Abstract
Aedes aegypti has 2 genes encoding xanthine dehydrogenase (XDH). We analyzed XDH1 and XDH2 gene expression by real-time quantitative PCR in tissues from sugar- and blood-fed females. Differential XDH1 and XDH2 gene expression was observed in tissues dissected throughout a time course. We next exposed females to blood meals supplemented with allopurinol, a well-characterized XDH inhibitor. We also tested the effects of injecting double-stranded RNA (dsRNA) against XDH1, XDH2, or both. Disruption of XDH by allopurinol or XDH1 by RNA interference significantly affected mosquito survival, causing a disruption in blood digestion, excretion, oviposition, and reproduction. XDH1-deficient mosquitoes showed a persistence of serine proteases in the midgut at 48 h after blood feeding and a reduction in the uptake of vitellogenin by the ovaries. Surprisingly, analysis of the fat body from dsRNA-XDH1-injected mosquitoes fell into 2 groups: one group was characterized by a reduction of the XDH1 transcript, whereas the other group was characterized by an up-regulation of several transcripts, including XDH1, glutamine synthetase, alanine aminotransferase, catalase, superoxide dismutase, ornithine decarboxylase, glutamate receptor, and ammonia transporter. Our data demonstrate that XDH1 plays an essential role and that XDH1 has the potential to be used as a metabolic target for Ae. aegypti vector control.—Isoe, J., Petchampai, N., Isoe, Y. E., Co, K., Mazzalupo, S., Scaraffia, P. Y. Xanthine dehydrogenase-1 silencing in Aedes aegypti mosquitoes promotes a blood feeding–induced adulticidal activity.
Keywords: ammonia, antioxidant genes, gene silencing, metabolic target
Female Aedes aegypti mosquitoes are responsible for the propagation of diseases of public health significance: yellow fever, dengue, chikungunya, and Zika virus (1–6). The unfortunate convergence of a lack of vaccines for dengue, chikungunya, and Zika viruses, the increase of global disease distribution due to climate change (7–9), and an increase in travel clearly demand better mosquito vector control strategies.
Ae. aegypti is an anautogenous mosquito species that requires a blood source to secure nutrients necessary for egg production. During blood meal digestion, only a small percentage of amino acids is retained for follicle development and maternal reserves, whereas the majority of amino acids are oxidized for metabolic needs and excreted as CO2 or other waste (10, 11). One of the by-products of blood digestion is ammonia, defined here as NH3, NH4+, or a combination of both. Previous studies have demonstrated that Ae. aegypti females have evolved strategies to efficiently detoxify ammonia via multiple metabolic pathways (12–18). Nevertheless, the interaction and regulation of these pathways remain poorly understood.
We have previously reported that silencing of arginase and/or urate oxidase reduces the expression of genes involved in ammonia metabolism, including xanthine dehydrogenase (XDH) -1 and -2, but does not affect the expression of pyrroline-5-carboxylate synthase and alanine aminotransferase (ALT)-1 and -2 (19). Chemical inhibition of ALT by high doses of l-cycloserine impairs motor activity and decreases Ae. aegypti survival (20), whereas reduced levels of ALT1 or ALT2 by RNA interference (RNAi) slightly impair motor activity without affecting mosquito survival. Silencing of ALT also causes a massive but temporary increase of uric acid in the midgut and a delay in digestion, excretion, and oviposition with a significant reduction in egg production (21). Additionally, knockdown of ALT1 or ALT2 causes a concomitant increase in the transcript levels of both the ammonia transporter Rhesus 50 glycoprotein (Rh50-1) and XDH1, possibly to avoid cell toxicity.
To further investigate the importance of XDH during mosquito nitrogen metabolism, we analyzed the effect of XDH inhibition on overall mosquito fitness using chemical and genetic approaches. We found that silencing XDH1 affects blood-fed mosquito survival by severely disrupting physiologic process including digestion, excretion, and reproduction. A decrease of XDH1 function using either a pharmacological agent or RNAi inhibits uric acid production and excretion, and impairs the antioxidant capacity of blood-fed females. Because depletion of XDH1 activity is lethal to blood-fed mosquitoes, researchers could target XDH1 and nitrogen metabolism for controlling populations of Ae. aegypti mosquitoes, which are vectors of public health threats.
MATERIALS AND METHODS
Reagents and antibodies
Bovine blood was obtained from Pel-Freeze Biologicals (Rogers, AR, USA). A uric acid kit was obtained from Pointe Scientific (Canton, MI, USA). Allopurinol, uric acid standard, pyridine, potassium hydroxide, sodium dithionite, sodium hydroxide, ATP, and custom-made primers were purchased from Sigma-Aldrich (St. Louis, MO, USA). Trizol reagent was ordered from Life Technologies (Carlsbad, CA, USA), reverse transcriptase and oligo-(dT)20 primer from Promega (Madison, WI, USA), and the reagents for real-time quantitative PCR (qPCR) from Quanta Biosciences (Gaithersburg, MD, USA). A rabbit polyclonal anti-XDH1 primary antibody was produced against Ae. aegypti peptide sequence VSSDQPNHDPIRRP through custom antibody services from GenScript Biotech (Piscataway Township, NJ, USA). This anti-XDH1 antibody detects only XDH1 in Ae. aegypti. Rabbit polyclonal antibodies against serine digestive proteases, 5G1 (AaSPVI), CxLT (AaSPII), and LT (AaLT) were provided by R. L. Miesfeld (University of Arizona, Tucson, AZ, USA). Anti-vitellogenin (Vg) antibody was provided by A. Raikhel (University of California, Riverside, Riverside, CA, USA). Anti-GAPDH antibody was obtained from Cell Signaling Technology (Danvers, MA, USA), and anti-α-tubulin antibody was purchased from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA, USA). Secondary antibodies IRDye 800CW goat anti-rabbit, IRDye 800CW goat anti-mouse, and IRDye 680RD donkey anti-mouse IgG were purchased from Li-Cor Biosciences (Lincoln, NE, USA).
Mosquitoes and blood feeding procedure
Ae. aegypti (NIH-Rockefeller strain) mosquitoes were maintained in Percival Intellus I-41VL incubators (Percival Scientific, Perry, IA, USA) and in a Caron 6015 Insect Growth Chamber connected to a Caron CRSY 102 condensate recirculating system (Caron Products and Services, Marietta, OH, USA) at 28°C and 75% relative humidity with a light–dark cycle of 16:8 h. Mosquitoes were reared as previously described (19). Females 3 to 4 d old were allowed to feed on either a bovine blood meal or a bovine blood meal supplemented with 3 mM allopurinol (the maximal concentration soluble in blood) for 15 min via an artificial blood feeder connected to a 37°C water bath. Only females that fed to repletion were used for experiments. All females were maintained on 3% sucrose before and after blood feeding. Experiments were replicated at least 3 times with 3 separate cohorts of mosquitoes.
Survival observations, oviposition, and egg production
Survival, oviposition, and fecundity were recorded as previously reported (21). Oviposition sites were provided for 168 h. Eggs from individual mosquitoes were counted using an optical microscope (Nikon, Tokyo, Japan).
Uric acid and heme assays
Mosquito excreta were collected at 24, 36, 48, and 72 h post–blood meal (PBM), whereas malpighian tubules were dissected without a buffer solution at 6, 12, 24, 36, 48, 72, and 96 h PBM. Uric acid and heme concentrations were quantified as previously described (21) with a minor modification for the heme assay: samples were completely dried using an Eppendorf Vacufuge plus concentrator (Hauppauge, NY, USA) and reconstituted with 33 μl of 0.1% KOH.
Microinjection of double-stranded RNA and real-time qPCR assays
Two genes encoding xanthine dehydrogenase (XDH1 and XDH2) were previously identified by Basic Local Alignment Search Tool (BLAST; National Center for Biotechnology Information, Bethesda, MD, USA; https://blast.ncbi.nlm.nih.gov/Blast.cgi) searches in Ae. aegypti (19), while Anopheles gambiae and Drosophila melanogaster have single copies of XDH in their respective genomes. Ae. aegypti XDH1 with 2 splicing variants (AAEL002683 and AAEL010630) encodes a protein of either 1348 or 1028 amino acids, and it shares 76 and 65% identity to An. gambiae and D. melanogaster, respectively, whereas Ae. aegypti XDH2 (AAEL014493) encodes a protein of 1343 residues and shares 69 and 62% identity to An. gambiae and D. melanogaster, respectively, suggesting that XDH2 arose from a gene duplication in the lineage leading to the Aedes genus. The methods utilized for the synthesis of double-stranded RNA (dsRNA) against XDH1, XDH2, and firefly luciferase (FL) control, dsRNA microinjection and real-time qPCR analysis have been previously described (19, 21). The relative mRNA levels for XDH1, XDH2, ammonia transporter (Rh50-1), glutamine synthetase (GS1 and GS2), ALT1, ALT2, NMDA receptor (NMDAR), and antioxidant genes including catalase (CAT1 and CAT2), thioredoxin reductase (TRX), superoxide dismutase (SOD1 and SOD2), and ornithine decarboxylase (ODC) in response to XDH knockdown were measured in mosquito tissues at 24 h PBM. Oligonucleotide primer sequences used in this study are shown in Table 1.
TABLE 1.
Gene-specific primers used for RNAi and real-time qPCR
| Gene | Accession no. | Primer, 5′–3′ |
PCR size (bp) | |
|---|---|---|---|---|
| Forward | Reverse | |||
| RNAi | ||||
| XDH1 | AAEL002683 | CAATCTGGTGCTCTTATCCACGT | AGCGTGAACAGTCCGTATCCTTG | 568 |
| XDH2 | AAEL014493 | GGCGCTCTGGTACATGTATA | CCCTGCATGAAAGCACCCTC | 554 |
| FL/pGL3-basic vector | U47295 | AGCACTCTGATTGACAAATACGA | AGTTCACCGGCGTCATCGTC | 548 |
| Real-time qPCR | ||||
| Ribosomal protein S7 | AAEL009496 | ACCGCCGTCTACGATGCCA | ATGGTGGTCTGCTGGTTCTT | 131 |
| XDH1 | AAEL002683 | GCGATTGACATTGGACAGATCGA | CCAGGAATGTCGGCGAAACC | 146 |
| XDH2 | AAEL014493 | GTTATGGACATTGGCTCTAGCCT | CCTGGACCTCTCGATAGCAGTGT | 140 |
| GS1 | AAEL001887 | CCTCTATGCTGGAGTTGACT | CGCATCTGCTTGGTGGAGA | 133 |
| GS2 | AAEL013458 | GAGGAGTTTGGCATCGTTG | GGTCGTAGGCTCGGATATG | 181 |
| ALT1 | AAEL009875 | CAACTGCCGGAGAAGGCAAT | TGATAGGTTCCATCCTTCTGACC | 140 |
| ALT2 | AAEL009872 | TTCTATGCATTCCAGCTGTTAGAGCA | ACGCCCGGAACATGTCGAG | 148 |
| ODC | AAEL007880 | CGCAGCATGAACCTAGACGT | TGCTTGGCGTAGTCGAACAG | 119 |
| Ammonia transporter | AAEL008046 | CTGAGCGAGGAAGATATGCACGA | CTGTGAAGAGACCGCCTACGAT | 130 |
| Glutamate receptor | AAEL008587 | GCATCTCTAGCAATGCAACGACA | TCCAGGCCATGCTCTTTCTGG | 135 |
| CAT1 | AAEL013407-PA | GCTGCTCGTGAGCGCATGAT | CGGCGTCCAAAGTCAGCATC | 116 |
| CAT2 | AAEL013407-PB | GATGAGGCTGCTCGCAAGCG | CGTCCAAAGTCAGCATCGAC | 119 |
| TRX | AAEL010777 | GACGAGTGCGAAGATCTGGC | CATCTCCAGCTTCTGGTCGTT | 120 |
| SOD1 | AAEL000274 | AGCGGAGTCGCCAAGGTCG | GCCCAAATCATCAGGATCGG | 108 |
| SOD2 | AAEL006271 | GGAAATGCTGGAGGCAGACT | CCTAGCACAACATGAATGGGTCT | 113 |
T7 promoter sequence (5′-TAATACGACTCACTATAGGGAGA-3′) was added in 5′ of each RNAi primer.
Protein extraction and Western blot analysis procedures
Fat body, thorax, malpighian tubules, ovary, and midgut proteins from wild-type sugar-fed (SF) and blood-fed mosquitoes were extracted and subjected to 12% SDS-PAGE separation and Western blot analysis as previously described (19). The dilutions of the primary antibodies were as follows: AaXDH1 (1:2000), Aa5G1 (1:1000), AaCxLT (1:1000), AaLT (1:1000), AaVg (1:5000), GAPDH (1:300), and α-tubulin (1:1000). After washing, blots were incubated with the appropriate secondary antibodies (1:10,000 dilution) for 1 h. The immunoreactive protein bands were visualized with an Odyssey infrared imaging system (Li-Cor Biosciences).
Statistical analysis
Survival analysis was performed by the Kaplan-Meier method, and differences were detected by the Wilcoxon test. An unpaired Student’s t test was used to measure the difference in egg production with a Mann-Whitney nonparametric analysis because the data were not normally distributed. One-way ANOVA and an unpaired Student’s t test were used for analyzing gene expression and nitrogen waste data. A value of P < 0.05 was considered significant. All the statistical analyses were carried out by GraphPad Prism 6.0 software for Mac OS X (GraphPad Software, La Jolla, CA, USA).
RESULTS
Differential XDH1 and XDH2 gene expression after blood feeding
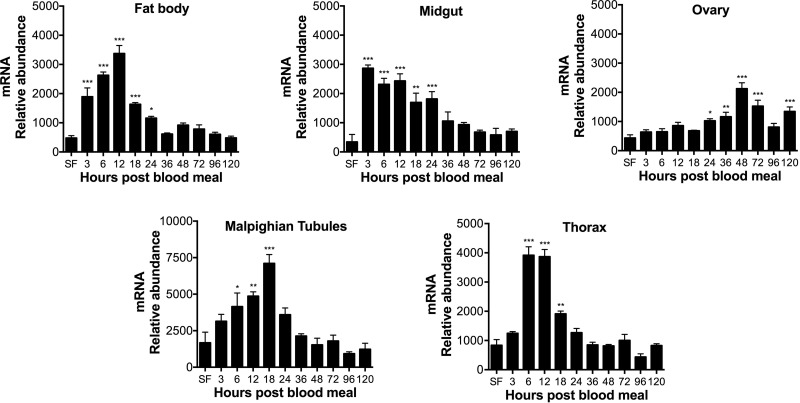
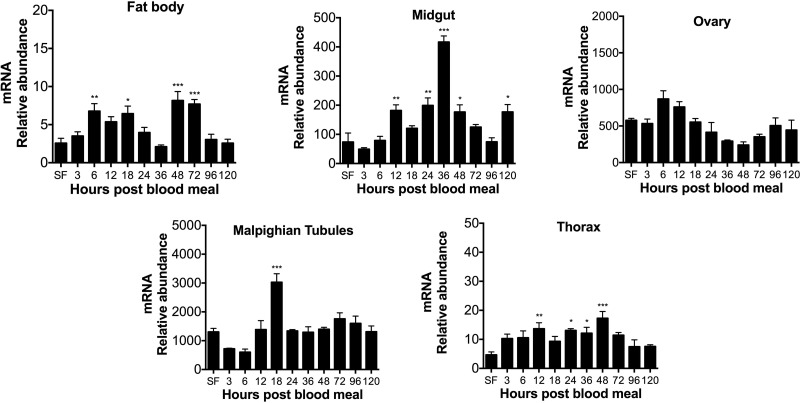
Previous work showed that XDH1 and XDH2 transcript levels decrease in the fat body of blood-fed Ae. aegypti females with a deficiency in arginase or/and urate oxidase (19). In contrast, XDH1 mRNA abundance increases in fat body, thorax, and malpighian tubules from mosquitoes with disrupted ALT expression (21). To investigate the expression pattern of genes encoding XDH in wild-type mosquitoes, we analyzed both XDH1 and XDH2 mRNA abundance by real-time qPCR in several tissues dissected before and after blood feeding. As shown in Fig. 1, XDH1 gene expression was immediately up-regulated after a blood meal in the fat body, midgut, and thorax. In the midgut, XDH1 transcript levels increased 10-fold by 3 h PBM compared to the sugar-fed mosquitoes and eventually returned to baseline levels over the 120-h time course. In malpighian tubules, the XDH1 mRNA levels increased after blood feeding, reaching a peak at 18 h PBM, while XDH1 transcript levels in the ovary were constitutively expressed during the first gonotrophic cycle, with a slight increase occurring between 24 and 48 h and a decrease thereafter. Transcripts for XDH2 were much less abundant compared to XDH1 in most of the tissues (Fig. 2). In the midgut and malpighian tubules, XDH2 mRNA levels increased over time, with peaks at 36 and 18 h, respectively. XDH2 gene expression in the fat body had peaks at 6, 18, 48, and 72 h PBM, whereas the XDH2 transcript levels in the thorax were significantly up-regulated at 12, 24, 36, and 48 h PBM. In the ovary, XDH2 gene expression did not change significantly during the first gonotrophic cycle.
Figure 1.
XDH1 mRNA expression patterns in Ae. aegypti tissues. Relative abundance of XDH1 transcript in mosquito tissues during first gonotrophic cycle. SF females were fed only on 3% sucrose. Blood-fed females were dissected at 3, 6, 12, 18, 24, 36, 48, 72, 96, and 120 h after blood feeding. XDH1 mRNA levels were normalized relative to mRNA levels of S7 ribosomal protein. Data are presented as means ± sem of 3 independent samples. Each cDNA replicate was prepared from pool of 10 mosquitoes. *P < 0.05, **P < 0.01, ***P < 0.001 compared to SF.
Figure 2.
XDH2 mRNA expression patterns in Ae. aegypti tissues. Relative abundance of XDH2 transcript in mosquito tissues during first gonotrophic cycle. SF females were fed only on 3% sucrose. Blood-fed females were dissected at 3, 6, 12, 18, 24, 36, 48, 72, 96, and 120 h after blood feeding. XDH2 mRNA levels were normalized relative to mRNA levels of S7 ribosomal protein. Data are presented as means ± sem of 3 independent samples. Each cDNA replicate was prepared from pool of 10 mosquitoes. *P < 0.05, **P < 0.01, ***P < 0.001 compared to SF.
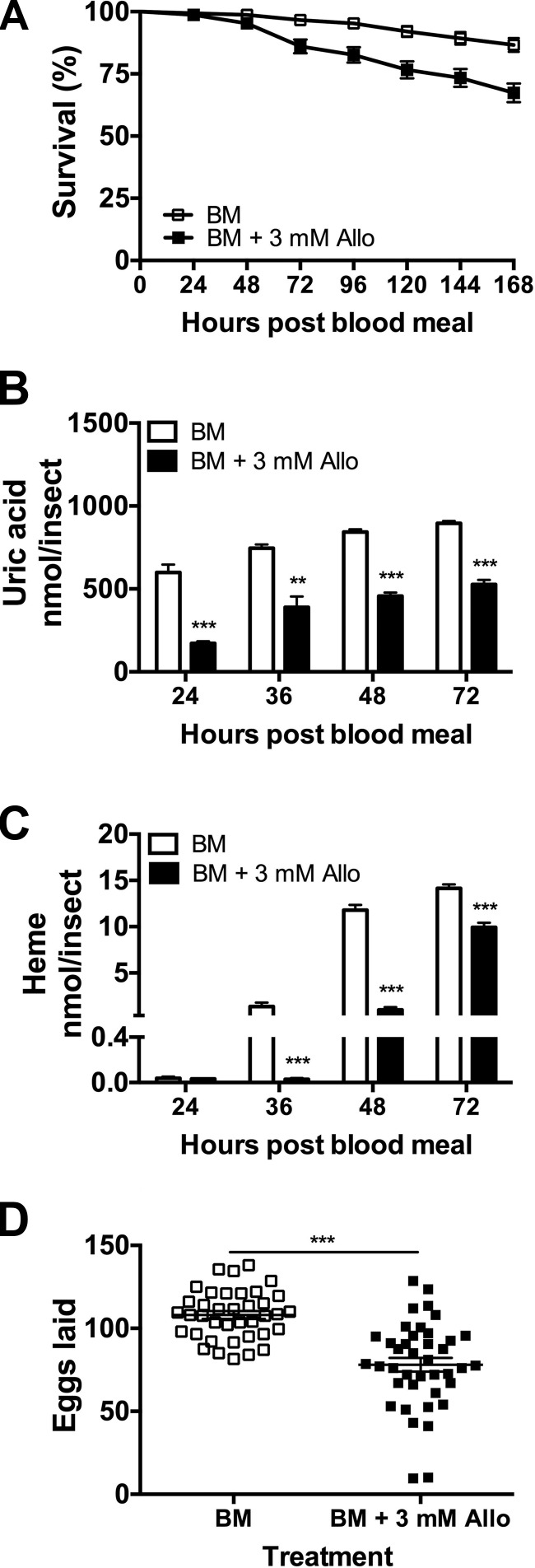
Allopurinol reduces survival, delays excretion and oviposition, and decreases egg production
Allopurinol ([1H]-pyrazolo[3,4-d]pyrimidin-4-ol) is a pharmacological inhibitor of XDH that has been studied in several hematophagous arthropods including kissing bugs (22), cattle ticks (23), and mosquitoes (15). To explore the effects of XDH inhibitor on Ae. aegypti survival, excretion, and oviposition, female mosquitoes were fed a blood meal supplemented with allopurinol (3 mM final concentration) and observed in individual containers. As shown in Fig. 3A, treated females had a higher mortality (33%) than in the control group (13%). This increase in mortality coincided with a decrease of waste markers compared to the control group. Uric acid concentration in the surviving allopurinol group decreased compared to the blood meal alone group at 24, 36, 48, and 72 h PBM (Fig. 3B). Surviving females also showed a significantly reduced amount of heme in their excreta at 36, 48, and 72 h PBM (Fig. 3C). This decrease indicates a disruption of digestive and excretory processes, likely as a result of XDH direct chemical inhibition. A delay in oviposition was also observed in surviving mosquitoes fed with the inhibitor. In the control group, females laid eggs on d 3 (92%) and 4 (8%) PBM while in the treated group, and surviving mosquitoes laid eggs on d 3 (43%), 4 (48%), and 5 (9%) PBM. Furthermore, females treated with allopurinol showed a significant reduction in fecundity (Fig. 3D).
Figure 3.
Effect of allopurinol on Ae. aegypti survival, excretion, and egg production. Females were fed blood meal supplemented with 3 mM allopurinol (Allo). A) Survival. Data are expressed as mean percentages ± sem. At least 30 females were used. B) Uric acid in excreta. C) Heme in excreta. Nitrogen waste data are expressed as means ± sem. At least 10 females were used. D) Egg production during first gonotrophic cycle. Each square represents number of eggs laid by individual female. Means ± sem are shown for each data set as horizontal lines. At least 30 females were used for each treatment. ***P < 0.001 compared to control.
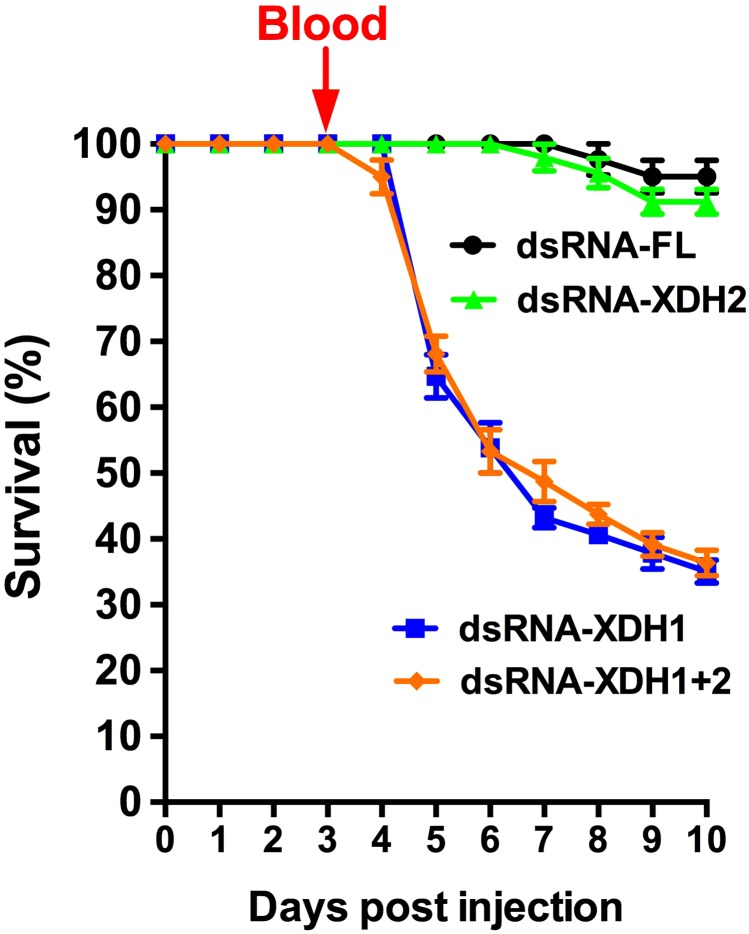
XDH1 knockdown critically impairs survival
In order to ascertain whether the effects of allopurinol on survival, waste production, excretion, and fecundity were due to a direct inhibition of XDH activity or an off-target drug effect, we next investigated the effect of XDH gene silencing on mosquito mortality. Mosquitoes were injected with dsRNA-XDH1, dsRNA-XDH2, dsRNA-XDH1+2, or dsRNA-FL control, given a blood meal on d 4, and then monitored for 10 d after injection. Both dsRNA-XDH1- and dsRNA-XDH1+2-injected females exhibited a dramatic decrease in survival at 48 h PBM, with a 65% survival rate (Fig. 4) compared to the control group. By 10 d after injection, only 35% of mosquitoes survived. In contrast, dsRNA-XDH2-injected mosquitoes expressed a similar mortality pattern to the dsRNA-FL-injected females, with a survival rate of around 90% by the end of the observed period, suggesting that allopurinol effects are likely due to XDH1 inhibition and that loss of XDH1 activity is lethal to blood-fed mosquitoes.
Figure 4.
Disruption of XDH1 is lethal to blood-fed mosquitoes. Females were injected with dsRNA-FL, dsRNA-XDH1, dsRNA-XDH2, or dsRNA-XDH1+2 and then fed blood meal. Mortality was observed for 10 d after injection. Data are expressed as mean ± sem percentages. At least 30 females were used for each dsRNA.
Distinct XDH1 protein expression in mosquito tissues
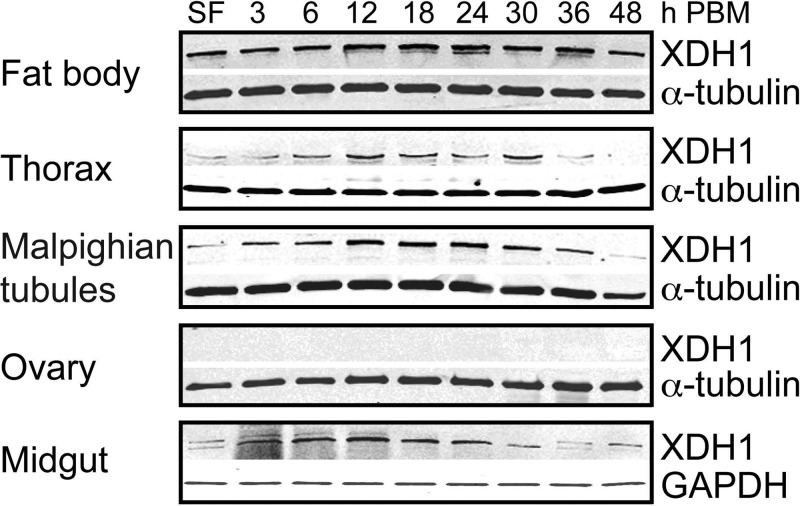
To further understand the XDH1 protein expression in Ae. aegypti tissues, we generated a rabbit polyclonal primary antibody against a XDH1 Ae. aegypti peptide sequence. An additional XDH2 antibody was not generated because RNAi against XDH2 showed no effect on mortality. Western blot analysis was used to monitor expression levels of the XDH1 protein in 5 tissues dissected from sugar- and blood-fed wild-type Ae. aegypti mosquitoes during the first gonotrophic cycle. As shown in Fig. 5, XDH1 was expressed in the fat body throughout the time course. In the thorax, protein expression was present until late oogenesis (48 h PBM). XDH1 increased over time in the malpighian tubules and the midgut with peaks at 24 and 12 h PBM, respectively, and declined gradually afterward. XDH1 protein levels synthesized in the ovary were below the level of detection for this assay.
Figure 5.
XDH1 protein expression patterns in Ae. aegypti tissues dissected from wild-type mosquitoes. XDH1 protein levels in fat body, thorax, malpighian tubules, ovary, and midgut at 3, 6, 12, 18, 24, 30, 36, and 48 h PBM were analyzed by Western blot analysis using anti-mosquito XDH1 primary antibody. SF females were fed only on 3% sucrose. Tissues of 10 females were pooled. Each lane contains 0.5 tissue equivalent of protein extracts. Anti-α-tubulin and anti-GAPDH antibodies are shown to confirm equal protein loading. Western blots are representative of 3 replicates.
Silencing of XDH1 reduces protein expression and delays synthesis and excretion of nitrogen waste
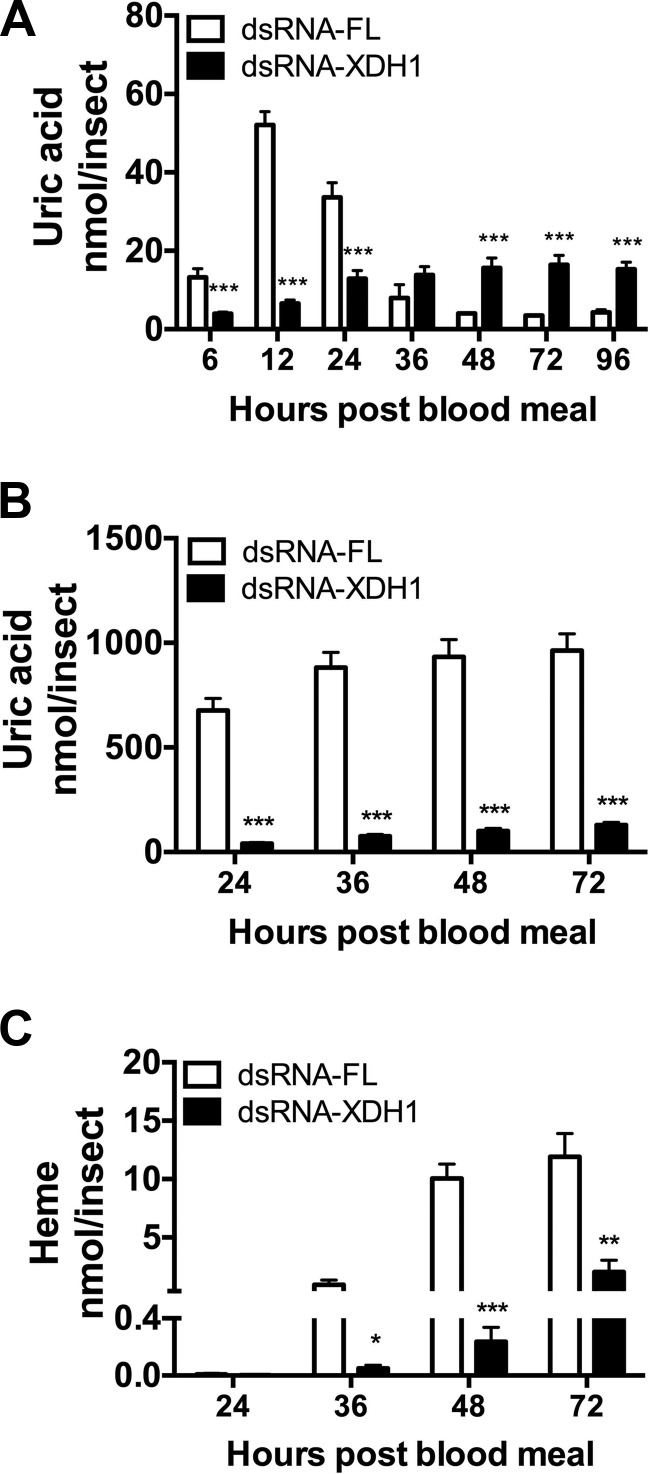
To verify the efficiency of the XDH1 mRNA knockdown, we monitored protein levels by Western blot analysis in the fat body, midgut, and malpighian tubules. Decreased XDH1 levels were seen in dsRNA-XDH1-injected mosquitoes compared to dsRNA-FL-injected control mosquitoes (Supplemental Fig. S1). Furthermore, to test the effect of XDH1 inhibition by RNAi on excretion, uric acid concentrations in the malpighian tubules and excreta were analyzed at 6, 12, 24, 36, 48, 72, and 96 h PBM and at 24, 36, 48, and 72 h PBM, respectively. As shown in Fig. 6A, malpighian tubules in dsRNA-XDH1-injected mosquitoes contained significantly less uric acid between 6 and 24 h PBM compared to the control group. Although uric acid concentrations in dsRNA-XDH1-injected mosquitoes were higher than the control levels at 48, 72, and 96 h PBM, they never exceeded 20 nmol per insect. In comparison, uric acid concentration in dsRNA-FL-injected mosquitoes averaged 55 nmol per insect at their peak around 12 h PBM. A delay was also seen when nitrogen waste was measured in excreta. As shown in Fig. 6B, dsRNA-XDH1-injected mosquitoes produced significantly less uric acid compared to the control group. Similarly, a significant decrease in heme excretion was seen at 36, 48, and 72 h PBM (Fig. 6C).
Figure 6.
Quantification of nitrogen waste in Ae. aegypti with XDH1 deficiency. A) Uric acid in malpighian tubules. B) Uric acid in excreta. C) Heme in excreta. malpighian tubules were dissected at 0, 6, 12, 24, 36, 48, 72, and 96 h PBM. Excreta were collected from dsRNA-FL or dsRNA-XDH1-injected mosquitoes at 24, 36, 48, and 72 h PBM. Data are presented as means ± sem. At least 10 females were used for each dsRNA-injection. ***P < 0.001 compared to dsRNA-FL.
XDH1 deficiency slows digestion, vitellogenesis, and oviposition, and reduces fecundity
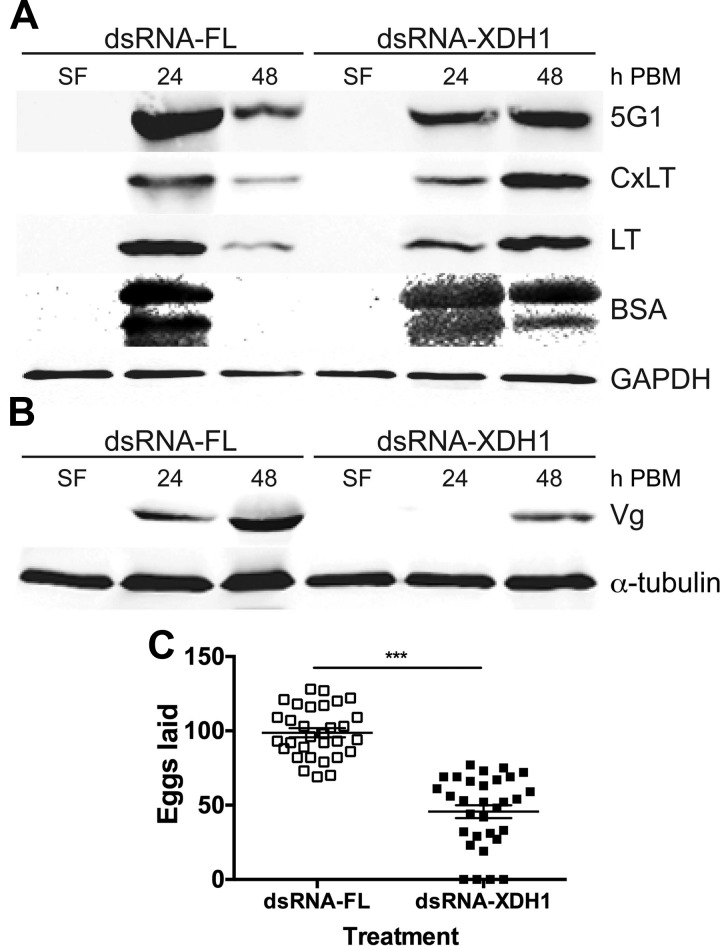
To validate whether a delay in excretion is the consequence of a delay in digestion among mosquitoes injected with dsRNA-XDH1, we first monitored levels of an abundant blood protein, bovine serum albumin (BSA), which disappears from the midgut as digestion progresses. Next, we assessed the expression levels for 3 major digestive serine proteases (5G1, CxLT, and LT) secreted from mosquito midgut epithelial cells. In a separate Western blot analysis, we examined the Vg protein in the ovaries in order to determine whether vitellogenesis was delayed in dsRNA-XDH1-injected mosquitoes. Western blot analysis showed a delay in the levels of midgut digestive enzymes in the XDH1 dsRNA-mediated knockdown group. In the dsRNA-FL control group, the protein levels of the 3 proteases and BSA were most abundant at 24 h PBM (Fig. 7A). By 48 h, PBM protease levels in the control group were greatly decreased, and BSA proteins in the midgut were no longer detectable. In comparison, protease levels were most abundant at 48 h PBM in the dsRNA-XDH1-injected mosquitoes, and intact BSA was still present in large amounts at 48 h PBM. In addition, we examined the effect of XDH silencing on egg development by measuring levels of Vg taken up by the ovaries. XDH1-knockdown mosquitoes exhibited a 24 h delay in Vg uptake compared to the control group (Fig. 7B). This delay correlated with a significant change in the timing of oviposition. Similar to the allopurinol results, dsRNA-FL-injected control females laid eggs on d 3 (97%) and 4 (3%) PBM. However, dsRNA-XDH1 females laid eggs on d 3 (52%), 4 (44%), and 5 (9%) PBM. There was an approximately 50% decrease in egg production with the dsRNA-XDH1-injected females relative to dsRNA-FL-injected mosquitoes (Fig. 7C).
Figure 7.
Effect of decreased XDH1 protein expression on digestion, vitellogenesis, and egg production. A) Expression of digestive serine proteases 5G1, CxLT, and LT and level of BSA in midgut of Ae. aegypti mosquitoes injected with dsRNA. Tissues were dissected before blood meal and at 24 and 48 h PBM. Tissues of 5 females were pooled. Total midgut protein extracts were subjected to SDS-PAGE and blotted onto nitrocellulose membranes. Each lane contains 0.25 midgut equivalent of protein extracts. Western blot analysis was performed using anti-5G1, CxLT, LT, and BSA primary antibodies. Anti-GAPDH antibody is shown to confirm equal protein loading. B) Expression of Vg in mosquitoes injected with dsRNA. Ovaries were dissected at 24 and 48 h PBM. Ovaries of 5 females were pooled. Each lane contains 0.3 ovary equivalent of protein extracts. Western blot analysis was performed using anti-AaVg primary antibodies. Anti-α-tubulin antibody is shown to confirm equal protein loading. Western blots are representative of 3 replicates. C) Egg production in dsRNA-injected Ae. aegypti. Females were injected with dsRNA-FL or dsRNA-XDH1, and number of eggs laid during first gonotrophic cycle was counted. Each square represents number of eggs laid by individual female. Means ± sem are shown for each data set as horizontal lines. At least 30 females were used for each treatment. ***P < 0.001 compared to dsRNA-FL.
XDH1 deficiency affects the transcriptional regulation of downstream genes
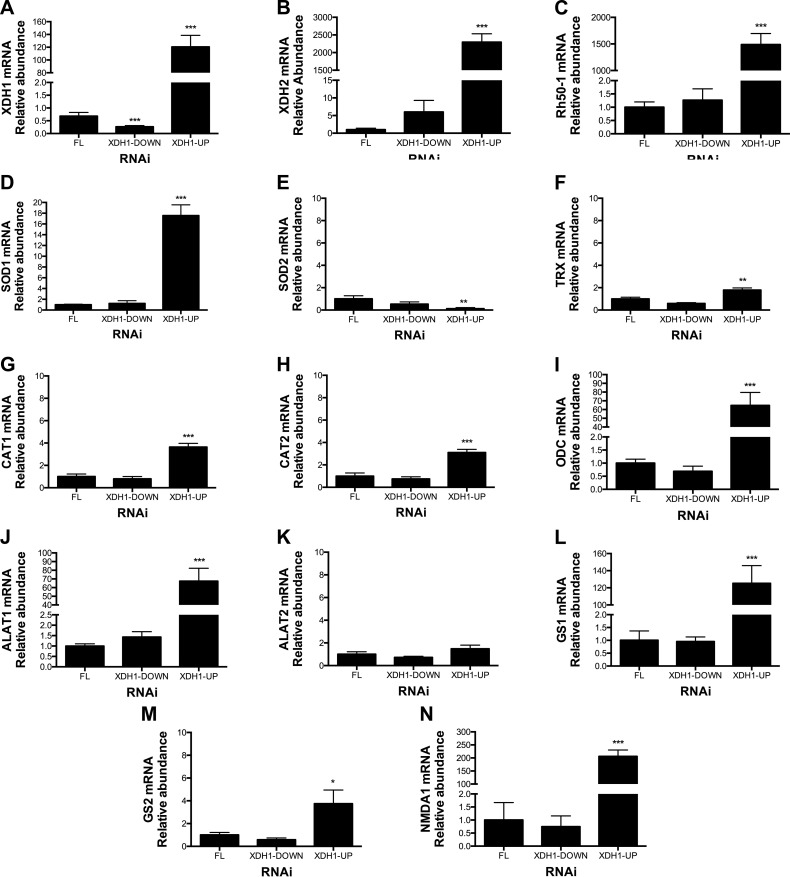
Because RNAi-mediated XDH1 silencing in blood-fed mosquitoes led to high mortality, we next examined the transcriptional response of Ae. aegypti mosquitoes to dsRNA treatment by real-time qPCR. Because the silencing effect of RNAi may differ among individual mosquitoes, the gene expression level for the dsRNA-injected mosquitoes at 24 h PBM was analyzed individually in the fat body, midgut, and malpighian tubules rather than by pooled dissected tissues. Complementary DNA (cDNA) was synthesized from the total RNA of dissected individual mosquitoes at 24 h PBM. We measured the expression levels of S7 ribosomal protein and XDH1 using gene-specific primers (Table 1). As expected with RNAi, we observed a significant reduction of the XDH1 transcripts in the midgut and malpighian tubules isolated from dsRNA-XDH1 individual mosquitoes. However, analysis of fat body mRNA from dsRNA injected mosquitoes showed that the transcriptional response of the fat body in dsRNA-XDH1-injected mosquitoes (22 tested) fell into 2 groups. One group (45%, XDH1-down) was characterized by a significant reduction of the XDH1 transcripts compared to the level observed in dsRNA-FL injected control mosquitoes. The other group (55%, XDH1-up) was characterized by an unexpected up-regulation of the XDH1 transcript level as measured by real-time qPCR: a 120-fold increase at 24 h PBM relative to the control mosquitoes (Fig. 8A).
Figure 8.
Changes in transcript levels in fat body from dsRNA-XDH1-injected mosquitoes. Relative abundance of mRNA levels for XDH1, XDH2, ammonia transporter (Rh50-1), SOD1, SOD2, TRX, CAT1, CAT2, ODC, ALT1, ALT2, GS1, GS2, and NMDAR were analyzed in fat body at 24 h PBM. As control, dsRNA-FL-injected mosquitoes were used. mRNA levels were normalized according to mRNA levels of S7 ribosomal protein. Data are presented as means ± sem of 10 or 12 individual mosquitoes. *P < 0.05, **P < 0.01, ***P < 0.001 compared to dsRNA-FL.
Because approximately half of the dsRNA-XDH1-injected mosquitoes significantly increased their XDH1 transcription in fat body, we evaluated the transcription level of other genes in both groups (up and down) of the dsRNA-XDH1 mosquitoes (Fig. 8B–N). We selected genes known to be involved in both ammonia metabolism and oxidative stress response. We measured the relative mRNA expression level of XDH1, XDH2, ammonia transporter (Rh50-1), GS1, GS2, ALT1, ALT2, NMDAR (also known as glutamate receptor), and several antioxidant genes, including catalase (CAT1 and CAT2), TRX, SOD1, SOD2, and ODC in fat body of individual mosquitoes (FL control, XDH1-down, and XDH1-up groups) by using gene-specific primers (Table 1). Except for XDH1 (Fig. 8A), the rest of the transcripts measured did not change when comparing the XDH1-down group to FL control (Fig. 8B–N). In contrast, the XDH1-up group had many differences relative to the control mosquitoes (Fig. 8B–N). Notably, XDH2 and Rh50-1 mRNAs increased about 2000- to 2500-fold compared to control (Fig. 8B, C), whereas ODC, ALT1, GS1, and NMDAR transcript levels increased approximately 100- to 300-fold (Fig. 8I, J, L, N). Unlike the transcriptional response observed in the fat body to XDH1 RNAi, significant transcriptional up-regulation was not observed for genes tested in the midgut and malpighian tubules of dsRNA-XDH1 mosquitoes (Supplemental Figs. S2 and S3).
DISCUSSION
Female mosquitoes have evolved to efficiently metabolize a blood meal in a manner that allows them to both acquire nutrients needed for egg production and remove excess nitrogen without building up toxic levels of ammonia. However, the mechanisms that mosquitoes use to maintain a nitrogen balance are still poorly understood. Notwithstanding the lack of a urea cycle (24) for ammonia disposal, mosquitoes can excrete or metabolize ammonia into uric acid and other nitrogen compounds (13–15, 19). Previous studies have demonstrated that XDH is involved in uric acid synthesis in Ae. aegypti mosquitoes (25, 26) and that XDH contributes to the clearance of surplus nitrogen (10, 15, 21, 25–27). Two genes encoding XDH were previously identified by BLAST searches in Ae. aegypti (19). In this report, we show that XDH1 and XDH2 have distinct spatiotemporal gene expression patterns in Ae. aegypti tissues during the first gonotrophic cycle, suggesting that each gene may encode proteins that play different physiologic roles. Pharmacologic and genetic inhibition of XDH by allopurinol and RNAi shortens mosquito life span and causes an impediment to blood digestion and excretion. Surviving female mosquitoes exhibited many deleterious phenotypes, including delayed digestion, excretion of nitrogen waste and ovipositon, and reduced fecundity compared to the control mosquitoes. Furthermore, we demonstrate that the depletion of XDH1, not XDH2, severely affected the survival of blood-fed female mosquitoes.
Unlike many other insects (e.g., fruit flies, moths, beetles), anautogenous female adult mosquitoes exhibit discontinuous feeding habits. Therefore, uric acid synthesis, transport, and excretion must be tightly regulated in tissues during blood meal digestion and vitellogenesis in order to optimize reproductive fitness. In blood-fed Ae. aegypti mosquitoes, uric acid can be excreted directly or further metabolized to urea through an amphibian-like uricolytic pathway (15). It was recently found that effective disposal of nitrogen waste in blood-fed Ae. aegypti mosquitoes requires ALT. The increase of Rh50-1 and XDH1 mRNA transcripts in several tissues of dsRNA-ALT-injected mosquitoes was associated with a survival strategy of ALT-deficient females to avoid ammonia toxicity and free radical damage (21).
Uric acid can play a dual role as a reservoir/end product for the excess ammonia and as a scavenger of free radicals during blood digestion in mosquitoes (13–15, 21, 25, 26). It has also been shown that uric acid administration enhances Plasmodium berghei development in Anopheles stephensi and decreases the mortality of P. berghei–infected mosquitoes by reducing reactive oxygen species (28, 29). Recently it was demonstrated that the induction of a reductive environment by the addition of uric acid, mimicking triatomine rectal environment, impairs epimastigote proliferation in vitro and stimulates Trypanosoma cruzi metacyclogenesis (30). Additionally, data from several insects including fruit fly D. melanogaster (31), anopheline larvae (32), click beetle Pyrearinus termitilluminans (33), and kissing bug Rhodnius prolixus (22, 34) indicate a role for endogenous uric acid as protection from free radical damage. Silencing of XDH gene expression in dsRNA-XDH-injected Lutzomyia longipalpis sandflies caused a reduction of life span coupled with a decrease of uric acid concentration in the whole body and excreta, suggesting that uric acid could also be an important free radical scavenger in L. longipalpis (35).
In Ae. aegypti, the knockdown of XDH1 by RNAi caused a reduction of XDH1 protein expression in multiple tissues (Supplemental Fig. S1). The single mosquito analysis rather than pooled samples allowed us to observe an unexpected transcriptional response of individual mosquitoes to XDH1 deficiency, whereby 55% of XDH1-injected mosquitoes had up-regulated transcript levels of not only XDH2 but also XDH1 itself in fat body at 24 h after blood feeding. Moreover, genes encoding NMDAR, antioxidant enzymes (CAT1, CAT2, SOD1, TRX, and ODC), and proteins involved in ammonia fixation, assimilation, excretion, and transport (GS1, GS2, ALT1, and Rh50-1) had increased transcript levels in fat body of these mosquitoes at 24 h after blood feeding. These significant changes in mRNA levels in fat body could indicate an acute response to a perturbed ammonia metabolism. However, we cannot exclude the possibility that the up-regulated versus down-regulated differences can be transcriptional behavior of dying cells. Before blood feeding, XDH1 mRNA and protein levels are reduced in female mosquitoes. We did not observe mortality among sugar-fed dsRNA-injected female mosquitoes, likely because of the minimum and manageable ammonia and free radical levels, and therefore low oxidative stress. Unlike dsRNA-FL control or wild-type mosquitoes, XDH1-deficient mosquitoes fail to produce uric acid as a reservoir for the excess ammonia and as a scavenger of free radicals upon blood feeding. Thus, fat body of these mosquitoes may sense a high level of oxidative stress and attempt to counterbalance this detrimental effect by increasing the transcriptional levels of the genes mentioned above. Because of genetic variations, it is possible that some mosquitoes are capable of enhancing their transcriptional response more efficiently under high oxidative damage and may be able to manage by delaying many physiologic processes and survive, while others eventually undergo morbidity and mortality as a result of their ineffective response.
Previous studies have shown that metabolic pathways involved in ammonia detoxification in mosquitoes (13–19), as well as ammonia transporter (Rh50-1), are finely regulated to avoid an imbalance to nitrogen metabolism (21). ODC catalyzes the first step of polyamine synthesis. When their blood meal was supplemented with 75 to 100 mM difluoromethylornithine (an ODC inhibitor), blood digestion and vitellogenesis in Ae. aegypti mosquitoes were disrupted (36). Additionally, it has been reported that polyamines can serve as antioxidants in several organisms (37–40), but their roles as scavengers of free radicals in mosquitoes are unreported. However, it is notable that the increase of ODC transcript levels in some dsRNA-XDH1 females is the most abundant among the antioxidant genes studied in this report. The increase in GS1 and NMDAR transcript levels observed in fat body from XDH1-up females is also well correlated with phenotypes associated with ammonia and free radical toxicity in other organisms. Hyperammonemia in vertebrates leads to an increase in GS activity (41), activation of NMDAR (42), overproduction of reactive oxygen species (43), and ammonia-induced death (44).
Even though XDH2 mRNA levels can be elevated 2500-fold in the fat body of some females injected with dsRNA-XDH1, relative to control group, we uncovered no evidence that XDH2 plays an essential role in preventing cell toxicity in blood-fed females. XDH2-deficient females have similar mortality rates to controls (Fig. 4) and do not exhibit any significant up-regulation of the XDH1 mRNA level or other transcripts examined in this study (Supplemental Fig. S4). In contrast, disruption of XDH1 severely impairs the nitrogen and antioxidant balance, causing high mortality in blood-fed Ae. aegypti females. Supporting our observations and previous studies, we found that dsRNA-XDH1-injected mosquitoes counterbalance the effects of ammonia and free radical damage by up-regulating specific mRNA transcripts in fat body. However, when the females can no longer convert ammonia to uric acid, they lose their ability not only to utilize it as an antioxidant but also to eliminate nitrogen excess through the excretion of uric acid. Our novel findings demonstrate that XDH1 silencing in Ae. aegypti promotes a blood feeding–induced adulticidal property and that mosquito-selected compounds that can be engineered to inhibit XDH1 enzymatic activity have the potential to be used for mosquito vector control.
ACKNOWLEDGMENTS
The authors thank the Louisiana Clinical and Translational Science Center (Baton Rouge, LA, USA) for statistical advice. This work was financially supported by Corine Adams Baines Professorship Award, and by the U.S. National Institutes of Health, National Institute of Allergy and Infectious Diseases (Grant R01AI088092 to P.Y.S.).
Glossary
- ALT
alanine aminotransferase
- BSA
bovine serum albumin
- CAT
catalase
- cDNA
complementary DNA
- dsRNA
double-stranded RNA
- FL
firefly luciferase
- GS
glutamine synthetase
- NMDAR
NMDA receptor
- ODC
ornithine decarboxylase
- PBM
post–blood meal
- qPCR
quantitative PCR
- Rh50-1
Rhesus 50 glycoprotein
- RNAi
RNA interference
- SF
sugar fed
- SOD
superoxide dismutase
- TRX
thioredoxin reductase
- Vg
vitellogenin
- XDH
xanthine dehydrogenase
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J. Isoe and P. Y. Scaraffia designed the research; J. Isoe, N. Petchampai, Y. E. Isoe, and K. Co performed the research; S. Mazzalupo contributed new reagents or analytic tools; J. Isoe, N. Petchampai, Y. E. Isoe, K. Co, S. Mazzalupo, and P. Y. Scaraffia analyzed data; and K. Co, S. Mazzalupo, and P. Y. Scaraffia wrote the paper.
REFERENCES
- 1.Kularatne S. A. (2015) Dengue fever. BMJ 351, h4661 [DOI] [PubMed] [Google Scholar]
- 2.Choumet V., Desprès P. (2015) Dengue and other flavivirus infections. Rev. Sci. Tech. 34, 473–478, 467–472 [PubMed] [Google Scholar]
- 3.Hrnjaković Cvjetković I. B., Cvjetković D., Patić A., Nikolić N., Stefan Mikić S., Milošević V. (2015) Chikungunya—a serious threat for public health. Med. Pregl. 68, 122–125 [DOI] [PubMed] [Google Scholar]
- 4.Pybus O. G., Tatem A. J., Lemey P. (2015) Virus evolution and transmission in an ever more connected world. Proc. Biol. Sci. 282, 20142878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attar N. (2016) Zika virus circulates in new regions. Nat. Rev. Microbiol. 14, 62 [Google Scholar]
- 6.Fauci A. S., Morens D. M. (2016) Zika virus in the Americas—yet another arbovirus threat. N. Engl. J. Med. 374, 601–604 [DOI] [PubMed] [Google Scholar]
- 7.Fredericks A. C., Fernandez-Sesma A. (2014) The burden of dengue and chikungunya worldwide: implications for the southern United States and California. Ann. Glob. Health 80, 466–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell L. P., Luther C., Moo-Llanes D., Ramsey J. M., Danis-Lozano R., Peterson A. T. (2015) Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370, 20140135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horwood P. F., Buchy P. (2015) Chikungunya. Rev. Off. Int. Epizoot. 34, 479–489 [DOI] [PubMed] [Google Scholar]
- 10.Briegel H. (1985) Mosquito reproduction: incomplete utilization of the blood meal protein for oögenesis. J. Insect Physiol. 31, 15–21 [Google Scholar]
- 11.Zhou G., Flowers M., Friedrich K., Horton J., Pennington J., Wells M. A. (2004) Metabolic fate of [14C]-labeled meal protein amino acids in Aedes aegypti mosquitoes. J. Insect Physiol. 50, 337–349 [DOI] [PubMed] [Google Scholar]
- 12.Scaraffia P. Y., Wells M. A. (2003) Proline can be utilized as an energy substrate during flight of Aedes aegypti females. J. Insect Physiol. 49, 591–601 [DOI] [PubMed] [Google Scholar]
- 13.Scaraffia P. Y., Isoe J., Murillo A., Wells M. A. (2005) Ammonia metabolism in Aedes aegypti. Insect Biochem. Mol. Biol. 35, 491–503 [DOI] [PubMed] [Google Scholar]
- 14.Scaraffia P. Y., Zhang Q., Wysocki V. H., Isoe J., Wells M. A. (2006) Analysis of whole body ammonia metabolism in Aedes aegypti using [15N]-labeled compounds and mass spectrometry. Insect Biochem. Mol. Biol. 36, 614–622 [DOI] [PubMed] [Google Scholar]
- 15.Scaraffia P. Y., Tan G., Isoe J., Wysocki V. H., Wells M. A., Miesfeld R. L. (2008) Discovery of an alternate metabolic pathway for urea synthesis in adult Aedes aegypti mosquitoes. Proc. Natl. Acad. Sci. USA 105, 518–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scaraffia P. Y., Zhang Q., Thorson K., Wysocki V. H., Miesfeld R. L. (2010) Differential ammonia metabolism in Aedes aegypti fat body and midgut tissues. J. Insect Physiol. 56, 1040–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petchampai N., Scaraffia P. Y. (2016) Nitrogen metabolism in mosquitoes: new insights into the nitrogen metabolism in blood-fed mosquitoes. In Advances in Insect Physiology: Progress in Mosquito Research (Raikhel A. S., ed.), Vol. 51, pp. 363–391, Elsevier Academic Press, London: [Google Scholar]
- 18.Scaraffia P. Y. (2016) Disruption of mosquito blood meal protein metabolism. In Genetic Control of Malaria and Dengue (Adelman Z. N., ed.), pp. 253–275, Elsevier Academic Press, San Diego, CA, USA [Google Scholar]
- 19.Isoe J., Scaraffia P. Y. (2013) Urea synthesis and excretion in Aedes aegypti mosquitoes are regulated by a unique cross-talk mechanism. PLoS One 8, e65393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belloni V., Scaraffia P. Y. (2014) Exposure to l-cycloserine incurs survival costs and behavioral alterations in Aedes aegypti females. Parasit. Vectors 7, 373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazzalupo S., Isoe J., Belloni V., Scaraffia P. Y. (2016) Effective disposal of nitrogen waste in blood-fed Aedes aegypti mosquitoes requires alanine aminotransferase. FASEB J. 30, 111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Souza A. V., Petretski J. H., Demasi M., Bechara E. J., Oliveira P. L. (1997) Urate protects a blood-sucking insect against hemin-induced oxidative stress. Free Radic. Biol. Med. 22, 209–214 [DOI] [PubMed] [Google Scholar]
- 23.Liyou N., Hamilton S., Mckenna R., Elvin C., Willadsen P. (2000) Localisation and functional studies on the 5′-nucleotidase of the cattle tick Boophilus microplus. Exp. Appl. Acarol. 24, 235–246 [DOI] [PubMed] [Google Scholar]
- 24.Krebs H. A., Henseleit K. (1932) Analysis concerning urea formation in animal bodies. Hoppe Seylers Z. Physiol. Chem. 210, 33–66 [Google Scholar]
- 25.Von Dungern P., Briegel H. (2001) Enzymatic analysis of uricotelic protein catabolism in the mosquito Aedes aegypti. J. Insect Physiol. 47, 73–82 [DOI] [PubMed] [Google Scholar]
- 26.Von Dungern P., Briegel H. (2001) Protein catabolism in mosquitoes: ureotely and uricotely in larval and imaginal Aedes aegypti. J. Insect Physiol. 47, 131–141 [DOI] [PubMed] [Google Scholar]
- 27.Sanders H. R., Evans A. M., Ross L. S., Gill S. S. (2003) Blood meal induces global changes in midgut gene expression in the disease vector, Aedes aegypti. Insect Biochem. Mol. Biol. 33, 1105–1122 [DOI] [PubMed] [Google Scholar]
- 28.Peterson T. M., Gow A. J., Luckhart S. (2007) Nitric oxide metabolites induced in Anopheles stephensi control malaria parasite infection. Free Radic. Biol. Med. 42, 132–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molina-Cruz A., DeJong R. J., Charles B., Gupta L., Kumar S., Jaramillo-Gutierrez G., Barillas-Mury C. (2008) Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J. Biol. Chem. 283, 3217–3223 [DOI] [PubMed] [Google Scholar]
- 30.Nogueira N. P., Saraiva F. M., Sultano P. E., Cunha P. R., Laranja G. A., Justo G. A., Sabino K. C., Coelho M. G., Rossini A., Atella G. C., Paes M. C. (2015) Proliferation and differentiation of Trypanosoma cruzi inside its vector have a new trigger: redox status. PLoS One 10, e0116712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilliker A. J., Duyf B., Evans D., Phillips J. P. (1992) Urate-null rosy mutants of Drosophila melanogaster are hypersensitive to oxygen stress. Proc. Natl. Acad. Sci. USA 89, 4343–4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benedict M. Q., Cohen A., Cornel A. J., Brummett D. L. (1996) Uric acid in Anopheles mosquitoes (Diptera: Culicidae): Effects of collarless, stripe, and white mutations. Ann. Entomol. Soc. Am. 89, 261–265 [Google Scholar]
- 33.Barros M. P., Bechara E. J. (2000) Luciferase and urate may act as antioxidant defenses in larval Pyrearinus termitilluminans (Elateridae: Coleoptera) during natural development and upon 20-hydroxyecdysone treatment. Photochem. Photobiol. 71, 648–654 [DOI] [PubMed] [Google Scholar]
- 34.Graça-Souza A. V., Maya-Monteiro C., Paiva-Silva G. O., Braz G. R., Paes M. C., Sorgine M. H., Oliveira M. F., Oliveira P. L. (2006) Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem. Mol. Biol. 36, 322–335 [DOI] [PubMed] [Google Scholar]
- 35.Sant’Anna M. R., Alexander B., Bates P. A., Dillon R. J. (2008) Gene silencing in phlebotomine sand flies: xanthine dehydrogenase knock down by dsRNA microinjections. Insect Biochem. Mol. Biol. 38, 652–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kogan P. H., Hagedorn H. H. (2000) Polyamines, and effects from reducing their synthesis during egg development in the yellow fever mosquito, Aedes aegypti. J. Insect Physiol. 46, 1079–1095 [DOI] [PubMed] [Google Scholar]
- 37.Ha H. C., Sirisoma N. S., Kuppusamy P., Zweier J. L., Woster P. M., Casero R. A. Jr (1998) The natural polyamine spermine functions directly as a free radical scavenger. Proc. Natl. Acad. Sci. USA 95, 11140–11145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minois N., Carmona-Gutierrez D., Bauer M. A., Rockenfeller P., Eisenberg T., Brandhorst S., Sigrist S. J., Kroemer G., Madeo F. (2012) Spermidine promotes stress resistance in Drosophila melanogaster through autophagy-dependent and -independent pathways. Cell Death Dis. 3, e401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Büttner S., Broeskamp F., Sommer C., Markaki M., Habernig L., Alavian-Ghavanini A., Carmona-Gutierrez D., Eisenberg T., Michael E., Kroemer G., Tavernarakis N., Sigrist S. J., Madeo F. (2014) Spermidine protects against α-synuclein neurotoxicity. Cell Cycle 13, 3903–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng D., Wang X., Li Z., Zhang Y., Peng Y., Li Y., He X., Zhang X., Ma X., Huang L., Yan Y. (2016) NO is involved in spermidine-induced drought tolerance in white clover via activation of antioxidant enzymes and genes. Protoplasma 253, 1243–1254 [DOI] [PubMed] [Google Scholar]
- 41.Ip Y. K., Chew S. F. (2010) Ammonia production, excretion, toxicity, and defense in fish: a review. Front. Physiol. 1, 134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hermenegildo C., Monfort P., Felipo V. (2000) Activation of N-methyl-d-aspartate receptors in rat brain in vivo following acute ammonia intoxication: characterization by in vivo brain microdialysis. Hepatology 31, 709–715 [DOI] [PubMed] [Google Scholar]
- 43.Murthy C. R., Rama Rao K. V., Bai G., Norenberg M. D. (2001) Ammonia-induced production of free radicals in primary cultures of rat astrocytes. J. Neurosci. Res. 66, 282–288 [DOI] [PubMed] [Google Scholar]
- 44.Monfort P., Montoliu C., Hermenegildo C., Muñoz M., Felipo V. (2000) Differential effects of acute and chronic hyperammonemia on signal transduction pathways associated to NMDA receptors. Neurochem. Int. 37, 249–253 [DOI] [PubMed] [Google Scholar]