Abstract
Activation of the C‐type lectin receptor Dectin‐1 by β‐glucans triggers multiple signals within DCs that result in activation of innate immunity. While these mechanisms can potently prime CD8+ cytotoxic T‐cell (CTL) responses without additional adjuvants, the Dectin‐1 effector pathways that control CTL induction remain unclear. Here we demonstrate that Dectin‐1‐induced CTL cross‐priming in mice does not require inflammasome activation but strictly depends on the adapter protein Card9 in vitro. In vivo, Dectin‐1‐mediated Card9 activation after vaccination drives both expansion and activation of Ag‐specific CTLs, resulting in long‐lasting CTL responses that are sufficient to protect mice from tumor challenge. This Dectin‐1‐induced antitumor immune response was independent of NK cell function and completely abrogated in Card9‐deficient mice. Thus, our results demonstrate that Dectin‐1‐triggered Card9 signaling but not inflammasome activation can potently cross‐prime Ag‐specific CTLs, suggesting that this pathway would be a candidate for immunotherapy and vaccine development.
Keywords: Card9, CD8+ cytotoxic T cells, Cross‐priming, Dectin‐1, Tumor immunity
Introduction
CD8+ cytotoxic T cells (CTLs) are central effectors of antitumor immunity. Owing to their strong cytolytic potency, CTL activation is tightly regulated and requires instruction by specialized APCs like DCs. DCs control uptake, processing, and presentation of exogenous (tumor‐derived) Ag in the context of MHC class I (MHC‐I), which enables them to prime CTLs 1. This process has been termed cross‐priming and is a prerequisite for the induction of CTL‐based antitumor immunity. Cross‐priming requires DC maturation with upregulation of MHC‐I and costimulatory molecules as well as the release of proinflammatory cytokines. Activation and maturation of DCs is mainly mediated by ligation of PRRs, which detect highly conserved PAMPs. Activation of PRRs triggers downstream signaling pathways that converge on nuclear translocation of different transcription factors like NF‐κB and IFN regulatory factors (IRFs) 2.
PAMPs associated with fungi such as β‐glucans have long been recognized not only to be important for the induction of antifungal immunity, but to have antitumor activity 3. Underlying mechanisms and molecular pathways were unclear but are beginning to emerge. β‐glucans are high molecular weight polymers of glucose, consisting of a linear β‐(1‐3)‐linked backbone and β‐(1‐6)‐linked side chains that can be detected by the prototypical C‐type lectin receptor (CLR) Dectin‐1 4. Ligation of plasma membrane bound Dectin‐1 on myeloid phagocytes like DCs by particulate β‐glucans such as Curdlan leads to formation of the “phagocytic synapse” necessary to induce diversified downstream signaling 5, 6. Recruitment of spleen tyrosine kinase (Syk) initiates the assembly of a multimolecular complex consisting of Card9, Bcl10, and Malt1, which triggers canonical activation of NF‐κB 7. A later study identified an additional Dectin‐1‐mediated, Card9‐independent pathway that leads to noncanonical NF‐κB activation via Syk, NF‐κB‐inducing kinase (NIK) and IKKα 8. Furthermore, Dectin‐1/Syk/Card9 signaling has been shown to activate the NLRP3 inflammasome for caspase 1 dependent processing of bioactive IL‐1β in both mice 9 and human macrophages 10.
In addition to its direct antifungal activity through induction of phagocytosis and production of ROS in phagocytes, Dectin‐1‐ligation results in Syk/Card9‐dependent activation of DCs with release of proinflammatory cytokines that promote the differentiation of murine TH1 and TH17 cells 11. Furthermore, Dectin‐1‐mediated DC maturation can trigger potent expansion and differentiation of Ag‐specific CTLs in mice 12. Also in human DCs, Curdlan‐mediated Dectin‐1 activation has been shown to promote the release of a variety of proinflammatory cytokines, TH1 and TH17 differentiation as well as expansion and increased cytolytic activity of CTLs 13.
Overall, CLR‐mediated interconnection of innate and adaptive immune activity is a cornerstone of immunity against numerous fungal pathogens including Candida albicans, Pneumocystis carinii, Cryptococcus neoformans, and Aspergillus fumigatus 14. In fact, polymorphisms in the gene encoding Dectin‐1 15 and mutations in Card9 16 are tightly associated with defective fungal clearance in humans. As inducers of all arms of adaptive immunity, CLR ligands, including those for Dectin‐1, are interesting candidates to enhance vaccine strategies and have been used successfully to trigger and improve Ag‐specific T‐cell responses 14. However, signaling pathways downstream of Dectin‐1/Syk that mediate CTL cross‐priming and vaccine results yet remain undefined. Using genetically deficient mice and targeted depletion of effector cells, we show here that Card9 is a critical signaling hub for Dectin‐1‐mediated cross‐priming of CTLs and that Card9‐mediated CTL induction is essential for control of tumor growth and long‐lasting antitumor immunity after Dectin‐1 ligand‐based prophylactic vaccination.
Results and discussion
Dectin‐1‐mediated cross‐priming of CD8+ T cells requires Card9 in DCs in vitro
To evaluate the molecular requirements for Dectin‐1‐mediated cross‐priming of CD8+ CTLs, we cocultured BM‐derived DCs with T cells derived from transgenic OT‐I mice. These CD8+ T cells have a uniform T‐cell receptor specific for the immune‐dominant OVA peptide (SIINFEKL). As described previously 11, stimulation with the Dectin‐1 agonist Curdlan resulted in rapid maturation of BM‐derived DCs (BMDCs) with upregulation of the costimulatory molecule CD80, a process that critically depended on Card9 (Fig. 1A). Following addition of OVA protein, Curdlan‐mediated activation of BMDCs induced Card9‐dependent proliferation and potent cross‐priming of cocultured Ag‐specific T cells (1B and Supporting Information Fig. 1). CTL priming with robust IFN‐γ release was mediated by DC‐intrinsic Dectin‐1 and downstream Card9 activation but did not require MyD88, a central adapter molecule for TLR signaling.
Figure 1.
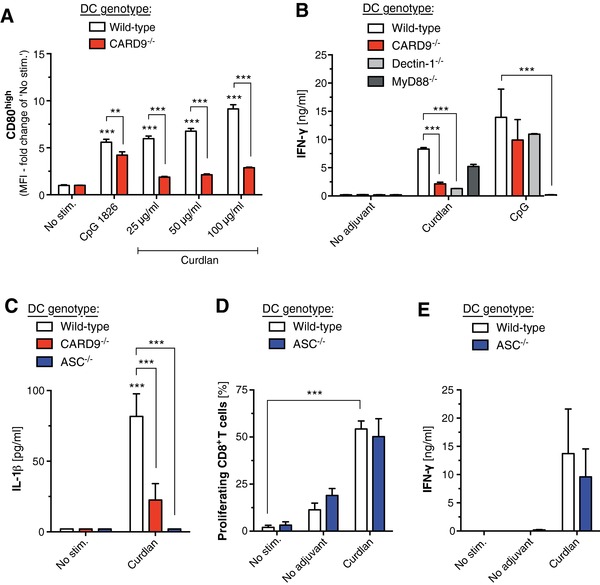
Dectin‐1‐mediated cross‐priming of CD8+ T cells in vitro requires Card9 signaling in DCs. (A) BM‐derived DCs (BMDCs) from WT and Card9‐deficient (Card9−/−) donor mice were stimulated with increasing concentrations of Curdlan or the TLR9 ligand CpG 1826. CD80 expression on BMDCs was analyzed by flow cytometry. An asterisk without brackets indicates comparison to the WT “No stim.” condition. (B) BMDCs from WT, Card9−/−‐deficient, Dectin‐1‐deficient (Dectin‐1−/−), and MyD88‐deficient (MyD88−/−) donor mice were stimulated as described above and were cocultured with magnetically purified, CFSE‐labeled CD8+ OT‐I T cells in the presence of OVA protein. IFN‐γ release into the supernatant was analyzed by ELISA. (C–E) BMDCs from WT, Dectin‐1−/−, or ASC‐deficient (ASC−/−) mice were stimulated as described above and (C) IL‐1β release was determined by ELISA. (D) After coculture with CD8+ OT‐I T cells in the presence of OVA protein, T‐cell proliferation was determined by flow cytometry and (E) IFN‐γ release into the supernatant was analyzed by ELISA. (A, B, D, and E) Data are shown as mean ± SEM of at least triplicate samples and are representative of at least two independent experiments. Data in (C) give mean values ± SEM of n = 4 individual samples (CpG stimulation in WT and Card9−/−, n = 7) that were pooled from four independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001; one‐way ANOVA with Bonferroni posttests.
During C. albicans infection, Dectin‐1 ligation can additionally result in the activation of the NLRP3 inflammasome and subsequent processing of pro‐IL‐1β into its bioactive form 9. Indeed, we observed that DCs that genetically lack the inflammasome adapter molecule apoptosis‐associated speck‐like protein containing a CARD (ASC) failed to produce IL‐1β in response to Curdlan‐mediated Dectin‐1 activation (Fig. 1C), but showed no defect in CTL cross‐priming (Fig. 1D and E). These findings are in line with a previous study demonstrating that NLRP3‐deficient mice showed regular CTL function following Curdlan‐based vaccination in vivo 17. Taken together, these data show that DC activation and subsequent cross‐priming of CTLs following Dectin‐1 activation by its specific ligand Curdlan is mediated via Card9 but is independent of MyD88 signaling or formation of the NLRP3 inflammasome.
Card9‐dependent CTL cross‐priming and antitumor immunity in vivo by Dectin‐1‐mediated vaccination
We next investigated the role of Card9 activity for cross‐priming of CTLs in vivo. As described previously 12, s.c. protein vaccination with OVA and the Dectin‐1 ligand Curdlan, but not OVA alone increased the frequency and cytolytic activity of MHC‐I‐SIINFEKL Tetramer+ CD8+ T cells in treated mice (Fig. 2A and B). Consistent with our in vitro findings, Dectin‐1‐induced, systemic expansion and activation of OVA‐specific CTLs was largely abrogated in Card9‐deficient mice. Compared to Card9‐independent controls, both frequency and cytolytic activity of Curdlan‐induced specific CTLs were inferior to those achieved by the TLR9 ligand CpG.
Figure 2.
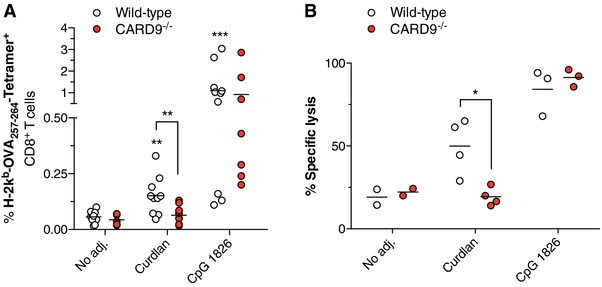
Robust cross‐priming of cytotoxic T cells following Dectin‐1 activation in vivo depends on Card9. WT and Card9−/− mice were injected s.c. with OVA protein in combination with different adjuvants (Curdlan or CpG). (A) Frequency of H‐2Kb‐SIINFEKL Tetramer+ (OVA‐specific) cytotoxic T cells in the peripheral blood was determined by flow cytometry. An asterisk without brackets indicates comparison to the WT “No adj.” group. (B) In vivo cytotoxic activity was measured by target cell elimination of fluorescently labeled, SIINFEKL peptide‐pulsed syngenic splenocytes. The graph shows mean specific target cell lysis in the draining LN of individual mice. (A and B) Each circle represents a single replicate. Data are pooled from two independent experiments (A) or show representative data from one of two identical experiments (B). * p < 0.05, ** p < 0.01, *** p < 0.001; one‐way ANOVA with Bonferroni posttests.
To investigate whether Card9‐mediated cross‐priming of CTLs would translate into antitumor immunity in a tumor prevention model, we immunized mice s.c. with a single dose of OVA protein either alone or mixed with different adjuvants. One week later, mice were challenged with OVA‐expressing B16 melanoma cells injected into the contralateral flank (Fig. 3A). We observed that Curdlan‐boosted vaccination resulted in significant tumor growth delay with complete rejection of OVA‐expressing tumors in 50% of the treated animals (Fig. 3B and Supporting Information Fig. 2A). In contrast, no protection from melanoma growth was observed in mice immunized with OVA Ag alone. The development of Dectin‐1‐ but not TLR9‐induced antitumor immunity was completely dependent on Card9, as Card9‐deficient recipient animals failed to control tumor growth following vaccination with Curdlan (Fig. 3B). Only in WT but not Card9−/− mice, Curdlan‐induced antitumor immunity was associated with prolonged survival of most treated animals (Fig. 3C).
Figure 3.
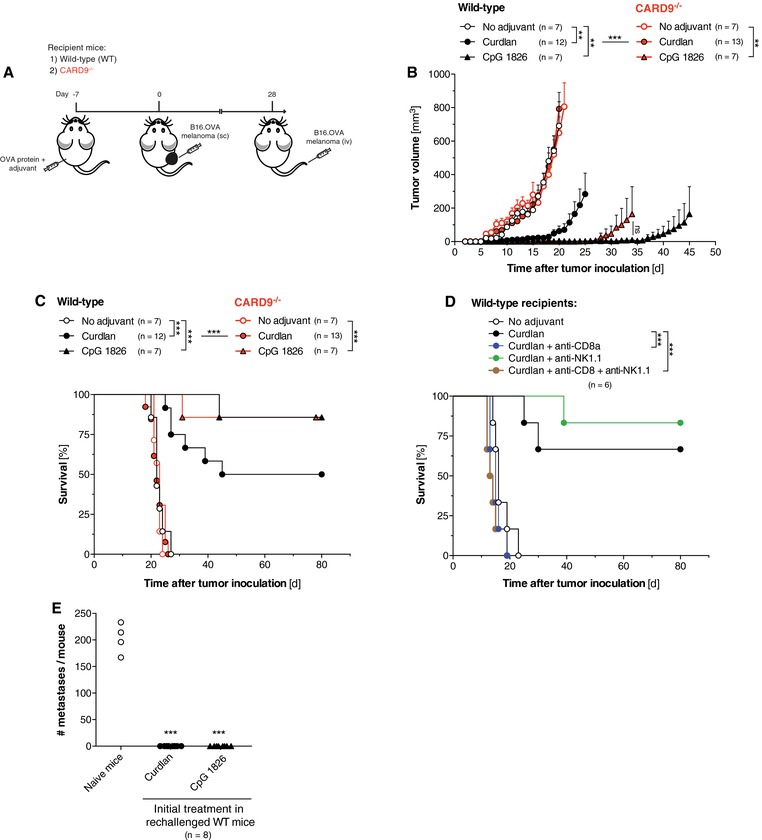
Curdlan‐based vaccination results in potent Card9‐dependent antitumor immunity. (A) Treatment scheme: WT and Card9−/− mice were vaccinated s.c. with OVA in combination with either Curdlan or CpG. One week later, B16.OVA melanoma cells were injected into the contralateral flank. (B) Tumor growth of WT and Card9−/− recipient mice was monitored daily. Data show mean tumor growth ± SEM from n = 7–13 individual mice. Mean tumor growth analysis was discontinued when the first animal per group succumbed to tumor progression. Complete tumor growth curves of each individual mouse are shown in the Supporting Information Fig. 2A. Statistical significance was calculated using one‐way ANOVA with Bonferroni posttests (* p < 0.05, ** p < 0.01, *** p < 0.001) based on the tumor volume at day 20, if not stated otherwise. (C) Survival of tumor‐bearing WT and Card9−/− mice. Survival was analyzed using the log‐rank test (*** p < 0.001). (D) WT mice were treated as described above. Some mice (n = 6) were additionally injected with anti‐CD8α‐ and/or anti‐NK1.1‐depleting antibodies, beginning 2 days prior to Curdlan‐based vaccination. *** p < 0.001; log‐rank test. (E) Four weeks after initial tumor induction, long‐term surviving animals were rechallenged by i.v. injection of B16.OVA cells. Pseudometastases in the lungs were counted on day 14. Treatment‐naive mice were used as controls for successful tumor induction. The graph shows mean number of pseudometastases of individual mice. Each circle represents a single replicate. Data are pooled from two independent experiments. *** p < 0.001; one‐way ANOVA with Bonferroni posttests.
Despite relatively rare occurrence and low cytolytic activity of OVA‐specific circulating CD8+ T cells in Curdlan‐vaccinated WT mice (Fig. 2A and B), Dectin‐1/Card9‐induced antitumor immunity was exclusively mediated by CTLs as Ab‐mediated CD8+ T cell but not NK cell depletion resulted in abrogation of Curdlan‐based antitumor immunity (Fig. 3D and Supporting Information Fig. 3). Emphasizing the crucial role of Ag‐specific CTLs for tumor control in our model, the frequency of circulating OVA‐specific CTLs correlated with the efficacy of the antitumor response. Independent of genotype and treatment, high CTL numbers were associated with reduced tumor volume and prolonged survival (Supporting Information Fig. 2B and C). Four weeks after initial tumor induction, all surviving WT animals were rechallenged i.v. with OVA‐expressing B16 melanoma cells. This resulted in rapid growth of macroscopically visible pseudometastases in the lungs of naïve, untreated WT mice (Fig. 3E). In contrast, recipient animals that had been vaccinated with protein in combination with either Curdlan or CpG 1826 and had thus rejected s.c. tumors were resistant to subsequent, systemic tumor rechallenge.
Taken together, our data identify Card9 as central regulator of Dectin‐1‐mediated cross‐priming of CTLs and subsequent antitumor immunity. Long‐lasting antitumor CTL responses critically depended on signaling through the Card9 pathway. We could exclude a dominant role of the inflammasome adapter ASC in Dectin‐1‐mediated CTL priming. This is in line with previous findings that cytolytic CTL function following a Curdlan‐based vaccine was independent of the NLRP3 inflammasome in vivo 17. Generally, type I IFNs (IFN‐α and IFN‐β) have been found to play a critical role in DC‐mediated cross‐priming of tumor‐specific CTLs 18, 19. We have previously shown that Dectin‐1 and downstream signaling via SYK/Card9 and the transcription factor IRF5 can induce potent IFN‐β production in DCs 20. Future studies will need to evaluate the impact of this pathway on Dectin‐1‐mediated CTL cross‐priming, especially in the context of antitumor immunity.
Dectin‐1 seems an attractive target for tumor immunotherapy, since Dectin‐1‐mediated cross‐priming pathways were shown to be active in human myeloid cells 13. In mice, intratumoral application of Curdlan was found to reprogram infiltrating DCs to become resistant to cancer‐derived thymic stromal lymphopoietin (TSLP), thus favoring the generation of beneficial Th1‐based immune responses 21. In contrast, during microbial infections, Dectin‐1 activation induced TSLP release by DCs, thus promoting Th2‐biased immunity 22. However, the importance of DC‐intrinsic TSLP production in the context of tumor immunity remains to be determined. Furthermore, Curdlan‐induced Dectin‐1 activation can block the immunosuppressive function of myeloid‐derived suppressor cells and thereby reduce tumor burden 23. These studies suggest that therapeutic targeting of Dectin‐1 may allow to coordinate several antitumoral immune mechanisms.
Robust Dectin‐1‐induced Ag‐specific antitumor immunity was mediated by relatively low frequencies of CTLs. These findings and a previous report that Dectin‐1 signaling in myeloid APCs can induce NK‐cell‐mediated tumor cell killing prompted the question whether NK or other immune cells may play a role here 24. However, targeted depletion demonstrated that antitumor immunity induced by Curdlan‐based vaccination entirely relied on CD8+ cells and was independent of NK cells in our model. However, the efficacy of Ag‐specific CTL induction by vaccination with either Curdlan or CpG was variable (our own data and 24) and the frequency of circulating specific CTLs clearly correlated with individual tumor volume reduction and survival increase. We therefore propose that combinatorial treatment strategies that target costimulatory molecules such as CD137 (4‐1BB) 25 or antagonize immune checkpoints 26 may potently synergize with agonists of the Dectin‐1/Syk/Card9 pathway and help to exploit the full potential of Card9‐related targeted antitumor immunity.
Concluding remarks
Overall, our data establish Card9 as a master regulator of Dectin‐1‐induced CTL priming and specific antitumor immunity. Card9 signaling allows to coordinate Dectin‐1‐induced CTL priming with a potent helper T‐cell response into broad, specific T‐cell immunity. Combining Dectin‐1‐induced T‐cell priming with recent immunomodulatory strategies such as checkpoint blockade seems a promising approach for future vaccine development to enhance cytotoxic immunity.
Materials and methods
Ethics statement
This study was carried out in strict accordance with the guidelines of the German animal protection law (TierSchG). All procedures including housing, treatment, and sacrifice by cervical dislocation following isoflurane anesthesia were performed as authorized by and were reported to the responsible state office Regierung von Oberbayern (Munich, Germany) per standard legal procedure (references 55.2.1.54‐2532‐26‐11 and 55.2‐1‐54‐2532‐111‐14).
Mice
Female C57BL/6 mice were purchased from Harlan–Winkelmann and Janvier. Card9‐ (Card9−/−) and ASC‐deficient (ASC−/−) mice have been described previously 7, 27. All mice were at least 6 wk of age at the onset of experiments.
Media, reagents, and cell lines
RPMI‐1640 medium (Invitrogen) and DMEM (Invitrogen) were supplemented with 10% v/v FCS (Hyclone), 3 mM l‐glutamine, 100 U/mL of penicillin, and 100 μg/mL of streptomycin (all from Sigma‐Aldrich). CpG 1826 and Curdlan were purchased from Invivogen and Wako Chemicals (Richmond, USA). The B16‐F10 murine melanoma cell line expressing full length chicken OVA (here referred to as B16.OVA) was cultured in complete DMEM medium supplemented with 400 μg/mL G418 (from Sigma‐Aldrich). CFSE and Celltracker Violet were purchased from Invitrogen.
Generation of BM‐derived DCs and cell culture
BM cells were harvested from murine femur and tibia and erythrocytes were lysed with ammonium chloride buffer (BD Biosciences, Heidelberg, Germany). BMDCs were generated by culturing BM cells in complete RPMI medium supplemented with 20 ng/mL GM‐CSF (from Immunotools, Friesoythe, Germay). On days 6 to 7, cells were harvested. CD8+ T cells from OT‐I splenocytes were purified with magnetic beads according to the manufacturer's protocol (Miltenyi Biotech, Bergisch Gladbach). For in vitro stimulation assays, cells were cultured in complete RPMI. DCs were stimulated with Curdlan (50 μg/mL), CpG 1826 (0.075 μM), or Pam3CSK4 (1 μg/mL), if not stated otherwise. For coculture experiments, 105 purified, CFSE‐labeled CD8+ OT‐I T cells were cultured in the presence of 25 000 stimulated DCs. After 48 h, T‐cell proliferation and IFN‐γ were analyzed.
Quantification of cytokines
Cell supernatants were analyzed for cytokine secretion by ELISA (BD, R&D Systems, or eBioscience) according to the manufacturer's protocol.
Flow cytometry
Cell suspensions were stained in PBS with 1% FCS. Fluorochrome‐coupled antibodies were purchased from eBioscience or BioLegend. The Fixable Viability Dye eFluor 506 (eBioscience) was used to stain and exclude dead cells. The iTAg MHC‐I murine tetramers detecting SIINFEKL‐specific CD8+ T cells were from MBL (Woburn, MA). For intracellular cytokine staining the Foxp3 Transcription Factor Fixation/Permeabilization Kit (eBioscience) was used. Data were acquired on a FACSCanto II (BD Biosciences) and analyzed using FlowJo software (TreeStar). Cells were gated as follows: DCs: singlets > Viability Dye− > CD11c+ > CD86 (MFI). T cells: singlets > Viability Dye− > CD3+ > CD8+ > CFSElow (proliferating) + IFN‐γ+.
In vivo cytotoxicity assay
In vivo cytotoxicity was evaluated 4 days after the final treatment as described previously 12. In brief, splenocytes from naïve syngenic donor mice were pulsed with SIINFEKL peptide at different concentrations (0 and 200 nM) for 30–45 min at 37°C. These two populations were then stained with different concentrations of Celltracker violet (CTv, 0.1 and 5 μM), washed, mixed in a 1:1 ratio, and were injected i.v. The next day, animals were sacrificed and the target cell frequency in the LN draining the initial vaccination site was determined by flow cytometry as follows: singlets > Viability Dye− > CTvlow versus CTvhigh. Specific killing was calculated using the formula 100 − (1 − %CTv peptide / %CTv no peptide) as described 12.
Tumor challenge and treatment
WT or Card9−/− mice were injected s.c. in the right flank with 100 μg OVA together with 25 μg CpG 1826 or 100 μg Curdlan. One week later, the frequency of Ag‐specific CD8+ T cells in the peripheral blood was analyzed with SIINFEKL‐H2Kb tetramers. Subsequently, mice were injected s.c. with 1 × 105 B16.OVA cells in the left flank and tumor growth was monitored continuously. According to standard legal procedure (responsible state office Regierung von Oberbayern), mice were euthanized when the maximum tumor diameter exceeded 15 mm. Treatment with anti‐CD8α (clone 2.43) or anti‐NK1.1 (clone PK136, both from BioXCell) depleting antibodies was initiated 2 days prior to vaccination (100 μg i.p.) and was repeated twice weekly (50 μg i.p.). For rechallenge experiments, mice were injected i.v. with 5 × 105 B16.OVA cells on day 28 after initial tumor induction. Fourteen days later, mice were sacrificed and macroscopically visible, superficial pulmonary pseudometastases were counted.
Statistics
All data are presented as mean + SEM if not stated otherwise. For multiple statistical comparison of a data set, the one‐way ANOVA test with Bonferroni posttest was used. Survival was analyzed using the log‐rank test. Significance was set at p‐values p < 0.05, p < 0.01, and p < 0.001 and was then indicated with asterisks (*, **, and ***). All statistical calculations were performed using Graphpad Prism (GraphPad Software, San Diego, USA).
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Abbreviations
- BMDCs
BM‐derived DCs
- CLR
C‐type lectin receptor
- CTL
cytotoxic T cell
- IRF
IFN regulatory factor
- MHC‐I
MHC class I
- Syk
Spleen tyrosine kinase
- TSLP
thymic stromal lymphopoietin
Supporting information
Supporting Information Figure 1: BMDCs from WT, Card9
Supporting Information Figure 2: WT and Card9‐deficient
Supporting Information Figure 3: WT mice were vaccinated with OVA
Supporting Information
Acknowledgments
This study was supported by research grants from the DFG (SFB 1054/B01 and RU 695/6‐1) and an ERC Advanced Grant (FP7, grant agreement No. 322865 to J.R.), Kommission für Klinische Forschung der Medizinischen Fakultät der Technischen Universität München (to T.H.), the Deutsche Forschungsgemeinschaft (PO 1575/3‐1 to H.P.), the Else‐Kröner‐Fresenius‐Stiftung (2012_A61 to H.P., EKFK to S.H.), and the Deutsche Krebshilfe e.V. (111620 to H.P and S.H.).
T.H., S.H., H.P., and J.R. designed the research, analyzed and interpreted the results. T.H., S.H., A.W. M.B, M.S., and S.B. did the experiments. S.H. and T.H. prepared the manuscript. C.P., J.C.F., G.E., and S.S. gave methodological support and conceptual advice. T.H., H.P., and J.R. guided the study.
References
- 1. Nair‐Gupta, P. and Blander, J. M. , An updated view of the intracellular mechanisms regulating cross‐presentation. Front. Immunol. 2013. 4: 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kawai, T. and Akira, S. , TLR signaling. Semin. Immunol. 2007. 19: 24–32. [DOI] [PubMed] [Google Scholar]
- 3. Chihara, G. , Hamuro, J. , Maeda, Y. , Arai, Y. and Fukuoka, F. , Fractionation and purification of the polysaccharides with marked antitumor activity, especially lentinan, from Lentinus edodes (Berk.) Sing. (an edible mushroom). Cancer Res. 1970, 30: 2776–2781. [PubMed] [Google Scholar]
- 4. Brown, G. D. and Gordon, S. , Immune recognition. A new receptor for beta‐glucans. Nature 2001. 413: 36–37. [DOI] [PubMed] [Google Scholar]
- 5. Geijtenbeek, T. B. and Gringhuis, S. I. , Signalling through C‐type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 2009. 9: 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goodridge, H. S. , Reyes, C. N. , Becker, C. A. , Katsumoto, T. R. , Ma, J. , Wolf, A. J. , Bose, N. et al., Activation of the innate immune receptor Dectin‐1 upon formation of a “phagocytic synapse.” Nature 2011. 472: 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gross, O. , Gewies, A. , Finger, K. , Schafer, M. , Sparwasser, T. , Peschel, C. , Foerster, I. et al., Card9 controls a non‐TLR signalling pathway for innate anti‐fungal immunity. Nature 2006. 442: 651–656. [DOI] [PubMed] [Google Scholar]
- 8. Gringhuis, S. I. , den Dunnen, J. , Litjens, M. , van der Vlist, M. , Wevers, B. , Bruijns, S. C. and Geijtenbeek, T. B. , Dectin‐1 directs T helper cell differentiation by controlling noncanonical NF‐kappaB activation through Raf‐1 and Syk. Nat. Immunol. 2009. 10: 203–213. [DOI] [PubMed] [Google Scholar]
- 9. Gross, O. , Poeck, H. , Bscheider, M. , Dostert, C. , Hannesschlager, N. , Endres, S. , Hartmann, G. et al., Syk kinase signalling couples to the Nlrp3 inflammasome for anti‐fungal host defence. Nature 2009. 459: 433–436. [DOI] [PubMed] [Google Scholar]
- 10. Kankkunen, P. , Teirilä, L. , Rintahaka, J. , Alenius, H. , Wolff, H. and Matikainen, S. , (1,3)‐β‐Glucans activate both Dectin‐1 and NLRP3 inflammasome in human macrophages. J. Immunol. 2010. 184: 6335–6342. [DOI] [PubMed] [Google Scholar]
- 11. LeibundGut‐Landmann, S. , Gross, O. , Robinson, M. J. , Osorio, F. , Slack, E. C. , Tsoni, S. V. , Schweighoffer, E. et al., Syk‐ and CARD9‐dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 2007. 8: 630–638. [DOI] [PubMed] [Google Scholar]
- 12. Leibundgut‐Landmann, S. , Osorio, F. , Brown, G. D. and Reis e Sousa, C. , Stimulation of dendritic cells via the dectin‐1/Syk pathway allows priming of cytotoxic T‐cell responses. Blood 2008. 112: 4971–4980. [DOI] [PubMed] [Google Scholar]
- 13. Agrawal, S. , Gupta, S. and Agrawal, A. , Human dendritic cells activated via dectin‐1 are efficient at priming Th17, cytotoxic CD8 T and B cell responses. PLoS One 2010. 5: e13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hardison, S. E. and Brown, G. D. , C‐type lectin receptors orchestrate antifungal immunity. Nat. Immunol. 2012. 13: 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferwerda, B. , Ferwerda, G. , Plantinga, T. S. , Willment, J. A. , van Spriel, A. B. , Venselaar, H. , Elbers, C. C. et al., Human dectin‐1 deficiency and mucocutaneous fungal infections. New Engl. J. Med. 2009. 361: 1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glocker, E. O. , Hennigs, A. , Nabavi, M. , Schaffer, A. A. , Woellner, C. , Salzer, U. , Pfeifer, D. et al., A homozygous CARD9 mutation in a family with susceptibility to fungal infections. New Engl. J. Med. 2009. 361: 1727–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kumar, H. , Kumagai, Y. , Tsuchida, T. , Koenig, P. A. , Satoh, T. , Guo, Z. , Jang, M. H. et al., Involvement of the NLRP3 inflammasome in innate and humoral adaptive immune responses to fungal beta‐glucan. J. Immunol. 2009. 183: 8061–8067. [DOI] [PubMed] [Google Scholar]
- 18. Diamond, M. S. , Kinder, M. , Matsushita, H. , Mashayekhi, M. , Dunn, G. P. , Archambault, J. M. , Lee, H. et al., Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 2011. 208: 1989–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fuertes, M. B. , Kacha, A. K. , Kline, J. , Woo, S. R. , Kranz, D. M. , Murphy, K. M. and Gajewski, T. F. , Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J. Exp. Med. 2011. 208: 2005–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. del Fresno, C. , Soulat, D. , Roth, S. , Blazek, K. , Udalova, I. , Sancho, D. , Ruland, J. et al., Interferon‐beta production via Dectin‐1‐Syk‐IRF5 signaling in dendritic cells is crucial for immunity to C. albicans . Immunity 2013. 38: 1176–1186. [DOI] [PubMed] [Google Scholar]
- 21. Wu, T. C. , Xu, K. , Banchereau, R. , Marches, F. , Yu, C. I. , Martinek, J. , Anguiano, E. et al., Reprogramming tumor‐infiltrating dendritic cells for CD103+ CD8+ mucosal T‐cell differentiation and breast cancer rejection. Cancer Immunol. Res. 2014. 2: 487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elder, M. J. , Webster, S. J. , Williams, D. L. , Gaston, J. S. and Goodall, J. C. , TSLP production by dendritic cells is modulated by IL‐1beta and components of the endoplasmic reticulum stress response. Eur. J. Immunol. 2016. 46: 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rui, K. , Tian, J. , Tang, X. , Ma, J. , Xu, P. , Tian, X. , Wang, Y. et al., Curdlan blocks the immune suppression by myeloid‐derived suppressor cells and reduces tumor burden. Immunol. Res. 2016. 64: 931–939. [DOI] [PubMed] [Google Scholar]
- 24. Chiba, S. , Ikushima, H. , Ueki, H. , Yanai, H. , Kimura, Y. , Hangai, S. , Nishio, J. et al., Recognition of tumor cells by Dectin‐1 orchestrates innate immune cells for anti‐tumor responses. eLife 2014. 3: e04177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sanchez‐Paulete, A. R. , Labiano, S. , Rodriguez‐Ruiz, M. E. , Azpilikueta, A. , Etxeberria, I. , Bolanos, E. , Lang, V. et al., Deciphering CD137 (4‐1BB) signaling in T‐cell costimulation for translation into successful cancer immunotherapy. Eur. J. Immunol. 2016. 46: 513–522. [DOI] [PubMed] [Google Scholar]
- 26. Topalian, S. L. , Drake, C. G. and Pardoll, D. M. , Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015. 27: 450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mariathasan, S. , Newton, K. , Monack, D. M. , Vucic, D. , French, D. M. , Lee, W. P. , Roose‐Girma, M. et al., Differential activation of the inflammasome by caspase‐1 adaptors ASC and Ipaf. Nature 2004. 430: 213–218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1: BMDCs from WT, Card9
Supporting Information Figure 2: WT and Card9‐deficient
Supporting Information Figure 3: WT mice were vaccinated with OVA
Supporting Information


