Abstract
Over 60% of lower extremity amputations are performed in patients with diabetes and peripheral arterial disease, and at least 25% require subsequent reamputation due to poor surgical site healing. The mechanisms underlying poor amputation stump healing in the setting of diabetes are not understood. N-acetylcysteine (NAC) is known to promote endothelial cell function and angiogenesis and may have therapeutic benefits in the setting of diabetes. We tested the hypothesis that NAC alters the vascular milieu to improve healing of amputation stumps in diabetes using a novel in vivo murine hindlimb ischemia-amputation model. Amputation stump tissue perfusion and healing were evaluated in C57BL/6J adult mice with streptozotocin-induced diabetes. Compared with controls, mice treated with daily NAC demonstrated improved postamputation stump healing, perfusion, adductor muscle neovascularization, and decreased muscle fiber damage. Additionally, NAC stimulated HUVEC migration and proliferation in a phospholipase C β–dependent fashion and decreased Gαq palmitoylation. Similarly, NAC treatment also decreased Gαq palmitoylation in ischemic and nonischemic hindlimbs in vivo. In summary, we demonstrate that NAC accelerates healing of amputation stumps in the setting of diabetes and ischemia. The underlying mechanism appears to involve a previously unrecognized effect of NAC on Gαq palmitoylation and phospholipase C β–mediated signaling in endothelial cells.—Zayed, M. A., Wei, X., Park, K., Belaygorod, L., Naim, U., Harvey, J., Yin, L., Blumer, K., Semenkovich, C. F. N-acetylcysteine accelerates amputation stump healing in the setting of diabetes.
Keywords: NAC, peripheral arterial disease, Gαq, arterial perfusion
Over 150,000 lower extremity amputations are performed in the United States each year (1). The majority (>60%) of amputations are performed in patients who have diabetes and peripheral arterial disease (1, 2). Appropriate healing does not occur in at least a quarter of these patients, leading to limb reamputation at a higher level (3, 4), with significant associated costs and patient morbidity (5, 6). The mechanisms underlying poor amputation stump healing are not understood, and there is currently no available therapy to augment amputation stump healing and perfusion in patients with diabetes.
N-acetylcysteine (NAC) is a thiol compound approved by the U.S. Food and Drug Administration for clinical use as a mucolytic (7). It is also commonly used as an effective treatment for acetaminophen toxicity by restoring hepatic glutathione reserves (8). NAC may have broader therapeutic potential, particularly in the setting of diabetes (9). In rodent models, NAC increases insulin sensitivity in the setting of diabetes (10, 11). In one report human subjects treated with NAC had improved glucose metabolism (12). Dogma holds that NAC is a hepatic antioxidant that forms glutathione molecules through covalent conjugation at its active sulfhydryl groups (7). Outside the liver, NAC appears to have additional effects through protein kinase B phosphorylation (13), NF-κB activation (14), and translocation of cytosolic proteins (15). NAC also activates endothelial cells (ECs) through unclear mechanisms (16).
Various GPCRs are expressed on the EC surface (17, 18). Similar to other distinct G proteins (such as Gs and Gi), Gq-coupled receptors are mostly membrane associated and palmitoylated (19). Activated Gαq induces phospholipase-C β (PLC-β) signaling in ECs and is essential for EC function, angiogenesis, and vascular permeability (18, 20–22). Palmitoylation is important for recycling signaling molecules to the membrane to facilitate proper cell signaling (23–25). In the setting of diabetes, palmitoylation dynamics are altered, and this is suspected to play an important role in tissue dysfunction and altered metabolism (25, 26). It is unknown whether Gαq palmitoylation can be pharmacologically targeted to affect EC function in vivo in the setting of diabetes.
We hypothesized that in the setting of diabetes, NAC can improve healing and perfusion at ischemic surgical amputation sites. Using a novel hindlimb ischemia-amputation murine model, we demonstrate that NAC improves perfusion and angiogenesis at ischemic amputation stumps in the context of altered Gαq palmitoylation and demonstrate that NAC-dependent EC migration is PLC-β dependent.
MATERIALS AND METHODS
Animal regulations
All murine housing, breeding, and experimental procedures (including novel hindlimb ischemia-amputation model) were performed in accordance with national guidelines and regulations and were approved by the Washington University School of Medicine Institutional Animal Care and Use Committee.
Murine hindlimb ischemia-amputation model
Male C57BL/6J mice, at least 8–10 wk old, were treated with a single intraperitoneal injection of streptozotocin (STZ) (150 mg/kg in 0.05 mol/l sodium citrate buffer) (27). Serial weekly serum glucose measurements were monitored for 1 mo, and mice with serum glucose >250 mg/dl underwent unilateral hindlimb ischemia and amputation. Mice were anesthetized with ketamine (80 mg/kg total body mass) and xylazine (15 mg/kg lean body mass) and maintained on a warming pad. Hair was removed from the bilateral hindquarters and hindlimbs using depilating cream; care was taken to avoid skin erythema. The femoral artery, proximal to the bifurcation of the lateral caudal femoral artery, was aseptically exposed, ligated with 6-0 silk sutures, and then transected. The wound was irrigated with sterile saline, and the incision was closed with interrupted sutures. Mice underwent daily intraperitoneal administration with either NAC (150 mg/kg) (28) or PBS control (Fig. 1A). Seven days after unilateral hindlimb ischemia, the recovered (yet still malperfused) limb underwent a guillotine amputation at a level just above the ankle (Fig. 1A). The tibia was resected more proximally, and ventral and dorsal tissue flaps were approximated with 3 interrupted 6-0 silk sutures. Buprenorphine (0.05 mg/kg twice a day) was administered for postoperative analgesia.
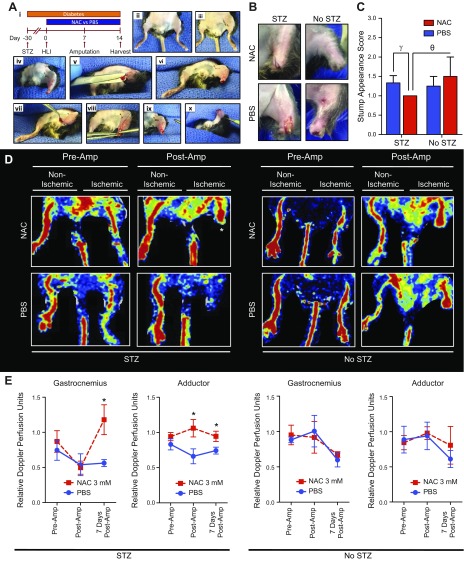
Figure 1.
NAC improves ischemic hindlimb stump perfusion and appearance. A) In a novel hindlimb ischemia-amputation model (i), mice were pretreated with STZ to induce hyperglycemia over a 1-mo period followed by staged unilateral hindlimb ischemia (HLI), infrageniculate amputation 7 d later, and tissue harvest 14 d later. HLI was performed through a small subcentimeter incision in the adductor region (ii, iii) After a 7-d recovery period, a guillotine transtibial amputation was performed under general anesthesia with aseptic technique (iv). Remnant proximal tibia was further resected to create an adequate ventral tissue rotational flap (v, vi). The ventral tissue flap was rotated over the amputation site to create a durable stump closure. The stump was closed using 3 interrupted 6-0 silk sutures (vii–x). B) Amputation stumps of diabetic and nondiabetic mice treated with NAC improved healing. There was no significant edema or inflammation in the NAC and PBS groups. C) Stump appearances were improved (lower appearance scores) in diabetic (10 NAC, 12 PBS) and nondiabetic (4 NAC, 4 PBS) mice 3 and 7 d after amputation. D) Diabetic and nondiabetic mice that were treated with NAC demonstrated higher amputation stump perfusion 7 d postamputation, particularly diabetic mice (indicated by an asterisk). E) Diabetic (7 NAC, 5 PBS) and nondiabetic (5 NAC, 3 PBS) mice demonstrated significantly increased perfusion in the gastrocnemius and adductor regions of healing ischemic hindlimb amputation stumps of mice treated with NAC for 14 d (d 1–14). *P < 0.05, γP = 0.06.
Hindlimb laser-doppler perfusion
Superficial hindlimb adductor thigh and gastrocnemius calf regions were serially monitored with noninvasive measurements obtained using a scanning laser-Doppler perfusion imager (model LD12-IR; Moor Instruments, Wilmington, DE, USA) modified for high resolution and depth of penetration (2 mm) with an 830-nm wavelength, infrared, 2.5-mW laser diode (100 μm beam diameter and 15 kHz bandwidth) (29). Before measurements were taken, anesthesia and mouse body temperature were controlled using the same techniques as with femoral artery ligation. Hindlimb adductor and gastrocnemius region measurements of ischemic and nonischemic limbs were performed before and immediately after hindlimb femoral artery ligation, 3 d after femoral artery ligation, before and immediately after hindlimb infrageniculate trans-tibial amputation at 7 d after femoral artery ligation, and 14 d after hindlimb femoral artery ligation. Hindlimb gastrocnemius and adductor regions of interest (ROIs) were drawn as previously described (29) to obtain average Doppler velocity measurements in blinded fashion to murine NAC vs. PBS treatment condition. Average velocity in an ROI was normalized to the area of the ROI due to unavoidable variations in animal positioning during Doppler scanning. Data were reported as a relative ratio of ligated-to-nonligated rate of Doppler blood perfusion in mice treated with NAC (n = 7) or PBS (n = 5).
Hindlimb amputation stump appearance scores
Amputation stump appearance was scored based on stump damage qualitative severity score: 1 = well healed, 2 = erythema/bruising, 3 = ulcer/bleeding, 4 = open wound. Amputation stump appearance scores were evaluated immediately after creation and in a blinded fashion at 3 and 7 d after amputation stump creation in mice that were treated with either NAC or PBS.
Hindlimb adductor angiography
Fourteen days after femoral artery ligation (7 d after amputation stump formation), mice underwent hindlimb angiography as previously described with some modifications (29). Mice underwent anesthesia as described above, and the thoracic and abdominal cavities were exposed. The right atrium was lacerated, and the left ventricle was cannulated to perfuse the arterial system with 30 ml of heparinized phosphate-buffered solution (heparin 2000 U in PBS; pH 7.4) at 100 mm Hg. The descending thoracic aorta was then cannulated, and mice were infused with a viscous barium sulfate (85% w/v) (Liquid Barospere; Lafayette Pharmaceuticals, Lafayette, IN, USA) suspension optimized for arterial filling. Arterial angiograms were then obtained by exposing specimens to an X-ray source (Kodak Image Station In-Vivo F/FX; Kodak, Rochester, NY, USA) for 10 s at 26 kV. As previously described, the number of adductor arterial collaterals in ischemic and nonischemic hindlimbs was derived from the hindlimb mid-zone in a blinded fashion to NAC and PBS treatment conditions.
Hindlimb adductor muscle histologic analysis
Adductor muscle segments of ischemic and nonischemic hindlimbs of mice treated with NAC and PBS were removed en bloc from the medial-most aspect of the thigh adductor muscle to the distal suprageniculate portion and evaluated with fluorescent microscopy. Muscle segments were dehydrated in graded 15 and 30% sucrose solutions followed by embedding in optimal cutting temperature blocks. At least 5 interrupted sections at 50-μm intervals were obtained. Adductor muscle fiber morphology and size were evaluated using hematoxylin and eosin staining. Relative muscle fiber area was evaluated in 3 ×20 images from 2 randomly selected sections (n = 3 per mouse group). Adductor arterial collateral vessel maturation and pericollateral collagen deposition were qualitatively evaluated using pentacrome staining of adductor muscle sections. Muscle microvessel density was evaluated with Alexa Fluor 594–conjugated Griffonia simplicifolia isolectin-1-B4 (1:100; Thermo Fisher Scientific, Waltham, MA, USA) and counterstained with DAPI for nuclear representation. Muscle reactive oxygen species (ROS) were evaluated with 2′,7′-dichlorodihydrofluroescein diacetate using an inverted fluorescent microscope (DFC 3000 G; Leica Microsystems, Wetzlar, Germany). Five ×20 images were collected from 2 randomly selected sections. Capillaries were identified as isolectin-1-B4–positive vessels with diameter <7 μm. ROS fluorescent signal was evaluated per adductor muscle fiber. ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used for morphometric analysis, microvessel density, and muscle fiber ROS signal intensity. Three independent experiments were performed for each condition (n = 3 per mouse group). Analyses were performed in a blinded fashion to mouse treatment conditions.
Reagents, lysate preparation, and Western blotting
Primary HUVECs (American Type Culture Collection, Manassas, VA, USA) were used for EC in vitro experiments and were maintained in endothelial basal medium culture according to the manufacturer’s instructions for no more than 3 passages (Cambrex, East Rutherford, NJ, USA). Cells were serum starved in basal medium (high-glucose DMEM supplemented with 0.1% bovine serum albumin and 20 U/L heparin). For lysis of all EC types, 5 × 105 cells were pelleted and lysed in modified Tris buffer [20 mM Hepes (pH 7.4), 0.1 5 M NaCl, 50 mM Tris, 50 mM NaF, 10 mM β-glycerophosphate, 1 mM each of CaCl2 and MgCl2, and Protease Inhibitors Cocktail Set III (Calbiochem, San Diego, CA, USA) (pH 7.4)] on ice for at least 30 min, and lysates were collected after centrifugation. Total protein samples were then separated by SDS-PAGE, transferred to a PVDF membrane, and subjected to Western blotting. Proteins were detected with antibodies to Gaq, Gα11, Gα13, Caveolin1, and R-Ras (all from Santa Cruz Biotechnology, Dallas, TX, USA).
ELISA
Adductor muscle tissue from ischemic and nonischemic hindlimbs of diabetic and nondiabetic mice were isolated. Muscle tissue was minced into small pieces and immersed for 30 min in 1 ml of lysis buffer per 10 mg of tissue. The resulting suspension underwent homogenization and centrifugation for 5 min at 1500 g. Tissue homogenate concentrations were then standardized by evaluating sample protein concentrations with a Bradford protein concentration assay. Tissue homogenate content of VEGF and inositol triphosphate (IP3) were evaluated using the manufacturer’s instructions (mouse VEGF ELISA, Thermo Fisher Scientific; IP3 ELISA, MyBioSource, San Diego, CA, USA).
BrdU proliferation assay
For BrdU incorporation, 1 × 104 HUVECs were seeded in a 96-well culture format precoated with 0.1% gelatin, followed by serum starvation for at least 6 h. Cells are maintained in basal media or supplemented with NAC at 3 mM with or without the PLC-β inhibitor U73122 at 1 μM. BrdU addition and detection at 12 h were performed according to the manufacturer’s instructions (Roche, Basel, Switzerland). For each condition, experiments were repeated at least 3 times, and absorbance was measured in a multiwell spectrophotometer at 450 nm (Spectra MaxPlus; Molecular Devices, Sunnyvale, CA, USA). HUVEC proliferation was expressed as the relative fold change in proliferation with different treatment conditions (n = 8).
Monolayer EC migration assay
EC cultures were grown to confluence in 10-cm culture plates. Cells were serum starved for 6 h, and wounds were created in the monolayer culture with a medium-sized pipette tip. Scratched monolayers were then supplemented with basal medium, NAC at 3 mM, or NAC at 3 mM and different concentrations of U73122 (1 or 7 μM). Monolayer scratches were then imaged at approximately the same areas of the same wounds 0, 4, and 6 h after monolayer wound induction. The percentage of wound area closure was measured using ImageJ software and expressed as a relative fold change in scratch migration (n = 6).
Click-chemistry palmitoylation assay
HUVECs were metabolically labeled after incubation with 50 μM of 17-octadecynoic acid (17-ODYA) for 12 h. To evaluate the effects of NAC on palmitoylation, 17-ODYA–labeled HUVECs where maintained in culture with and without NAC at 3 mM. Total 17-ODYA–labeled proteins were evaluated by click chemistry conjugation to rhodamine-azide. Samples were then separated by SDS-PAGE and subjected to gel-fluorescent imaging (Typhoon; GE Healthcare, Waukesha, WI, USA). Protein loading was evaluated by Western blotting after gel transfer to a PDVF membrane blotted with antibodies to Gαq, Gα11, Gα13, and Caveolin1. To evaluate Gαq palmitoylation, 17-ODYA–labeled HUVEC lysates were immunoprecipitated with Gαq antibody and then conjugated with rhodamine-azide. Gαq-immunoprecipitated fluorescent signals were evaluated relative to total Gαq as determined by Western blotting. Samples were performed in triplicate, and averages were collected from 3 separate experiments (n = 3).
S-acyl resin-assisted capture assay
This procedure was performed as described with some modifications (30, 31). HUVECs were treated for different time periods (2, 4, 6, and 12 h) and with different concentrations (1, 3, and 5 mM) of NAC. Treated cell cultures were harvested, pelleted, and solubilized using the previously described lysis buffer solution. Lysates were standardized based on Bradford protein concentrations. Two equal portions of lysates were made for +HA (hydroxylamine) and –HA samples. Aliquots of each sample were separated by SDS-PAGE and Western blotting to evaluate total protein expression levels of Gαq and caveolin. Streptavidin-agarose beads were added to the remainder of the +HA and –HA samples at room temperature for 90 min in an end-over-end rotation. Bound proteins were reduced using lysis buffer containing 0.1% SDS, 0.2% Triton X-100, and 1% β-mercaptoethanol for 15 min at 37°C with gentle mixing. Final +HA and –HA samples were then separated using SDS-PAGE and Western blotting for Gαq and Caveolin1. Relative differences in palmitoylated protein:total protein were estimated using band densitometry analysis (ImageJ). Averages were collected from 3 different experiments (n = 3).
Statistical analysis
Hindlimb ischemia perfusion and histology analyses were evaluated using 2-way ANOVA and unpaired 2-way Student’s t test for comparisons across animal groups. Comparisons between individual HUVEC treatment conditions were also evaluated using 2-way ANOVA and unpaired 2-way Student’s t test. Similarly, ELISA assays were evaluated using 2-way ANOVA. Values of P < 0.05 were considered to be significant.
RESULTS
NAC improves amputation stump healing and perfusion
To model human pathology, we designed a novel in vivo murine chronic hindlimb ischemia-amputation model (Fig. 1A). After distal hindlimb amputation, diabetic mice receiving a daily regimen of NAC injections tended to have less stump ecchymosis and breakdown (Fig. 1B) and improved appearance (Fig. 1C). Mice treated with NAC demonstrated significantly improved Doppler perfusion of the ischemic amputation stump gastrocnemius region (P = 0.03) (Fig. 1D, E) and of the hindlimb adductor region (P = 0.03) (Fig. 1D, E). These findings demonstrate that NAC improves healing and perfusion in amputation stumps, particularly in diabetic mice.
NAC decreases muscle damage and increases angiogenesis and arteriogenesis in a hindlimb ischemia-amputation model
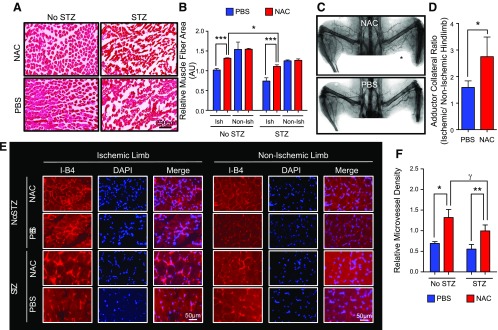
Adequate healing and perfusion of an amputation stump in a chronically ischemic limb is dependent on adequate revascularization via angiogenesis and arteriogenesis. Ischemic adductor muscle segments in diabetic mice that were treated with NAC demonstrated improved muscle fiber area (P < 0.001) (Fig. 2A, B), increased angiographic collateral formation (P = 0.05) (Fig. 2C, D), increased pericollateral collagen deposition and maturation (Supplemental Fig. 1), and a higher density of microvessels (P = 0.01) (Fig. 2E, F). This demonstrates that NAC augments arteriogenesis and angiogenesis in the ischemic hindlimbs of diabetic mice.
Figure 2.
NAC decreased muscle fiber damage and increased angiogenesis/arteriogenesis in adductor regions of mice that underwent hindlimb ischemia and amputation. A, B) Diabetic mice treated with NAC demonstrated preserved muscle fiber size (μm3) and morphology. C, D) X-ray angiography demonstrated a significantly higher density of mature arterial collaterals in the adductor hindlimb region of diabetic mice treated with NAC (n = 7) compared with PBS (n = 11). E, F) Ischemic adductor muscle segment microvessel density was significantly increased in diabetic and nondiabetic mice after NAC treatment. *P < 0.05, **P < 0.01, ***P < 0.001, γP = 0.2.
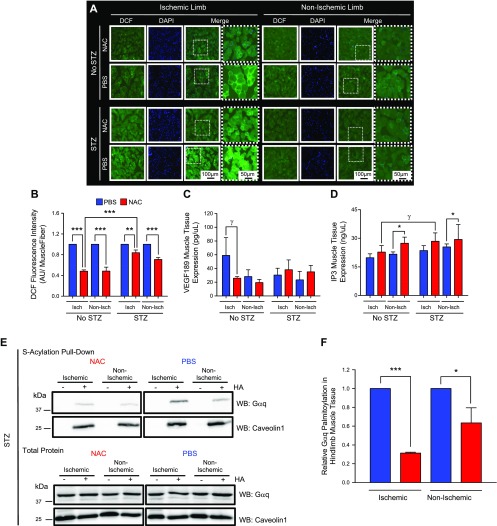
NAC decreases muscle ROS and increases muscle IP3
NAC is known to blunt tissue ROS (7), which can improve postischemia angiogenesis (32). Consistent with this notion, we observed that NAC decreases ROS in the ischemic adductor hindlimb muscle tissue of both diabetic and nondiabetic mice (P < 0.01 and P < 0.001, respectively) (Fig. 3A, B). Compared with nondiabetic mice, ROS inhibition in ischemic hindlimbs of diabetic mice was less profound after NAC (Fig. 3B), suggesting that additional signaling mechanisms explain the differences observed in hindlimb perfusion, angiogenesis, and arteriogenesis of diabetic mice treated with NAC (Figs. 1 and 2). NAC did not significantly affect VEGF in ischemic hindlimbs of diabetic mice (Fig. 3C) but increased IP3 content in hindlimbs of diabetic and nondiabetic mice (2-way ANOVA, P < 0.05) (Fig. 3D). These results demonstrate that, in addition to blunting ROS in ischemic hindlimbs, NAC can alter tissue IP3 content.
Figure 3.
NAC blunts ischemic muscle tissue ROS and decreases Gαq palmitoylation. A) 2′,7′-Dichlorodihydrofluroescein diacetate–positive ischemic adductor hindlimbs demonstrated higher ROS compared with nonischemic hindlimbs. B) Diabetic mice treated with NAC demonstrated a less robust decrease in adductor muscle ROS compared with nondiabetic controls (n = 3). C) NAC does not stimulate VEGF expression in ischemic and/or nonischemic hindlimbs of diabetic mice. In nondiabetic mice, NAC appears to blunt VEGF expression in ischemic hindlimbs of diabetic mice (n = 3). D) NAC increases IP3 in nonischemic hindlimbs of diabetic and nondiabetic mouse hindlimbs (n = 3; 2-way ANOVA, P < 0.03). E, F) NAC does not affect total protein expression of Gαq or Caveolin1 (E); however, NAC decreased Gαq palmitoylation in ischemic and nonischemic hindlimbs. –HA samples served as negative controls for S-acyl capture assay (F). The difference in Gαq palmitoylation is greater in the ischemic tissue relative to the nonischemic tissue (E, F). Caveolin1 palmitoylation is not affected by NAC treatment in either ischemic or nonischemic hindlimb tissue (E). γP = 0.08, *P < 0.05, ***P < 0.001.
Hindlimb Gαq palmitoylation is altered by NAC
IP3 signaling is dependent on Gαq signaling, and post-translational Gαq protein palmitoylation is essential for EC function and angiogenesis (22, 33). NAC treatment decreased Gαq palmitoylation in ischemic and nonischemic adductor hindlimb muscle tissue of STZ-pretreated diabetic mice. Specifically, NAC treatments reduced Gαq palmitoylation by 41.3% in nonischemic adductor muscle segments (P = 0.05) (Fig. 3E, F) and reduced Gαq palmitoylation by 69% in ischemic muscle segments (P < 0.001) (Fig. 3E, F). Similarly, NAC treatment decreased Gαq palmitoylation in ischemic hindlimb muscle tissue of wild-type and db/db mice (Supplemental Fig. 2A, B). These findings provide initial evidence that exposure to NAC alters the palmitoylation dynamics of Gαq.
NAC reduced Gαq palmitoylation in ECs
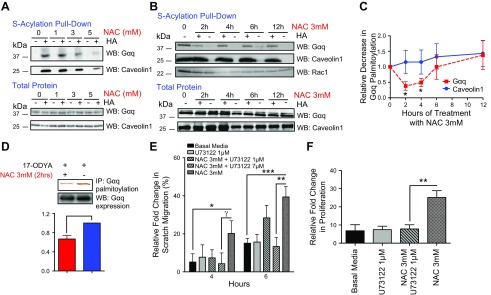
Because NAC treatment decreased Gαq palmitoylation in hindlimb adductor muscle tissue, we next evaluated whether NAC can specifically affect Gαq palmitoylation in ECs in vitro. In HUVEC cultures, NAC decreased Gαq palmitoylation in a dose-dependent fashion (Fig. 4A). NAC did not alter global palmitoylation and did not affect expression of Gαq, Gα11, or Gα13 (Supplemental Fig. 3A, B). However, using an S-acylation exchange pull-down assay, we observed decreases in Gαq palmitoylation of 62 and 51% at 2 and 4 h after treatment of HUVECs with NAC (P = 0.01 and 0.02, respectively) (Fig. 4B, C), with no change in palmitoylation of Caveolin1 at the same time points. This effect was transient, and Gαq palmitoylation returned to baseline levels by 12 h after NAC treatment (Fig. 4B, C). We confirmed this novel NAC-associated effect using an additional click chemistry approach. This technique also demonstrated that NAC decreased Gαq palmitoylation at 2 h by 43.5% (P < 0.008) (Fig. 4D). Therefore, our findings demonstrate that NAC treatment transiently decreases Gαq palmitoylation in ECs in vitro.
Figure 4.
NAC decreases Gαq palmitoylation in HUVECs and promotes EC function in a PLC-β–dependent fashion. A) S-acylation pull down assay demonstrates that, relative to total protein levels, NAC can decrease Gαq palmitoylation in a dose-dependent fashion and does not affect Caveolin1 or Rac1 palmitoylation in HUVECs. B, C) Gαq palmitoylation is decreased with maximal effect at 2 and 4 h after treatment (n = 3). D) Click-chemistry assay also demonstrates a significant decrease in Gαq palmitoylation (n = 3). E) EC monolayer scratches have increased closure after treatment with NAC 3 mM. Cotreatment with PLC-β inhibitor U73122 1 and 7 μM inhibited EC monolayer scratch closure at 4 and 6 h after treatment (n = 6). F) EC proliferation was inhibited with NAC 3 mM and U73122 1 μM cotreatment (n = 8). *P < 0.05, **P < 0.01.
NAC affects EC function in a PLC-β–dependent fashion
EC migration and proliferation are critical angiogenic functions that facilitate adequate hindlimb recovery during ischemia. Gαq-induced PLC-β activation is essential for normal EC function and IP3 signaling (34). We therefore evaluated whether NAC-induced EC migration and proliferation are PLC-β dependent. Chemical inhibition of PLC-β with U73122 abolished NAC-induced EC migration (Supplemental Fig. 4; quantified in Fig. 4E) in a dose-dependent fashion (P < 0.01) (Fig. 4E). PLC-β inhibition also robustly decreased NAC-induced EC proliferation (P < 0.002) (Fig. 4F). These results suggest that NAC-induced proliferation and migration are PLC-β dependent.
DISCUSSION
The primary objective of this study was to determine whether NAC can augment healing and perfusion of amputation stumps in the setting of ischemia, which is a common clinical problem that affects patients with diabetes. Using a novel hindlimb-ischemia amputation model, we provide evidence that NAC increases ischemic hindlimb neovascularization and adductor collateral formation. We also demonstrate that NAC selectively affects the palmitoylation of Gαq and alters EC function in a PLC-β–dependent fashion. These findings suggest that NAC has broader effects than previously realized in the setting of tissue regeneration in diabetes and identify a novel mechanism of action of NAC at the endothelium.
Poor surgical site healing is associated with significant morbidity and mortality in people with diabetes (1, 35). Patients with diabetes and peripheral arterial disease are 28 times more likely than nondiabetic patients to undergo lower extremity amputations, and nearly 25% of these individuals will have impaired healing of their amputation stumps (1, 5, 36). This complication disrupts health care outcomes and imposes substantial socioeconomic burdens (6). Targeted therapies to improve amputation stump healing in this patient population could transform vascular care. We developed a novel diabetic murine hindlimb ischemia-amputation model relevant to human amputated limb pathophysiology to explore mechanisms for augmenting tissue arterial perfusion and rapid healing.
Using this novel ischemia-amputation model, we demonstrate that NAC stimulates in vivo hindlimb adductor arteriogenesis (maturation of existing arterial collaterals; Fig. 2C, D) and hindlimb distal gastrocnemius muscle tissue angiogenesis (formation of new capillaries from existing arterial branches; Fig. 2G, H) proximal to the amputation stump. This enhanced arterial inflow recovery in ischemic amputated hindlimbs after NAC administration appears to preserve muscle tissue integrity (Fig. 2A, B) and to augment distal stump perfusion and healing, particularly in diabetic animals that manifest increased tissue ROS (Fig. 3A, B) but comparable stump healing (Fig. 1B, C) and neovascularization (Fig. 2E, F) compared with nondiabetic mice. These findings are consistent with and build upon prior studies that demonstrate that NAC administration in experimental diabetes improves insulin metabolism and blunts NF-κB signaling (37), normalizes endothelium-dependent responses (38), and augments in vivo neovascularization (a process that is presumably impaired in the peripheral arterial system in the setting of diabetes) (32).
NAC is commonly used to treat acetaminophen poisoning by restoring hepatic reserves of the antioxidant glutathione (8). However, beyond its well-characterized antioxidant properties, NAC has other important pleotropic effects and affects various cell types (7). Its original U.S. Food and Drug Administration–approved role as a mucolytic agent stems from its capacity as a thiol to reduce disulfide bonds connecting mucin monomers (39). NAC competitively binds to other sulfhydryl-reactive sites and was recently found to reduce intrachain disulfide bonds (-S-S-) of soluble plasma von Willebrand factor multimers (40). In the current study, we confirmed NAC effects on ROS (Fig. 3A, B) and tissue VEGF production (Fig. 3C), but these effects alone are unlikely to account for the differences we observed in tissue perfusion and neovascularization observed in diabetic hindlimb amputation stumps. Additional benefits are likely to derive from our novel observation that NAC affects post-translational S-palmitoylation (the process of reversible conjugation of fatty acids to protein cysteine residues via a liable thioester bond) (41). Up to 150 endothelial proteins undergo S-palmitoylation, which help inhibit or augment various essential EC functions, including insulin signaling, cell survival, permeability, and migration (26, 42–45). Compared with potent deacylases (2-bromopalmitate and tunicamycin) (46), NAC does not appear to globally disrupt palmitoylation in ECs (Supplemental Fig. 3). Instead, our work supports that concept that NAC specifically disrupts palmitoylation of important EC signaling proteins, such as the heterotrimeric Gαq protein subunit. Presumably, the extent to which NAC affects palmitoylation of specific molecules may depend on the number of palmitoylation sites and how readily accessible their palmitoylation sites are on the surface of the signaling molecule.
Cellular activation of Gα proteins accelerates palmitate turnover by nearly 50-fold (47). The balance between palmitoylated and depalmitoylated Gαq affects its structure and downstream signaling via PLC-β and IP3 (24, 25). Gα proteins and their regulators have been implicated in maintenance of vascular perfusion and cardiovascular function (34). NAC is known to preserve EC functions via PLC-β–mediated signaling and in turn to counteract the effect of toxins on the endothelium (16, 48, 49). We observed that NAC promotes EC migration and proliferation (functions essential for robust neovascularization) in a PLC-β–dependent fashion (Fig. 4E, F). We also observed that the content of IP3, a second messenger downstream of PLC-β, is altered after NAC administration. We did not observe substantial differences in IP3 content in ischemic hindlimbs of diabetic and nondiabetic mice. This is expected due to the transient half-life of IP3 in tissue, which may not allow for a robust difference in vivo in ischemic tissue. In short, our findings provide the first clues that NAC appears to affect all of the components of the Gαq-PLC-β-IP3 signaling cascade, which could contribute to the robust improvement in perfusion and healing in ischemic amputation stumps in vivo with diabetes. Future studies will help determine whether NAC-induced Gαq depalmitoylation also plays a central role in EC function.
By augmenting tissue neovascularization, NAC can improve perfusion and healing of extremity amputation stumps with chronic ischemia and diabetes. NAC alters Gαq palmitoylation and PLC-β–dependent EC functions, which are known to be critical for tissue healing. Future studies will evaluate the molecular and biochemical consequences of NAC-induced changes in palmitoylation as well as the potential clinical use of NAC for surgical site healing in ischemic limbs of patients with diabetes.
ACKNOWLEDGMENTS
This work was supported by the Vascular Cures Foundation Wylie Scholar Award (to M.A.Z.), Society for Vascular Surgery Foundation Research Seed Grant (to M.A.Z.), Washington University Diabetes Research Center grant (P30 DK020579), the U.S. Department of Veterans Affairs (VA-STL-151-16-01), and U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grants DK076729, DK101392, and DK056341. The authors thank Dr. Sangeeta Adak, Dr. Larry Spears, and Malik Darwech (all from Washington University) for their scientific and technical input; the Washington University Diabetes Models Phenotyping Core for assistance with murine morphometric studies; and Dr. Luis Sanchez and Dr. Timothy Eberlein (all from Washington University) for institutional support.
Glossary
- 17-ODYA
17-octadecynoic acid
- EC
endothelial cell
- IP3
inositol triphosphate
- NAC
N-acetylcysteine
- PLC-β
phospholipase-C β
- ROI
region of interest
- ROS
reactive oxygen species
- STZ
streptozotocin
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
M. Zayed, X. Wei, J. Harvey, K. Blumer, and C. Semenkovich designed the research and analyzed and interpreted the data; M. Zayed, X. Wei, K. Park, L. Belaygorod, U. Naim, J. Harvey, and L. Yi were involved in the data acquisition and analysis; M. Zayed, X. Wei, and C. Semenkovich drafted the manuscript; M. Zayed was responsible for the integrity of the work as a whole; and all authors revised the manuscript critically for important intellectual content and approved the final version to be published.
REFERENCES
- 1.Zayed M., Bech F., Hernandez-Boussard T. (2014) National review of factors influencing disparities and types of major lower extremity amputations. Ann. Vasc. Surg. 28, 1157–1165 [DOI] [PubMed] [Google Scholar]
- 2.Gregg E. W., Li Y., Wang J., Burrows N. R., Ali M. K., Rolka D., Williams D. E., Geiss L. (2014) Changes in diabetes-related complications in the United States, 1990-2010. N. Engl. J. Med. 370, 1514–1523https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24738668&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 3.Ebskov B., Josephsen P. (1980) Incidence of reamputation and death after gangrene of the lower extremity. Prosthet. Orthot. Int. 4, 77–80 [DOI] [PubMed] [Google Scholar]
- 4.Kanade R., van Deursen R., Burton J., Davies V., Harding K., Price P. (2007) Re-amputation occurrence in the diabetic population in South Wales, UK. Int. Wound J. 4, 344–352 https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17961158&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sen C. K., Gordillo G. M., Roy S., Kirsner R., Lambert L., Hunt T. K., Gottrup F., Gurtner G. C., Longaker M. T. (2009) Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 17, 763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dillingham T. R., Pezzin L. E., Shore A. D. (2005) Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch. Phys. Med. Rehabil. 86, 480–486https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15759232&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 7.Samuni Y., Goldstein S., Dean O. M., Berk M. (2013) The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta 1830, 4117–4129https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23618697&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 8.Prescott L. F., Park J., Ballantyne A., Adriaenssens P., Proudfoot A. T (1977) Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet 2, 432–434 [DOI] [PubMed] [Google Scholar]
- 9.Lasram M. M., Dhouib I. B., Annabi A., El Fazaa S., Gharbi N. (2015) A review on the possible molecular mechanism of action of N-acetylcysteine against insulin resistance and type-2 diabetes development. Clin. Biochem. 48, 1200–1208 [DOI] [PubMed] [Google Scholar]
- 10.Ismael M. A., Talbot S., Carbonneau C. L., Beauséjour C. M., Couture R. (2008) Blockade of sensory abnormalities and kinin B(1) receptor expression by N-acetyl-L-cysteine and ramipril in a rat model of insulin resistance. Eur. J. Pharmacol. 589, 66–72 [DOI] [PubMed] [Google Scholar]
- 11.Kaneto H., Kajimoto Y., Miyagawa J., Matsuoka T., Fujitani Y., Umayahara Y., Hanafusa T., Matsuzawa Y., Yamasaki Y., Hori M. (1999) Beneficial effects of antioxidants in diabetes: possible protection of pancreatic beta-cells against glucose toxicity. Diabetes 48, 2398–2406 [DOI] [PubMed] [Google Scholar]
- 12.Ammon H. P., Müller P. H., Eggstein M., Wintermantel C., Aigner B., Safayhi H., Stützle M., Renn W. (1992) Increase in glucose consumption by acetylcysteine during hyperglycemic clamp: a study with healthy volunteers. Arzneimittelforschung 42, 642–645 [PubMed] [Google Scholar]
- 13.Wang T., Mao X., Li H., Qiao S., Xu A., Wang J., Lei S., Liu Z., Ng K. F., Wong G. T., Vanhoutte P. M., Irwin M. G., Xia Z. (2013) N-Acetylcysteine and allopurinol up-regulated the Jak/STAT3 and PI3K/Akt pathways via adiponectin and attenuated myocardial postischemic injury in diabetes. Free Radic. Biol. Med. 63, 291–303 [DOI] [PubMed] [Google Scholar]
- 14.Kim H., Seo J. Y., Roh K. H., Lim J. W., Kim K. H. (2000) Suppression of NF-kappaB activation and cytokine production by N-acetylcysteine in pancreatic acinar cells. Free Radic. Biol. Med. 29, 674–683 [DOI] [PubMed] [Google Scholar]
- 15.Meyer M., Schreck R., Baeuerle P. A (1993) H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 12, 2005–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atkins K. B., Lodhi I. J., Hurley L. L., Hinshaw D. B. (2000) N-acetylcysteine and endothelial cell injury by sulfur mustard. J. Appl. Toxicol. 20(Suppl 1), S125–S128 [DOI] [PubMed] [Google Scholar]
- 17.Pierce K. L., Premont R. T., Lefkowitz R. J. (2002) Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 3, 639–650 [DOI] [PubMed] [Google Scholar]
- 18.Hubbard K. B., Hepler J. R. (2006) Cell signalling diversity of the Gqalpha family of heterotrimeric G proteins. Cell. Signal. 18, 135– 150 [DOI] [PubMed] [Google Scholar]
- 19.Wedegaertner P. B., Chu D. H., Wilson P. T., Levis M. J., Bourne H. R. (1993) Palmitoylation is required for signaling functions and membrane attachment of Gq alpha and Gs alpha. J. Biol. Chem. 268, 25001–25008 [PubMed] [Google Scholar]
- 20.Smrcka A. V., Hepler J. R., Brown K. O., Sternweis P. C. (1991) Regulation of polyphosphoinositide-specific phospholipase C activity by purified Gq. Science 251, 804–807 [DOI] [PubMed] [Google Scholar]
- 21.Al Suleimani Y. M., Hiley C. R (2015) Characterization of calcium signals provoked by lysophosphatidylinositol in human microvascular endothelial cells. Physiol Res. 65, 53–62 [DOI] [PubMed] [Google Scholar]
- 22.Sivaraj K. K., Li R., Albarran-Juarez J., Wang S., Tischner D., Grimm M., Swiercz J. M., Offermanns S., Wettschureck N. (2015) Endothelial Gαq/11 is required for VEGF-induced vascular permeability and angiogenesis. Cardiovasc. Res. 108, 171–180 [DOI] [PubMed] [Google Scholar]
- 23.Aicart-Ramos C., Valero R. A., Rodriguez-Crespo I. (2011) Protein palmitoylation and subcellular trafficking. Biochim. Biophys. Acta 1808, 2981–2994 [DOI] [PubMed] [Google Scholar]
- 24.Linder M. E., Deschenes R. J. (2007) Palmitoylation: policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 8, 74–84 [DOI] [PubMed] [Google Scholar]
- 25.Resh M. D. (2006) Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci. STKE, 359, re14. [DOI] [PubMed] [Google Scholar]
- 26.Wei X., Song H., Semenkovich C. F (2014) Insulin-regulated protein palmitoylation impacts endothelial cell function. Arterioscler. Thromb. Vasc. Biol. 34, 346–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang A. Y., Ehrhardt A., Xu H., Kay M. A. (2007) Adenovirus transduction is required for the correction of diabetes using Pdx-1 or Neurogenin-3 in the liver. Mol. Ther. 15, 255–263 [DOI] [PubMed] [Google Scholar]
- 28.Victor V. M., Rocha M., De la Fuente M. (2003) N-acetylcysteine protects mice from lethal endotoxemia by regulating the redox state of immune cells. Free Radic. Res. 37, 919–929 [DOI] [PubMed] [Google Scholar]
- 29.Zayed M. A., Yuan W., Leisner T. M., Chalothorn D., McFadden A. W., Schaller M. D., Hartnett M. E., Faber J. E., Parise L. V. (2007) CIB1 regulates endothelial cells and ischemia-induced pathological and adaptive angiogenesis. Circ. Res. 101, 1185–1193 [DOI] [PubMed] [Google Scholar]
- 30.Wan J., Roth A. F., Bailey A. O., Davis N. G. (2007) Palmitoylated proteins: purification and identification. Nat. Protoc. 2, 1573–1584 [DOI] [PubMed] [Google Scholar]
- 31.Forrester M. T., Hess D. T., Thompson J. W., Hultman R., Moseley M. A., Stamler J. S., Casey P. J. (2011) Site-specific analysis of protein S-acylation by resin-assisted capture. J. Lipid Res. 52, 393–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ebrahimian T.G., Heymes C., You D., Blanc-Brude O., Mees B., Waeckel L., Duriez M., Vilar J., Brandes R. P., Levy B. I., Shah A. M., Silvestre J. S. (2006) NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. Am. J. Pathol. 169, 719–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S., Iring A., Strilic B., Albarran Juarez J., Kaur H., Troidl K., Tonack S., Burbiel J. C., Müller C. E., Fleming I., Lundberg J. O., Wettschureck N., Offermanns S. (2015) P2Y(2) and Gq/G(1)(1) control blood pressure by mediating endothelial mechanotransduction. J. Clin. Invest. 125, 3077–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimple A. J., Bosch D. E., Giguere P. M., Siderovski D. P (2011) Regulators of G-protein signaling and their Galpha substrates: promises and challenges in their use as drug discovery targets. Pharmacol. Rev. 63, 728–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mangram A. J., Horan T. C., Pearson M. L., Silver L. C., Jarvis W. R.; Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee (1999) Guideline for prevention of surgical site infection, 1999. Am. J. Infect. Control 27, 97–132, quiz 133–134, discussion 96 [PubMed] [Google Scholar]
- 36.Wanivenhaus F., Mauler F., Stelzer T., Tschopp A., Böni T., Berli M. C. (2016) Revision rate and risk factors after lower extremity amputation in diabetic or dysvascular patients. Orthopedics 39, e149–e154 [DOI] [PubMed] [Google Scholar]
- 37.Ho E., Chen G., Bray T. M. (1999) Supplementation of N-acetylcysteine inhibits NFkappaB activation and protects against alloxan-induced diabetes in CD-1 mice. FASEB J. 13, 1845–1854 [PubMed] [Google Scholar]
- 38.Pieper G. M., Siebeneich W. (1998) Oral administration of the antioxidant, N-acetylcysteine, abrogates diabetes-induced endothelial dysfunction. J. Cardiovasc. Pharmacol. 32, 101–105 [DOI] [PubMed] [Google Scholar]
- 39.Sheffner A. L., Medler E. M., Jacobs L. W., Sarett H. P. (1964) The in vitro reduction in viscosity of human tracheobronchial secretions by acetylcysteine. Am. Rev. Respir. Dis. 90, 721–729 [DOI] [PubMed] [Google Scholar]
- 40.Chen J., Reheman A., Gushiken F. C., Nolasco L., Fu X., Moake J. L., Ni H., López J. A. (2011) N-acetylcysteine reduces the size and activity of von Willebrand factor in human plasma and mice. J. Clin. Invest. 121, 593–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salaun C., Greaves J., Chamberlain L. H (2010) The intracellular dynamic of protein palmitoylation. J. Cell. Biol. 191, 1229–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burgoyne J. R., Haeussler D. J., Kumar V., Ji Y., Pimental D. R., Zee R. S., Costello C. E., Lin C., McComb M. E., Cohen R. A., Bachschmid M. M. (2012) Oxidation of HRas cysteine thiols by metabolic stress prevents palmitoylation in vivo and contributes to endothelial cell apoptosis. FASEB J. 26, 832–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marin E. P., Derakhshan B., Lam T. T., Davalos A., Sessa W. C (2012) Endothelial cell palmitoylproteomic identifies novel lipid-modified targets and potential substrates for protein acyl transferases. Circ. Res. 110, 1336–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernandez-Hernando C., Fukata M., Bernatchez P. N., Fukata Y., Lin M. I., Bredt D. S., (2006) Identification of Golgi-localized acyl transferases that palmitoylate and regulate endothelial nitric oxide synthase. J. Cell. Biol. 174, 369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Cardena G., Oh P., Liu J., Schnitzer J. E., Sessa W. C (1996) Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc. Natl. Acad. Sci. USA 93, 6448–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Resh M. D. (2006) Use of analogs and inhibitors to study the functional significance of protein palmitoylation. Methods 40, 191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wedegaertner P. B., Bourne H. R. (1994) Activation and depalmitoylation of Gs alpha. Cell 77, 1063–1070 [DOI] [PubMed] [Google Scholar]
- 48.Xia Z., Liu M., Wu Y., Sharma V., Luo T., Ouyang J., McNeill J. H. (2006) N-acetylcysteine attenuates TNF-alpha-induced human vascular endothelial cell apoptosis and restores eNOS expression. Eur. J. Pharmacol. 550, 134–142 [DOI] [PubMed] [Google Scholar]
- 49.Shih R. H., Cheng S. E., Hsiao L. D., Kou Y. R., Yang C. M (2011) Cigarette smoke extract upregulates heme oxygenase-1 via PKC/NADPH oxidase/ROS/PDGFR/PI3K/Akt pathway in mouse brain endothelial cells. J. Neuroinflammation 8, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]