Abstract
Tetraspanins (TSPANs) comprise a large family of 4-transmembrane domain proteins. The importance of TSPANs in vascular smooth muscle cells (VSMCs) is unexplored. Given that TGF-β1 and myocardin (MYOCD) are potent activators for VSMC differentiation, we screened for TGF-β1 and MYOCD/serum response factor (SRF)–regulated TSPANs in VSMC by using RNA-seq analyses and RNA-arrays. TSPAN2 was found to be the only TSPAN family gene induced by TGF-β1 and MYOCD, and reduced by SRF deficiency in VSMCs. We also found that TSPAN2 is highly expressed in smooth muscle–enriched tissues and down-regulated in in vitro models of VSMC phenotypic modulation. TSPAN2 expression is attenuated in mouse carotid arteries after ligation injury and in failed human arteriovenous fistula samples after occlusion by dedifferentiated neointimal VSMC. In vitro functional studies showed that TSPAN2 suppresses VSMC proliferation and migration. Luciferase reporter and chromatin immunoprecipitation assays demonstrated that TSPAN2 is regulated by 2 parallel pathways, MYOCD/SRF and TGF-β1/SMAD, via distinct binding elements within the proximal promoter. Thus, we identified the first VSMC-enriched and MYOCD/SRF and TGF-β1/SMAD-dependent TSPAN family member, whose expression is intimately associated with VSMC differentiation and negatively correlated with vascular disease. Our results suggest that TSPAN2 may play important roles in vascular disease.—Zhao, J., Wu, W., Zhang, W., Lu, Y. W., Tou, E., Ye, J., Gao, P., Jourd'heuil, D., Singer, H. A., Wu, M., Long, X. Selective expression of TSPAN2 in vascular smooth muscle is independently regulated by TGF-β1/SMAD and myocardin/serum response factor.
Keywords: differentiation, tetraspanins, phenotypic modulation, arteriovenous fistula
Mature/contractile vascular smooth muscle cells (VSMCs) are dominant components of the vasculature, and principally function to contract to control blood pressure and blood flow. VSMCs possess intrinsic phenotypic plasticity, which allows them to switch their quiescent contractile phenotype to a synthetic one in response to diverse pathophysiologic cues, such as injury and growth factors (1–3). Because synthetic VSMCs are proinflammatory, highly proliferative, and migratory, they have been demonstrated to be the main contributors of neointima formation, which underlies the pathogenesis of various important vascular disorders, such as atherosclerosis and restenosis (2, 4, 5). VSMC phenotypic modulation is governed by a closely interactive network of growth factors, cell-matrix interactions, signal transducers, transcriptional factors, and noncoding RNAs (6–8); however, detailed mechanisms remain elusive, particularly with respect to how extracellular signals are transduced via cell membranes to intracellular compartments, then converging upon the genome for transcriptional regulation. Therefore, it is of great interest to look for chief upstream signal molecules, particularly VSMC-selective cell membrane regulators.
VSMC-specific genes are primarily regulated by a key transcription factor, serum response factor (SRF), which physically associates with its coactivator, myocardin (MYOCD), at conserved CArG elements within the promoter or intronic region of the majority of VSMC-specific genes (2, 9, 10). MYOCD/SRF target genes range from contractile filament genes, such as MYH11, ACTA2, and TAGLN, to a number of regulatory genes, including ion channels, noncoding RNAs, and signal transducers, which together confer a fully executed VSMC contractile phenotype (11–13). Thus far, although several signaling-related genes have predicted CArG boxes, only a handful of them, such as Hic5 and CRP1, have been formally identified as direct transcriptional targets of MYOCD/SRF/CArG triad (14, 15). Furthermore, information on the functional roles of these signal genes in VSMC phenotypic modulation and vascular disease is largely obscure.
Tetraspanins (TSPANs) are 4-transmembrane domain family proteins. There are 33 TSPAN family members in humans, all of which share structural similarities (16). TSPANs frequently exert their regulatory roles via a unique signaling platform, termed tetraspanin-enriched microdomains, which are organized in cis with other membrane partners (16, 17). TSPANs are emerging as important regulators in cancer malignancy, the immune system, fertilization, and infectious diseases. The importance of TSPANs as they relate to the cardiovascular system is only now being appreciated (18). For example, TSPAN12 promotes Frizzled-4 (FZD4)/β-catenin signaling and governs retinal vascular development. Another family member, the widely expressed CD82 (TSPAN27), was shown to limit pathologic angiogenesis via changing lipid raft clustering and CD44 trafficking in endothelial cells (19, 20). In addition, CD9 (TSPAN29) is abundantly expressed on the surface of platelets and acts as a repressor of integrin αIIbβ3 activation in platelets (18). Most of these studies are confined to endothelial cells and platelets; however, little is known about TSPANs in VSMC. TSPANs play vital roles in fundamental biologic processes, such as cell adhesion, proliferation, differentiation, and migration, all of which can be attributes of VSMCs (16, 18, 21). It is therefore possible that important TSPAN genes also exist to signal key regulators of VSMC phenotypic modulation and thus influence the onset or progression of vascular disease.
TSPAN2 is a newly identified TSPAN family member that suppresses inflammation in the CNS (22), regulates cell motility in lung cancer (23), and promotes glucotoxic apoptosis in human pancreatic β cells (24). More recently, a genome-wide association study (GWAS) identified a single-nucleotide polymorphism (SNP) that is located in the regulatory region of TSPAN2 and is strongly associated with atherosclerosis in large arteries (25). In light of the role of VSMCs in atherosclerosis and the newfound SNP that associates TSPAN2 and atherosclerosis, we sought to characterize the expression and regulation of TSPAN2 in VSMCs. In this study, we showed that TSPAN2 is markedly induced by both MYOCD and TGF-β1, which are 2 potent activators of the VSMC contractile phenotype. We found that TSPAN2 is highly abundant in VSMCs within the vasculature and that expression of TSPAN2 is sharply reduced when VSMCs undergo phenotypic transition. Finally, we demonstrated that transcription of TSPAN2 in VSMCs is regulated by 2 parallel pathways, MYOCD/SRF and TGF-β1/SMAD, via distinct binding sites in the vicinity of the TSPAN2 promoter. Therefore, we identified the first VSMC-enriched TSPAN family member, whose expression levels are intimately associated with VSMC differentiation and negatively correlated with vascular disease. Our work supports the aforementioned GWAS studies that indicate that VSMC-specific TSPAN2 might play important roles in vascular disease, such as atherosclerosis.
MATERIALS AND METHODS
Human tissue and cell culture
RNA samples for human tissue expression profile were purchased from Zyagen (San Diego, CA, USA). Arteriovenous fistula (AVF) human samples were derived from patients who underwent surgical revision of failed AVFs at Albany Medical College under the approved institutional review board protocol (No. 3733), as previously described (26). Segments of the revision of the failed AVF vein and replacement vein were frozen-fixed before immunofluorescent staining. Primary human coronary artery smooth muscle cells (HCASMCs) were purchased from Thermo Fisher Scientific (Waltham, MA, USA) and maintained according to manufacturer instructions. Human aortic SMCs (HASMCs) and mouse aortic SMCs (MASMCs) were prepared by the cell culture core in the Department of Molecular and Cellular Physiology at Albany Medical College. Human leiomysarcoma uterine SMC cell line (SK-LMS) and 10T1/2 cells were cultured in DMEM that was supplemented with 10% fetal bovine serum.
RNA-seq in HCASMCs and RNA-array in MASMCs
RNA-seq analysis of HCASMCs with forced expression of MYOCD was described previously (26). Growing HCASMCs were transduced with adenovirus that carried MYOCD (Ad-MYOCD) for 72 h, or starved HCASMCs were induced by TGF-β1 (2 ng/ml) for 24 h. RNA was isolated by using an miRNeasy extraction kit (Qiagen, Valenica, CA, USA) and submitted for RNA-seq to the University of Rochester Medical Center’s Genomics Research Center. Polyadenylated RNA was selected for RNA-seq with a depth of 20 million reads per replicate using the Illumina HiSeq 2500 (Illumina, San Diego, CA, USA). Detailed information of library construction was described previously (27). The expression value of all transcripts was presented as FPKM (fragments per kilobase of exon per million fragments mapped). RNA-seq data were deposited in the Gene Expression Omnibus (GEO; National Center for Biotechnology Information, Bethesda MD, USA; https://www.ncbi.nlm.nih.gov/geo/) (GSE77120 for MYOCD overexpression, GSE85910 for TGF-β1 induction). MASMCs from wild-type (WT) or VSMC-specific SRF-knockout mice were transduced with Ad-MYOCD or Ad-Control for 3 d. Microarray analysis of RNA samples was performed by Arraystar (Rockville, MD, USA). Arraystar Mouse LncRNA Microarray V3.0 was used for global profiling of mouse mRNA transcripts, including 24,881 protein coding genes. RNA sample labeling and hybridization were both performed according to the Agilent One-Color Microarray-Based Gene Expression Analysis protocol (Agilent Technology, Santa Clara, CA, USA). Each RNA sample was transcribed into fluorescent cRNA along the entire transcripts. Labeled cRNAs were purified, fragmented, and hybridized for 17 h at 65°C. Hybridized arrays were washed, fixed, and scanned. Agilent Feature Extraction software (v11.0.1.1) was used to analyze all images. Quantile normalization and subsequent data processing were both performed by using GeneSpring GX v11.5.1 software (Agilent Technologies). Differentially expressed mRNAs were identified via fold change filtering between samples (GEO accession no. of the RNA-array data: GSE85930).
Growth factor and conditioned medium treatment in cultured VSMCs
Subconfluent HCASMCs or HASMCs were serum starved overnight, followed by treatment with different growth factors for 24 h, as previously described (26). To induce VSMC differentiation, subconfluent, growing HCASMCs were switched to medium 231 that contained smooth muscle differentiation supplement (Thermo Fisher Scientific). Smooth muscle differentiation supplement contained 1% (v/v) of fetal bovine serum and 30 µg/ml of heparin. Cells were induced for 72 h before RNA extraction.
RNA microarray GEO data analysis
Deposited RNA microarray data for carotid arteries at 5 d after complete ligation were retrieved from the GEO database (accession number: GSE70410) (28). Raw CEL files for ligated and unligated WT carotid arteries (n = 4) were normalized by using Affymetrix Expression Console (build 1.4.1.46; Santa Clara, CA, USA) with Sketch-Quantile algorithm and summarized with median polish method (Robust multiarray average). Differential gene expression was statistically determined by 1-way ANOVA, and P values were corrected for multiple comparisons by using Benjamini-Hochberg false discovery rate with Affymetrix Transcriptome Analysis Console (v3.0)
Mouse complete ligation
Mouse complete ligation surgery was performed in accordance to the protocol approved by the Albany Medical College Institutional Animal Care and Use Committee. C57BL/6 male mice (age 10–12 wk) were anesthetized with ketamine (0.1 mg/g, i.p.) and xylazine (0.01 mg/g, i.p.). Complete ligation was performed with the left carotid artery immediately proximal to the carotid bifurcation after a midline incision of the neck. The left injured and right uninjured carotid arteries were collected for RNA extraction 2 wk after surgery.
RNA isolation and quantitative RT-PCR
Total RNA from cultured cells or homogenized tissues was isolated by using a miRNeasy Kit. cDNA was synthesized by using an iScript cDNA kit (Bio-Rad, Hercules, CA, USA). IQ SYBR Green–based quantitative RT-PCR was performed in a MyiQ real-time PCR detection system (Bio-Rad), as previously described. Triplicates of each sample were examined and data were representative of multiple independent experiments (n ≥ 3). Average raw CT value of genes assayed throughout the studies is included in Supplemental Table 1.
TSPAN2 lentivirus construction and viral transduction
Constructs using for building TSPAN2 lentivirus were generously provided by Masato Enari according to the related publication (23). Lentivirus that carried the human TSPAN2 transcript and vector control lentivirus were generated in HEK293FT cells as described in Otsubo, et al. (23). Viral particles were harvested at 48 h after plasmids transfection, then concentrated with Amicon Ultra-15 Centrifugal Filter Units (Millipore, Billerica, MA, USA). Lentivirus was titrated with lenti-X quantitative RT-PCR titration kit (Clontech, Mountain View, CA, USA) before viral transduction for gain of function studies. For lentivirus transduction, growing HCASMCs were seeded in 6-well plates and transduced with 1 ml of fresh medium that contained lentivirus and polybrene (5 µg/ml) per well. Cells were refreshed with growth medium 24 h after transduction.
VSMC proliferation and migration assays
Growing HCASMCs were seeded in triplicate at a density of 40% in 6-well plates for each condition. Cells were treated with the same amount of lentivirus that carried human TSPAN2 cDNA (Lenti-TSPAN2) vs. lentivirus vector control (Lenti-control), or small interfering RNA (siRNA) to human TSPAN2 (siTSPAN2) vs. scramble siRNA negative control (siControl). Cells were refreshed the next day. After overnight serum starvation, cells were stimulated with growth medium for 4 d, followed by cell number counting by using a hemocytometer. Wound scratch assay was used to assess cell migration, as described in Zhao et al. (26). In brief, HCASMCs were transfected with equal amounts of siTSPAN2 or siControl. Cells were starved for 3 d, followed by creation of the scratch wound. Dynamic images were captured by time-lapse microscopy system (Leica DMI 6000B with Leica DFC camera 420C; Leica, Wetzlar, Germany). Six images for each group at indicated time points were randomly selected to quantitate cell migration.
Chromatin immunoprecipitation assay for SRF and SMAD2/3
Chromatin immunoprecipitation (ChIP) assay was carried out by using a ChIP-IT High Sensitivity kit (Active Motif, Carlsbad, CA, USA). HCASMCs or MASMCs (2 × 107) were cross-linked with 1% formaldehyde for 15 min, then quenched with glycine for 5 min. Cells were lysed by a lysis buffer that was supplemented with a protein inhibitor complex and PMSF on ice for 10 min. Chromatin was sonicated with Bioruptor UCD-200 (Diagenode, Denville, NJ, USA) at high magnitude for 22 min (30 s on, 30 s off) to obtain chromatin fragments of 300–1000 bp in length. One tenth of total chromatin was included as input. Chromatin complexes were precipitated with either SRF Ab (G-20, sc-335 X; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or SMAD2/3 Ab (Cell Signaling Technology, Danvers, MA, USA). The same amount of ChIP-grade rabbit IgG control (Abcam, Cambridge, MA, USA) was used as negative control for both the SRF and SMAD2 Abs. After reverse cross-linking and column purification, DNA samples were subjected to either semiquantitative PCR or quantitative PCR that amplified the putative CArG box or each individual SMAD-binding element (SBE)–containing fragment within the −1-kb promoter region of the TSPAN2 gene. PCR primers are included in Supplemental Table 2.
Luciferase assays and mutagenesis
The −2-kb and serial truncated TSPAN2 promoters were PCR amplified from HCASMC genomic DNA and cloned into the pGL3 basic luciferase vector. The predicted CArG box was mutated by using a QuikChange Site-Directed Mutagenesis Kit (Stratagene, San Diego, CA, USA). Primers for the PCR clone and mutagenesis are listed in Supplemental Table 2. Sequence validation of all constructs was performed by Cornell University Life Sciences Core Laboratories Center. Luciferase assays were performed in SKLMS and 10T1/2 cells, as described previously. Cells were seeded in 24-well plates and grown to 80% confluency. Cells were then cotransfected with reporter plasmids, expression plasmids, either SRF-VP16 or MYOCD, and internal control Renilla using Lipofectamine 2000. For TGF-β1 activation, WT and SBE-truncated TSPAN2 reporters were transfected to 10T1/2 cells for 6 h and cultured in growth medium for 24 h. After overnight serum starvation, cells were stimulated with TGF-β1 (5 ng/ml) for 24 h before luciferase activity assessment. Luciferase activity was evaluated by using a Dual Luciferase Assay Kit per vendor protocol (Promega, Madison, WI, USA).
siRNA-mediated gene knockdown
The source of siRNAs for human SRF and SMAD4 were obtained, as described previously (29). siRNA to mouse Srf (s74392 and s74391) and siTSPAN2 (S19653) were purchased from Thermo Fisher Scientific. siRNAs were delivered to HCASMCs or MASMCs by using Lipofectamine 2000 at a concentration of 25 nM for 3 d before RNA extraction.
Double staining for TSPAN2 transcripts and ACTA2 protein
Freshly harvested mouse aortas, carotid arteries, livers, and brains were immediately fixed in 4% paraformaldehyde at 4°C for 24 h. Frozen sections (10 µm) were prepared for experiments. In situ hybridization was carried out using RNAscope 2.5 Chromogenic Assay according to manufacturer instruction (Advanced Cell Diagnostics, Newark, CA, USA). Slides were baked at 60°C for 30 min and rinsed with PBS. Slides were then sequentially incubated with the following reagents before hybridization: hydrogen peroxide at room temperature for 10 min, boiling target retrieval solution for 5 min, and RNAscope Protease Plus (Acdbio, Newark, CA, USA) at 40°C for 30 min. Slides were then hybridized with a TSPAN2 probe (sequence available upon request) at 40°C for 2 h. The signal was amplified by AMP solution under the indicated conditions. Color was developed via incubation with a Fast Red solution at room temperature for an optimal time period. Slides were finally incubated with a 1/100 dilution of ACTA2 Ab (Dako, Carpinteria, CA, USA) at 4°C overnight, followed by incubation with a 1/1000 dilution of secondary Ab Alexa Fluor 488 for 1 h to stain for smooth muscle contractile protein. Nuclei were then counterstained with DAPI (Molecular Probes, Foster City, CA, USA) before image acquisition by confocal microscope.
Immunofluorescence staining of human AVF samples
Frozen sections of control veins and failed AVF samples were prepared for immunofluorescent staining. In brief, veins were embedded in optimum cutting temperature compound, cryosectioned, and fixed in ice-cold acetone for 10 min. A 1/200 dilution of rabbit anti-MYH11 (Alfa Aesar, Haverhill, MA, USA), a 1/300 dilution of TSPAN2 (Proteintech, Chicago, IL, USA), and a 1/300 goat anti-rabbit IgG Alexa Fluor (Abcam) were used to detect MYH11 and TSPAN2. A concentration-matched rabbit IgG served as negative control. Cells were counterstained with a 1/10,000 dilution of DAPI before microscopic observation. Fluorescent signal was captured by a confocal microscope and processed by Photoshop (Adobe, San Jose, CA, USA) under the same conditions.
Protein extraction and Western blotting
HCASMCs were rinsed in cold PBS twice and total protein was extracted with ice-cold lysis buffer (Cell Signaling Technology) that was supplemented with a protease inhibitor cocktail (1%; Sigma-Aldrich, St. Louis, MO, USA), PMSF (1 mM; Sigma-Aldrich), Na3VO4 (1 mM), and NaF (50 mM). Protein concentration was measured by a detergent-compatible protein assay kit (Bio-Rad). Equal amounts of protein were resolved in 10% SDS-PAGE gel for Western blotting, as described previously (29). Primary Abs used were as follows: SRF (Santa Cruz Biotechnology), pSMAD2 (Cell Signaling Technology), SMAD2/3 (Cell Signaling Technology), and glyceraldehyde 3-phosphate dehydrogenase (Sigma-Aldrich).
Statistical analysis
All experiments were repeated separately at least 3 times. Data in graphs are presented as means ± sd. Statistical analysis was conducted by unpaired 2-tailed Student’s t test using GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA). A value of P < 0.05 was considered statistically significant.
RESULTS
TSPAN2 is a novel TSPAN family member that is activated by TGF-β1 and SRF/MYOCD
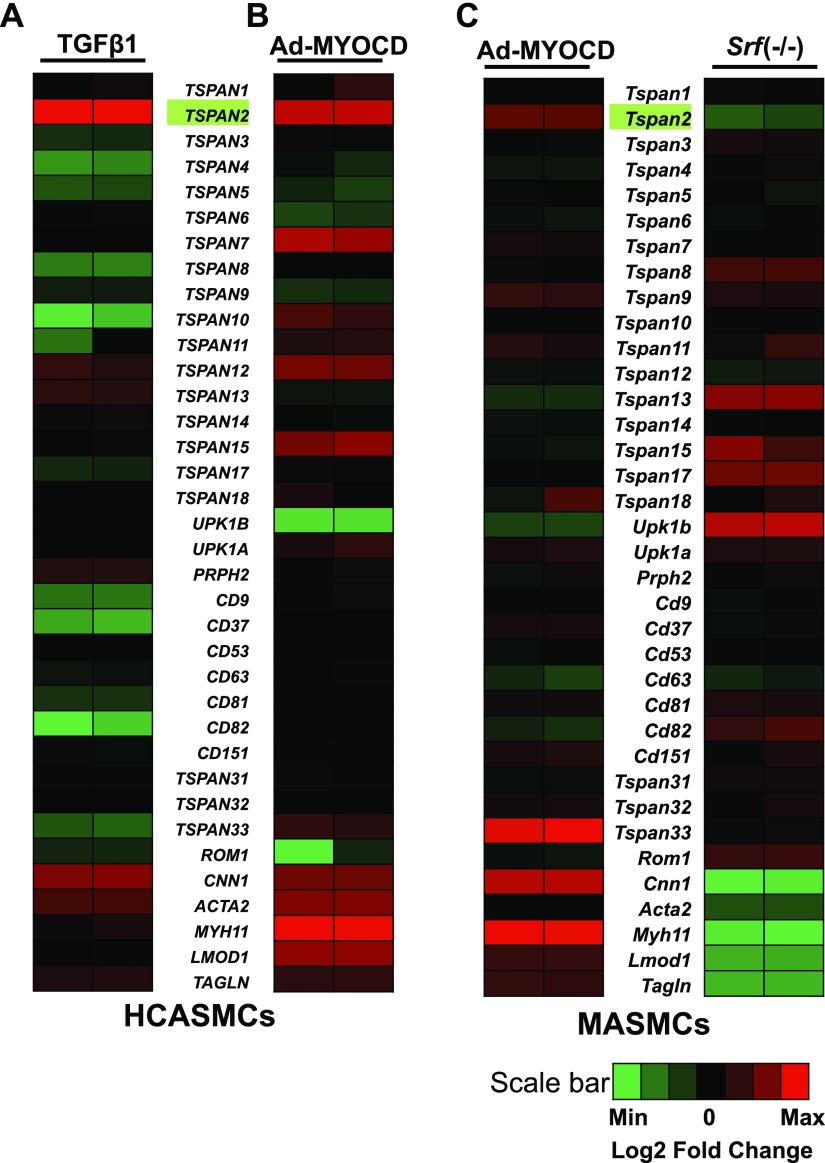
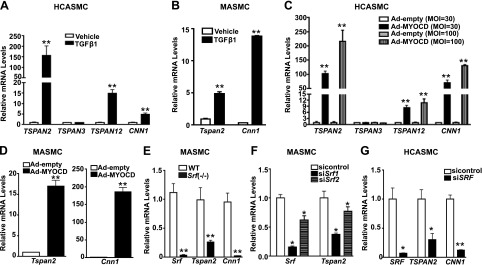
The human genome harbors 33 distinct TSPAN family genes. Thus far, none of them has been implicated as VSMC-selective genes. To systematically screen for TSPAN genes that are potentially important in the VSMC contractile phenotype, we performed RNA-seq analyses in HCASMCs after induction with TGF-β1, a potent activator of VSMC differentiation. As expected, TGF-β1 markedly induced gene expression of VSMC contractile genes, such as CNN1, ACTA2, and TAGLN. Of interest, more than one half of TSPAN family genes showed significant reduction upon TGF-β1 stimulation. Only 4 TSPAN genes (TSPAN2, TSPAN12, TSPAN13, and PRPH2/TSPAN22) exhibited up-regulation by TGF-β1, among which TSPAN2 showed the most dramatic change, with FPKM fold induction >144 (Fig. 1A and GEO18021004). Many TGF-β1–induced genes in VSMCs are linked to differentiation and are also activated by MYOCD, the master regulator of VSMC differentiation. To test whether induction of TSPAN2 could also be achieved through MYOCD, we performed RNA-seq in HCASMCs with forced expression of MYOCD. We found 8 TSPAN genes that were markedly increased by MYOCD and, once again, TSPAN2 showed the greatest up-regulation, with FPKM fold induction >300 (Fig. 1B and GSE77120). To extend this finding to mice, we examined RNA-array results derived from MASMCs that overexpressed MYOCD. Only 2 TSPAN genes, Tspan2 and Tspan33, showed significant induction by MYOCD (fold induction >2; Fig 1C, left, and GSE85930). To ascertain whether MYOCD-regulated Tspan genes are SRF dependent, we analyzed RNA array data that were obtained with MASMCs derived from VSMC-specific Srf-knockout mice. Consistently, mRNA levels of most mouse Tspan genes showed no difference between WT and Srf-knockout cells. Only Tspan2 and Cd63 (TSPAN30) were down-regulated in Srf-knockout cells (Fig 1C, right, and GSE85930). Thus, on the basis of RNA-seq and gene array studies, only TSPAN2 showed TGF-β1 induction as well as both MYOCD and SRF dependence in VSMCs, consistent across species. We next performed quantitative RT-PCR to validate the findings from RNA-seq and RNA-arrays. We found that TGF-β1 caused a 150-fold increase in TSPAN2 mRNA in HCASMCs (Fig. 2A). Similar inductions were observed in MASMCs (Fig. 2B). We also validated by quantitative RT-PCR the observation that MYOCD markedly up-regulated TSPAN2 mRNA in HCASMCs and MASMCs (Fig. 2C, D), and we confirmed a significant reduction in Tspan2 mRNA in Srf−/− MASMCs compared with WT counterparts (Fig. 2E). Finally, we confirmed that both TGF-β1 and MYOCD can induce protein levels of TSPAN2 in HCASMs (Supplemental Fig. 1A). To further confirm the SRF-dependent expression of TSPAN2, we applied siRNA studies. Two separate siRNAs that were targeted to mouse Srf consistently caused a significant decrease of Tspan2 mRNA in MASMCs (Fig. 2F). This reduction was also observed in vivo as Tspan2 mRNA was significantly attenuated in bladders from Srf-knockout mice compared with WT counterparts (Supplemental Fig. 1B). Furthermore, knockdown of SRF decreased TSPAN2 mRNA in HCASMC isolates (Fig. 2G). Taken together, we demonstrated that TSPAN2 is activated by both TGF-β1 and SRF/MYOCD in VSMCs, which indicates that TSPAN2 could be a novel cell membrane gene linked to the VSMC contractile phenotype.
Figure 1.
RNA-seq analyses and RNA-arrays for TSPAN family genes that are modulated by TGF-β1 and MYOCD/SRF in VSMCs. Heat map illustrates log2-transformed fold change of the transcript levels of TSPAN family members compared with their levels under basal condition. Contractile genes served as positive controls. Duplicate samples were included for each condition. A) Subconfluent HCASMCs were starved overnight, followed by TGF-β1 treatment for 24 h before total RNA was isolated for RNA-seq. Heat map shown illustrates the relative expression of each individual TSPAN gene of duplicate samples of vehicle or TGF-β1–treated HCASMCs. B) HCASMCs were transduced with adenovirus that carried MYOCD (Ad-MYOCD) or empty control adenovirus (Ad-Control) for 72 h before isolating RNA for RNA-seq. Heat map depicts the relative expression of TSPANs of the duplicate samples of HCASMCs treated with Ad-Control or Ad-MYOCD. C) MASMCs were transduced with Ad-MYOCD or Ad-Control for 72 h, and RNA was subjected to RNA-array analysis. Heat map shows the relative expression of each individual mouse Tspan gene of the duplicate MASMCs treated with Ad-Control or Ad-MYOCD (left). MASMCs were isolated from VSMC-specific Srf-knockout (Srf −/−) or WT littermates. RNA was isolated for RNA array. Heat map illustrates the relative expression of each individual Tspan gene of duplicate samples of Srf−/− compared with WT aortic MASMCs (right).
Figure 2.
Quantitative RT-PCR validation of TSPAN2 gene expression regulated by TGF-β1 and MYOCD/SRF in VSMCs. A) HCASMCs were starved overnight, followed by TGF-β1 treatment for 24 h. RNA was isolated for quantitative RT-PCR analysis of indicated TSPAN genes and the positive control CNN1. (Robust induction of TSPAN2 was seen after treatment of TGF-β1.) B) Quantitative RT-PCR analysis of Tspan2 and Cnn1 gene expression in MASMCs treated with TGF-β1 (5 ng/ml) for 24 h. C) HCASMCs were transduced with adenovirus that carried MYOCD (Ad-MYOCD) or empty control adenovirus (Ad-Control) with indicated doses for 72 h before RNA was isolated for quantitative RT-PCR of indicated genes. D) MASMCs were transduced with Ad-MYOCD or control virus, as previously described. RNA was isolated for assessment of gene expression of Tspan2 and Cnn1. E) Quantitative RT-PCR of indicated genes in WT versus Srf-knockout MASMCs. F) Srf-knockdown effect on Tspan2 gene expression in MASMCs using 2 separate siRNAs. G) Effect of SRF knockdown on TSPAN2 gene expression in HCASMCs. MOI, multiplicity of infection. Values are means ± sd of at least 3 repeats. *P < 0.05, **P < 0.01.
TSPAN2 expression is enriched in VSMCs
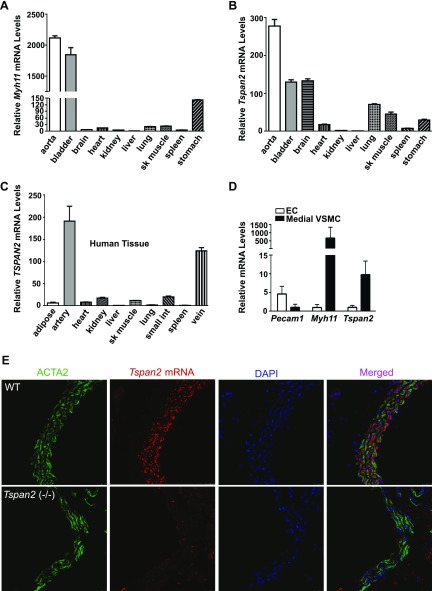
Similar to Myh11, the most definitive marker gene of the contractile VSMC phenotype, Tspan2 expression is abundant in smooth muscle–enriched mouse tissues, such as the aorta and bladder (Fig. 3A, B). We also observed high levels of TSPAN2 in the brain, a result that is consistent with the abundance of Tspan2 in oligodendrocytes (30, 31). VSMC-enriched TSPAN2 gene expression was confirmed in human tissues, with human arteries and veins displaying the highest TSPAN2 mRNA levels across all tissues tested (Fig. 3C). To more precisely assess whether TSPAN2 is selective to VSMCs within the vasculature, we purified endothelial cells (ECs) and medial VSMCs from mouse aortas and compared TSPAN2 mRNA levels. Myh11 mRNA levels in the isolated medial layer of VSMCs were 100-fold higher than those in ECs from the same aortas. Conversely, mRNA levels of Pecam1, an EC-specific marker gene, were ≥5-fold higher in ECs compared with medial layer VSMCs, which indicated high purity in both VSMCs and ECs from the isolation. Quantitative RT-PCR demonstrated that Tspan2 mRNA in medial VSMCs was 10-fold higher than that in ECs, which confirmed enrichment of TSPAN2 in VSMCs (Fig. 3D). Since commercially available TSPAN2 Abs failed to detect endogenous mouse TSPAN2 protein by immunostaining and Western blot, we performed in situ hybridization to confirm the expression pattern of Tspan2 in mouse tissues. Consistently, double staining showed that Tspan2 transcripts were only detected in ACTA2 (protein)-positive VSMCs and not in ECs and adventitial cells in the aorta and carotid artery (Fig. 3E). Strong and no signals of Tspan2 mRNA were consistently observed in the brain and liver, respectively (Supplemental Fig. 2). No signal was detected in aortas from Tspan2-knockout mice (Fig. 3E), which confirmed the authenticity of Tspan2 mRNA signals revealed by in situ hybridization; therefore, we demonstrated that TSPAN2 is enriched in smooth muscle within the vascular system.
Figure 3.
TSPAN2 is enriched in VSMCs. A, B) Quantitative RT-PCR analysis of Myh11 (A) and Tspan2 (B) mRNA levels across different mouse tissues. C) Quantitative RT-PCR analysis of TSPAN2 mRNA levels in different human tissues. Relative mRNA levels of mouse Myh11, Tspan2, and human TSPAN2 were normalized to levels of the liver (set to 1). Representative data are shown from 3 independent experiments. D) ECs and medial layer VSMCs were purified from mouse aortas. RNA was then isolated for quatitative RT-PCR assessment of indicated genes. Values are means ± sd (n = 5). E) Double staining for Tspan2 mRNA by in situ hybridization and ACTA2 protein by immunofluorescence staining in aorta from WT and Tspan2−/− mice. (Note: Tspan2 transcripts (red) and ACTA2 protein (green) are colocalized in the medial layer VSMCs in mouse aortas.) sk muscle, skeletal muscle; small int, small intestine. Results are representative of ≥3 separate experiments.
TSPAN2 expression is closely correlated with VSMC differentiation and decreased in diseased human vessels
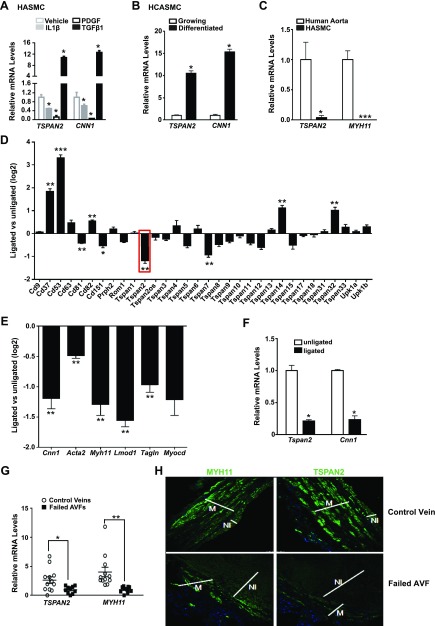
The strong induction by TGF-β1 and MYOCD/SRF, as well as the VSMC-enriched gene expression profile, suggest that TSPAN2 could be a novel gene associated with the VSMC contractile phenotype. To test this, we examined TSPAN2 gene expression in several distinct models of VSMC phenotypic modulation. Similar to CNN1, mRNA levels of TSPAN2 were strongly increased by TGF-β1, but were reduced by both PDGF and IL-1β, 2 growth factors that promote VSMC dedifferentiation (Fig. 4A) (32, 33). TSPAN2 mRNA was elevated in differentiated HCASMCs induced by a commercially conditioned VSMC differentiation medium compared with growing HCASMCs (Fig. 4B) (34). Medial layer VSMCs residing in healthy vessels represent contractile/differentiated VSMCs, whereas cultured VSMCs display dedifferentiated phenotype. Similar to MYH11, TSPAN2 mRNA was reduced in cultured HASMCs compared with freshly isolated human aortic medial VSMCs from the same vessel (Fig. 4C). Tspan2 is the most down-regulated gene among all Tspan family members at 5 d after injury in the mouse carotid artery complete ligation model (28), which is similar to VSMC contractile genes and MYOCD (Fig. 4D, E). We have previously shown that the VSMC contractile program is compromised at 2 wk after injury in mouse carotid artery ligation model (35). Similar to Cnn1, we found that Tspan2 mRNA is markedly down-regulated in ligated vessels at 2 wk after injury in this model (Fig. 4F),
Figure 4.
TSPAN2 expression is down-regulated during VSMC phenotypic modulation and in diseased vessels. A) HASMCs were starved overnight, followed by treatment of indicated growth factors for 24 h. RNA was extracted for quantitative RT-PCR of TSPAN2 and CNN1. *P < 0.05. B) Quantitative RT-PCR analysis of TSPAN2 and CNN1 in growing vs. differentiated HCASMCs induced by conditioned medium for 72 h. *P < 0.05. C) Quantitative RT-PCR analysis of TSPAN2 and MYH11 in the medial layer VSMCs of human aortas compared with primary culture of HASMCs isolated from the same vessels. Values are means ± sd of at least 3 repeats. *P < 0.05, ***P < 0.001. D, E) Relative expression of Tspan family members (D) and the indicated VSMC contractile genes and Myocd (E) determined by RNA microarray of mouse carotid arteries at 5 d after complete ligation (28). Gene expression fold-change (log2) comparing ligated with unligated carotid arteries is shown as mean ± sem (n = 4). False discovery rate–adjusted P values for the comparison are reported. *P ≤ 0.05, **P ≤ 0.01; ***P ≤ 0.001. F) Tspan2 and Cnn1 mRNA levels were analyzed by quantitative RT-PCR in unligated and ligated mouse carotid arteries 2 wk after complete ligation surgery. Values are means ± sd (n = 4). *P < 0.05. G) Quantitative RT-PCR assessment of TSPAN2 and MYH11 mRNA in replacement control veins vs. failed AVF samples. Relative expression was normalized to the average mRNA value of the examined failed arteriovenous fistula (AVF) samples (set to 1). The source of AVF samples was described previously (26), and AVF samples were the unidentified discarded segments from patients with chronic kidney disease who underwent surgical revision of failed AVFs. *P < 0.05; **P < 0.01. H) Immunofluorescence staining of MYH11 (left) and TSPAN2 (right) proteins in the replacement control veins and failed AVF samples. [Note: higher levels of TSPAN2 protein were observed in control veins; medial layer VSMCs (M) have relatively higher levels of TSPAN2 protein compared with neointima (NI) cells in failed AVF samples.] Results are representative of 3 separate experiments.
Neointimal hyperplasia has been found to be a major characteristic of human AVF failure involving VSMC dedifferentiation. In agreement with this, we found that mRNA levels of TSPAN2 were much lower in samples that were derived from failed human AVF samples compared with control vein samples obtained at the time of AVF placement (Fig. 4G). Immunofluorescence staining using a human TSPAN2 Ab (20463-1-AP; Proteintech) confirmed that, similar to MYH11, TSPAN2 was abundantly expressed in normal veins, but was notably reduced in the neointima compared with medial VSMC layer in failed AVF samples (Fig. 4H). Together, we demonstrated that TSPAN2 is positively associated with the VSMC contractile phenotype and is decreased under pathologic conditions in which the contractile phenotype is compromised.
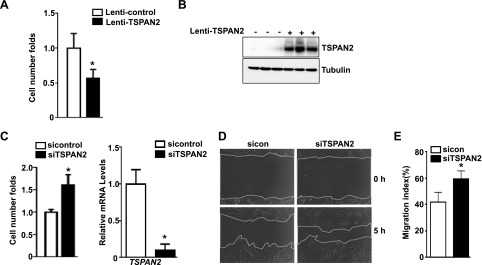
TSPAN2 suppresses VSMC proliferation and migration
Positive correlation of TSPAN2 gene expression to VSMC contraction phenotype and its down-regulation in diseased vessels suggest that TSPAN2 may affect VSMC proliferation and migration, both of which underlie the pathogenesis of some prominent occlusive vascular diseases. Indeed, forced expression of TSPAN2 via lentiviral transduction in HCASMCs showed that TSPAN2 significantly suppresses cell proliferation at 4 d after lentivirus transduction (Fig. 5A). Consistently, siRNA-mediated TSPAN2 gene silencing achieves >80% knockdown efficiency in HCASMCs, which leads to a significant increase in cell proliferation (Fig. 5C). To assess whether loss of TSPAN2 function has an effect on cell migration, we transfected HCASMCs with siTSPAN2 for 3 d, then conducted scratch wound assay. Time-lapse microscopy showed that siTSPAN2 promotes VSMC migration at 5 h after creation of the scratch wound (Fig. 5D, E). Together, these data suggest that TSPAN2 is a suppressor for VSMC proliferation and migration at least in vitro, and that decreased TSPAN2 gene expression under pathologic conditions may contribute to the pathogenesis of various occlusive vascular diseases, such as atherosclerosis, postangioplasty-caused restenosis, and allograft vasculopathy.
Figure 5.
Effect of TSPAN2 on VSMC proliferation and migration. A) Growing HCASMCs were transduced with lentivirus that carried TSPAN2 (Lenti-TSPAN2) or equal amounts of lenti-vector control (Lenti-control). Cells were starved overnight, then stimulated with growth medium. Cells were counted by using a hemocytometer at 4 d after serum stimulation. Cell proliferation was defined as fold change to Lenti-control group (set to 1). Values are means ± sd, and data are representative of 3 separate experiments (n = 3). B) Protein was isolated from cells in panel A for Western blot analysis of TSPAN2; representative results are shown. C) Growing HCASMCs were transfected with siRNA to TSPAN2 (siTSPAN2) or the same amount of negative control siRNA (sicontrol). Cell proliferation analysis was performed as in panel A (left). Quantitative RT-PCR was performed to assess TSPAN2 knockdown efficiency (right). Values are means ± sd, and data are representative of 3 separate experiments (n = 3). D) HCASMCs were treated with siTSPAN2 or sicontrol as in panel C. Scratch wound was created, and cell migration was assessed by time-lapse microscopy. Representative images at 5 h after creation of the scratch wound are shown. E) Quantitative analysis of panel D. Migration index was defined as the percentage of the area covered by migrating cells to the original wound area (n = 6). *P < 0.05.
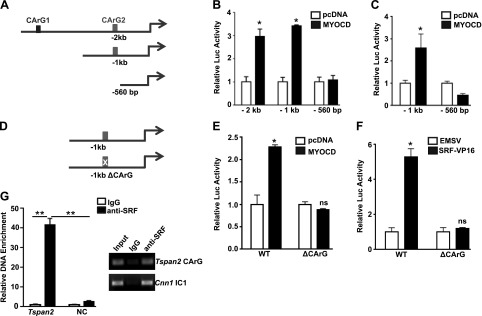
TSPAN2 is a direct transcriptional target of SRF/MYOCD/CArG triad
Most VSMC-specific genes possess one or more conserved CArG boxes and are direct transcriptional targets of SRF/MYOCD (36). Comparative genomics analysis predicted 2 CArG boxes within the −2-kb promoter region of human TSPAN2. The proximal element is a consensus CArG box and is conserved in mice, whereas the distal element is degenerate and human specific. We constructed −2-kb, −1-kb, and −560-bp luciferase reporters, which encompass both CArG boxes—the proximal CArG box only and no CArG box, respectively (Fig. 6A). The −2- and −1-kb luciferase reporters were similarly activated, whereas the −560-bp promoter was resistant to MYOCD stimulation in 10T1/2 cells, which indicated that the distal CArG box is not necessary for MYOCD-induced TSPAN2 promoter activity (Fig. 6B). A similar response to MYOCD was observed with the −1-kb and −560-bp reporters in SKLMS cells, which express high levels of VSMC contractile genes (Fig. 6C). A point mutation in the conserved CArG box abolished activation induced by both MYOCD and SRF, which confirmed that the activation of the −1-kb reporter by MYOCD and SRF is through this element (Fig. 6E, F). Finally, we performed ChIP assay for SRF binding and found there was an enrichment of the DNA region that flanks the conserved CArG box. In contrast, no obvious enrichment was observed in a negative control region (intron 2 of Dhx32) without predicted CArG box (Fig. 6G). These data, together with Figs. 1 and 2, demonstrated that TSPAN2 is a direct transcriptional target of the MYOCD/SRF/CArG triad.
Figure 6.
TSPAN2 is a direct transcriptional target for SRF/MYOCD. A) Schematic of truncated versions of TSPAN2 luciferase reporters. B, C) Indicated TSPAN2 reporters were transfected in 10T1/2 cells (B) or SKLMS cells (C) in the presence of either control pcDNA vector or MYOCD expression plasmid for 36 h before assessment of luciferase activity. Luciferase activity was normalized to the internal control reporter Renilla. MYOCD-dependent activation of the TSPAN2 promoter was defined as the fold increase to the pcDNA control group (set to 1). D) Schematic of the −1-kb WT reporter and its CArG mutant. E, F) Both reporters were transfected in SKLMS cells for MYOCD-mediated (E) or SRF-VP16–mediated (F) activation, as described in panel C. G) ChIP assays were carried out in growing MASMCs for analysis of SRF binding to the putative CArG box. Signals of amplified DNA were normalized to the input control. Relative enrichment of the CArG box–containing fragment was expressed as the fold increase to the IgG control (set to 1). Primers to a region close to Dhx32 without any predicted CArG box (NC) and primers to CArG1 in the intron1 (IC1) of mouse Cnn1 were used as negative and positive controls, respectively. EMSV, empty vector; NC, negative control; ns, not significant. Representative data are shown from 3 independent experiments. Values are means ± sd, and data are representative of at least 3 separate experiments. *P < 0.05, **P < 0.01.
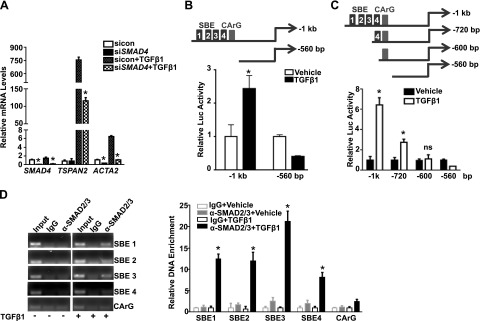
TGF-β1/SMAD transcriptionally activates TSPAN2 promoter
To further delineate the regulation of TGF-β1 on TSPAN2 gene expression, we determined whether the induction by TGF-β1 was dependent on classic SMAD signaling. Similar to ACTA2, siRNA-mediated suppression of endogenous SMAD4—the common mediator SMAD—caused a dramatic decrease of TSPAN2 mRNA after stimulation of TGF-β1 in HCASMCs (Fig. 7A). This indicates that the classic SMAD pathway is involved in TGF-β1–induced TSPAN2 gene expression. Sequence analysis of the −1-kb TSPAN2 promoter predicted 4 putative SBEs, which suggested that TGF-β1 could directly activate the TSPAN2 promoter via these 4 SBEs. We compared luciferase activity between the −1-kb TSPAN2 promoter (with all 4 SBEs) and the truncated −560-bp reporter (without SBE) in 10T1/2 cells induced by TGF-β1. An obvious induction of the luciferase activity was observed in the −1-kb promoter, whereas there was no induction in the −560-bp reporter, which indicated that activation of TSPAN2 promoter by TGF-β1 is through the predicted 4 SBEs (Fig. 7B). To further ascertain whether the specific SBEs are critical for TGF-β1 activation, we constructed 2 more truncated TSPAN2 luciferase reporters with serial deletions of the putative SBEs. The −1-kb WT reporter displayed the greatest promoter activity induced by TGF-β1 among all reporters tested. The −720-bp reporter that contained only SBE4 was activated by TGF-β1; however, its activation was significantly reduced compared with the −1-kb reporter. The −600- and −560-bp reporters, both lacking all predicted SBEs, were not activated by TGF-β1, which demonstrated that activation of the TSPAN2 promoter by TGF-β1 is via the 4 putative SBEs (Fig. 7C). Finally, we performed ChIP assays to determine the binding activity of SMAD2/3 to the putative SBEs in HCASMCs. As expected, SMAD2/3 failed to bind any of the predicted SBEs under the basal conditions. Upon TGF-β1 activation, there was enrichment of the DNA flanking each of the predicted SBEs after immunoprecipitation with the SMAD2/3 Ab. Of interest, SBE3 exhibited the strongest binding affinity. We did not observe enrichment in a negative control region that encompassed the CArG box but not SBE, which indicated the specific binding of SMAD2/3 to SBEs (Fig. 7D). These results showed that TSPAN2 is a direct transcriptional target of the classic TGF-β1/SMAD pathway via the 4 putative SBEs within its proximal promoter region.
Figure 7.
TSPAN2 is a direct transcriptional target for TGF-β1/SMAD. A) HCASMCs were transfected with siRNA to SMAD4 (siSMAD4) for 24 h. Cells were then starved overnight, followed by treatment of TGF-β1 (4 ng/ml) for another 24 h before RNA was isolated for quantitative RT-PCR analysis of indicated genes. B) The −1-kb TSPAN2 WT luciferase reporter and –560-bp truncated version (with all 4 SBEs deleted), as depicted (top), were transfected in 10T1/2 cells. Cells were then starved overnight and stimulated by TGF-β1 (5 ng/ml) for 24 h before assessment of luciferase activity. TGF-β1 activation was defined as fold increase to the vehicle-treated control group (set to 1). Representative data from 3 separate experiments are expressed as the average of triplicates. C) Schematic of the truncated TSPAN2 luciferase reporters, as indicated (top). Luciferase activity of the indicated reporters in 10T1/2 cells treated with vehicle or TGF-β1, as described in panel B (bottom). D) ChIP assays were performed in starved HCASMCs or HCASMCs that were stimulated with TGF-β1 (5 ng/ml) for 5 h to examine the binding of SMAD2/3 to each individual putative SBE, denoted as SBE1, SBE2, SBE3, and SBE4. Semiquantitative PCR (left) and quantitative PCR (right) of the enrichment of each individual putative SBE-containing fragment is shown. Amplified DNA signal was normalized to the input control, and the relative enrichment of the individual SBE-containing fragment was expressed as fold increases to its IgG control (set to 1). ns, not significant. Values are means ± sd, and data are representative of 3 separate experiments. *P < 0.05.
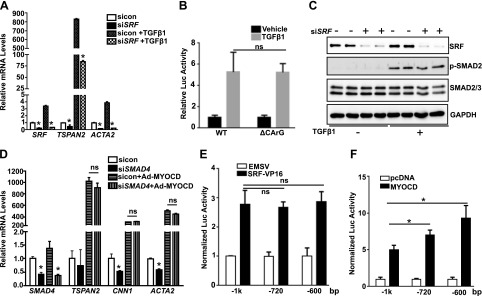
SRF/MYOCD and TGF/SMAD independently regulate TSPAN2 expression
Having confirmed the transcriptional regulation of TSPAN2 by both the SRF/MYOCD and TGF/SMAD pathways, we sought to determine whether these pathways function independently. First, we depleted SRF in HCASMCs, then treated cells with TGF-β1. Unlike ACTA2, where TGF-β1 induction was completely dependent upon SRF, depletion of SRF only partially attenuated TGF-β1 induction of TSPAN2 mRNA (Fig. 8A); however, there was no significant difference between WT and CArG mutant TSPAN2 reporters in response to TGF-β1 treatment, which indicated that the influence of SRF on TGF-β1–induced TSPAN2 gene expression is CArG independent (Fig. 8B). Depletion of SRF in HCASMCs also showed no effect on TGF-β1–induced SMAD2 phosphorylation at 5 h after TGF-β1 treatment, which suggested that SRF is not necessary for the activation of SMAD pathway. Conversely, knockdown of SMAD4 did not impact MYOCD-induced TSPAN2 gene expression (Fig. 8D). WT reporter (with all 4 putative SBEs and the CArG box), the −720-bp reporter (with the proximal SBE only and the CArG box), and −600-bp reporter (with the CArG box but lacking all 4 predicted SBEs) displayed similar responses to SRF, which suggested that the putative SBEs are not necessary for the transactivation of TSPAN2 promoter by SRF (Fig. 8E). Both the −720- and −600-bp truncated reporters showed marginally increased luciferase activity to MYOCD compared with WT reporter (Fig. 8F). This suggests a negative effect of the putative SBEs on the transactivity of MYOCD to TSPAN2 promoter, which is likewise SRF/CArG independent. Overall, our results demonstrated that 2 distinct pathways, SRF/MYOCD/CArG and TGF-β1/SMAD/SBE, transcriptionally activate TSPAN2 gene expression.
Figure 8.
SRF/MYOCD and TGF-β1/SMAD regulate TSPAN2 transcription in a parallel way. A) HCASMCs were transfected with siRNA to SRF (siSRF) for 24 h, and cells were starved overnight before treatment of TGF-β1 for another 24 h. RNA was subjected to quantitative RT-PCR analysis of indicated genes. B) Luciferase assays for indicated reporters were performed in 10T1/2 cells, followed by TGF-β1 treatment, as described above. C) Growing HCASMCs were transfected with siSRF for 48 h, followed by serum starvation overnight. Cells were then stimulated with TGF-β1 for 5 h before protein extraction for Western blot analysis of the indicated proteins. D) Growing HCASMCs were transfected with siRNA to SMAD4 (siSMAD4) for 24 h. Cells were then transduced with Ad-MYOCD or Ad-empty for 48 h before RNA was extracted for quantitative RT-PCR analysis of indicated genes. E, F) Indicated TSPAN2 reporters were cotransfected with either SRF-VP16 vs. vector control EMSV (E) or MYOCD vs. pcDNA vector control (F) in SKLMS cells, and luciferase activity was assessed, as described in Fig. 6. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; ns, not significant. Values are means ± sd and data are representative of 3 separate experiments. EMSV, empty vector. *P < 0.05.
DISCUSSION
Intensive studies have established an essential role for VSMC phenotypic modulation in the pathogenesis of a number of vascular diseases, such as atherosclerosis, restenosis, transplant arteriopathy, and hypertension (1, 2, 37). A hallmark of VSMC phenotypic modulation is a compromised VSMC contractile phenotype, which is characterized by down-regulation of VSMC contractile genes, such as MYH11, CNN1, and TAGLN, and an enhanced rate of VSMC proliferation, migration, and synthetic activity (38–40). We previously identified KCNMB1 and MYOSLID as the first SRF/MYOCD-dependent ion channel and long noncoding RNA gene, respectively, both of which elicit their unique regulatory roles to reinforce the VSMC contractile phenotype (12, 26). In this study, we demonstrate for the first time, to our knowledge, that TSPAN2 is a novel cell membrane gene that is directly targeted by TGF-β/SMAD and MYOCD/SRF. We show that TSPAN2 is selectively enriched in SMCs within the vasculature. We provide strong evidence that TSPAN2 is intimately associated with the VSMC contractile phenotype and drastically down-regulated in diseased vessels wherein the contractile VSMC phenotype is compromised. Of importance, we provide in vitro evidence that demonstrates that TSPAN2 suppresses VSMC proliferation and migration. Our results support a recent GWAS study, wherein an important SNP located in the regulatory region of TSPAN2 has been highly associated with atherosclerosis in big arteries. Our findings, together with this GWAS study, indicate that VSMC-TSPAN2 could play critical roles in governing VSMC phenotypic transition, and that dysregulated TSPAN2 might contribute to multiple important occlusive vascular pathologies, such as atherosclerosis, postangioplasty caused restenosis, and allograft vasculopathy.
RNA-seq analysis in HCASMCs and RNA-arrays in MASMCs uncovered several highly abundant TSPAN family members, such as CD9, CD63, CD151, and CD81 in VSMCs, all of which have broad-spectrum gene expression profiles (18, 21, 41). These ubiquitously expressed TSPAN genes are resistant to both TGF-β1 and MYOCD stimulation in VSMCs, which indicates that they might not be connected to the VSMC contractile phenotype. In contrast, although the basal level of TSPAN2 is low in cultured VSMCs, it can be markedly induced by both TGF-β1 and MYOCD in a fashion similar to most VSMC-specific genes. This indicates that the transcriptional regulation and mode of action of TSPAN2 in VSMCs are distinct from other TSPAN family members. Of note, TSPAN2 was originally identified as a gene enriched in oligodendrocytes (31). Recent studies also have shown that TSPAN2 can be derepressed by a p53 mutation in lung cancer to contribute to lung cancer progression (23). High glucose-induced TSPAN2 expression promotes glucotoxic apoptosis in β1-cells (24). These studies would indicate that TSPAN2 is not a definitive SMC marker gene, in contrast to other VSMC contractile genes, such as MYH11 and CNN1. Our finding and the above studies underscore the fact that disparate regulatory circuits govern cell and context-dependent TSPAN2 gene expression, which also indicates a functional diversity of TSPAN2 under different circumstances; therefore, we speculate that TSPAN2 has a regulatory role in VSMCs distinct from that in other fields, such as the CNS and cancer cells.
A major finding of the current study is the identification of the SRF/MYOCD and TGF/SMAD pathways as important regulators of TSPAN2 expression. The majority of VSMC marker genes harbor one or more conserved CArG boxes in their regulatory region that complex with SRF and its cofactor, MYOCD, which confers VSMC-specific gene transcription (9, 10, 42). In corroboration with its transcriptional activation by SRF/MYOCD, we demonstrated that TSPAN2 is selectively expressed in VSMCs within the vessel wall. This was supported by results that were derived from complementary approaches, including quantitative RT-PCR analysis of TSPAN2 mRNA across different mouse and human tissues, purified medial SMC layers vs. ECs from the same vessel source, and in situ hybridization. Although we could not conclude that TSPAN2 is a VSMC marker, our data clearly show that it is enriched in VSMCs only within the vessel wall.
Most VSMC-specific genes are also regulated by the multifaceted TGF-β1/SMAD signal pathway. This raises an important question: do most VSMC marker genes coincidently possess responsive SBEs required by the canonical TGF-β1/SMAD pathway, or does this relate to the crosstalk between the SRF/MYOCD and TGF-β1/SMAD pathways? We and others previously reported that TGF-β1, per se, induces expression of MYOCD and SRF (29, 43), which might, at least in part, if not completely, account for the general induction of SMC gene expression elicited by TGF-β1 signaling. In line with this, we found that depletion of SRF in VSMCs partially attenuated TGF-β1–induced TSPAN2 mRNA levels, which seems to be CArG independent as the CArG mutation failed to influence TGF-β1–induced TSPAN2 promoter activity. In contrast, depletion of SRF totally abolished TGF-β1–induced ACTA2 expression, which indicated that SRF is indispensable for TGF-β1– induced ACTA2 expression in VSMCs. These results would also suggest that the detailed mechanism that underlies the role of SRF in TGF-β1–induced VSMC gene expression might differ between individual SMC genes. In contrast, depletion of SMAD4 exhibited no effect on MYOCD-induced TSPAN2 and ACTA2 gene expression. Loss of the putative SBEs did not alter the transactivity of SRF to the TSPAN2 promoter, which is in keeping with the disparate transcriptional activation of TSPAN2 mediated by the SRF/CArG and TGF-β/SMAD pathways. Of interest, we consistently observed a moderate but significant increase in the transactivity of MYOCD to the SBE mutants compared with WT TSPAN2 reporter. It has been demonstrated that MYOCD can regulate SMC-specific gene expression (SM22α) via an SBE-dependent, but CArG-independent, pathway (44, 45). In this context, we surmise that the putative SBEs could negatively impact the MYOCD-dependent and CArG-independent transactivation of the TSPAN2 promoter, though this awaits further investigation. It will also be interesting to see whether those in vitro functional CArG and SBEs behave similarly in vivo by using CRISP-CAS–mediated genome editing technology in mouse.
In the present study, results derived from vascular injury models, diseased human AVF samples, and cultured VSMCs strongly support the conclusion that TSPAN2 is closely associated with the VSMC contractile phenotype and is sharply down-regulated in the course of VSMC phenotypic modulation. This suggests that TSPAN2 could play a critical role in the pathogenesis of vascular disease. The drastic reduction of TSPAN2 expression during VSMC phenotypic modulation may be directly attributed to the down-regulation of MYOCD and aberrant TGF-β/SMAD signaling (36, 46), though we could not exclude other unknown mechanisms. Emerging evidence indicates that MYOCD and the TGF-β/SMAD pathway are indispensable to vascular development and homeostasis (36, 46–51). The deficiency of key components of either pathway, such as MYOCD, SMAD4, or TGFβR in VSMCs, causes defects in VSMC differentiation, vascular development, and arterial diseases, such as aneurysms (51–54). It will be important to elucidate whether these phenotypes are secondary to the perturbation in VSMC differentiation, or if they relate to other distinct roles of MYOCD and TGF-β signaling in inflammation and cell death. Delineating novel upstream signaling targets that are common to SRF/MYOCD and TGF-β/SMAD might lead to a better understanding of the role of MYOCD and TGF-β in vascular biology and disease. An elegant recent report showed that one important TSPAN family member, CD82, modulates TGF-β1/Smad3 signaling, which leads to the induction of CDK inhibitors and cell-cycle inhibition. This ultimately promotes a quiescent phenotype of long-term hematopoietic stem cells (55). Similarly, we speculate that VSMC-TSPAN2 might interplay with MYOCD/SRF and TGF-β/SMAD in controlling the VSMC contractile phenotype and vascular disease.
In summary, we have demonstrated that TSPAN2 is a novel VSMC-enriched, SRF/MYOCD- and TGF/SMAD-dependent cell membrane gene and seems to be a suppressor of VSMC proliferation and migration. We conclude that expression of TSPAN2 is tightly controlled by VSMC phenotypic transition and negatively associated with vascular disease. Our study, together with the recent GWAS report, suggests that TSPAN2 could be an important diagnostic marker for vascular diseases, and that aberrant expression of TSPAN2 might play important roles in the pathogenesis of occlusive vascular diseases, such as injury caused arterial restenosis, AVF failure, and atherosclerosis.
ACKNOWLEDGMENTS
This work was supported by American Heart Association Scientist Development Grant 10SDG3670036, U.S. National Institutes of Health/National Heart, Lung, and Blood Institute (NIH/NHLBI) Grant R01-HL122686, Paul Teschan Research Fund #2015_04, and Albany Medical College faculty startup funding (to X.L.); American Heart Association Scientist Development Grant 13SDG16920099 and NIH/NHLBI Grant R01-HL121700 (to M.W.); NIH/NHLBI Grant R01-HL49426 (to H.A.S.); and American Heart Association Grant-in-Aid 16GRNT31280002 (to D.J.). The authors declare no conflicts of interest.
Glossary
- AVF
arteriovenous fistula
- ChIP
chromatin immunoprecipitation
- EC
endothelial cell
- FPKM
fragments per kilobase of exon per million fragments mapped
- GEO
Gene Expression Omnibus
- GWAS
genome-wide association study
- HASMC
human aortic smooth muscle cell
- HCASMC
human coronary artery smooth muscle cell
- MASMC
mouse aortic smooth muscle cell
- MYOCD
myocardin
- SBE
SMAD-binding element
- siRNA
small interfering RNA
- SNP
single-nucleotide polymorphism
- SRF
serum response factor
- TSPAN
tetraspanin
- VSMC
vascular smooth muscle cell
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J. Zhao, W. Wu, and X. Long designed and performed research, analyzed data, and wrote the paper; W. Zhang, Y. W. Lu, E. Tou, J. Ye, and P. Gao performed experiments and analyzed data; and D. Jourd'heuil, H. A. Singer, and M. Wu designed the research and edited the paper.
REFERENCES
- 1.Thyberg J. (1998) Phenotypic modulation of smooth muscle cells during formation of neointimal thickenings following vascular injury. Histol. Histopathol. 13, 871–891 [DOI] [PubMed] [Google Scholar]
- 2.Owens G. K., Kumar M. S., Wamhoff B. R. (2004) Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 84, 767–801 [DOI] [PubMed] [Google Scholar]
- 3.Herring B. P., Hoggatt A. M., Burlak C., Offermanns S. (2014) Previously differentiated medial vascular smooth muscle cells contribute to neointima formation following vascular injury. Vasc. Cell 6, 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pauletto P., Sarzani R., Rappelli A., Chiavegato A., Pessina A. C., Sartore S. (1994) Differentiation and growth of vascular smooth muscle cells in experimental hypertension. Am. J. Hypertens. 7, 661–674 [DOI] [PubMed] [Google Scholar]
- 5.Bennett M. R., Sinha S., Owens G. K. (2016) Vascular smooth muscle cells in atherosclerosis. Circ. Res. 118, 692–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albinsson S., Sessa W. C. (2011) Can microRNAs control vascular smooth muscle phenotypic modulation and the response to injury? Physiol. Genomics 43, 529–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miano J. M., Long X. (2015) The short and long of noncoding sequences in the control of vascular cell phenotypes. Cell. Mol. Life Sci. 72, 3457–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Y., Urs S., Boucher J., Bernaiche T., Venkatesh D., Spicer D. B., Vary C. P., Liaw L. (2010) Notch and transforming growth factor-beta (TGFbeta) signaling pathways cooperatively regulate vascular smooth muscle cell differentiation. J. Biol. Chem. 285, 17556–17563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z., Wang D. Z., Pipes G. C., Olson E. N. (2003) Myocardin is a master regulator of smooth muscle gene expression. Proc. Natl. Acad. Sci. USA 100, 7129–7134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J., Kitchen C. M., Streb J. W., Miano J. M. (2002) Myocardin: a component of a molecular switch for smooth muscle differentiation. J. Mol. Cell. Cardiol. 34, 1345–1356 [DOI] [PubMed] [Google Scholar]
- 11.Cordes K. R., Sheehy N. T., White M. P., Berry E. C., Morton S. U., Muth A. N., Lee T. H., Miano J. M., Ivey K. N., Srivastava D. (2009) miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460, 705–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long X., Tharp D. L., Georger M. A., Slivano O. J., Lee M. Y., Wamhoff B. R., Bowles D. K., Miano J. M. (2009) The smooth muscle cell-restricted KCNMB1 ion channel subunit is a direct transcriptional target of serum response factor and myocardin. J. Biol. Chem. 284, 33671–33682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsson E., McLean S. E., Mecham R. P., Lindahl P., Nelander S. (2008) Do two mutually exclusive gene modules define the phenotypic diversity of mammalian smooth muscle? Mol. Genet. Genomics 280, 127–137 [DOI] [PubMed] [Google Scholar]
- 14.Wang X., Hu G., Betts C., Harmon E. Y., Keller R. S., Van De Water L., Zhou J. (2011) Transforming growth factor-β1-induced transcript 1 protein, a novel marker for smooth muscle contractile phenotype, is regulated by serum response factor/myocardin protein. J. Biol. Chem. 286, 41589–41599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lilly B., Olson E. N., Beckerle M. C. (2001) Identification of a CArG box-dependent enhancer within the cysteine-rich protein 1 gene that directs expression in arterial but not venous or visceral smooth muscle cells. Dev. Biol. 240, 531–547 [DOI] [PubMed] [Google Scholar]
- 16.Hemler M. E. (2008) Targeting of tetraspanin proteins--potential benefits and strategies. Nat. Rev. Drug Discov. 7, 747–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delos Santos R. C., Garay C., Antonescu C. N. (2015) Charming neighborhoods on the cell surface: plasma membrane microdomains regulate receptor tyrosine kinase signaling. Cell. Signal. 27, 1963–1976 [DOI] [PubMed] [Google Scholar]
- 18.Zhang F., Kotha J., Jennings L. K., Zhang X. A. (2009) Tetraspanins and vascular functions. Cardiovasc. Res. 83, 7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Junge H. J., Yang S., Burton J. B., Paes K., Shu X., French D. M., Costa M., Rice D. S., Ye W. (2009) TSPAN12 regulates retinal vascular development by promoting Norrin- but not Wnt-induced FZD4/beta-catenin signaling. Cell 139, 299–311 [DOI] [PubMed] [Google Scholar]
- 20.Wei Q., Zhang F., Richardson M. M., Roy N. H., Rodgers W., Liu Y., Zhao W., Fu C., Ding Y., Huang C., Chen Y., Sun Y., Ding L., Hu Y., Ma J. X., Boulton M. E., Pasula S., Wren J. D., Tanaka S., Huang X., Thali M., Hämmerling G. J., Zhang X. A. (2014) CD82 restrains pathological angiogenesis by altering lipid raft clustering and CD44 trafficking in endothelial cells. Circulation 130, 1493–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemler M. E. (2001) Specific tetraspanin functions. J. Cell Biol. 155, 1103–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Monasterio-Schrader P., Patzig J., Möbius W., Barrette B., Wagner T. L., Kusch K., Edgar J. M., Brophy P. J., Werner H. B. (2013) Uncoupling of neuroinflammation from axonal degeneration in mice lacking the myelin protein tetraspanin-2. Glia 61, 1832–1847 [DOI] [PubMed] [Google Scholar]
- 23.Otsubo C., Otomo R., Miyazaki M., Matsushima-Hibiya Y., Kohno T., Iwakawa R., Takeshita F., Okayama H., Ichikawa H., Saya H., Kiyono T., Ochiya T., Tashiro F., Nakagama H., Yokota J., Enari M. (2014) TSPAN2 is involved in cell invasion and motility during lung cancer progression. Cell Rep. 7, 527–538 [DOI] [PubMed] [Google Scholar]
- 24.Hwang I. H., Park J., Kim J. M., Kim S. I., Choi J. S., Lee K. B., Yun S. H., Lee M. G., Park S. J., Jang I. S. (2016) Tetraspanin-2 promotes glucotoxic apoptosis by regulating the JNK/β-catenin signaling pathway in human pancreatic β cells. FASEB J. 30, 3107–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NINDS Stroke Genetics Network (SiGN); International Stroke Genetics Consortium (ISGC) (2015) Loci associated with ischaemic stroke and its subtypes (SiGN): a genome-wide association study. Lancet Neurol. 15, 174–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao J., Zhang W., Lin M., Wu W., Jiang P., Tou E., Xue M., Richards A., Jourd’heuil D., Asif A., Zheng D., Singer H. A., Miano J. M., Long X. (2016) MYOSLID is a novel serum response factor-dependent long noncoding RNA that amplifies the vascular smooth muscle differentiation program. Arterioscler. Thromb. Vasc. Biol. 36, 2088–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell R. D., Long X., Lin M., Bergmann J. H., Nanda V., Cowan S. L., Zhou Q., Han Y., Spector D. L., Zheng D., Miano J. M. (2014) Identification and initial functional characterization of a human vascular cell-enriched long noncoding RNA. Arterioscler. Thromb. Vasc. Biol. 34, 1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiene L. S., Homann S., Suvorava T., Rabausch B., Müller J., Kojda G., Kretschmer I., Twarock S., Dai G., Deenen R., Hartwig S., Lehr S., Köhrer K., Savani R. C., Grandoch M., Fischer J. W. (2016) Deletion of hyaluronan synthase 3 inhibits neointimal hyperplasia in mice. Arterioscler. Thromb. Vasc. Biol. 36, e9–e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long X., Miano J. M. (2011) Transforming growth factor-beta1 (TGF-beta1) utilizes distinct pathways for the transcriptional activation of microRNA 143/145 in human coronary artery smooth muscle cells. J. Biol. Chem. 286, 30119–30129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dugas J. C., Tai Y. C., Speed T. P., Ngai J., Barres B. A. (2006) Functional genomic analysis of oligodendrocyte differentiation. J. Neurosci. 26, 10967–10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cahoy J. D., Emery B., Kaushal A., Foo L. C., Zamanian J. L., Christopherson K. S., Xing Y., Lubischer J. L., Krieg P. A., Krupenko S. A., Thompson W. J., Barres B. A. (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28, 264–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida T., Gan Q., Shang Y., Owens G. K. (2007) Platelet-derived growth factor-BB represses smooth muscle cell marker genes via changes in binding of MKL factors and histone deacetylases to their promoters. Am. J. Physiol. Cell Physiol. 292, C886–C895 [DOI] [PubMed] [Google Scholar]
- 33.Alexander M. R., Murgai M., Moehle C. W., Owens G. K. (2012) Interleukin-1β modulates smooth muscle cell phenotype to a distinct inflammatory state relative to PDGF-DD via NF-κB-dependent mechanisms. Physiol. Genomics 44, 417–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai Y., Nagel D. J., Zhou Q., Cygnar K. D., Zhao H., Li F., Pi X., Knight P. A., Yan C. (2015) Role of cAMP-phosphodiesterase 1C signaling in regulating growth factor receptor stability, vascular smooth muscle cell growth, migration, and neointimal hyperplasia. Circ. Res. 116, 1120–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long X., Cowan S. L., Miano J. M. (2013) Mitogen-activated protein kinase 14 is a novel negative regulatory switch for the vascular smooth muscle cell contractile gene program. Arterioscler. Thromb. Vasc. Biol. 33, 378–386 [DOI] [PubMed] [Google Scholar]
- 36.Miano J. M. (2015) Myocardin in biology and disease. J. Biomed. Res. 29, 3–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross R., Glomset J. A. (1973) Atherosclerosis and the arterial smooth muscle cell: proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science 180, 1332–1339 [DOI] [PubMed] [Google Scholar]
- 38.Geary R. L., Wong J. M., Rossini A., Schwartz S. M., Adams L. D. (2002) Expression profiling identifies 147 genes contributing to a unique primate neointimal smooth muscle cell phenotype. Arterioscler. Thromb. Vasc. Biol. 22, 2010–2016 [DOI] [PubMed] [Google Scholar]
- 39.Halayko A. J., Solway J. (2001) Molecular mechanisms of phenotypic plasticity in smooth muscle cells. J. Appl. Physiol. (1985) 90, 358–368 [DOI] [PubMed] [Google Scholar]
- 40.Zhang C., van der Voort D., Shi H., Zhang R., Qing Y., Hiraoka S., Takemoto M., Yokote K., Moxon J. V., Norman P., Rittie L., Kuivaniemi H., Atkins G. B., Gerson S. L., Shi G. P., Golledge J., Dong N., Perbal B., Prosdocimo D. A., Lin Z. (2016) Matricellular protein CCN3 mitigates abdominal aortic aneurysm. J. Clin. Invest. 126, 1282–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herr M. J., Mabry S. E., Jennings L. K. (2014) Tetraspanin CD9 regulates cell contraction and actin arrangement via RhoA in human vascular smooth muscle cells. PLoS One 9, e106999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du K. L., Ip H. S., Li J., Chen M., Dandre F., Yu W., Lu M. M., Owens G. K., Parmacek M. S. (2003) Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol. Cell. Biol. 23, 2425–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao Q., Luo Z., Pepe A. E., Margariti A., Zeng L., Xu Q. (2009) Embryonic stem cell differentiation into smooth muscle cells is mediated by Nox4-produced H2O2. Am. J. Physiol. Cell Physiol. 296, C711–C723 [DOI] [PubMed] [Google Scholar]
- 44.Kitchen C. M., Cowan S. L., Long X., Miano J. M. (2013) Expression and promoter analysis of a highly restricted integrin alpha gene in vascular smooth muscle. Gene 513, 82–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu P., Ritchie R. P., Fu Z., Cao D., Cumming J., Miano J. M., Wang D. Z., Li H. J., Li L. (2005) Myocardin enhances Smad3-mediated transforming growth factor-beta1 signaling in a CArG box-independent manner: Smad-binding element is an important cis element for SM22alpha transcription in vivo. Circ. Res. 97, 983–991 [DOI] [PubMed] [Google Scholar]
- 46.Chen P. Y., Qin L., Li G., Tellides G., Simons M. (2016) Smooth muscle FGF/TGFβ cross talk regulates atherosclerosis progression. EMBO Mol. Med. 8, 712–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ackers-Johnson M., Talasila A., Sage A. P., Long X., Bot I., Morrell N. W., Bennett M. R., Miano J. M., Sinha S. (2015) Myocardin regulates vascular smooth muscle cell inflammatory activation and disease. Arterioscler. Thromb. Vasc. Biol. 35, 817–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li S., Wang D. Z., Wang Z., Richardson J. A., Olson E. N. (2003) The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc. Natl. Acad. Sci. USA 100, 9366–9370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miano J. M., Ramanan N., Georger M. A., de Mesy Bentley K. L., Emerson R. L., Balza R. O. Jr., Xiao Q., Weiler H., Ginty D. D., Misra R. P. (2004) Restricted inactivation of serum response factor to the cardiovascular system. Proc. Natl. Acad. Sci. USA 101, 17132–17137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang J., Cheng L., Li J., Chen M., Zhou D., Lu M. M., Proweller A., Epstein J. A., Parmacek M. S. (2008) Myocardin regulates expression of contractile genes in smooth muscle cells and is required for closure of the ductus arteriosus in mice. J. Clin. Invest. 118, 515–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang P., Hou S., Chen J., Zhang J., Lin F., Ju R., Cheng X., Ma X., Song Y., Zhang Y., Zhu M., Du J., Lan Y., Yang X. (2016) Smad4 deficiency in smooth muscle cells initiates the formation of aortic aneurysm. Circ. Res. 118, 388–399 [DOI] [PubMed] [Google Scholar]
- 52.Huang J., Wang T., Wright A. C., Yang J., Zhou S., Li L., Yang J., Small A., Parmacek M. S. (2015) Myocardin is required for maintenance of vascular and visceral smooth muscle homeostasis during postnatal development. Proc. Natl. Acad. Sci. USA 112, 4447–4452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mao X., Debenedittis P., Sun Y., Chen J., Yuan K., Jiao K., Chen Y. (2012) Vascular smooth muscle cell Smad4 gene is important for mouse vascular development. Arterioscler. Thromb. Vasc. Biol. 32, 2171–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu J. H., Wei H., Jaffe M., Airhart N., Du L., Angelov S. N., Yan J., Allen J. K., Kang I., Wight T. N., Fox K., Smith A., Enstrom R., Dichek D. A. (2015) Postnatal deletion of the type II transforming growth factor-β receptor in smooth muscle cells causes severe aortopathy in mice. Arterioscler. Thromb. Vasc. Biol. 35, 2647–2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hur J., Choi J. I., Lee H., Nham P., Kim T. W., Chae C. W., Yun J. Y., Kang J. A., Kang J., Lee S. E., Yoon C. H., Boo K., Ham S., Roh T. Y., Jun J. K., Lee H., Baek S. H., Kim H. S. (2016) CD82/KAI1 maintains the dormancy of long-term hematopoietic stem cells through interaction with DARC-expressing macrophages. Cell Stem Cell 18, 508–521 [DOI] [PubMed] [Google Scholar]