Abstract
Biopsies of suspected drug-induced liver injury pose a particular challenge to the pathologist that requires a careful and systematic approach. The initial evaluation should be as objective as possible, setting aside consideration of the medical history. Histological changes are catalogued with attention to the hepatic architecture, noting the intensity and character of inflammation, cholestasis, apoptosis and necrosis. Bile ducts, portal vessels, hepatocytes, sinusoidal lining cells and vessels are each examined for evidence of injury. The assessment culminates with the determination of the overall pattern of injury. Armed with this information, the pathologist correlates the findings with the patient’s medical and pharmacological history. Drug induced liver injury is always a diagnosis of exclusion, so the emphasis should be on identification of potential competing causes of injury. If alternate etiologies of injury can be eliminated, the histological injury pattern can be compared to the known patterns of injury of suspect medications. The histological changes can also shed light on the potential mechanism of injury in situations where the suspect agents are new and do not yet have reports of hepatotoxicity. The pathological findings, the histological differential diagnosis and expert interpretation are part of a complete biopsy assessment and provide information that is of greatest value in patient management.
Keywords: hepatotoxicity, acute liver failure, acute hepatitis, hepatic necrosis, cholestasis
Introduction
Evaluation of a liver biopsy in a suspected case of drug-induced liver injury (DILI) can be a daunting experience. Unlike the well-defined and commonly encountered patterns of chronic hepatitis and fatty liver disease, a biopsy in a case of DILI can show a wide variety of histological findings: inflammation, necrosis, cholestasis, fibrosis, nodular regeneration, vascular injury, and duct destruction among others. These histological lesions can be arranged in combinations that can be difficult to classify into recognizable patterns of liver injury. Nevertheless, the determination that a drug is or is not involved in liver injury has real clinical consequences and a liver biopsy can provide a wealth of information on both the pattern of injury as well as its severity, guiding both determination of the etiology of the injury as well as subsequent clinical decision-making.
Because of the inherent complexity of the pathology, the pathologist must approach the biopsy with a systematic evaluation plan. This review will outline one possible method, beginning with objective assessment of the extent and pattern of hepatic injury, followed by correlation with the clinical history and laboratory findings and a final assessment of the both the likelihood and specific etiology of DILI. Although most of the discussion relates to evaluation of injury related to prescription and nonprescription medications, these same principles apply to the evaluation of injury related to environmental and occupational toxins and injury due to herbal and dietary supplements. Therefore, while it is not explicitly stated in every instance, the term “DILI” should also be understood to include these other etiologies as appropriate.
Use of the Liver Biopsy in Drug-Induced Liver Injury
A liver biopsy is not required to evaluate a patient with suspected DILI. In the U.S. Drug-Induced Liver Injury Network (DILIN), only 50% of patients enrolled in the prospective protocol underwent liver biopsy during the course of their evaluation 1. Unlike autoimmune hepatitis, in which the published algorithms incorporate liver biopsy as part of the diagnosis 2,3, the most widely used clinical algorithm for DILI determination (the RUCAM) 4 does not have a place for including the findings of liver biopsies. Nevertheless, when a liver biopsy is performed, there are several questions the pathologist may be asked to address: Are the patient’s liver abnormalities due to DILI or some other etiology of liver disease? If DILI is likely, can the liver biopsy help define which drug is causing the patient’s injury? How severe is the injury and does the inflammatory pattern suggest steroid-responsiveness by analogy to autoimmune hepatitis? Can it inform us with respect to mechanism of injury or prognosis?
Once the clinical decision to perform a biopsy has been made, it is important that a plan for biopsy evaluation be made prior to the procedure. A portion of the biopsy may need to be sent for culture or for viral PCR testing. If mitochondrial injury is suspected, a 1 to 2 mm segment may be fixed in glutaraldehyde and sent for ultrastructural examination. Saving a piece frozen for cryostat sections is unlikely to be necessary as most specialized tests can be performed on the formalin-fixed tissue. If staining for fat is desired (as in the case of microvesicular steatosis), a formalin-fixed piece can be cut on a cryostat prior to processing and stained with oil red O or Sudan black. Contacting the pathologist prior to the biopsy can be helpful to decide how best to triage the specimen.
Clearly the more clinical questions that need to be addressed, the more critical it is to have an adequate biopsy to work with, both for the separate specialized testing outlined above and for routine histological assessment. There have been not been studies of biopsy adequacy in DILI, but some answers can be inferred from studies of biopsy adequacy in chronic viral hepatitis and fatty liver disease. Most of these studies have focused on the effects of biopsy size on the staging and grading of chronic hepatitis C. Sampling error is increased with shorter biopsies as well as with those taken with a narrow gauge needle with a significant underestimation of both grade and stage in biopsies less than 1.5 cm in length or 10 portal areas 5–7. Studies of biopsy size in fatty liver disease have shown similar findings 8. It should be remembered that these studies were performed to identify size limitations with respect to specific biopsy features or for making a specific diagnosis (steatohepatitis). In biopsies performed to evaluate a broad clinical differential diagnosis, these biopsy size estimates should be considered as lower estimates. In order to adequately evaluate injury to ducts 9 and veins, 10 to 20 complete portal areas and a similar number of central veins may be necessary. Given the dependence of observing complete structures on the width of the biopsy and the total number of structures on the biopsy length 10,11 it would be reasonable to follow the guidance of AASLD position paper on liver biopsy and obtain at least 3 cm of core using a 16 gauge needle 12. Biopsies obtained using a transvenous approach using narrower gauge needles may require additional length of biopsy.
Considerations in the histological assessment of DILI
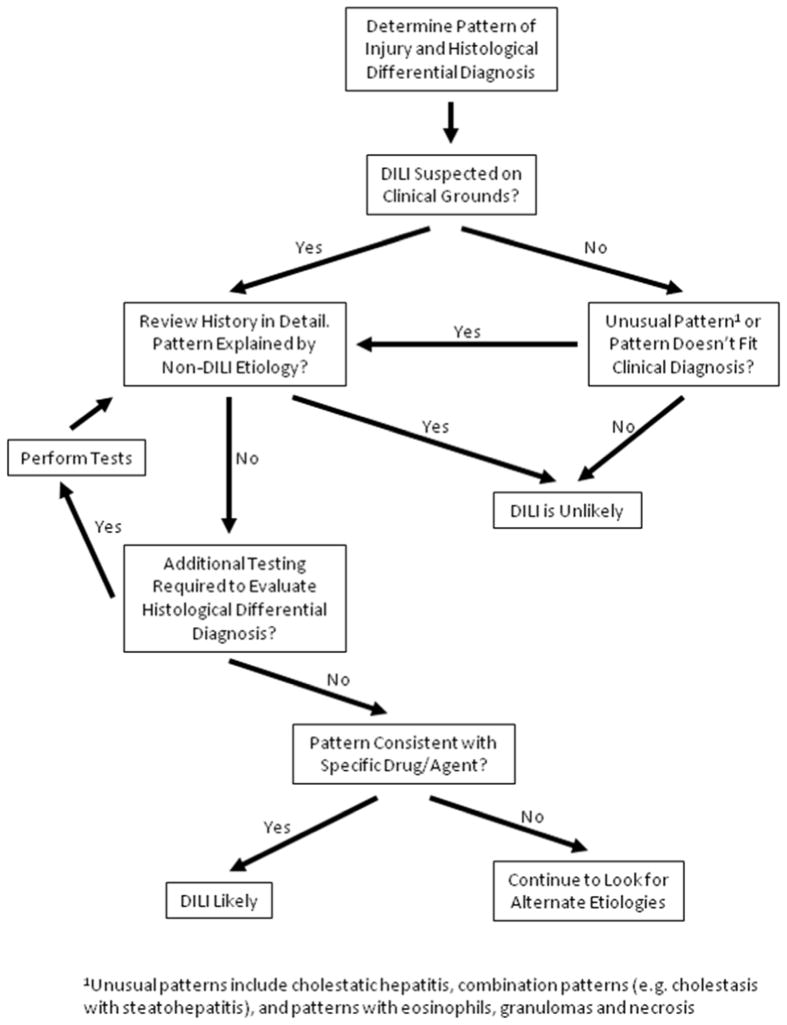
Figure 1 outlines a general approach to the evaluation of liver biopsies in DILI. The initial review should be as objective as possible, without regard to clinical information. True blinded review, in which the biopsy is evaluated in the absence of any clinical information, has the greatest chance to identify subtle unexpected findings but is difficult to achieve in a typical practice setting. The pathologist should be ready to use all of the available histochemical and immunohistochemical tools so as to provide as much information as possible. In cases in which the etiology of liver injury is unclear or potentially multifactorial, the evaluation begins with examination of multiple levels stained with hematoxylin and eosin and includes routine special stains (Table 1). Masson trichrome and reticulin stains are used to assess hepatic architecture and fibrosis. Staining for iron helps to distinguish the various pigments in the liver as well as to assess iron overload. While Wilson’s disease may sometimes enter the differential diagnosis of DILI, the copper stain is better used to identify evidence of chronic cholestasis. Periodic acid-Schiff (PAS) staining performed following diastase digestion is mainly useful for identifying clusters of macrophages that may remain as the only evidence of injury following an episode of acute hepatitis, although occult storage diseases such as alpha-1-antitrypsin deficiency may also be revealed. Stains for bile (if not clearly seen on the other stains) and infectious organisms can be used as needed. Fat stains are only useful on tissue that has not undergone tissue processing, although as noted above, the tissue can be formaldehyde fixed prior to cryostat sectioning and still be stained for fat.
Figure 1.
Algorithm for Biopsy Evaluation in Suspected Drug-Induced Liver Injury.
From Kleiner DE. Liver histology in the diagnosis and prognosis of drug-induced liver injury. Clinical Liver Disease 2014;4(1):12–6; with permission.
Table 1.
Special Histochemical Stains and Immunoperoxidase Stains Useful in the Evaluation of Liver Injury
| Core Stains (used in all cases in which the etiology is unclear or the clinical scenario is complex) | |
| Masson Trichrome | Distinguishes necrotic collapse from fibrosis and identifies early perisinusoidal fibrosis; Helps to identify occluded or narrowed veins (portal and central) |
| Reticulin | Allows assessment of the hepatocyte plate architecture, including detection of nodular regenerative hyperplasia, collapse of reticulin network in necrosis, peliotic dilations |
| Iron | Identifies iron deposits and helps to distinguish pigments (iron, lipofuscin, bile) |
| Copper (rhodanine) | Identifies copper accumulation in chronic cholestasis and copper storage diseases |
| Periodic acid-Schiff with diastase | Highlight macrophages containing cell debris as well as glycoprotein/glycolipid storage diseases. Also stains some forms of bile and lipofuscin |
| Discretionary Histochemical Stains (optional stains for particular applications) | |
| Oil red O, Sudan black | Fat stains – must be performed on fixed or frozen tissue prior to processing |
| Bile | Directly identifies bile |
| Periodic acid-Schiff | Dark purple stain highlights hepatocytes against areas of necrosis or fibrosis as well as outlining hepatocellular fat vacuoles, secondary stain for fungi |
| Elastin | Identifies vessels and elastin deposits in fibrosis |
| Acid fast (Ziehl-Nielsen or Fite) | Identifies mycobacteria |
| Methanamine silver | Identifies fungal organisms |
| Warthin-Starry, Steiner and Dieterle | Identifies atypical bacterial infection |
| Immunoperoxidase Stains (used to identify specific proteins) | |
| Keratin 7 | Bile ducts and ductules, cholestatic hepatocytes |
| Keratin 19 | Bile ducts and ductules, Canals of Hering |
| CD34 | Normally stains endothelium of arteries and veins and aberrantly expressed in sinusoidal endothelium exposed to increased arterial blood flow |
| Ubiquitin | Mallory-Denk bodies |
| Hepatitis B surface and core antigens | Detection of hepatitis B in viral carriers |
| CMV, HSV, Adenovirus | Identification of specific viral infection |
| In-Situ Hybridization | |
| EBER | Identification of EBV-infected lymphocytes |
Immunoperoxidase stains can also be helpful in the evaluation of suspected DILI. Acute viral infection with herpes simplex virus (HSV) or adenovirus can mimic toxic acute hepatitis and the viral inclusions can be difficult to identify without specific stains. Similarly, patients with acute reactivation of hepatitis B secondary to immunosuppressive medications will often have positive reactions to immunostains for hepatitis B. The immunostains for keratin 7 and 19 as well as for the endothelial cell marker CD34 may be the most useful. The keratin stains can help identify residual ductal epithelial cells in portal areas with marked inflammation and duct injury. The absence of staining can confirm the loss of bile ducts. In cases with chronic cholestatic injury, keratin 7 may be expressed in periportal hepatocytes13. CD34 is normally not expressed in the sinusoidal endothelial cells, but in situations in which arterial blood flow is increased relative to portal vein flow, the sinusoidal endothelial cells will express CD34 aberrantly 14. Epstein-Barr virus (EBV) is detected through the use of an in-situ hybridization reaction, allowing EBV-related hepatitis to be detected or excluded from consideration.
Characteristic Patterns of Injury
The result of this initial evaluation should be an accurate and detailed description of the histological lesions as well as characterization of the injury into one or more of the stereotypical patterns of hepatic injury. While drugs and herbals have been associated with all types of liver injury, any individual agent has a limited range of injury patterns 15. For example, the combination drug amoxicillin-clavulanate most often causes a cholestatic hepatitis with mild to moderate inflammation, prominent cholestasis and duct injury 16,17. It sometimes causes a chronic cholestatic injury with bile duct loss 18 and has only rarely been implicated in cases of true acute hepatitis. It does not cause fatty liver disease of any sort and has not been associated with vascular injury patterns or cirrhosis. Thus, classification of the injury pattern can be correlated with the known range of injury patterns for particular suspect agents, allowing the pathologist to limit the differential diagnosis and comment on the likelihood that a particular agent caused the patient’s liver injury.
Despite the wide range of potential patterns, most suspected drug injuries that come to biopsy seem to fall into one of the necroinflammatory or cholestatic patterns of injury (Table 2). In Hans Popper’s landmark paper on drug and toxin induced liver injury, acute viral hepatitis-like injury and cholestastic hepatitis accounted for 39% and 32% of the cases, respectively 19. A more recent analysis of biopsies from 249 cases of suspected drug and herbal-induced liver injury by the U.S. DILIN found that over half of the biopsies could be classified into one of six necroinflammatory and cholestatic injury patterns. These patterns included cholestatic hepatitis (29%), acute hepatitis (21%), chronic hepatitis (14%), chronic cholestasis (10%), and acute cholestasis (9%) 15. Zonal necrosis, the typical pattern of acetaminophen injury, accounted for only 3% of cases, probably because cases of acetaminophen DILI were excluded from enrollment in the DILIN.
Table 2.
Common Necroinflammatory and Cholestatic Injury Patterns
| Pattern | Characteristic features | Non-DILI differential | ALT/ULN1 | AP/ULN1 | Frequency2 |
|---|---|---|---|---|---|
| Acute (Lobular) Hepatitis | Lobular-dominant inflammation with/without confluent or bridging necrosis; no cholestasis | Acute viral hepatitis, acute autoimmune hepatitis | 13 to 27 | 1 to 3 | 21% |
| Chronic (Portal) Hepatitis | Portal-dominant inflammation, interface hepatitis (also includes mononucleosis pattern), with or without portal-based fibrosis; no cholestasis | Chronic viral or autoimmune diseases, early PBC/PSC, mononucleosis-associated hepatitis, reactive hepatitis from systemic disease, common variable immunodeficency | 3 to 10 | 1 to 2 | 14% |
| Zonal Coagulative Necrosis | Zone 3 or 1 coagulative necrosis, usually without significant inflammation | Hypoxic-ischemic injury (zone 3) | 6 to 47 | 1 to 2 | 3% |
| Cholestatic Hepatitis | Acute or chronic hepatitis pattern plus zone 3 cholestasis | Acute viral hepatitis, large duct obstruction, graft-vs-host disease | 2 to 13 | 1 to 3 | 29% |
| Acute Cholestasis (Intrahepatic, Canalicular) | Hepatocellular and/or canalicular cholestasis in zone 3; may show duct injury, but little inflammation | Sepsis, acute large duct obstruction, benign recurrent intrahepatic cholestasis | 2 to 10 | 1 to 4 | 9% |
| Chronic Cholestasis | Periportal cholate stasis, periportal fibrosis, copper accumulation, duct sclerosis or injury, duct loss | PSC, PBC, chronic cholestatic injury/AIH overlap, chronic large duct obstruction, idiopathic adulthood ductopenia, ductopenic GVHD, IgG4 related systemic sclerosis | 3 to 12 | 2 to 8 | 10% |
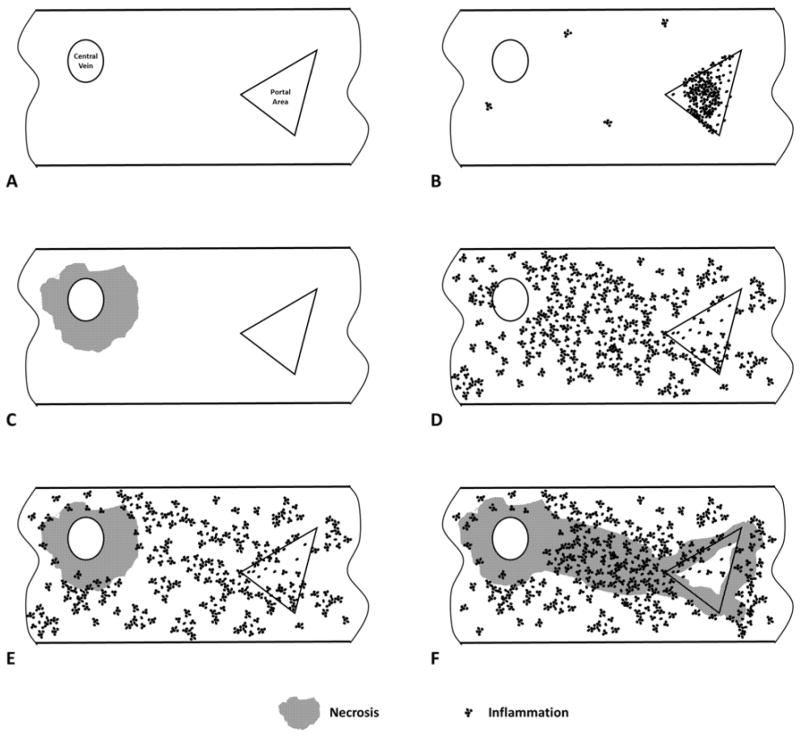
For most cases, an initial categorization of the inflammatory pattern into acute hepatitis-like or chronic hepatitis-like can be made at low magnification. Figure 2 shows schematic diagrams of combinations of inflammation and necrosis that can be seen. Acute hepatitis-like inflammation predominantly affects the lobular parenchyma and can be associated with either zonal or non-zonal necrosis (Figure 3). Foci of lobular inflammation, composed of small aggregates of lymphocytes and macrophages, can be so numerous that they disrupt the normal lobular sinusoidal architecture, a phenomenon known as lobular disarray. The inflammation is often accompanied by evidence of cytologic injury of hepatocytes, including cytoplasmic swelling, clumping and clearing, as well as apoptosis or necrosis. There may be evidence of hepatocyte regeneration, including variation in the size of hepatocytes, hepatocyte rosette formation and reactive nuclear changes. Portal inflammation and interface hepatitis are often present as well, but they do not dominant the inflammatory pattern. Portal dominant inflammation with only mild to moderate lobular inflammation is more characteristic of chronic hepatitis-like injury, such as is seen in cases of chronic viral or autoimmune hepatitis (Figure 4). Other features may also help in this categorization. Necrosis, particularly large areas of confluent necrosis or hepatocyte drop-out, is more consistent with acute hepatitis while advanced fibrosis (more than fibrotic expansion of portal areas) would be more consistent with chronic hepatitis. It should be noted that, in the context of DILI, a patient presenting with “acute hepatitis” may show a chronic hepatitis pattern of inflammation. For example, both minocycline 20,21 and the statins 22,23 may show portal predominant inflammation similar to chronic viral hepatitis, yet present with an acute onset of aminotransferase elevations and jaundice. Other features, including microgranulomas and mild to moderate steatosis may be present without affecting the categorization as either acute or chronic hepatitis. The presence of hepatocellular or canalicular bile accumulation should prompt consideration of one of the cholestatic patterns of injury, discussed below. The differential diagnosis for both acute and chronic hepatitis patterns mainly includes the hepatitis viruses and autoimmune hepatitis, but consideration should also be given to less common causes of hepatitis, including Epstein-Barr related hepatitis 24, hepatitis associated with collagen vascular diseases25 and hepatitis associated with immunodeficiencies 26.
Figure 2.
Necroinflammatory patterns. A. Normal liver biopsy with portal area (triangle) and central vein (oval). B. Chronic hepatitis pattern with portal predominant inflammation. C. Zone 3 necrosis without significant portal or parenchymal inflammation. D. Acute hepatitis with diffuse inflammation but no confluent necrosis. E. Acute hepatitis with zone 3 necrosis. F. Acute hepatitis with bridging necrosis involving portal areas and central veins.
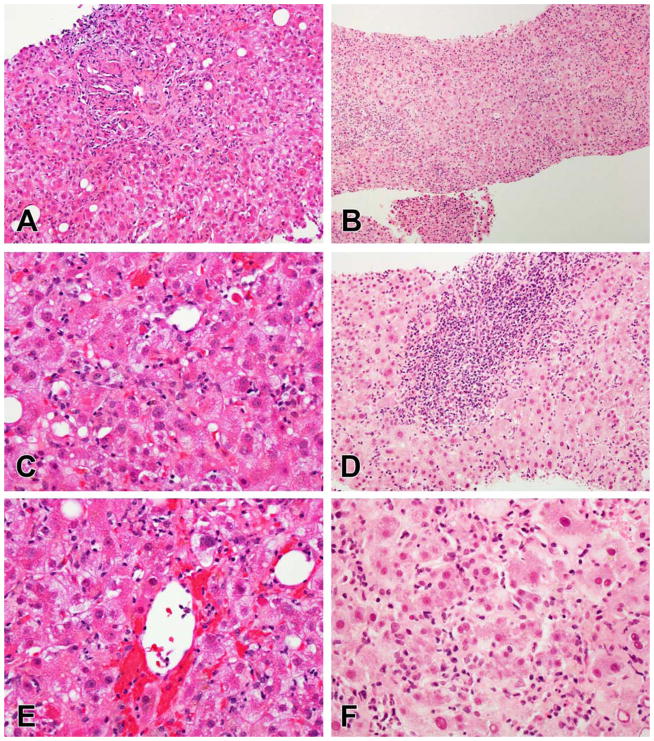
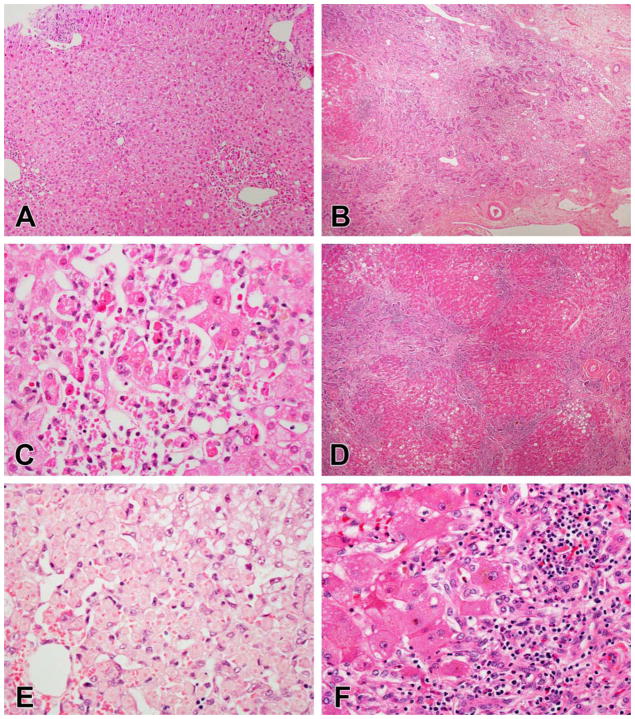
Figure 3.
Examples of acute hepatitis. A, C, E. Acute hepatitis due to atorvastatin. The portal areas show sparse inflammation although the portal-parenchymal interface is disrupted (A). Numerous foci of lobular inflammation as well as scattered acidophil bodies are present (C). Hemorrhage was present around the central veins (E). B, D, F. Acute hepatitis due to ipilimumab. At low magnification the biopsy looks cellular, mainly from infiltrates of inflammatory cells. The portal areas do not stand out in panel (B) but a few showed dense lymphocytic inflammation with circumferential interface hepatitis (D). At high magnification there is extensive disruption of the hepatocyte plates and the sinusoidal architecture by inflammation (F).
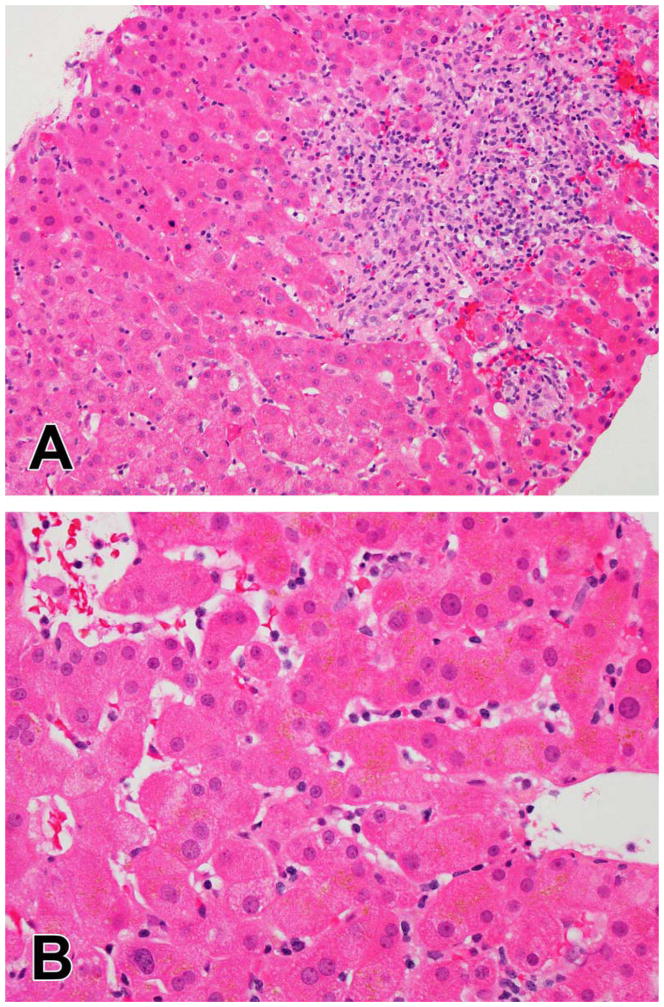
Figure 4.
Chronic hepatitis-like injury due to 6-mercaptopurine. The inflammation is mainly in the portal areas, with only occasional foci of lobular inflammation (A). Sinusoidal lymphocytosis is present, but the sinusoidal architecture is intact.
When large epithelioid granulomas dominate the inflammation, the pattern should be classified as granulomatous hepatitis. This pattern was uncommon in the DILIN series accounting for only 1% of the cases, although epithelioid granulomas were noted in almost 5% of cases 15. Granulomas may be an indication of a hypersensitivity-type of drug reaction and their presence has been associated with a better prognosis 15. Certain drugs have granulomas or granulomatous hepatitis as a typical pattern of injury. These drugs include the sulfonamides, dapsone, allopurinol and phenytoin all of which have been associated with systemic syndrome of rash, eosinophilia and other systemic symptoms of hypersensitivity 27. The differential diagnosis includes infection (including unusual bacterial and rickettsial infections as well and those from fungi and mycobacteria), sarcoidosis and primary biliary cholangitis. While drug induced granulomatous injury can mimic the discrete round well-formed granulomas of sarcoidosis (e.g. with interferon alfa 28), they are usually less well-defined, irregular aggregates of epithelioid histiocytes admixed with lymphocytes and eosinophils.
Confluent necrosis centered around the central vein (zone 3) that mimics hypoxic-ischemic liver injury is the characteristic injury pattern of acetaminophen. Figure 5 contrasts this zonal type of necrosis with the more irregular necrosis that follows severe acute hepatitis. Although the diagnosis is usually clinically apparent, the pathologist may see a biopsy when the diagnosis is not suspected or when there are other potential causes of liver injury. Early in the injury there is coagulative necrosis of hepatocytes in zone 3 extending to involve zones 2 and 1 as the injury becomes more severe. The sinusoidal cells remain mainly intact as the toxic injury is specific to the hepatocytes. Macrophages and neutrophils may be present, particularly within and around the edges of the necrotic zone 29,30. Although uncommon, similar patterns of zonal necrosis without an inflammatory infiltrate suggestive of acute hepatitis may be seen with other drugs and toxins. Necrosis may also be observed in veno-occlusive disease (VOD)/sinusoidal obstruction syndrome (SOS) 31, so careful review of central veins for occlusive lesions is necessary because the treatments are very different. The main non-toxic cause of bland zonal necrosis is hypoxic/ischemic injury. Irregular patches of non-zonal necrosis should prompt a search for viral inclusions at the edges of the necrotic foci and immunostains for herpes simplex and adenovirus may be helpful.
Figure 5.
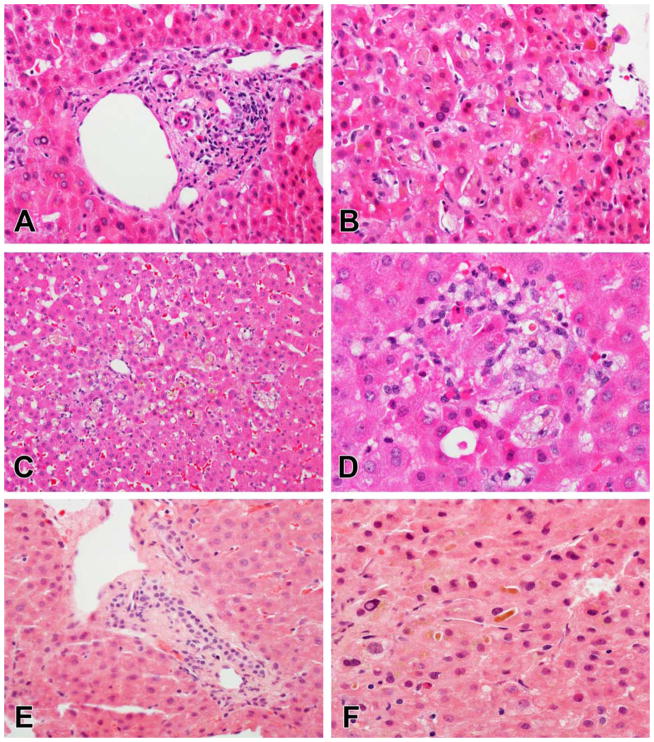
Necrosis patterns in DILI. A, C, E. Zonal necrosis due to mithramycin (A, C) and acetaminophen (E). In both of these examples there is some combination of coagulative necrosis and apoptosis in zone 3 without much inflammation beyond an infiltrate of macrophages. In the case of acetaminophen, the coagulative necrosis mainly affects the hepatocytes as the sinusoidal lining cells remain intact (E). B, D, F. Sub-massive necrosis due to isoniazid injury. The necrosis in this case is irregular, with large areas of complete multiacinar necrosis (B) next to areas that show some necrosis along with regenerative nodules (D). Inflammation remains in residual portal areas and along the edges of the regenerative nodules (F)
The cholestatic patterns of injury include acute (or bland) cholestasis, cholestatic hepatitis and chronic cholestasis. Cholestatic hepatitis was a common pattern of injury in both Popper’s original study and the DILIN study, accounting for 32% and 29% of cases respectively 15,19. In the DILIN study, this diagnostic category included all cases with a combination of inflammation and cholestasis that was visible on routine stains. The inflammatory component in cholestatic hepatitis varies from very mild portal and lobular inflammation to patterns that mimic acute and chronic hepatitis (Figure 6A–D). Bile plugs are seen in canaliculi and often can be recognized in hepatocyte cytoplasm or in sinusoidal macrophages. The presence of canalicular bile is diagnostic, but bile pigment in hepatocytes and macrophages must be distinguished from iron and lipofuscin. Bile accumulates first in zone 3, and may be accompanied by hepatocyte swelling and infiltration of foamy macrophages in the sinusoids. While duct injury is a common finding (seen in 52% of cases), it may also be seen in acute hepatitis (40%) and chronic hepatitis (24%) patterns in which no bile stasis was identified 15. Cholestatic hepatitis is the main pattern of injury for multiple drugs, including most antibiotics and psychotropic drugs. As the degree of inflammation becomes very mild, cholestastic hepatitis patterns merge with acute cholestatic injury (Figure 6E and F). In acute cholestatic injury (also called bland or intrahepatic cholestasis), there is zone 3 cholestasis as described above, but with little or no inflammatory reaction either in the portal areas or parenchyma. Acute cholestatic injury is the characteristic injury pattern of the anabolic steroids and oral contraceptives 32. Such injury from oral contraceptives has become less common as the doses of estrogens and progestins have been reduced in more recent formulations, but anabolic steroid jaundice remains a clinical problem, particularly among young men using body building supplements 33–35. These supplements can be easily obtained without a prescription and often contain steroid derivatives 36. The differential diagnosis for cholestatic hepatitis and acute cholestasis depends heavily on the other histological features in the biopsy, particularly the inflammatory pattern. With severe acute hepatitis-like inflammation and hepatocellular injury, the differential mainly includes acute viral hepatitis. Fulminant variants of autoimmune hepatitis are also a consideration, particularly if there is prominent portal inflammation and plasma cells. With acute cholestasis and mild cholestatic hepatitis, acute large duct obstruction and sepsis/post-operative cholestasis should be considered. Cholangiolar cholestasis (bile plugs within dilated ductules) strongly suggests sepsis or sepsis-like inflammatory syndromes 37. Cholestatic hepatitis with moderate degrees of inflammation has a more limited differential diagnosis, but other types of inflammation associated cholestasis may show this pattern 38. Cholestatic hepatitis may also be the result if more than one etiology is present, for example chronic viral hepatitis with acute large duct obstruction.
Figure 6.
Cholestatic injury patterns. A, B, C, D. Cholestatic hepatitis due to amoxicillin-clavulanate (A, B) and azathioprine (C, D). In the amoxicillin-clavulanate case, the portal areas show mild inflammation and duct injury (A). Canalicular and hepatocellular cholestasis is present in zone 3, associated with mild lobular inflammation (B). In the azathioprine case, there is zone 3 cholestasis with clusters of pigmented macrophages in the sinuses (C). Scattered foci of inflammation with hepatocyte apoptosis are also present (D), while the portal areas had little inflammation (not shown). E, F. Acute cholestasis due to anabolic steroids. In this case the portal areas are normal without duct injury or loss (E). There is prominent canalicular cholestasis with bile plugs in zone 3, but little associated inflammation or hepatocyte injury (F).
Chronic cholestatic injury (Figure 7) is less common than cholestatic hepatitis, accounting for only about 10% of cases 15, but it is clinically important as it is the most common pattern of injury in patients with evidence of chronic liver injury due to drugs 39. Chronic cholestatic injury is recognized by the characteristic hepatocellular change of periportal cholatestasis (pseudoxanthomatous change) often accompanied by copper accumulation or keratin 7 expression in periportal hepatocytes. Duct injury is common, found in 78% of cases while a third will have some degree of bile duct paucity 15. There may be prominent ductular reaction, but this is not a specific finding. The histological changes of duct injury are variable, from reactive epithelial changes to infiltration of ducts by inflammatory cells to periductal sclerosis. Although there are some specific drugs that cause chronic cholestasis, most notably hepatic arterial infusion with floxuridine 40, chronic cholestasis is minor pattern of injury for many drugs that cause cholestatic hepatitis. Vanishing bile duct syndrome may present acutely or may be found on follow-up biopsies in patients with prolonged jaundice. Recent additions to the list of drugs causing VBDS include multiple members from the fluoroquinolone antibiotics 41,42 and the oncological agent temozolomide 43. The latter drug appears to cause VBDS as its primary pattern, although it is possible that there is bias towards biopsy of only severe prolonged injury in cancer patients. The differential diagnosis of chronic cholestatic DILI mainly includes chronic large duct obstruction, primary biliary cholangitis and the various non-drug etiologies of sclerosing cholangitis.
Figure 7.
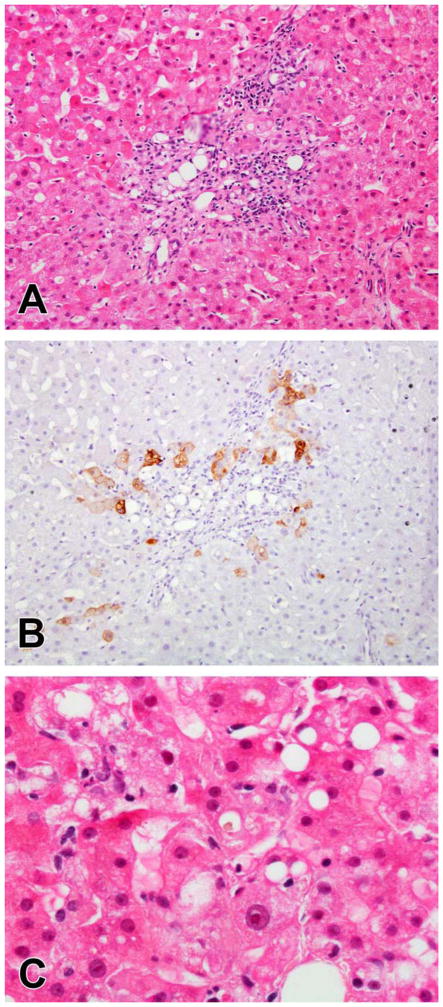
Chronic cholestatic injury with bile duct loss due to lenolidomide. The portal areas have mild inflammation and lack either a bile duct or ductular reaction (A). The absence of duct structures is confirmed by keratin 7 staining, which also demonstrates the aberrant expression of keratin 7 in periportal hepatocytes that occurs in chronic cholestasis (B). As with the other cholestatic patterns, there is often cholestasis in zone 3 (C)
Drug induced fatty liver disease is well-reported but because the non-drug etiologies of fatty liver disease are very common, caution should be taken before ascribing steatosis to DILI. There are three basic patterns of fatty liver disease caused by drug and other agents: Macrovesicular steatosis (with or without inflammation and fibrosis), steatohepatitis and microvesicular steatosis. In drug induced macrovesicular steatosis and steatohepatitis, the changes may be very similar to the changes of non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Specific drugs may cause histological changes that are subtly different than common NAFLD and NASH. Methotrexate can be associated with portal fibrosis 44 and lack the ballooning injury of typical NASH, although the typical pattern of steatohepatitis can also be observed 45. Of note, alcohol use, obesity and diabetes all raise the risk of methotrexate injury suggesting synergistic injury 46,47. In amiodarone-related injury, the Mallory-Denk bodies tend to be periportal rather than perivenular 48. On the other hand, tamoxifen associated steatohepatitis is indistinguishable from NASH 49. Steatosis and steatohepatitis may also result from secondary drug effects, such as weight gain or drug associated lipodystrophy.
The pattern of microvesicular steatosis should be distinguished from other forms of fatty liver disease because of its clinical and prognostic significance. Microvesicular steatosis is not merely small vacuole fat, but a foamy change of the hepatocyte cytoplasm resulting from innumerable small fat vacuoles (Figure 8). Cells with larger fat vacuoles may be present but the foamy change should dominate the histological picture to classify the case as microvesicular steatosis. This pattern is almost always associated with mitochondrial injury, typically with uncoupling of oxidative phosphorylation (aspirin) 50 or damage to mitochondrial DNA (fialuridine) 51,52. A distinctive clinical syndrome of lactic acidosis and hepatic failure results from the mitochondrial injury. The mere presence of patches of microvesicular steatosis (without the full pattern) was associated with worse outcome in the DILIN study 15. The list of agents in clinical use associated with microvesicular steatosis is short, but includes the anti-HIV medications zidovudine, didanosine, stavudine and indinivir 53–55 as well as the antibiotic linezolid 56. The differential diagnosis of diffuse microvesicular steatosis is short, including only fatty liver of pregnancy. It should be assumed that diffuse microvesicular steatosis is due to a drug or toxin unless proven otherwise.
Figure 8.
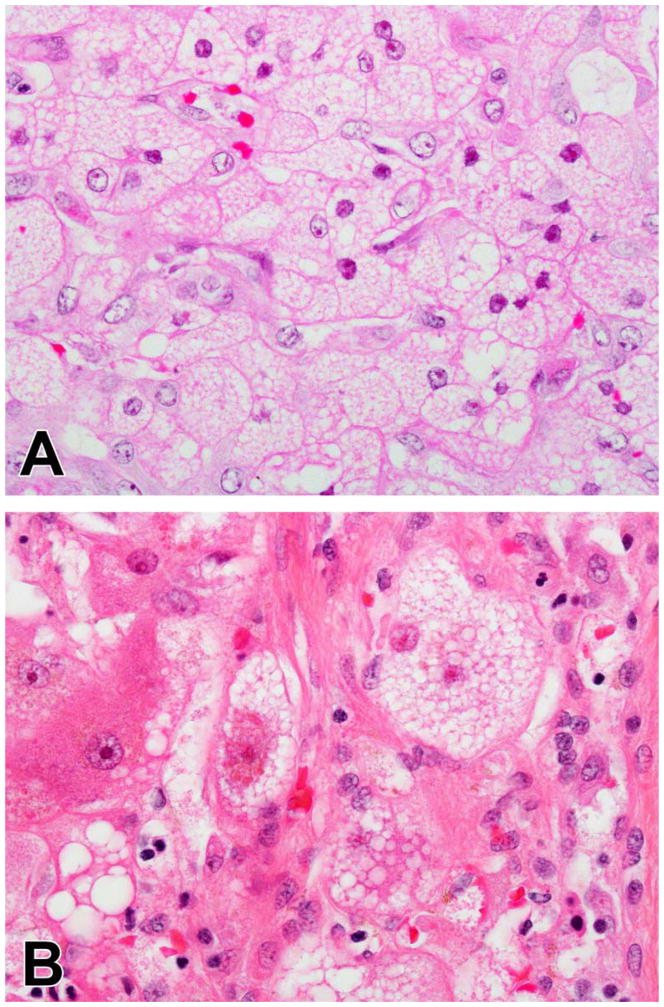
Microvesicular steatosis in DILI. In fialuridine injury there is diffuse microvesicular steatosis because the drug interferes with mitochondrial DNA replication in all of the hepatocytes. The cells have a uniform, foamy appearance (A). Microvesicular steatois can also be seen as a focal injury in other forms of DILI. In (B) this small cluster of foamy cells is seen in a case of isoniazid injury.
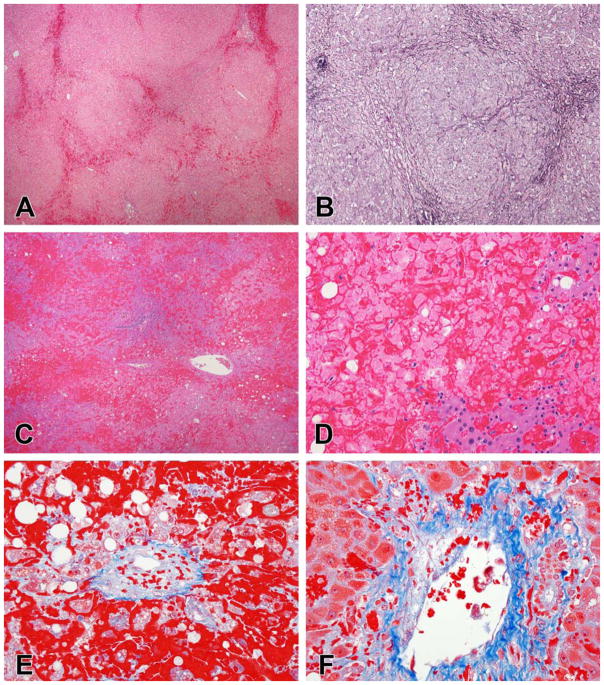
A variety of vascular injury patterns have been attributed to drugs and some, like VOD/SOS, are almost always due to a drug or toxin (Figure 9). Among the drugs commonly associated with vascular injury are the oncotherapeutic agents and immunosuppressive agents (particularly the purine analogues). The anabolic and contraceptive steroids have also been associated with vascular injury 32, although the frequency of such injury is probably less than the cholestatic injury caused by these steroids. It is most helpful to think of the various vascular injuries by the part of the hepatic vasculature affected. Budd-Chiari syndrome results from thrombosis of the major veins. VOD/SOS affects the small veins and distal sinusoids. Peliosis hepatis and sinusoidal dilation are sinusoidal injuries; sinusoidal dilation may also be the result of hepatoportal sclerosis. Nodular regenerative hyperplasia is observed in the parenchyma but probably results from injury to portal veins. Hepatoportal sclerosis is defined by alterations and loss of small and medium-sized portal veins. True arteritis is rare in the liver, but has been reported as part of a syndrome drug induced systemic vasculitis 57,58. The other changes in the liver observed in the liver with vascular injury vary from subtle in the case of hepatoportal sclerosis and nodular regenerative hyperplasia to dramatic, with extensive hemorrhage and necrosis in the case of VOD/SOS and Budd-Chiari. The differential diagnosis varies with the particular vascular pattern. Budd-Chiari may be caused by hypercoaguable states, central venous stasis and is associated with a variety of systemic disorders. VOD/SOS is mainly observed in the context of stem cell transplantation or exposure to toxins like pyrrolizidine alkaloids 59 and is rarely seen in non-drug/toxin contexts. Nodular regenerative hyperplasia and hepatoportal sclerosis have been associated with collagen-vascular diseases 60, lymphoproliferative diseases 61 and some immunodeficiency states like common variable immunodeficiency 26 independent of the drugs used to treat these conditions.
Figure 9.
Examples of vascular injury. A., B. Nodular regenerative hyperplasia due to oxaliplatin injury. There is congestion of dilated sinuses between the regenerative nodules (A). The reticulin stain shows the irregular liver cells plates more clearly, with widened, 2-cell thick plates of enlarged hepatocytes within the nodules and compressed plates of atrophic hepatocytes between the nodules (B). C, D, E, F. VOD/SOS following hematopoietic stem cell transplant. There is extensive hemorrhage and necrosis (C), with only a few groups of residual hepatocytes (D), mainly in zone 1. Trichrome stains show that the central veins are narrowed by loose, pale staining connective tissue (E). Some of the portal veins also showed partial occlusion with loose connective tissue (F).
Sometimes the changes in the liver biopsy are relatively unremarkable. There may be very mild degrees of portal or lobular inflammation, rare apoptotic bodies or mild steatosis. This may reflect the timing of the biopsy—if the liver biopsy is done because the injury is slow to resolve, there may be only a little residual injury left. Some patterns of injury, like hepatoportal sclerosis or nodular regenerative hyperplasia may be very subtle, with nearly normal architecture and no inflammation or fibrosis to suggest injury. VBDS may also be subtle, with no inflammation or ductular reaction and only minimal cholestasis. Resolving acute hepatitis may leave little evidence of its passage. Clusters of pigmented, PAS-positive macrophages may be the only remaining evidence of prior injury. Other patterns of injury may be characterized mainly by an alteration of hepatocyte cytoplasm such as inclusions, lipofuscin accumulation or glycogenosis. Glycogenosis may be associated with high dose corticosteroid therapy and high aminotransferase levels may prompt a biopsy. The differential diagnosis includes poorly controlled type I diabetes 62 and glycogen storage diseases.
Difficult Differentials
Once the biopsy has been thoroughly reviewed for the pattern and severity of injury as described the pathologist must consider the histological changes in light of the patient’s history (Figure 1). There may already be a differential diagnosis that the clinical team would like assessed and the pathology may suggest other possibilities. It may be, after consideration of the history, that the pathological changes are not only consistent with injury from a drug, but that the particular agent can be identified with the pathology providing an even greater level of certainty for the diagnosis. On the other hand, the histological changes may be inconsistent with the agent(s) under consideration or may even suggest an alternate etiology for the injury. More testing may be required to exclude possible alternate explanations. It is important to remember that the diagnosis of DILI is always a diagnosis of exclusion, so a conclusion of DILI should be reached only after careful consideration of other possibilities.
If the diagnosis of DILI seems obvious on clinical grounds alone, it is probably less likely that the team will require a liver biopsy merely to confirm their suspicions. It is more likely that a point has been reached in the clinical evaluation where the findings need clarification and so a decision to perform a liver biopsy is made. Table 3 lists some of the possible reasons to perform a liver biopsy in a case of suspected DILI. The situations of known underlying liver disease and autoimmune hepatitis deserve further consideration.
Table 3.
Possible reasons to perform a liver biopsy in DILI
| Multiple candidates as the etiologic agent |
| Experimental agent or agent for which there is little prior record for injury |
| Gain insight into potential mechanism of injury |
| Assessment of the severity of injury to enable clinical decision |
| Known underlying liver disease |
| Alternate possible etiologies (e.g. sepsis, graft-vs-host disease) |
| Exclusion of autoimmune hepatitis |
Given the prevalence of chronic viral hepatitis and fatty liver disease (both alcoholic and non-alcoholic), it is not unusual for patients with a possible drug injury to have a known (or suspected) underlying liver disease. After an objective review of the histological findings the pathologist must decide whether there are histological features present that are not consistent with the underlying liver disease. For example, neither NASH nor chronic viral hepatitis should have canalicular or hepatocellular bile stasis unless there is advanced cirrhosis. While cholestasis is clearly out of place in most early stage chronic liver diseases there may be other findings, such as obvious duct injury or loss, granulomas, vascular injury or microvesicular steatosis that might suggest a superimposed injury. It may even be possible to distinguish a superimposed acute hepatitis from the underlying disease. These “aberrant” features can be considered in light of the drug history. Prior biopsies can be very helpful in defining a pre-existing level of disease severity that can be compared to the current biopsy. One possible outcome is that no histological features can be identified that are not explained by the known underlying liver disease. While it is still possible that DILI may be present, the pathologist must conclude that the histological changes do not support a diagnosis of DILI.
Autoimmune hepatitis (AIH) presents a particularly difficult challenge to both the clinician and the pathologist. Since there are no definitive tests for AIH, this diagnosis is also a diagnosis of exclusion. In fact, even in the absence of suspected DILI, a liver biopsy is often performed to confirm the clinical suspicion of AIH. Drug-induced AIH (DIAIH) has been reported in association with a number of drugs and also has been the subject of larger clinical studies 20,63. Drugs that have been associated with an AIH-like syndrome include nitrofurantoin, minocycline, hydralazine, methyldopa and the statins. In a study of 261 Mayo Clinic patients with AIH, 24 were thought have evidence of DIAIH 63. Nitrofurantoin and minocycline were the main drugs implicated in this study. There were few histological differences between DIAIH cases and AIH cases. Both showed evidence of chronic hepatitis of similar grade and stage, but there were no cases of cirrhosis in the DIAIH group. Both groups responded similarly to corticosteroid therapy, although the DIAIH patients could be weaned from steroids more successfully than the AIH patients. A study from the DILIN analyzed cases of DILI from patients taking drugs typically associated with DIAIH, including nitrofurantoin, minocycline, hydralazine and methyldopa 20. Clinical features of AIH were found in most of the cases of nitrofurantoin and minocycline and about half of the cases of hydralazine and methyldopa injury. A variety of patterns of injury were observed on histological examination, including acute hepatitis (43%) and cholestatic hepatitis (29%). Cholestasis is an unusual finding in idiopathic AIH and a separate blinded evaluation of cases of AIH and DIAIH found that cholestasis was helpful in diagnosing DIAIH 64. Liver biopsies performed to diagnose graft versus host disease (GVHD) offer similar difficulties. GVHD, like AIH, is a diagnosis of exclusion and often the diagnosis that must be excluded is DILI. The main histological change in GVHD is bile duct injury, often without ductular reaction and little inflammation, but hepatitic variants also exist 65. Although this differential diagnosis has not been subjected to rigorous study, recent work has been presented that may eventually prove useful 66.
Final Analysis and Consultative Opinion
The assessment should not stop with the assessment of pattern and severity, as Figure 1 shows. After the initial evaluation, the pathologist should proceed to interpret the histological findings in light of the patient’s medical history, laboratory tests and available imaging. Dr. Irey, a toxicological pathologist at the Armed Forces Institute of Pathology, outlined a series of considerations that are important when evaluating any histological injury related to a drug, herbal supplement or toxic agent 67. These may be divided into factors purely related to the patient’s history and the agents in question, such as temporal eligibility and toxicological analysis, and factors in which the pathological changes may play a role. Temporal eligibility refers to the fact that drugs have windows of exposure during which they are most likely to cause injury. For most agents this window is between a few days and several months prior to the onset of injury. Some drugs however, may cause injury even after many months to years of exposure. In particular, the agents associated with autoimmune hepatitis-like reactions, such as nitrofurantoin, minocycline and the statins, fall into this category 20,22. The injury may also not be recognized until weeks after the drug has been stopped. This is especially true of antibiotics, which are often given in limited courses. Amoxicillin-clavulanate, typically given as a 10-day course, is associated with a cholestatic hepatitis that may not been seen for one to three weeks after the drug is stopped 16. A single dose of cefazolin, given as antibacterial prophylaxis prior to surgery, is associated with cholestatic injury that manifests up to three weeks later 68. Toxicological analysis may be helpful in the follow-up evaluation of DILI, but with the exception of tests for acetaminophen adducts, may not be available in real time.
The remaining factors require the pathologist to consider clinical information in light of what is seen under the microscope. Key among these factors is the exclusion of competing causes of injury. No matter how suggestive the history, the pathologist should assume that there is some alternate, non-drug explanation for the injury. The histological findings may eliminate some possibilities among the clinical differential diagnoses and raise other possibilities that need to be excluded by additional testing. This is particularly true of the common patterns of injury, chronic hepatitis and fatty liver disease, as well as cases in which the injury is relatively mild and etiologically non-specific. With these patterns there may always remain some uncertainty as to etiology. The pathologist should be alert to overlapping patterns of injury, particularly if the patient has a known underlying liver disease, such as viral hepatitis or NASH. Once competing causes of injury have been thoroughly evaluated, consideration can turn to the patient’s list of medications, including any over-the-counter drug and herbal or nutritional supplements. A detailed history of such agents may be lacking, in which case the pathologist may need to contact the patient’s physician for additional information. Each agent should be considered in turn for the likelihood of causing the particular histological injury observed. Agents that cause similar histological patterns of injury can be stratified based on their overall propensity to cause injury 69, but even rare causes of DILI cause injury sometimes.
There are a variety of resources available to help the pathologist deal with the complexity of evaluating potential cases of DILI. The hepatotoxicity website maintained by the U.S. National Library of Medicine, LiverTox, (http://livertox.nlm.nih.gov/) can be helpful as both a summary of clinical presentations by individual and as a key to the primary literature. Tables of injury caused by particular drugs can found in DILI-specific chapters of the major textbooks of liver pathology as well as the pathology chapters of hepatotoxicity references. The results of the analysis outlined above should be reflected in the pathologist’s report. The report should include not only information on the histological changes and pattern of injury but should comment on the histological differential diagnosis and the likelihood that the injury is related to particular agents.
Final Thoughts
A liver biopsy is not like a simple laboratory test or even an imaging evaluation. A biopsy is informative because it provides a comprehensive and direct view of the physical relationships of all of the cell types and pathologic processes in the biopsied organ. The hepatic pathologist is a true expert medical consultant, whose job includes the careful assessment of the patient’s clinical history in light of these complex histological changes and to provide an interpretation based on their understanding of hepatic pathophysiology. Additional clinical testing may be required to exclude non-DILI etiologies that are suggested by the biopsy. The hepatic pathologist may be asked to disentangle contributions to injury from multiple etiologies—a difficult task requiring experience in the biopsy changes over a wide range of liver diseases. The final report should therefore contain an assessment of pathologic changes in light of the patient’s drug exposures as well as whatever clinical evaluation has been performed to exclude competing causes of injury. DILI may be the price paid for pharmacological progress19, but to an individual patient it is an unwanted and potentially lethal complication of therapy. When a liver biopsy is performed in these circumstances, the pathologist should make full use of the opportunity this provides to demonstrate the power of careful histological analysis to illuminate difficult clinical problems.
Table 4.
Factors to Consider in the Histological Evaluation of DILI
| Factor | Questions |
|---|---|
| Temporal Eligibility | What agents were taken and over what time period? Do those exposures match the known risk profile of the agents? |
| Exclusion of Competing Causes | Given the histological pattern of injury, have all the appropriate clinical tests been performed to exclude other possibilities? |
| Known Potential for Injury | What is the evidence that the agents in question cause liver injury? Are the potential agents common or rare causes of injury? |
| Precedent for Pathologic Injury Pattern | Given the histological changes, how does that compare to the reported injury patterns of the agents in question? If the agent’s injury pattern is unknown because the agent is novel or recently introduced, can the injury pattern be explained by the agent’s mechanism of action or by relation to other agents in the same class? |
| De-challenge/Re-challenge | What is the natural history of the injury associated with the agents in question? Are the histological changes consistent with ongoing/resolving injury? |
| Toxicologic Analysis | Are there laboratory studies that can be done to check for drug levels or accumulation of toxic by-products? |
Key Points.
Hepatic pathology in drug induced liver injury is complex, but may be approached systematically.
Biopsy assessment begins with objective evaluation of character and severity of histological changes
The histological findings are summarized as a pattern of injury that generates the
histological differential diagnosis
The pathologist provides an expert interpretation of the findings in light of the patient’s medical and drug history.
Summary.
The evaluation of liver biopsies performed in cases of suspected drug induced liver injury (DILI) can be complex. However, the biopsy may be approached systematically, first by careful identification of histological lesions and then by identification of the overall pattern of injury. These findings lead directly to the histological differential diagnosis. Potential DILI must be separated from concomitant non-DILI liver disease. Once competing causes of injury have been excluded, the findings can be analyzed with respect to the various prescription and nonprescription medications and dietary supplements under suspicion to provide a complete interpretation of the findings.
Footnotes
Disclosure/Acknowledgement: This review was supported by the Intramural Research Program of the NIH, National Cancer Institute. Dr. Kleiner prepared the manuscript, including all figures and tables.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135(6):1924–1934. 1934 e1921–1924. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31(5):929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 3.Hennes EM, Zeniya M, Czaja AJ, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48(1):169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 4.Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46(11):1323–1330. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 5.Fontana RJ, Kleiner DE, Bilonick R, et al. Modeling hepatic fibrosis in African American and Caucasian American patients with chronic hepatitis C virus infection. Hepatology. 2006;44(4):925–935. doi: 10.1002/hep.21335. [DOI] [PubMed] [Google Scholar]
- 6.Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39(2):239–244. doi: 10.1016/s0168-8278(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 7.Schiano TD, Azeem S, Bodian CA, et al. Importance of specimen size in accurate needle liver biopsy evaluation of patients with chronic hepatitis C. Clin Gastroenterol Hepatol. 2005;3(9):930–935. doi: 10.1016/s1542-3565(05)00541-0. [DOI] [PubMed] [Google Scholar]
- 8.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128(7):1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 9.Moreira RK, Chopp W, Washington MK. The concept of hepatic artery-bile duct parallelism in the diagnosis of ductopenia in liver biopsy samples. Am J Surg Pathol. 2011;35(3):392–403. doi: 10.1097/PAS.0b013e3182082ef6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crawford AR, Lin XZ, Crawford JM. The normal adult human liver biopsy: a quantitative reference standard. Hepatology. 1998;28(2):323–331. doi: 10.1002/hep.510280206. [DOI] [PubMed] [Google Scholar]
- 11.Rocken C, Meier H, Klauck S, Wolff S, Malfertheiner P, Roessner A. Large-needle biopsy versus thin-needle biopsy in diagnostic pathology of liver diseases. Liver. 2001;21(6):391–397. doi: 10.1034/j.1600-0676.2001.210605.x. [DOI] [PubMed] [Google Scholar]
- 12.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD American Association for the Study of Liver D. Liver biopsy. Hepatology. 2009;49(3):1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 13.Pai RK, Hart JA. Aberrant expression of cytokeratin 7 in perivenular hepatocytes correlates with a cholestatic chemistry profile in patients with heart failure. Mod Pathol. 2010;23(12):1650–1656. doi: 10.1038/modpathol.2010.175. [DOI] [PubMed] [Google Scholar]
- 14.Delladetsima I, Sakellariou S, Kokkori A, Tiniakos D. Atrophic hepatocytes express keratin 7 in ischemia-associated liver lesions. Histol Histopathol. 2016;31(10):1089–1094. doi: 10.14670/HH-11-738. [DOI] [PubMed] [Google Scholar]
- 15.Kleiner DE, Chalasani NP, Lee WM, et al. Hepatic histological findings in suspected drug-induced liver injury: systematic evaluation and clinical associations. Hepatology. 2014;59(2):661–670. doi: 10.1002/hep.26709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.deLemos AS, Ghabril M, Rockey DC, et al. Amoxicillin-Clavulanate-Induced Liver Injury. Dig Dis Sci. 2016 doi: 10.1007/s10620-016-4121-6. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larrey D, Vial T, Micaleff A, et al. Hepatitis associated with amoxycillin-clavulanic acid combination report of 15 cases. Gut. 1992;33(3):368–371. doi: 10.1136/gut.33.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith LA, Ignacio JR, Winesett MP, et al. Vanishing bile duct syndrome: amoxicillin-clavulanic acid associated intra-hepatic cholestasis responsive to ursodeoxycholic acid. J Pediatr Gastroenterol Nutr. 2005;41(4):469–473. doi: 10.1097/01.mpg.0000178086.44155.73. [DOI] [PubMed] [Google Scholar]
- 19.Popper H, Rubin E, Cardiol D, Schaffner F, Paronetto F. Drug-Induced Liver Disease: a Penalty for Progress. Arch Intern Med. 1965;115:128–136. doi: 10.1001/archinte.1965.03860140008003. [DOI] [PubMed] [Google Scholar]
- 20.de Boer YS, Kosinski AS, Urban TJ, et al. Features of Autoimmune Hepatitis in Patients With Drug-induced Liver Injury. Clin Gastroenterol Hepatol. 2016 doi: 10.1016/j.cgh.2016.05.043. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gough A, Chapman S, Wagstaff K, Emery P, Elias E. Minocycline induced autoimmune hepatitis and systemic lupus erythematosus-like syndrome. BMJ. 1996;312(7024):169–172. doi: 10.1136/bmj.312.7024.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo MW, Hoofnagle JH, Gu J, et al. Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatology. 2014;60(2):679–686. doi: 10.1002/hep.27157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alla V, Abraham J, Siddiqui J, et al. Autoimmune hepatitis triggered by statins. J Clin Gastroenterol. 2006;40(8):757–761. doi: 10.1097/00004836-200609000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Suh N, Liapis H, Misdraji J, Brunt EM, Wang HL. Epstein-Barr virus hepatitis: diagnostic value of in situ hybridization, polymerase chain reaction, and immunohistochemistry on liver biopsy from immunocompetent patients. Am J Surg Pathol. 2007;31(9):1403–1409. doi: 10.1097/PAS.0b013e31802ffdd5. [DOI] [PubMed] [Google Scholar]
- 25.Abraham S, Begum S, Isenberg D. Hepatic manifestations of autoimmune rheumatic diseases. Ann Rheum Dis. 2004;63(2):123–129. doi: 10.1136/ard.2002.001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuss IJ, Friend J, Yang Z, et al. Nodular regenerative hyperplasia in common variable immunodeficiency. Journal of clinical immunology. 2013;33(4):748–758. doi: 10.1007/s10875-013-9873-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169(5):1071–1080. doi: 10.1111/bjd.12501. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann RM, Jung MC, Motz R, et al. Sarcoidosis associated with interferon-alpha therapy for chronic hepatitis C. J Hepatol. 1998;28(6):1058–1063. doi: 10.1016/s0168-8278(98)80357-7. [DOI] [PubMed] [Google Scholar]
- 29.Antoniades CG, Quaglia A, Taams LS, et al. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology. 2012;56(2):735–746. doi: 10.1002/hep.25657. [DOI] [PubMed] [Google Scholar]
- 30.Cover C, Liu J, Farhood A, et al. Pathophysiological role of the acute inflammatory response during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2006;216(1):98–107. doi: 10.1016/j.taap.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Shulman HM, Fisher LB, Schoch HG, Henne KW, McDonald GB. Veno-occlusive disease of the liver after marrow transplantation: histological correlates of clinical signs and symptoms. Hepatology. 1994;19(5):1171–1181. [PubMed] [Google Scholar]
- 32.Ishak KG. Hepatic lesions caused by anabolic and contraceptive steroids. Semin Liver Dis. 1981;1(2):116–128. doi: 10.1055/s-2008-1040724. [DOI] [PubMed] [Google Scholar]
- 33.Ampuero J, Garcia ES, Lorenzo MM, Calle R, Ferrero P, Gomez MR. Stanozolol-induced bland cholestasis. Gastroenterol Hepatol. 2014;37(2):71–72. doi: 10.1016/j.gastrohep.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 34.El Sherrif Y, Potts JR, Howard MR, et al. Hepatotoxicity from anabolic androgenic steroids marketed as dietary supplements: contribution from ATP8B1/ABCB11 mutations? Liver Int. 2013;33(8):1266–1270. doi: 10.1111/liv.12216. [DOI] [PubMed] [Google Scholar]
- 35.Elsharkawy AM, McPherson S, Masson S, Burt AD, Dawson RT, Hudson M. Cholestasis secondary to anabolic steroid use in young men. BMJ. 2012;344:e468. doi: 10.1136/bmj.e468. [DOI] [PubMed] [Google Scholar]
- 36.Nieschlag E, Vorona E. Doping with anabolic androgenic steroids (AAS): Adverse effects on non-reproductive organs and functions. Rev Endocr Metab Disord. 2015;16(3):199–211. doi: 10.1007/s11154-015-9320-5. [DOI] [PubMed] [Google Scholar]
- 37.Lefkowitch JH. Bile ductular cholestasis: an ominous histopathologic sign related to sepsis and “cholangitis lenta”. Hum Pathol. 1982;13(1):19–24. doi: 10.1016/s0046-8177(82)80134-2. [DOI] [PubMed] [Google Scholar]
- 38.Crawford JM, Boyer JL. Clinicopathology conferences: inflammation-induced cholestasis. Hepatology. 1998;28(1):253–260. doi: 10.1002/hep.510280133. [DOI] [PubMed] [Google Scholar]
- 39.Fontana RJ, Hayashi PH, Barnhart H, et al. Persistent Liver Biochemistry Abnormalities Are More Common in Older Patients and those With Cholestatic Drug Induced Liver Injury. Am J Gastroenterol. 2015;110(10):1450–1459. doi: 10.1038/ajg.2015.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ludwig J, Kim CH, Wiesner RH, Krom RA. Floxuridine-induced sclerosing cholangitis: an ischemic cholangiopathy? Hepatology. 1989;9(2):215–218. doi: 10.1002/hep.1840090209. [DOI] [PubMed] [Google Scholar]
- 41.Levine C, Trivedi A, Thung SN, Perumalswami PV. Severe Ductopenia and Cholestasis from Levofloxacin Drug-Induced Liver Injury: A Case Report and Review. Semin Liver Dis. 2014;34(2):246–251. doi: 10.1055/s-0034-1375964. [DOI] [PubMed] [Google Scholar]
- 42.Orman ES, Conjeevaram HS, Vuppalanchi R, et al. Clinical and histopathologic features of fluoroquinolone-induced liver injury. Clin Gastroenterol Hepatol. 2011;9(6):517–523. e513. doi: 10.1016/j.cgh.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grant LM, Kleiner DE, Conjeevaram HS, Vuppalanchi R, Lee WM. Clinical and histological features of idiosyncratic acute liver injury caused by temozolomide. Dig Dis Sci. 2013;58(5):1415–1421. doi: 10.1007/s10620-012-2493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dahl MG, Gregory MM, Scheuer PJ. Liver damage due to methotrexate in patients with psoriasis. Br Med J. 1971;1(5750):625–630. doi: 10.1136/bmj.1.5750.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rau R, Karger T, Herborn G, Frenzel H. Liver biopsy findings in patients with rheumatoid arthritis undergoing longterm treatment with methotrexate. J Rheumatol. 1989;16(4):489–493. [PubMed] [Google Scholar]
- 46.Nyfors A, Poulsen H. Liver biopsies from psoriatics related to methotrexate therapy. 2. Findings before and after methotexate therapy in 88 patients. A blind study. Acta Pathol Microbiol Scand [A] 1976;84(3):262–270. [PubMed] [Google Scholar]
- 47.Yeo CM, Chong VH, Earnest A, Yang WL. Prevalence and risk factors of methotrexate hepatoxicity in Asian patients with psoriasis. World J Hepatol. 2013;5(5):275–280. doi: 10.4254/wjh.v5.i5.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis JH, Mullick F, Ishak KG, et al. Histopathologic analysis of suspected amiodarone hepatotoxicity. Hum Pathol. 1990;21(1):59–67. doi: 10.1016/0046-8177(90)90076-h. [DOI] [PubMed] [Google Scholar]
- 49.Oien KA, Moffat D, Curry GW, et al. Cirrhosis with steatohepatitis after adjuvant tamoxifen. Lancet. 1999;353(9146):36–37. doi: 10.1016/S0140-6736(05)74872-8. [DOI] [PubMed] [Google Scholar]
- 50.Fromenty B, Pessayre D. Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity. Pharmacol Ther. 1995;67(1):101–154. doi: 10.1016/0163-7258(95)00012-6. [DOI] [PubMed] [Google Scholar]
- 51.Kleiner DE, Gaffey MJ, Sallie R, et al. Histopathologic changes associated with fialuridine hepatotoxicity. Mod Pathol. 1997;10(3):192–199. [PubMed] [Google Scholar]
- 52.Lewis W, Levine ES, Griniuviene B, et al. Fialuridine and its metabolites inhibit DNA polymerase gamma at sites of multiple adjacent analog incorporation, decrease mtDNA abundance, and cause mitochondrial structural defects in cultured hepatoblasts. Proc Natl Acad Sci U S A. 1996;93(8):3592–3597. doi: 10.1073/pnas.93.8.3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arenas-Pinto A, Grant AD, Edwards S, Weller IV. Lactic acidosis in HIV infected patients: a systematic review of published cases. Sex Transm Infect. 2003;79(4):340–343. doi: 10.1136/sti.79.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mokrzycki MH, Harris C, May H, Laut J, Palmisano J. Lactic acidosis associated with stavudine administration: a report of five cases. Clin Infect Dis. 2000;30(1):198–200. doi: 10.1086/313594. [DOI] [PubMed] [Google Scholar]
- 55.Lichterfeld M, Fischer HP, Spengler U, Rockstroh JK. Fatty liver and increased serum lactate in a woman with HIV. Dtsch Med Wochenschr. 2003;128(3):81–84. doi: 10.1055/s-2003-36654. [DOI] [PubMed] [Google Scholar]
- 56.De Bus L, Depuydt P, Libbrecht L, et al. Severe drug-induced liver injury associated with prolonged use of linezolid. J Med Toxicol. 2010 doi: 10.1007/s13181-010-0047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gaffey CM, Chun B, Harvey JC, Manz HJ. Phenytoin-induced systemic granulomatous vasculitis. Arch Pathol Lab Med. 1986;110(2):131–135. [PubMed] [Google Scholar]
- 58.Mullick FG, Ishak KG. Hepatic injury associated with diphenylhydantoin therapy. A clinicopathologic study of 20 cases. Am J Clin Pathol. 1980;74(4):442–452. doi: 10.1093/ajcp/74.4.442. [DOI] [PubMed] [Google Scholar]
- 59.Gao H, Li N, Wang JY, Zhang SC, Lin G. Definitive diagnosis of hepatic sinusoidal obstruction syndrome induced by pyrrolizidine alkaloids. J Dig Dis. 2012;13(1):33–39. doi: 10.1111/j.1751-2980.2011.00552.x. [DOI] [PubMed] [Google Scholar]
- 60.Reynolds WJ, Wanless IR. Nodular regenerative hyperplasia of the liver in a patient with rheumatoid vasculitis: a morphometric study suggesting a role for hepatic arteritis in the pathogenesis. J Rheumatol. 1984;11(6):838–842. [PubMed] [Google Scholar]
- 61.Al-Mukhaizeem KA, Rosenberg A, Sherker AH. Nodular regenerative hyperplasia of the liver: an under-recognized cause of portal hypertension in hematological disorders. Am J Hematol. 2004;75(4):225–230. doi: 10.1002/ajh.20024. [DOI] [PubMed] [Google Scholar]
- 62.Torbenson M, Chen YY, Brunt E, et al. Glycogenic hepatopathy: an underrecognized hepatic complication of diabetes mellitus. Am J Surg Pathol. 2006;30(4):508–513. doi: 10.1097/00000478-200604000-00012. [DOI] [PubMed] [Google Scholar]
- 63.Bjornsson E, Talwalkar J, Treeprasertsuk S, et al. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010;51(6):2040–2048. doi: 10.1002/hep.23588. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki A, Brunt EM, Kleiner DE, et al. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug-induced liver injury. Hepatology. 2011;54(3):931–939. doi: 10.1002/hep.24481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stift J, Baba HA, Huber E, et al. Consensus on the histopathological evaluation of liver biopsies from patients following allogeneic hematopoietic cell transplantation. Virchows Arch. 2014;464(2):175–190. doi: 10.1007/s00428-013-1528-8. [DOI] [PubMed] [Google Scholar]
- 66.Stueck AE, Schiano T, Fiel MI. A Novel Histologic Diagnostic Algorithm for Hepatic Graft Versus Host Disease. Mod Pathol. 2016;29(S2):426A. doi: 10.1038/modpathol.2017.151. [DOI] [PubMed] [Google Scholar]
- 67.Irey NS. Teaching monograph. Tissue reactions to drugs. Am J Pathol. 1976;82(3):613–647. [PMC free article] [PubMed] [Google Scholar]
- 68.Alqahtani SA, Kleiner DE, Ghabril M, et al. Identification and Characterization of Cefazolin-Induced Liver Injury. Clin Gastroenterol Hepatol. 2015;13(7):1328–1336. e1322. doi: 10.1016/j.cgh.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bjornsson ES, Hoofnagle JH. Categorization of drugs implicated in causing liver injury: Critical assessment based on published case reports. Hepatology. 2016;63(2):590–603. doi: 10.1002/hep.28323. [DOI] [PubMed] [Google Scholar]
- 70.Kleiner DE. Liver histology in the diagnosis and prognosis of drug-induced liver injury. Clinical Liver Disease. 2014;4(1):12–16. doi: 10.1002/cld.371. [DOI] [PMC free article] [PubMed] [Google Scholar]