Abstract
Introduction:
Insulin remains the cornerstone of therapy in a substantial number of patients with type 2 diabetes mellitus (T2DM). Inadequate knowledge regarding insulin usage is likely to influence its acceptance and adherence, and outcome of therapy, underscoring great need to investigate knowledge, attitude, and practice of insulin usage in patients with T2DM.
Methodology:
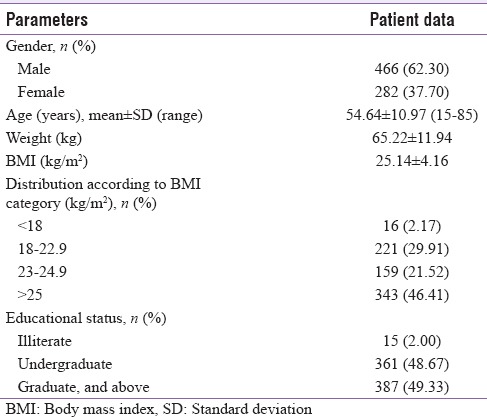
A cross-sectional registry-based retrospective study analyzed data collected from 748 respondents (male: 466, female: 282), mostly from high or middle economic status, who were enrolled as outpatient in a referral clinic during last 10 years (2006–2016), to assess the general characteristics of patients with type 2 diabetes and their baseline knowledge, attitude, and practice of insulin usage and injection practices.
Results:
Mean ± standard deviation (SD) of duration of diabetes was 12.24 ± 7.60 years and mean ± SD duration of insulin therapy was 3.42 ± 4.18 years, which was initiated after a mean ± SD diabetes duration of 8.80 ± 6.42 years. Mean insulin dose per kilogram of body weight/day was 0.51 ± 0.27 units. Total daily dose of insulin was 33.36 ± 18.44 units and number of injections/day (mean ± SD) was 2.06 ± 0.73. Among the respondents, 58.96% were on human insulin and 35.70% were on analog insulin. Pen devices were used by 66.08% of the population whereas 31.76% used insulin syringes. The prevalence of lipohypertrophy (LH) was 12.57%, which was significantly (P < 0.001) associated with wrong technique with regard to injection angle (10.45% vs. 23.02%), site of injection (7.00% vs. 30.51%), rotation of site of injection (0.88% vs. 17.66%), and reuse of needle (5.77% vs. 15.19%). LH was also significantly (P < 0.05) associated with the use of human (14.74%) compared to analog insulin (8.24%).
Conclusion:
The current study highlights the unique patterns of insulin usage and associated high prevalence of LH among insulin users in India.
Keywords: Diabetes, injection technique, insulin, lipodystrophy, lipohypertrophy
INTRODUCTION
Although oral antidiabetic drugs (OADs) remain the mainstay in the treatment of type 2 diabetes mellitus (T2DM), insulin therapy becomes inevitable in a substantial number of patients as the disease progresses. According to recent estimates, roughly 4 of 10 patients with T2DM in India and Gulf countries are using insulin alone or in combination with OADs at any given point of time.[1,2] Insulin therapy is an essential part of diabetes management; all type 1 and most type 2 diabetes patients require insulin at some stage. As injectable therapies such as human insulin, insulin analogs, and glucagon-like peptide-1 receptor agonists are used to manage diabetes, correct injection technique is vital for achievement of glycemic control. Specific recommendations to collate and explain best practices in insulin injecting technique are needed for people with diabetes as well as health-care providers (HCPs).[3] Inadequate knowledge regarding insulin usage is likely to influence its acceptance and adherence, and outcome of therapy, underscoring a great need to investigate knowledge, attitude, and practice of insulin use in patients with diabetes, type1 and type 2 alike.
Insulin site reactions are common local adverse events of insulin therapy. Lipodystrophy (LD), often caused by repeated reuse of needles, manifests as a localized lesion at the repeated injection site. Chronic reuse of needles and injections at the same site are known contributing factors. LD are divided to two subgroups: lipohypertrophy (LH) and lipoatrophy (LA). LH is a thick soft to firm swelling with “rubbery” consistency, and LA is a scarring lesion with depression.[3,4,5,6] There was a strong relationship between the presence of LH and improper or lack of rotation of sites, as found in studies encompassing a detail analysis of execution of insulin injection process and their implications.[7] LH retards insulin absorption significantly and to a sufficient magnitude to have an adverse effect on diabetes control.[8] Proper rotation of injection site and avoidance of needle reuse can prevent LH, or reduce its size which in turn improves glucose control.[4]
The primary objective of this study was to assess the knowledge and practice of patients with T2DM on insulin treatment regarding vital parameters encompassing the self-injection process and to correlate these parameters with hard end points such as LD, self-reported hypoglycemia, and a glycemic status as indicated by glycated hemoglobin (HbA1c). Major secondary objectives were to assess patients' awareness and execution of therapeutic lifestyle management parameters and self-monitoring of blood glucose (SMBG), analysis of usage of concomitant OADs.
METHODOLOGY
This cross-sectional registry-based retrospective trial was conducted in an urban tertiary referral clinic, which uses a customized software to collect relevant information at the 1st point of contact and also during follow-up through pro forma-based interview conducted by trained diabetic counselors under the constant supervision of specialist physician/endocrinologist. The electronic pro forma are built into a customized user-friendly software called Diabetes, Endocrinology and Metabolic Disease Information Management System (DEMIMS©). Data entries which are made during the years 2006–2016 were reviewed and filtered through a two-tier screening process. Appropriateness for inclusion was based on the following points: (i) ongoing insulin usage for at least previous 3 months till the registration in the clinic (1st point of contact), (ii) having full comprehensive data addressing the insulin injection process, (iii) having full set of anthropometric data, and (iv) a HbA1c level at registration or either before or after 3 weeks of the 1st point of contact. Once a particular patient's data were selected for enrollment in the study, the data entry team comprising a data entry operator and a diabetic counselor each would transfer the same to a Microsoft Excel master-sheet, which when completed was submitted to the biostatistician.
Hypoglycemia' was defined as the occurrence of one or more typical symptoms, such as palpitations, tiredness, sweating, strong hunger, dizziness, and tremor, which were reverted by oral intake of carbohydrate or parenteral administration of glucose with or without a confirmed blood glucose reading of ≤70 mg/dL, by glucometer or incidental laboratory report.
Statistical analyses were performed using GraphPad Prism, Version 6.01 (GraphPad Software, La Jolla, CA, USA). Chi-square test with Yates' correction was used to measure association. The effect of Yates' correction is to prevent overestimation of statistical significance for small data. The logic of Yates' correction rests upon the fact that because contingency table analyses are based on dichotomous data, and the statistical Chi-square distribution is continuous (rather than dichotomous), an adjustment must be applied to contingency table analyses, so as to obtain more accurate results.[9]
RESULTS
Baseline characteristics, demographic and anthropometric profile
The analyzed dataset comprised a total number of 748 individuals with T2DM who has been getting insulin. Mean (±standard deviation [SD]) age was 54.64 ± 10.97 years. Median age was 61 years with a range of 15–85 years. Of 748 study participants, 466 (62.30%) were male whereas 282 (37.70%) were female. Mean ± SD body weight was 65.22 ± 11.94 kg. Median weight with range was 64 (36–126) years. Almost 2/3rd (67.93%) of the participants were either overweight or obese by Asian criteria.[10]
More than half of the participants were well educated with 67.78% having completed secondary education, and 51.7% having acquired at least a college graduation [Table 1].
Table 1.
Baseline characteristics, demographic and anthropometric profile
Awareness of diet, exercise, and self-monitoring
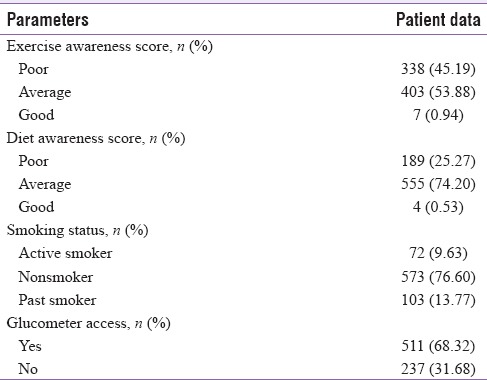
Table 2 highlights patient's awareness as categorized by the interviewers (counselors) during the 1st clinic visit. Only a few patients (<1%) showed optimal awareness when two lifestyle parameters namely exercise and diet were considered. One-third of them did not have access to glucometer despite being on insulin therapy. Self-reported hypoglycemia during preceding 6 months was 9.20% among patients performing SMBG using glucometer whereas the same was reported by only 4.21% of patients not performing SMBG. There was statistically significant (P < 0.05) association between SMBG performed and hypoglycemia episodes.
Table 2.
Summary of behavioral profiles with respect to therapeutic lifestyle modification, smoking habits, and self-monitoring
Concomitant usage of oral antidiabetic drugs
While 47% of patients were treated with insulin alone, rest (53%) of the patients had been treated with insulin plus any one or more classes of OADs. As regards the concomitant usage of OADs, metformin usage remained the highest followed closely by sulfonylureas (SU), while the rest of classes were used minimally [Figure 1].
Figure 1.
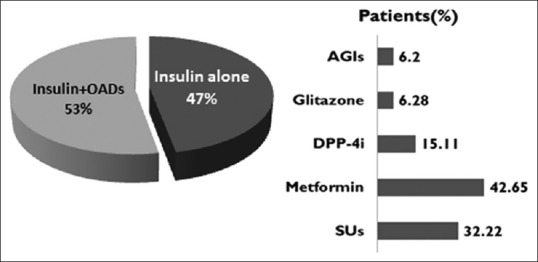
Summary of concomitant OAD usage. AGI: Alpha-glucosidase inhibitors, DPP-4i: Dipeptidyl peptidase-4 inhibitor, SU: Sulfonylurea, OAD: Oral antidiabetic drug
Reasons for insulin initiation
All total 20 different reasons either alone or in combination(s) were identified which described the major causes of insulin initiation. Figure 2 gives a breakup of all indications to 7 major categories of indication for starting insulin in the primary or secondary level.
Figure 2.
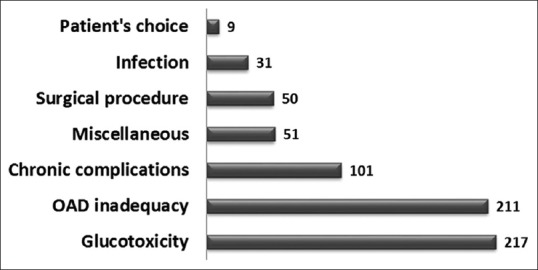
Reasons for initiating insulin treatment. In 78 (10.43%) patients, more than 1 indication was noted. OAD: Oral antidiabetic drug
Durations of disease and insulin treatment
At enrollment, the patients' mean diabetes duration was 12.24 ± 7.60 years and they were on insulin for a mean period of 3.42 ± 4.18 years. They had spent 8.80 ± 6.42 years of diabetes without insulin, i.e., before initiation. Mean ± SD HbA1c% of the entire group was 9.12 ± 2.12, with 15.2% of participants achieving a target of HbA1c <7%. The percentage of participants achieving HbA1c goal of <7% as regards the durations of diabetes spent with (after initiation) or without (before initiation) insulin therapy are shown in Tables 3a and b, respectively.
Table 3a.
Status of glycemic control with regards to duration of diabetes when insulin was initiated
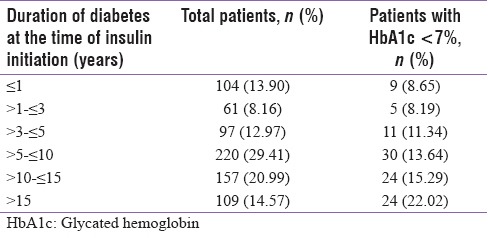
Table 3b.
Showing how glycaemic control varies in different subgroups according to the total duration of ongoing insulin treatment
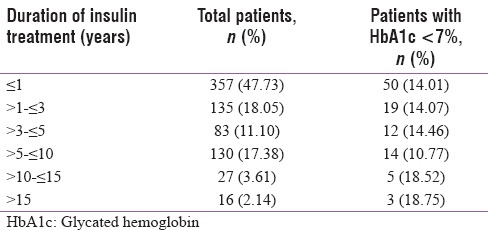
Mean total daily dose (TDD) of insulin was 33.36 ± 18.44 units and mean daily dose per kilogram body weight was 0.51 ± 0.27 units. Mean number of insulin injections per day was 2.06 ± 0.73. Among our participants, 16.84% received 1 injection, 66.98% received 2 injections, while 10.03% received 3 injections per day, and only 5.75% received 4 injections per day.
Among the respondents, 58.96% were on human insulin, 35.70% were on analog insulin, and 5.35% patients were using both human and analog insulin. Insulin pen devices were used by 66.08%, whereas 31.76% used insulin syringes, and 2.15% were using both insulin pens and syringes.
Type of insulin regimen
The collected dataset provided insight to contemporary practice among primary and secondary level physicians in choosing a type of insulin on the basis of action both individually and as a part of more complex regimen. These observations are depicted in Table 4. Premix insulin remains the predominant choice, followed distantly by basal.
Table 4.
Types and regimens of insulin on the basis of action as prescribed by primary level and secondary level physicians. Predominance of premix insulin much above the others is noteworthy
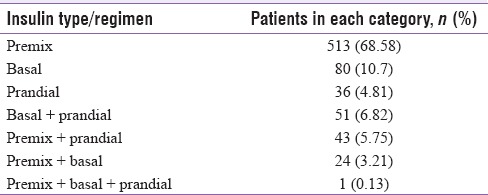
Insulin injection techniques
In this study, correct insulin injection techniques were analyzed with regard to parameters such as angle, site selection, site rotation, storage, gap between meal and injection, shaking/mixing, hygiene, and timely needle change [Table 5]. Injection technique appeared to be optimal with regard to the most parameters, namely, angle, site selection, time gap between meal and injection, hygiene, and storage of insulin, in majority of our patients. However, significantly higher numbers of patients (69.65%) were not rotating the injection site properly. A huge proportion (72.19%) of patients reused the same needle for injecting for more than 3 times.
Table 5.
The proportion of patients executing different parameters of insulin injection technique either rightly or wrongly
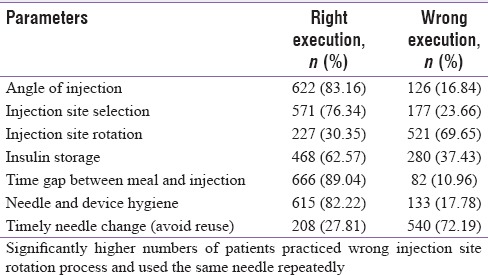
Hypoglycemia
A total of 57 (7.6%) of the 748 interviewed participants reported experiencing at least one episode of hypoglycemia during previous 6 months. There was no statistically significant association between hypoglycemic episodes with type of insulin. Hypoglycemia was reported by 6.74% of patients using analog insulin, 7.71% of patients using human insulin, and 12.50% of patients using both human and analog insulin. However, significant association was observed between improper time gap between meal and insulin injection and hypoglycemic episodes. While only 6.90% of patients maintaining proper gap between insulin injection and meal had experienced hypoglycemia, the same was experienced by as much as 13.41% of patients not maintaining proper gap (P < 0.05). We did not find significant difference in incidental hypoglycemia with regard to right versus wrong execution of parameters such as injection site selection, site rotation, angle, needle reuse, storage, and also device used (pen vs. syringe) in our study. Quite interestingly, significantly higher (P < 0.05) numbers of patients performing SMBG through glucometer (9.20%) had reported hypoglycemia in the last 6 months whereas the same was reported by only 4.21% of patients not performing SMBG.
Lipohypertrophy
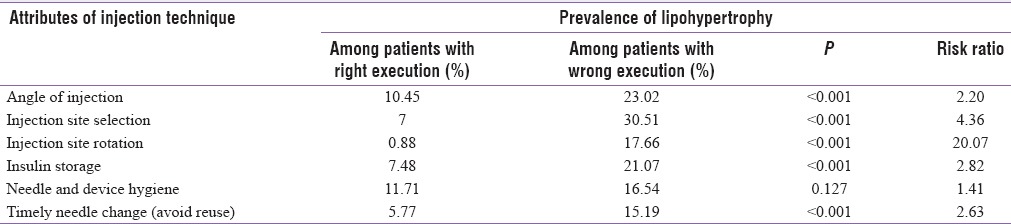
A total of 94 (12.57%) patients were found to have LH vide visual examination and manual palpation. We found a significant association of LH with parameters such as angle of injection, site of injection, periodic injection site rotation, insulin storage, and timely needle change, while injection hygiene was insignificantly associated [Table 6]. The most robust association was found with wrong technique of rotation. LH was also significantly (P < 0.05) associated with the use of different humans (14.74%) compared to analog insulin (8.24%).
Table 6.
Attributes of injection techniques and their relationship with the prevalence of lipohypertrophy
The association of LH with hypoglycemia and hyperglycemia was also examined in this study [Table 7]. There was a trend of worsened glycemic status among patients having LH, without statistical significance. Similarly, patients with LH had worse mean HbA1c (9.34 ± 2.28) than their peers without LH (9.08 ± 2.10). However, this difference was not significant.
Table 7.
Describes how lipohypertrophy is related to different parameters of insulin therapy, glycemic control, and hypoglycemia as reported by patients
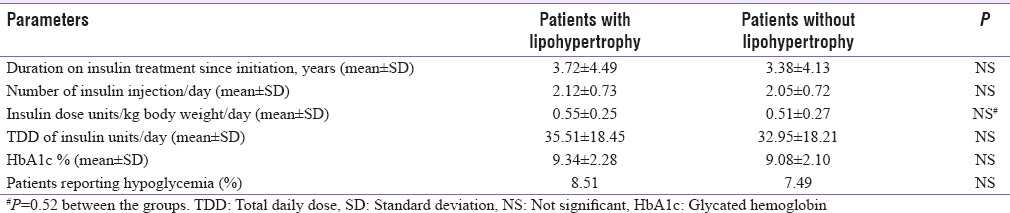
There was no difference in mean ± SD of duration of insulin treatment, number of injections per day, and total dose of insulin per day between patients with or without LH. The mean insulin dose per kilogram body weight per day appeared to be more in the patients with LH, but without any statistical difference [Table 7].
DISCUSSION
Glycemic control is a key focus in the management of T2DM and is associated with both micro- and macro-vascular benefits. The treatment of T2DM has evolved with our understanding of the pathophysiology of this complex disease. Although a wide range of oral drug treatments, characterized by different mechanisms of action, are available to achieve glycemic control in patients with T2DM, glycemic control often fails even after a combination of them due to the progressive nature of the disease, eventually necessitating insulin treatment.[11] Acceptance and adherence to insulin treatment can be hindered by concerns such as perceived interference with the quality of life, worsening of diabetes condition, guilt or feeling of failure of self-management, and daily injection burden. Correct execution of each attribute of the injection process is a key to achieving success in insulin treatment.[3]
The mean age and duration of diabetes were somewhat similar to the baseline data from the Indian cohort in the multinational prospective observational study(MOSAIc) addressing similar issues amongst T2DM patients on insulin with or without OADs.[12] We had lesser numbers of female patients (32.7%) compared to the MOSAIc cohort (44%) which could be due to referral bias, or gender discrimination in healthcare-seeking behavior.
At least half of the patients had reasonable educational exposure with 51.7% having college graduation or higher qualifications, and 67.78% having high school graduation or an equivalent degree. There were 57% high school educated patients in the MOSAIc Indian cohort 57%.[12] In that study, 6% of enrolled Indian patients were active smoker as against their global figure of 14%.[12] We found that 9% of our patients were active smoker by their own admission.
Any study looking at knowledge, attitude, and practice of patient living with a chronic ailment like diabetes requires the respondents (patients) to have sufficient educational background and socioeconomic stability to minimize major confounding factors. To this end, our study seems to have enrolled the ideal types of patients with >90% being literate and >50% being highly educated. Such a phenomenon is quite expected in the current Indian scenario in an urban referral center, notwithstanding the fact that the same may influence the outcome of such a study which looks at patients' behavioral aspect in depth. However, despite having such advantageous background, only a negligible numbers of patients (<1%) showed optimal awareness in the two lifestyle parameters, i.e., exercise and diet when assessed using a simple response tool. MOSAIc investigators used a unique Diabetes Knowledge Test score, in a scale ranging from 0 (no questions correct) to 9 (all questions correct). In that study, Indian patients stood somewhere in the middle with a mean score of 4 ± 2, modestly lower than the global average (5 ± 2).[12] Such a phenomenon underscores the huge mismatch between general educational and socioeconomic background and diabetes awareness, and urgent need to address such a lacuna.
The American Diabetic Association has statutory recommendations for SMBG using glucometer in all diabetic patients on insulin.[13] However, one-third of our study patients did not have access to glucometer despite being on insulin therapy for fairly long periods. Other studies from developing countries like Kenya also have reported low utilization and adherence.[14] A large Chinese study found that only 1/5th patients did a optimally structured SMBG while 1/3rd never performed it at all. Male sex and poor socioeconomic status were among major associated factors for not performing SMBG.[15] In our study, we did not perform any regression analysis to find out such associations. There was no statistically significant association between patients achieving HbA1c target (<7%) with educational qualification, income status, exercise and diet awareness score, and types of insulin. Moreover, patients having glucometer access did not show better glycemic control compared to patients not having an access to glucometer. Interestingly, the incidence of self-reported hypoglycemia during preceding 6 months in our study was significantly more in the group performing SMBG compared to the group not opting for SMBG group (9.20% vs. 4.21%, P < 0.05). Such findings indicate that patients performed SMBG during a panic situation associated with an attack of hypoglycemia, rather than using it in a structured manner to plan judicious treatment to achieve targets of control. Such a pattern of increased blood glucose monitoring as a direct outcome of hypoglycemia experienced during insulin therapy was also observed in a cohort from South Asia in a multinational study.[16] These points underscore the need for imparting proper education and motivation to ourinsulin treated patients for performing a proactive, structured SMBG.
Recently conducted DiabCare Asia trials in India and Gulf countries showed a mean ± SD HbA1c% of 8.9 ± 2.1 (19.7% patients with <7%) and 8.3 ± 2.0 (24% patients with <7%), respectively. In our cohort, we found a higher HbA1c (9.12 ± 2.12), with only15.2% of patients achieving a target of HbA1c <7%. This is probably due to relatively longer mean duration of diabetes in our patients (12.2 years), compared to 6.8 years and 8.7 years in the two DiabCare Asia cohorts, respectively.[1,2] A substantial proportion of patients in the DiabCare groups were on OADs alone, signifying less complex nature of their disease.[1,2] Our cohort consisted of patients referred to a tertiary endocrine care facility; hence, the percentage of complex patients may be higher.
Although the UK Prospective Diabetes Study showed the benefits of combining (ongoing) OADs along with (newly initiated) insulin, many experts in the Western countries have been debating the rationale behind such combinations.[17,18] However, it is not difficult to find many prescriptions in Asian countries containing such combinations.[1,2,19,20] While 47% of our studied cohort were treated with insulin alone, rest (53%) of the patients had been treated with insulin plus one or more OADs. In a large worldwide survey comprising over 13,000 patients, higher numbers (56.5%) of participants were on insulin, while 40.5% of participants took both insulin and OADs, and minimal numbers used other drugs.[21] Metformin was the highest prescribed OAD (42.65%) which is, however, little less then expected, given that we included only T2DM patients on insulin. Surprisingly, substantial numbers of patients still received SUs in spite of their long duration of diabetes and concomitant insulin prescription indicating its popularity among primary and secondary level practitioners, even at the risk of causing more hypoglycemia. As this study used database of earlier years (2006 onward), the number of prescriptions for dipeptidyl peptidase-4 inhibitor was probably less. Concomitant prescriptions of glitazones (thiazolidinedione) were much less in number (6.28%), probably due to fear of weight gain, an adverse event attributed to insulin treatment also. As an individual agent, alpha-glucosidase inhibitors (AGIs) seems to be an underutilized group in such combination (6.2% only), given the fact that Indians use large portions of carbohydrate in their major meals. One explanation could be the preference of our practitioners for premix insulin to tackle postprandial hyperglycemia over AGIs as the later agent relatively modest benefit in this regard. In contrast, a large survey of Japanese general practitioners' prescriptions revealed thiazolidinedione as the most common concomitant OAD prescribed in addition to insulin, followed by SUs (18.2%), metformin (9.6%), and AGIs (5.3%). One in every 4 patients was prescribed ≥2 OADs.[20] The DiabCare study from Gulf countries revealed that along with insulin 1, 2, and 3 OAD classes were prescribed in 15.9%, 15.5% and 6.2% patients respectively. In a West Asian T2DM population inclusive of both insulin treated or untreated patients, the prescription trends for OADs was completely different from the present study, with metformin (83.5%) remaining at the top, followed by SUs (58.6%), thiazolidinedione (28%), and AGIs (4.9%).[2] A survey of similar unselected population from India revealed higher numbers of prescription of SUs (71.6%), even more than that of metformin (61.6%).[1] There appears to be a country-wise variation of prescription trends with thiazolidinedione remaining predominant choice in Japan, and SUs in India, and metformin in other countries.
We tried to look at various reasons for initiation of insulin treatment from patients' history or medical records. The most common reason was severe hyperglycemia alone, which has been included under the broad heading of glucotoxicity. This was followed by failure to achieve desired glycemic targets despite adequate intensification with multiple OADs (secondary OAD failure). These trends are in line with recently published major national guidelines for insulin initiation.[22,23] In our study, mean duration of diabetes was 12.24 ± 7.60 years at enrollment, and patients were on insulin for the mean period of 3.42 ± 4.18 years, having spent 8.80 ± 6.42 years of diabetes without insulin, i.e., before initiation. In the MOSAIc study cohort from both India and rest of the World, insulin was initiated after a mean disease duration of 12 ± 8 years.[12] In the background of ongoing insulin ± OAD treatment, T2DM patients are expected to have long-standing disease characterized by advanced beta cell failure. The mean duration of insulin treatment was 3 years in both the DiabCare India and DiabCare Gulf survey, a finding, quite similar to our cohort (3.42 ± 4.18 years). It was observed that number of patients achieving HbA1c target of <7% did not differ when patients were categorized according duration in years of insulin treatment [Table 3b]. Although there was no definite trend to suggest that longer duration of treatment with insulin favored a better glycemic control in majority of patients, the numbers were not large in some subgroups to show significant difference. The duration of diabetes in years at the time of initiating insulin, however, appeared to be inversely related to the likelihood of patients achieving HbA1c target of <7% [Table 3a]. We found that longer the diabetes had been managed successfully with OADs and without insulin therapy, there was more likelihood of achieving HbA1c target <7%. This seems paradoxical, as it is generally believed that early insulin initiation would provide better glycemic control in more number of patients. It may be due to the fact that many T2DM patients, who require insulin quite early during diabetes actually belong to the special category called latent autoimmune diabetes in adult, which is characterized by advanced autoimmune destruction of pancreatic beta cell.[24] In the context of T2DM, a hypothetical explanation is that if a patient requires insulin at a very early phase of diabetes, the disease pattern is bound to be much more complex than that of a patient who can be managed without insulin for many years after the diagnosis of T2DM. In our future studies, we shall try to examine this matter more extensively. TDD of insulin (U/day) was 33.36 ± 18.34 in our study, which was similar to DiabCare India survey which reported 32.1 ± 17.0. While the practitioners from Gulf countries reported higher mean daily dose (U/day) of 57.5 ± 30.4, their Japanese counterpart used relatively lower dose of 25.8 ± 22.9 U/day. Such finding may indicate racial variations in daily requirement of insulin. The same can also represent a pattern of practice amongst practitioners of a particular country or region. Majority of our patients (66.98%) were on two injections per day while 16.84% were on 1 injection and 10.03% were on 3 injections per day respectively. Similar trend was also observed in DiabCare India survey with 65% on 2 injection, 17.6% on 1 injection, and 13.5% on 3 injections per day.[1] In MOSAIc study, majority among Indian and Chinese cohorts were prescribed 2 injections daily, while for rest of the world, prescribing single injection was the most common practice.[12] Such a behavior is explained by huge popularity of premix which is generally prescribed twice a day over basal or basal bolus regimen in India and China.
We found slightly higher uses of analog insulin among our patients. In our study, 58.96% used only human insulin, 35.70% only analog insulin, while a small numbers (5.35%) even used both. The comparative figures for human versus analog were 71% versus 32% in the DiabCare India study, 26% versus 85% in DiabCare Gulf study, and 68.3% versus 31.7% in the Japanese GP study.[1,2,20] Such variations of choice may have been influenced mainly by factors like socioeconomic conditions, affordability, availability etc., rather than by superiority of a particular type of insulin.
Insulin pen device was used by 66.08% of the patients, whereas 31.76% patients used insulin syringes, and 2.15% were using both Insulin pen as well as syringes. Our findings were similar to that of the DiabCare India study where 65.6% used pens, 32.0% used syringes.[1] Data from the multinational MOSAIc revealed that majority used pen device over syringes in countries like China (100%), Germany (95%), India (59%), Russia (93%), Saudi Arabia (63%), where the trend was just reverse in Mexico (33%) and USA (45%).[12] Data from a recent large worldwide survey indicated that insulin pen alone was used by 85.6% of patients, while 9.6% used a syringe alone, 2.8% used both, and 1.4% used a pen and another device (usually an insulin pump).[21] Thus, it seems economic condition is a important, but not the sole factor responsible for physicians'/patients' choice and familiarity in using modern devices during insulin therapy.
Over 2/3rd of our patient were prescribed premix insulin (human or analog), a trend supported by evidence from previous epidemiological surveys, real world data, and consensus statements of key opinion leaders.[1,22,23,25,26] This trend continuing with modern co-formulations.[27] Only 1 of 6 patients were on basal insulin (alone or in combination with prandial). There were few other interesting permutations and combinations, used less commonly, indicated probably in complex situations. In the multinational MOSAIc study, countries such as Russia, Mexico, Saudi-Arabia, and the USA had predominant basal insulin recipients (from 2/3rd to 3/4th), while in China and India, premix was predominant insulin type (from 3/5th to 2/3rd).[12] Compared to 68.58% of premix users in our study, the figure for DiabCare India survey was less (52%), but it (premix) came out as the most commonly used insulin type.[1] In yet another Asian cohort from Japan, general practitioners prescribed premix insulin in more than 50% of patients when used as monotherapy.[20] Premix insulin remained the predominant choice for specialists also, though to a slightly lesser extent. Only about 1/4th to 1/6th patients were put on basal insulin alone in that study. Thus it is quite apparent that unlike Western countries, premix remains the predominant choice among Asian physicians. A major reason for such a choice could be the ease of initiation and intensification using the same insulin in T2DM.[28] Others have attributed such a trend to specific disease pattern, genetics, access to resources, ethnicity, and lifestyle.[29,30] With regard to injection technique, certain parameters such as angle, site selection, time gap between injection and meal, hygiene, and storage of insulin were correctly executed in majority of patients [Table 5]. These findings were similar to a that of a recent survey from tertiary care institute from South India where majority were correct in angle of injection, storage, and site selection and hygiene, but not the time gap.[31] However, one common area of concern was lack of timely change of needle to avoid reusing (>1 injection) in 72.19% patients in our study and 96.89% in the South India study.[31] Frid et al. found needle reuse(>1prick) in approximately half of their patients worldwide, almost a third using the same needle up to 6 times. Cost of needle was cited most frequently reason for such practice which had major impact on occurrence of LH (discussed later).[21] In contrast, more than 4/5th patients were found to be (rightfully) avoiding needle reuse in developed nation like Italy.[32] This remains a contentious issue in resource-limited country like India. Although the recent guidelines have strongly discouraged needle reuse, the optimal cutoff for maximum number of injection per needle has not been definitively set.[3,33] Overall, lack of counseling on the part of HCP is more important than the patient's' affordability, and we opine that counselors should highlight the importance of using of needle only once, and explain how this step can minimize the long-term adverse effect of reuse. The same interventional study from Italy also found that only 40.1% of patients considered other attributes of injection technique as very important at baseline which increased to 64.6% after 3 months counseling.[32] This underscores the importance of counseling in this hitherto neglected aspect of diabetes management. Another major issue as regards the injection technique was improper site rotation by an overwhelming majority (69.67%). Rotation appeared to be a major problem in other cohorts from previously described studies as well.[34,35,36,37]
Studies have reported that repeated injections of insulin in the same skin region induce local reactions of the subcutaneous adipose layer, broadly known as LD, but most often manifest as LH.[7] The knowledge about factors triggering the subcutaneous tissue to develop firm growths of adipose tissue is limited. However, it appears that such lesions are the result of a multifactorial process, such as repeated injections/infusions with needles/catheters at the same body site, and local growth promoting action of insulin molecule or even insulin excipients. With insulin injection, reuse of needles may increase local tissue trauma and hypertrophic responses.[34,35] Surprisingly, well-designed and dedicated studies addressing this practical, highly relevant issue of LH as major source of variability in glycemic control has not been done by medical community, either clinicians, academicians, insulin or device manufacturers. Few studies have shown that injecting into lipohypertrophic sites may result in significantly unpredictable and delayed absorption which can lead to hyperglycemia and/or hypoglycemia.[36]
The prevalence of LH in our study was 12.57% which was quite similar to another study from India, but much less compared to a study carried out on an Italian population (48.7%), and a worldwide survey (30.8%) using visual inspection and manual palpation.[31,32,37] Another study from Spain enrolling both T1DM and T2DM patients with 90% pen device users reported LH in 2/3rd.[7] The following confounders may be responsible for lower prevalence of LH in our study: (i) ours was not a prospective study (ii) mean duration of insulin treatment and mean number of injection per day were comparatively lower in our cohort. One weakness of our study was our inability to detect and characterize LH using ultrasonography which can easily distinguish the affected tissue from adjacent normal SC tissue. In ultrasonography, appearance of homogeneous hyperechogenic densities filling part or all of the SC tissue at injection sites characterizes LH.[38] There could be underreporting of such lesions if combined visual inspection and manual palpation (by diabetic counselors) remains the only modality of detection, which can partly explain lower prevalence in our study.
LD was significantly (P < 0.05) associated with the use of different Human (14.74%) compared to Analogue insulin (8.24%). Like a recent survey from South India, we found overwhelmingly robust association of LH (risk ratio 20.07, P < 0.001) with wrong/improper rotation of injection site, while the same for confounders like repeated use of same needle is less robust but nevertheless significant [Table 6]. Frid et al., using logistic regression analysis found incorrect rotation and duration on insulin treatment to be the most important factors associated with LH (P < 0.001).[37] Less robust but nevertheless significant correlation between the presence of LH and the reuse of needles, numbers of injections per day, an earlier age at diagnosis of DM, a longer duration of having DM and taking insulin have also been documented in that large survey.[37] Unlike the Worldwide survey, in our study, the duration of insulin treatment, numbers of injections per day, TDD of insulin, and daily insulin dose per kilogram body weight were marginally but insignificantly higher in patients having LH [Table 7]. These may be due to lesser numbers of patients in our cohort. In a Spanish cohort, among patients with LH, 61% reported needle reuse, reversely those who reused needles, of them 70% had LH.[7] Such findings can have huge clinical implication as they can provide the right direction to intervention strategies to avoid LH in patients treated with insulin. Importance of educating the patients to self-examine and detect early changes leading to LH and LA has already been emphasized by others.[39]
The international study by Frid et al. found that the mean HbA1c level was 0.55% higher in patients with LH than in those without LH (P < 0.05). A trend of worsened glycemic status among patients having LH was seen in our cohort also. However, the change was without statistical significance [Table 7]. The smaller and insignificant difference may be due to smaller size of the study cohort and other dominant factors influencing glycemic excursions. Another prospective interventional study has shown 6.8% reduction in HbA1c, and 2 unit absolute reduction of total daily insulin requirement within 3 months of counseling to avoid areas with LH and rotating properly while injecting insulin.[32] The strong link between hypoglycemia and LH has been well documented. The main reasons could be impaired and variable insulin absorption and action when the insulin is injected into the affected tissue.[40] Frid et al. have documented significantly higher occurrence of unexpected hypoglycemia and glucose variability in patients who had LH and incorrect rotation of site.[37] In a Spanish cohort, 39.1% of patients with LH had unexplained hypoglycemia compared to only 5.9% in those without LH (P < 0.01).[7] In our study, also, patients with LH had more hypoglycemic episodes compared to those without LH, though the difference did not achieve a statistical significance [Table 7]. This may be due to low absolute number of patients with LH, and underreporting of hypoglycemia due to retrospective data collection. At times, patients may have missed out on detecting minor hypoglycemia. Lack of access to glucometer in majority of patients also deprived them of the opportunity to correlate their subtle symptoms with blood glucose. Or, there may be dominant factors other than LH such as insulin dose, concomitant drugs or dietary indiscipline, etc., influencing glycemic excursions.
CONCLUSION
Our work adds much-needed information to our knowledge about diabetes care, insulin use, and injection practices. The current study reveals the unique patterns of insulin usage and injection practices in India, highlights the high prevalence of LH in insulin users, and identifies the factors associated with this complication. The results of this study provide real-life data, which will help formulate newer strategies to improve delivery of diabetes care.
Financial support and sponsorship
Mr. Saptarshi Bose is currently employed by Biocon Limited, Bengaluru. However, the views expressed in this article are his personal.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are thankful to Mr. Santosh Anand for his assistance in preparing the draft, and to Ms. Smritisikha Bora and Mr. Jatin Bora for helping in the data extraction. The cooperation of Diabetes Care team and the registered patients of Excel Centre, Guwahati, and guidance from Ms. Vandita Gupta and Mr. Sautik Roy is also acknowledged.
REFERENCES
- 1.Mohan V, Shah SN, Joshi SR, Seshiah V, Sahay BK, Banerjee S, et al. Current status of management, control, complications and psychosocial aspects of patients with diabetes in India: Results from the DiabCare India 2011 Study. Indian J Endocrinol Metab. 2014;18:370–8. doi: 10.4103/2230-8210.129715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Omar MS, Khudada K, Safarini S, Mehanna S, Nafach J. DiabCare survey of diabetes management and complications in the Gulf countries. Indian J Endocrinol Metab. 2016;20:219–27. doi: 10.4103/2230-8210.176347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tandon N, Kalra S, Balhara YP, Baruah MP, Chadha M, Chandalia HB, et al. Forum for injection technique (FIT), India: The Indian recommendations 2.0, for best practice in insulin injection technique, 2015. Indian J Endocrinol Metab. 2015;19:317–31. doi: 10.4103/2230-8210.152762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thow JC, Johnson AB, Marsden S, Taylor R, Home PD. Morphology of palpably abnormal injection sites and effects on absorption of isophane(NPH) insulin. Diabet Med. 1990;7:795–9. doi: 10.1111/j.1464-5491.1990.tb01494.x. [DOI] [PubMed] [Google Scholar]
- 5.Richardson T, Kerr D. Skin-related complications of insulin therapy: Epidemiology and emerging management strategies. Am J Clin Dermatol. 2003;4:661–7. doi: 10.2165/00128071-200304100-00001. [DOI] [PubMed] [Google Scholar]
- 6.Holstein A, Stege H, Kovacs P. Lipoatrophy associated with the use of insulin analogues: A new case associated with the use of insulin glargine and review of the literature. Expert Opin Drug Saf. 2010;9:225–31. doi: 10.1517/14740330903496402. [DOI] [PubMed] [Google Scholar]
- 7.Blanco M, Hernández MT, Strauss KW, Amaya M. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39:445–53. doi: 10.1016/j.diabet.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Overland J, Molyneaux L, Tewari S, Fatouros R, Melville P, Foote D, et al. Lipohypertrophy: Does it matter in daily life? A study using a continuous glucose monitoring system. Diabetes Obes Metab. 2009;11:460–3. doi: 10.1111/j.1463-1326.2008.00972.x. [DOI] [PubMed] [Google Scholar]
- 9. [Browsed on 2016 Sep 17]. Available from: http://www.how2stats.net/2011/09/yates-correction.html .
- 10.Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–70. [PubMed] [Google Scholar]
- 11.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet. 2005;365:1333–46. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 12.Polinski JM, Kim SC, Jiang D, Hassoun A, Shrank WH, Cos X, et al. Geographic patterns in patient demographics and insulin use in 18 countries, a global perspective from the multinational observational study assessing insulin use: Understanding the challenges associated with progression of therapy (MOSAIc) BMC Endocr Disord. 2015;15:46. doi: 10.1186/s12902-015-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Standards of medical care in diabetes-2010. Diabetes Care. 2010;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wambui Charity K, Kumar AM, Hinderaker SG, Chinnakali P, Pastakia SD, Kamano J. Do diabetes mellitus patients adhere to self-monitoring of blood glucose (SMBG) and is this associated with glycemic control? Experiences from a SMBG program in Western Kenya. Diabetes Res Clin Pract. 2016;112:37–43. doi: 10.1016/j.diabres.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Ji L, Su Q, Feng B, Shan Z, Hu R, Xing X, et al. Glycemic control and self-monitoring of blood glucose in Chinese patients with type 2 diabetes on insulin: Baseline results from the COMPASS study. Diabetes Res Clin Pract. 2016;112:82–7. doi: 10.1016/j.diabres.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Aronson R, Galstyan G, Goldfracht M, Alsifri S, Elliot L, Kapur R, et al. Health economic impact of hypoglycaemia in a global population of patients with insulin-treated diabetes. Poster Number 0713-PD, IDF. 2015 doi: 10.1016/j.diabres.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: Progressive requirement for multiple therapies (UKPDS 49).UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005–12. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 18.Bailey CJ. The current drug treatment landscape for diabetes and perspectives for the future. Clin Pharmacol Ther. 2015;98:170–84. doi: 10.1002/cpt.144. [DOI] [PubMed] [Google Scholar]
- 19.Raheja BS, Kapur A, Bhoraskar A, Sathe SR, Jorgensen LN, Moorthi SR, et al. DiabCare Asia – India study: Diabetes care in India – Current status. J Assoc Physicians India. 2001;49:717–22. [PubMed] [Google Scholar]
- 20.Arai K, Takai M, Hirao K, Matsuba I, Matoba K, Takeda H, et al. Present status of insulin therapy for type 2 diabetes treated by general practitioners and diabetes specialists in Japan: Third report of a cross-sectional survey of 15,652 patients. J Diabetes Investig. 2012;3:396–401. doi: 10.1111/j.2040-1124.2012.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frid AH, Hirsch LJ, Menchior AR, Morel DR, Strauss KW. Worldwide injection technique questionnaire study: Population parameters and injection practices. Mayo Clin Proc. 2016;91:1212–23. doi: 10.1016/j.mayocp.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Premix Insulin: Initiation and Continuation Guideline for Management of Diabetes in Primary Care: Indian National Consensus Group, J. Assoc Physic India. 2009;57(Suppl):42–6. [Google Scholar]
- 23.Kumar A, Sharma SK, Rajput R, Unnikrishnan AG. Initiating therapy or switching to biphasic insulin aspart improves glycaemic control in type 2 diabetes: An Indian experience from the A1chieve study. J Assoc Physicians India. 2013;61(1 Suppl):16–20. [PubMed] [Google Scholar]
- 24.Pozzilli P, Di Mario U. Autoimmune diabetes not requiring insulin at diagnosis (latent autoimmune diabetes of the adult): Definition, characterization, and potential prevention. Diabetes Care. 2001;24:1460–7. doi: 10.2337/diacare.24.8.1460. [DOI] [PubMed] [Google Scholar]
- 25.Das AK, Sahay BK, Seshiah V, Mohan V, Muruganathan A, Kumar A, et al. INCG Group. Indian National Consensus Group: National Guidelines on Initiation and Intensification of Insulin Therapy with Premixed Insulin Analogs. API India, Medicine Update; Ch. 51. 2013. [Last accessed on 2014 Feb 14]. pp. 227–36. Available from: http://www.apiindia.org/medicine_update_2013/chap51.pdf .
- 26.Unnikrishnan AG, Tibaldi J, Hadley-Brown M, Krentz AJ, Ligthelm R, Damci T, et al. Practical guidance on intensification of insulin therapy with BIAsp 30: A consensus statement. Int J Clin Pract. 2009;63:1571–7. doi: 10.1111/j.1742-1241.2009.02192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalra S, Baruah MP. Insulin degludec aspart: One-year real world experience. Indian J Endocrinol Metab. 2016;20:369–71. doi: 10.4103/2230-8210.177416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalra S, Balhara YP, Sahay BK, Ganapathy B, Das AK. Why is premixed insulin the preferred insulin? Novel answers to a decade-old question. J Assoc Physicians India. 2013;61(1 Suppl):9–11. [PubMed] [Google Scholar]
- 29.John M, Kalra S, Unnikrishnan AG, Ganapathy B, Baruah MP, Sahay RK. Recommendations for insulin initiation based on ethnicity. Med Hypotheses. 2011;77:460–1. doi: 10.1016/j.mehy.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Kalra S, Gupta Y. Insulin initiation: bringing objectivity to choice. J Diabetes Metab Disord. 2015;14:17. doi: 10.1186/s40200-015-0146-1. doi: 10.1186/s40200-015-0146-1. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patil M, Sahoo J, Kamalanathan S, Selviambigapathy J, Balachandran K, Kumar R, et al. Assessment of insulin injection techniques among diabetes patients in a tertiary care centre. Diabetes Metab Syndr. 2016:pii: S1871-402130211-9. doi: 10.1016/j.dsx.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Grassi G, Scuntero P, Trepiccioni R, Marubbi F, Strauss K. Optimizing insulin injection technique and its effect on blood glucose control. J Clin Transl Endocrinol. 2014;1:145–50. doi: 10.1016/j.jcte.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalra S, Balhara YP, Baruah MP, Chadha M, Chandalia HB, Chowdhury S, et al. Forum for injection techniques, India: The first Indian recommendations for best practice in insulin injection technique. Indian J Endocrinol Metab. 2012;16:876–85. doi: 10.4103/2230-8210.102929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hauner H, Stockamp B, Haastert B. Prevalence of lipohypertrophy in insulin-treated diabetic patients and predisposing factors. Exp Clin Endocrinol Diabetes. 1996;104:106–10. doi: 10.1055/s-0029-1211431. [DOI] [PubMed] [Google Scholar]
- 35.Vardar B, Kizilci S. Incidence of lipohypertrophy in diabetic patients and a study of influencing factors. Diabetes Res Clin Pract. 2007;77:231–6. doi: 10.1016/j.diabres.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 36.Frid A, Hirsch L, Gaspar R, Hicks D, Kreugel G, Liersch J, et al. New injection recommendations for patients with diabetes. Diabetes Metab. 2010;36(Suppl 2):S3–18. doi: 10.1016/S1262-3636(10)70002-1. [DOI] [PubMed] [Google Scholar]
- 37.Frid AH, Hirsch LJ, Menchior AR, Morel DR, Strauss KW. Worldwide injection technique questionnaire study: Injecting complications and the role of the professional. Mayo Clin Proc. 2016;91:1224–30. doi: 10.1016/j.mayocp.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Catambing I, Villa M. Ultrasonographic measurement of skin and subcutaneous thickness at insulin injection sites among adult Filipinos with diabetes. J ASEAN Fed Endocr Soc. 2014;29:24–32. [Google Scholar]
- 39.Gentile S, Strollo F, Ceriello A. AMD-OSDI Injection Technique Study Group. Lipodystrophy in insulin-treated subjects and other injection-site skin reactions: Are we sure everything is clear? Diabetes Ther. 2016;7:401–9. doi: 10.1007/s13300-016-0187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Famulla S, Hövelmann U, Fischer A, Coester HV, Hermanski L, Kaltheuner M, et al. Insulin injection into lipohypertrophic tissue: Blunted and more variable insulin absorption and action and impaired postprandial glucose control. Diabetes Care. 2016;39:1486–92. doi: 10.2337/dc16-0610. [DOI] [PubMed] [Google Scholar]


