Abstract
Background:
Bacterial infections are responsible for great number of mortality in Intensive Care Unit (ICU). Knowledge about prevalence of bacterial infections and their antibiotic-resistance pattern would be a great step for their treatment and management.
Materials and Methods:
Data about nosocomial infections in ICUs of Alzahra Hospital (referral hospital in Isfahan, center of Iran) were gathered during the years 2007–2010. A questionnaire was fulfilled for any specific patient with nosocomial infection containing demographic data of patient and also characteristics of the infection.
Results:
Out of all patients, 707 individuals (65.6%) were male and 370 (34.4%) were female. Our data revealed that Pseudomonas aeruginosa (13.9%), Klebsiella (11%), and Escherichia coli (6.4%) were the most prevalent bacterial infections. The most common sites of nosocomial infections in the ICU were respiratory system (399 cases, 37%), urinary system (230 cases, 21.4%), and blood (102 cases, 9.5%). The antibiotic-resistance of each bacteria in ICU ward was assessed and data were categorized in a table. There were less documentary about bacterial cultures in the year 2007 when compared with the next years.
Conclusion:
We found some differences (such as bacterial prevalence in ICU wards which caused nosocomial infections) in our local prevalence of nosocomial infections and also in their resistance pattern compared to other centers. Knowing about our data will help physicians to administer the most suitable antibiotics for treatment of nosocomial infections in our area.
Keywords: Antibiotic-resistance, Intensive Care Unit, nosocomial infection
Introduction
Drug resistant bacterial infections are of the most causes of mortality in Intensive Care Unit (ICU).[1,2,3] These infections can be acquired from the community or caused by hospital-dependent factors (infection in the site of surgery, infection caused by venous catheter, ventilator associated infection, etc.).[2,4,5] Microorganisms which are responsible for these infections are transferred by hospital's staff, means, and ventilating system.[6,7] Kind of surgery and duration of hospitalization are important factors which are related to incidence of nosocomial infections in ICU ward.[8] Neonates and pediatrics are more susceptible to get mentioned infection because of their lower degree of immunity and also unsuitable diet.[9] The most organs of which might be involved by infection are respiratory system and urinary system, and the prevalent microorganisms in these infections are Pseudomonas aeruginosa, Klebsiella pneumonia, Escherichia coli, and Candida spp.[10]
Ventilator-associated pneumonia (VAP) is the most common way of infection in ICU and bacteria associated with that infection are antibiotic-resistant P. aeruginosa and Acinetobacter baumannii.[1,3,11,12,13] Internal factors (bacterial colonization in pharynx and stomach) and external factors (hospital's mean and staff) are ways of bacterial transmission related to VAP.[9,14,15,16,17]
In the most cases, treatment of bacterial infections is empiric and based on epidemiologic and experimental data. Thus, it is an important issue to recognize bacteria responsible for nosocomial infection.[1,18] Therefore, if we have no enough data about the prevalence of bacteria and their pattern of antimicrobial resistant, it will be possible to prescribe several antibiotics in so much steps[1] and this kind of antibiotic prescription not only has no benefit in the way of treatment, but also make some complication for patients such as Clostridium difficile induced diarrhea caused by immethodical consumption of antibiotics.[1,18]
As mentioned above, understanding about the pattern of bacterial prevalence and drug resistance in ICU is the first step to treat the infections. In this study, we aimed to determine bacterial prevalence of nosocomial infections and also the pattern of antibiotic-resistance of the most prevalent germs in ICUs of our local are.
Materials and Methods
All patients hospitalized in the ICU of Alzahra Hospital (referral hospital of Isfahan, center of Iran) during the years 2007–2010 who were complicated by nosocomial infections were included into the study. According to previous definition, nosocomial infection is defined as those infections which involve patient who is hospitalized more than 48 h.[19]
Our data about ICU contained all of the associated wards in the hospital (Special ICU, Neurology ICU, Neonates ICU, Trauma ICU, Phase 3 ICU, Central ICU, and Pediatric ICU).
Data of mentioned patient were gathered from hospital's archive retrospectively. A questionnaire contained patient demographics including data about sex, age, duration of hospitalization, bacteria species responsible for infection (based on microbial culture, done in the Clinical Pathology Laboratory of Hospital), site of infection, and antimicrobial-resistance pattern was fulfilled for every individual.
Finally, all the gathered data were entered into SPSS-18 software (SPSS Inc., Chicago, Illinois, USA) and data were analyzed using ANOVA and Chi-square test. Results with P < 0.05 assumed to be statistically meaningful.
Results
Data were collected on patients in ICUs between January 2007 and 2010. Out of all the patients, 707 individuals (65.6%) were male and 370 (34.4%) were female. Pearson Chi-square test did not reveal any significant relationship between sex and type of bacteria causing nosocomial infection (P = 0.635)
Our data showed 1077 cases of nosocomial infection in mentioned wards (any patient might complicated with more than one bacteria); Table 1 showed prevalence of bacterial infection in years.
Table 1.
Distribution of bacterial infection in ICU by years
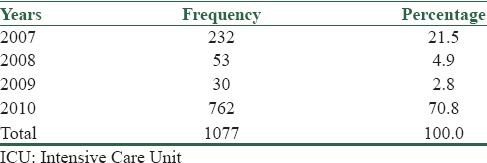
Incidence of nosocomial infection in ICU was different between months; the most prevalent months of incidence were Aban (equal to 23 October to 21 November) and Esfand (equal to 20 February to 20 March) with the rate of 12.8% (138 cases) and 12.1% (130 cases), respectively. The minimum was seen in Farvardin (equal to 21 March to 20 April).
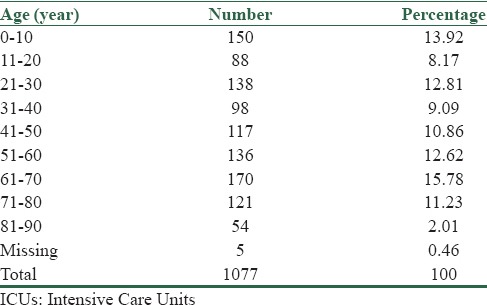
There were 97 cases (9% of all) of patients with age <1 year. Regarding Table 2, there was no relation between age and type of bacteria causing nosocomial infection (P = 0.182).
Table 2.
Distribution of nosocomial infections in ICUs, by age
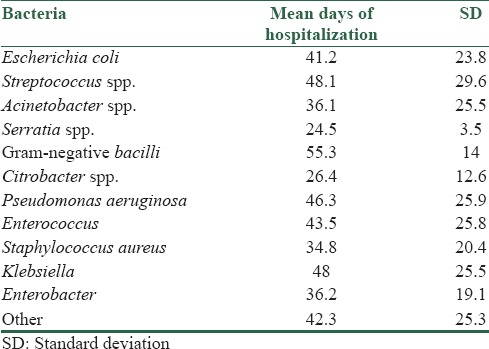
The minimum days of hospitalization in ICU were 2 and maximum days were 159 with mean days of 42.9 ± 25.3 (outlying data were excluded during statistical analysis). Bacteria were categorized in the archive of the hospital as the following: E. coli, Streptococcus spp., Acinetobacter, Serratia spp., Gram-negative bacilli, Citrobacter spp., P. aeruginosa, Enterococcus spp., Staphylococcus aureus, Klebsiella, Enterobacter spp., and others.
As seen in Table 3, patients with nosocomial infections which caused by Gram-negative bacilli had most days of hospitalization (55.3 ± 14 days) and after that Streptococcus spp. caused the longest days of hospitalization (48.1 ± 29.6 days) between other nosocomial infections. ANOVA test revealed statistical relationship between duration of hospitalization (by days) and type of bacteria caused nosocomial infections (P = 0.005).
Table 3.
Duration of hospitalization and kind of bacterial infection
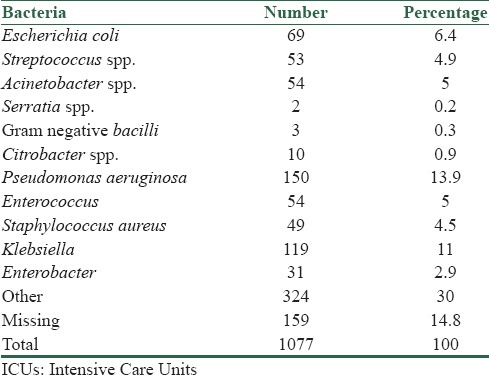
The most common sites of nosocomial infections in the ICU were respiratory system (399 cases, 37%), urinary system (230 cases, 21.4%), and blood (102 cases, 9.5%). Group of “other” bacteria had the most incidences, after that Pseudomonas aeruginosa and Klebsiella had higher rate of incidence in nosocomial infection. Table 4 shows the prevalence of bacteria responsible for nosocomial infection in ICU. There were 159 cases of nosocomial infection in this study which had no bacterial culture in their files (Said as missing data in Table 4).
Table 4.
Prevalence of commonly reported bacteria from patients in ICUs
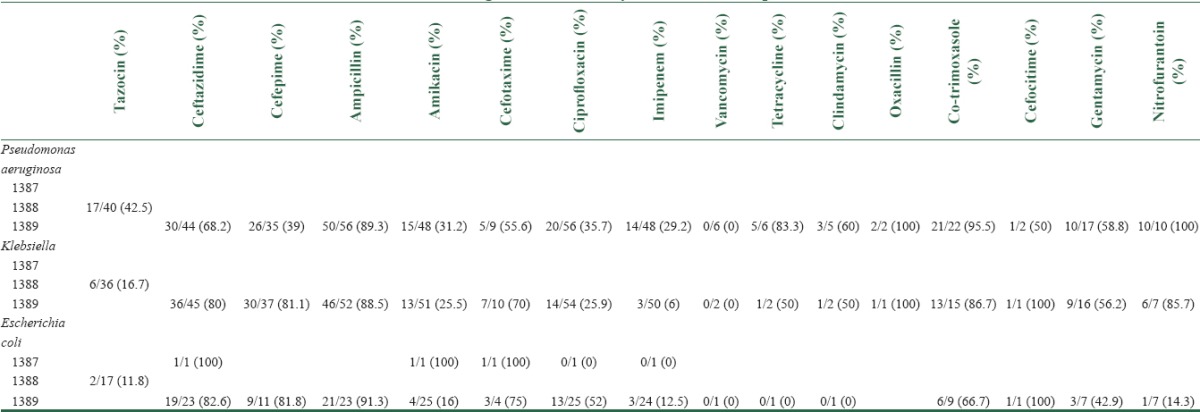
Antimicrobial agent resistance in years for the most prevalent bacteria could be seen in Table 5. There are missing data in the year 1387 Hijri-Shamsi as we searched in the computerized search system of the hospital.
Table 5.
Antimicrobial agent resistance in years for the most prevalent bacteria
Discussion
Previous studies reported higher rate of infections in ICU and this caused by severity of illness and also some procedures which are life-saving.[20] Morbidity and mortality are significant causes of infections in ICU, and multi resistant Gram-negative bacilli (e.g., Acinetobacter and Pseudomonas species) were reported to be most prevalent germs responsible for infections.[21] Our data showed that most prevalent bacteria causing infections are Pseudomonas aeruginosa, Klebsiella spp., and E. coli. A Chinese study reported Pseudomonas aeruginosa, E. coli, and A. baumannii as the most prevalent germs consistent with nosocomial infection.[22] Another study performed in Shanghai revealed A. baumannii and Pseudomonas aeruginosa as the most prevalent germs related with device-associated and healthcare-associated infections, respectively.[23]
Urinary tract and respiratory system are reported to be the most common sites of infection in ICU.[20,24] Our data revealed that respiratory system is the most common site of infection in ICU, after that urinary system and blood circulating system have higher rates in this topic. A recent study also showed that respiratory tract infections are the most commonly reported site of nosocomial infections in ICU.[25]
We did not find any significant relationship between age and sex of patients and incidence of nosocomial infections. A study by Chelazzi et al. revealed similar data to ours, they showed that there was no relationship between age, sex, or body mass index and incidence of nosocomial infections.[25] As we reported, prevalence of nosocomial infection went higher from the year 2007 to year 2010 and this topic may cause by improving a better reporting system for nosocomial infections during years and also more usage of microbial culture and laboratory data instead of empirical treatment of nosocomial infections.
Our data showed that mean days of hospitalization is significantly related with type of bacteria causing nosocomial infection in ICU. For example, Serratia spp. (24.5 ± 13.5 days) and Citrobacter spp. (26.4 ± 12.6 days) are acquired more soon compared with some bacteria such as Gram-negative bacilli (55.3 ± 14 days) or Streptococcus spp. (48.1 ± 29.6 days). A previous study showed that there was no relationship between ICU length of stay or days of mechanical ventilation and incidence of nosocomial infections.[25]
Data about antimicrobial resistance of microorganisms revealed that there was an improvement in the use of microbial culture and antibiogram from the year 1386 to 1389. Herein we talk about most prevalent germs in ICU.
P. aeruginosa's antibiograms showed that the most antibiotic-resistances were ampicillin, oxacillin, co-trimoxazole, and nitrofurantoin. Vancomycin, imipenem, and ciprofloxacin had reported to have the least resistance. Comparison of our data with a recently published article showed that there are some differences between our local antimicrobial resistance; for example, they found that 88% of P. aeruginosa's were resistant to cefepime,[26] but our data showed that just 39% of them are resistant to cefepime. Ceftazidime considered as a drug in which 82% of P. aeruginosa's were resistant to it,[26] although there were 68% of our cases which was resistant to ceftazidime. Imipenem and vancomycin had the least resistance pattern for Klebsiella. Cefotaxime, ampicillin, and ceftazidime showed the most resistance for E. coli. Although we gathered all data of bacterial prevalence and their antibiotic-resistance patterns, there were limited data about antibiotic-resistance, especially in previous years. Future studies with greater deal of antibiograms can help to investigate the antimicrobial resistance pattern. We did not collect risk factors apart from device use, which limits comparison of rates between units. For example, we did not consider the days of mechanical ventilation or type of procedures and medical interventions in any individual case of nosocomial infections.
Conclusion
We found some differences in our local prevalence of nosocomial infections and also in their resistance pattern compared to other centers. Knowing about our data will help physicians to administer the most suitable antibiotics for the treatment of nosocomial infections in our area.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Al Johani SM, Akhter J, Balkhy H, El-Saed A, Younan M, Memish Z. Prevalence of antimicrobial resistance among gram-negative isolates in an adult intensive care unit at a tertiary care center in Saudi Arabia. Ann Saudi Med. 2010;30:364–9. doi: 10.4103/0256-4947.67073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharya S, Mondal AS. Clinical microbiology in the intensive care unit: Strategic and operational characteristics. Indian J Med Microbiol. 2010;28:5–10. doi: 10.4103/0255-0857.58720. [DOI] [PubMed] [Google Scholar]
- 3.Kallel H, Dammak H, Bahloul M, Ksibi H, Chelly H, Ben Hamida C, et al. Risk factors and outcomes of intensive care unit-acquired infections in a Tunisian ICU. Med Sci Monit. 2010;16:PH 69–75. [PubMed] [Google Scholar]
- 4.Lizan-Garcia M, Peyro R, Cortina M, Crespo MD, Tobias A. Nosocomial infection surveillance in a surgical intensive care unit in Spain, 1996-2000: A time-trend analysis. Infect Control Hosp Epidemiol. 2006;27:54–9. doi: 10.1086/499167. [DOI] [PubMed] [Google Scholar]
- 5.Nicastri E, Petrosillo N, Martini L, Larosa M, Gesu GP, Ippolito G INF-NOS Study Group. Prevalence of nosocomial infections in 15 Italian hospitals: First point prevalance study for the INF-NOS project. Infection. 2003;31:10–5. [PubMed] [Google Scholar]
- 6.Baraibar J, Correa H, Mariscal D, Gallego M, Vallés J, Rello J. Risk factors for infection by Acinetobacter baumannii in intubated patients with nosocomial pneumonia. Chest. 1997;112:1050–4. doi: 10.1378/chest.112.4.1050. [DOI] [PubMed] [Google Scholar]
- 7.Mayank D, Anshuman M, Singh RK, Afzal A, Baronia AK, Prasad KN. Nosocomial cross-transmission of Pseudomonas aeruginosa between patients in a tertiary intensive care unit. Indian J Pathol Microbiol. 2009;52:509–13. doi: 10.4103/0377-4929.56143. [DOI] [PubMed] [Google Scholar]
- 8.Jung JY, Park MS, Kim SE, Park BH, Son JY, Kim EY, et al. Risk factors for multi-drug resistant Acinetobacter baumannii bacteremia in patients with colonization in the intensive care unit. BMC Infect Dis. 2010;10:228. doi: 10.1186/1471-2334-10-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassan KA, Hasan MK, Chowdhury MG, Akhter H. Aspects of infection in intensive care unit – Prevention and control. Mymensingh Med J. 2010;19:474–6. [PubMed] [Google Scholar]
- 10.Akhtar N. Hospital acquired infections in a medical intensive care unit. J Coll Physicians Surg Pak. 2010;20:386–90. [PubMed] [Google Scholar]
- 11.Drews MB, Ludwig AC, Leititis JU, Daschner FD. Low birth weight and nosocomial infection of neonates in a neonatal intensive care unit. J Hosp Infect. 1995;30:65–72. doi: 10.1016/0195-6701(95)90250-3. [DOI] [PubMed] [Google Scholar]
- 12.Fischer JE, Allen P, Fanconi S. Delay of extubation in neonates and children after cardiac surgery: Impact of ventilator-associated pneumonia. Intensive Care Med. 2000;26:942–9. doi: 10.1007/s001340051285. [DOI] [PubMed] [Google Scholar]
- 13.Gaynes RP, Edwards JR, Jarvis WR, Culver DH, Tolson JS, Martone WJ. Nosocomial infections among neonates in high-risk nurseries in the United States. National Nosocomial Infections Surveillance System. Pediatrics. 1996;98:357–61. [PubMed] [Google Scholar]
- 14.Bergmans DC, Bonten MJ, van Tiel FH, Gaillard CA, van der Geest S, Wilting RM, et al. Cross-colonisation with Pseudomonas aeruginosa of patients in an intensive care unit. Thorax. 1998;53:1053–8. doi: 10.1136/thx.53.12.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 16.Safdar N, Crnich CJ, Maki DG. The pathogenesis of ventilator-associated pneumonia: Its relevance to developing effective strategies for prevention. Respir Care. 2005;50:725–39. [PubMed] [Google Scholar]
- 17.Torres A, El-Ebiary M, Soler N, Montón C, Fàbregas N, Hernández C. Stomach as a source of colonization of the respiratory tract during mechanical ventilation: Association with ventilator-associated pneumonia. Eur Respir J. 1996;9:1729–35. doi: 10.1183/09031936.96.09081729. [DOI] [PubMed] [Google Scholar]
- 18.Bobo LD, Dubberke ER. Recognition and prevention of hospital-associated enteric infections in the intensive care unit. Crit Care Med. 2010;38:S324–34. doi: 10.1097/CCM.0b013e3181e69f05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulvatunyou N, Boonbarwornrattanakul A, Soonthornkit Y, Kocharsanee C, Lertsithichai P. Incidence of ventilator-associated pneumonia (VAP) after the institution of an educational program on VAP prevention. J Med Assoc Thai. 2007;90:89–95. [PubMed] [Google Scholar]
- 20.Jarvis WR, Edwards JR, Culver DH, Hughes JM, Horan T, Emori TG, et al. Nosocomial infection rates in adult and pediatric intensive care units in the United States. National Nosocomial Infections Surveillance System. Am J Med. 1991;91:185S–191S. doi: 10.1016/0002-9343(91)90367-7. [DOI] [PubMed] [Google Scholar]
- 21.Doyle JS, Buising KL, Thursky KA, Worth LJ, Richards MJ. Epidemiology of infections acquired in intensive care units. Semin Respir Crit Care Med. 2011;32:115–38. doi: 10.1055/s-0031-1275525. [DOI] [PubMed] [Google Scholar]
- 22.Xie DS, Xiong W, Xiang LL, Fu XY, Yu YH, Liu L, et al. Point prevalence surveys of healthcare-associated infection in 13 hospitals in Hubei Province, China, 2007-2008. J Hosp Infect. 2010;76:150–5. doi: 10.1016/j.jhin.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Tao L, Hu B, Rosenthal VD, Gao X, He L. Device-associated infection rates in 398 intensive care units in Shanghai, China: International Nosocomial Infection Control Consortium (INICC) findings. Int J Infect Dis. 2011;15:e774–80. doi: 10.1016/j.ijid.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Visnegarwala F, Iyer NG, Hamill RJ. Ventilator-associated pneumonia. Int J Antimicrob Agents. 1998;10:191–205. doi: 10.1016/s0924-8579(98)00037-5. [DOI] [PubMed] [Google Scholar]
- 25.Chelazzi C, Pettini E, Villa G, De Gaudio AR. Epidemiology, associated factors and outcomes of ICU-acquired infections caused by Gram-negative bacteria in critically ill patients: An observational, retrospective study. BMC Anesthesiol. 2015;15:125. doi: 10.1186/s12871-015-0106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed NH, Hussain T, Biswal I. Antimicrobial resistance of bacterial isolates from respiratory secretions of ventilated patients in a multi-specialty hospital. Avicenna J Med. 2015;5:74–8. doi: 10.4103/2231-0770.160233. [DOI] [PMC free article] [PubMed] [Google Scholar]


