Abstract
Background:
Since epithelial cell adhesion molecule glycoprotein (EPCAM) is associated with the development and metastasis of colon adenocarcinoma, it can be helpful in predicting the tumor stage before surgery. In this study, we investigated EPCAM glycoprotein expression in colon adenocarcinoma and its relationship with tumor staging.
Materials and Methods:
This study was done on formalin-fixed and paraffin-embedded tissues of 71 patients diagnosed with colon adenocarcinoma, together with normal tissues around them, which were available at the archive of pathology lab of Al-Zahra hospital, Isfahan. Hematoxylin and eosin (HandE) and immunohistochemistry (IHC) staining methods for EPCAM marker were performed on paraffin-embedded blocks.
Results:
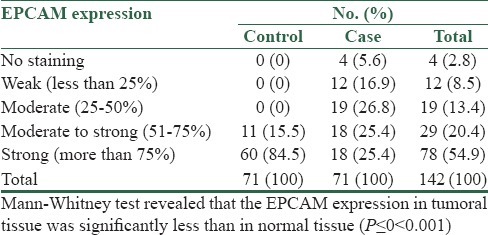
The percentage of staining of EPCAM glycoprotein in the tumoral and normal tissues of 71 patients with colon adenocarcinoma was studied and compared. In normal tissue, 84.5% showed strong staining, 15.5% showed moderate to strong, and none of the patients showed moderate, weak, or no staining at all. In the tumoral tissue, 25.4% had strong, 25.4% had moderate to strong, 26.8% showed moderate, 16.9% showed weak and 5.6% had no staining at all. EPCAM expression was significantly less in tumoral tissue than in normal.
Conclusion:
There was an inverse relationship between tumor staging and the percentage of staining in EPCAM glycoprotein so this marker can be used for predicting the tumor stage.
Keywords: Colon adenocarcinoma, epithelial cell adhesion molecule glycoprotein, TNM staging
Introduction
Colon cancer is one of the prevalent cancers around the world. In Iran, during recent years, colon cancer prevalence has increased as it is the third widespread cancer in men (8.3 per 100,000 people) and the fourth prevalent cancer in women (6.5 per 100,000 people).[1]
It is the third widespread and second cause of death among cancers in the United States. Colon cancer is the most prevalent treatable cancer among gastrointestinal (GI) cancers. The incidence of disease in both men and women is the same and the average age of incidence is 62 years. Standard treatment for colon adenocarcinoma is surgery, and its 5 years of survival after tumor resection is about 40–60%. Patients with higher stage of disease, who have tumor local progression or metastasis, benefit from adjuvant therapy before the surgery. Therefore, radiotherapy or chemotherapy before the operation in these patients leads to reduction of tumor recurrence and survival enhancement.[2]
TNM staging is an important prognostic factor, but it can only be evaluated after the operation, and determining the stage before surgery in colon carcinoma is difficult. Thus, it is necessary to figure out reliable prognostic factors before the surgery to identify those patients who should benefit from radiotherapy and chemotherapy beforehand. It has been proved that in case of colon adenocarcinoma, the expression of some immunohistochemical markers is related to tumor development and metastasis. Epithelial cell adhesion molecule glycoprotein (EPCAM) is one of those markers.[3]
EPCAM is a membranous glycoprotein with the molecular weight of 40 kDa and is coded by GA733-2 gene. This gene exists in the basolateral surface in most of the normal epithelial tissues of the body and its role is connecting the cells together by means of calcium. The expression of this marker increases in benign and malignant tumors that arise from epithelial tissues. Thus, this gene is used to differentiate epithelial from non-epithelial tissues and segregate normal epithelial cells from tumoral ones. A better way for treating cancer is to prevent metastasis. Accordingly, it is important to predict the capability of primary tumors to metastasize. The first step in metastasis process is separating the cancerous cells from primary tumors. So, EPCAM could have an impact in tumor progression at this stage. Due to EPCAM over-expression in most of tumors, its role in tumor progression is still questionable and there are a lot of controversies in different researches conducted in this area. The function of this marker could be related to environmental condition of the cell, tissue, and tumor.[4]
Despite advanced methods in surgery and adjuvant therapy, GI cancers, such as colon adenocarcinoma, still lead to increased mortality. Immunotherapy is a successful method of treating colon adenocarcinoma. Monoclonal antibodies are used against protein receptor in this method. Since EPCAM glycoprotein expresses on the surface of the cells of colon cancer, it can be used as a target for cancer treatment.[5] Anti-EPCAM antibodies are used for treating this tumor in cases of colon adenocarcinoma which there is EPCAM over-expression.[4]
The objective of this research was to determine the amount of expression of this marker and its relationship with colon adenocarcinoma staging. We conducted this study because of the following reasons: Lack of enough studies on EPCAM expression in colon cancer and its relationship with tumor staging, great controversy in this field, prevalence of colon cancer, inability to determine tumor stage and therapy before the operation, the effect of this tumor on the quality of life and rate of patients’ survival, and the importance of EPCAM as a potential target for adjuvant therapy in colon adenocarcinoma.
Therefore, if there is EPCAM over-expression in this tumor, we could start treatment and restrain metastasis before surgery using anti-EPCAM antibodies.[6] In addition, having figured out the relationship between the rate of expression of this marker and tumor staging, we can help patients to benefit from adjuvant therapy before the surgery by predicting the tumor stage beforehand.
Materials and Methods
This descriptive-analytical, cross-sectional study was conducted during 2006–2011 on paraffin-embedded tissues of 71 patients diagnosed with colon adenocarcinoma, along with normal tissue around them, which were obtained from Al-Zahra hospital archive, Isfahan, Iran. Sampling was done through non-probability simple and consecutive method by which the slides were filed and the respective paraffin blocks were picked. The slides were reviewed carefully and tumor TNM stage was determined according to the respective protocol. This was followed by these steps: 1) The paraffin-embedded tissue blocks were sectioned in 3-μm-thick slices and mounted on slides covered with poly-L-lysine; 2) the slides were placed inside an oven at 60°C for 45 min, then in xylol, and in graded alcohol; 3) they were incubated in proteinase K enzyme for 12 min until they were deparaffinized and the antigens were recovered; 4) they were then incubated in H2O2 solution for 10 min; 5) anti-EPCAM (MOC31) antibody was added and incubated for 1 h (the characteristics of EPCAM are isotype: IgG1, clonality: Monoclonal, manufacturer: Roche, USA); 6) polymer envision (secondary antibody) was added and incubated for 30 min; and 7) DAB (chromogen) was added and incubated for 5 min, followed by hematoxylin staining for 1 min.
Between all the above-mentioned stages, there was a stage of rinsing with phosphate-buffered saline (PBS). Then, all slides were covered with cover slips. Negative control was selected from the paraffin-embedded tissue blocks with the diagnosis of lymphoma [Figure 1], and the blocks that had normal colon tissue with membranous staining for EPCAM were chosen as positive controls [Figure 2].[7]
Figure 1.
Negative control
Figure 2.
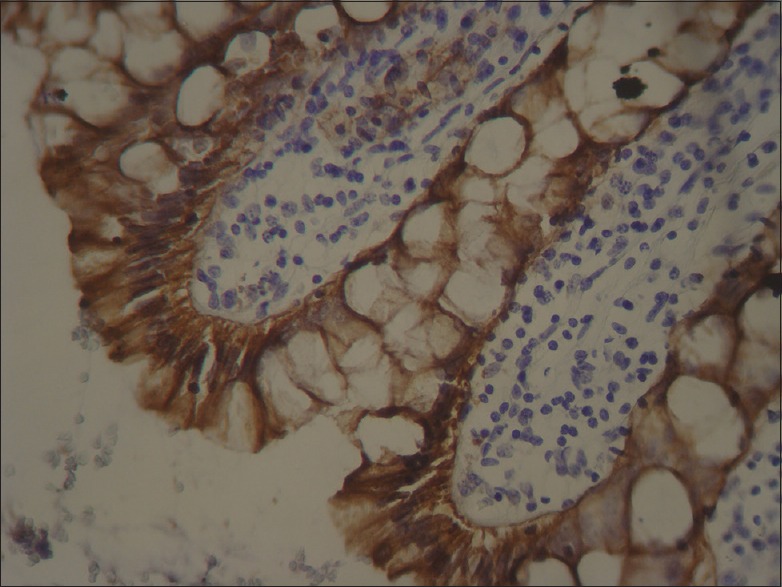
Positive control
After being stained by immunohistochemical method, the slides of each block were evaluated by light microscopy and the amount of stained cells was determined based on the following pattern:
Negative: No staining [Figure 3]
Weak (less than 25%)
Moderate (25–50%)
Moderate to strong (51–75%)
Strong (more than 75%) [Figure 4].
Figure 3.
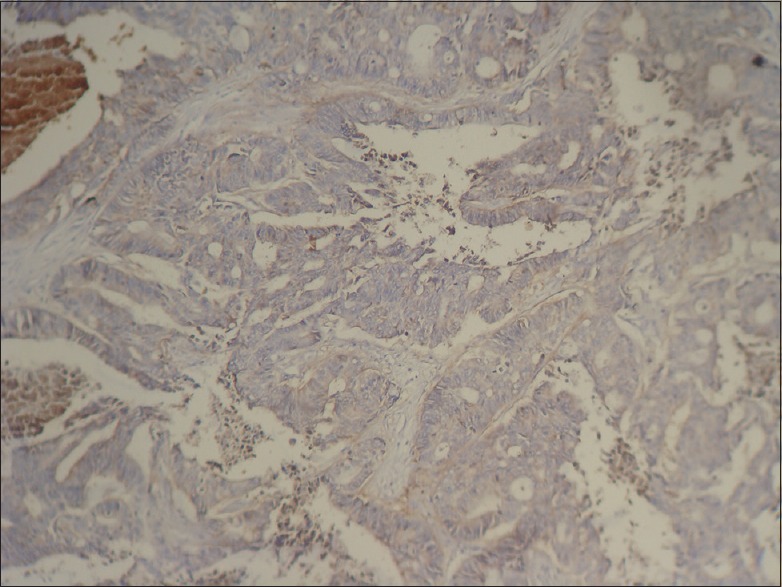
Colon adenocarcinoma, negative staining
Figure 4.
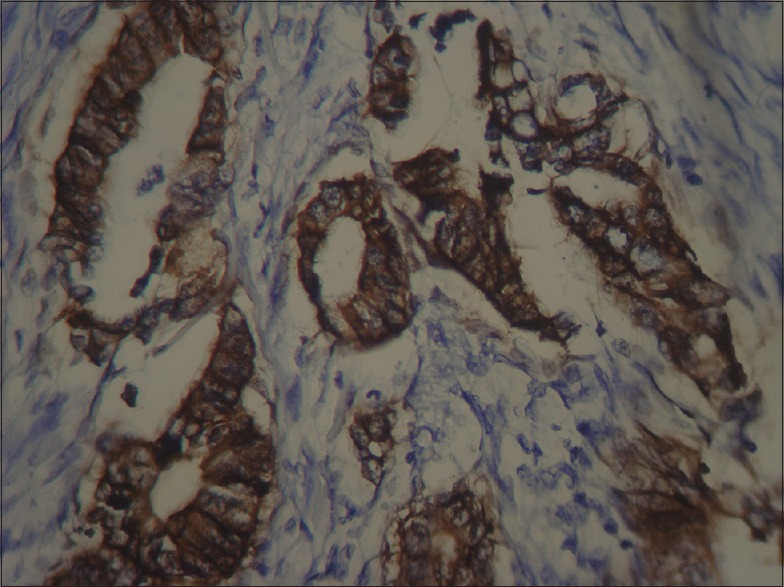
Colon adenocarcinoma, positive staining
Next, the results obtained on checking EPCAM staining were entered in a data collection form, along with other collected data including patient's age, gender, and tumor stage. Spearman correlation test was used to analyze the data. Besides, Mann–Whitney test was applied to compare the staining percentage of normal and tumoral tissue. P value of less than 0.05 was considered as statistically significant.
Results
The present study was conducted on paraffin-embedded tissues of 71 patients suffering from colon adenocarcinoma, together with normal tissue around it. Thirty-five patients were women (49.3%) and 36 were men (50.7%). Their age range was 30–85 years with a mean age of 62.3 ± 2.6 years.
EPCAM expression in the tumoral tissue and the normal tissue around it was determined and the results are presented in Table 1.
Table 1.
EPCAM expression in the tumoral tissue and the surrounding normal tissue
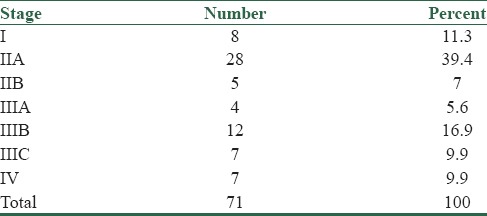
Frequency of various tumor stages in colon adenocarcinoma are presented in Table 2.
Table 2.
Frequency of various tumor stages in colon adenocarcinoma
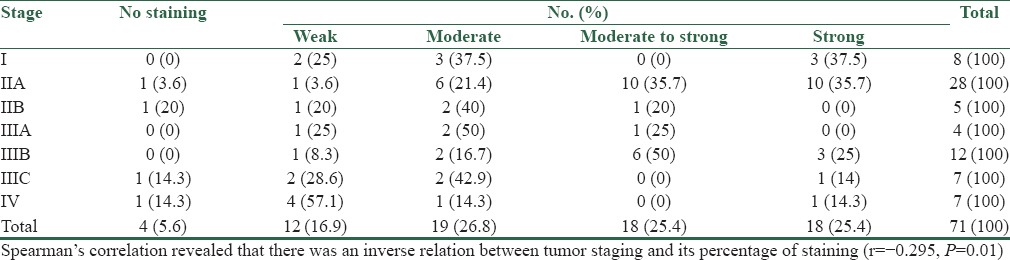
In Table 3, the EPCAM expression in different stages of tumor are presented.
Table 3.
EPCAM expression in different stages of tumor
Discussion
In the present study, 71 patients diagnosed with colon adenocarcinoma were investigated for the severity of EPCAM glycoprotein expression. The percentage of staining in the tumoral tissue, in the normal tissue around it, and the different stages of tumor were defined and compared.
According to the obtained results of the research, percentage of staining in the tumoral tissue was significantly less than in the normal tissue. Also, there was an inverse relation between tumor stage and its percentage of staining. Previous studies show that EPCAM is over-expressed in primary stages of cancer, but its expression decreases during disease progression stage. Lack of marker, along with aggressive cancer and poor prognosis lead to reduced lifetime of patients. However, due to the existing controversy in results, it has been reported that EPCAM over-expression in some of the cancers, including colon cancer, may be associated with poor prognosis.[4] Likewise, in a study, the expression of EPCAM in colorectal cancer was said to be more than 98%. According to this research, this marker does not vanish by tumor progress or metastasis.[8]
Went et al. conducted a research to verify EPCAM expression in colon, stomach, prostate, and lung cancer; the utmost expression was observed in colon cancer. The amount of expression was 97.7%, 90.7%, 87.2%, and 63.2%, respectively. In this study, in well-differentiated and poorly differentiated colon cancer, there was no relationship with tumor grade and staging, and survival of patients; but in cases of moderate differentiation, there was a strong relationship, so that patients with moderate differentiation and negative EPCAM had very low survival. Among these patients, those with high differentiation showed the most and severe EPCAM expression; but in patients with poor differentiation of cancer, the amount and severity of EPCAM expression were high.[9] In a survey conducted by Kuhn et al., it was found out that there is no relationship between EPCAM expression in colon cancer and tumor grading and staging.[10] Another research revealed that there is coincidence between EPCAM over-expression in primary and metastatic tumors, which is 76% in case of colorectal cancer. In 10% of cases of this tumor, there was a change from EPCAM over-expression of primary tumor to EPCAM over-expression of metastatic tumors. Furthermore, this study proved that in colorectal cancers with metastasis to lymph nodes, 89% of cells show over-expression and 10% show less expression for EPCAM.[11]
One of the limitations of this research was its small sample size. Further research needs to be done in this field using a larger sample size.
Conclusion
The present study revealed a reverse correlation between severity of EPCAM glycoprotein expression and tumor staging. It was figured out that it is possible, to some extent, to predict tumor staging before the surgery through the amount of EPCAM expressed in tumor.
Financial support and sponsorship
Isfahan University of Medical Sciences, Vice-chancellery for research.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This study was a research project done in Isfahan University of Medical Sciences, Isfahan, Iran. The authors acknowledge the support and contribution of university chancellery in approving and supplying the project financially. They are also thankful to Mr. Nasr and Mrs. Zeid, the immunohistochemistry (IHC) technologists of the Al-Zahra hospital pathology lab, Isfahan, Iran, who were in charge of IHC staining part. They appreciate Mr. Akbar Hassanzade's efforts in entering and analyzing the data.
References
- 1.Malekzadeh R, Bishehsari F, Mahdavinia M, Ansari R. Epidemiology and molecular Genetics of Colorectal Cancer in Iran: A Review. Arch Iran Med. 2009;12:161–9. [PubMed] [Google Scholar]
- 2.Rosai J. Rosai and Ackerman's surgical pathology. New York, NY, USA: Elsevier; 2011. pp. 768–9. [Google Scholar]
- 3.Songun I, Litvinov SV, Vandevelde C, Vals S, Hermans J. Loss of EPCAM (CD 17-1A) expression predicts survival in patients with gastric cancer. Br J Cancer. 2005;92:1767–72. doi: 10.1038/sj.bjc.6602519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiah SH-G, Tai K-Y, Wu CH-W. Epigenic regulation of EPCAM in tumor invasion and metastasis. J Cancer Moecules. 2008;3:165–8. [Google Scholar]
- 5.Chaudry MA, Sales K, Ruf P, Lindhofer H, Winslet MC. EPCAM an immunotherapeutic target for gastrointestinal malignancy: Current experience and future challenges. Br J Cancer. 2007;96:1013–9. doi: 10.1038/sj.bjc.6603505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauerle P. EPCAM as a target in cancer therapy. J Clin Oncol. 2010;28:239–40. doi: 10.1200/JCO.2009.26.8540. [DOI] [PubMed] [Google Scholar]
- 7.Went PT, Luqli A, Meier S, Bundi M, Mirlacher M, Sauter G, et al. Frequent EPCAM protein expression in human carcinoma. Hum Pathol. 2004;35:122–8. doi: 10.1016/j.humpath.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Baurele P, Ruettinger D. EPCAM's Renaissance. Drug Discovery and Development. 2009 Apr [Google Scholar]
- 9.Went P, Vasei M, Bubendorf L, Terracciano L, Tornillo L, Reide V, et al. Frequent high-level expression of the immunotherapeutic target EPCAM in colon, stomach, prostate and lung cancer. Br J Cancer. 2006;94:128–35. doi: 10.1038/sj.bjc.6602924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhn S, Koch M, Nübel T, Ladwein M, Antolovic D, Klingbeil P, et al. A complex of EPCAM, claudin-7, CD44 variant isoforms and Tetraspanins promotes colorectal cancer progression. Mol Cancer Res. 2007;5:553–67. doi: 10.1158/1541-7786.MCR-06-0384. [DOI] [PubMed] [Google Scholar]
- 11.Spizzo G, Fong D, Wurn M, Ensinger CH, Obrist P, Hofer C, et al. EPCAM expression in primary tumor tissue and metastases: An immunohistochemical analysis. J Clin Pathol. 2011;64:415–20. doi: 10.1136/jcp.2011.090274. [DOI] [PMC free article] [PubMed] [Google Scholar]


