Abstract
Alveolar epithelial cell (AEC) mitochondrial dysfunction and apoptosis are important in idiopathic pulmonary fibrosis and asbestosis. Sirtuin 3 (SIRT3) detoxifies mitochondrial reactive oxygen species, in part, by deacetylating manganese superoxide dismutase (MnSOD) and mitochondrial 8-oxoguanine DNA glycosylase. We reasoned that SIRT3 deficiency occurs in fibrotic lungs and thereby augments AEC mtDNA damage and apoptosis. Human lungs were assessed by using immunohistochemistry for SIRT3 activity via acetylated MnSODK68. Murine AEC SIRT3 and cleaved caspase-9 (CC-9) expression were assayed by immunoblotting with or without SIRT3 enforced expression or silencing. mtDNA damage was measured by using quantitative PCR and apoptosis via ELISA. Pulmonary fibrosis after asbestos or bleomycin exposure was evaluated in 129SJ/wild-type and SIRT3-knockout mice (Sirt3−/−) by using fibrosis scoring and lung collagen levels. Idiopathic pulmonary fibrosis lung alveolar type II cells have increased MnSODK68 acetylation compared with controls. Asbestos and H2O2 diminished AEC SIRT3 protein expression and increased mitochondrial protein acetylation, including MnSODK68. SIRT3 enforced expression reduced oxidant-induced AEC OGG1K338/341 acetylation, mtDNA damage, and apoptosis, whereas SIRT3 silencing promoted these effects. Asbestos- or bleomycin-induced lung fibrosis, AEC mtDNA damage, and apoptosis in wild-type mice were amplified in Sirt3−/− animals. These data suggest a novel role for SIRT3 deficiency in mediating AEC mtDNA damage, apoptosis, and lung fibrosis.—Jablonski, R. P., Kim, S.-J., Cheresh, P., Williams, D. B., Morales-Nebreda, L., Cheng, Y., Yeldandi, A., Bhorade, S., Pardo, A., Selman, M., Ridge, K., Gius, D., Budinger, G. R. S., Kamp, D. W. SIRT3 deficiency promotes lung fibrosis by augmenting alveolar epithelial cell mitochondrial DNA damage and apoptosis.
Keywords: sirtuin 3, oxidative stress, pulmonary fibrosis
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrotic lung disease with increasing mortality (1) and a median life expectancy of <5 yr after diagnosis (2). Although 2 agents have been shown to slow disease progression (3, 4), there are no therapies that stop the loss of or restore lung function, which highlights the need for additional treatments. Careful research by our group and others has utilized asbestos fibers, an important cause of lung fibrosis (asbestosis), to unravel the molecular events that promote fibrosis to identify targets for therapy. Our investigation has focused on alveolar type II (AT2) cell mitochondrial dysfunction, DNA damage, and apoptosis that are evident in patients with IPF and asbestosis (for review, see refs. 5–12).
Accumulating evidence implicates exaggerated lung aging as a hallmark of IPF and asbestosis (8, 13). Many of the 9 proposed hallmarks of the aging phenotype are present in fibrotic lungs, including genomic instability, telomere shortening, epigenetic alterations, abnormal proteostasis, dysregulated nutrient sensing, cellular senescence/apoptosis, stem cell depletion, and mitochondrial dysfunction (for review, see refs. 8–17). Mitochondria are causally linked to age-related diseases, including IPF and asbestosis, as they are both the source and target of reactive oxygen species (ROS), which results in mtDNA damage (10, 18–20). We showed that an mtDNA base excision repair (BER) enzyme, 8-oxoguanine DNA glycosylase 1 (OGG1), prevents oxidant-induced alveolar epithelial cell (AEC) apoptosis by preventing mtDNA damage (21), and that asbestos-induced lung fibrosis is increased in Ogg1−/− mice (22). Collectively, these data support a link between mtDNA damage and apoptosis in the pathophysiology of IPF.
Sirtuin 3 (SIRT3) is a mitochondrial member of the sirtuin family of NAD-dependent deacetylases that direct cell fate and aging (for review, see refs. 23, 24). Mice that are deficient in one of the sirtuin genes develop pathology that parallels that in aged humans. Murine Sirt3-knockout (Sirt3−/−) studies (25–31) have established that SIRT3 is the primary mitochondrial deacetylase (32), and that SIRT3 deficiency leads to acetylation and inactivation of numerous mitochondrial proteins that are critical for mitochondrial integrity, including manganese superoxide dismutase (MnSOD) at lysine residue 68 (MnSODK68) (28, 33–35) and most members of the electron transport chain and tricarboxylic acid cycle (23, 24). Moreover, Sirt3−/− mice develop spontaneous fibrosis in a number of organs, including the lungs, in part as a result of increased acetylation of glycogen synthase kinase-β, which leads to increased TGF-β1 synthesis (31). Furthermore, Sirt3−/− mice are more susceptible to bleomycin-induced lung fibrosis, in part, via effects of fibroblasts (36). Of note, 2 groups have recently shown that mitochondrial OGG1 is a crucial SIRT3 deacetylation target in nonpulmonary cells, and that SIRT3 depletion augments acetylation of OGG1 residues, K338 and K341 (OGG1K338/K341), thereby reducing mtDNA BER and increasing mtDNA damage and apoptosis (37, 38). We reasoned that SIRT3 depletion plays an important role in the pathobiology of IPF by promoting AT2 cell acetylation of MnSOD and OGG1, which results in AT2 cell mtDNA damage and apoptosis.
In this study, we show that, compared with normal human lungs, patients with IPF have increased acetylation of key SIRT3 target MnSODK68, including in AT2 cells, which is consistent with deficient AEC SIRT3 activity. We also demonstrate that SIRT3 modulates acetylation of mitochondrial proteins—MnSOD and OGG1—that are important for maintaining mtDNA integrity and mitigating apoptosis. Furthermore, compared with wild-type (WT) mice, Sirt3−/− mice have increased lung fibrosis and a greater degree of AEC mtDNA damage and apoptosis. Taken together, these findings suggest a critical role for deficient AEC SIRT3 deacetylase activity in promoting pulmonary fibrosis by increasing acetylation of mitochondrial proteins that augment oxidant-induced AEC mtDNA damage and apoptosis.
MATERIALS AND METHODS
Lung tissue from humans
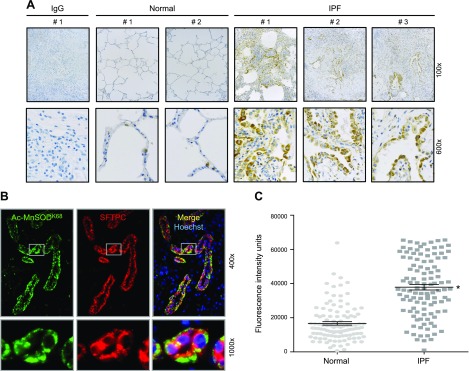
For this pilot study of human lung tissue, institutional review board–approved, deidentified lung tissue from patients with normal lungs (n = 3) and with IPF (n = 6) were sectioned for immunohistochemical (IHC) analysis of acetylated MnSODK68 (Cell Signaling Technology, Danvers, MA, USA). Diagnosis of IPF was established by experienced pulmonary pathologists (A.Y., A.P., and M.S.) according to American Thoracic Society and European Respiratory Society guidelines (39). Colocalization of acetylated MnSODK68 with AT2 cells [surfactant protein C (SFPTC)] was performed with immunofluorescence and analyzed semiquantitatively as described (22). Images were acquired by using a Zeiss Axioskop using identical detector settings (Zeiss, Jena, Germany). Cytosolic green fluorescence (representing the MnSODK68 signal) of AT2 cells (those displaying red fluorescence/SFPTC positivity) was quantified via ImageJ (National Institutes of Health, Bethesda, MD, USA) on unadjusted images by drawing 1 identically sized region on the cytosol of each double-positive cell. Per-cell green fluorescence, representing MnSOD acetylation, of 121 normal and 110 IPF lung AT2 cells was graphed by using GraphPad Prism (GraphPad Software, La Jolla, CA, USA).
Reagents
We used crocidolite and amosite amphibole asbestos, which were Union International Centere le Cancer (UICC) reference standards and kindly supplied by Dr. Andy Ghio (Environmental Protection Agency, Washington D.C., USA), as prepared and characterized previously (40). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise specified.
Animals
The Institutional Animal Care and Use Committee at Northwestern University and the Jesse Brown Veterans Affairs Medical Center approved all animal studies herein. Male and female (age 8–10 wk) 129SJ WT mice (The Jackson Laboratory, Bar Harbor, ME, USA) and Sirt3−/− (129SJ background) were used for lung fibrosis studies (22, 41). Intratracheal instillation of asbestos or bleomycin to induce pulmonary fibrosis, or their respective negative controls TiO2 and saline, was performed as described (42, 43). In brief, after tracheal intubation with a 20-gauge angiocathether, 100 µg crocidolite asbestos or TiO2 control (each suspended in 50 µl sterile PBS) or 0.25 U bleomycin in 50 µl normal saline were administered in 2 equal aliquots given 2 min apart. After each aliquot, mice were placed in the right, then left, decubitus position for 10–15 s. Lungs were harvested 21 d after instillation of asbestos, bleomycin, or control. The right lung was fixed in paraffin and subject to staining for hematoxylin and eosin, Masson’s trichrome, or CC-3 and pro-SPFTC via IHC. The left lung was homogenized and subject to the Sircol assay for soluble collagen on the basis of the modified Picosirius red collagen precipitation assay previously described by our group.
Cell culture
The A549 (a malignant lung AEC line with AT2-like properties) and MLE-12 (a nonmalignant murine lung epithelial) cell lines were purchased from American Type Culture Collection (Manassas, VA, USA) and maintained in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) with 2 mM l-glutamine supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 U/ml). Cells were plated in 6-well plates or 100-mm dishes and grown to confluency before adding amphibole asbestos or H2O2 [for up to 24 h as previously described (44)] and harvesting protein extracts for Western blotting or, in separate experiments, obtaining nuclear and mitochondrial DNA for DNA fragmentation and a PCR-based DNA damage assay. Primary murine and male Sprague-Dawley rat (Harlan Laboratories, Indianapolis, IN, USA) AT2 cells were isolated and maintained as previously described by our group and others (22, 45–47).
SIRT3 enforced expression and gene silencing studies
SIRT3 enforced expression (SIRT3-EE) studies were performed by using transient transfection of AECs with Flag-tagged Sirt3 and empty vector control plasmid constructs with Lipofectamine 2000 (Thermo Fisher Scientific). After transfection for 48 h, cells were exposed to experimental conditions for variable periods (0–24 h) and cell lysates were collected for Western blotting, DNA fragmentation, and mtDNA damage assays. For SIRT3 gene-silencing studies, Sirt3-specific small interfering RNA (siRNA) (Thermo Fisher Scientific; target sequence: CCCAGAGGUUCUUGCAUGUGGU) and negative scramble siRNA controls were transiently transfected into A549 and MLE-12 cells by using Lipofectamine RNAiMax (Thermo Fisher Scientific) and tested as above after 48 h.
DNA fragmentation assay
Apoptosis was evaluated by using the Cell Death Detection ELISA Plus kit (Roche Diagnostics, Indianapolis, IN, USA) as previously described (21, 40, 44) according to the manufacturer’s protocol.
mtDNA damage assay
Nuclear and mtDNA damage was assessed by quantitative PCR as described (21, 48). In brief, genomic DNA was extracted by using the Qiagen Genomic-Tip 20G and Qiagen DNA Buffer Set (Qiagen, Gaithersberg, MD, USA). PCR was performed by using elongase enzyme (Thermo Fisher Scientific) to amplify both short and long forms of a mtDNA fragment and nuclear DNA (β-globin). Each DNA was quantified by Pico-Green (Thermo Fisher Scientific) by using the FL600 microplate fluorescence reader (Tecan, Mannendorf, Switzerland) with excitation and emission wavelengths of 485 and 530 nm, respectively. Data obtained from the mitochondrial small fragment was used to normalize results of the mitochondrial long fragment. The number of mitochondrial lesions was calculated by using the following equation: D = (1 – 2−(Δlong−Δshort)) × 10,000 (bp)/size of the long fragment (bp).
Quantitative PCR with reverse transcription
Total RNA was extracted from A549 cells by using Trizol (Thermo Fisher Scientific) according to manufacturer’s specifications. One microgram of RNA was reverse transcribed by using SuperScript III reverse transcriptase (Thermo Fisher Scientific) to generate first-strand complimentary DNA. Real-time quantitative PCR was performed by using the TaqMan Expression PrimeTime Standard quantitative PCR Assay (Integrated DNA Technologies, Coralville, IA, USA) with specific primers (human SIRT3; forward: 5′-TGCAGAAGTAGCAGTTCAGTG-3′, reverse: 5′-GCTTCCTCTAGTGACACTGTTAG-3′; human β-actin; forward: 5′-GTCACCGGAGTCCATCAC-3′, reverse: 3′-GCCATGTACGTTGCTATCCA-5′).
Western blot
Cell lysates and mitochondrial fractions were collected and immunoblotted as described (44) by using Abs (Cell Signaling Technology, unless indicated) that were directed against SIRT3, CC-9, p53 (Santa Cruz Biotechnology, Dallas, TX, USA), total acetyl-lysine, Mn-SODK68, OGG1K338/K341 (Abcam, Cambridge, MA, USA), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and cytochrome oxidase IV. Protein concentration was quantified by using the bicinchoninic acid protein assay kit (Thermo Fischer Scientific). Protein bands were visualized by enhanced luminol-based detection using Amersham ECL Western Blotting Detection Reagent and quantified by densitometry using ImageJ.
Statistical analysis
The results of each experimental in vivo condition were determined from the mean of duplicate or triplicate trials. Data were expressed as means ± sem (n = 3 unless otherwise stated). For in vivo studies, 6 animals were used per group unless otherwise noted. An independent sample 2-tailed Student’s t test was used to assess significance between 2 groups. Analysis of variance was used when comparing more than 2 groups with a single control. Differences between 2 groups within the set were analyzed by Fisher’s protected least significant differences test. A value of P < 0.05 was considered significant.
RESULTS
AT2 cells of patients with IPF have increased acetylated Mn-SODK68 expression
To investigate whether fibrotic human lung tissue is associated with functional SIRT3 deficiency, we performed a pilot study using archived, deidentified lung tissue from participants with normal lung (n = 3) and patients with IPF (n = 6). Because we were interested in SIRT3 deacetylase function, we used IHC analysis of acetylated Mn-SODK68 expression as an established surrogate marker of mitochondrial SIRT3 function as used by others (28, 33, 35, 49). We detected minimal amounts of acetylated MnSODK68 expression in normal lungs (Fig. 1A and Supplemental Fig. 1A). In contrast, fibrotic regions of IPF lungs had markedly increased expression of acetylated Mn-SODK68, primarily in the lung epithelium (Fig. 1A). Of note, colocalization immunofluorescence studies confirmed that the majority of acetylated MnSODK68-expressing cells from patients with IPF lungs (green fluorescence) were AT2 cells as assessed by colocalization with SFPTC staining (red fluorescence), whereas AT2 cell MnSOD acetylation was negligible in control lungs (Fig. 1B and Supplemental Fig. 1B). Semiquantitative analysis confirmed a statistically significant near doubling of per-cell acetylated MnSODK68 expression in AT2 cells from patients with IPF compared with normal controls (Fig. 1C). Collectively, these data suggest deficient AT2 cell SIRT3 activity in patients with IPF compared with participants with normal lung.
Figure 1.
Tissue from patients with IPF has increased acetylation of MnSODK68, a known SIRT3 deacetylase target, which is localized to AT2 cells. A) Explanted lungs from patients with end-stage IPF were evaluated via IHC by using Abs that were targeted to a negative control IgG (fibrotic lung) and Ac-MnSODK68 from normal controls and fibrotic lungs. Shown is a representative panel of 2 normal and 3 fibrotic lungs (for the remainder, please see Supplemental Fig. 1). B) Dual immunofluorescence (IF) staining was used to colocalize expression of acetylated MnSODK68 (green fluorescence) and SFTPC (red fluorescence, used as a marker for AT2 cells). C) Semiquantitative analysis, measured by per-cell fluorescence intensity units as described in Materials and Methods, was used to compare AcMnSODK68 expression in AT2 cells of fibrotic (n = 110) and normal control (n = 121) lungs. Bars shown indicate means ± sem. *P < 0.05 vs. normal AT2 cells.
Oxidative stress reduces AEC SIRT3 protein expression and activity
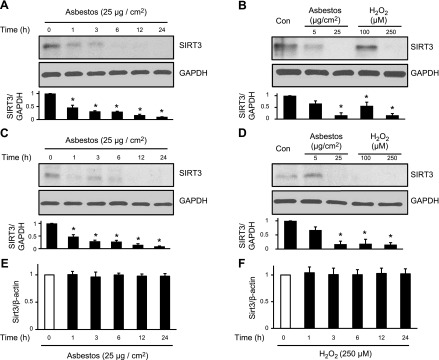
We next performed mechanistic studies to investigate whether SIRT3 deficiency is causally involved in mediating lung fibrosis by using cultured AECs in vitro and murine lung fibrosis models (asbestos and bleomycin). To determine whether oxidative stress alters SIRT3 expression, AECs were exposed to variable doses of amosite asbestos (5–25 µg/cm2) or H2O2 (100–250 µM) for up to 24 h. Asbestos and H2O2 reduced A549 and MLE-12 cell SIRT3 protein expression in a time- and dose-dependent manner (Fig. 2A–D), with reductions as early as 1 h after exposure that persisted at 24 h; however, A549 SIRT3 mRNA expression was unchanged after 24 h of asbestos and H2O2 exposure (Fig. 2E, F). We observed the greatest reduction in SIRT3 protein expression after treatment with asbestos (25 µg/cm2) and H2O2 (200 µM), and used these doses for the remaining in vitro experiments unless otherwise specified.
Figure 2.
SIRT3 expression is decreased in a time- and dose-dependent manner in AECs that are exposed to asbestos and H2O2, whereas SIRT3 mRNA expression is unchanged. A–E) A549 cells (A, B, E, F) and MLE-12 cells (C, D) were exposed to asbestos (5–25 µg/cm2) or H2O2 (100–250 µM) for 0–24 h. Total cellular protein was extracted, and SIRT3 and GAPDH levels were assessed via Western blot (A–D). Shown is a representative Western blot from a total of 3 experiments with the densitometric analysis of SIRT3/GAPDH relative to untreated controls shown below. Total RNA was extracted from A549 cells and quantitative PCR was performed (E, F) to determine relative SIRT3 mRNA copy number normalized to β-actin (n = 3). *P < 0.05 vs. 0 h control.
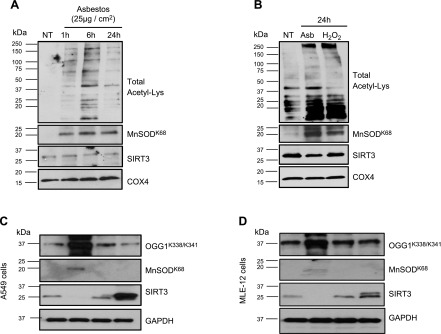
To investigate whether oxidative stress impacts SIRT3 deacetylase function, we assessed acetylation levels of known SIRT3 targets (MnSODK68 and OGG1K338/K341) (33, 35, 37, 38). Amosite asbestos increased MLE-12 cell global mitochondrial protein lysine acetylation in a time-dependent manner (Fig. 3A), which was first evident at 1 h and persisting at 24 h. The increase in mitochondrial protein lysine acetylation was associated with increased MnSODK68 acetylation and reduced SIRT3 protein expression. Of note, asbestos and H2O2 increased primary rat AT2 cell mitochondrial protein lysine acetylation and Mn-SODK68 and reduced SIRT3 protein expression (Fig. 3B). The finding of increased asbestos- and H2O2-induced acetylation that was greater than changes in SIRT3 protein levels suggests that modification of SIRT3 function, rather than SIRT3 protein expression, is a more important marker of SIRT3 deficiency. Consistent with a study that showed that SIRT3 regulates OGG1 acetylation (38), SIRT3-EE reduced OGG1K338/K341 and MnSODK68 acetylation in A549 (Fig. 3C) and MLE-12 (Fig. 3D) cells, whereas SIRT3 silencing augmented OGG1K338/K341 and MnSODK68 acetylation compared with their respective negative controls. Taken together, these data convincingly show that both endogenous (H2O2) and exogenous (asbestos) oxidative stress markedly decrease AEC SIRT3 deacetylase activity, whereas SIRT3 protein expression is reduced to a lesser extent.
Figure 3.
Total AEC mitochondrial protein, OGG1, and MnSOD show increased acetylation after oxidative stress or SIRT3 silencing. A, B) MLE-12 cells (A) were exposed to asbestos (25 µg/cm2) for variable periods, and primary isolated rat AT2 cells (B) were exposed to asbestos (25 µg/cm2) or H2O2 (200 µM) for 24 h, with mitochondrial protein isolated. Global mitochondrial protein lysine acetylation, acetylated MnSODK68, SIRT3, and GAPDH expression were assessed by Western blotting. Shown are representative blots from a total of 3 experiments. C, D) A549 (C) and MLE-12 (D) cells were transfected with siRNA that was targeted to SIRT3 (siRNA) or enforced expression of a SIRT3 WT plasmid (SIRT3-WT) for 48 h, then total cellular protein was extracted and expression of acetylated OGG1K338/K341, acetylated MnSODK68, SIRT3, and GAPDH was assessed by Western blotting. Shown is a representative blot from a total of 3 experiments. COX4, cytochrome oxidase IV; NT, no treatment.
SIRT3 expression is necessary for mitigating oxidant-induced mtDNA damage and apoptosis
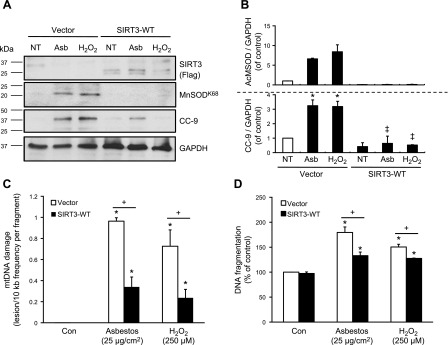
To establish whether SIRT3 is important for the prevention of AEC mtDNA damage and apoptosis in the setting of oxidative stress, we performed genetic SIRT3-EE and silencing studies. First, we overexpressed SIRT3 in A549 cells and assessed markers of oxidant-induced mtDNA damage and intrinsic apoptosis (via CC-9 expression and DNA fragmentation). Compared with empty vector controls, SIRT3-EE reduced mtDNA damage (Fig. 4C), CC-9 and MnSODK68 protein expression (Fig. 4A, B), and attenuated DNA fragmentation (Fig. 4D) after amosite asbestos or H2O2 exposure. SIRT3-EE also prevented oxidant-induced reductions in aconitase 2 expression (data not shown), which we had previously shown to be important for preventing mtDNA damage, mitochondrial p53 localization, and apoptosis in the setting of oxidative stress (21, 44).
Figure 4.
Enforced expression of SIRT3 protects against oxidant-induced AEC mtDNA damage and apoptosis. SIRT3 was transiently overexpressed in A549 cells by using either an empty vector control (vector) or a SIRT3 WT plasmid construct (SIRT3-WT) via Lipofectamine. Cells were exposed to asbestos (25 µg/cm2) or H2O2 (250 µM) for 24 h. A) SIRT3, acetylated MnSODK68, CC-9, and GAPDH in total cell lysate were assessed by Western blot. B) Shown is a representative figure from a total of 3 experiments that were assessed by densitometric quantifications of the MnSODK68/GAPDH and CC-9/GAPDH expression normalized to untreated controls. C, D) Asbestos- or H2O2-induced A549 cell mtDNA damage (C) and DNA fragmentation (D) were measured as described; n = 3. *P < 0.05 vs. control [no treatment (NT)], +P < 0.05 vs. vector control, ‡P < 0.05 vs. vector/Asb or H2O2.
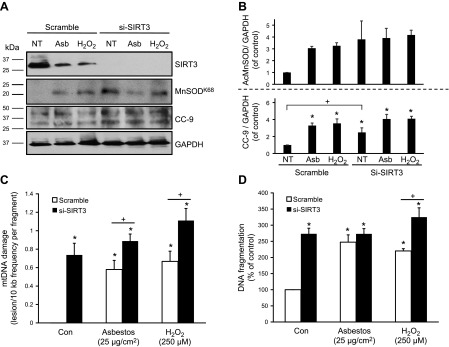
Next, to investigate whether loss of SIRT3 augments oxidant-induced mtDNA damage and apoptosis, A549 cells were transiently transfected with SIRT3 siRNA. Compared with cells that were treated with a scramble siRNA negative control, SIRT3 silencing blocked SIRT3 protein expression (Fig. 5A, B) and increased mtDNA damage (Fig. 5C) after amosite asbestos or H2O2 exposure. Compared with negative controls, SIRT3 siRNA-treated A549 cells demonstrated increased baseline apoptosis as measured by a 2.5-fold increase in DNA fragmentation (Fig. 5D) and CC-9 expression as well as increased acetylation of MnSODK68 (Fig. 5A, B). Together, these data show that SIRT3-EE mitigates oxidant-induced AEC mtDNA damage and intrinsic apoptosis at baseline, whereas loss of SIRT3 promotes these deleterious effects.
Figure 5.
SIRT3 deficiency enhances AEC mtDNA damage and apoptosis after exposure to oxidative stress. A549 cells were transiently transfected with siRNA that was targeted to SIRT3 (si-SIRT3) or a universal scramble negative control (scramble) using RNAiMax for 48 h, then cells were exposed to asbestos (25 µg/cm2) or H2O2 (250 µM) for 24 h. A) SIRT3, MnSODK68, CC-9, and GAPDH in total cell lysate were assessed by Western blot. B) Shown is a representative blot from a total of 3 experiments that were assessed by a densitometric analysis of the CC-9/GAPDH and MnSODK68/GAPDH expression normalized to untreated controls. C, D) MtDNA damage (C) and DNA fragmentation (D) were measured after 24 h of oxidant stress as described; n = 3 for both. *P < 0.05 vs. control [no treatment (NT)], +P < 0.05 vs. scramble.
Asbestos- and bleomycin-induced pulmonary fibrosis is augmented in Sirt3−/− mice
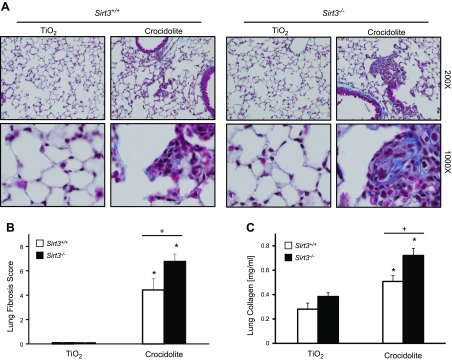
To determine whether SIRT3 deficiency augments lung fibrosis, we studied WT and Sirt3−/− mice by using both asbestos and bleomycin models of lung fibrosis as previously described (22, 50). As expected, crocidolite asbestos (100 µg in 50 µl PBS) induces prominent bronchoalveolar duct (BAD) junction–centered lung fibrosis in WT mice at 3 wk compared with an inert control particle (100 µg TiO2 in 50 µl PBS; Fig. 6A). These changes are associated with increased fibrosis scores (Fig. 6B) and lung collagen levels (Fig. 6C). Similar to WT mice, Sirt3−/− mice that were exposed to a control particle had preservation of normal lung architecture and negligible increases in fibrosis score and lung collagen (Fig. 6). Of note, asbestos-induced lung fibrosis was increased in Sirt3−/− mice as assessed by histopathology, fibrosis scoring, and lung collagen assay (Fig. 6). To test the generalizability of our findings, we used the bleomycin model of lung fibrosis and similarly found that a single intratracheal instillation of bleomycin (0.01 IU in 50 µl PBS) significantly increased pulmonary fibrosis in Sirt3−/− mice as measured histologically, by fibrosis score, and lung collagen levels (Supplemental Fig. 2). These findings demonstrate that Sirt3−/− mice are more susceptible to asbestos- and bleomycin-induced lung fibrosis compared with their WT counterparts.
Figure 6.
SIRT3-deficient mice are more susceptible to asbestos-induced pulmonary fibrosis. A, B) Three weeks after intratracheal instillation with TiO2 (100 μg in 50 μλ PBS) or crocidolite asbestos (100 μg in 50 μl PBS), serial mouse lung sections were stained with Masson’s trichrome stain (A) or scored for fibrosis (B); n = 6 for TiO2/Sirt3+/+; n = 7 for TiO2/Sirt3−/−; n = 4 for crocidolite/Sirt3+/+; and n = 9 for crocidolite/Sirt3−/−. C) Whole-mouse lungs were treated as described and subjected to the Sircol assay; n = 6 for TiO2/Sirt3+/+; n = 7 for TiO2/Sirt3−/−; n = 5 for crocidolite/Sirt3+/+; n = 9 for crocidolite/Sirt3−/−. *P < 0.05 vs. TiO2,+P < 0.05 vs. Sirt3+/+.
Asbestos-induced AEC mtDNA damage and apoptosis are increased in Sirt3−/− mice
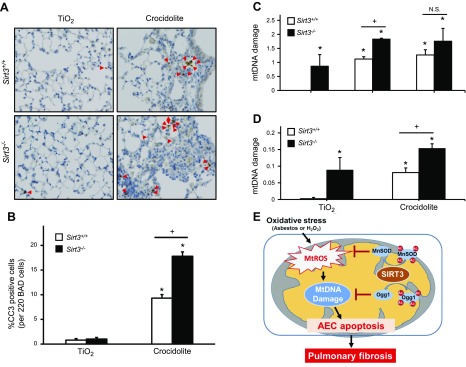
Asbestos-induced fibrotic changes and AEC apoptosis are first evident in cells, including AT2 cells, at the BAD junction, where asbestos fiber deposition occurs (22, 51). To determine whether Sirt3−/− mice have more apoptotic cells at the BAD junction compared with WT mice, we evaluated CC-3 staining by using IHC and semiquantitative analysis of serial mouse lung sections as previously described (22). As expected, TiO2 induced negligible increases in CC-3–positive cells at the BAD junctions in WT and Sirt3−/− mice (0.8 vs. 1.0% positive per 220 BAD junction cells, respectively; P = not significant); however, asbestos significantly increased CC-3–positive cells at the BAD junction in WT mice (P < 0.05 vs. TiO2), and this was augmented in Sirt3−/− animals (9.3 vs. 17.8% positive per 220 BAD junction cells, respectively; P < 0.05 vs. WT; Fig. 7A). Furthermore, IHC apoptosis colocalization studies using CC-3 and SPFTC confirmed that the presence of apoptotic AT2 cells in fibrotic areas of asbestos-exposed WT mice was augmented in Sirt3−/− mice, though this difference did not reach statistical significance (2.9 vs. 5.1% copositive cells per 220 BAD junction cells, respectively; n = 3; Supplemental Fig. 3).
Figure 7.
Asbestos-induced lung cell apoptosis and AEC mtDNA damage are increased in Sirt3−/− mice. A) Lungs from WT and Sirt3−/− mice were harvested 3 wk after intratracheal instillation of TiO2 or crocidolite asbestos, as described in Fig. 6, and subjected to CC-3 or SFTPC IHC on serial sections. Shown is a representative panel. Red arrows indicate double-positive (CC-3 and SFTPC) cells. B) Semiquantitative analysis of 220 cells at the BAD junctions show a significant increase in CC-3 positive cells in WT mice that were treated with crocidolite, which is further amplified in Sirt3−/− mice. C) Primary AT2 cells obtained from WT and Sirt3−/− mice were exposed to asbestos (25 µg/cm2) or H2O2 (200 µM) for 24 h; n = 3. D) mtDNA damage was assessed in AT2 cells that were isolated from WT and Sirt3−/− mice 3 wk after intratracheal instillation with a single dose of TiO2 or crocidolite asbestos as described above; n = 3. E) Hypothetical model. N.S., not significant. *P < 0.05 vs. control or TiO2, +P < 0.05 vs. Sirt3+/+.
We previously reported a causal role for oxidant-induced AEC mtDNA damage in mediating asbestos-induced intrinsic AEC apoptosis in vitro (21) and in vivo (22). Research by others in nonlung cells suggests that SIRT3 deficiency promotes mtDNA damage (37, 38). To directly test whether SIRT3 deficiency augments asbestos-induced AT2 cell mtDNA damage, we isolated primary AT2 cells from WT and Sirt3−/− mice and exposed them in vitro to amosite asbestos (25 µg/cm2) and H2O2 (250 µM) for 24 h. Murine primary AT2 cells from WT mice had increased mtDNA damage after treatment with asbestos or H2O2 as expected. Of interest, these deleterious effects were amplified in AT2 cells from Sirt3−/− mice (Fig. 7C), though the differences between AT2 cells from WT and Sirt3−/− mice after H2O2 exposure did not reach statistical significance. As an alternative in vivo approach, we exposed WT and Sirt3−/− mice to intratracheally instilled TiO2 or crocidolite asbestos for 3 wk, then evaluated mtDNA damage in primary isolated AT2 cells. As expected, Sirt3−/− mice had increased mtDNA damage under control conditions and, compared with WT, asbestos doubled the level of mtDNA damage in Sirt3−/− mice (0.075 vs. 0.15 lesions/10-kb frequency per fragment; P < 0.05). Collectively, these data show that loss of SIRT3 amplifies mtDNA damage under basal conditions and that asbestos fibers further enhance AT2 cell mtDNA damage.
DISCUSSION
AEC mitochondrial dysfunction and apoptosis are important in the pathogenesis of pulmonary fibrosis. We explored the role of SIRT3 in the pathobiology of lung fibrosis given that SIRT3-mediated deacetylation regulates mitochondrial function and integrity. Our results show that lung tissue from patients with IPF demonstrates decreased AT2 cell SIRT3 function. As previously observed (33, 35), minor changes in SIRT3 protein expression as a result of oxidative stress can be associated with large alterations in deacetylase activity; therefore, we sought to evaluate acetylation of the well-characterized SIRT3 target MnSODK68 as a surrogate of SIRT3 activity. We also show that AEC SIRT3 activity and expression are altered by oxidative stress, whereas gain and loss of function SIRT3 expression studies establish the role of SIRT3 in limiting AEC mitochondrial protein acetylation, mtDNA damage, and intrinsic apoptosis (as measured by CC-9 expression and DNA fragmentation ELISA). Finally, compared with WT mice, Sirt3−/− mice are more susceptible to pulmonary fibrosis, and this is associated with increased AT2 cell mtDNA damage and apoptosis. Collectively, these data support a novel role for AEC SIRT3 deficiency in the pathogenesis of lung fibrosis.
An important finding of our pilot human lung tissue study is that, compared with normal tissue, all 6 patients with fibrotic lung disease had marked increases in MnSODK68 acetylation, which is an established marker of SIRT3 dysfunction (33–35). Colocalization studies show MnSODK68 acetylation occurs primarily in SFPTC-positive cells, which establishes the prominent involvement of AT2 cells (Fig. 1). Because mitochondrial SIRT3 protein expression assessed by semiquantitative analysis of IHC samples is technically limited by the availability of suitable Abs (data not shown), and as we were primarily interested in evaluating SIRT3 activity, we focused our experiments in human lung tissues on MnSODK68 as a well-established marker of SIRT3 activity, as used by others (28, 33, 35, 49, 52). Our findings that show a loss of SIRT3 function in patients with IPF are in agreement with studies that have shown that SIRT3 is deficient in other fibrotic and age-related diseases, including hearing loss (53), neurodegenerative disease (24), metabolic syndrome (54, 55), cancer (56), cardiac hypertrophy/fibrosis (25, 37, 57), and systemic sclerosis (52). Our data in humans with IPF showing SIRT3 deficiency are in agreement with other studies that have shown that Sirt3−/− mice spontaneously develop fibrosis with age, including in the lungs, in part, as a result of enhanced activity of profibrotic TGF-β (31). Studies of skeletal muscle mtDNA from elderly and young patients, as well as 3 generations of women who share the same mtDNA sequences, have documented decreases in mtDNA copy number and SIRT3 protein expression and deacetylase function with age (58, 59). Our results demonstrating AT2 cell SIRT3 deacetylase deficiency in lung fibrosis may also contribute to the increased risk of lung cancers noted in patients with IPF and asbestosis (10, 13, 17). Of interest, SIRT3 can act as a tumor suppressor (27), and reduced SIRT3 function is regarded as a biomarker of poor cancer outcome in many (60–63), but not all, patients with cancer (64). These human data provide the rationale for further studies that will explore the role of SIRT3 in IPF and other fibrotic lung diseases (i.e., asbestosis), lung cancer, and the aging lung.
By using cultured AEC and transgenic murine lung fibrosis models, we provide several lines of evidence that implicate a causal role for deficient SIRT3 function in promoting lung fibrosis. First, we find that AEC SIRT3 activity and expression are altered by oxidative stress resulting from asbestos and H2O2 exposure. SIRT3 alterations reported here are in accord with other studies that involved oxidative stress in the liver (ionizing radiation) (65), heart (administration of doxorubicin, transverse aortic constriction, or ischemia-reperfusion injury) (25, 37, 66), and kidney (cisplatin) (67). Second, we use gain and loss of AEC SIRT3 expression studies to demonstrate the importance of SIRT3 in limiting AEC mitochondrial protein acetylation (i.e., total mitochondrial protein, MnSODK68, OGG1), mtDNA damage, and apoptosis. Our findings in AECs parallel those observed in other cell types as noted above. Finally, we demonstrate that mice that are deficient in SIRT3 show increased asbestos- and bleomycin-induced lung fibrosis compared with their WT counterparts that is associated with increased AT2 cell mtDNA damage and apoptosis. Our lung fibrosis data add to the accumulating evidence that Sirt3−/− mice, which do not display a fibrotic phenotype at age 8–12 wk when we study them, are more susceptible to organ fibrosis after environmental exposures, such as in this study, and spontaneously develop age-related diseases, including lung fibrosis with aging (31). Collectively, our findings suggest an innovative function of AEC SIRT3 deficiency in promoting AEC mtDNA damage and apoptosis that can promote lung fibrosis.
Although the mechanisms that underlie lung fibrosis in the setting of SIRT3 deficiency are not fully established, our studies identify several possibilities. First, we explored the role of mtDNA damage on the basis of our prior studies that suggested that mtDNA damage is crucial in promoting AEC apoptosis and lung fibrosis after asbestos exposure (21, 22) and because mitochondrial OGG1 is a SIRT3 deacetylation target that is important for mtDNA repair (37, 38). Our in vitro and in vivo AEC studies presented here demonstrate that SIRT3 function is critical for preserving AEC mtDNA integrity and reducing apoptosis. Of note, mice that are deficient in SIRT3 have AT2 cells with increased mtDNA damage at baseline, and this is augmented further by oxidative stress. Second, we explored the role of mitochondrial OGG1, a key SIRT3 deacetylase target that regulates mtDNA integrity via its role in mtDNA repair. Deacetylation of OGG1K338/K341 by SIRT3 maintains its BER activity in glioma, renal epithelial tumor, and cardiac cells (37, 38). Here, we demonstrate that decreased SIRT3 exposure owing to oxidative stress or SIRT3 silencing augments acetylation of OGG1. By using SIRT3-EE and silencing studies we show a direct relationship between AEC SIRT3 expression and deacetylation of OGG1K338/K341 (Fig. 3C, D), which we infer preserves AEC mtDNA integrity. Third, our findings that demonstrate increased AEC MnSODK68 acetylation, a well-established marker of SIRT3 deficiency (33, 35), provide a role for increased mitochondrial ROS in mediating AEC mtDNA damage and apoptosis in patients with IPF and asbestosis (7, 8, 10), which is in accordance with existing data that demonstrate the pathogenic role of mtROS in asbestos- and bleomycin-induced AEC injury and apoptosis (44, 68, 69).
There are some limitations in this study. We recognize that unraveling the direct effect of SIRT3 from indirect changes, especially in light of the diverse number of SIRT3 targets reported in the literature, is an ongoing challenge in the field of sirtuin research. We addressed this by using both in vitro and in vivo genetic approaches to better understand the effect of SIRT3 deficiency on AEC mtDNA damage and apoptosis, important end points in the pathogenesis of lung fibrosis. Although the present study, along with our earlier work, implicates mitochondrial OGG1 and MnSOD as crucial SIRT3 targets in the pathogenesis of AEC mtDNA damage and apoptosis, we recognize that SIRT3 may function via altering other mitochondrial targets (e.g., IDH2, Ku70, OPAI) or cell types (e.g., fibroblasts, macrophages) (36, 52, 70), and that other sirtuin family members, especially SIRT1 and SIRT6 (71), may play important roles in the pathogenesis of pulmonary fibrosis (23, 24, 26, 72). It is possible that the differences in SIRT3 expression and MnSODK68 acetylation noted in our human lung IHC pilot experiments may be a result of other factors, such as age, smoking status, or other medical comorbidities, and future prospective studies should address this question through evaluation of a larger sample size of patients. However, the finding of altered SIRT3 expression and acetylation in the lungs of patients with scleroderma-associated interstitial lung disease supports a critical role of SIRT3 in fibrotic lung disease (52). Because of the uncertain nature of altered SIRT3 expression and function in malignant cells, we used both transformed cell lines (A549 and MLE-12) and primary AT2 cells to assess the generalizability of our findings, which were corroborated in human IPF lungs (Fig. 1). Although Sirt3−/− mice demonstrate an increased susceptibility to fibrosis of multiple organs with age (37), we focused on young mice to avoid the confounding effect of aging. Furthermore, the critical lysine residues on OGG1 that are deacetylated by SIRT3 await further structure-function studies, a subject of ongoing analysis in our lab. We feel, however, that these limitations do not undermine our primary conclusions that implicate SIRT3 deficiency in promoting oxidant-induced AEC mtDNA damage and apoptosis that mediate lung fibrosis.
In summary, we demonstrate that AT2 cells from patients with advanced lung fibrosis have increased acetylation of the known SIRT3 target MnSODK68, which suggests that SIRT3 deficiency occurs in human IPF. We show that loss of SIRT3 augments oxidant-induced mtDNA damage, mitochondrial protein acetylation, and apoptosis in a variety of AECs, and that these deleterious effects are mitigated by SIRT3-EE. Finally, mice that are deficient in SIRT3 are more susceptible to asbestos- and bleomycin-induced lung fibrosis, and their AT2 cells show increased mtDNA damage compared with their WT counterparts. As illustrated in our hypothetical model (Fig. 7D), we reason that the OGG1–MnSOD–SIRT3 axis is crucial for maintaining the AT2 cell mtDNA integrity necessary for preventing apoptosis that drives lung fibrotic signaling and may lead to potential therapeutic targets for IPF and asbestosis.
ACKNOWLEDGMENTS
This work was supported by U.S. Veteran’s Administration Merit Grant 2I01BX000786-05A2 (to D.W.K.), U.S. National Institutes of Health (NIH) National Institute of Environmental Health Sciences Grant R01-ES020357 (to D.W.K.), and NIH National Heart, Lung, and Blood Institute Training Grant 2T32HL076139-11A1 (to R.P.J.). D.G. was supported by NIH grants 2R01CA152601-A1, 1R01CA152799-01A1, 1R01CA168292-01A1, and 1R01CA214025-01; the Avon Foundation for Breast Cancer Research; the Lynn Sage Cancer Research Foundation; the Zell Family Foundation; and the Chicago Biomedical Consortium; as well as support from the Searle Funds at The Chicago Community Trust.
Glossary
- AEC
alveolar epithelial cell
- AT2
alveolar type II cell
- BAD
bronchoalveolar duct
- BER
base excision repair
- CC-9
cleaved caspase 9
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- IHC
immunohistochemical
- IPF
idiopathic pulmonary fibrosis
- MnSOD
manganese superoxide dismutase
- OGG1
8-oxoguanine DNA glycosylase 1
- ROS
reactive oxygen species
- SFPTC
surfactant protein C
- siRNA
small interfering RNA
- SIRT3
sirtuin 3
- SIRT3-EE
SIRT3 enforced expression
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
R. P. Jablonski, S.-J. Kim, P. Cheresh, A. Yeldandi, D. Gius, G. R. S. Budinger, and D. W. Kamp conceived of and designed the research; R. P. Jablonski, S.-J. Kim, P. Cheresh, D. B. Williams, L. Morales-Nebreda, Y. Cheng, A. Yeldandi, S. Bhorade, A. Pardo, M. Selman, K. Ridge, D. Gius, G. R. S. Budinger, and D. W. Kamp acquired, analyzed, and interpreted data; and R. P. Jablonski, S.-J. Kim, and D. W. Kamp drafted the manuscript for important intellectual content.
REFERENCES
- 1.Olson A. L., Swigris J. J., Lezotte D. C., Norris J. M., Wilson C. G., Brown K. K. (2007) Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am. J. Respir. Crit. Care Med. 176, 277–284 [DOI] [PubMed] [Google Scholar]
- 2.Raghu G., Rochwerg B., Zhang Y., Garcia C. A., Azuma A., Behr J., Brozek J. L., Collard H. R., Cunningham W., Homma S., Johkoh T., Martinez F. J., Myers J., Protzko S. L., Richeldi L., Rind D., Selman M., Theodore A., Wells A. U., Hoogsteden H., Schünemann H. J.; American Thoracic Society; European Respiratory society; Japanese Respiratory Society; Latin American Thoracic Association (2015) An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am. J. Respir. Crit. Care Med. 192, e3–e19 [DOI] [PubMed] [Google Scholar]
- 3.King T. E. Jr., Bradford W. Z., Castro-Bernardini S., Fagan E. A., Glaspole I., Glassberg M. K., Gorina E., Hopkins P. M., Kardatzke D., Lancaster L., Lederer D. J., Nathan S. D., Pereira C. A., Sahn S. A., Sussman R., Swigris J. J., Noble P. W.; ASCEND Study Group (2014) A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 370, 2083–2092 [DOI] [PubMed] [Google Scholar]
- 4.Richeldi L., du Bois R. M., Raghu G., Azuma A., Brown K. K., Costabel U., Cottin V., Flaherty K. R., Hansell D. M., Inoue Y., Kim D. S., Kolb M., Nicholson A. G., Noble P. W., Selman M., Taniguchi H., Brun M., Le Maulf F., Girard M., Stowasser S., Schlenker-Herceg R., Disse B., Collard H. R.; INPULSIS Trial Investigators (2014) Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 370, 2071–2082 [DOI] [PubMed] [Google Scholar]
- 5.Kuwano K., Kunitake R., Kawasaki M., Nomoto Y., Hagimoto N., Nakanishi Y., Hara N. (1996) P21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 154, 477–483 [DOI] [PubMed] [Google Scholar]
- 6.Korfei M., Ruppert C., Mahavadi P., Henneke I., Markart P., Koch M., Lang G., Fink L., Bohle R. M., Seeger W., Weaver T. E., Guenther A. (2008) Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 178, 838–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel A. S., Song J. W., Chu S. G., Mizumura K., Osorio J. C., Shi Y., El-Chemaly S., Lee C. G., Rosas I. O., Elias J. A., Choi A. M., Morse D. (2015) Epithelial cell mitochondrial dysfunction and PINK1 are induced by transforming growth factor-beta1 in pulmonary fibrosis. PLoS One 10, e0121246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selman M., Pardo A. (2014) Revealing the pathogenic and aging-related mechanisms of the enigmatic idiopathic pulmonary fibrosis. An integral model. Am. J. Respir. Crit. Care Med. 189, 1161–1172 [DOI] [PubMed] [Google Scholar]
- 9.Brody A. R., Overby L. H. (1989) Incorporation of tritiated thymidine by epithelial and interstitial cells in bronchiolar-alveolar regions of asbestos-exposed rats. Am. J. Pathol. 134, 133–140 [PMC free article] [PubMed] [Google Scholar]
- 10.Liu G., Cheresh P., Kamp D. W. (2013) Molecular basis of asbestos-induced lung disease. Annu. Rev. Pathol. 8, 161–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uhal B. D., Nguyen H. (2013) The Witschi Hypothesis revisited after 35 years: genetic proof from SP-C BRICHOS domain mutations. Am. J. Physiol. Lung Cell. Mol. Physiol. 305, L906–L911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bueno M., Lai Y. C., Romero Y., Brands J., St Croix C. M., Kamga C., Corey C., Herazo-Maya J. D., Sembrat J., Lee J. S., Duncan S. R., Rojas M., Shiva S., Chu C. T., Mora A. L. (2015) PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J. Clin. Invest. 125, 521–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thannickal V. J., Murthy M., Balch W. E., Chandel N. S., Meiners S., Eickelberg O., Selman M., Pardo A., White E. S., Levy B. D., Busse P. J., Tuder R. M., Antony V. B., Sznajder J. I., Budinger G. R. (2015) Blue journal conference. Aging and susceptibility to lung disease. Am. J. Respir. Crit. Care Med. 191, 261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mossman B. T., Lippmann M., Hesterberg T. W., Kelsey K. T., Barchowsky A., Bonner J. C. (2011) Pulmonary endpoints (lung carcinomas and asbestosis) following inhalation exposure to asbestos. J. Toxicol. Environ. Health B Crit. Rev. 14, 76–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.López-Otín C., Blasco M. A., Partridge L., Serrano M., Kroemer G. (2013) The hallmarks of aging. Cell 153, 1194–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fingerlin T. E., Murphy E., Zhang W., Peljto A. L., Brown K. K., Steele M. P., Loyd J. E., Cosgrove G. P., Lynch D., Groshong S., Collard H. R., Wolters P. J., Bradford W. Z., Kossen K., Seiwert S. D., du Bois R. M., Garcia C. K., Devine M. S., Gudmundsson G., Isaksson H. J., Kaminski N., Zhang Y., Gibson K. F., Lancaster L. H., Cogan J. D., Mason W. R., Maher T. M., Molyneaux P. L., Wells A. U., Moffatt M. F., Selman M., Pardo A., Kim D. S., Crapo J. D., Make B. J., Regan E. A., Walek D. S., Daniel J. J., Kamatani Y., Zelenika D., Smith K., McKean D., Pedersen B. S., Talbert J., Kidd R. N., Markin C. R., Beckman K. B., Lathrop M., Schwarz M. I., Schwartz D. A. (2013) Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat. Genet. 45, 613–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang S. X., Jaurand M. C., Kamp D. W., Whysner J., Hei T. K. (2011) Role of mutagenicity in asbestos fiber-induced carcinogenicity and other diseases. J. Toxicol. Environ. Health B Crit. Rev. 14, 179–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos J. H., Hunakova L., Chen Y., Bortner C., Van Houten B. (2003) Cell sorting experiments link persistent mitochondrial DNA damage with loss of mitochondrial membrane potential and apoptotic cell death. J. Biol. Chem. 278, 1728–1734 [DOI] [PubMed] [Google Scholar]
- 19.De Souza-Pinto N. C., Bohr V. A. (2002) The mitochondrial theory of aging: involvement of mitochondrial DNA damage and repair. Int. Rev. Neurobiol. 53, 519–534 [DOI] [PubMed] [Google Scholar]
- 20.Bohr V. A., Stevnsner T., de Souza-Pinto N. C. (2002) Mitochondrial DNA repair of oxidative damage in mammalian cells. Gene 286, 127–134 [DOI] [PubMed] [Google Scholar]
- 21.Kim S. J., Cheresh P., Williams D., Cheng Y., Ridge K., Schumacker P. T., Weitzman S., Bohr V. A., Kamp D. W. (2014) Mitochondria-targeted Ogg1 and aconitase-2 prevent oxidant-induced mitochondrial DNA damage in alveolar epithelial cells. J. Biol. Chem. 289, 6165–6176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheresh P., Morales-Nebreda L., Kim S. J., Yeldandi A., Williams D. B., Cheng Y., Mutlu G. M., Budinger G. R., Ridge K., Schumacker P. T., Bohr V. A., Kamp D. W. (2015) Asbestos-induced pulmonary fibrosis is augmented in 8-oxoguanine DNA glycosylase knockout mice. Am. J. Respir. Cell Mol. Biol. 52, 25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y., Fu L. L., Wen X., Wang X. Y., Liu J., Cheng Y., Huang J. (2014) Sirtuin-3 (SIRT3), a therapeutic target with oncogenic and tumor-suppressive function in cancer. Cell Death Dis. 5, e1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kincaid B., Bossy-Wetzel E. (2013) Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front. Aging Neurosci. 5, 48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sundaresan N. R., Gupta M., Kim G., Rajamohan S. B., Isbatan A., Gupta M. P. (2009) Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J. Clin. Invest. 119, 2758–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shulga N., Pastorino J. G. (2010) Ethanol sensitizes mitochondria to the permeability transition by inhibiting deacetylation of cyclophilin-D mediated by sirtuin-3. J. Cell Sci. 123, 4117–4127 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Kim H. S., Patel K., Muldoon-Jacobs K., Bisht K. S., Aykin-Burns N., Pennington J. D., van der Meer R., Nguyen P., Savage J., Owens K. M., Vassilopoulos A., Ozden O., Park S. H., Singh K. K., Abdulkadir S. A., Spitz D. R., Deng C. X., Gius D. (2010) SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell 17, 41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao R., Coleman M. C., Pennington J. D., Ozden O., Park S. H., Jiang H., Kim H. S., Flynn C. R., Hill S., Hayes McDonald W., Olivier A. K., Spitz D. R., Gius D. (2010) Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol. Cell 40, 893–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li S., Banck M., Mujtaba S., Zhou M. M., Sugrue M. M., Walsh M. J. (2010) p53-induced growth arrest is regulated by the mitochondrial SirT3 deacetylase. PLoS One 5, e10486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawamura Y., Uchijima Y., Horike N., Tonami K., Nishiyama K., Amano T., Asano T., Kurihara Y., Kurihara H. (2010) Sirt3 protects in vitro-fertilized mouse preimplantation embryos against oxidative stress-induced p53-mediated developmental arrest. J. Clin. Invest. 120, 2817–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sundaresan N. R., Bindu S., Pillai V. B., Samant S., Pan Y., Huang J. Y., Gupta M., Nagalingam R. S., Wolfgeher D., Verdin E., Gupta M. P. (2015) SIRT3 blocks aging-associated tissue fibrosis in mice by deacetylating and activating glycogen synthase kinase 3β. Mol. Cell. Biol. 36, 678–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lombard D. B., Alt F. W., Cheng H. L., Bunkenborg J., Streeper R. S., Mostoslavsky R., Kim J., Yancopoulos G., Valenzuela D., Murphy A., Yang Y., Chen Y., Hirschey M. D., Bronson R. T., Haigis M., Guarente L. P., Farese R. V. Jr., Weissman S., Verdin E., Schwer B. (2007) Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol. Cell. Biol. 27, 8807–8814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y., Zhang J., Lin Y., Lei Q., Guan K. L., Zhao S., Xiong Y. (2011) Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 12, 534–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tao R., Vassilopoulos A., Parisiadou L., Yan Y., Gius D. (2014) Regulation of MnSOD enzymatic activity by Sirt3 connects the mitochondrial acetylome signaling networks to aging and carcinogenesis. Antioxid. Redox Signal. 20, 1646–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu J., Cheng K., Zhang B., Xu H., Cao Y., Guo F., Feng X., Xia Q. (2015) Novel mechanisms for superoxide-scavenging activity of human manganese superoxide dismutase determined by the K68 key acetylation site. Free Radic. Biol. Med. 85, 114–126 [DOI] [PubMed] [Google Scholar]
- 36.Sosulski M. L., Gongora R., Feghali-Bostwick C., Lasky J. A., Sanchez C. G. (2016) Sirtuin 3 deregulation promotes pulmonary fibrosis. J. Gerontol. A Biol. Sci. Med. Sci. pii, glw151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pillai V. B., Bindu S., Sharp W. W., Fang Y. H., Kim G. H., Gupta M., Samant S., Gupta M. P. (2016) Sirt3 protects mitochondrial DNA damage and blocks the development of doxorubicin-induced cardiomyopathy in mice. Am. J. Physiol. Heart Circ. Physiol. 310, H962–H972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng Y., Ren X., Gowda A. S., Shan Y., Zhang L., Yuan Y. S., Patel R., Wu H., Huber-Keener K., Yang J. W., Liu D., Spratt T. E., Yang J. M. (2013) Interaction of Sirt3 with OGG1 contributes to repair of mitochondrial DNA and protects from apoptotic cell death under oxidative stress. Cell Death Dis. 4, e731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raghu G., Collard H. R., Egan J. J., Martinez F. J., Behr J., Brown K. K., Colby T. V., Cordier J. F., Flaherty K. R., Lasky J. A., Lynch D. A., Ryu J. H., Swigris J. J., Wells A. U., Ancochea J., Bouros D., Carvalho C., Costabel U., Ebina M., Hansell D. M., Johkoh T., Kim D. S., King T. E. Jr., Kondoh Y., Myers J., Müller N. L., Nicholson A. G., Richeldi L., Selman M., Dudden R. F., Griss B. S., Protzko S. L., Schünemann H. J.; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis (2011) An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 183, 788–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panduri V., Weitzman S. A., Chandel N., Kamp D. W. (2003) The mitochondria-regulated death pathway mediates asbestos-induced alveolar epithelial cell apoptosis. Am. J. Respir. Cell Mol. Biol. 28, 241–248 [DOI] [PubMed] [Google Scholar]
- 41.Urich D., Soberanes S., Burgess Z., Chiarella S. E., Ghio A. J., Ridge K. M., Kamp D. W., Chandel N. S., Mutlu G. M., Budinger G. R. (2009) Proapoptotic Noxa is required for particulate matter-induced cell death and lung inflammation. FASEB J. 23, 2055–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fattman C. L., Tan R. J., Tobolewski J. M., Oury T. D. (2006) Increased sensitivity to asbestos-induced lung injury in mice lacking extracellular superoxide dismutase. Free Radic. Biol. Med. 40, 601–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murthy S., Adamcakova-Dodd A., Perry S. S., Tephly L. A., Keller R. M., Metwali N., Meyerholz D. K., Wang Y., Glogauer M., Thorne P. S., Carter A. B. (2009) Modulation of reactive oxygen species by Rac1 or catalase prevents asbestos-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L846–L855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panduri V., Liu G., Surapureddi S., Kondapalli J., Soberanes S., de Souza-Pinto N. C., Bohr V. A., Budinger G. R., Schumacker P. T., Weitzman S. A., Kamp D. W. (2009) Role of mitochondrial hOGG1 and aconitase in oxidant-induced lung epithelial cell apoptosis. Free Radic. Biol. Med. 47, 750–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corti M., Brody A. R., Harrison J. H. (1996) Isolation and primary culture of murine alveolar type II cells. Am. J. Respir. Cell Mol. Biol. 14, 309–315 [DOI] [PubMed] [Google Scholar]
- 46.Beck J. M., Preston A. M., Wilcoxen S. E., Morris S. B., Sturrock A., Paine R. III (2009) Critical roles of inflammation and apoptosis in improved survival in a model of hyperoxia-induced acute lung injury in Pneumocystis murina-infected mice. Infect. Immun. 77, 1053–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mercer P. F., Johns R. H., Scotton C. J., Krupiczojc M. A., Königshoff M., Howell D. C., McAnulty R. J., Das A., Thorley A. J., Tetley T. D., Eickelberg O., Chambers R. C. (2009) Pulmonary epithelium is a prominent source of proteinase-activated receptor-1-inducible CCL2 in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 179, 414–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santos J. H., Mandavilli B. S., Van Houten B. (2002) Measuring oxidative mtDNA damage and repair using quantitative PCR. Methods Mol. Biol. 197, 159–176 [DOI] [PubMed] [Google Scholar]
- 49.Ozden O., Park S. H., Wagner B. A., Yong Song H., Zhu Y., Vassilopoulos A., Jung B., Buettner G. R., Gius D. (2014) SIRT3 deacetylates and increases pyruvate dehydrogenase activity in cancer cells. Free Radic. Biol. Med. 76, 163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lam A. P., Herazo-Maya J. D., Sennello J. A., Flozak A. S., Russell S., Mutlu G. M., Budinger G. R., DasGupta R., Varga J., Kaminski N., Gottardi C. J. (2014) Wnt coreceptor Lrp5 is a driver of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 190, 185–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang L. Y., Overby L. H., Brody A. R., Crapo J. D. (1988) Progressive lung cell reactions and extracellular matrix production after a brief exposure to asbestos. Am. J. Pathol. 131, 156–170 [PMC free article] [PubMed] [Google Scholar]
- 52.Akamata K., Wei J., Bhattacharyya M., Cheresh P., Bonner M. Y., Arbiser J. L., Raparia K., Gupta M. P., Kamp D. W., Varga J. (2016) SIRT3 is attenuated in systemic sclerosis skin and lungs, and its pharmacologic activation mitigates organ fibrosis. Oncotarget 7, 69321–69336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Someya S., Yu W., Hallows W. C., Xu J., Vann J. M., Leeuwenburgh C., Tanokura M., Denu J. M., Prolla T. A. (2010) Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143, 802–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rato L., Duarte A. I., Tomás G. D., Santos M. S., Moreira P. I., Socorro S., Cavaco J. E., Alves M. G., Oliveira P. F. (2014) Pre-diabetes alters testicular PGC1-α/SIRT3 axis modulating mitochondrial bioenergetics and oxidative stress. Biochim. Biophys. Acta 1837, 335–344 [DOI] [PubMed] [Google Scholar]
- 55.Hirschey M. D., Shimazu T., Jing E., Grueter C. A., Collins A. M., Aouizerat B., Stančáková A., Goetzman E., Lam M. M., Schwer B., Stevens R. D., Muehlbauer M. J., Kakar S., Bass N. M., Kuusisto J., Laakso M., Alt F. W., Newgard C. B., Farese R. V. Jr., Kahn C. R., Verdin E. (2011) SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol. Cell 44, 177–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haigis M. C., Deng C. X., Finley L. W., Kim H. S., Gius D. (2012) SIRT3 is a mitochondrial tumor suppressor: a scientific tale that connects aberrant cellular ROS, the Warburg effect, and carcinogenesis. Cancer Res. 72, 2468–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pillai V. B., Samant S., Sundaresan N. R., Raghuraman H., Kim G., Bonner M. Y., Arbiser J. L., Walker D. I., Jones D. P., Gius D., Gupta M. P. (2015) Honokiol blocks and reverses cardiac hypertrophy in mice by activating mitochondrial Sirt3. Nat. Commun. 6, 6656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hebert S. L., Marquet-de Rougé P., Lanza I. R., McCrady-Spitzer S. K., Levine J. A., Middha S., Carter R. E., Klaus K. A., Therneau T. M., Highsmith E. W., Nair K. S. (2015) Mitochondrial aging and physical decline: insights from three generations of women. J. Gerontol. A Biol. Sci. Med. Sci. 70, 1409–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joseph A. M., Adhihetty P. J., Buford T. W., Wohlgemuth S. E., Lees H. A., Nguyen L. M., Aranda J. M., Sandesara B. D., Pahor M., Manini T. M., Marzetti E., Leeuwenburgh C. (2012) The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell 11, 801–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mahjabeen I., Kayani M. A. (2016) Loss of mitochondrial tumor suppressor genes expression is associated with unfavorable clinical outcome in head and neck squamous cell carcinoma: data from retrospective study. PLoS One 11, e0146948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang B., Fu X., Shao L., Ding Y., Zeng D. (2014) Aberrant expression of SIRT3 is conversely correlated with the progression and prognosis of human gastric cancer. Biochem. Biophys. Res. Commun. 443, 156–160 [DOI] [PubMed] [Google Scholar]
- 62.Zhang C. Z., Liu L., Cai M., Pan Y., Fu J., Cao Y., Yun J. (2012) Low SIRT3 expression correlates with poor differentiation and unfavorable prognosis in primary hepatocellular carcinoma. PLoS One 7, e51703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McGlynn L. M., McCluney S., Jamieson N. B., Thomson J., MacDonald A. I., Oien K., Dickson E. J., Carter C. R., McKay C. J., Shiels P. G. (2015) SIRT3 & SIRT7: potential novel biomarkers for determining outcome in pancreatic cancer patients. PLoS One 10, e0131344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan S. M., Han X., Han P. J., Chen H. M., Huang L. Y., Li Y. (2014) SIRT3 is a novel prognostic biomarker for esophageal squamous cell carcinoma. Med. Oncol. 31, 103 [DOI] [PubMed] [Google Scholar]
- 65.Coleman M. C., Olivier A. K., Jacobus J. A., Mapuskar K. A., Mao G., Martin S. M., Riley D. P., Gius D., Spitz D. R. (2014) Superoxide mediates acute liver injury in irradiated mice lacking sirtuin 3. Antioxid. Redox Signal. 20, 1423–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Porter G. A., Urciuoli W. R., Brookes P. S., Nadtochiy S. M. (2014) SIRT3 deficiency exacerbates ischemia-reperfusion injury: implication for aged hearts. Am. J. Physiol. Heart Circ. Physiol. 306, H1602–H1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morigi M., Perico L., Rota C., Longaretti L., Conti S., Rottoli D., Novelli R., Remuzzi G., Benigni A. (2015) Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J. Clin. Invest. 125, 715–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim S. J., Cheresh P., Jablonski R. P., Morales-Nebreda L., Cheng Y., Hogan E., Yeldandi A., Chi M., Piseaux R., Ridge K., Michael Hart C., Chandel N., Scott Budinger G. R., Kamp D. W. (2016) Mitochondrial catalase overexpressed transgenic mice are protected against lung fibrosis in part via preventing alveolar epithelial cell mitochondrial DNA damage. Free Radic. Biol. Med. 101, 482–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brar S. S., Meyer J. N., Bortner C. D., Van Houten B., Martin W. J. II (2012) Mitochondrial DNA-depleted A549 cells are resistant to bleomycin. Am. J. Physiol. Lung Cell. Mol. Physiol. 303, L413–L424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bindu S., Pillai V. B., Kanwal A., Samant S., Mutlu G. M., Verdin E., Dulin N., Gupta M. P. (2017) SIRT3 blocks myofibroblast differentiation and pulmonary fibrosis by preventing mitochondrial DNA damage. Am. J. Physiol. Lung Cell. Mol. Physiol. 312, L68–L78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Minagawa S., Araya J., Numata T., Nojiri S., Hara H., Yumino Y., Kawaishi M., Odaka M., Morikawa T., Nishimura S. L., Nakayama K., Kuwano K. (2011) Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-β-induced senescence of human bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 300, L391–L401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sundaresan N. R., Samant S. A., Pillai V. B., Rajamohan S. B., Gupta M. P. (2008) SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol. Cell. Biol. 28, 6384–6401 [DOI] [PMC free article] [PubMed] [Google Scholar]