Abstract
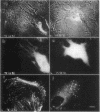
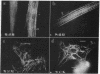
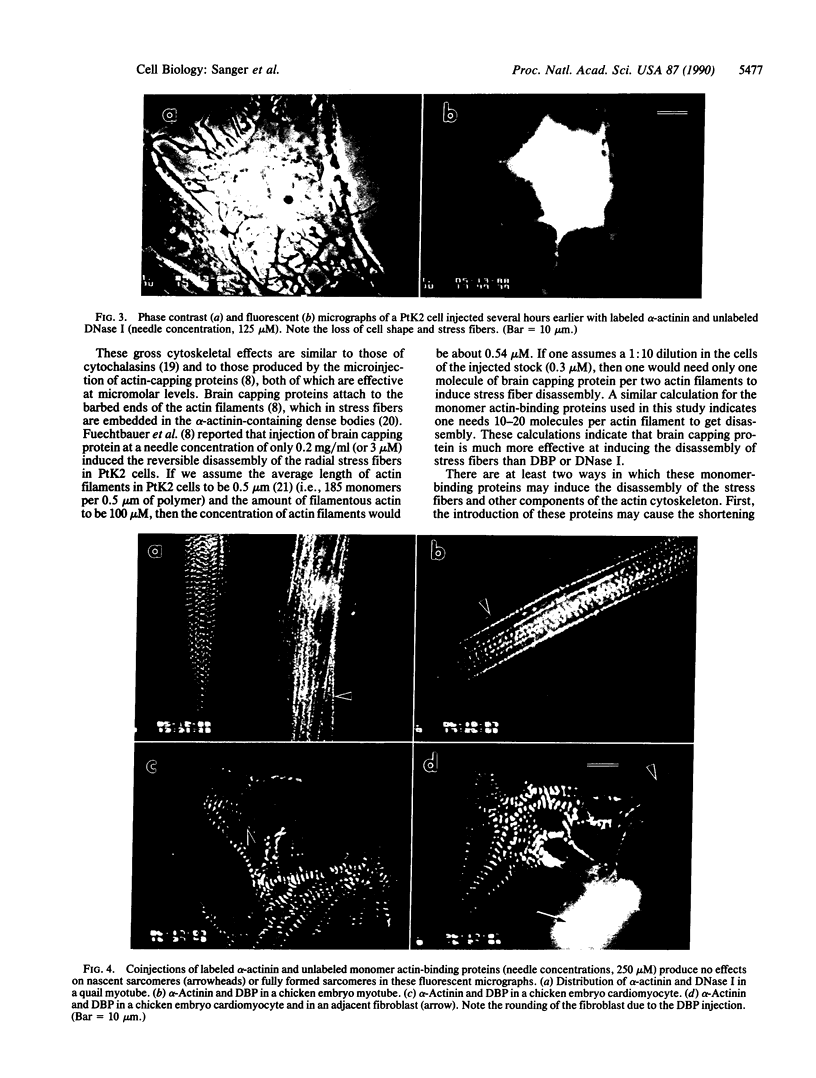
Plasma vitamin D-binding protein (DBP), which binds to monomeric actin, causes the breakdown of stress fibers when it is microinjected into nonmuscle cells. Disruption of the stress fiber network is also accompanied by shape changes in the cell that resemble those seen after cytochalasin treatment. When DBP was coinjected with fluorescently labeled alpha-actinin, no fluorescent stress fibers or attachment plaques were visible 30 min after injection. Twelve hours later the cells regained their flattened shape and their stress fibers. Fluorescently labeled DBP causes the same reversible changes in cell shape as the unlabeled protein. Upon injection, the labeled DBP diffuses throughout the cytoplasm, becoming localized by 12 hr in a punctate pattern, presumably due to lysozomal sequestration. Similar injections of DBP into skeletal myotubes and cardiac myocytes did not lead to shape changes or breakdown of nascent and/or fully formed myofibrils, even though DBP has a 2-fold higher binding affinity for muscle actin over that of the nonmuscle isoactins. Similar differential effects in nonmuscle cells were also observed after the microinjection of DNase I, another protein capable of binding monomer actin. The effects of these microinjected monomer actin-binding proteins imply that an accessible pool of monomer actin is needed to maintain stress fiber integrity in nonmuscle cells but not the integrity of the nascent or fully formed myofibrils in muscle cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARONSON J. Sarcomere size in developing muscles of a tarsonemid mite. J Biophys Biochem Cytol. 1961 Oct;11:147–156. doi: 10.1083/jcb.11.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blikstad I., Eriksson S., Carlsson L. alpha-Actinin promotes polymerization of actin from profilactin. Eur J Biochem. 1980 Aug;109(2):317–323. doi: 10.1111/j.1432-1033.1980.tb04797.x. [DOI] [PubMed] [Google Scholar]
- Byers H. R., White G. E., Fujiwara K. Organization and function of stress fibers in cells in vitro and in situ. A review. Cell Muscle Motil. 1984;5:83–137. doi: 10.1007/978-1-4684-4592-3_2. [DOI] [PubMed] [Google Scholar]
- Cooke N. E., Haddad J. G. Vitamin D binding protein (Gc-globulin). Endocr Rev. 1989 Aug;10(3):294–307. doi: 10.1210/edrv-10-3-294. [DOI] [PubMed] [Google Scholar]
- Dlugosz A. A., Antin P. B., Nachmias V. T., Holtzer H. The relationship between stress fiber-like structures and nascent myofibrils in cultured cardiac myocytes. J Cell Biol. 1984 Dec;99(6):2268–2278. doi: 10.1083/jcb.99.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dome J. S., Mittal B., Pochapin M. B., Sanger J. M., Sanger J. W. Incorporation of fluorescently labeled actin and tropomyosin into muscle cells. Cell Differ. 1988 Mar;23(1-2):37–52. doi: 10.1016/0045-6039(88)90035-8. [DOI] [PubMed] [Google Scholar]
- Füchtbauer A., Jockusch B. M., Maruta H., Kilimann M. W., Isenberg G. Disruption of microfilament organization after injection of F-actin capping proteins into living tissue culture cells. 1983 Jul 28-Aug 3Nature. 304(5924):361–364. doi: 10.1038/304361a0. [DOI] [PubMed] [Google Scholar]
- Glacy S. D. Pattern and time course of rhodamine-actin incorporation in cardiac myocytes. J Cell Biol. 1983 Apr;96(4):1164–1167. doi: 10.1083/jcb.96.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad J. G., Harper K. D., Guoth M., Pietra G. G., Sanger J. W. Angiopathic consequences of saturating the plasma scavenger system for actin. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1381–1385. doi: 10.1073/pnas.87.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad J. G., Kowalski M. A., Sanger J. W. Actin affinity chromatography in the purification of human, avian and other mammalian plasma proteins binding vitamin D and its metabolites (Gc globulins). Biochem J. 1984 Mar 15;218(3):805–810. doi: 10.1042/bj2180805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. S., McKenna N. M., Wang Y. Association of microinjected myosin and its subfragments with myofibrils in living muscle cells. J Cell Biol. 1988 Dec;107(6 Pt 1):2213–2221. doi: 10.1083/jcb.107.6.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko H., Okamoto M., Goshima K. Structural change of myofibrils during mitosis of newt embryonic myocardial cells in culture. Exp Cell Res. 1984 Aug;153(2):483–498. doi: 10.1016/0014-4827(84)90615-3. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Actin polymerization and its regulation by proteins from nonmuscle cells. Physiol Rev. 1982 Apr;62(2):672–737. doi: 10.1152/physrev.1982.62.2.672. [DOI] [PubMed] [Google Scholar]
- Kreis T. E., Winterhalter K. H., Birchmeier W. In vivo distribution and turnover of fluorescently labeled actin microinjected into human fibroblasts. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3814–3818. doi: 10.1073/pnas.76.8.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees A., Haddad J. G., Lin S. Brevin and vitamin D binding protein: comparison of the effects of two serum proteins on actin assembly and disassembly. Biochemistry. 1984 Jun 19;23(13):3038–3047. doi: 10.1021/bi00308a030. [DOI] [PubMed] [Google Scholar]
- Lind S. E., Janmey P. A., Chaponnier C., Herbert T. J., Stossel T. P. Reversible binding of actin to gelsolin and profilin in human platelet extracts. J Cell Biol. 1987 Aug;105(2):833–842. doi: 10.1083/jcb.105.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannherz H. G., Goody R. S., Konrad M., Nowak E. The interaction of bovine pancreatic deoxyribonuclease I and skeletal muscle actin. Eur J Biochem. 1980 Mar;104(2):367–379. doi: 10.1111/j.1432-1033.1980.tb04437.x. [DOI] [PubMed] [Google Scholar]
- Mc Leod J. F., Kowalski M. A., Haddad J. G., Jr Interactions among serum vitamin D binding protein, monomeric actin, profilin, and profilactin. J Biol Chem. 1989 Jan 15;264(2):1260–1267. [PubMed] [Google Scholar]
- Mittal B., Sanger J. M., Sanger J. W. Visualization of myosin in living cells. J Cell Biol. 1987 Oct;105(4):1753–1760. doi: 10.1083/jcb.105.4.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosawa M., Shimaoka S., Funatsu T., Ishiwata S., Maruyama K. Beta-actinin is not distinguishable from an actin barbed-end capping protein in chicken breast muscle. J Biochem. 1987 Jun;101(6):1481–1483. doi: 10.1093/oxfordjournals.jbchem.a122018. [DOI] [PubMed] [Google Scholar]
- Pochapin M. B., Sanger J. M., Sanger J. W. Microinjection of Lucifer yellow CH into sea urchin eggs and embryos. Cell Tissue Res. 1983;234(2):309–318. doi: 10.1007/BF00213770. [DOI] [PubMed] [Google Scholar]
- Sanger J. M., Mittal B., Pochapin M. B., Sanger J. W. Myofibrillogenesis in living cells microinjected with fluorescently labeled alpha-actinin. J Cell Biol. 1986 Jun;102(6):2053–2066. doi: 10.1083/jcb.102.6.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. M., Mittal B., Pochapin M., Sanger J. W. Observations of microfilament bundles in living cells microinjected with fluorescently labelled contractile proteins. J Cell Sci Suppl. 1986;5:17–44. doi: 10.1242/jcs.1986.supplement_5.2. [DOI] [PubMed] [Google Scholar]
- Sanger J. M., Sanger J. W. Banding and polarity of actin filaments in interphase and cleaving cells. J Cell Biol. 1980 Aug;86(2):568–575. doi: 10.1083/jcb.86.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. W., Mittal B., Sanger J. M. Analysis of myofibrillar structure and assembly using fluorescently labeled contractile proteins. J Cell Biol. 1984 Mar;98(3):825–833. doi: 10.1083/jcb.98.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. W., Mittal B., Sanger J. M. Formation of myofibrils in spreading chick cardiac myocytes. Cell Motil. 1984;4(6):405–416. doi: 10.1002/cm.970040602. [DOI] [PubMed] [Google Scholar]
- Sanger J. W., Sanger J. M., Jockusch B. M. Differences in the stress fibers between fibroblasts and epithelial cells. J Cell Biol. 1983 Apr;96(4):961–969. doi: 10.1083/jcb.96.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. W., Sanger J. M. The cytoskeleton and cell division. Methods Achiev Exp Pathol. 1979;8:110–142. [PubMed] [Google Scholar]
- Sanger J. W. The use of cytochalasin B to distinguish myoblasts from fibroblasts in cultures of developing chick striated muscle. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3621–3625. doi: 10.1073/pnas.71.9.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Chaponnier C., Ezzell R. M., Hartwig J. H., Janmey P. A., Kwiatkowski D. J., Lind S. E., Smith D. B., Southwick F. S., Yin H. L. Nonmuscle actin-binding proteins. Annu Rev Cell Biol. 1985;1:353–402. doi: 10.1146/annurev.cb.01.110185.002033. [DOI] [PubMed] [Google Scholar]