Abstract
Background:
Despite abundance of sunshine in India, Vitamin D deficiency is common and therefore there is an increasing trend toward taking Vitamin D supplements either as prescription medicine or as a nutritional supplement. Studies have suggested that duration of sun exposure may influence serum lipid profile.
Objectives:
To study the effect of increased sunlight exposure versus Vitamin D supplementation on Vitamin D status and lipid profile in individuals with Vitamin D deficiency (25-hydroxyvitamin-D [25OHD] <50 nmol/L).
Design:
A prospective, randomized open-label trial was carried out in apparently healthy Indian men (40–60 years). Based on 25OHD concentrations, individuals were divided into control (>50 nmol/L, n = 50) and intervention (<50 nmol/L, n = 100) groups. Individuals from intervention group were randomly allocated to two groups; either “increased sunlight exposure group” (n = 50, received at least 20 min sunlight exposure to forearms and face between 11 a.m. and 3 p.m. over and above their current exposure) or “cholecalciferol supplement group” (n = 50, received oral cholecalciferol 1000 IU/day).
Results:
Significant increase in 25OHD concentrations was seen in both intervention groups (P < 0.01). Significant decrease in total cholesterol (TC), high-density-lipoprotein cholesterol (HDL-C), and low-density-lipoprotein cholesterol (LDL-C) was seen in individuals with increased sunlight exposure (P < 0.05). Cholecalciferol supplement group showed a significant increase in TC and HDL-C (P < 0.05) and insignificant increase in LDL-C.
Conclusions:
Increase in Vitamin D concentrations through sunlight exposure significantly reduced TC, LDL-C, and HDL-C concentrations, and cholecalciferol supplementation increased TC and HDL-C concentrations.
Keywords: Cholecalciferol, cholesterol, Indian, sunlight, Vitamin D
INTRODUCTION
Vitamin D deficiency is common worldwide, even in sun-rich countries such as India and the Middle East.[1] Suboptimal concentrations of Vitamin D have been reported in over 50% of the Indian population possibly due to changing lifestyles, leading to reduced effective sunlight exposure.[2] Epidemiological evidence suggests a positive association of sunlight exposure with 25-hydroxyvitamin D (25OHD) concentrations.[3,4]
Cholesterol and cholecalciferol (Vitamin D3) are synthesized in a common metabolic pathway from a common substrate 7-dehydrocholesterol (7-DHC). 7-DHC is converted to cholesterol by the enzyme 7-DHC reductase and to cholecalciferol by ultra-violet B radiation from sunlight.[5] Thus, it is likely that duration of sunlight exposure apart from influencing Vitamin D synthesis may also influence cholesterol synthesis. Based on epidemiological data, Grimes et al. have shown that population means of blood cholesterol concentrations increase with increasing distance from the Equator and reduction in duration of sunlight received. There are also reports of seasonal variations in the population mean serum cholesterol concentrations. Grimes et al. have also shown that the mean cholesterol concentrations for each month were below the annual mean during the summer months and above the annual mean during the winter months.[6]
Apart from other lifestyle changes, reduced sunlight exposure in populations migrated from higher sunlight to lower sunlight geographies seems to have an unfavorable effect on the lipid metabolism, leading to increased atherosclerosis. The British Heart Foundation Statistics reports that the death rate from coronary heart disease is highest in South Asian men and women than in the UK population as a whole.[7] It has also been shown that the UK-based Indian cohort had a greater body mass index (BMI), systolic blood pressure and serum cholesterol concentrations, apolipoprotein B; lower high-density lipoprotein cholesterol; and higher fasting blood glucose than their siblings in Punjab, India.[8]
As a result of an increased awareness in the medical community and also in the general population, there is an increasing trend of consuming Vitamin D supplements either as prescription medicines or as a nutritional supplement. Although increased sunlight exposure may be a physiological alternative to oral supplementation in sun-rich countries, it is very infrequently advised possibly due to difficulty in implementing lifestyle modifications and perceived risk of skin cancer.[9] To the best of our knowledge, the effect of increased casual sunlight exposure on Vitamin D concentrations and lipid profile has not been previously studied.
Thus, the specific objective of this study was to assess the effect of increased sunlight exposure, in comparison with Vitamin D supplementation, on Vitamin D status and lipid profile in Indian men (aged 40–60 years) with Vitamin D deficiency (25OHD <50 nmol/L) in a randomized control trial.
METHODOLOGY
Sample selection
This was prospective, balanced, randomized (1:1), open-label, parallel group study conducted at Pune, India. Apparently healthy men (40–60 years) from different institutes, hospitals, nongovernmental organizations, offices, colleges, and residential areas in Pune (Western India, located at 18.52°N, 73.86°E) were invited to voluntarily participate in the study. Out of approached 15 sites, 11 showed interest, and from these, 6 sites were selected randomly using lottery method. Inclusion criteria were apparently healthy men aged between 40 and 60 years who were willing and able to provide consent to participate in the study. From the six selected sites, out of eligible individuals, a total of 300 men were randomly selected by computer-generated random number sequence and were offered to participate in the study. Two hundred and twenty men provided consent to take part in the study. Clinical examination, biochemical assessments, and electrocardiogram (ECG) were performed on these 220 men. Exclusion criteria were disorders known to interfere with lipid parameters and Vitamin D metabolism such as diabetes mellitus (fasting blood sugar [FBS] concentration >125 mg/dl),[10] dyslipidemia (low-density lipoprotein cholesterol [LDL-C] >130),[11] liver dysfunction (serum glutamic pyruvic transaminase [SGPT] >65 IU/L), renal dysfunction (serum creatinine [CR] >1.2 mg/dl), thyroid disorders (by history and clinical assessment), or cardiac disorders (by history and ECG). After applying exclusion criteria, 203 men were enrolled in the study [Figure 1]. Enrollment was done between June 2013 and December 2013. Ethical approval for the study was obtained from the Institutional Ethics Committee.
Figure 1.
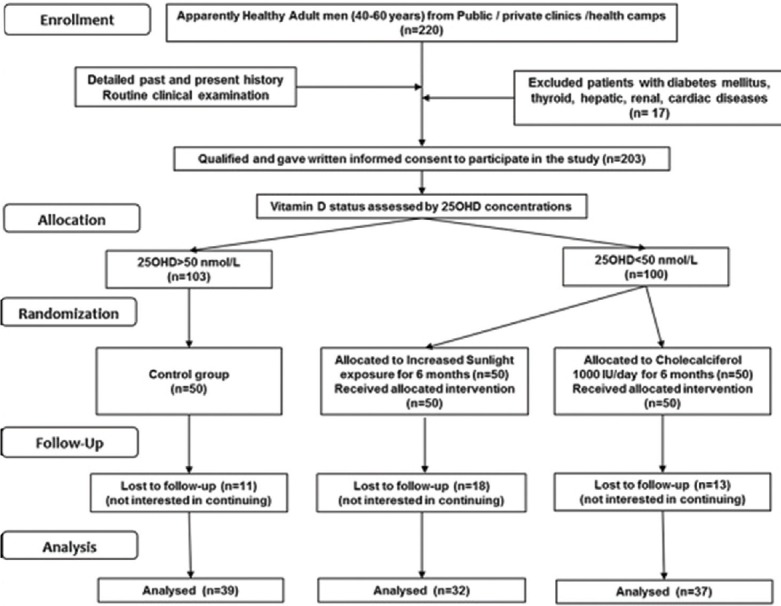
Consort diagram
Study groups
The individuals were assessed for their Vitamin D status. Based on 25OHD, individuals were allocated to the “control group (25OHD >50 nmol/L)” and “intervention group (25OHD <50 nmol/L).” The individuals from intervention group were further randomly allocated into two groups to receive an intervention of either “increased sunlight exposure” or “cholecalciferol supplement.” Randomization was performed by generating a random sequence using web-based application at randomizer.org.[12] The random sequence generation and allocation of participants to the two treatment groups were done by a statistician.
Increased sunlight exposure group
Individuals in this group were advised to receive 20 min sunlight exposure to the forearms and face between 11 a.m. and 3 p.m. over and above their current exposure duration for 6 months. Individuals were advised to go outdoors for a 20-min stroll on work premises or nearby.
Cholecalciferol supplement group
Individuals were prescribed cholecalciferol 1000 IU to be taken orally after dinner every day for 6 months.
Controls group
Out of 103 individuals with 25OHD concentration >50 nmol/L, fifty individuals were randomly selected.
Sample size for each group was based on observed variations reported in adults for main study outcome parameter, i.e., LDL-C.[13] For overall power of the study to be 80%, with Type 1 error probability of 5%, desired sample size was estimated to be 35 in each intervention group. Considering possible defaulters and dropouts, fifty individuals were recruited in each group.
Clinical examination of all study individuals was performed by a physician to assess their health status. Detailed past and present medical histories were recorded.
Biochemical estimations
A venous blood sample (8 ml) was collected between 7 a.m. and 9 a.m. from each individual after an overnight fast for more than 12 h using Vacutainers (BD, Franklin Lakes, NJ, USA). Serum was separated after centrifugation at 2500 rpm for 15 min at room temperature within 2 h of collection. FBS, serum CR, and SGPT (alanine transaminase) were measured for exclusion criteria screening (glucose was performed by hexokinase method, CR by Jaffe method without deproteinization, and SGPT by an enzymatic method). Serum 25OHD concentrations were measured by ELISA (DLD Diagnostics, Germany; intraassay coefficient of variation [CV] 4.9, interassay CV 7.8%). High-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), and triglycerides (TG) were measured enzymatically. LDL-C and very LDL-C concentrations were calculated using Friedewald equation.
Sunlight exposure assessment
For assessment of duration of sunlight exposure, a validated structured questionnaire was administered by the same investigator to all individuals with detailed interview of the subjects. Information regarding nature of job/work, sunlight exposure in minutes (between 7 a.m. to 11 a.m., 11 a.m. to 3 p.m., and 3 p.m. to 7 p.m. separately, average 12 h day divided into three equal periods), clothing pattern, mode of travel, average distance traveled each day, use of hat or helmet, and use of sunscreens was recorded. Solar radiation between 7 a.m. and 11 a.m. and between 3 p.m. and 7 p.m. is approximately 40% of radiation between 11 a.m. and 3 p.m.[14] Hence, exposure between 7 a.m. and 11 a.m. and between 3 p.m. and 7 p.m. was converted to 40% and added to sunlight exposure duration between 11 a.m. and 3 p.m. Based on these calculated durations, individuals were further grouped into three groups: (a) exposure <1 h, (b) exposure between 1 and 2 h, and (c) exposure >2 h.[15]
Anthropometric and body composition measurements
Height was measured to the nearest 0.1 cm using Leicester height meter, Child Growth Foundation, UK (range 60–207 cm); weight was measured on an electronic digital scale to the nearest 0.1 kg. BMI was computed as weight in kg/height in m2. Body composition was measured using Lunar DPX-Pro total body pencil beam Densitometer (GE Healthcare, WI) using a medium mode scan (Software Encore 2005 version 9.30.044). The precision of the DPX-Pro for repeat measurements in adults is 1.89% for total fat percentage.[16]
Compliance
Compliance of the intervention was assessed by monthly phone calls and in person during follow-up at 3 and 6 months. Weekly reminders were also sent through text messages on mobile phones.
Follow-up and outcome parameters
HDL-C, LDL-C, TC, TG, and 25OHD were estimated at 6 months in all individuals
Validated structured questionnaires were administered to assess sunlight exposure at the end of 6 months.
Statistical methods
Data were analyzed using the SPSS Software for Windows (version 16.0, 2001, SPSS Inc., Chicago, IL, USA). Normality of the variables was tested using one-sample Kolmogorov–Smirnov test before performing statistical analysis. Continuous variables are presented as mean with standard deviation for normally distributed variables and median with interquartile range for nonnormally distributed variables.
One-way ANOVA was used to examine differences in means at baseline and end line parameters between groups. Paired t-test was performed in intervention-related data.
RESULTS
At the end of 6 months, 39, 32, and 37 individuals completed the study in control, increased sunlight exposure, and cholecalciferol group, respectively. The individuals who completed the study showed 95% compliance to the regimen and were similar in both intervention groups (P > 0.1). There was no intolerance or adverse reaction to cholecalciferol supplements or additional sunlight exposure in any subjects. Remaining individuals discontinued intervention prematurely as they were not interested in continuing the intervention.
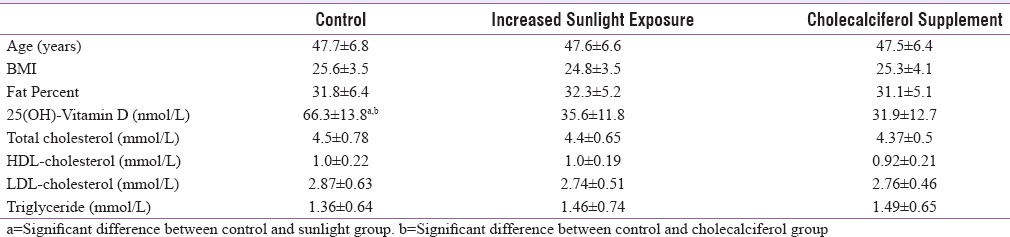
At baseline, there were no significant differences in age, BMI, body fat percentage, and lipid parameters between the three groups (P > 0.1). Mean 25OHD concentrations in the control group were significantly higher than that of the two intervention groups (P < 0.05), but no significant differences were observed between the two intervention groups [P > 0.1, Table 1].
Table 1.
Important baseline parameters in three groups
At baseline, in all the three groups, a significant increasing trend in mean serum 25OHD concentrations was observed with the increasing duration of sunlight exposure [P < 0.05, Figure 2]. Our results confirm positive association between duration of sunlight exposure and 25OHD concentrations at baseline (r = 0.20, P < 0.05), which persisted after intervention with increased sunlight exposure for 6 months. Table 2 illustrates the distribution of individuals with duration of sunlight exposure before and after intervention. In the control and cholecalciferol supplemented groups, there was no change in sunlight exposure levels. In the increased sunlight group, there was a significant shift of subjects from low to higher duration of sunlight exposure (P < 0.05). After 6 months intervention, a significant increase in 25OHD concentrations was observed in the “increased sunlight exposure” and in “cholecalciferol groups” (P < 0.01). The increase of 25OHD concentrations in cholecalciferol supplemented group was significantly higher than in increased sunlight exposure group [P < 0.05, Table 3].
Figure 2.
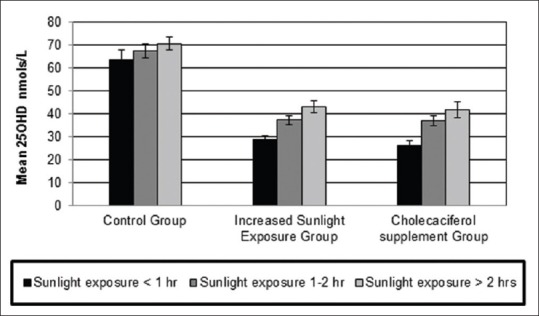
Mean 25-hydroxyvitamin D concentrations in each group subdivided as per sunlight exposure duration at baseline
Table 2.
Distribution of subjects as per sunlight exposure duration before and after intervention
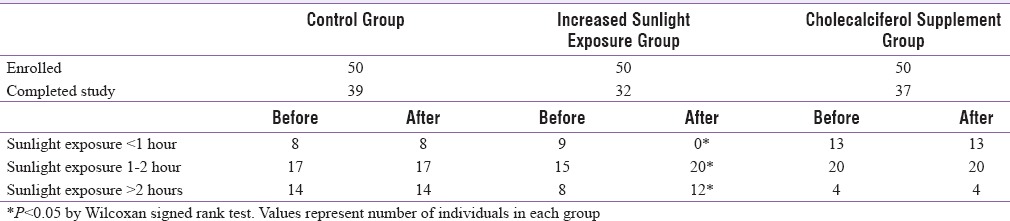
Table 3.
Effect of intervention (increased sunlight exposure or cholecalciferol supplementation) on mean Vitamin D and lipid profile compared to control group
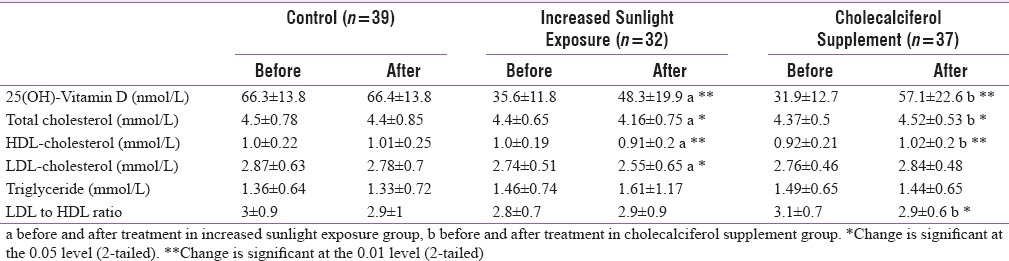
There was significant decline in TC, HDL-C, and LDL-C concentrations in individuals who had increased sunshine exposure (P < 0.05). Those in cholecalciferol supplemented group showed a significant (P < 0.05) increase in TC and HDL-C concentrations, while increase in LDL-C concentrations was not statistically significant. LDL-C to HDL-C ratio increased in increased sunlight exposure group while it significantly decreased in cholecalciferol supplement group (P < 0.05). No change was observed in TG concentrations in either of the intervention groups [Table 3].
When expressed as percentage change over baseline after 6 months, the cholecalciferol group showed twice the increase in 25OHD concentrations over increased sunlight group (10% vs. 5%) (P < 0.01). Mean 25OHD concentrations were unchanged in the control group [Figure 3]. In the lipid profile, a small decrease in TC (3.8%) and LDL-C (3.5%) and a negligible change in HDL-C were observed in the control group (P > 0.05). A significant decrease in TC (9.4%) and LDL-C (7.2%) (P < 0.05) and a small decrease in HDL-C (3.3%) were observed in the increased sunlight exposure group. On the other hand, the cholecalciferol group exhibited a significant increase in TC (5.5%), HDL-C (3.6%), and LDL-C (3.4%) [P < 0.05, Figure 3].
Figure 3.
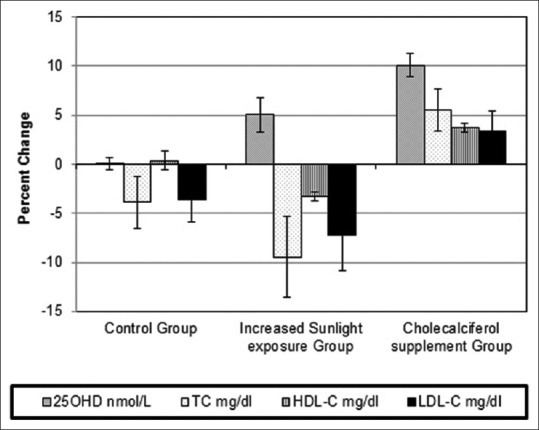
Percent change in serum 25-hydroxyvitamin D and lipid parameters after intervention in the three groups
DISCUSSION
Our study demonstrates that increased exposure to sunlight over a 6-month period significantly increased 25OHD and also resulted in a significant reduction in TC, LDL-C, and HDL-C. Orally administered Vitamin D also increased 25OHD concentrations significantly; however, there was an increase in the TC, HDL-C, and LDL-C though the latter increase was not significant.
Our results in cholecalciferol supplementation group are in agreement with earlier intervention studies. Placebo-controlled randomized trials analyzing effects of Vitamin D supplementation on lipids have consistently shown an increase in LDL-C concentrations, but changes in HDL-C and TC are variable. In a study by Ponda et al., repletion of 25OHD concentrations correlated with a significant increase in LDL-C.[17] In a similar study by Zittermann et al., Vitamin D supplementation also significantly increased LDL-C concentrations (15.4%) compared with placebo (−2.5%), but no significant change was seen in HDL-C concentrations.[18] In a meta-analysis of ten randomized clinical trials on the influence of Vitamin D supplementation on plasma lipid profiles, Wang et al. concluded that Vitamin D supplementation could increase LDL-C concentrations but does not appear to significantly affect TC, HDL-C, and TG. The pooled estimate of effect for Vitamin D supplementation on LDL-C was 3.23 mg/dl (95% confidence interval, 0.55–5.90 mg/dl).[13] These studies however have used different formulations (cholecalciferol, ergocalciferol, calcitriol) and doses and had variable duration of supplementation (8 weeks to 1 year) and therefore are difficult to compare.
Results in increased sunlight exposure intervention group are similar to those seen in epidemiological studies. Reduction in HDL-C can be explained on the basis that common substrate 7-DHC in skin is possibly used for synthesis of Vitamin D due to increased sunlight exposure and thus reduces cutaneous cholesterol production and consequently HDL-C concentration. Our earlier cross-sectional study has also shown inverse relationship between 25OHD and HDL-C in higher sunlight exposure group as against a positive relationship in low sunlight exposure group.[15] Increase in HDL-C in cholecalciferol supplemented group could possibly be explained with same logic, wherein with oral supplementation, lesser 7-DHC is used for Vitamin D production.
Various other mechanisms have been suggested to explain the effect of Vitamin D and its active metabolites on lipid metabolism. In an in vitro study by Gupta et al., it has been reported that Vitamin D and its metabolites inhibited HMG-CoA-reductase and lanosterol 14a-demethylase enzyme activity in various rat cell lines which are important enzymes in cholesterol synthesis.[19] Although lower LDL-C concentrations were seen in sunlight exposure group, cholecalciferol supplemented showed opposite results, mechanisms for this are unclear. It is not known whether sunlight exposure affects cholesterol metabolism by improving Vitamin D status or is independent of it.
Although there are a few studies which have investigated effect of Vitamin D supplementation on lipid profile, to the best of our knowledge, there are no published studies in medical literature of randomized control trials that have examined the effect of increased sunlight exposure and oral cholecalciferol supplementation on lipid profile.
One of the limitations of the present study was the dropout rate at the follow-up after 6 months. However, no significant difference was observed between the initial sample and the follow-up group of individuals for all study parameters; i.e., age, body measurements, Vitamin D and lipid profile across the three study groups at baseline (P > 0.1). The individuals who completed the study showed 95% compliance to the regimen. Second, this study was carried out in working Indian middle-aged men and current observations may not apply on other age and gender groups.
CONCLUSION
Our study demonstrates that with increase in sunlight exposure, there is improvement in Vitamin D concentrations and lipid profile, while, in comparison, orally administered Vitamin D had an adverse effect on lipid profile. Our observations have implications for public health advice on improving Vitamin D status and lipid profile of the population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Holick MF, Chen TC. Vitamin D deficiency: A worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–6S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 2.Harinarayan CV, Joshi SR. Vitamin D status in India – Its implications and remedial measures. J Assoc Physicians India. 2009;57:40–8. [PubMed] [Google Scholar]
- 3.Kadam NS, Chiplonkar SA, Khadilkar AV, Fischer PR, Hanumante NM, Khadilkar VV. Modifiable factors associated with low bone mineral content in underprivileged premenarchal Indian girls. J Pediatr Endocrinol Metab. 2011;24:975–81. doi: 10.1515/jpem.2011.405. [DOI] [PubMed] [Google Scholar]
- 4.Kadam N, Chiplonkar S, Khadilkar A, Divate U, Khadilkar V. Low bone mass in urban Indian women above 40 years of age: Prevalence and risk factors. Gynecol Endocrinol. 2010;26:909–17. doi: 10.3109/09513590.2010.487604. [DOI] [PubMed] [Google Scholar]
- 5.Kuan V, Martineau AR, Griffiths CJ, Hyppönen E, Walton R. DHCR7 mutations linked to higher Vitamin D status allowed early human migration to Northern latitudes. BMC Evol Biol. 2013;13:144. doi: 10.1186/1471-2148-13-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimes DS, Hindle E, Dyer T. Sunlight, cholesterol and coronary heart disease. [Last accessed on 2015 Dec 20];QJM. 1997 90:153–4. doi: 10.1093/qjmed/89.8.579. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9068807 . [DOI] [PubMed] [Google Scholar]
- 7.Balarajan R. Ethnic differences in mortality from ischaemic heart disease and cerebrovascular disease in England and Wales. BMJ. 1991;302:560–4. doi: 10.1136/bmj.302.6776.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatnagar D, Anand IS, Durrington PN, Patel DJ, Wander GS, Mackness MI, et al. Coronary risk factors in people from the Indian subcontinent living in West London and their siblings in India. Lancet. 1995;345:405–9. doi: 10.1016/s0140-6736(95)90398-4. [DOI] [PubMed] [Google Scholar]
- 9.Gilchrest B. Sun exposure and vitamin D sufficiency. Am J Clin Nutr. 2008;88:570–7. doi: 10.1093/ajcn/88.2.570S. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl. 1):62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institutes of Health. ATP III At-A-Glance: Quick Desk Reference. 2001. [Last cited on 2016 Jan 18]. Available from: http://www.nhlbi.nih.gov/health.pro/guidelines/current/cholesterol-guidelines/quick-desk-reference-html#Step8 .
- 12. [Last accessed on 2014 Mar 10]. Available from: https://www.randomizer.org/
- 13.Wang H, Xia N, Yang Y, Peng DQ. Influence of Vitamin D supplementation on plasma lipid profiles: A meta-analysis of randomized controlled trials. Lipids Health Dis. 2012;11:42. doi: 10.1186/1476-511X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diffey BL. Sources and measurement of ultraviolet radiation. Methods. 2002;28:4–13. doi: 10.1016/s1046-2023(02)00204-9. [DOI] [PubMed] [Google Scholar]
- 15.Patwardhan VG, Khadilkar AV, Chiplonkar SA, Mughal ZM, Khadilkar VV. Varying relationship between 25-hydroxy-vitamin D, high density lipoprotein cholesterol, and serum 7-dehydrocholesterol reductase with sunlight exposure. J Clin Lipidol. 2015;9:652–7. doi: 10.1016/j.jacl.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Kiebzak GM, Leamy LJ, Pierson LM, Nord RH, Zhang ZY. Measurement precision of body composition variables using the lunar DPX-L densitometer. J Clin Densitom. 2000;3:35–41. doi: 10.1385/jcd:3:1:035. [DOI] [PubMed] [Google Scholar]
- 17.Ponda MP, Dowd K, Finkielstein D, Holt PR, Breslow JL. The short-term effects of Vitamin D repletion on cholesterol: A randomized, placebo-controlled trial. Arterioscler Thromb Vasc Biol. 2012;32:2510–5. doi: 10.1161/ATVBAHA.112.254110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zittermann A, Frisch S, Berthold HK, Götting C, Kuhn J, Kleesiek K, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89:1321–7. doi: 10.3945/ajcn.2008.27004. [DOI] [PubMed] [Google Scholar]
- 19.Gupta AK, Sexton RC, Rudney H. Effect of Vitamin D3 derivatives on cholesterol synthesis and HMG-CoA reductase activity in cultured cells. J Lipid Res. 1989;30:379–86. [PubMed] [Google Scholar]


