Abstract
Background
Studies demonstrate that most arm motor recovery occurs within 3 months after stroke, when measured with standard clinical scales. Improvements on these measures, however, reflect a combination of recovery in motor control, increases in strength, and acquisition of compensatory strategies.
Objective
To isolate and characterize the time course of recovery of arm motor control over the first year post-stroke.
Methods
Longitudinal study of 18 participants with acute ischemic stroke. Motor control was evaluated kinematically using a 2-D reaching task designed to minimize the need for anti-gravity strength and prevent compensation. Arm impairment was evaluated with the Fugl-Meyer assessment for upper extremity (FMA-UE), activity limitation with the Action Research Arm Test (ARAT), and strength with biceps dynamometry. Assessments were conducted at: 1.5, 5, 14, 27, and 54 weeks post-stroke.
Results
Motor control in the paretic arm improved up to week 5, with no further improvement beyond this time point. In contrast, improvements in the FMA-UE, ARAT, and biceps dynamometry continued beyond 5 weeks, with a similar magnitude of improvement between weeks 5 and 54 as between weeks 1.5 and 5.
Conclusions
Recovery after stroke plateaued much earlier for arm motor control, isolated with a global kinematic measure, compared to motor function assessed with clinical scales. This dissociation between the time courses of kinematic and clinical measures of recovery may be due to the contribution of strength improvement to the latter. Novel interventions, focused on the first month post-stroke, will be required to exploit the narrower window of spontaneous recovery for motor control.
INTRODUCTION
Arm paresis is present in 50–80% of stroke survivors.1–3 Clinical studies have shown that recovery from it is largely complete by 6 months post-stroke, but the precise time course depends on the outcome measure chosen.4–6 A reason for this is that activity limitation assessments, such as the Action Research Arm Test (ARAT), cannot reliably distinguish restitution from compensation.1,7,8 Restitution entails a return towards premorbid patterns of motor control and normal levels of strength, whereas compensation refers to using alternative strategies to accomplish a task.9–12 Post-stroke paresis comprises deficits in both strength and motor control, which we define as the ability to make coordinated, accurate, goal-directed movements.13,14 Motor control, and not just strength, is essential for skilled use of the limb and may determine how much it is used in everyday activities. The Fugl-Meyer assessment of upper extremity (FMA-UE), an impairment scale, is largely immune to compensation but has significant anti-gravity strength requirements.1,15 Consequently, its score blends strength with motor control, which have been shown to have dissociable recovery time courses.16–18 Recently, 3-D kinematics has been used to assess the recovery of motor control post-stroke; however, these tasks also require anti-gravity strength.19,20
Here we sought to isolate and characterize the time course of recovery of motor control in the paretic and non-paretic arms over the first year post-stroke. Motor control was assayed with a 2-D reaching task, which we have previously used to separate motor control from anti-gravity strength requirements and minimize compensation.21,22 This task allows precise measurement of kinematics, which increases specificity and sensitivity to post-stroke motor control changes.21–25 The time course of recovery of arm motor control was compared to recovery as measured by clinical scales of arm motor impairment, activity limitation, and strength.
METHODS
Study Design
The Study of Motor Acute Recovery Time course after Stroke (SMARTS) was a multicenter, longitudinal investigation of motor recovery of the upper extremity. Participating centers were: Columbia University, Johns Hopkins University, and University of Zurich. Here we report the arm kinematics sub-study.
Participants were scheduled to undergo testing at five post-stroke intervals: Visit-1 within the first two weeks (± 5 days), Visit-2 at 4 weeks (± 7 days), Visit-3 at 12 weeks (± 14 days), Visit-4 at 24 weeks (± 14 days), and Visit-5 at 52 weeks (± 14 days). Participants were enrolled up to Visit-2. All local ethics boards approved this study. Informed consent was obtained for all study procedures.
Study Participants
Eligible patients had MRI-confirmed first-time ischemic stroke with arm paresis. Exclusion criteria were: FMA-UE > 63, hemorrhagic stroke, traumatic brain injury, visual field deficit greater than quadrantanopsia (assessed with item 3 in NIH stroke scale), preexisting condition affecting arm function, additional neurological/psychiatric illness affecting motor performance or recovery, and inability to give informed consent.
We recruited participants from Johns Hopkins Hospital, New York Presbyterian Hospital, and affiliated institutions, between March 2012 and January 2014. Participants who completed at least two visits were included; the total sample size was 18. Demographics and clinical characteristics are summarized in Table 1.
Table 1.
Participant demographic and clinical characteristics at enrollment.
| Age, years, M (SD) | 55 (12.9) |
| Male/Female | 9/9 |
| Handedness | 14 right, 4 left |
| Lesion side | 6 dominant, 12 non-dominant |
| Stroke type | 5 cortical, 7 subcortical, 6 mixed |
| Time from stroke to enrollment, days, M (SD) | 13.13 (13.23) |
| FMA-UE, M (SD) | 42.50 (17.44) |
| ARAT, M (SD) | 34.22 (20.8) |
| Barthel Index, M (SD) | 83.06 (17.67) |
| NIHSS, M (SD) | 3.44 (2.04) |
| MoCA, M (SD) | 24.1 (3.34) |
| Star Cancellation (Neglect <44), Range | 47 – 54 |
FMA-UE: Fugl-Meyer Assessment of the Upper Extremity, ARAT: Action Research Arm Test, NIHSS: National Institutes of Health stroke scale, MoCA: Montreal Cognitive Assessment, M: Mean, SD: Standard deviation.
Twelve neurologically healthy volunteers with a similar age distribution as stroke participants (mean age = 58.4 years) underwent arm kinematic testing at a single visit. This control group was used as the reference population for our analysis.
Clinical Measures
FMA-UE and ARAT were used to assess arm motor impairment and activity limitation, respectively.7,8,15 Both scales are ordinal, with a maximum score of 66 for FMA-UE, and of 57 for ARAT. Both measures have shown good reliability, validity, and sensitivity to post-stroke motor changes.26–28 We tracked strength recovery of biceps brachii using dynamometry (MicroFET2, Pro Med Products, Atlanta, GA). Biceps brachii was chosen as it is a muscle used in our kinematic task. Maximum voluntary elbow flexion was measured while holding the dynamometer stationary against the participant’s wrist.29 The average of three trials was transformed into a Z-score using a normative dataset.30
Kinematic task
Arm motor control was assayed with a 2-D reaching task using the Kinereach™ apparatus, which is designed to decrease strength requirements by providing anti-gravity support and reducing friction (Fig. 1A).31
Figure 1.
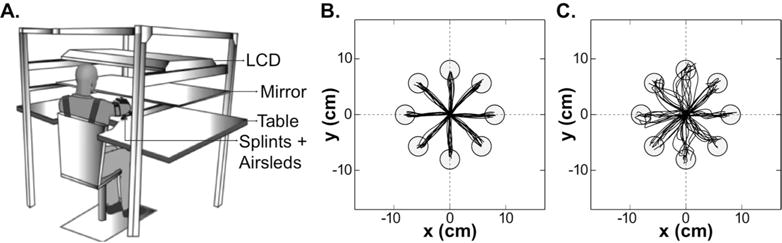
A. Experimental kinematic apparatus (modified from Przybyla et al.), and sample trajectories for B. the dominant right arm of a healthy control subject (AMD2= 3.95) and C. the paretic dominant right arm of a stroke participant (AMD2= 33.86, FMA-UE= 58).
Participants sat at a glass-surface table with their trunk secured to a high-back chair, thereby minimizing truncal compensation. Seat height was adjusted to bring shoulder, elbow, and wrist close to the same horizontal plane. The hand and forearm were splinted, only allowing shoulder and elbow movements. Frictionless movements were attained via an air-sled system. To prevent visualization of the arm, hand position was represented by a cursor on an LCD. This display was only visible through its reflection on a mirror, which lay above the forearm.
Participants were directed to make 80 straight movements with the cursor to eight circular targets with 1 cm radius, arrayed radially at 8 cm from a central start position. After holding the start position for 0.5 s individual targets appeared on a balanced pseudo-randomized order. Once a target appeared, participants had 3 s to complete the movement. Targets turned green making a pleasant ding for movements ending inside them with a peak speed between 10–40 cm/s. Hand position was tracked at 130 Hz with the Flock of Birds system (Ascension Technology, USA).
Kinematic analysis
Hand position data were analyzed using IGOR Pro (Wavemetrics, USA). Position time-series were low-pass filtered (8 Hz Butterworth), and differentiated to yield speed and acceleration. Left hand data were horizontally flipped, thus allowing grouping of movements directed towards targets that required similar joint configurations. For each movement, we identified the peak speed at the first zero-crossing of acceleration above a threshold of 10 cm/s. Movement-start was defined as the time, before peak speed, when speed surpassed 2 cm/s. Movement-end was defined as the time, after peak speed, when speed remained below 2 cm/s for more than 0.1 s. As we have done previously21,22,33, in order to decrease contamination from target guesses, incomplete movements due to late reaction to target appearance, and involuntary movements due to failure to fully stabilize the arm in the starting position, we applied the following exclusion criteria: direction at peak speed ≥ 90° away from target direction, or ending ≤30% of target distance. However, for sake of completeness, we still conducted all analyses with the full dataset.
Reaching kinematics were characterized using functional principal components analysis (FPCA), a generalization of traditional PCA to time-series data. This technique dramatically reduces the dimension of the analysis problem while retaining the major patterns that differ across movements.32 This analysis strategy precludes a priori choice of specific kinematic variables, such as directional error, smoothness, or endpoint accuracy, which bias the analysis towards specific components of motor control. Instead, FPCA compares distributions of movements at a global level and is sensitive to changes in overall movement quality. We computed the squared Mahalanobis distance (MD2) with respect to the reaching trajectories from the dominant arm of the reference population. Subject-specific average squared Mahalanobis distances (AMD2) were computed for each subject at each target for each time-point. Details of this analysis have been reported previously and are provided in Supplemental Materials.22,33
Statistical Analysis
For each outcome variable (FMA-UE, ARAT, biceps dynamometry Z-score, and AMD2), linear mixed models were used to examine changes over time; this analytic framework extends repeated measures ANOVA to allow more general tests of association. Subject-specific random intercepts were used to account for within-subject correlations for all outcome variables. Because AMD2 was measured for each target, additional subject/visit-specific intercepts were used to account for correlations across targets at each visit. Visit was treated as a categorical predictor with 5 levels. This mixed-model analysis uses all available data to estimate Visit means, so that missing visits do not cause the remainder of a subject’s data to be omitted. Non-constant residual variance across visits was addressed by using weighted least squares; alternative methods to account for non-constant variance, including square root and log transformations, yielded similar results. In this model framework, we make comparisons of interest (e.g. Visit-1 vs. Visit-2, Visit-2 vs. Visit-5) using Wald tests for contrasts in coefficients.
Based on our recent work, the sample size was deemed to be powered to detect changes in motor control similar to those observed in chronic stroke patients undergoing therapy.22
RESULTS
From the cohort of 18 stroke participants, 13 were enrolled at Visit-1 and 5 at Visit-2. Nine participants completed all five visits. Four dropped out of the study (one after Visit-2, one after Visit-3, and two after Visit-4) due to lack of interest. One participant was too paretic to perform the kinematic task at Visit-1. Combining late enrollment, dropouts, and inability to perform the task, 13% of the data were missing. We conducted a sensitivity analysis on missing data by removing an additional 13% of the data at random and repeating our model fitting and testing steps, and obtained significant results for the same comparisons as in the analysis presented here. Additionally, applying our pre-specified kinematic criteria we excluded: 4.56% of movements in Visit-1, 0.58% in Visit-2, 0.61% in Visit-3, 0.78% in Visit-4, and 0.34% in Visit-5. Analysis of the whole dataset (without excluding any movements) did not change the main finding (see Supplemental Materials).
Median time-after-stroke, in weeks, for each visit was: 1.6 for Visit-1 (SD = 2.7 days), 5.3 for Visit-2 (SD = 6.2 days), 13.9 for Visit-3 (SD = 5.6 days), 27 for Visit-4 (SD = 12.1 days), and 53.7 for Visit-5 (SD = 10 days). Henceforth, all results are reported using the rounded median time-after-stroke for each visit: Visit-1 = Week 1.5, Visit-2 = Week 5, Visit-3 = Week 14, Visit-4 = Week 27, and Visit-5 = Week 54.
The primary analysis focused on changes in the paretic arm during early recovery (between Weeks 1.5 and 5), and late recovery (between Weeks 5 and 54). For each of the outcome variables, FMA-UE, ARAT, biceps dynamometry Z-score, and average squared Mahalanobis distance (AMD2), we computed the difference from the regression model values between two given visits (delta), and the significance level for each comparison (adjusted p-values reflecting multiple comparisons for primary outcomes appear in Supplementary Table 3, and agree with the conclusions presented here). AMD2 is a measure of the statistical distance between entire reaching trajectories made by each stroke participant and healthy controls; hence it is a global kinematic measure.
Recovery of motor control of the arm reached a plateau at week 5 post-stroke
Stroke participants had abnormal reaching trajectories (Fig. 1B–C). Most notably, we found evidence that recovery of arm motor control plateaued after 5 weeks (Fig. 2). During early recovery, AMD2 in the paretic arm showed a significant decrease, i.e., movements became more like those made by healthy controls (delta = −18.39, p < 0.001). During late recovery, AMD2 in the paretic arm showed no significant change (delta = 1.65, p = 0.50). Comparison of the paretic arm AMD2 with that of healthy controls showed significant differences across all visits (largest p<0.01), thereby excluding the possibility of a ceiling effect for the kinematic task. Analyses for the non-paretic arm also showed a significant decrease in AMD2 in early recovery (delta = −3.54, p < 0.001), with no significant difference in late recovery (delta = 0.68, p = 0.08).
Figure 2.
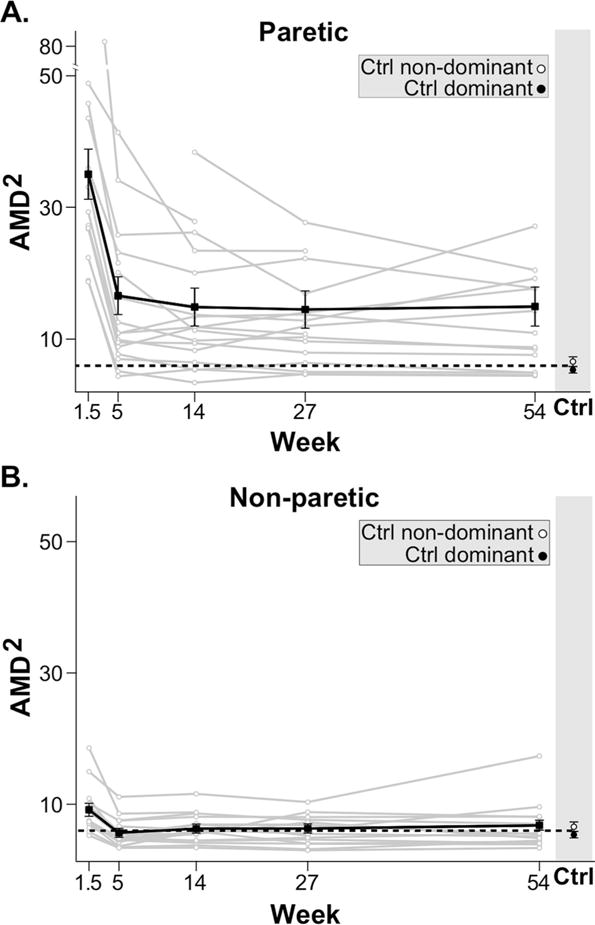
Time-course of motor control (AMD2) for paretic (A.) and non-paretic (B.) arms for individual stroke participants (grey) and mean regression linear model (black) with standard error bars. The horizontal black dotted lines indicate the average AMD2 of both dominant and non-dominant arms in healthy control subjects. For both arms there was clear improvement in motor control between week 1.5 and 5. No further improvement in motor control was seen beyond week 5.
Comparisons between contiguous visits in the late recovery period showed no significant changes in AMD2 in either arm. Paretic arm deltas were: Week 5 to 14 = −1.72 (p = 0.463), Week 14 to 27 = −0.37 (p = 0.87), and Week 27 to 54 = 0.44 (p = 0.85). Non-paretic deltas were: Week 5 to 14 = 0.6 (p = 0.28), Week 14 to 27 = 0.02 (p = .97), and Week 27 to 54 = 0.49 (p = 0.46).
Lastly, we conducted a secondary analysis motivated by the hypothesis that a subset of stroke participants, specifically those with severe motor control impairment at week 5, may continue to improve to Week 14 or beyond. For this purpose, subjects were split into “high” and “low” AMD2 using their Week 5 value in the paretic arm; the median was used as the threshold to define the two groups. Within each group we then repeated the primary comparisons (Fig. 3). For the “low” group, we found a significant decrease in AMD2 during early recovery (delta = −7.66, p < 0.001) and a smaller non-significant increase during late recovery (delta = 1.35, p = 0.23). For the “high” group, the early recovery decrease in AMD2 was large and significant (delta = −31.59, p <0.001), while the decrease during late recovery was smaller and non-significant (delta = −6.89, p = 0.08).
Figure 3.
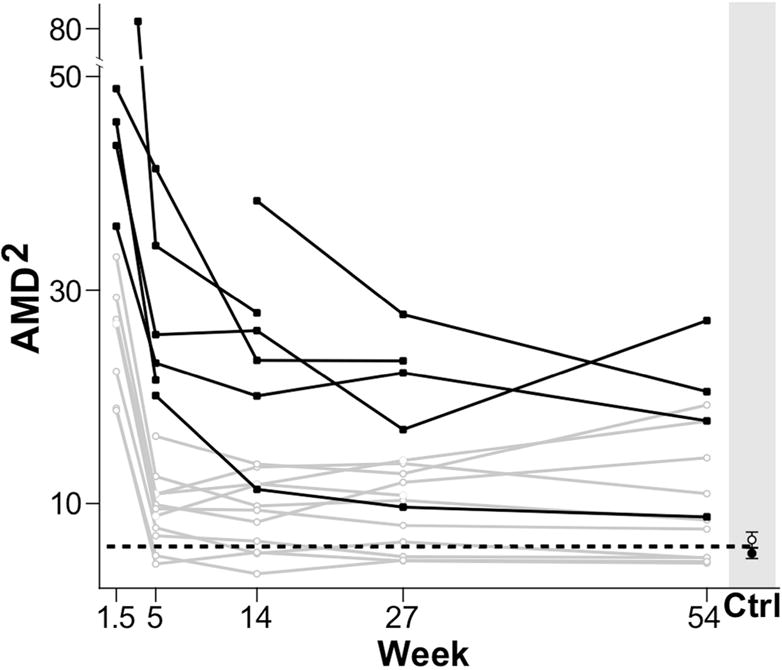
Severity of impairment and time-course of motor control (AMD2) for the paretic arm of all stroke participants. Participants were divided in two groups based on their motor control impairment severity (AMD2 at 5 weeks): in black, participants above the AMD2 median value (representing the more severely impaired), and in grey, participants below the AMD2 median value (representing the less impaired.) The black dotted line indicates the average AMD2 of both dominant and non-dominant arms in healthy control subjects. Although not significant, we observed some recovery of motor control beyond week 5 for subjects in the more severely impaired group.
In conclusion, motor control improved in the paretic and non-paretic arms during early recovery (up to Week 5 post-stroke), with no significant further improvement during late recovery (beyond Week 5). An early plateau in recovery of motor control was observed regardless of initial impairment severity, although it should be emphasized that our sample size could have been underpowered to detect a possible interaction between initial impairment severity and time to plateau. Still, any changes beyond 5 weeks would only represent a small fraction of those seen before that time point.
Clinical motor scores continued to improve beyond week 5 post-stroke
FMA-UE, ARAT, and biceps dynamometry Z-scores were used to track arm motor impairment, activity limitation, and arm strength, respectively (Fig. 4). All three measures showed significant improvements during early recovery, FMA-UE delta = 8.2, p = 0.010; ARAT delta = 9.7, p = 0.019; biceps dynamometry Z-score delta = 0.57, p = 0.008. During late recovery, all these clinical measures also showed significant improvements, FMA-UE delta = 10.1, p < 0.001; ARAT delta = 10.0, p < 0.001; biceps dynamometry Z-score delta = 0.94, p = 0.003. Analysis of the correlation between these clinical measures is included in Supplementary Materials (see Supplemental Figure 1).
Figure 4.
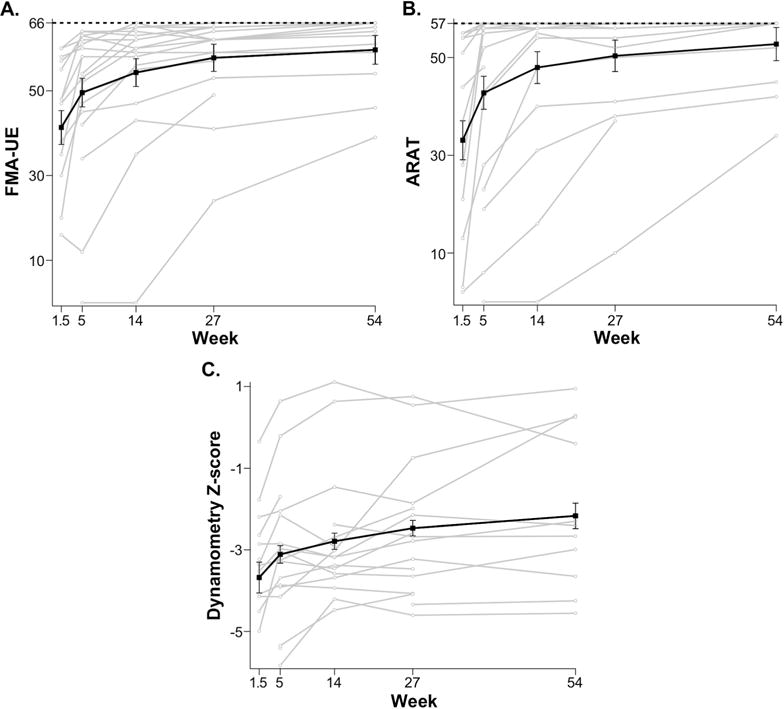
Time-course of clinical scales: FMA-UE (A.), ARAT (B.), and Biceps dynamometry Z-score (C.) for individual stroke participants (grey), and mean regression linear model (black) with standard error bars. The horizontal black dotted lines in A. and B. indicate the maximum possible scores for both measures (i.e: ARAT= 57, FMA-UE=66). For all clinical measures, the biggest improvement was seen between week 1.5 and week 5. Improvement in all clinical measures continued beyond week 5.
Figures 2 and 4 suggest distinct time courses for motor recovery depending on the measure used. To emphasize these differences in the recovery curves, the normalized values of FMA-UE, ARAT, biceps dynamometry Z-scores, and AMD2 are plotted on a single axis (Fig. 5). Normalization was accomplished by subtracting the mean value for each measure at week 1.5 and dividing by the difference in means at week 5 and at week 1.5. This normalization results in an average of zero at week 1.5 and an average of one at week 5; normalized values are scaled by the average delta between week 1.5 and week 5. Qualitative assessment of Figure 5, in addition to the statistical analyses above, support the hypothesis that recovery of motor control, measured using AMD2, plateaued much earlier than improvements in FMA-UE, ARAT, and biceps dynamometry Z-score. Repeating all analyses using log and square root transformations of clinical and kinematic measures did not qualitatively alter Figure 5 or its interpretation.
Figure 5.
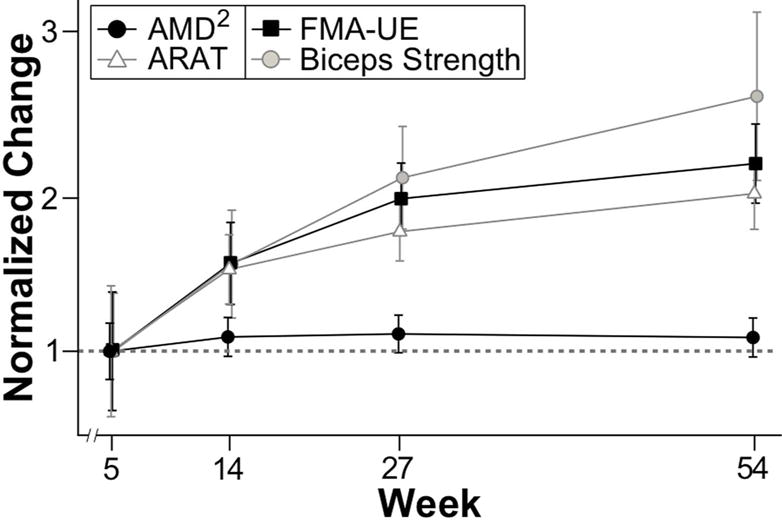
Normalized time-course for all outcome variables: AMD2, ARAT, FMA-UE, and Biceps dynamometry (strength) Z-score. The horizontal gray dotted line indicates the normalized value of the recovery achieved between the first and the second visit for each measure, which is one. AMD2 plateaued at week 5, while all clinical measures continued to improve through the first year after stroke.
Figure 5 emphasizes that for each clinical measure the delta from week 5 to week 54 is roughly 100% of the delta from week 1.5 to week 5. This is not the case for the motor control measure (AMD2) in the paretic arm, where the delta from week 5 to week 54 is roughly 10% of the delta from week 1.5 to week 5. We conducted an analysis to determine the power to detect a delta between week 5 and week 54 in AMD2 analogous to the one found in the clinical measures, the results indicated >99% power to detect such delta. In summary, qualitative comparison of the normalized time courses of the outcome measures also suggest that FMA-UE, ARAT, and biceps dynamometry Z-score improved as much during late recovery (between weeks 5 and 54) as they did during early recovery (between weeks 1.5 and 5), whereas AMD2 did not improve beyond Week 5.
DISCUSSION
Here we sought to determine the time course of recovery of motor control in the paretic and non-paretic arms after stroke using a 2-D reaching task that largely isolates motor control from the contaminating effects of weakness and compensation. The global kinematic measure of motor control was derived from the comparison between stroke participants’ reaching trajectories and normal trajectories from the dominant arm of healthy controls. The main finding was that improvement in arm motor control is almost all over by 5 weeks, whereas FMA-UE and ARAT scores continued to show robust improvements up to 14 weeks post-stroke. Furthermore, these two clinical measures, along with arm strength, continued to show improvements through the first year post-stroke. The results presented here not only suggest that there is a limited time-period for recovery of motor control, but also that this recovery process is distinct from what is captured by clinical measures of motor impairment, activity limitation, or strength.
The critical question raised by our results is why, at the group-level, did recovery of motor control plateau at 5 weeks, while FMA-UE and ARAT continued to show improvement beyond this point. Although this result is consistent with our a priori hypotheses about the potential for dissociation between control, strength, and compensation, it is important to first address the concern that perhaps the kinematic measure, being new and less validated, failed to detect the improvements seen with the other three clinical measures. There are several reasons that make this unlikely. In a recent study in over 200 stroke participants, a composite measure of arm kinematics was obtained using a robot. This kinematic measure was found to be more sensitive than FMA-UE in detecting motor recovery over the first 3 months post-stroke.34 Furthermore, we have recently shown that our global kinematic measure can detect changes after robotic therapy in chronic stroke that were undetectable with either FMA-UE or ARAT.22 Thus continuous 2-D kinematic data are in fact more sensitive to small differences in motor behavior than standard clinical measures.22,33 Moreover, it is also highly unlikely that the AMD2 improved in parallel with the clinical measures but went undetected. Analyses indicate >99% power to detect a change in AMD2 of the same percent magnitude as was seen for the clinical measures beyond week 5.
We speculate that the dissociation between the time courses of recovery of motor control and the clinical measures is mainly due to the improvements in strength beyond week 5. This is based on the observation that FMA-UE, ARAT, and biceps dynamometry followed similar time courses of recovery during the later recovery period (between week 5 and 54). Previous work in humans and non-human primates has shown that there are descending pathways that can explain return of strength without parallel returns in motor control.35–38 That fractionated control of the proximal upper extremity can be affected by stroke is consistent with the demonstration of short-latency excitation in deltoid muscles in healthy humans, which strongly suggests that monosynaptic corticomotoneuronal projections exist for proximal muscles of the contralateral arm.39 The authors of this study commented that bilateral organization of additional medium-latency projections to these same proximal arm muscles might explain why strength is relatively spared.
Functional imaging has also shown that humans have extensive representational maps across all of M1 even when they are only moving individual fingers.40 Loss of a piece of this motor cortical neural representation or of its output due to stroke would lead to a concomitant decrease in signal-to-noise, compromising motor control. Supporting this view, a recent study in mice showed that blocking intra-cortical synaptic transmission in motor cortex abolished complex movement trajectories elicited by long duration stimulation (500 ms), whereas map topography for initial movement direction elicited with 10 ms pulses remained intact, presumably because corticofugal output was spared.41 This result suggests that even when considering motor cortex without invoking alternative descending pathways, a mechanism for dissociation between control and strength can be envisaged.
Also, It is of interest that there was a small but transient worsening in motor control in the ipsilateral arm. Previous studies have reported motor abnormalities in the post-stroke ipsilateral upper extremity.42,43 The underlying mechanism seems to be mainly driven by the disruption of bihemispheric circuitry, which has been shown to be involved during the planning and execution of complex motor tasks.44–46 The alternative explanation of a disruption in non-decussating corticospinal fibers is not supported by recent evidence.38
Our study has some limitations. Given the strict inclusion criteria, our cohort represents a specific subset of the general stroke population. Another limitation is the sample size of 18 participants. Larger studies would be needed to definitively address the effect of initial severity and other variables of interest, such as lesion location, on the time course of recovery of motor control. Our comparison between “high” (more severely impaired) versus “low” motor control impairment groups, was motivated in part by a recent study by Semrau and colleagues, in which they showed that all their kinematic measures returned to normal within 6 weeks in mildly affected patients but some of the measures continued to show improvement beyond 6 weeks in severely affected patients.47 Several points should be made about the comparison between our results and the results by Semrau and colleagues. First, they found an interaction between severity and time to maximum recovery using kinematic measures, which is consistent with previous natural history studies using clinical measures.4–6,12,48,49 Due to our limited sample size, we were neither able to confirm nor refute the presence of this interaction: the decline in AMD2 after five weeks among the “high” (more severely impaired) group was not statistically significant, but visual inspection suggests modest recovery beyond week 5 for subjects in this group. Thus it is very likely that a small but nevertheless significant fraction of recovery of motor control is prolonged beyond 5 weeks in patients with more severe hemiparesis. Second, our global measure represents a mathematical distance from normal trajectories, which makes it more robust to compensatory contamination than any individual kinematic measure. That is to say, we constrained recovery of motor control to mean similarity to normal movements, whereas it is conceivable that single variables like initial directional error and speed could reflect recovery via compensation and strength. Third, the main point of our study was not to determine the absolute time at which recovery of motor control occurs but to contrast its time course with motor recovery measured with standard clinical scales.
Another potential concern is that any continued improvements of motor control in the hand and wrist, which were not directly measured, could contribute to the prolonged time course of recovery seen with clinical scales. We think this is unlikely, however, since this would explain neither the prolonged improvement of biceps strength nor the fact that after removing the hand and wrist components from the FMA-UE, its time course remained unchanged (see Supplemental Figure 2).
In conclusion, motor control shows maximal improvement in the first 5 weeks post-stroke, whereas clinical measures of motor impairment, activity limitation, and strength continued to improve beyond this point. We postulate that improvements in strength may be the underlying basis for the continued improvement in clinical measures, based both on their similar time courses of recovery and the correlations between them. In a recent study that measured kinematics in a 3-D reaching task, which necessarily requires anti-gravity strength, trajectory smoothness improved substantially beyond 5 weeks up to 8 weeks post-stroke.19 This result is consistent with the idea that strength improvements may give the appearance of a more extended time course of recovery for motor control. The distinct time course for motor control recovery suggests that more precise kinematic measures could better isolate the underlying process of spontaneous biological recovery. Strength training and learning of compensatory strategies can happen at any time, whereas spontaneous biological recovery only occurs early after stroke. Tracking motor control could enable us to identify and exploit the limited time window for spontaneous biological recovery in stroke survivors, as it may also be a period of heightened behavioral responsiveness to training.50–59
Our results highlight the urgency of implementing novel interventions to improve arm motor control very early on during the acute post-stroke period. It may be asked why rehabilitation should target motor control specifically. The answer is that motor control, and not just strength, is essential to use the upper limb in everyday complex tasks, for example typing and writing. One potential candidate treatment for improving motor control is robotics. In a recent study in chronic stroke patients, we showed that robotic arm therapy improved motor control on the same 2-D reaching task as was used in the current study.22 The changes were small but robust. It is possible that administering robotic therapy in the first month post-stroke, coinciding with the period of spontaneous biological recovery, might lead to larger improvements in motor control than those seen in chronic stroke.
Supplementary Material
References
- 1.World Health Organization. Towards a common language for functioning, disability and health. ICF; 2002. [Google Scholar]
- 2.Rathore SS, Hinn AR, Cooper LS, Tyroler HA, Rosamond WD. Characterization of incident stroke signs and symptoms. Stroke; a journal of cerebral circulation. 2002;33(11):2718–2721. doi: 10.1161/01.str.0000035286.87503.31. 2002/11/01/ [DOI] [PubMed] [Google Scholar]
- 3.Heller A, Wade DT, Wood VA, Sunderland A, Hewer RL, Ward E. Arm function after stroke: measurement and recovery over the first three months. Journal of Neurology, Neurosurgery, and Psychiatry. 1987;50(6):714. doi: 10.1136/jnnp.50.6.714. 1987/06// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skilbeck CE, Wade DT, Hewer RL, Wood VA. Recovery after stroke. Journal of Neurology, Neurosurgery, and Psychiatry. 1983;46(1):5–8. doi: 10.1136/jnnp.46.1.5. 1983/01// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke; a journal of cerebral circulation. 1992;23(8):1084–1089. doi: 10.1161/01.str.23.8.1084. 1992/08/01/ [DOI] [PubMed] [Google Scholar]
- 6.Nakayama H, Jørgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen stroke study. Arch Phys Med Rehabil. 1994;75(4):394–398. doi: 10.1016/0003-9993(94)90161-9. 1994/04/01/ [DOI] [PubMed] [Google Scholar]
- 7.Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4(4):483–492. doi: 10.1097/00004356-198112000-00001. 1981/12// [DOI] [PubMed] [Google Scholar]
- 8.Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the action research arm test. Neurorehabil Neural Repair. 2008;22(1):78–90. doi: 10.1177/1545968307305353. 2008/01/01/ [DOI] [PubMed] [Google Scholar]
- 9.Roby-Brami A, Feydy A, Combeaud M, Biryukova EV, Bussel B, Levin MF. Motor compensation and recovery for reaching in stroke patients. Acta Neurologica Scandinavica. 2003;107(5):369–381. doi: 10.1034/j.1600-0404.2003.00021.x. 2003/05/01/ [DOI] [PubMed] [Google Scholar]
- 10.Levin MF, Kleim JA, Wolf SL. What do motor recovery and “compensation” mean in patients following stroke? Neurorehabil Neural Repair. 2008 doi: 10.1177/1545968308328727. 2008/12/31/ [DOI] [PubMed] [Google Scholar]
- 11.Raghavan P, Santello M, Gordon AM, Krakauer JW. Compensatory motor control after stroke: an alternative joint strategy for object-dependent shaping of hand posture. J Neurophysiol. 2010;103(6):3034–3043. doi: 10.1152/jn.00936.2009. 2010/06// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF. Getting neurorehabilitation right what can be learned from animal models? Neurorehabil Neural Repair. 2012;26(8):923–931. doi: 10.1177/1545968312440745. 2012/10/01/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krakauer JW. Arm function after stroke: from physiology to recovery. Semin Neurol. 2005;25(4):384–395. doi: 10.1055/s-2005-923533. 2005/12// [DOI] [PubMed] [Google Scholar]
- 14.Wagner JM, Lang CE, Sahrmann SA, et al. Relationships between sensorimotor impairments and reaching deficits in acute hemiparesis. Neurorehabil Neural Repair. 2006;20(3):406–416. doi: 10.1177/1545968306286957. 2006/09// [DOI] [PubMed] [Google Scholar]
- 15.Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med 1975. 1975;7(1):13–31. [PubMed] [Google Scholar]
- 16.Noskin O, Krakauer JW, Lazar RM, et al. Ipsilateral motor dysfunction from unilateral stroke: implications for the functional neuroanatomy of hemiparesis. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79(4):401–406. doi: 10.1136/jnnp.2007.118463. 2008/04// [DOI] [PubMed] [Google Scholar]
- 17.Lang CE, Edwards DF, Birkenmeier RL, Dromerick AW. Estimating minimal clinically important differences of upper-extremity measures early after stroke. Arch Phys Med Rehabil. 2008;89(9):1693–1700. doi: 10.1016/j.apmr.2008.02.022. 2008/09// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barak S, Duncan PW. Issues in selecting outcome measures to assess functional recovery after stroke. NeuroRx. 2006;3(4):505–524. doi: 10.1016/j.nurx.2006.07.009. 2006/10// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Kordelaar J, van Wegen E, Kwakkel G. Impact of time on quality of motor control of the paretic upper limb after stroke. Arch Phys Med Rehabil. 2014;95(2):338–344. doi: 10.1016/j.apmr.2013.10.006. 2014/02// [DOI] [PubMed] [Google Scholar]
- 20.van Kordelaar J, van Wegen EE, Nijland RH, Daffertshofer A, Kwakkel G. Understanding adaptive motor control of the paretic upper limb early poststroke: the EXPLICIT-stroke program. Neurorehabil Neural Repair. 2013 Nov-Dec;27(9):854–863. doi: 10.1177/1545968313496327. [DOI] [PubMed] [Google Scholar]
- 21.Kitago T, Liang J, Huang VS, et al. Improvement after constraint-induced movement therapy: recovery of normal motor control or task-specific compensation? Neurorehabil Neural Repair. 2013;27(2):99–109. doi: 10.1177/1545968312452631. 2013/02// [DOI] [PubMed] [Google Scholar]
- 22.Kitago T, Goldsmith J, Harran M, et al. Robotic therapy for chronic stroke: general recovery of impairment or improved task-specific skill? J Neurophysiol. 2015;114(3):1885–1894. doi: 10.1152/jn.00336.2015. 2015/09// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krebs HI, Aisen ML, Volpe BT, Hogan N. Quantization of continuous arm movements in humans with brain injury. Proc Natl Acad Sci USA. 1999;96(8):4645–4649. doi: 10.1073/pnas.96.8.4645. 1999/04/13/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rohrer B, Fasoli S, Krebs HI, et al. Movement smoothness changes during stroke recovery. J Neurosci. 2002;22(18):8297–8304. doi: 10.1523/JNEUROSCI.22-18-08297.2002. 2002/09/15/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lum PS, Burgar CG, Kenney DE, Van der Loos HF. Quantification of force abnormalities during passive and active-assisted upper-limb reaching movements in post-stroke hemiparesis. IEEE Trans Biomed Eng. 1999;46(6):652–662. doi: 10.1109/10.764942. 1999/06// [DOI] [PubMed] [Google Scholar]
- 26.Hsieh Y-w, Wu C-y, Lin K-c, Chang Y-f, Chen C-l, Liu J-s. Responsiveness and validity of three outcome measures of motor function after stroke rehabilitation. Stroke; a journal of cerebral circulation. 2009;40(4):1386–1391. doi: 10.1161/STROKEAHA.108.530584. 2009/04// [DOI] [PubMed] [Google Scholar]
- 27.Lang CE, Wagner JM, Dromerick AW, Edwards DF. Measurement of upper-extremity function early after stroke: properties of the action research arm test. Arch Phys Med Rehabil. 2006;87(12):1605–1610. doi: 10.1016/j.apmr.2006.09.003. 2006/12// [DOI] [PubMed] [Google Scholar]
- 28.Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63(10):1606–1610. doi: 10.1093/ptj/63.10.1606. 1983/10// [DOI] [PubMed] [Google Scholar]
- 29.Bohannon RW. Make tests and break tests of elbow flexor muscle strength. Phys Ther. 1988;68(2):193–194. doi: 10.1093/ptj/68.2.193. 1988/02// [DOI] [PubMed] [Google Scholar]
- 30.Andrews AW, Thomas MW, Bohannon RW. Normative values for isometric muscle force measurements obtained with hand-held dynamometers. Phys Ther. 1996;76(3):248–259. doi: 10.1093/ptj/76.3.248. 1996/03// [DOI] [PubMed] [Google Scholar]
- 31.Przybyla N, Good D, Sainburg R. Virtual reality arm supported training reduces motor impairment In two patients with severe hemiparesis. J Neurol Transl Neurosci. 2013;1(2):1018. 2013/09/15/ [PMC free article] [PubMed] [Google Scholar]
- 32.Yao F, Müller H-G, Wang J-L. Functional data analysis for sparse longitudinal data. Journal of the American Statistical Association. 2005;100(470):577–590. 2005/06/01/ [Google Scholar]
- 33.Goldsmith J, Kitago T. Assessing systematic effects of stroke on motorcontrol by using hierarchical function-on-scalar regression. J R Stat Soc Ser C Appl Stat. 2016;65(2):215–236. doi: 10.1111/rssc.12115. 2016/02// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krebs HI, Krams M, Agrafiotis DK, et al. Robotic measurement of arm movements after stroke establishes biomarkers of motor recovery. Stroke; a journal of cerebral circulation. 2014;45(1):200–204. doi: 10.1161/STROKEAHA.113.002296. 2014/01// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118(Pt 2):495–510. doi: 10.1093/brain/118.2.495. 1995/04// [DOI] [PubMed] [Google Scholar]
- 36.Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci. 2009;29(15):4993–4999. doi: 10.1523/JNEUROSCI.3720-08.2009. 2009/04/15/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol (Lond) 2011;589(Pt 23):5603–5612. doi: 10.1113/jphysiol.2011.215160. 2011/12/01/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain. 2012;135(Pt 7):2277–2289. doi: 10.1093/brain/aws115. 2012/07// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colebatch JG, Rothwell JC, Day BL, Thompson PD, Marsden CD. Cortical outflow to proximal arm muscles in man. Brain. 1990;113(Pt 6):1843–1856. doi: 10.1093/brain/113.6.1843. 1990/12// [DOI] [PubMed] [Google Scholar]
- 40.Diedrichsen J, Wiestler T, Krakauer JW. Two distinct ipsilateral cortical representations for individuated finger movements. Cereb Cortex. 2013;23(6):1362–1377. doi: 10.1093/cercor/bhs120. 2013/06// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harrison TC, Ayling OGS, Murphy TH. Distinct cortical circuit mechanisms for complex forelimb movement and motor map topography. Neuron. 2012;74(2):397–409. doi: 10.1016/j.neuron.2012.02.028. 2012/04/26/ [DOI] [PubMed] [Google Scholar]
- 42.Schaefer SY, Haaland KY, Sainburg RL. Ipsilesional motor deficits following stroke reflect hemispheric specializations for movement control. Brain. 2007;130(Pt 8):2146–2158. doi: 10.1093/brain/awm145. 2007/08// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haaland KY, Schaefer SY, Knight RT, et al. Ipsilesional trajectory control is related to contralesional arm paralysis after left hemisphere damage. Exp Brain Res. 2009;196(2):195–204. doi: 10.1007/s00221-009-1836-z. 2009/06// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haslinger B, Erhard P, Weilke F, et al. The role of lateral premotor-cerebellar-parietal circuits in motor sequence control: a parametric fMRI study. Brain Res Cogn Brain Res. 2002;13(2):159–168. doi: 10.1016/s0926-6410(01)00104-5. 2002/04// [DOI] [PubMed] [Google Scholar]
- 45.Poldrack RA, Sabb FW, Foerde K, et al. The neural correlates of motor skill automaticity. J Neurosci. 2005;25(22):5356–5364. doi: 10.1523/JNEUROSCI.3880-04.2005. 2005/06/01/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Filippi M, Rocca MA, Mezzapesa DM, et al. Simple and complex movement-associated functional MRI changes in patients at presentation with clinically isolated syndromes suggestive of multiple sclerosis. Human brain mapping. 2004 Feb;21(2):108–117. doi: 10.1002/hbm.10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Semrau JA, Herter TM, Scott SH, Dukelow SP. Examining differences in patterns of sensory and motor recovery after stroke with robotics. Stroke; a journal of cerebral circulation. 2015;46(12):3459–3469. doi: 10.1161/STROKEAHA.115.010750. 2015/12// [DOI] [PubMed] [Google Scholar]
- 48.Kwakkel G, Kollen BJ, Grond Jvd, Prevo AJH. Probability of regaining dexterity in the flaccid upper limb. Stroke; a journal of cerebral circulation. 2003;34(9):2181–2186. doi: 10.1161/01.STR.0000087172.16305.CD. 2003/09/01/ [DOI] [PubMed] [Google Scholar]
- 49.Prabhakaran S, Zarahn E, Riley C, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair. 2008;22(1):64–71. doi: 10.1177/1545968307305302. 2008/01/01/ [DOI] [PubMed] [Google Scholar]
- 50.Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008;63(3):272–287. doi: 10.1002/ana.21393. 2008/03/01/ [DOI] [PubMed] [Google Scholar]
- 51.Zeiler SR, Krakauer JW. The interaction between training and plasticity in the poststroke brain. Current Opinion in Neurology. 2013;26(6):609–616. doi: 10.1097/WCO.0000000000000025. 2013/12// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeiler SR, Hubbard R, Gibson EM, et al. Paradoxical motor recovery from a first stroke after induction of a second stroke reopening a postischemic sensitive period. Neurorehabil Neural Repair. 2016;30(8):794–800. doi: 10.1177/1545968315624783. 2016/09/01/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10(12):861–872. doi: 10.1038/nrn2735. 2009/12// [DOI] [PubMed] [Google Scholar]
- 54.Nudo RJ. Mechanisms for recovery of motor function following cortical damage. Current Opinion in Neurobiology. 2006;16(6):638–644. doi: 10.1016/j.conb.2006.10.004. 2006/12// [DOI] [PubMed] [Google Scholar]
- 55.Buma F, Kwakkel G, Ramsey N. Understanding upper limb recovery after stroke. Restorative Neurology & Neuroscience. 2013;31(6):707–722. doi: 10.3233/RNN-130332. 2013/12// [DOI] [PubMed] [Google Scholar]
- 56.Witte OW, Stoll G. Delayed and remote effects of focal cortical infarctions: secondary damage and reactive plasticity. Adv Neurol 1997. 1997;73:207–227. [PubMed] [Google Scholar]
- 57.Biernaskie J, Chernenko G, Corbett D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J Neurosci. 2004;24(5):1245–1254. doi: 10.1523/JNEUROSCI.3834-03.2004. 2004/02/04/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barbay S, Plautz EJ, Friel KM, et al. Behavioral and neurophysiological effects of delayed training following a small ischemic infarct in primary motor cortex of squirrel monkeys. Exp Brain Res. 2005;169(1):106–116. doi: 10.1007/s00221-005-0129-4. 2005/11/05/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dobkin BH. Rehabilitation after stroke. New England Journal of Medicine. 2005;352(16):1677–1684. doi: 10.1056/NEJMcp043511. 2005/04/21/ [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


