Abstract
Month-season of birth (M-SOB) is a risk factor in multiple chronic diseases, including multiple sclerosis (MS), where the lowest and greatest risk of developing MS coincide with the lowest and highest birth rates, respectively. To determine whether M-SOB effects in such chronic diseases as MS can be experimentally modeled, we examined the effect of M-SOB on susceptibility of C57BL/6J mice to experimental autoimmune encephalomyelitis (EAE). As in MS, mice that were born during the M-SOB with the lowest birth rate were less susceptible to EAE than mice born during the M-SOB with the highest birth rate. We also show that the M-SOB effect on EAE susceptibility is associated with differential production of multiple cytokines/chemokines by neuroantigen-specific T cells that are known to play a role in EAE pathogenesis. Taken together, these results support the existence of an M-SOB effect that may reflect seasonally dependent developmental differences in adaptive immune responses to self-antigens independent of external stimuli, including exposure to sunlight and vitamin D. Moreover, our documentation of an M-SOB effect on EAE susceptibility in mice allows for modeling and detailed analysis of mechanisms that underlie the M-SOB effect in not only MS but in numerous other diseases in which M-SOB impacts susceptibility.—Reynolds, J. D., Case, L. K., Krementsov, D. N., Raza, A., Bartiss, R., Teuscher, C. Modeling month-season of birth as a risk factor in mouse models of chronic disease: from multiple sclerosis to autoimmune encephalomyelitis.
Keywords: autoimmunity, cytokines, EAE, month of birth, seasonal variation
Month-season of birth (M-SOB) has been identified as a risk factor in the development of multiple chronic diseases later in life. These include behavioral, psychiatric, cardiovascular, neurologic, reproductive, endocrine, and immune/inflammatory diseases as well as longevity (1–22). In this regard, a recent phenome-wide study that examined the effect of M-SOB in 1699 diseases that involve more than 1.7 million individuals identified 55 illnesses that showed a significant association between M-SOB and disease susceptibility, with distinct incidence patterns across disease categories. Of these, 39 have been previously reported to show an association with M-SOB, whereas 16 diseases have not (23). A follow-up study that linked seasonally varying biofactors with 7 M-SOB–dependent diseases highlighted 3 biologic networks associated with M-SOB effects (24).
With respect to inflammatory neurologic disorders, numerous groups have identified M-SOB as a risk factor in multiple sclerosis (MS) (25–30). This is thought to be primarily a function of seasonal changes in developmental exposure to sunlight and/or vitamin D (VitD) status (31–34); however, it has been suggested that the relationship between MS susceptibility and M-SOB may be spurious because several studies may not have adequately controlled for confounders, such as patterns of live births in the general population, year of birth, latitude, and region (35–37). In this regard, a recent study found that the M-SOB effect in MS persists even when adjusting for these variables, with the lowest and highest birth rates coinciding with the M-SOB that predicts the lowest and greatest risk of developing MS, respectively (38).
The finding that the M-SOB effect on reproduction correlates with the M-SOB effect in MS susceptibility suggests that the 2 phenotypes may be related. In this regard, we have previously shown that the female-biased sexual dimorphism in MS susceptibility—as in systemic lupus erythematosus (39, 40)—is highly correlated with female-biased sibling sex ratios in families with disease-affected probands (41). We found a similar sex ratio distortion in favor of females within families of probands affected with rheumatoid arthritis and pauciarticular-onset juvenile arthritis, but not within families of probands affected with nonsexually dimorphic autoimmune diseases, such systemic-onset juvenile arthritis and type 1 diabetes. Moreover, the major histocompatibility complex has been found to influence reproduction in a variety of ways in both mice and humans (42–50).
To address whether the association between the M-SOB effect on MS susceptibility and birth rate can be modeled, we performed a retrospective study to examine the effect of M-SOB on susceptibility to experimental autoimmune encephalomyelitis (EAE), the principal autoimmune model of MS (51, 52). By using C57BL/6J (B6/J) myelin oligodendrocyte glycoprotein peptide 35–55 (MOG35-55) model of EAE (MOG-EAE) (53), we report that, as in MS, mice that are born in months with the lowest birth rates are significantly less susceptible to EAE, whereas mice that are born in months with the highest birth rates are more susceptible to EAE independent of external stimuli. In addition, we found that the M-SOB effect on EAE susceptibility correlates with differential production of cytokines/chemokines by MOG35-55–specific T cells, which suggests that M-SOB may shape the neonatal immune system and subsequent adaptive immune responses to self-antigens during adulthood, independent of sunlight and VitD exposure.
MATERIALS AND METHODS
Animals
B6/J mice used in this study were either purchased from The Jackson Laboratory (Bar Harbor, ME, USA) or generated in the vivarium of the University of Vermont by using B6/J breeding stock purchased from The Jackson Laboratory. Animals were maintained in accordance with the Animal Welfare Act and the Public Health Service Policy on the Humane Care and Use of Laboratory Animals. Animals were maintained on standard laboratory chow and water ad libitum under standard environmental conditions, including controlled temperature, humidity, and a 12-h light/dark cycle. In addition, infectious disease status of the colony was monitored serologically by using a standard sentinel program. No change in the serologic profile of animals was observed throughout the course of experiments. Seasonal birth rates for B6/J mice born in the Norther Hemisphere were obtained from the Trudeau Institute (54) and from the Animal Resources Center (Caning Vale, WA, Australia) for the Southern hemisphere (55).
Induction and evaluation of EAE
Mice were immunized for induction of EAE by using either MOG35–55 + complete Freund’s adjuvant (CFA) double-inoculation protocol (56) or MOG35–55 + CFA + pertussis toxin (PTX) single inoculation protocol (57). For the 2× injection protocol, mice were injected subcutaneously with a sonicated emulsion of 100 μg MOG35–55 and an equal volume of CFA that contained 200 μg Mycobacterium tuberculosis H37RA (Difco Laboratories, Detroit, MI, USA) in the posterior right and left flank. One week later, all mice received the same injection at 2 sites on the right and left flank anterior to the initial injection sites. Animals that were immunized using the 1× MOG35–55 + CFA + PTX single-inoculation protocol received a sonicated emulsion of 200 μg MOG35–55 and an equal volume of CFA that contained 200 μg M. tuberculosis H37RA by subcutaneaous injections distributed equally in the posterior right and left flank and scruff of the neck. Immediately thereafter, each animal received 200 ng PTX (List Biologic Laboratories, Campbell, CA, USA) by intravenous injection. Mice were scored daily starting at d 5 after injection, as previously described (57): 0, no clinical expression of disease; 1, flaccid tail without hind-limb weakness; 2, hind-limb weakness; 3, complete hind-limb paralysis and floppy tail; 4, hind-limb paralysis accompanied by a floppy tail and urinary or fecal incontinence; and 5, moribund. Clinical quantitative trait variables, including incidence, cumulative disease score, peak score, and severity index, were generated as previously described (56). Severity of clinical disease course was quantified as the area under the disease course curve.
Cytokine/chemokine measurement
Mice were immunized by using the 2× EAE protocol, and spleens and draining lymph nodes were harvested 10 d later. Single-cell suspensions of 1 × 106 cells/ml were cultured with 50 μg/ml MOG35–55. After 72 h, levels of IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12(p40), IL-12 (p70), IL-13, G-CSF, GM-CSF, Eotaxin/CCL11, KC/CXCL1 (murine IL-8 homolog), MCP-1/CCL2, MIP-1α/CCL3, MIP-1β/CCL4, RANTES/CCL5, and TNF-α in culture supernatants were quantified by Bio-Plex multiplex assay (Bio-Rad, Hercules, CA, USA) as described by manufacturer protocol. IL-17 and IFN-γ levels were analyzed by ELISA as previously described (58, 59).
Statistics
Statistical analyses were performed by using either SAS System for Windows, ver. 8.1 (SAS Institute, Cary, NC, USA) or GraphPad Prism (ver. 6.07; GraphPad Software, La Jolla, CA, USA).
RESULTS
M-SOB influences MOG-EAE susceptibility
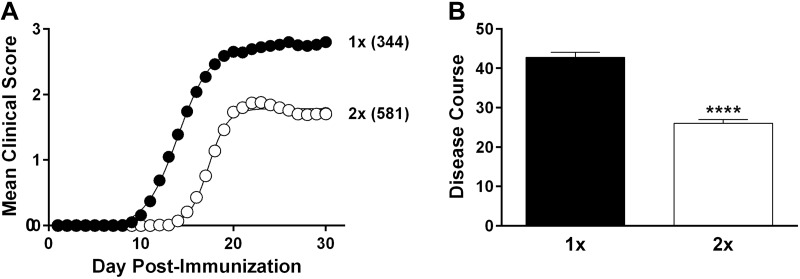
A total of 925 B6/J mice were immunized at different times during the year from 2004 to 2013. Mice ranged in age from 6 to 22 wk at the time of immunization, with an average age of 12.0 ± 3.4 wk. Male and female mice were represented equally (χ2 = 0.6; P = 0.4). EAE was induced by immunization with MOG35-55 in CFA by using 2 distinct protocols, one that included PTX as an ancillary adjuvant with a single (1×) MOG35-55 immunization, and one that did not use PTX, but included double (2×) immunizations (see Materials and Methods). Of 925 mice, 581 were immunized by using the 2× MOG35-55 + CFA protocol, whereas 344 were immunized by using the 1× MOG35-55 + CFA + PTX protocol. Compared with 2× MOG35-55 + CFA immunized mice, the severity of clinical disease course was significantly greater in 1× MOG35-55 + CFA + PTX immunized mice (Fig. 1A, B).
Figure 1.
Inclusion of PTX as an ancillary adjuvant elicits a more severe EAE clinical disease course in B6/J mice. EAE was elicited in B6/J mice by using a 1× MOG35-55 + CFA + PTX or 2× MOG35-55 + CFA–immunization protocol. A) Regression analysis revealed that the clinical disease course fits a variable slope sigmoid dose-response curve (shown as solid lines) that was significantly different (F = 1805.0; P < 0.0001) between 1- and 2×-immunized mice as assessed by the extra sum-of-squares F test. Number in parentheses are the number of animals per immunization cohort. B) Differences in overall severity of clinical disease course were also quantified by averaging the area under the curve of both the 1- and 2×-immunized mice. Significance of observed differences was determined by using the unpaired Student’s t test (t = 10.3). ****P < 0.0001.
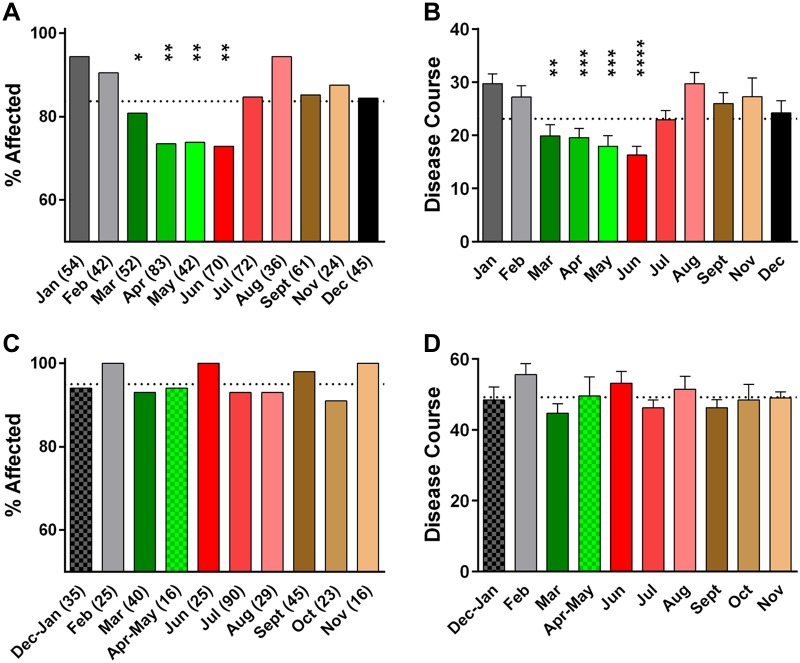
To assess the potential role of an M-SOB effect on EAE susceptibility, we stratified results from the 2 immunization cohorts by month of birth. A significant effect of M-SOB was observed among 2× MOG35-55 + CFA immunized mice, with mice born in March, April, May, and June exhibiting lower disease susceptibility (Fig. 2A) and severity (Fig. 2B) compared with mice born in January, February, July, August, September, November, and December. In contrast, a significant effect of M-SOB was not detected for 1× MOG35-55 + CFA + PTX–immunized B6/J mice (Fig. 2C, D). Results of logistic regression analyses for effects of MOG35-55, CFA, and M. tuberculosis H37RA stocks, as well as investigator, did not detect a significant effect of any of these variables on M-SOB effect (P > 0.05 for all). Taken together, these results support the existence of a significant M-SOB effect on susceptibility to MOG-EAE elicited by using the 2× protocol.
Figure 2.
SOB influences EAE susceptibility of 2× MOG35-55 + CFA–immunized B6/J mice. A, C) 2×- and 1×-immunized mice were stratified by month of birth (MOB). Numbers in parentheses indicate number of animals. Significance of observed differences in incidence of 2- (A) and 1×- (C) immunized mice as a function of MOB were determined by using Fisher’s exact test with January (A) and December to January (C) as reference variables, respectively. Animals were considered affected if clinical scores ≥ 1 were apparent for at least 2 consecutive days. B, D) Significance of observed differences in disease severity of 2- (B) and 1×- (D) immunized mice as a function of MOB were determined by 1-way ANOVA followed by Dunn’s multiple comparison test with January (B) and December to January (D) as reference variables, respectively. A significant effect of MOB was detected with 2×-immunized mice (F = 5.4; P < 0.0001) but not for 1×-immunized mice (F = 1.0; P = 0.4). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P < 0.0001.
M-SOB influences MOG35-55–specific T-cell effector responses
To address whether M-SOB influences T-cell effector responses related to EAE susceptibility, we studied MOG35-55–specific T-cell responses of age, sex, and M-SOB matched cohorts of B6/J mice that were immunized using the 1× and 2× protocols. For the 1× protocol, seventy-one 6- to 12-wk-old mice were studied, with 11 females and 11 males born in December and January immunized in February, and 22 females and 27 males born in April and May immunized in June. For the 2× protocol, eighty-eight 6- to 12-wk-old mice were studied, with 22 females and 22 males born in December and January immunized in February, and 22 females and 22 males born in April and May immunized in June. To study the M-SOB effect on MOG35-55–specific T-cell effector responses, we quantified the production of 23 cytokines/chemokines at d 10 after immunization in peripheral lymphoid organs—spleen and draining lymph nodes—by Bio-Plex and ELISA after ex vivo MOG35-55 restimulation.
Using a Bonferroni P value that was corrected for multiple testing (P = 0.001), we identified among the 1×-immunized mice a significant M-SOB effect on the production of IL-1β, IL-17, and GM-CSF (13% of all cytokines/chemokines examined) with decreased production of IL-17 and Eotaxin and increased production of GM-CSF in April to June (Table 1). Strikingly, in contrast to the 1×-immunized mice, 65% of the cytokines/chemokines measured in the 2×-immunized mice exhibited a significant M-SOB effect (Table 1), with greater production of IL-1α, IL-1β, IL-3, IL-4, IL-5, IL-9, IL-10, IL-12(p70), IL-13, IL-17, G-CSF, GM-CSF, KC/CXCL1, and MIP-1α/CCL3 observed in December to February compared with April to June. TNF-α was the only cytokine/chemokine that exhibited increased production in April to June compared with December to February. An M-SOB effect on the production of IL-2, IL-6, IL-12(p40), IFNγ, Eotaxin, MCP-1/CCL2, MIP-1β/CCL4, and RANTES/CCL5 was not observed at P ≤ 0.001. These data support the existence of an M-SOB effect on the production of cytokines/chemokines by MOG35-55–specific T cells that correlates with the M-SOB effect on EAE susceptibility. Of importance, the production of many of the cytokines/chemokines influenced by M-SOB in 2×-immunized mice have been implicated in EAE and/or MS pathogenesis.
TABLE 1.
SOB markedly influences cytokine/chemokine production by MOG35-55–specific T cells in 2× MOG35-55 + CFA–immunized B6/J mice
| Cytokine/ chemokine | 1× |
2× |
||||
|---|---|---|---|---|---|---|
| December–February | April–June | P | December–February | April–June | P | |
| IL-1α | 40.1 ± 4.3 (22) | 31.3 ± 2.1 (49) | 46.5 ± 3.2 (44) | 32.3 ± 1.3 (44) | 0.0001 ↓ | |
| IL-1β | 43.9 ± 3.2 | 60.4 ± 3.2 | 64.9 ± 2.3 | 18.4 ± 0.9 | <0.0001 ↓ | |
| IL-2 | 409.0 ± 31.7 | 386.6 ± 18.8 | 204.4 ± 22.9 | 128.0 ± 9.2 | ||
| IL-3 | 723.5 ± 77.3 | 823.0 ± 69.4 | 674.8 ± 54.4 | 270.8 ± 25.6 | <0.0001 ↓ | |
| IL-4 | 20.4 ± 2.1 | 17.6 ± 1.3 | 14.3 ± 0.9 | 9.8 ± 0.9 | 0.0006 ↓ | |
| IL-5 | 46.4 ± 7.7 | 41.2 ± 4.6 | 35.5 ± 3.2 | 10.3 ± 1.0 | <0.0001 ↓ | |
| IL-6 | 839.2 ± 101.6 | 899.8 ± 49.2 | 1120.8 ± 218.8 | 499.3 ± 75.4 | ||
| IL-9 | 263.1 ± 19.9 | 228.7 ± 13.1 | 397.5 ± 16.1 | 192.5 ± 11.9 | <0.0001 ↓ | |
| IL-10 | 215.3 ± 20.0 | 277.3 ± 16.0 | 263.3 ± 16.2 | 157.2 ± 10.4 | <0.0001 ↓ | |
| IL-12 (p40) | 155.0 ± 9.5 | 127.1 ± 4.8 | 225.5 ± 12.7 | 220.8 ± 8.6 | ||
| IL-12 (p70) | 129.0 ± 11.8 | 119.9 ± 6.2 | 542.3 ± 28.1 | 267.1 ± 12.0 | <0.0001 ↓ | |
| IL-13 | 1102.0 ± 114.8 | 987.8 ± 75.8 | 930.2 ± 60.3 | 682.6 ± 48.5 | 0.0002 ↓ | |
| IL-17 | 1486.0 ± 144.4 | 454.8 ± 45.4 | <0.0001 ↓ | 2349.7 ± 232.1 | 391.5 ± 41.1 | <0.0001 ↓ |
| IFN-γ | 2279.0 ± 376.2 | 1861.0 ± 152.9 | 4518.2 ± 405.4 | 2714.5 ± 385.7 | ||
| TNF-α | 39.6 ± 4.2 | 47.1 ± 3.3 | 32.3 ± 1.9 | 75.8 ± 4.9 | <0.0001 ↑ | |
| G-CSF | 26.2 ± 2.9 | 27.0 ± 1.6 | 74.7 ± 7.4 | 16.5 ± 1.9 | <0.0001 ↓ | |
| GM-CSF | 1175.0 ± 50.9 | 2879.0 ± 214.8 | <0.0001 ↑ | 1867.7 ± 168.2 | 959.4 ± 127.0 | <0.0001 ↓ |
| Eotaxin/CCL11 | 1336.0 ± 98.4 | 510.2 ± 35.6 | <0.0001 ↓ | 1318.0 ± 119.9 | 1600.0 ± 186.9 | |
| KC/CXCL1 | 46.5 ± 8.2 | 86.7 ± 9.4 | 40.0 ± 3.0 | 19.2 ± 1.9 | <0.0001 ↓ | |
| MCP-1/CCL2 | 7770.0 ± 489.6 | 8605.0 ± 480.8 | 2340.6 ± 223.3 | 1591.1 ± 108.7 | ||
| MIP-1α/CCL3 | 308.6 ± 15.4 | 281.4 ± 14.0 | 163.3 ± 5.8 | 115.9 ± 5.1 | <0.0001 ↓ | |
| MIP-1β/CCL4 | 1746.0 ± 100.9 | 1686.0 ± 114.0 | 1317.7 ± 141.8 | 1812.4 ± 156.6 | ||
| RANTES/CCL5 | 2304.0 ± 188.5 | 3529.0 ± 246.0 | 3723.3 ± 219.6 | 2923.9 ± 180.7 | ||
Values in parenthesis indicate number of animals studied. P values passing a Bonferroni corrected threshold corrected for multiple testing (0.05/46 = 0.001) are listed.
M-SOB effect on EAE severity and cytokine/chemokine production by MOG35-55–specific T cells correlate with seasonal variation in birth rates
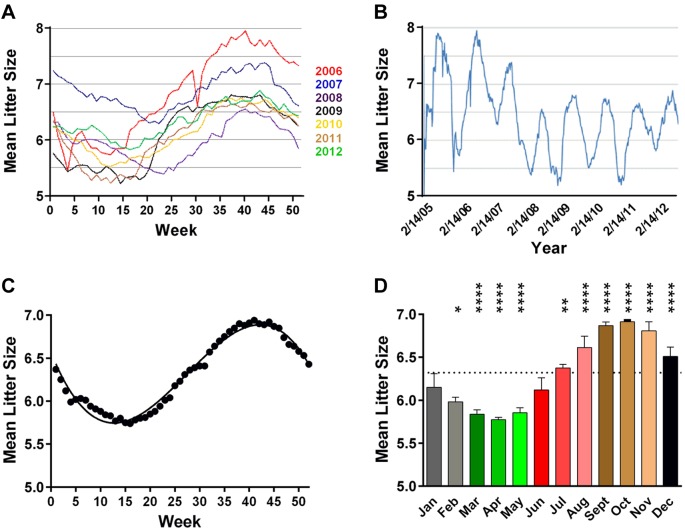
Given that reproductive circannual rhythms persist in domesticated rats and mice in the absence of classic zeitgebers (50–69), and that seasonal variability in birth rates is observed in human populations (70, 71), with the lowest birthrates coinciding with the M-SOB that predicts the lowest risk of developing MS (38), we compared birth rates of B6/J mice with the M-SOB effect on EAE susceptibility. In this regard, B6/J mice that are born in the Northern hemisphere exhibit seasonal variation in the number of pups born per litter per week across the year (Fig. 3A) with a similar periodicity across the years studied (Fig. 3B, C) (54). When stratified by month of birth, fewer pups are born in February, March, April, and May (Fig. 3D). Seasonal birth rate data from the southern hemisphere was based on the 3-mo running averages for the percent total number of mice weaned (Fig. 4A). To compare birth rates between the northern and southern hemispheres, we generated the 3-mo running averages for the number of B6/J mice born per litter per month (Fig. 4B) and regressed the 2 variables against each other (Fig. 4C). A highly significant association between birth rates in the northern and southern hemisphere was detected (F = 56.2; P < 0.0001; r = 0.84; P < 0.0001), which suggested that the seasonal birth pattern for B6/J mice may reflect a free-running circannual rhythm (72) or exposure to uncontrolled external environmental factors that vary by season in both the northern and southern hemispheres.
Figure 3.
B6/J mice exhibit seasonal variation in birthrates. A) Mean number of pups per litter from 2006 to 2012. B) Annual variation in birthrates from 2006 to 2012. C) Mean number of progeny per litter for all calendar years from 2006 to 2012. Data were fitted to a third-order polynomial function (R2 = 0.47). D) Monthly number of B6/J mice born per litter differs significant across the year. Significance of observed differences for the monthly number of B6/J mice born per litter was determined by 1-way ANOVA (F = 107.3; P < 0.0001) followed by Dunn’s multiple comparison test with January as reference variable. *P ≤ 0.05; **P ≤ 0.01; ****P < 0.0001.
Figure 4.
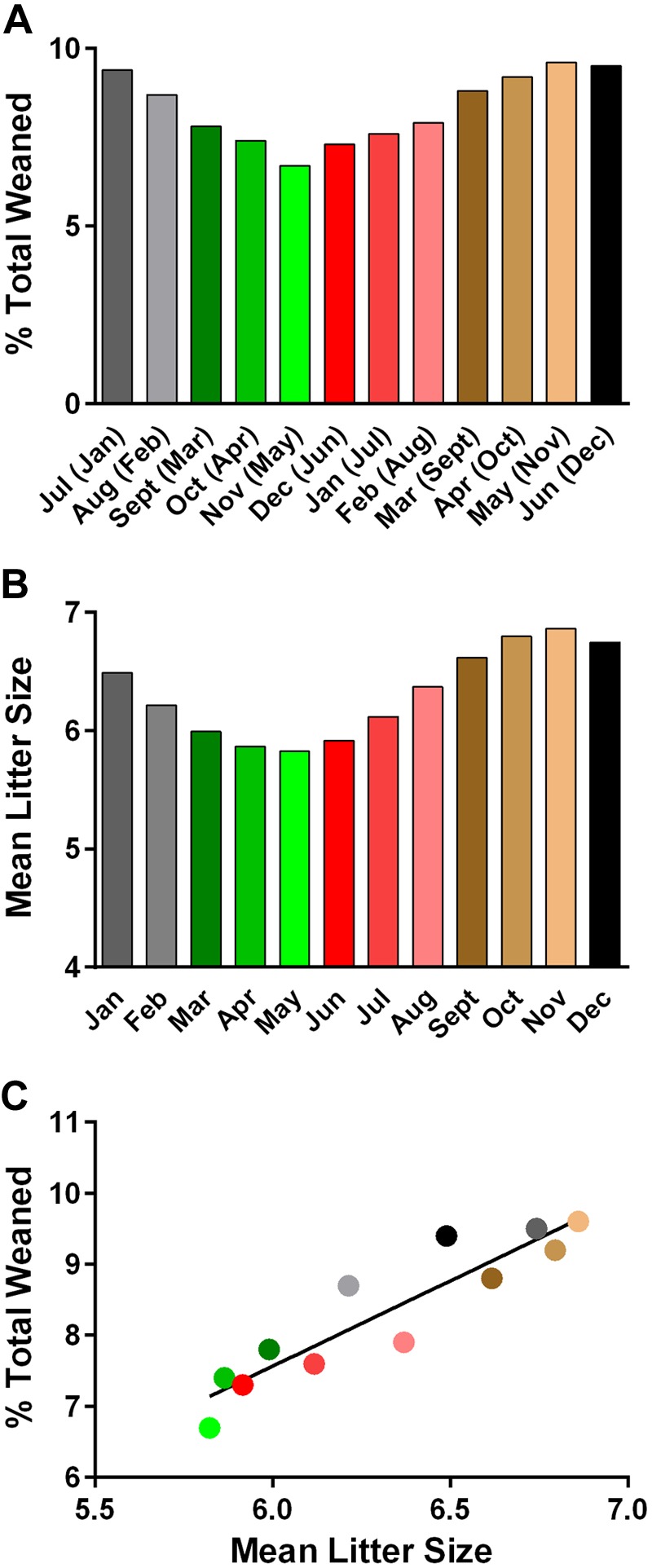
Similar patterns of seasonal birth rates for B6/J mice are observed in the Northern and Southern hemispheres. A) Three-month running averages for the number of B6/J mice weaned per month in the Southern hemisphere. Months in parentheses reflect the corresponding months in the Northern hemisphere. B) Three-month running averages for the number of B6/J mice born per litter per month in the Northern hemisphere. A χ2 goodness-of-fit test was used to examine whether the percent total number of animals weaned and mean litter size were uniformly distributed across the 12 mo. Results indicate that the number of animals weaned per month in the Southern hemisphere (P < 0.0001) and mean litter size per month in the Northern hemisphere (P < 0.0001) do not conform to a uniform distribution. Rather, both the number of animals weaned per month and litter size were less than expected in March (September), April (October), May (November), and June (December), and greater than expected during July (January), August (February), September (March), October (April), November (May), December (June), January (July), and February (August). C) A significant association between 3-mo running averages for the percent total B6/J mice weaned per month in the Southern hemisphere and 3-mo running average for the number of B6/J mice born per litter per month in the Northern hemisphere was detected (F = 56.2; P < 0.0001). Significance of association was determined by linear regression.
To assess the relationship between birth rates and the M-SOB effect on EAE severity, we regressed incidence, severity of clinical disease course, cumulative disease score, peak score, and severity index against the monthly mean litter size, 3-mo running average for litter size, and 3-mo running averages for percent total pups weaned (Table 2). A highly significant positive association between all EAE quantitative trait variables and birth rate variables was detected, with lower and greater disease susceptibility correlating with birth during low and high birth rate seasons, respectively.
TABLE 2.
Seasonal variation in birth rates positively correlate with seasonal variation in EAE susceptibility
| EAE quantitative trait variable | F | P |
|---|---|---|
| Incidence vs. | ||
| Monthly mean litter size | 6.5 | 0.01 |
| 3-mo running average litter size | 12.2 | 0.0005 |
| 3-mo running average % weaned | 12.6 | 0.0004 |
| Disease course vs. | ||
| Monthly mean litter size | 15.6 | <0.0001 |
| 3-mo running average litter size | 31.2 | <0.0001 |
| 3-mo running average % weaned | 31.6 | <0.0001 |
| Cumulative disease score vs. | ||
| Monthly mean litter size | 14.7 | 0.0001 |
| 3-mo running average litter size | 30.1 | <0.0001 |
| 3-mo running average % weaned | 30.9 | <0.0001 |
| Peak score vs. | ||
| Monthly mean litter size | 17.4 | <0.0001 |
| 3-mo running average litter size | 33.8 | <0.0001 |
| 3-mo running average % weaned | 34.8 | <0.0001 |
| Severity index vs. | ||
| Monthly mean litter size | 21.9 | <0.0001 |
| 3-mo running average litter size | 35.2 | <0.0001 |
| 3-mo running average % weaned | 32.3 | <0.0001 |
For incidence, animals were considered affected if clinical scores ≥ 1 were apparent for at least 2 consecutive days. Severity of disease course was quantified by averaging the area under the curve of each immunized mouse. Cumulative disease score is the average of the summation of daily scores. Peak score is the average of the highest score attained across the 30-d observation. Severity index is calculated by dividing the cumulative disease score by the number of days affected. Significance of association was determined by linear regression.
DISCUSSION
M-SOB has been reported to be a risk factor in susceptibility to a variety of chronic human disease (1–22), including MS (25–30). A recent study has indicated that the M-SOB effect in MS susceptibility correlates with M-SOB effects on birth rates, with the lowest and greatest risk of developing MS coinciding with the M-SOB that exhibits the lowest and highest birth rates, respectively (38). Taken together, this finding suggests that the 2 phenotypes may be functionally related. In this study, we tested the hypothesis that the positive correlation between the M-SOB effect on birth rates and MS susceptibility can be experimentally modeled. By using the B6/J MOG35-55 model of EAE, we report the identification of an M-SOB effect on disease susceptibility of 2× MOG35-55 + CFA–immunized mice that is not seen with 1× MOG35-55 + CFA + PTX–immunized B6/J mice. The finding that the M-SOB effect is not observed in 1× MOG35-55 + CFA + PTX–immunized B6/J mice suggests that the penetrance of the M-SOB effect can be modified by exposure to additional environmental inflammatory stimuli that influence disease susceptibility (73–75). In this regard, epidemiologic data strongly implicate MS environmental risk factors (MS-ERFs) that act upon the genetically susceptible background at the population level not only during adulthood but during gestation, development, and early life (31, 76). Importantly, there are studies that indicate that MS-ERFs can act synergistically, with the risk of MS in individuals exposed to more than one factor combining additively or multiplicatively (77, 78). Moreover, our results establish that the 2×-immunization protocol compared with the 1×-protocol is more appropriate for modeling the effects of MS-ERFs on EAE susceptibility.
In addition, we show that the M-SOB effect on EAE susceptibility is associated with differential production of multiple cytokines/chemokines that are produced by MOG35-55–specific T cells known to be involved in MS and EAE pathogenesis. Of interest, the M-SOB effect on the production of these cytokines/chemokines by MOG35-55–specific T cells parallels the M-SOB effect on the human neonatal immune system where spring/summer newborns present with the lowest levels of immune cells of all types and cytokine/chemokine levels; fall newborns exhibit higher levels of activated T cells and mucosal IL-12p70, TNF-α, IL-13, IL-10, and IL-2; and winter newborns have the highest levels of innate immune cells, IL-5, IL-17–related immune mediators, and activated T cells (79). In this regard, M-SOB has been reported to influence the numbers of signal joint T-cell receptor excision circles in CD4 and CD8 T cells (80). Such developmental differences may impact the ability to handle microbial exposure at mucosal surfaces in early life and subsequent development of immune-mediated diseases later in life. Whether these seasonal differences are a result of low-grade maternal inflammation or intrauterine exposure to bacteria and viruses (81–83); external environmental zeitgebers, such as nutritional status (84, 85); UV radiation/photoperiod acting alone or via VitD production (86, 87); climate, including humidity and temperature (88); or a free-running physiologic rhythm (72, 89–91) is unknown. In MS, M-SOB effect is thought to be a result of seasonal variation in UV exposure and vitamin D levels (31–34); however, our results suggest that other mechanisms may be involved as nutritional status, UV radiation/photoperiod, VitD exposure, and climate are all controlled in mouse experiments. Of interest, a similar periodicity is observed for seasonal variation in immune cell frequency and gene expression in adult humans (92–94).
Lastly, our finding that the M-SOB effects on birthrates and EAE susceptibility are highly positively correlated recapitulates the finding in MS, where the lowest and highest birthrates coincide seasonally with predicted MS risk (38), and establishes an association between reproduction and susceptibility to CNS autoimmune disease. Whether the M-SOB effect on reproduction is mechanistically related to the M-SOB effect on EAE susceptibility is unknown. It is possible that the same mechanism that influences birth rates mediates the M-SOB effect on EAE susceptibility. For example, seasonal changes in sex-hormonal status persist in domesticated rodents, including mice, that are housed in windowless vivaria under controlled lighting (12-h light/dark), temperature (21 ± 1°C), and humidity (50–60%) (66, 68, 95), and have been shown to be affected by M-SOB (96, 97). Consequently, M-SOB–dependent sex-hormonal changes may contribute to the seasonal differences in reproduction and susceptibility to EAE and cytokine/chemokine production by MOG35-55–specific T cells in B6/J mice.
In this regard, it is well recognized that seasonal variation in phytoestrogen levels (genistein, daidzein, and glycitein) and/or Fusarium mycotoxin contamination (aphlatoxin, deoxynivalenol, and zearalenone) of soybean/alfalfa/corn/grain meal–based diets vary depending on seasonal mill dates (98, 99), producing significant effects on reproductive, toxicologic, and comparative estrogenic or hormonal end points (98, 100). Of importance, both genistein and daidzein are known to have immunomodulatory activity (101–103) and impact EAE susceptibility (104–106). In addition, there is a gut microbiome–dependent mechanism whereby daidzin and daidzein are converted to equol by colonic bacteria that have specialized enzymes (107, 108). In this regard, equol-producing and nonproducing bacterial genera have been found to differ between gut microbiomes of patients with MS and healthy controls (109), and there is growing evidence that diet may be a risk factor in MS (110). Equol binds to both estrogen receptor-α and -β, with significant impacts on both estrogen- and androgen-regulated responses, including fertility (111–115), and has been shown to demonstrate anti-inflammatory properties (116). Similarly, free mycotoxins have both endocrine disruptor and immunomodulatory activity (117–121), and their masked conjugated forms can be converted to free toxin by fecal microbiota (122, 123). Additional external environmental factors with the potential to influence reproduction and disease susceptibility in a seasonally dependent fashion are bedding, particularly corncob-based bedding (99), and water (124–127), including use of nonacidified vs. acidified water (128–130), which is used to prevent growth of micro-organisms.
Alternatively, distinct mechanisms with the same periodicity may uniquely influence seasonal variation in immunity and reproduction, particularly as immunologic circannual rhythms also persist under controlled conditions in domesticated rats and mice (131–138). Notwithstanding, our documentation that M-SOB effects in chronic human diseases can be modeled in mice will allow for detailed analysis of mechanisms that are associated with M-SOB effects in not only MS, but numerous other human diseases in which M-SOB impacts disease susceptibility during their lifetime. Of importance, the periodicity of the M-SOB effect in chronic diseases in humans is recapitulated by the periodicity observed for birth rates in B6/J mice, with some diseases showing an association with high-risk M-SOB and correspondingly greater protection against other diseases (23). Consequently, the periodicity of the M-SOB effect on both types of chronic human diseases can be modeled in B6/J mice with respect to seasonal variation in birth rates and reproductive performance.
ACKNOWLEDGMENTS
This work was supported by the U.S. National Institutes of Health, National Institute of Neurological Disorders and Stroke Grants NS061014, AI041747, NS060901, NS036526, and NS069628 (all to C.T.).
Glossary
- B6/J
C57BL/6J
- CFA
complete Freund’s adjuvant
- EAE
experimental autoimmune encephalomyelitis
- M-SOB
month-season of birth
- MOG35-55
myelin oligodendrocyte glycoprotein peptide 35-55
- MOG-EAE
myelin oligodendrocyte glycoprotein induced experimental autoimmune encephalomyelitis
- MS
multiple sclerosis
- MS-ERF
multiple sclerosis environmental risk factor
- PTX
pertussis toxin
- VitD
vitamin D
AUTHOR CONTRIBUTIONS
J. D. Reynolds and A. Raza collated data; L. K. Case, D. N. Krementsov, and R. Bartiss conducted experiments, acquired data, and assisted C. Teuscher in analyzing data; and C. Teuscher designed research studies and wrote the manuscript.
REFERENCES
- 1.Korsgaard J., Dahl R. (1983) Sensitivity to house dust mite and grass pollen in adults. Influence of the month of birth. Clin. Allergy 13, 529–535 [DOI] [PubMed] [Google Scholar]
- 2.Doblhammer G., Vaupel J. W. (2001) Lifespan depends on month of birth. Proc. Natl. Acad. Sci. USA 98, 2934–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huber S., Fieder M., Wallner B., Moser G., Arnold W. (2004) Brief communication: birth month influences reproductive performance in contemporary women. Hum. Reprod. 19, 1081–1082 [DOI] [PubMed] [Google Scholar]
- 4.Huber S., Fieder M. (2009) Strong association between birth month and reproductive performance of Vietnamese women. Am. J. Hum. Biol. 21, 25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn H. S., Morgan T. M., Case L. D., Dabelea D., Mayer-Davis E. J., Lawrence J. M., Marcovina S. M., Imperatore G.; SEARCH for Diabetes in Youth Study Group (2009) Association of type 1 diabetes with month of birth among U.S. youth: the SEARCH for Diabetes in Youth Study. Diabetes Care 32, 2010–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGrath J. J., Eyles D. W., Pedersen C. B., Anderson C., Ko P., Burne T. H., Norgaard-Pedersen B., Hougaard D. M., Mortensen P. B. (2010) Neonatal vitamin D status and risk of schizophrenia: a population-based case-control study. Arch. Gen. Psychiatry 67, 889–894 [DOI] [PubMed] [Google Scholar]
- 7.Kemkes A. (2010) The impact of maternal birth month on reproductive performance: controlling for socio-demographic confounders. J. Biosoc. Sci. 42, 177–194 [DOI] [PubMed] [Google Scholar]
- 8.Huber S., Fieder M. (2011) Perinatal winter conditions affect later reproductive performance in Romanian women: intra and intergenerational effects. Am. J. Hum. Biol. 23, 546–552 [DOI] [PubMed] [Google Scholar]
- 9.Disanto G., Chaplin G., Morahan J. M., Giovannoni G., Hyppönen E., Ebers G. C., Ramagopalan S. V. (2012) Month of birth, vitamin D and risk of immune-mediated disease: a case control study. BMC Med. 10, 69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halldner L., Tillander A., Lundholm C., Boman M., Långström N., Larsson H., Lichtenstein P. (2014) Relative immaturity and ADHD: findings from nationwide registers, parent- and self-reports. J. Child Psychol. Psychiatry 55, 897–904 [DOI] [PubMed] [Google Scholar]
- 11.Gardener H., Gao X., Chen H., Schwarzschild M. A., Spiegelman D., Ascherio A. (2010) Prenatal and early life factors and risk of Parkinson’s disease. Mov. Disord. 25, 1560–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Disanto G., Handel A. E., Para A. E., Ramagopalan S. V., Handunnetthi L. (2011) Season of birth and anorexia nervosa. Br. J. Psychiatry 198, 404–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salib E., Cortina-Borja M. (2006) Effect of month of birth on the risk of suicide. Br. J. Psychiatry 188, 416–422 [DOI] [PubMed] [Google Scholar]
- 14.Messias E., Mourao C., Maia J., Campos J. P., Ribeiro K., Ribeiro L., Kirkpatrick B. (2006) Season of birth and schizophrenia in Northeast Brazil: relationship to rainfall. J. Nerv. Ment. Dis. 194, 870–873 [DOI] [PubMed] [Google Scholar]
- 15.Javaras K. N., Austin S. B., Field A. E. (2011) Season of birth and disordered eating in a population-based sample of young U.S. females. Int. J. Eat. Disord. 44, 630–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levitan R. D., Kaplan A. S., Davis C., Lam R. W., Kennedy J. L. (2010) A season-of-birth/DRD4 interaction predicts maximal body mass index in women with bulimia nervosa. Neuropsychopharmacology 35, 1729–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brewerton T. D., Dansky B. S., O’Neil P. M., Kilpatrick D. G. (2012) Seasonal patterns of birth for subjects with bulimia nervosa, binge eating, and purging: results from the National Women’s Study. Int. J. Eat. Disord. 45, 131–134 [DOI] [PubMed] [Google Scholar]
- 18.Vassallo M. F., Banerji A., Rudders S. A., Clark S., Mullins R. J., Camargo C. A. Jr (2010) Season of birth and food allergy in children. Ann. Allergy Asthma Immunol. 104, 307–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagamine S., Sonohata M., Kitajima M., Kawano S., Ogawa K., Mawatari M., Hotokebuchi T. (2011) Seasonal trends in the incidence of hip osteoarthritis in Japanese patients. Open Orthop. J. 5, 134–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusunoki T., Asai K., Harazaki M., Korematsu S., Hosoi S. (1999) Month of birth and prevalence of atopic dermatitis in schoolchildren: dry skin in early infancy as a possible etiologic factor. J. Allergy Clin. Immunol. 103, 1148–1152 [DOI] [PubMed] [Google Scholar]
- 21.Kuo C. L., Chen T. L., Liao C. C., Yeh C. C., Chou C. L., Lee W. R., Lin J. G., Shih C. C. (2016) Birth month and risk of atopic dermatitis: a nationwide population-based study. Allergy 71, 1626–1631 [DOI] [PubMed] [Google Scholar]
- 22.Poltavskiy E., Spence J. D., Kim J., Bang H. (2016) Birth month and cardiovascular disease risk association: is meaningfulness in the eye of the beholder? Online J. Public Health Inform. 8, e186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boland M. R., Shahn Z., Madigan D., Hripcsak G., Tatonetti N. P. (2015) Birth month affects lifetime disease risk: a phenome-wide method. J. Am. Med. Inform. Assoc. 22, 1042–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boland M. R., Tatonetti N. P. (2016) In search of ‘birth month genes’: using existing data repositories to locate genes underlying birth month-disease relationships. AMIA Jt. Summits Transl. Sci. Proc. 2016, 189–198 [PMC free article] [PubMed] [Google Scholar]
- 25.Willer C. J., Dyment D. A., Sadovnick A. D., Rothwell P. M., Murray T. J., Ebers G. C.; Canadian Collaborative Study Group (2005) Timing of birth and risk of multiple sclerosis: population based study. BMJ 330, 120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huber S., Didham R., Fieder M. (2008) Month of birth and offspring count of women: data from the Southern hemisphere. Hum. Reprod. 23, 1187–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staples J., Ponsonby A. L., Lim L. (2010) Low maternal exposure to ultraviolet radiation in pregnancy, month of birth, and risk of multiple sclerosis in offspring: longitudinal analysis. BMJ 340, c1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torkildsen O., Grytten N., Aarseth J., Myhr K. M., Kampman M. T. (2012) Month of birth as a risk factor for multiple sclerosis: an update. Acta Neurol. Scand. Suppl. 195, 58–62 [DOI] [PubMed] [Google Scholar]
- 29.Grytten N., Torkildsen Ø., Aarseth J. H., Benjaminsen E., Celius E. G., Dahl O. P., Holmøy T., Løken-Amsrud K., Midgard R., Myhr K. M., Risberg G., Vatne A., Kampman M. T. (2013) Month of birth as a latitude-dependent risk factor for multiple sclerosis in Norway. Mult. Scler. 19, 1028–1034 [DOI] [PubMed] [Google Scholar]
- 30.Torkildsen O., Aarseth J., Benjaminsen E., Celius E., Holmøy T., Kampman M. T., Løken-Amsrud K., Midgard R., Myhr K. M., Riise T., Grytten N. (2014) Month of birth and risk of multiple sclerosis: confounding and adjustments. Ann. Clin. Transl. Neurol. 1, 141–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Handel A. E., Giovannoni G., Ebers G. C., Ramagopalan S. V. (2010) Environmental factors and their timing in adult-onset multiple sclerosis. Nat. Rev. Neurol. 6, 156–166 [DOI] [PubMed] [Google Scholar]
- 32.Krementsov D. N., Teuscher C. (2013) Environmental factors acting during development to influence MS risk: insights from animal studies. Mult. Scler. 19, 1684–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ascherio A., Munger K. L., Simon K. C. (2010) Vitamin D and multiple sclerosis. Lancet Neurol. 9, 599–612 [DOI] [PubMed] [Google Scholar]
- 34.McDowell T. Y., Amr S., Langenberg P., Royal W., Bever C., Culpepper W. J., Bradham D. D. (2010) Time of birth, residential solar radiation and age at onset of multiple sclerosis. Neuroepidemiology 34, 238–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hintzen R. (2013) Multiple sclerosis: month of birth effect in MS--fact or artefact? Nat. Rev. Neurol. 9, 489–490 [DOI] [PubMed] [Google Scholar]
- 36.Fiddes B., Wason J., Kemppinen A., Ban M., Compston A., Sawcer S. (2013) Confounding underlies the apparent month of birth effect in multiple sclerosis. Ann. Neurol. 73, 714–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiddes B., Wason J., Sawcer S. (2014) Confounding in association studies: month of birth and multiple sclerosis. J. Neurol. 261, 1851–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodríguez Cruz P. M., Matthews L., Boggild M., Cavey A., Constantinescu C. S., Evangelou N., Giovannoni G., Gray O., Hawkins S., Nicholas R., Oppenheimer M., Robertson N., Zajicek J., Rothwell P. M., Palace J. (2016) Time- and region-specific season of birth effects in multiple sclerosis in the United Kingdom. JAMA Neurol. 73, 954–960 [DOI] [PubMed] [Google Scholar]
- 39.Moorthy L. N., Peterson M. G., Onel K. B., Lehman T. J. (2008) Do children with lupus have fewer male siblings? Lupus 17, 128–131 [DOI] [PubMed] [Google Scholar]
- 40.Aggarwal R., Sestak A. L., Chakravarty E. F., Harley J. B., Scofield R. H. (2013) Excess female siblings and male fetal loss in families with systemic lupus erythematosus. J. Rheumatol. 40, 430–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Case L. K., Wall E. H., Osmanski E. E., Dragon J. A., Saligrama N., Zachary J. F., Lemos B., Blankenhorn E. P., Teuscher C. (2015) Copy number variation in Y chromosome multicopy genes is linked to a paternal parent-of-origin effect on CNS autoimmune disease in female offspring. Genome Biol. 16, 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wedekind C., Chapuisat M., Macas E., Rülicke T. (1996) Non-random fertilization in mice correlates with the MHC and something else. Heredity (Edinb) 77, 400–409 [DOI] [PubMed] [Google Scholar]
- 43.Ober C. L., Martin A. O., Simpson J. L., Hauck W. W., Amos D. B., Kostyu D. D., Fotino M., Allen F. H. Jr (1983) Shared HLA antigens and reproductive performance among Hutterites. Am. J. Hum. Genet. 35, 994–1004 [PMC free article] [PubMed] [Google Scholar]
- 44.Ober C., Hyslop T., Elias S., Weitkamp L. R., Hauck W. W. (1998) Human leukocyte antigen matching and fetal loss: results of a 10 year prospective study. Hum. Reprod. 13, 33–38 [DOI] [PubMed] [Google Scholar]
- 45.Ober C., Elias S., Kostyu D. D., Hauck W. W. (1992) Decreased fecundability in Hutterite couples sharing HLA-DR. Am. J. Hum. Genet. 50, 6–14 [PMC free article] [PubMed] [Google Scholar]
- 46.Ober C., Aldrich C. L., Chervoneva I., Billstrand C., Rahimov F., Gray H. L., Hyslop T. (2003) Variation in the HLA-G promoter region influences miscarriage rates. Am. J. Hum. Genet. 72, 1425–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burrows C. K., Kosova G., Herman C., Patterson K., Hartmann K. E., Velez Edwards D. R., Stephenson M. D., Lynch V. J., Ober C. (2016) Expression quantitative trait locus mapping studies in mid-secretory phase endometrial cells identifies HLA-F and TAP2 as fecundability-associated genes. PLoS Genet. 12, e1005858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mika K. M., Lynch V. J. (2016) An ancient fecundability-associated polymorphism switches a repressor into an enhancer of endometrial TAP2 expression. Am. J. Hum. Genet. 99, 1059–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson C. A., Boucher G., Lees C. W., Franke A., D’Amato M., Taylor K. D., Lee J. C., Goyette P., Imielinski M., Latiano A., Lagacé C., Scott R., Amininejad L., Bumpstead S., Baidoo L., Baldassano R. N., Barclay M., Bayless T. M., Brand S., Büning C., Colombel J. F., Denson L. A., De Vos M., Dubinsky M., Edwards C., Ellinghaus D., Fehrmann R. S., Floyd J. A., Florin T., Franchimont D., Franke L., Georges M., Glas J., Glazer N. L., Guthery S. L., Haritunians T., Hayward N. K., Hugot J. P., Jobin G., Laukens D., Lawrance I., Lémann M., Levine A., Libioulle C., Louis E., McGovern D. P., Milla M., Montgomery G. W., Morley K. I., Mowat C., Ng A., Newman W., Ophoff R. A., Papi L., Palmieri O., Peyrin-Biroulet L., Panés J., Phillips A., Prescott N. J., Proctor D. D., Roberts R., Russell R., Rutgeerts P., Sanderson J., Sans M., Schumm P., Seibold F., Sharma Y., Simms L. A., Seielstad M., Steinhart A. H., Targan S. R., van den Berg L. H., Vatn M., Verspaget H., Walters T., Wijmenga C., Wilson D. C., Westra H. J., Xavier R. J., Zhao Z. Z., Ponsioen C. Y., Andersen V., Torkvist L., Gazouli M., Anagnou N. P., Karlsen T. H., Kupcinskas L., Sventoraityte J., Mansfield J. C., Kugathasan S., Silverberg M. S., Halfvarson J., Rotter J. I., Mathew C. G., Griffiths A. M., Gearry R., Ahmad T., Brant S. R., Chamaillard M., Satsangi J., Cho J. H., Schreiber S., Daly M. J., Barrett J. C., Parkes M., Annese V., Hakonarson H., Radford-Smith G., Duerr R. H., Vermeire S., Weersma R. K., Rioux J. D. (2011) Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat. Genet. 43, 246–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franke A., McGovern D. P., Barrett J. C., Wang K., Radford-Smith G. L., Ahmad T., Lees C. W., Balschun T., Lee J., Roberts R., Anderson C. A., Bis J. C., Bumpstead S., Ellinghaus D., Festen E. M., Georges M., Green T., Haritunians T., Jostins L., Latiano A., Mathew C. G., Montgomery G. W., Prescott N. J., Raychaudhuri S., Rotter J. I., Schumm P., Sharma Y., Simms L. A., Taylor K. D., Whiteman D., Wijmenga C., Baldassano R. N., Barclay M., Bayless T. M., Brand S., Büning C., Cohen A., Colombel J. F., Cottone M., Stronati L., Denson T., De Vos M., D’Inca R., Dubinsky M., Edwards C., Florin T., Franchimont D., Gearry R., Glas J., Van Gossum A., Guthery S. L., Halfvarson J., Verspaget H. W., Hugot J. P., Karban A., Laukens D., Lawrance I., Lemann M., Levine A., Libioulle C., Louis E., Mowat C., Newman W., Panés J., Phillips A., Proctor D. D., Regueiro M., Russell R., Rutgeerts P., Sanderson J., Sans M., Seibold F., Steinhart A. H., Stokkers P. C., Torkvist L., Kullak-Ublick G., Wilson D., Walters T., Targan S. R., Brant S. R., Rioux J. D., D’Amato M., Weersma R. K., Kugathasan S., Griffiths A. M., Mansfield J. C., Vermeire S., Duerr R. H., Silverberg M. S., Satsangi J., Schreiber S., Cho J. H., Annese V., Hakonarson H., Daly M. J., Parkes M. (2010) Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat. Genet. 42, 1118–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simmons S. B., Pierson E. R., Lee S. Y., Goverman J. M. (2013) Modeling the heterogeneity of multiple sclerosis in animals. Trends Immunol. 34, 410–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson A. P., Harp C. T., Noronha A., Miller S. D. (2014) The experimental autoimmune encephalomyelitis (EAE) model of MS: utility for understanding disease pathophysiology and treatment. Handb. Clin. Neurol. 122, 173–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller S. D., Karpus W. J. (2007) Experimental autoimmune encephalomyelitis in the mouse. Curr. Protoc. Immunol. 15.1, 1–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bartiss R. (2015) Predicting seasonal breeding variation in a colony of C57BL/6J mice. 56th Annual Short Course on Medical and Experimental Mammalian Genetics, At Jackson Laboratory, 1, 1 [Google Scholar]
- 55. Animal Resources Center. Accessed July 19, 2015 at: https://www.researchgate.net/publication/280683615_Predicting_Seasonal_Breeding_Variation_in_a_Colony_of_C57BL6J_Mice.
- 56.Butterfield R. J., Sudweeks J. D., Blankenhorn E. P., Korngold R., Marini J. C., Todd J. A., Roper R. J., Teuscher C. (1998) New genetic loci that control susceptibility and symptoms of experimental allergic encephalomyelitis in inbred mice. J. Immunol. 161, 1860–1867 [PubMed] [Google Scholar]
- 57.Teuscher C., Noubade R., Spach K., McElvany B., Bunn J. Y., Fillmore P. D., Zachary J. F., Blankenhorn E. P. (2006) Evidence that the Y chromosome influences autoimmune disease in male and female mice. Proc. Natl. Acad. Sci. USA 103, 8024–8029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noubade R., Milligan G., Zachary J. F., Blankenhorn E. P., del Rio R., Rincon M., Teuscher C. (2007) Histamine receptor H1 is required for TCR-mediated p38 MAPK activation and optimal IFN-gamma production in mice. J. Clin. Invest. 117, 3507–3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu C., Diehl S. A., Noubade R., Ledoux J., Nelson M. T., Spach K., Zachary J. F., Blankenhorn E. P., Teuscher C. (2010) Endothelial histamine H1 receptor signaling reduces blood-brain barrier permeability and susceptibility to autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 107, 18967–18972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanson F. B., Sholes F. N. (1924) Seasonal differences in sex ratio, litter size and birth weight of the albino rat under uniform laboratory conditions. Genetics 9, 363–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parkes A. S. (1927) Studies on the sex-ratio and related phenomena. (9) Observations on fertility and sex-ratio in mice, 1922-5. Brit. J. Exp. Biol. 4, 93–104 [Google Scholar]
- 62.Ohzu E., Sato A. (1963) Breeding experiments of white rats and mice. IX. Seasonal variations of birth rate and litter size in several strains of laboratory rat. Zool. Mag. (Toyko) 72, 139–145 [Google Scholar]
- 63.Schlager G., Roderick T. H. (1968) Secondary sex ratio in mice. J. Hered. 59, 363–365 [DOI] [PubMed] [Google Scholar]
- 64.Pennycuik P. R. (1972) Seasonal changes in reproductive productivity, growth rate, and food intake in mice exposed to different regimes of day length and environmental temperature. Aust. J. Biol. Sci. 25, 627–635 [DOI] [PubMed] [Google Scholar]
- 65.Drickamer L. C. (1977) Seasonal variation in litter size, bodyweight and sexual maturation in juvenile female house mice (Mus musculus). Lab. Anim. 11, 159–162 [DOI] [PubMed] [Google Scholar]
- 66.Mock E. J., Kamel F., Wright W. W., Frankel A. I. (1975) Seasonal rhythm in plasma testosterone and luteinising hormone of the male laboratory rat. Nature 256, 61–63 [DOI] [PubMed] [Google Scholar]
- 67.Pelikan J. (1981) Patterns of reproduction in the house mouse. Symp. Zool. Soc. Lond. 47, 205–229 [Google Scholar]
- 68.Wong C. C., Döhler K. D., Atkinson M. J., Geerlings H., Hesch R. D., von zur Mühlen A. (1983) Circannual variations in serum concentrations of pituitary, thyroid, parathyroid, gonadal and adrenal hormones in male laboratory rats. J. Endocrinol. 97, 179–185 [DOI] [PubMed] [Google Scholar]
- 69.Drickamer L. C. (1990) Seasonal variation in fertility, fecundity and litter sex ratio in laboratory and wild stocks of house mice (Mus domesticus). Lab. Anim. Sci. 40, 284–288 [PubMed] [Google Scholar]
- 70.Roenneberg T., Aschoff J. (1990) Annual rhythm of human reproduction: I. Biology, sociology, or both? J. Biol. Rhythms 5, 195–216 [DOI] [PubMed] [Google Scholar]
- 71.Roenneberg T. (2004) The decline in human seasonality. J. Biol. Rhythms 19, 193–195, discussion 196–197 [DOI] [PubMed] [Google Scholar]
- 72.Paul M. J., Zucker I., Schwartz W. J. (2008) Tracking the seasons: the internal calendars of vertebrates. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 341–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blankenhorn E. P., Butterfield R. J., Rigby R., Cort L., Giambrone D., McDermott P., McEntee K., Solowski N., Meeker N. D., Zachary J. F., Doerge R. W., Teuscher C. (2000) Genetic analysis of the influence of pertussis toxin on experimental allergic encephalomyelitis susceptibility: an environmental agent can override genetic checkpoints. J. Immunol. 164, 3420–3425 [DOI] [PubMed] [Google Scholar]
- 74.Spach K. M., Noubade R., McElvany B., Hickey W. F., Blankenhorn E. P., Teuscher C. (2009) A single nucleotide polymorphism in Tyk2 controls susceptibility to experimental allergic encephalomyelitis. J. Immunol. 182, 7776–7783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saligrama N., Case L. K., Krementsov D. N., Teuscher C. (2014) Histamine H2 receptor signaling × environment interactions determine susceptibility to experimental allergic encephalomyelitis. FASEB J. 28, 1898–1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burrell A. M., Handel A. E., Ramagopalan S. V., Ebers G. C., Morahan J. M. (2011) Epigenetic mechanisms in multiple sclerosis and the major histocompatibility complex (MHC). Discov. Med. 11, 187–196 [PubMed] [Google Scholar]
- 77.Disanto G., Morahan J. M., Ramagopalan S. V. (2012) Multiple sclerosis: risk factors and their interactions. CNS Neurol. Disord. Drug Targets 11, 545–555 [DOI] [PubMed] [Google Scholar]
- 78.Van der Mei I., Lucas R. M., Taylor B. V., Valery P. C., Dwyer T., Kilpatrick T. J., Pender M. P., Williams D., Chapman C., Otahal P., Ponsonby A. L. (2016) Population attributable fractions and joint effects of key risk factors for multiple sclerosis. Mult. Scler. 22, 461–469 [DOI] [PubMed] [Google Scholar]
- 79.Thysen A. H., Rasmussen M. A., Kreiner-Moller E., Larsen J. M., Folsgaard N. V., Bonnelykke K., Stokholm J., Bisgaard H., Brix S. (2016) Season of birth shapes neonatal immune function. J. Allergy Clin. Immunol. 137, 1238–1246.e1-13 [DOI] [PubMed] [Google Scholar]
- 80.Disanto G., Watson C. T., Meier U. C., Ebers G. C., Giovannoni G., Ramagopalan S. V. (2013) Month of birth and thymic output. JAMA Neurol. 70, 527–528 [DOI] [PubMed] [Google Scholar]
- 81.Stensballe L. G., Ravn H., Kristensen K., Agerskov K., Meakins T., Aaby P., Simões E. A. (2009) Respiratory syncytial virus neutralizing antibodies in cord blood, respiratory syncytial virus hospitalization, and recurrent wheeze. J. Allergy Clin. Immunol. 123, 398–403 [DOI] [PubMed] [Google Scholar]
- 82.Baschat A. A., Towbin J., Bowles N. E., Harman C. R., Weiner C. P. (2003) Prevalence of viral DNA in amniotic fluid of low-risk pregnancies in the second trimester. J. Matern. Fetal Neonatal Med. 13, 381–384 [DOI] [PubMed] [Google Scholar]
- 83.Payne C. M., Ray C. G., Borduin V., Minnich L. L., Lebowitz M. D. (1986) An eight-year study of the viral agents of acute gastroenteritis in humans: ultrastructural observations and seasonal distribution with a major emphasis on coronavirus-like particles. Diagn. Microbiol. Infect. Dis. 5, 39–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prasad M., Lumia M., Erkkola M., Tapanainen H., Kronberg-Kippilä C., Tuokkola J., Uusitalo U., Simell O., Veijola R., Knip M., Ovaskainen M. L., Virtanen S. M. (2010) Diet composition of pregnant Finnish women: changes over time and across seasons. Public Health Nutr. 13, 939–946 [DOI] [PubMed] [Google Scholar]
- 85.Watson P. E., McDonald B. W. (2007) Seasonal variation of nutrient intake in pregnancy: effects on infant measures and possible influence on diseases related to season of birth. Eur. J. Clin. Nutr. 61, 1271–1280 [DOI] [PubMed] [Google Scholar]
- 86.Norval M., McLoone P., Lesiak A., Narbutt J. (2008) The effect of chronic ultraviolet radiation on the human immune system. Photochem. Photobiol. 84, 19–28 [DOI] [PubMed] [Google Scholar]
- 87.Hart P. H., Gorman S., Finlay-Jones J. J. (2011) Modulation of the immune system by UV radiation: more than just the effects of vitamin D? Nat. Rev. Immunol. 11, 584–596 [DOI] [PubMed] [Google Scholar]
- 88.Stevenson T. J., Visser M. E., Arnold W., Barrett P., Biello S., Dawson A., Denlinger D. L., Dominoni D., Ebling F. J., Elton S., Evans N., Ferguson H. M., Foster R. G., Hau M., Haydon D. T., Hazlerigg D. G., Heideman P., Hopcraft J. G., Jonsson N. N., Kronfeld-Schor N., Kumar V., Lincoln G. A., MacLeod R., Martin S. A., Martinez-Bakker M., Nelson R. J., Reed T., Robinson J. E., Rock D., Schwartz W. J., Steffan-Dewenter I., Tauber E., Thackeray S. J., Umstatter C., Yoshimura T., Helm B. (2015) Disrupted seasonal biology impacts health, food security and ecosystems. Proc. Biol. Sci. 282, 20151453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gwinner E. (1986) Circannual Rhythms, Springer, Heidelberg, Germany: [Google Scholar]
- 90.Helm B., Ben-Shlomo R., Sheriff M. J., Hut R. A., Foster R., Barnes B. M., Dominoni D. (2013) Annual rhythms that underlie phenology: biological time-keeping meets environmental change. Proc. Biol. Sci. 280, 20130016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Visser M. E., Caro S. P., van Oers K., Schaper S. V., Helm B. (2010) Phenology, seasonal timing and circannual rhythms: towards a unified framework. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 3113–3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dopico X. C., Evangelou M., Ferreira R. C., Guo H., Pekalski M. L., Smyth D. J., Cooper N., Burren O. S., Fulford A. J., Hennig B. J., Prentice A. M., Ziegler A. G., Bonifacio E., Wallace C., Todd J. A. (2015) Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat. Commun. 6, 7000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paynter S., Ware R. S., Sly P. D., Williams G., Weinstein P. (2015) Seasonal immune modulation in humans: observed patterns and potential environmental drivers. J. Infect. 70, 1–10 [DOI] [PubMed] [Google Scholar]
- 94.Goldinger A., Shakhbazov K., Henders A. K., McRae A. F., Montgomery G. W., Powell J. E. (2015) Seasonal effects on gene expression. PLoS One 10, e0126995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mock E. J., Frankel A. I. (1978) A seasonal influence on testes weight and serum gonadotropin levels of the mature male laboratory rat. Biol. Reprod. 18, 772–778 [DOI] [PubMed] [Google Scholar]
- 96.Mock E. J., Frankel A. I. (1978) A shifting circannual rhythm in serum testosterone concentration in male laboratory rats. Biol. Reprod. 19, 927–930 [DOI] [PubMed] [Google Scholar]
- 97.Mock E. J., Frankel A. I. (1980) Influence of month of birth on the serum hormone concentrations and weights of the accessory sex organs and testes during maturation of the male laboratory rat. Biol. Reprod. 22, 119–133 [DOI] [PubMed] [Google Scholar]
- 98.Thigpen J. E., Setchell K. D., Padilla-Banks E., Haseman J. K., Saunders H. E., Caviness G. F., Kissling G. E., Grant M. G., Forsythe D. B. (2007) Variations in phytoestrogen content between different mill dates of the same diet produces significant differences in the time of vaginal opening in CD-1 mice and F344 rats but not in CD Sprague-Dawley rats. Environ. Health Perspect. 115, 1717–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thigpen J. E., Setchell K. D., Kissling G. E., Locklear J., Caviness G. F., Whiteside T., Belcher S. M., Brown N. M., Collins B. J., Lih F. B., Tomer K. B., Padilla-Banks E., Camacho L., Adsit F. G., Grant M. (2013) The estrogenic content of rodent diets, bedding, cages, and water bottles and its effect on bisphenol A studies. J. Am. Assoc. Lab. Anim. Sci. 52, 130–141 [PMC free article] [PubMed] [Google Scholar]
- 100.Barnard D. E., Lewis S. M., Teter B. B., Thigpen J. E. (2009) Open- and closed-formula laboratory animal diets and their importance to research. J. Am. Assoc. Lab. Anim. Sci. 48, 709–713 [PMC free article] [PubMed] [Google Scholar]
- 101.Tyagi A. M., Srivastava K., Sharan K., Yadav D., Maurya R., Singh D. (2011) Daidzein prevents the increase in CD4+CD28null T cells and B lymphopoesis in ovariectomized mice: a key mechanism for anti-osteoclastogenic effect. PLoS One 6, e21216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nakaya M., Yamasaki M., Miyazaki Y., Tachibana H., Yamada K. (2003) Estrogenic compounds suppressed interferon-gamma production in mouse splenocytes through direct cell-cell interaction. In Vitro Cell. Dev. Biol. Anim. 39, 383–387 [DOI] [PubMed] [Google Scholar]
- 103.Mohammad-Shahi M., Haidari F., Rashidi B., Saei A. A., Mahboob S., Rashidi M. R. (2011) Comparison of the effects of genistein and daidzein with dexamethasone and soy protein on rheumatoid arthritis in rats. Bioimpacts 1, 161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.De Paula M. L., Rodrigues D. H., Teixeira H. C., Barsante M. M., Souza M. A., Ferreira A. P. (2008) Genistein down-modulates pro-inflammatory cytokines and reverses clinical signs of experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 8, 1291–1297 [DOI] [PubMed] [Google Scholar]
- 105.Castro S. B., Junior C. O., Alves C. C., Dias A. T., Alves L. L., Mazzoccoli L., Mesquita F. P., Figueiredo N. S., Juliano M. A., Castañon M. C., Gameiro J., Almeida M. V., Teixeira H. C., Ferreira A. P. (2012) Immunomodulatory effects and improved prognosis of experimental autoimmune encephalomyelitis after O-tetradecanoyl-genistein treatment. Int. Immunopharmacol. 12, 465–470 [DOI] [PubMed] [Google Scholar]
- 106.Razeghi Jahromi S., Arrefhosseini S. R., Ghaemi A., Alizadeh A., Moradi Tabriz H., Togha M. (2014) Alleviation of experimental allergic encephalomyelitis in C57BL/6 mice by soy daidzein. Iran. J. Allergy Asthma Immunol. 13, 256–264 [PubMed] [Google Scholar]
- 107.Setchell K. D., Clerici C. (2010) Equol: history, chemistry, and formation. J. Nutr. 140, 1355S–1362S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rafii F. (2015) The role of colonic bacteria in the metabolism of the natural isoflavone daidzin to equol. Metabolites 5, 56–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen J., Chia N., Kalari K. R., Yao J. Z., Novotna M., Soldan M. M., Luckey D. H., Marietta E. V., Jeraldo P. R., Chen X., Weinshenker B. G., Rodriguez M., Kantarci O. H., Nelson H., Murray J. A., Mangalam A. K. (2016) Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 6, 28484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jörg S., Grohme D. A., Erzler M., Binsfeld M., Haghikia A., Müller D. N., Linker R. A., Kleinewietfeld M. (2016) Environmental factors in autoimmune diseases and their role in multiple sclerosis. Cell. Mol. Life Sci. 73, 4611–4622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bennetts H. W., Underwood E. J., Shier F. L. (1946) A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust. Vet. J. 22, 2–12 [DOI] [PubMed] [Google Scholar]
- 112.Lightfoot R. J., Croker K.P., Neil H. G. (1968) Failure of sperm transport in relation to ewe infertility following prolonged grazing on oestrogenic pastures. Aust. J. Agric. Res. 18, 755–765 [Google Scholar]
- 113.Biggers J. D., Curnow D. H. (1954) Oestrogenic activity of subterranean clover. I. The oestrogenic activity of genistein. Biochem. J. 58, 278–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Braden A. H. N., Lamberton J. (1967) The estrogenic activity and metabolism of certain isoflavones in sheep. Aust. J. Agric. Res. 18, 335–348 [Google Scholar]
- 115.Pope G. S. (1954) The importance of pasture plant oestrogens in the reproduction and lactation of grazing animals. Dairy Sci. Abstr. 16, 334–355 [Google Scholar]
- 116.Blay M., Espinel A. E., Delgado M. A., Baiges I., Bladé C., Arola L., Salvadó J. (2010) Isoflavone effect on gene expression profile and biomarkers of inflammation. J. Pharm. Biomed. Anal. 51, 382–390 [DOI] [PubMed] [Google Scholar]
- 117.Corrier D. E. (1991) Mycotoxicosis: mechanisms of immunosuppression. Vet. Immunol. Immunopathol. 30, 73–87 [DOI] [PubMed] [Google Scholar]
- 118.Bondy G. S., Pestka J. J. (2000) Immunomodulation by fungal toxins. J. Toxicol. Environ. Health B Crit. Rev. 3, 109–143 [DOI] [PubMed] [Google Scholar]
- 119.Oswald I. P., Marin D. E., Bouhet S., Pinton P., Taranu I., Accensi F. (2005) Immunotoxicological risk of mycotoxins for domestic animals. Food Addit. Contam. 22, 354–360 [DOI] [PubMed] [Google Scholar]
- 120.Hueza I. M., Raspantini P. C., Raspantini L. E., Latorre A. O., Górniak S. L. (2014) Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound. Toxins (Basel) 6, 1080–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cano P. M., Seeboth J., Meurens F., Cognie J., Abrami R., Oswald I. P., Guzylack-Piriou L. (2013) Deoxynivalenol as a new factor in the persistence of intestinal inflammatory diseases: an emerging hypothesis through possible modulation of Th17-mediated response. PLoS One 8, e53647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Berthiller F., Crews C., Dall’Asta C., Saeger S. D., Haesaert G., Karlovsky P., Oswald I. P., Seefelder W., Speijers G., Stroka J. (2013) Masked mycotoxins: a review. Mol. Nutr. Food Res. 57, 165–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Alizadeh A., Braber S., Akbari P., Kraneveld A., Garssen J., Fink-Gremmels J. (2016) Deoxynivalenol and its modified forms: are there major differences? Toxins (Basel) 8, E334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reddersen K., Heberer T., Dünnbier U. (2002) Identification and significance of phenazone drugs and their metabolites in ground- and drinking water. Chemosphere 49, 539–544 [DOI] [PubMed] [Google Scholar]
- 125.Stackelberg P. E., Furlong E. T., Meyer M. T., Zaugg S. D., Henderson A. K., Reissman D. B. (2004) Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking-water-treatment plant. Sci. Total Environ. 329, 99–113 [DOI] [PubMed] [Google Scholar]
- 126.Donald D. B., Cessna A. J., Sverko E., Glozier N. E. (2007) Pesticides in surface drinking-water supplies of the northern Great Plains. Environ. Health Perspect. 115, 1183–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Delgado L. F., Charles P., Glucina K., Morlay C. (2012) The removal of endocrine disrupting compounds, pharmaceutically activated compounds and cyanobacterial toxins during drinking water preparation using activated carbon--a review. Sci. Total Environ. 435-436, 509–525 [DOI] [PubMed] [Google Scholar]
- 128.Langgartner D., Foertsch S., Füchsl A. M., Reber S. O. (2016) Light and water are not simple conditions: fine tuning of animal housing in male C57BL/6 mice. [E-pub online ahead of print] Stress [DOI] [PubMed] [Google Scholar]
- 129.Wolf K. J., Daft J. G., Tanner S. M., Hartmann R., Khafipour E., Lorenz R. G. (2014) Consumption of acidic water alters the gut microbiome and decreases the risk of diabetes in NOD mice. J. Histochem. Cytochem. 62, 237–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sofi M. H., Gudi R., Karumuthil-Melethil S., Perez N., Johnson B. M., Vasu C. (2014) pH of drinking water influences the composition of gut microbiome and type 1 diabetes incidence. Diabetes 63, 632–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brock M. A. (1983) Seasonal rhythmicity in lymphocyte blastogenic responses of mice persists in a constant environment. J. Immunol. 130, 2586–2588 [PubMed] [Google Scholar]
- 132.Pati A. K., Florentin I., Chung V., De Sousa M., Levi F., Mathe G. (1987) Circannual rhythm in natural killer activity and mitogen responsiveness of murine splenocytes. Cell. Immunol. 108, 227–234 [DOI] [PubMed] [Google Scholar]
- 133.Planelles D., Hernández-Godoy J., Montoro A., Montoro J., González-Molina A. (1994) Seasonal variation in proliferative response and subpopulations of lymphocytes from mice housed in a constant environment. Cell Prolif. 27, 333–341 [DOI] [PubMed] [Google Scholar]
- 134.Haus E., Lakatua D. J., Swoyer J., Sackett-Lundeen L. (1983) Chronobiology in hematology and immunology. Am. J. Anat. 168, 467–517 [DOI] [PubMed] [Google Scholar]
- 135.Nelson R. J., Demas G. E. (1996) Seasonal changes in immune function. Q. Rev. Biol. 71, 511–548 [DOI] [PubMed] [Google Scholar]
- 136.Płytycz B., Seljelid R. (1997) Rhythms of immunity. Arch. Immunol. Ther. Exp. (Warsz.) 45, 157–162 [PubMed] [Google Scholar]
- 137.Haus E., Smolensky M. H. (1999) Biologic rhythms in the immune system. Chronobiol. Int. 16, 581–622 [DOI] [PubMed] [Google Scholar]
- 138.Martinez-Bakker M., Helm B. (2015) The influence of biological rhythms on host-parasite interactions. Trends Ecol. Evol. (Amst.) 30, 314–326 [DOI] [PubMed] [Google Scholar]