C. R. (2012) Temporal lobe epilepsy: quantitative MR volumetry in detection of hippocampal atrophy. Radiology 264, 542–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamolodchikov D., Strickland S. (2016) A possible new role for Aβ in vascular and inflammatory dysfunction in Alzheimer’s disease. Thromb. Res. 141, S59–S61 [DOI] [PubMed] [Google Scholar]
- 6.Mandolesi G., Gentile A., Musella A., Fresegna D., De Vito F., Bullitta S., Sepman H., Marfia G. A., Centonze D. (2015) Synaptopathy connects inflammation and neurodegeneration in multiple sclerosis. Nat. Rev. Neurol. 11, 711–724 [DOI] [PubMed] [Google Scholar]
- 7.Vezzani A., Aronica E., Mazarati A., Pittman Q. J. (2013) Epilepsy and brain inflammation. Exp. Neurol. 244, 11–21 [DOI] [PubMed] [Google Scholar]
- 8.Hainke U., Thomas K., Ziemssen T. (2016) Laquinimod in the treatment of relapsing remitting multiple sclerosis. Expert Opin. Drug Metab. Toxicol. 12, 701–709 [DOI] [PubMed] [Google Scholar]
- 9.Constantinescu S. E., Constantinescu C. S. (2016) Laquinimod (ABR-215062) for the treatment of relapsing multiple sclerosis. Expert Rev. Clin. Pharmacol. 9, 49–57 [DOI] [PubMed] [Google Scholar]
- 10.Kappos L., Radue E. W., O’Connor P., Polman C., Hohlfeld R., Calabresi P., Selmaj K., Agoropoulou C., Leyk M., Zhang-Auberson L., Burtin P.; FREEDOMS Study Group (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 362, 387–401 [DOI] [PubMed] [Google Scholar]
- 11.Chataway J., Schuerer N., Alsanousi A., Chan D., MacManus D., Hunter K., Anderson V., Bangham C. R., Clegg S., Nielsen C., Fox N. C., Wilkie D., Nicholas J. M., Calder V. L., Greenwood J., Frost C., Nicholas R. (2014) Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet 383, 2213–2221 [DOI] [PubMed] [Google Scholar]
- 12.McGavern D. B., Murray P. D., Rivera-Quiñones C., Schmelzer J. D., Low P. A., Rodriguez M. (2000) Axonal loss results in spinal cord atrophy, electrophysiological abnormalities and neurological deficits following demyelination in a chronic inflammatory model of multiple sclerosis. Brain 123, 519–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGavern D. B., Murray P. D., Rodriguez M. (1999) Quantitation of spinal cord demyelination, remyelination, atrophy, and axonal loss in a model of progressive neurologic injury. J. Neurosci. Res. 58, 492–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denic A., Johnson A. J., Bieber A. J., Warrington A. E., Rodriguez M., Pirko I. (2011) The relevance of animal models in multiple sclerosis research. Pathophysiology 18, 21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pirko I., Johnson A. J., Chen Y., Lindquist D. M., Lohrey A. K., Ying J., Dunn R. S. (2011) Brain atrophy correlates with functional outcome in a murine model of multiple sclerosis. Neuroimage 54, 802–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDole J., Johnson A. J., Pirko I. (2006) The role of CD8+ T-cells in lesion formation and axonal dysfunction in multiple sclerosis. Neurol. Res. 28, 256–261 [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez M., David C. S. (1985) Demyelination induced by Theiler’s virus: influence of the H-2 haplotype. J. Immunol. 135, 2145–2148 [PubMed] [Google Scholar]
- 18.Brahic M., Bureau J. F., Michiels T. (2005) The genetics of the persistent infection and demyelinating disease caused by Theiler’s virus. Annu. Rev. Microbiol. 59, 279–298 [DOI] [PubMed] [Google Scholar]
- 19.Altintas A., Cai Z., Pease L. R., Rodriguez M. (1993) Differential expression of H-2K and H-2D in the central nervous system of mice infected with Theiler’s virus. J. Immunol. 151, 2803–2812 [PubMed] [Google Scholar]
- 20.Azoulay-Cayla A., Syan S., Brahic M., Bureau J. F. (2001) Roles of the H-2Db and H-Kb genes in resistance to persistent Theiler’s murine encephalomyelitis virus infection of the central nervous system. J. Gen. Virol. 82, 1043–1047 [DOI] [PubMed] [Google Scholar]
- 21.Lin X., Njenga M. K., Johnson A. J., Pavelko K. D., David C. S., Pease L. R., Rodriguez M. (2002) Transgenic expression of Theiler’s murine encephalomyelitis virus genes in H-2b mice inhibits resistance to virus-induced demyelination. J. Virol. 76, 7799–7811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendez-Fernandez Y. V., Johnson A. J., Rodriguez M., Pease L. R. (2003) Clearance of Theiler’s virus infection depends on the ability to generate a CD8+ T cell response against a single immunodominant viral peptide. Eur. J. Immunol. 33, 2501–2510 [DOI] [PubMed] [Google Scholar]
- 23.Pavelko K. D., Mendez-Fernandez Y., Bell M. P., Hansen M. J., Johnson A. J., David C. S., Rodriguez M., Pease L. R. (2012) Nonequivalence of classical MHC class I loci in ability to direct effective antiviral immunity. PLoS Pathog. 8, e1002541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson A. J., Njenga M. K., Hansen M. J., Kuhns S. T., Chen L., Rodriguez M., Pease L. R. (1999) Prevalent class I-restricted T-cell response to the Theiler’s virus epitope Db:VP2121-130 in the absence of endogenous CD4 help, tumor necrosis factor alpha, gamma interferon, perforin, or costimulation through CD28. J. Virol. 73, 3702–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azoulay A., Brahic M., Bureau J. F. (1994) FVB mice transgenic for the H-2Db gene become resistant to persistent infection by Theiler’s virus. J. Virol. 68, 4049–4052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsunoda I., Fujinami R. S. (2010) Neuropathogenesis of Theiler’s murine encephalomyelitis virus infection, an animal model for multiple sclerosis. J. Neuroimmune Pharmacol. 5, 355–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pavelko K. D., Girtman M. A., Mitsunaga Y., Mendez-Fernandez Y. V., Bell M. P., Hansen M. J., Allen K. S., Rodriguez M., Pease L. R. (2011) Theiler’s murine encephalomyelitis virus as a vaccine candidate for immunotherapy. PLoS One 6, e20217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmittgen T. D., Livak K. J. (2008) Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108 [DOI] [PubMed] [Google Scholar]
- 29.Robb R. A., Barillot C. (1989) Interactive display and analysis of 3-D medical images. IEEE Trans. Med. Imaging 8, 217–226 [DOI] [PubMed] [Google Scholar]
- 30.Johnson H. L., Chen Y., Jin F., Hanson L. M., Gamez J. D., Pirko I., Johnson A. J. (2012) CD8 T cell-initiated blood-brain barrier disruption is independent of neutrophil support. J. Immunol. 189, 1937–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDole J. R., Danzer S. C., Pun R. Y., Chen Y., Johnson H. L., Pirko I., Johnson A. J. (2010) Rapid formation of extended processes and engagement of Theiler’s virus-infected neurons by CNS-infiltrating CD8 T cells. Am. J. Pathol. 177, 1823–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altman J. D., Moss P. A., Goulder P. J., Barouch D. H., McHeyzer-Williams M. G., Bell J. I., McMichael A. J., Davis M. M. (1996) Phenotypic analysis of antigen-specific T lymphocytes. Science 274, 94–96 [DOI] [PubMed] [Google Scholar]
- 33.Reed B. K., Chopp L. B., Malo C. S., Renner D. N., Van Keulen V. S., Girtman M. A., Nevala W. N., Pavelko K. D., Gil D., Schrum A. G., Johnson A. J., Pease L. R. (2015) A versatile simple capture assay for assessing the structural integrity of MHC multimer reagents. PLoS One 10, e0137984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pirko I., Suidan G. L., Rodriguez M., Johnson A. J. (2008) Acute hemorrhagic demyelination in a murine model of multiple sclerosis. J. Neuroinflammation 5, 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pavelko K. D., Pease L. R., David C. S., Rodriguez M. (2007) Genetic deletion of a single immunodominant T-cell response confers susceptibility to virus-induced demyelination. Brain Pathol. 17, 184–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cumba Garcia L. M., Huseby Kelcher A. M., Malo C. S., Johnson A. J. (2016) Superior isolation of antigen-specific brain infiltrating T cells using manual homogenization technique. J. Immunol. Methods 439, 23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson A. J., Upshaw J., Pavelko K. D., Rodriguez M., Pease L. R. (2001) Preservation of motor function by inhibition of CD8+ virus peptide-specific T cells in Theiler’s virus infection. FASEB J. 15, 2760–2762 [DOI] [PubMed] [Google Scholar]
- 38.Mendez-Fernandez Y. V., Hansen M. J., Rodriguez M., Pease L. R. (2005) Anatomical and cellular requirements for the activation and migration of virus-specific CD8+ T cells to the brain during Theiler’s virus infection. J. Virol. 79, 3063–3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stadelmann C. (2011) Multiple sclerosis as a neurodegenerative disease: pathology, mechanisms and therapeutic implications. Curr. Opin. Neurol. 24, 224–229 [DOI] [PubMed] [Google Scholar]
- 40.Dell’Orco J. M., Wasserman A. H., Chopra R., Ingram M. A., Hu Y. S., Singh V., Wulff H., Opal P., Orr H. T., Shakkottai V. G. (2015) Neuronal atrophy early in degenerative ataxia is a compensatory mechanism to regulate membrane excitability. J. Neurosci. 35, 11292–11307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siffrin V., Vogt J., Radbruch H., Nitsch R., Zipp F. (2010) Multiple sclerosis - candidate mechanisms underlying CNS atrophy. Trends Neurosci. 33, 202–210 [DOI] [PubMed] [Google Scholar]
- 42.Miller S. D., Eagar T. N. (2001) Functional role of epitope spreading in the chronic pathogenesis of autoimmune and virus-induced demyelinating diseases. Adv. Exp. Med. Biol. 490, 99–107 [DOI] [PubMed] [Google Scholar]
- 43.Elmer B. M., McAllister A. K. (2012) Major histocompatibility complex class I proteins in brain development and plasticity. Trends Neurosci. 35, 660–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee H., Brott B. K., Kirkby L. A., Adelson J. D., Cheng S., Feller M. B., Datwani A., Shatz C. J. (2014) Synapse elimination and learning rules co-regulated by MHC class I H2-Db. Nature 509, 195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Penn A. A., Riquelme P. A., Feller M. B., Shatz C. J. (1998) Competition in retinogeniculate patterning driven by spontaneous activity. Science 279, 2108–2112 [DOI] [PubMed] [Google Scholar]
- 46.Boulanger L. M. (2009) Immune proteins in brain development and synaptic plasticity. Neuron 64, 93–109 [DOI] [PubMed] [Google Scholar]
- 47.Shatz C. J. (2009) MHC class I: an unexpected role in neuronal plasticity. Neuron 64, 40–45 [DOI] [PMC free article] [PubMed] [Google Scholar]