Abstract
Aims
To identify different types of models used in economic evaluations of smoking cessation, analyse the quality of the included models examining their attributes and ascertain their transferability to a new context.
Methods
A systematic review of the literature on the economic evaluation of smoking cessation interventions published between 1996 and April 2015, identified via Medline, EMBASE, National Health Service (NHS) Economic Evaluation Database (NHS EED), Health Technology Assessment (HTA). The checklist‐based quality of the included studies and transferability scores was based on the European Network of Health Economic Evaluation Databases (EURONHEED) criteria. Studies that were not in smoking cessation, not original research, not a model‐based economic evaluation, that did not consider adult population and not from a high‐income country were excluded.
Findings
Among the 64 economic evaluations included in the review, the state‐transition Markov model was the most frequently used method (n = 30/64), with quality adjusted life years (QALY) being the most frequently used outcome measure in a life‐time horizon. A small number of the included studies (13 of 64) were eligible for EURONHEED transferability checklist. The overall transferability scores ranged from 0.50 to 0.97, with an average score of 0.75. The average score per section was 0.69 (range = 0.35–0.92). The relative transferability of the studies could not be established due to a limitation present in the EURONHEED method.
Conclusion
All existing economic evaluations in smoking cessation lack in one or more key study attributes necessary to be fully transferable to a new context.
Keywords: Economic evaluation, modelling, smoking, systematic review, tobacco, transferability
Introduction
The core strategies in reducing smoking prevalence are to prevent people from starting smoking, to reduce the number of smokers and to decrease the chances of relapse. This can be achieved by implementing population‐based tobacco control policies (e.g. legislations and mass media campaigns) and smoking cessation programmes (e.g. drug or behavioural therapies) targeted at current smokers. However, due to the increasing number of interventions now available, decision‐makers face difficulties in deciding which intervention to implement. Given scarce resources, relative costs and benefits of those interventions are one of the key decision‐making criteria, thus making the importance of economic evaluations rise in recent years 1, 2.
Economic evaluations combine the outcomes of interventions with their costs, in order to determine which intervention provides the best value for money 3. Such evaluations, for example, have shown that treatment with varenicline 4, 5 or behavioural support by mobile phone 6 can be cost‐effective. Model‐based economic evaluations are especially appropriate to extrapolate the benefits beyond clinical trials and when a single primary source of data is not sufficient 7. In addition, a model‐based economic evaluation has the ability to adapt itself to a new context, making the process of executing economic evaluations less time‐consuming and thus less costly 8, 9. Unfortunately, such evaluations often originate in affluent societies. The number of lives that can be saved from the use of such evidence elsewhere (e.g. countries in Central and Eastern Europe) is potentially enormous. Sadly, those countries often have too limited research resources to study cost‐effectiveness of such interventions in their own context, highlighting the importance of transferability assessments 9, 10.
The notion of transferability of evidence from one context to others varies widely in the literature. ‘Transferability’, ‘generalizability’ and ‘external validity’ are the concepts used to assess the ability of a study to be relevant to the decision maker's context to the extent the findings could actually be used 11, 12, 13, 14, 15. However, a distinction also exists between what is feasible/applicable and what is generalizable/transferable. Applicability refers to ‘how can I replicate the intervention in my own decision context?’ (the process question) and generalizability refers to ‘whether the effectiveness will be similar to that in the original context?’ (the outcome question) 12, 13, 15, 16. Therefore, these two underlying questions seem to have defined transferability in the literature.
Transferability assessments to date have focused mainly on the way in which a model is constructed and populated, as modelling provides a well‐defined structure helping us to recognize the limitations and their implications for generalizability of the results 7, 17, 18, 19. There has not been a systematic enquiry in to the transferability of economic evaluations in smoking cessation, although a few systematic reviews in this area exist 20, 21. The review by Kirsch et al. 21, for instance, limits itself to a narrow definition of study population and to a specific type of economic model. In this paper, we therefore set out to: (i) identify different types of models used in economic evaluations of smoking cessation; (ii) analyse the quality of the included models examining their attributes; and (iii) ascertain their transferability to a new context.
Methods
Search strategy and implementation
A systematic search was conducted to identify all relevant models used for economic evaluation in smoking cessation on the following databases: National Health Service (NHS) Economic Evaluation Database (NHS EED), Health Technology Assessment (HTA), Medline and EMBASE. They were searched for publications in English language between 1996 and April 2015. The search strategy was based on related published systematic reviews 20, 22, 23, 24, leading to the final search terms ‘smoking’, ‘nicotine’ and ‘tobacco’ in NHS EED and HTA. Medline and EMBASE required additional terms related to model‐based economic evaluation, which were based on Wilczynski et al. 25 and McKinlay 26 to acquire high sensitivity as well as high specificity 27. Supporting information, Table S1 shows an overview of the search strategies used by databases. All results were exported to EndNote (Thomson Reuters) version X7, where duplications were removed automatically and remaining duplicates checked manually.
Exclusion criteria and screening
Title and abstract screening for the first 50 papers was performed independently by two reviewers (M.H. and M.B.) based on the following exclusion criteria: (1) topic not in smoking cessation (as the focus was on the interventions to reduce tobacco use), (2) no original research (to avoid inclusion of review of evidence or opinion pieces), (3) no model‐based economic evaluation (to avoid inclusion of other designs, e.g. trial‐based evaluations), (4) no adult general population (to focus on adults, rather than children), (5) no high‐income country (to reduce study heterogeneity by including comparable, industrialized countries based on their income levels) and (6) not available in the English language (practicality reasons mainly to address resource constraints). No differences in exclusion/inclusion were observed between both reviewers; only minor discrepancies were recorded in the reason of exclusion. The inter‐rater reliability (IRR) gave a Cohen's kappa of 0.912, meaning almost perfect agreement 28. Remaining discrepancies were discussed, leading to full agreement. Screening of the remaining papers was then completed by one researcher (M.B.). Full text screening was performed independently by two reviewers (M.B. and K.L.C. or M.H.). There were only minor discrepancies between the reviewers, which led to full agreement after discussion. Supporting information, Tables S2 and S3 show an extended list of exclusion criteria for full‐text screening.
Data extraction
Data on the following items were extracted using an Excel template adapted from published studies 20, 29, 30 and included: study attributes (type of evaluation, interventions, comparator and country); model (type, transition or health states, time horizon and perspective); effectiveness (outcome and discount rate, primary measure of effectiveness and utility valuations); costs (perspective, categories, resource, index year and discount rate); uncertainty (type and outcome of sensitivity analysis); and results and major limitations.
As data from some included studies were already extracted by the University of York's Centre for Reviews and Dissemination (CRD) (n = 39 of 64), only one researcher (M.B.) extracted data independently on those studies and compared with the CRD extraction. The CRD database contains clear and structured summaries of the economic analyses by experts, and therefore it was deemed sufficient to compare the results of data extraction to these summaries. For the remaining studies that were not included in the CRD database, the data were extracted independently by two reviewers (M.B. and one of the following: M.H., K.L.C., R.D.K. and P.K). Any disagreement between the reviewers was resolved by consensus with a third reviewer.
Quality appraisal
In order to appraise the quality, 10% of the included studies were first assessed independently by M.B. and M.H., using a quality checklist and corresponding classification from the National Institute for Health and Care Excellence (NICE) Methodology Guide with the aim to filter out quality‐poor studies 31. The quality checklist was based on three major criteria: (1) the study was conducted from a relevant perspective (i.e. at least payer or health‐care perspective; (2) the study was a cost–utility or cost–benefit analysis with cost/quality adjusted life years (QALY) or benefit–cost ratio reported; and (3) limitations, either stated in the original study or identified by the reviewers during data extraction stage. Once the overall assessment using these criteria was completed, the studies were assigned to one of the following three classifications: (i) a study with minor limitations (ML); (ii) a study with potentially serious limitations (PSL); or (iii) a study with very serious limitations (VSL). As full agreement on quality classification was reached in the 10% of the included studies, M.B. then completed the quality appraisal of the remaining studies.
Transferability assessment
The studies appraised as the one with minor limitations (ML) were considered to be of sufficient quality to be included for transferability assessment applying the EURONHEED checklist 9. Two independent researchers (M.B. and one of the following: M.H., K.L.C., R.D.K. and P.K.) applied the checklist. The EURONHEED checklist was developed originally by Boulenger et al. 9 and described and updated further with guidelines by Nixon et al. 32. It consists of 42 questions, 26 of which relate to overall methodological quality and internal validity, and 16 questions relate to transferability. An overview of all questions is provided in Supporting information, Table S4. Every question can be answered by ‘yes/partially/no or not applicable (NA)’, assigning a score of 1, 0.5 and 0, respectively. While each item in the checklist is treated equally (but implicitly giving more weight to 16 of the 42 items), the assigned score to each question thus additionally provides another weight to reflect the extent to which each item was reported in the study being assessed 32. The combination of the questions generates an overall summary score 9, 10. We calculated two summary scores: the total summary score including all 42 items and the transferability score including the 16 items. The summary scores were calculated using the following formula; , in which n is the number of questions, x is the number of questions for which the response was NA and S is the score of each question 9. The summary scores reflect how thoroughly key methodological items are reported as the quality of reporting is paramount for generalizability/transferability 32. In addition to this, we calculated the scored percentage of the total score possible per section. This showed us what sections within model‐based economic evaluations were of sufficient quality and which needed further improvement. For example, a score of 0.75 means that 75% of this section is of sufficient quality.
Results
Search outcomes
The systematic literature search yielded 1925 references. After removing duplicates, 1500 studies were included for title and abstract screening which led to a total of 101 studies selected for full text screening. On applying the exclusion criteria, 64 studies were judged to be eligible for data extraction. Thirteen of the 64 studies were included for transferability assessment. An overview of the process is provided in Fig. 1.
Figure 1.
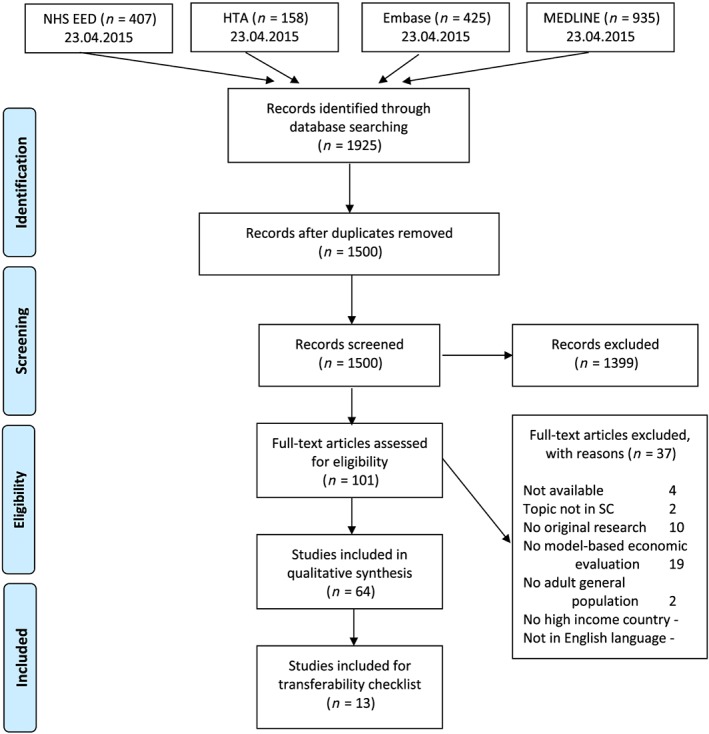
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram, based on National Health Service Economic Evaluation Database (NHS EED) and Health Technology Assessment Database (HTA). [Colour figure can be viewed at wileyonlinelibrary.com]
Overview of studies
An overview of the identified models is shown in Table 1. Most studies originated from Europe (n = 30 of 64) and the United States (n = 24 of 64), followed by Australia (n = four of 64) and Asia (n = two of 64). Three of 64 studies were multi‐continental.
Table 1.
Overview of studies by population, intervention, comparators and outcome.
| Author, year | Country | Population | Intervention | Comparator | Outcome |
|---|---|---|---|---|---|
| Ahmad, 2005a | CA, USA | General Californian population | Raising legal smoking age from 18 to 21 | Legal smoking age 18, 19, 20 | QALY |
| Ahmad, 2005b | USA | General American population | Raising legal smoking age from 18 to 21 | No intervention | LY gained and QALY |
| Annemans, 2015 | Belgium | 18+ Belgian smokers | Varenicline in retreatment | No treatment, and retreatment with bupropion or NRT | QALY |
| Annemans, 2009 | Belgium | 18+ Belgian smokers | Varenicline | Pharmacotherapies, brief counselling and unaided cessation | LY gained and QALY |
| Athanasakis, 2012 | Greece | 18+ Greek smokers | Varenicline | Bupropion, NRT and unaided cessation | QALY |
| Bae, 2009 | South Korea | General Korean population | Varenicline | NRT, bupropion and no drugs | QALY |
| Bauld, 2011 | Scotland | Not reported | One‐to‐one counselling or group‐based support programme | No intervention | QALY |
| Bertram, 2007 | Australia | Australian smokers aged 20–79 | NRT or bupropion | No intervention | DALY |
| Bolin, 2006 | Sweden | Swedish smokers aged 35+ | Bupropion tablets with four nurse visits for motivational support | NRT | QALY |
| Bolin, 2008 | Sweden | Swedish smokers aged 18+ | Varenicline | Bupropion | QALY |
| Bolin, 2009a | Sweden | Swedish adult population | 12‐week varenicline treatment expanded with 12 weeks of maintenance with varenicline | 12 weeks of varenicline +12 weeks of placebo | QALY |
| Bolin, 2009b | Belgium, France, Sweden | Not reported | Varenicline | NRT | QALY |
| Boyd, 2009 | UK | Glasgow smoking population | ‘Starting fresh’ and ‘Smoking concerns’ | Self‐quit | QALY |
| Brown, 2014 | England | 16+ without having quit successfully in the last month | Stoptober | Usual situation for all other months | LY gained and QALY |
| Cantor, 2015 | USA, Texas | Physicians and pharmacists from 16 communities in Texas Participants: 18+ | The health‐care team approach to smoking cessation: ETOEP | Usual practice | QALY |
| Chevreul, 2014 | France | Insured current French smokers aged 15–75 years | Full coverage of the medical management of smoking cessation | Current situation | ICER per LY gained |
| Cornuz, 2006 | Canada, France, Spain, Switzerland, UK, USA | Smokers smoking > 20 cigarettes per day | Four NRTs (gum, patch, spray, inhaler) and bupropion, given as adjunct to cessation counselling | Not Reported | LY gained |
| Cornuz, 2003 | A European country (some data used from Switzerland) | Smokers smoking > 20 cigarettes per day | Four NRTs (gum, patch, spray, inhaler) and bupropion, given as adjunct to cessation counselling | GP counselling during routine visit | Incremental cost per LY gained |
| Croghan, 1997 | USA, Rochester | Smokers aged 18+ | Non‐physician smoking cessation counselling | No intervention | LY gained |
| Dino, 2008 | USA | Adolescents aged 17–25 years | American Lung Association's Not On Tobacco national teen smoking cessation programme | Brief intervention | Discounted LY |
| Feenstra, 2005 | The Netherlands | Dynamic population | Face‐to‐face smoking cessation interventions | Current situation | LY gained and QALY |
| Fiscella, 1996 | USA | Not reported | Nicotine patches as an adjunct to physician‐based counselling | Physician‐based counselling | QALY |
| Guerriero, 2013 | UK | Smokers aged 16+ | Text‐based support in adjunct to current practice | Current situation | LY gained and QALY |
| Halpern, 2007a | USA | Not reported | Varenicline | Nicotine patch, bupropion, and no pharmacotherapy | ROI, IRR, B–C‐ratio |
| Halpern, 2007b | USA | Reflection of US population | Work‐place smoking cessation coverage | No coverage | IRR, ROI |
| Heitjan, 2008 | USA | American whites | Nicotine patch, bupropion, varenicline and tailored therapy based on genetic testing | No intervention | Residual LY |
| Hill, 2006 | USA | Not reported | NRT (gum, patch, inhaler, nasal spray), Zyban or combinations | No intervention | ICER |
| Hojgaard, 2011 | Denmark | General Danish population | Smoking cessation programme and a smoking ban | Current situation | LY gained |
| Hoogendoorn, 2008 | The Netherlands | General Dutch population | Varenicline | No intervention, bupropion, nortriptyline or NRT | Number of quitters, LY gained, and QALY |
| Howard, 2008 | USA | US adult 18+ population | Varenicline | Bupropion, NRT, and unaided quitting | QALY |
| Hurley, 2008 | Australia | General Australian population | Australian National Tobacco Campaign | Current situation | LY gained and QALY |
| Igarashi, 2009 | Japan | Japanese smokers aged 20+ smoking >20 cigarettes per day | Varenicline combined with counselling | Counselling | QALY |
| Jackson, 2007 | USA | Not reported | Varenicline | Bupropion | Net benefit |
| Knight, 2010 | USA | General American population making single quit attempt | Varenicline 12 + 12 weeks | Bupropion, NRT and unaided cessation | QALY |
| Lai, 2007 | Estonia | Estonian smokers aged 15–59 | Increase of tax, clean indoor air law enforcement, and NRT | No intervention (do‐nothing counterfactual) | DALY |
| Lal, 2014 | Australia | Smokers aged 35–100 | Telephone counselling | Self‐help | DALY |
| Levy, 2006 | USA | Employees aged 18–64 | Four coverage scenarios | No coverage | Changes in medical expenditures |
| Levy, 2002 | USA | Hypothetical cohort of smokers | Coverage of costs of different combinations of treatment, and brief interventions by care providers | No intervention | Quit rates |
| Linden, 2010 | Finland | Finnish adult smokers making a first quit attempt | Varenicline | Prescribed medicine, bupropion or unaided cessation | LY gained and QALY |
| McGhan, 1996 | Not reported | Not reported | Self‐care, behavioural therapy, group withdrawal clinic or nicotine patch | Not reported | Net benefit |
| Nielsen, 2000 | USA | Smokers enrolled on a smoking cessation programme | Nicotine patch, bupropion, or combination | Placebo | Net benefit |
| Nohlert, 2013 | Sweden | General Swedish population | Low and high intensity smoking cessation program | No intervention | QALY |
| O'Donnell, 2011 | USA | Dynamic population | Cold turkey, behavioural therapy, medication therapy or combinations | No intervention | LY gained |
| Olsen, 2006 | Denmark | General Danish population | Group courses, individual courses or quick interventions | No intervention | LY gained |
| Ong, 2005 | USA, Minnesota | Minnesota population of smokers | Free NRT | State‐wide campaign of smoke‐free work‐places | QALY |
| Over, 2014 | The Netherlands | Dutch smokers aged 25–80 | Tax increase or reimbursement | Current situation | QALY |
| Pinget, 2007 | Switzerland | Swiss smokers | Physician training in smoking cessation counselling | Physician training in dyslipidaemia management | LY gained |
| Ranson, 2002 | 139 countries | Current smokers in 1995 | Tobacco control policies (price increases, NRT, non‐price interventions) | No tobacco control policy | DALY saved |
| Shearer, 2006 | Australia | General Australian population | Brief advice, telephone counselling, NRT or bupropion | No intervention, brief advice, counselling or pharmacotherapies | ICER |
| Simpson, 2013 | USA | New York State aged 18+ | New York Tobacco Control Programme | No intervention | Smoking costs avoided |
| Song, 2002 | UK | Hypothetical cohort of smokers | Advice plus NRT, advice plus bupropion or advice plus NRT and bupropion | Advice or counselling only | LY gained |
| Stapleton, 1999 | UK | Smokers in general | Transdermal nicotine patches with GP counselling | GP counselling | LY gained |
| Stapleton, 2012 | Data used from USA and UK | Smokers in general | Cytisine, Agency for Health Care Policy and Research Guideline for smoking cessation, NICE appraisal of NRT, or effect size given as an odds ratio or relative rate | Placebo | LY gained |
| Taylor, 2011 | UK | Hypothetical cohort of smokers who recently initiated quit attempts | NRT, bupropion or varenicline | No drug therapy | QALY |
| Tran, 2002 | USA, Virginia | Smokers aged 21–70 who tried (at least once) to quit smoking | Cold turkey, nicotine patch, nicotine gum or bupropion | Self‐quit | QALY |
| Van Baal, 2007 | The Netherlands | Dynamic population | Tobacco tax increase | Current situation | LY gained and QALY |
| Van Genugten, 2003 | The Netherlands | Dutch population | Policy measures (‘Don't start’, ‘quit’, ‘tax’) | Future smoking prevalence is based on trend extrapolation | DALY |
| Vemer, 2010a | The Netherlands, Belgium, Germany, Sweden, France, and UK | Smokers aged 18+ in the Netherlands, Belgium, Germany, Sweden, France and the UK | NRT, bupropion or varenicline | Unaided quit attempt | QALY |
| Vemer, 2010b | The Netherlands | Dutch smokers aged 18+ | Smoking cessation support | Current situation | QALY |
| Von Wartburg, 2014 | Canada, France, Spain, Switzerland, UK, USA | Cohort representative of Canadian demographics, smokers who seriously consider quitting within the next 30 days | Standard 12 weeks of varenicline, or 12 + 12 weeks of varenicline | Bupropion, NRT, or unaided cessation | QALY |
| Warner, 1996 | USA | Hypothetical cohort of blue‐collar workers | Work‐site smoking‐cessation programme | No intervention | LY gained, medical expenditures saved |
| Welton, 2008 | UK | Not reported | Genetic testing of DRD2 Taq1ANRT, bupropion, their combination, or standard care | Brief advice or individual counselling | Incremental net benefit |
| Xenakis, 2009 | USA | Not reported | Varenicline with counselling | Counselling + bupropion or placebo | Incremental costs |
| Xu, 2014 | USA | US adult 18+ population | Anti‐smoking campaign | No campaign | LY gained and QALY |
NRT = nicotine replacement therapy; QALY = quality adjusted life years; DALY = disability adjusted life years; NICE = National Institute for Health and Care Excellence; GP general practitioner; ICER = incremental cost‐effectiveness ratio; LY = life years; IRR = inter‐rater reliability; ROI = return on investment; B–C = benefit–cost.
The populations in the analyses were described mainly as the general adult population of smokers. In three studies the populations were described further as smoking 20 cigarettes per day or more 33, 34, 35, making or considering a single or first quit attempt 36, 37, 38, 39 or had recently tried to quit smoking 40, 41. In five studies the population was described only as a dynamic and/or hypothetical cohort 42, 43, 44, 45, 46 and in nine studies the population was not reported at all 47, 48, 49, 50, 51, 52, 53, 54, 55.
A significant part of the intervention was smoking cessation programmes, either pharmacotherapy 4, 5, 36, 37, 38, 40, 41, 48, 50, 51, 53, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, behavioural therapy 6, 42, 47, 66, 67, 68, 69 or a combination of these 33, 34, 35, 43, 45, 46, 49, 52, 54, 70, 71, 72, 73, 74, 75. Several studies evaluated wider tobacco control interventions 39, 44, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, whereas five studies included both smoking cessation programmes and tobacco control interventions 89, 90, 91, 92, 93.
In a number of studies, the authors selected ‘no intervention’ or ‘current situation’ as comparator. All other studies described the comparators in more detail (Table 1).
The main measure of outcome used is the QALY. In total, 23 of 64 studies reported QALY as their main outcome 5, 35, 38, 40, 41, 47, 48, 49, 56, 58, 59, 61, 62, 63, 65, 69, 70, 76, 78, 81, 86, 88, 94, followed by life years (LY) gained (n = nine of 64) 33, 43, 46, 66, 67, 68, 73, 74, 89 or a combination of these (n = 12 of 64) 4, 6, 35, 36, 37, 39, 42, 44, 57, 77, 80, 83. Five of 64 studies reported disability adjusted life years (DALY) as their main outcome 60, 82, 90, 91, 92, and only four of 64 (incremental) net benefit 52, 53, 55, 71. There were two of 64 studies reporting only the intermediate outcomes of the intervention 85, 93 (Table 1).
Overview of economic models
Table 2 shows the main model attributes used in the included studies. Thirty of 64 studies used a Markov model, 12 of which used a specific type called the benefits of smoking cessation on outcomes (BENESCO) model 4, 5, 36, 37, 48, 56, 57, 58, 59, 61, 62, 65. Decision‐tree models 41, 43, 52, 55, 63, 71, 75, 83, 93, discrete‐event simulations (DES) 45, 54, the chronic disease model (RIVM‐CDM) 44, 81, 88, the tobacco policy model (TPM) 76, 77, the quit benefits model (QBM) 80, the World Health Organization (WHO) model 90, the global health outcomes model (GHO model) 70 and the abstinent‐contingent treatment model (ABT model) 73 were also used. Twelve of 64 studies did not report explicitly the model used, reporting only decision analysis modelling or simulation modelling 39, 50, 51, 66, 69, 72, 74, 78, 86 or limiting the description to only dynamic or static modelling 42, 82, 92.
Table 2.
Characteristics showed per model and summary of most reported characteristics.
| Type of model | Study | Characteristics | ||||||
|---|---|---|---|---|---|---|---|---|
| Transition/health statesa | Time‐horizon | Perspective | Discounting | Analysis | ||||
| Effects | Costs | Primary measure of effectiveness | Sensitivity analysisb | |||||
| Markov (n = 30) | Annemans, 2015 | 4 | Life‐time | Health‐care payer | 1.5 and 3% | 1.5 and 3% | Abstinence rates | USA and PSA |
| Annemans, 2009 | 4 + 6 | Life‐time | Health‐care payer | 1.5% | 3% | Continuous abstinence rates | USA and PSA | |
| Athanasakis, 2012 | 5 | Life‐time | Societal | 3% | 3% | Continuous abstinence rates | PSA | |
| Bae, 2009 | NR | Life‐time | NR | 5% | 5% | Quit rates | USA and PSA | |
| Bertram, 2007 | 3 | Life‐time | Health‐care system | 3% | 3% | Quit rates | PSA | |
| Bolin, 2008 | NR | 20 and 50 years | Health‐care and societal | 3% | 3% | Probability of cessation | DSA and PSA | |
| Bolin, 2009a | NR | 50 years | NR | 3% | 3% | Smoking prevalence and quit rates | USA, MSA, and PSA | |
| Bolin, 2009b | SC intervention +4 | Life‐time | Health‐care system | 3.5% | 3.5% | Continuous abstinence rates | PSA, MSA, and DSA | |
| Chevreul, 2014 | 3 | Life‐time | Social Health Insurance | 3% | 3% | Quit rates | PSA | |
| Cornuz, 2006 | NR | Life‐time | NR | NR | 3% | Odds ratio for quitting | USA | |
| Cornuz, 2003 | NR | NR | Third‐party payer | 3% | 3% | Odds ratio for quitting | NR | |
| Dino, 2008 | Current smoker, quit, reduce, stay smoker | Life‐time | School | 3% | 3% | Quit rates | MSA and ECA | |
| Fiscella, 1996 | NR | NR | Health‐care payer | 3% | 3% | Cessation rates | USA and PSA | |
| Guerriero, 2013 | 3 + MI, CHD, stroke, lung cancer, COPD | Life‐time | Health service (UK NHS) | 3.5% | 3.5% | Relative risk of quitting, relapse rates | DSA and PSA | |
| Heitjan, 2008 | NR | NR | NR | NR | 3% | Initiation rates and successful quit attempts | USA and ECA | |
| Hojgaard, 2011 | 2 | 10 years and life‐time | Societal | 3.5% | 3.5% | Quit and relapse rates | ECA | |
| Hoogendoorn, 2008 | 4 + 6 | Life‐time | Health‐care payer | 1.5% | 4% | Abstinence rates | USA and PSA | |
| Howard, 2008 | 4 + 6 | Life‐time | Health‐care system | 3% | 3% | Continuous abstinence rates | USA and PSA | |
| Igarashi, 2009 | Success‐alive, failure‐alive, sick‐smoke, sick‐non‐smoke, death | Until age 90 | Health‐care payer | 3% | 3% | Abstinence rates | USA, MSA, and PSA | |
| Knight, 2010 | NR | Life‐time | NR | 3% | 3% | Quit rates | USA and PSA | |
| Lal, 2014 | 3 + Mortality due to: cancer, COPD, CHD, stroke, other diseases | Life‐time | Health sector | 3% | 5% | Quit rates | PSA | |
| Levy, 2006 | NR | 20 years | Employer | NR | 5% | Probability of smoking cessation | DSA | |
| Linden, 2010 | 4 + 6 | Life‐time | Societal | 5% | 5% | Continuous abstinence rates | USA, MSA, and PSA | |
| Olsen, 2006 | 3 | Life‐time | Payer | 3.5% | 3.5% | Abstinence rates | USA and PSA | |
| Pinget, 2007 | NR | 1 year | Third‐party payer | NR | 3% | Point abstinence at 1 year | USA | |
| Simpson, 2013 | Quit or continue smoking | 20 years | NR | 3% | 3% | Rates for media awareness and quitline and (NYTCP) NRT utilization rates | NR | |
| Taylor, 2011 | Recent quitter, smoker (lung CA, CHD, MI, stroke, COPD), former smoker (lung CA, CHD, MI, stroke, COPD), dead | Life‐time | Health service (UK NHS) | 3.5% | 3.5% | Abstinence rates | USA | |
| Vemer, 2010a | 4 | Life‐time | Health‐care system | 0–5.0% | 3.0–5.0% | Change in incremental net monetary benefits | NR | |
| Von Wartburg, 2014 | Exclusive health states as a function of their demographics and smoking status. | Life‐time | Health‐care system and societal | NR | 5% | Quit rates | USA and PSA | |
| Welton, 2008 | NR | Life‐time | Health service (UK NHS) | Not discounted | Not required | Abstinence rates | MSA and PSA | |
| Most reported | NR (n = 11), 4 (n = 3) and combined with 6 (n = 4) | Life‐time (n = 21) | Health‐care system/payer (n = 17) | 3% (n = 12) | 3% (n = 16) | Quit/abstinence rates (n = 24) | USA with PSA (n = 9) | |
| Decision‐tree model (n = 9) | Boyd, 2009 | NR | 4 or 52 weeks | Health service (UK NHS) | NR | NR | Quit rates | USA and MSA |
| Levy, 2002 | Quit attempt or no quit attempt, quit or fail | 1 year | Health‐care payer | NR | Not required | Predicted quit rates | USA and MSA | |
| McGhan, 1996 | NR | NR | Employer | NR | NR | Quit rates | NR | |
| Nielsen, 2000 | NR | NR | Employer | NR | 3% | Quit rates | USA | |
| Song, 2002 | NR | NR | Health service (UK NHS) | NR | Not required | Quit rates | ECA | |
| Tran, 2002 | NR | 1 year | Payer | 3% | Not required | Continuous abstinence rates | USA | |
| Halpern, 2007b | Quit attempt or no quit attempt, quit or fail, resume | 2, 5, 10 or 20 years | NR | NR | 3% | Quit rates | NR | |
| Jackson, 2007 | Quit or continue smoking | 1 year | Employer | NR | Not required | Continuous abstinence rates | NR | |
| Xu, 2014 | Current smoker, quit attempt or continue smoking | NR | Funding agency | 3% | 3% | Quit rates | USA | |
| Most reported | Quit attempt or no quit attempt, (quit or fail) (n = 4) | Short‐term (n = 5) | Health‐care system/payer (n = 4) | 3% (n = 2) | 3% (n = 3) | Quit/abstinence rates (n = 9) | USA (n = 3) or in combination with MSA (n = 2) | |
| Remaining models reported (n = 25) | ||||||||
| Markov & Monte Carlo | Bauld, 2011 | Ex‐smoker, smoker, death and smoking‐related death | 1 year or life‐time | Health service (UK NHS) | 3.5% | NR | Continuous abstinence rates | DSA |
| DES | Warner, 1996 | NR | 50 years | Societal and employer | NR | 3%, 3.5%, 4% | Quit rates | USA and ECA |
| Xenakis, 2009 | NR | 1 year | Health‐care payer | NR | Not required | Continuous abstinence rates | USA | |
| CDM | Over, 2014 | 1 + age, gender, SES | 75 years | Health‐care system | NR | 1.5% and 4% | Quit rates | USA and MSA |
| Van Baal, 2007 | 1 + 14‐smoking related chronic diseases | 100 years | Health‐care system | 1.5% | 4% | Price elasticity of tobacco consumption | USA | |
| Vemer, 2010b | NR | 20 years and life‐time | Health‐care system | 1.5% | 4% | Additional number of successful quitters | NR | |
| TPM | Ahmad, 2005a | 1 | 50 years | Societal | 3% | 3% | Initiation rates | NR |
| Ahmad, 2005b | 1 | 50 years | Societal | 3% | 3% | Initiation rates | USA | |
| QBM | Hurley, 2008 | NR | Life‐time | NR | 3% | 3% | Reduction in smoking prevalence | DSA, MSA, and PSA |
| WHO model | Lai, 2007 | NR | 100 years | Societal | 3% | 3% | Change in disease incidence | ECA |
| GHO | Bolin, 2006 | 4 | 20 years | Health‐care and societal | 3% | 3% | QALY | USA, MSA, and PSA |
| ACT | Stapleton, 1999 | NR | Life‐time | Health service (UK NHS) | 1.75% | Not required | Additional number of LY saved | USA |
| Decision analytical/simulation modelling | Brown, 2014 | NR | Until age 65 | NR | 3.5% | NR | Increase in quit attempts | USA |
| Cantor, 2015 | Short term: quit or no‐quit. Long term: alive or dead | 1 year or life‐time | Health‐care provider | 3% | 3% | Quit rates | USA and MSA | |
| Croghan, 1997 | NR | Life‐time | NR | 0%, 3%, 5% | Not required | Abstinence rates | USA | |
| Halpern, 2007a | Continued cessation, relapse, resume smoking, continued smoking | 10 years | NR | NR | 3% | Quit rates | NR | |
| Hill, 2006 | NR | 6 months | Texas government | NR | Not required | % individuals not smoking at 6 months | USA and MSA | |
| Nohlert, 2013 | NR | Until age 85 | Societal | 3% | 3% | Abstinence rates | USA, MSA, and PSA | |
| Ong, 2005 | NR | 1 year | NR | 3% | Not required | Sustained quitters generated | MSA and PSA | |
| Shearer, 2006 | NR | NR | Government | NR | Not required | Continuous abstinence rates | MSA | |
| Stapleton, 2012 | NR | Life‐time | Health service | 3.5% | 1.5–3.5% | Abstinence rates | Various possible | |
| Dynamic/static modelling (n = 3) | Feenstra, 2005 | 1 | 75 years | Societal | 4% | 4% | Abstinence rates | USA and MSA |
| Ranson, 2002 | NR | NR | NR | 3.0–10.0% | 3.0–10.0% | Number of deaths averted | ECA | |
| Van Genugten, 2003 | Current or former smoker. Lung cancer, CHD, stroke, and COPD | Period 1998–2050 | NR | NR | NR | Total number of life‐years lost as the sum of the remaining life expectancy at the age of death | MSA | |
| SmokingPaST Framework (n = 1) | O'Donnell, 2011 | NR | NR | NR | NR | NR | Quit attempts | NR |
| Most reported | Not reported (n = 15), 1 (n = 3) | Life‐time (n = 7) | Health‐care system/payer (n = 10) | Not reported (n = 8), 3% (n = 8) | 3% (n = 8) | Quit/abstinence rates (n = 13) | USA (n = 6) or combinations with USA (n = 7) | |
This refers to the states considered in the model and may include: (1) never smoker, current smoker, former smoker; (2) never smoker, current smoker, ex‐smoker, death; (3) current smoker, former smoker, death; (4) current smoker, recent quitter, long‐term quitter; (5) no morbidity, chronic obstructive pulmonary disease (COPD) or lung cancer, coronary heart disease (CHD) or stroke first event, CHD or stroke subsequent event, death from CHD/stroke, death from COPD/lung cancer, death (all cause); (6) no current morbidity, asthma exacerbation, CHD or stroke: post first event, COPD or lung cancer, CHD or stroke: post subsequent event, death (CHD or stroke), death (COPD or lung cancer), death (all cause).
Uncertainty analysis: USA = univariate sensitivity analysis; MSA = multivariate sensitivity analysis; ECA = extreme case analysis; PSA = probabilistic sensitivity analysis; DSA = deterministic sensitivity analysis; NRT = nicotine replacement therapy; NYTCP = New York Tobacco Control Program; SES = socio‐economic status; MI = minor limitations; SC = ; NR = not reported; QALY = quality adjusted life years.
Several (18 of 30) studies based on Markov models provided sufficient information on transition or health states used in the model. The most frequently used transition states were current smoker, former smoker or death, while health states included asthma exacerbation, coronary heart disease (CHD), stroke, chronic obstructive pulmonary disease (COPD) and lung cancer. In decision‐tree models (n = nine of 64) the most reported transition states were quit attempt or no quit attempt, often combined with success to quit or failure to quit.
The majority of the Markov models used a life‐time horizon (n = 22 of 30) while decision‐tree models considered a time between 1 and 50 years. Most of the studies based on other models lacked sufficient information, or reported a time‐horizon of 50 years. Most evaluations used a health‐care and/or payer perspective (n = 50 of 64). Twelve of 64 used a societal perspective. The reported primary measure of effectiveness in all models was quit rate or its variants (e.g. continuous abstinence rates).
The majority of the studies (n = 55 of 64) performed sensitivity analyses to account for uncertainties in their estimates. Markov model‐based studies performed mainly both univariate and probabilistic sensitivity analyses, decision‐tree models used univariate sensitivity analyses often in combination with multivariate sensitivity analyses (n = five of nine), and the other models (n = 25 of 64) conducted univariate sensitivity analyses (n = 13 of 25).
Quality assessment and transferability
Of the 64 included studies assessed for quality, 15 were excluded based on the first criteria (no health‐care perspective), 12 based on the second (no cost benefit or cost–utility analysis) and 24 on the final criteria (having major limitations). As shown in Table 3, 13 of 64 studies were then classified as having minor limitations, 35 as having potentially serious limitations and 16 as having very serious limitations.
Table 3.
Results of the quality assessment.
| Classification | Studies |
|---|---|
| Minor limitations | Annemans, 2015; Annemans, 2009; Athanasakis, 2012; Bolin, 2006; Bolin, 2008; Bolin, 2009b; Boyd, 2009; Cornuz, 2003; Guerriero, 2013; Hoogendoorn, 2008; Howard, 2008; Over, 2014; Stapleton, 1999 |
| Potentially serious limitations | Ahmad, 2005a; Ahmad, 2005b; Bae, 2009; Bauld, 2011; Bolin, 2009a; Brown, 2014; Cantor, 2015; Chevreul, 2014; Cornuz, 2006; Feenstra, 2005; Fiscella, 1996; Halpern, 2007b; Heitjan, 2008; Hill, 2006; Hojgaard, 2011; Hurley, 2008; Igarashi, 2009; Linden, 2010; Levy, 2002; Nohlert, 2013; Ong, 2005; Pinget, 2007; Shearer, 2006; Simpson, 2013; Song, 2002; Stapleton, 2012; Taylor, 2011; Tran, 2002; Van Baal, 2007; Vemer, 2010a; Vemer, 2010b; Von Wartburg, 2014; Warner, 1996; Welton, 2008; Xenakis, 2009 |
| Very serious limitations | Bertram, 2007; Croghan, 1997; Dino, 2008; Halpern, 2007a; Knight, 2010; Lai, 2007; Lal, 2014; Levy, 2006; McGhan, 1996; Nielsen, 2000; Olsen, 2006; Ranson, 2002; Van Genugten, 2003; Xu, 2014; Jackson, 2007; O'Donnell, 2011 |
Table 4 provides an overview of the scoring per question on the EURONHEED checklist for the 13 studies judged as having sufficient quality including the summary scores. The studies’ total scores varied between 57 and 87% and the scores of the transferability checklist from 50 to 97%.
Table 4.
Results of the European Network of Health Economic Evaluation Databases (EURONHEED) checklist.
| 1 = yes, 0.5 = partially, 0 = no/no information, NA = not Applicable | Annemans, (2015) | Annemans, (2009) | Athanasa‐kis, (2012) | Bolin, (2006) | Bolin, (2008) | Bolin, (2009b) | Boyd, (2008) | Cornuz, (2003) | Guerriero, (2013) | Hoogen‐doorn, (2008) | Howard, (2008) | Over, (2014) | Stapleton, (1999) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q2 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| HT1 | 0.5 | 0 | 0.5 | 0.5 | 1 | 0 | 1 | 1 | 1 | 1 | 0.5 | 1 | 0.5 |
| HT2 | 0.5 | 0 | 0.5 | 0.5 | 0.5 | 1 | 0 | 1 | 1 | 1 | 0.5 | 1 | 0.5 |
| SE1 | 0.5 | 0.5 | 1 | 1 | 1 | 0 | 1 | 1 | 0.5 | 0 | 0 | 1 | 1 |
| SE2 | 0.5 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | 1 |
| P1 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| SP1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 | 1 |
| SP2 | 0.5 | 0.5 | 0.5 | 1 | 1 | 1 | 1 | 0 | 0.5 | 1 | 0.5 | 1 | 0 |
| SP3 | 0 | 0.5 | 0.5 | NA | 1 | NA | 0.5 | NA | 0 | 0.5 | 0.5 | NA | 0 |
| SP4 | 0 | 0 | 0 | 1 | 1 | 0.5 | 0 | 0.5 | 1 | 0.5 | 0.5 | NA | 0 |
| M1 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 1 | NA | 1 | 1 | 1 | NA | 0.5 |
| M2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 | NA |
| E1 | NA | NA | NA | 0.5 | 1 | 1 | 0 | NA | 0.5 | NA | NA | NA | 1 |
| E2 | NA | NA | NA | NA | 1 | 1 | 0.5 | NA | 0.5 | NA | NA | NA | 1 |
| E3 | 0 | 0 | 0 | 0 | 0 | 0 | NA | 0.5 | NA | 0 | 0 | 0 | NA |
| E4 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| E5 | 1 | 0.5 | 0.5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| E6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E7 | NA | NA | NA | 0.5 | 0.5 | 1 | 0 | NA | 1 | 1 | NA | 0 | 0 |
| B1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| B2 | 0 | 0 | 0 | 0.5 | 0.5 | 0 | 0 | NA | 0.5 | NA | 1 | 0 | NA |
| B3 | 1 | 1 | 1 | 0.5 | 0.5 | 0 | 0 | NA | 0.5 | NA | 0 | 0 | NA |
| B4 | 0 | 0 | 0 | NA | NA | NA | NA | NA | NA | NA | 0 | 0 | NA |
| B5 | 1 | 0.5 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0.5 |
| C1 | 1 | 0.5 | 0.5 | 1 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 1 | 1 | 1 |
| C2 | 0.5 | 0.5 | 0.5 | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| C3 | 1 | 1 | 1 | 1 | 0.5 | 0 | 1 | 1 | 0.5 | 1 | 1 | 0 | 1 |
| C4 | 1 | 1 | 0.5 | 1 | 0.5 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| C5 | 0.5 | 0.5 | 1 | 0.5 | 1 | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | 1 |
| C6 | 0 | 0 | 0 | 0.5 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 1 | 0 | 1 |
| C7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| C8 | 0.5 | 0.5 | 0.5 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| C9 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| C10 | NA | NA | NA | NA | NA | 0.5 | NA | 1 | NA | NA | NA | NA | NA |
| C11 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0.5 | 1 | 1 | 0 | 0 |
| D1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| D2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | NA | 1 | 1 | 1 | 1 | NA |
| D3 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0.5 |
| D4 | 1 | 0 | 05 | 0.5 | 0.5 | 0.5 | 0 | 0 | 0 | 0.5 | 0.5 | 0 | 0 |
| S1 | 0 | 0 | 0 | 0 | 0 | 1 | 0.5 | 0.5 | 0 | 1 | 1 | 0.5 | 0 |
| O1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 |
| Summary scoresa (%) | |||||||||||||
| Totalb | 61 | 57 | 64 | 74 | 79 | 67 | 70 | 77 | 76 | 87 | 78 | 59 | 69 |
| Transferabilityc | 60 | 50 | 63 | 73 | 81 | 80 | 88 | 75 | 81 | 97 | 90 | 67 | 66 |
Full items of the EURONHEED checklist are described in Supporting information, Table S4. Items comprising the transferability subchecklist are shown in bold type.
Average of the total summary score: 71%; average of the transferability summary score: 75%.
Summary scores were calculated using the formula as in EURONHEED checklist: .
Total summary score, number of questions = 42.
Transferability summary score, number of questions = 16.
The average score per section presented as the percentage of the total score are shown in Fig. 2. The average score per section was 0.69 (range = 0.35–0.92). The sections that scored below the average (69%) were: health technology assessment study population, effectiveness, benefit measure, variability and generalizability.
Figure 2.
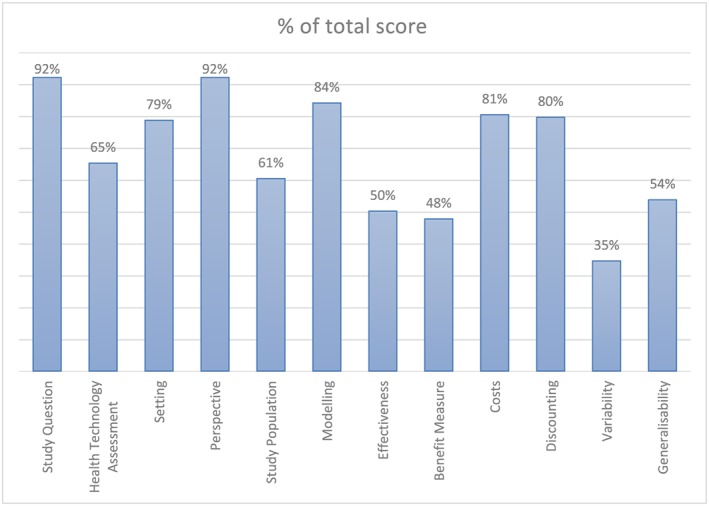
Percentage of total score per section. Calculated as the average of the% of total score of subitems. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
Key findings
Markov‐based state transition models with QALY as the outcome measure were the most frequently used technique in evaluating the cost‐effectiveness of smoking cessation interventions. However, the majority of the studies were reported poorly, making it hard to assess their transferability using the existing checklist‐based method. Where such assessment was possible, studies showed a wide variation in transferability scores, driven mainly by the method of selecting populations, assessing effectiveness and outcomes and estimating variability and generalizability of their own findings.
Relative transferability
The EURONHEED method assumes that without a quality score it would be impossible to transfer a study to another setting 9, 32, 95. Therefore, the explicit assessment using this method resulted in some studies being more favourable candidates than others. However, on average, all studies lacked in some attributes for full transferability. One of the main differences between a high score and a low score is how differently the studies scored on the questions on costs. For example, Annemans et al. (2009), with a score of 0.50, addressed most of the cost questions only partially, whereas Hoogendoorn et al. (2008), with a score of 0.97, did so fully. Therefore, costs are important determinants of the transferability assessment 9. Our review also highlighted other determinants; namely, selection of study population, intervention and comparator descriptions, effectiveness and benefit measures and variability/generalizability analyses—all scoring below the overall average score. Without a threshold, it was not possible to rank the assessed studies on their relative transferability, and this will be explored further below.
Comparison to current literature
Several systematic reviews are available on the cost‐effectiveness of smoking cessation 22, 23, 24, but only one systematic review looking at model‐based economic evaluations 20. Most of the studies included in their review used the Markov model with long‐term time horizons, included comparable health states and reported the similar measures of effectiveness and outcomes as ours, and common weaknesses included poor reporting of the modelling details. However, a key difference from our review is that they did not build on their findings to evaluate the extent to which such models could be transferable from the original context to others, for wider benefits 9, 10, 17. In areas outside smoking cessation, Korber has evaluated physical activity interventions for their transferability 96. Consistent with our findings, she also found that a very few included studies explored variability from place to place and discussed caveats regarding the generalizability of results, ‘leading to a wide variation in the transferability of the study results ranging from “low” to “very high” with everything in between’ 96. Another study 97 found that population and methodological characteristics were poorly reported—a finding that echoes our own results on the weaknesses of the models.
Implications of this review
Despite the availability of several guidelines on how to conduct and report adequately on economic evaluations 29, 31, there is still a considerable variation in the quality of published economic evaluations in smoking cessation. Arguably, this may limit the use of such evidence in other contexts. Some authors argue that the factors affecting the perception of applicability (the process question) and transferability (the outcome question) together might be broader than the factors associated with external validity 13. Notwithstanding this difference, the EURONHEED method relies heavily upon the quality of reporting to ascertain transferability 32. Therefore, such scores can be limited in use by the end‐users for two reasons. First, a poorly constructed model could have been reported well scoring high on the transferability scale and vice versa. Secondly, without a threshold score, it is hard to judge a study or to rank and compare across the studies. Nixon et al. 32 argue that the EURONHEED score should, rather, be used as a general guide in making decisions, but also note that the explicit assessment of transferability using this method will introduce an educational element, helping researchers to improve the design, conduct and reporting of future studies.
This review highlights the educational element noted above. Transparency in the model building and subsequent analysis and results, which can be captured by the quality of reporting, can enhance our understanding of the underlying process and outcome questions. However, a robust method would require more analyses based on the model outputs (as opposed to the checklists), backed up by the perceptions of actual stakeholders (including decision makers) as to what is relevant, adaptable, valid and transferable to them 13, 16. The European study on Quantifying Utility of Investment in Protection from Tobacco (EQUIPT) 98 provides some promise to that end by encompassing both model‐based analyses (e.g. on the parameter importance and variability) and the analysis of the stakeholder views (e.g. on the importance of interventions and intention to use economic evidence in policymaking) 99, 100, in addition to the systematic reviews based on the published models such as this. Although the final results of the EQUIPT study are yet to be published, this comprehensive framework appears to provide the end‐users with an understanding of a key transferability attribute—what changes in the economic model would make it transferable to their own settings and why 15.
This review also reiterates the already identified challenge in terms of the way in which economic evaluations in broader public health are designed, conducted and reported 101. The finding that only one‐fifth of the included study met quality classification for transferability implies that policymakers, researchers and journal editors need to work together in enhancing the quality of new economic evaluations and making it more transferable. The guidelines used by economic evaluation community and journals such as this are helpful to that end 102. However, such guidelines should also emphasize the need for the authors to assess and report transferability of their models to the new contexts. This would ensure that future studies could consider adding model‐based analysis of transferability on to the checklist‐based evaluation, backed up by, where possible, analysis of the views of stakeholders.
Limitations
A major limitation of this review has been the limitation embedded in the existing method of transferability assessment 9, 32. Future research may overcome this limitation by adopting a comprehensive assessment as discussed above. In addition, limiting the search to English language only might have excluded some studies. However, we identified more model‐based economic evaluations than a previous similar review 22. The use of three quality criteria 31 for inclusion of studies in the transferability assessment could potentially have introduced some bias, as it was based on the overall assessment, as opposed to some standard checklists such as those by Drummond 103 or Philips 104. However, the variety of items included in our data extraction form as outlined in the best practice guidelines 102 were very similar to the Drummond or Philips checklists, implying the possibility of such bias to be minimal. Finally, exclusion of low‐/middle‐income countries to reduce study heterogeneity could have limited this review in its primary focus (i.e. evidence transferability to less‐affluent countries).
Conclusion
Existing economic evaluations in smoking cessation vary in quality, resulting mainly from the way in which they selected their populations, measured costs and effects and assessed the variability and generalizability of their own findings. All studies lacked one or more key study attributes for full transferability. A robust design, coupled with comprehensive reporting of key study attributes, could make economic evaluations transferable to a new context.
Declaration of interests
None.
Funding
S.P. and P.K.'s time in this research was funded partly by the European Union's Seventh Framework Programme for research, technological development and demonstration under grant agreement no. 602270 (EQUIPT).
Supporting information
Table S1 Search strategy.
Table S2 Exclusion criteria.
Table S3 List of high‐income countries available at: http://data.worldbank.org/about/country‐and‐lending‐groups
Table S4 EURONHEED checklist.
Acknowledgements
We would like to thank Teresa Jones for facilitating searches and providing access to full text materials from the Brunel Library systems. The first version of this paper was presented to an internal seminar at the Health Economics Research Group (HERG), Brunel University London. The feedback received from HERG members is gratefully acknowledged.
Berg, M. L. , Cheung, K. L. , Hiligsmann, M. , Evers, S. , de Kinderen, R. J. A. , Kulchaitanaroaj, P. , and Pokhrel, S. (2017) Model‐based economic evaluations in smoking cessation and their transferability to new contexts: a systematic review. Addiction, 112: 946–967. doi: 10.1111/add.13748.
References
- 1. McFarland A. Economic evaluation of interventions in health care. Nurs Stand 2014; 29: 49–58. [DOI] [PubMed] [Google Scholar]
- 2. Anderson R. Systematic reviews of economic evaluations: utility or futility? Health Econ 2010; 19: 350–364. [DOI] [PubMed] [Google Scholar]
- 3. Goodman C. S. Introduction to Health Technology Assessment. Falls Church, VA: The Lewin Group; 2004. [Google Scholar]
- 4. Hoogendoorn M., Welsing P., Rutten‐van Molken M. P. Cost‐effectiveness of varenicline compared with bupropion, NRT, and nortriptyline for smoking cessation in the Netherlands. Curr Med Res Opin 2008; 24: 51–61. [DOI] [PubMed] [Google Scholar]
- 5. Howard P., Knight C., Boler A., Baker C. Cost–utility analysis of varenicline versus existing smoking cessation strategies using the BENESCO simulation model: application to a population of US adult smokers. Pharmacoeconomics 2008; 26: 497–511. [DOI] [PubMed] [Google Scholar]
- 6. Guerriero C., Cairns J., Roberts I., Rodgers A., Whittaker R., Free C. The cost‐effectiveness of smoking cessation support delivered by mobile phone text messaging: Txt2stop. Eur J Health Econ 2013; 14: 789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Urdahl H., Manca A., Sculpher M. J. Assessing generalisability in model‐based economic evaluation studies: a structured review in osteoporosis. Pharmacoeconomics 2006; 24: 1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Essers B. A., Seferina S. C., Tjan‐Heijnen V. C., Severens J. L., Novak A., Pompen M. et al. Transferability of model‐based economic evaluations: the case of trastuzumab for the adjuvant treatment of HER2‐positive early breast cancer in the Netherlands. Value Health 2010; 13: 375–380. [DOI] [PubMed] [Google Scholar]
- 9. Boulenger S., Nixon J., Drummond M., Ulmann P., Rice S., de Pouvourville G. Can economic evaluations be made more transferable? Eur J Health Econ 2005; 6: 334–346. [DOI] [PubMed] [Google Scholar]
- 10. Goeree R., He J., O'Reilly D., Tarride J.‐E., Xie F., Lim M. et al. Transferability of health technology assessments and economic evaluations: a systematic review of approaches for assessment and application. ClinicoEconomics Outcomes Res 2011; 3: 89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sculpher M. J., Pang F. S., Manca A., Drummond M. F., Golder S., Urdahl H. et al. Generalisability in economic evaluation studies in health‐care: a review and case studies. Health Technol Assess 2004; 8: 1. [DOI] [PubMed] [Google Scholar]
- 12. Wang S., Moss J. R., Hiller J. E. Applicability and transferability of interventions in evidence‐based public health. Health Promot Int 2006; 21: 76–83. [DOI] [PubMed] [Google Scholar]
- 13. Burchett H. E. D., Mayhew S. H., Lavis J. N., Dobrow M. J. When can research from one setting be useful in another? Understanding perceptions of the applicability and transferability of research. Health Promot Int 2013; 28: 418–430. [DOI] [PubMed] [Google Scholar]
- 14. Buffett C., Ciliska D., Thomas H. Can I use this evidence in my program decision? Assessing applicability and transferability of evidence: Citeseer. Hamilton, ON: National Collaborating Centre for Methods and Tools; 2007. [Google Scholar]
- 15. Mason J. M., Mason A. R. The generalisability of pharmacoeconomic studies. Issues and challenges ahead. Pharmacoeconomics 2006; 24: 937–945. [DOI] [PubMed] [Google Scholar]
- 16. Burchett H. E. D., Dobrow M. J., Lavis J. N., Mayhew S. H. The applicability and transferability of public health research from one setting to another: a survey of maternal health researchers. Glob Health Promot 2013; 20: 16–24. [DOI] [PubMed] [Google Scholar]
- 17. Spath H. M., Carrere M. O., Fervers B., Philip T. Analysis of the eligibility of published economic evaluations for transfer to a given health care system. Methodological approach and application to the French health care system. Health Policy 1999; 49: 161–177. [DOI] [PubMed] [Google Scholar]
- 18. Evers S., Goossens M., de Vet H., van Tulder M., Ament A. Criteria list for assessment of methodological quality of economic evaluations: consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005; 21: 240–245. [PubMed] [Google Scholar]
- 19. Welte R., Feenstra T., Jager H., Leidl R. A decision chart for assessing and improving the transferability of economic evaluation results between countries. Pharmacoeconomics 2004; 22: 857–876. [DOI] [PubMed] [Google Scholar]
- 20. Bolin K. Economic evaluation of smoking‐cessation therapies: a critical and systematic review of simulation models. Pharmacoeconomics 2012; 30: 551–564. [DOI] [PubMed] [Google Scholar]
- 21. Kirsch F. A systematic review of quality and cost‐effectiveness derived from Markov models evaluating smoking cessation interventions in patients with chronic obstructive pulmonary disease. Expert Rev Pharmacoecon Outcomes Res 2015; 15: 301–316. [DOI] [PubMed] [Google Scholar]
- 22. Mahmoudi M., Coleman C. I., Sobieraj D. M. Systematic review of the cost‐effectiveness of varenicline vs. bupropion for smoking cessation. Int J Clin Pract 2012; 66: 171–182. [DOI] [PubMed] [Google Scholar]
- 23. Hoogendoorn M., Feenstra T. L., Hoogenveen R. T., Rutten‐van Mölken M. P. M. H. Long‐term effectiveness and cost‐effectiveness of smoking cessation interventions in patients with COPD. Thorax 2010; 65: 711–718. [DOI] [PubMed] [Google Scholar]
- 24. Ronckers E. T., Groot W., Ament A. J. H. A. Systematic review of economic evaluations of smoking cessation: standardizing the cost‐effectiveness. Med Decis Making 2005; 25: 437–448. [DOI] [PubMed] [Google Scholar]
- 25. Wilczynski N. L., Haynes R. B., Lavis J. N., Ramkissoonsingh R., Arnold‐Oatley A. E. Optimal search strategies for detecting health services research studies in MEDLINE. Can Med Assoc J 2004; 171: 1179–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McKinlay R. J., Wilczynski N., Haynes R. B., Hedges Team . Optimal search strategies for detecting cost and economic studies in EMBASE. BMC Health Serv Res 2006; 6: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Glanville J., Kaunelis D., Mensinkai S. How well do search filters perform in identifying economic evaluations in MEDLINE and EMBASE. Int J Technol Assess Health Care 2009; 25: 522–529. [DOI] [PubMed] [Google Scholar]
- 28. Landis J. R., Koch G. G. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–174. [PubMed] [Google Scholar]
- 29. Husereau D., Drummond M., Petrou S., Carswell C., Moher D., Greenberg D. et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013; 16: 231–250. [DOI] [PubMed] [Google Scholar]
- 30. Goehler A., Geisler B., Manne J., Jahn B., Conrads‐Frank A., Gazelle G. S. et al. Decision‐analytic models to simulate health outcomes and costs in heart failure. Pharmacoeconomics 2011; 29: 753–769. [DOI] [PubMed] [Google Scholar]
- 31. National Institute for Health and Care Excellence (NICE) . Methods for the development of NICE public health guidance (third edition). NICE; 2012. Available at: https://www.nice.org.uk/process/pmg4/chapter/appendix-i-quality-appraisal-checklist-economic-evaluations (accessed 25 April 2015) (Archived at http://www.webcitation.org/6o7eXq2dr on 8 February 2017).
- 32. Nixon J., Rice S., Drummond M., Boulenger S., Ulmann P., de Pouvourville G. Guidelines for completing the EURONHEED transferability information checklists. Eur J Health Econ 2009; 10: 157–165. [DOI] [PubMed] [Google Scholar]
- 33. Cornuz J., Gilbert A., Pinget C., McDonald P., Slama K., Salto E. et al. Cost‐effectiveness of pharmacotherapies for nicotine dependence in primary care settings: a multinational comparison. Tob Control 2006; 15: 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cornuz J., Pinget C., Gilbert A., Paccaud F. Cost‐effectiveness analysis of the first‐line therapies for nicotine dependence. Eur J Clin Pharmacol 2003; 59: 201–206. [DOI] [PubMed] [Google Scholar]
- 35. Igarashi A., Takuma H., Fukuda T., Tsutani K. Cost–utility analysis of varenicline, an oral smoking‐cessation drug, in Japan. Pharmacoeconomics 2009; 27: 247–261. [DOI] [PubMed] [Google Scholar]
- 36. Knight C., Howard P., Baker C. L., Marton J. P. The cost‐effectiveness of an extended course (12 + 12 weeks) of varenicline compared with other available smoking cessation strategies in the United States: an extension and update to the BENESCO model. Value Health 2010; 13: 209–214. [DOI] [PubMed] [Google Scholar]
- 37. Linden K., Jormanainen V., Linna M., Sintonen H., Wilson K., Kotomaki T. Cost effectiveness of varenicline versus bupropion and unaided cessation for smoking cessation in a cohort of Finnish adult smokers. Curr Med Res Opin 2010; 26: 549–560. [DOI] [PubMed] [Google Scholar]
- 38. von Wartburg M., Raymond V., Paradis P. E. The long‐term cost‐effectiveness of varenicline (12‐week standard course and 12 + 12‐week extended course) vs. other smoking cessation strategies in Canada. Int J Clin Pract 2014; 68: 639–646. [DOI] [PubMed] [Google Scholar]
- 39. Brown K., Michie S., Walmsley W. How effective and cost‐effective was the national mass media smoking cessation campaign ‘stoptober’? Drug Alcohol Depend 2013; 135: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Taylor M., Leonardi‐Bee J., Agboola S., McNeill A., Coleman T. Cost effectiveness of interventions to reduce relapse to smoking following smoking cessation. Addiction 2011; 106: 1819–1826. [DOI] [PubMed] [Google Scholar]
- 41. Tran M. T., Holdford D. A., Kennedy D. T., Small R. E. Modeling the cost‐effectiveness of a smoking‐cessation program in a community pharmacy practice. Pharmacotherapy 2002; 22: 1623–1631. [DOI] [PubMed] [Google Scholar]
- 42. Feenstra T. L., Hamberg‐van Reenen H. H., Hoogenveen R. T., Rutten‐van Molken M. P. Cost‐effectiveness of face‐to‐face smoking cessation interventions: a dynamic modeling study. Value Health 2005; 8: 178–190. [DOI] [PubMed] [Google Scholar]
- 43. Song F., Raftery J., Aveyard P., Hyde C., Barton P., Woolacott N. Cost‐effectiveness of pharmacological interventions for smoking cessation: a literature review and a decision analytic analysis. Med Decis Making 2002; 22: S26–37. [DOI] [PubMed] [Google Scholar]
- 44. van Baal P. H., Brouwer W. B., Hoogenveen R. T., Feenstra T. L. Increasing tobacco taxes: a cheap tool to increase public health. Health Policy 2007; 82: 142–152. [DOI] [PubMed] [Google Scholar]
- 45. Warner K. E., Smith R. J., Smith D. G., Fries B. E. Health and economic implications of a work‐site smoking‐cessation program: a simulation analysis. J Occup Environ Med 1996; 38: 981–992. [DOI] [PubMed] [Google Scholar]
- 46. O'Donnell M. P., Roizen M. F. The SmokingPaST Framework: illustrating the impact of quit attempts, quit methods, and new smokers on smoking prevalence, years of life saved, medical costs saved, programming costs, cost effectiveness, and return on investment. Am J Health Promot 2011; 26: e11–23. [DOI] [PubMed] [Google Scholar]
- 47. Bauld L., Boyd K. A., Briggs A. H., Chesterman J., Ferguson J., Judge K. et al. One‐year outcomes and a cost‐effectiveness analysis for smokers accessing group‐based and pharmacy‐led cessation services. Nicotine Tob Res 2011; 13: 135–145. [DOI] [PubMed] [Google Scholar]
- 48. Bolin K., Wilson K., Benhaddi H., de Nigris E., Marbaix S., Mork A. C. et al. Cost‐effectiveness of varenicline compared with nicotine patches for smoking cessation: results from four European countries. Eur J Public Health 2009b; 19: 650–654. [DOI] [PubMed] [Google Scholar]
- 49. Fiscella K., Franks P. Cost‐effectiveness of the transdermal nicotine patch as an adjunct to physicians’ smoking cessation counseling. JAMA 1996; 275: 1247–1251. [PubMed] [Google Scholar]
- 50. Halpern M. T., Dirani R., Schmier J. K. The cost effectiveness of varenicline for smoking cessation. Manag Care Interface 2007; 20: 18–25. [PubMed] [Google Scholar]
- 51. Hill A. A cost‐effectiveness evaluation of single and combined smoking cessation interventions in Texas. Tex Med 2006; 102: 50–55. [PubMed] [Google Scholar]
- 52. McGhan W. F., Smith M. D. Pharmacoeconomic analysis of smoking‐cessation interventions. Am J Health Syst Pharm 1996; 53: 45–52. [DOI] [PubMed] [Google Scholar]
- 53. Welton N. J., Johnstone E. C., David S. P., Munafo M. R. A cost‐effectiveness analysis of genetic testing of the DRD2 Taq1A polymorphism to aid treatment choice for smoking cessation. Nicotine Tob Res 2008; 10: 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xenakis J. G., Kinter E. T., Ishak K. J., Ward A. J., Marton J. P., Willke R. J. et al. A discrete‐event simulation of smoking‐cessation strategies based on varenicline pivotal trial data. Pharmacoeconomics 2011; 29: 497–510. [DOI] [PubMed] [Google Scholar]
- 55. Jackson K. C., Nahoopii R., Said Q., Dirani R., Brixner D. An employer‐based cost–benefit analysis of a novel pharmacotherapy agent for smoking cessation. J Occup Environ Med 2007; 49: 453–460. [DOI] [PubMed] [Google Scholar]
- 56. Annemans L., Marbaix S., Nackaerts K., Bartsch P. Cost‐effectiveness of retreatment with varenicline after failure with or relapse after initial treatment for smoking cessation. Prev Med Rep 2015; 2: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Annemans L., Nackaerts K., Bartsch P., Prignot J., Marbaix S. Cost effectiveness of varenicline in Belgium, compared with bupropion, nicotine replacement therapy, brief counselling and unaided smoking cessation: a BENESCO Markov cost‐effectiveness analysis. Clin Drug Invest 2009; 29: 655–665. [DOI] [PubMed] [Google Scholar]
- 58. Athanasakis K., Igoumenidis M., Karampli E., Vitsou E., Sykara G., Kyriopoulos J. Cost‐effectiveness of varenicline versus bupropion, nicotine‐replacement therapy, and unaided cessation in Greece. Clin Ther 2012; 34: 1803–1814. [DOI] [PubMed] [Google Scholar]
- 59. Bae J. Y., Kim C. H., Lee E. K. Evaluation of cost–utility of varenicline compared with existing smoking cessation therapies in South Korea. Value Health 2009; 12: S70–3. [DOI] [PubMed] [Google Scholar]
- 60. Bertram M. Y., Lim S. S., Wallace A. L., Vos T. Costs and benefits of smoking cessation aids: making a case for public reimbursement of nicotine replacement therapy in Australia. Tob Control 2007; 16: 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bolin K., Mork A. C., Willers S., Lindgren B. Varenicline as compared to bupropion in smoking‐cessation therapy: cost–utility results for Sweden 2003. Respir Med 2008; 102: 699–710. [DOI] [PubMed] [Google Scholar]
- 62. Bolin K., Mork A. C., Wilson K. Smoking‐cessation therapy using varenicline: the cost–utility of an additional 12‐week course of varenicline for the maintenance of smoking abstinence. J Eval Clin Pract 2009a; 15: 478–485. [DOI] [PubMed] [Google Scholar]
- 63. Boyd K. A., Briggs A. H. Cost‐effectiveness of pharmacy and group behavioural support smoking cessation services in Glasgow. Addiction 2009; 104: 317–325. [DOI] [PubMed] [Google Scholar]
- 64. Heitjan D. F., Asch D. A., Ray R., Rukstalis M., Patterson F., Lerman C. Cost‐effectiveness of pharmacogenetic testing to tailor smoking‐cessation treatment. Pharm J 2008; 8: 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vemer P., Rutten‐van Molken M. P. Crossing borders: factors affecting differences in cost‐effectiveness of smoking cessation interventions between European countries. Value Health 2010; 13: 230–241. [DOI] [PubMed] [Google Scholar]
- 66. Croghan I. T., Offord K. P., Evans R. W., Schmidt S., Gomez‐Dahl L. C., Schroeder D. R. et al. Cost‐effectiveness of treating nicotine dependence: the Mayo Clinic experience. Mayo Clin Proc 1997; 72: 917–924. [DOI] [PubMed] [Google Scholar]
- 67. Pinget C., Martin E., Wasserfallen J. B., Humair J. P., Cornuz J. Cost‐effectiveness analysis of a European primary‐care physician training in smoking cessation counseling. Eur J Cardiovasc Prev Rehabil 2007; 14: 451–455. [DOI] [PubMed] [Google Scholar]
- 68. Olsen K. R., Bilde L., Juhl H. H., Kjaer N. T., Mosbech H., Evald T. et al. Cost‐effectiveness of the Danish smoking cessation interventions: subgroup analysis based on the Danish Smoking Cessation Database. Eur J Health Econ 2006; 7: 225–264. [DOI] [PubMed] [Google Scholar]
- 69. Nohlert E., Helgason A. R., Tillgren P., Tegelberg A., Johansson P. Comparison of the cost‐effectiveness of a high and a low‐intensity smoking cessation intervention in Sweden: a randomized trial. Nicotine Tob Res 2013; 15: 1519–1527. [DOI] [PubMed] [Google Scholar]
- 70. Bolin K., Lindgren B., Willers S. The cost utility of bupropion in smoking cessation health programs: simulation model results for Sweden. Chest 2006; 129: 651–660. [DOI] [PubMed] [Google Scholar]
- 71. Nielsen K., Fiore M. C. Cost–benefit analysis of sustained‐release bupropion, nicotine patch, or both for smoking cessation. Prev Med 2000; 30: 209–216. [DOI] [PubMed] [Google Scholar]
- 72. Shearer J., Shanahan M. Cost effectiveness analysis of smoking cessation interventions. Aust NZ J Public Health 2006; 30: 428–434. [DOI] [PubMed] [Google Scholar]
- 73. Stapleton J. A., Lowin A., Russell M. A. Prescription of transdermal nicotine patches for smoking cessation in general practice: evaluation of cost‐effectiveness. Lancet 1999; 354: 210–215. [DOI] [PubMed] [Google Scholar]
- 74. Stapleton J. A., West R. A direct method and ICER tables for the estimation of the cost‐effectiveness of smoking cessation interventions in general populations: application to a new cytisine trial and other examples. Nicotine Tob Res 2012; 14: 463–471. [DOI] [PubMed] [Google Scholar]
- 75. Halpern M. T., Dirani R., Schmier J. K. Impacts of a smoking cessation benefit among employed populations. J Occup Environ Med 2007; 49: 11–21. [DOI] [PubMed] [Google Scholar]
- 76. Ahmad S. The cost‐effectiveness of raising the legal smoking age in California. Med Decis Making 2005; 25: 330–340. [DOI] [PubMed] [Google Scholar]
- 77. Ahmad S. Closing the youth access gap: the projected health benefits and cost savings of a national policy to raise the legal smoking age to 21 in the United States. Health Policy 2005; 75: 74–84. [DOI] [PubMed] [Google Scholar]
- 78. Cantor S. B., Deshmukh A. A., Luca N. S., Nogueras‐Gonzalez G. M., Rajan T., Prokhorov A. V. Cost‐effectiveness analysis of smoking‐cessation counseling training for physicians and pharmacists. Addict Behav 2015; 45: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chevreul K., Cadier B., Durand‐Zaleski I., Chan E., Thomas D. Cost effectiveness of full coverage of the medical management of smoking cessation in France. Tob Control 2014; 23: 223–230. [DOI] [PubMed] [Google Scholar]
- 80. Hurley S. F., Matthews J. P. Cost‐effectiveness of the Australian National Tobacco Campaign. Tob Control 2008; 17: 379–384. [DOI] [PubMed] [Google Scholar]
- 81. Over E. A., Feenstra T. L., Hoogenveen R. T., Droomers M., Uiters E., van Gelder B. M. Tobacco control policies specified according to socioeconomic status: health disparities and cost‐effectiveness. Nicotine Tob Res 2014; 16: 725–732. [DOI] [PubMed] [Google Scholar]
- 82. Van Genugten M. L., Hoogenveen R. T., Mulder I., Smit H. S., Jansen J., De Hollander A. E. Future burden and costs of smoking‐related disease in the Netherlands: a dynamic modeling approach. Value Health 2003; 6: 494–499. [DOI] [PubMed] [Google Scholar]
- 83. Xu X., Alexander R. J., Simpson S. A., Goates S., Nonnemaker J. M., Davis K. C. et al. A cost‐effectiveness analysis of the first federally funded antismoking campaign. Am J Prev Med 2015; 48: 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Simpson S. A., Nonnemaker J. M. New York tobacco control program cessation assistance: costs, benefits, and effectiveness. Int J Environ Res Public Health 2013; 10: 1037–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Levy D. E. Employer‐sponsored insurance coverage of smoking cessation treatments. Am J Manag Care 2006; 12: 553–562. [PubMed] [Google Scholar]
- 86. Ong M. K., Glantz S. A. Free nicotine replacement therapy programs vs implementing smoke‐free workplaces: a cost‐effectiveness comparison. Am J Public Health 2005; 95: 969–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dino G., Horn K., Abdulkadri A., Kalsekar I., Branstetter S. Cost‐effectiveness analysis of the Not On Tobacco program for adolescent smoking cessation. Prev Sci 2008; 9: 38–46. [DOI] [PubMed] [Google Scholar]
- 88. Vemer P., Rutten‐van Molken M. P., Kaper J., Hoogenveen R. T., van Schayck C. P., Feenstra T. L. If you try to stop smoking, should we pay for it? The cost utility of reimbursing smoking cessation support in the Netherlands. Addiction 2010; 105: 1088–1097. [DOI] [PubMed] [Google Scholar]
- 89. Hojgaard B., Olsen K. R., Pisinger C., Tonnesen H., Gyrd‐Hansen D. The potential of smoking cessation programmes and a smoking ban in public places: comparing gain in life expectancy and cost effectiveness. Scand J Public Health 2011; 39: 785–796. [DOI] [PubMed] [Google Scholar]
- 90. Lai T., Habicht J., Reinap M., Chisholm D., Baltussen R. Costs, health effects and cost‐effectiveness of alcohol and tobacco control strategies in Estonia. Health Policy 2007; 84: 75–88. [DOI] [PubMed] [Google Scholar]
- 91. Lal A., Mihalopoulos C., Wallace A., Vos T. The cost‐effectiveness of call‐back counselling for smoking cessation. Tob Control 2014; 23: 437–442. [DOI] [PubMed] [Google Scholar]
- 92. Ranson M. K., Jha P., Chaloupka F. J., Nguyen S. N. Global and regional estimates of the effectiveness and cost‐effectiveness of price increases and other tobacco control policies. Nicotine Tob Res 2002; 4: 311–319. [DOI] [PubMed] [Google Scholar]
- 93. Levy D. T., Friend K. A simulation model of policies directed at treating tobacco use and dependence. Med Decis Making 2002; 22: 6–16. [DOI] [PubMed] [Google Scholar]
- 94. Knight A., Finkelstein J., Cha E., Brotman D. A mobile computer‐assisted education system to promote smoking cessation for hospitalized patients. J Hosp Med 2010; 5: 43–44. [Google Scholar]
- 95. Nixon J., Ulmann P., Glanville J., Boulenger S., Drummond M., de Pouvourville G. The European Network of Health Economic Evaluation Databases (EURO NHEED) Project. Eur J Health Econ 2004; 5: 183–187. [DOI] [PubMed] [Google Scholar]
- 96. Korber K. Potential transferability of economic evaluations of programs encouraging physical activity in children and adolescents across different countries—a systematic review of the literature. Int J Environ Res Public Health 2014; 11: 10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wolfenstetter S. B., Wenig C. M. Economic evaluation and transferability of physical activity programmes in primary prevention: a systematic review. Int J Environ Res Public Health 2010; 7: 1622–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pokhrel S., Evers S., Leidl R., Trapero‐Bertran M., Kalo Z., Vries H. et al. EQUIPT: protocol of a comparative effectiveness research study evaluating cross‐context transferability of economic evidence on tobacco control. BMJ Open 2014; 4: e006945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cheung K. L., Evers S. M., Hiligsmann M., Voko Z., Pokhrel S., Jones T. et al. Understanding the stakeholders’ intention to use economic decision‐support tools: a cross‐sectional study with the tobacco return on investment tool. Health Policy 2016; 120: 46–54. [DOI] [PubMed] [Google Scholar]
- 100. Voko Z., Cheung K. L., Jozwiak‐Hagymasy J., Wolfenstetter S., Jones T., Munoz C. et al. Similarities and differences between stakeholders’ opinions on using Health Technology Assessment (HTA) information across five European countries: results from the EQUIPT survey. Health Res Policy Syst 2016; 14: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Edwards R. T., Charles J. M., Lloyd‐Williams H. Public health economics: a systematic review of guidance for the economic evaluation of public health interventions and discussion of key methodological issues. BMC Public Health 2013; 13: 1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Walker D. G., Wilson R. F., Sharma R., Bridges J., Niessen L., Bass E. B. et al Best Practices for Conducting Economic Evaluations in Health Care: A Systematic Review of Quality Assessment Tools. Methods Research Report (prepared by Johns Hopkins University Evidence‐based Practice Center under contract no. 290‐2007‐10061‐I.). 2012/12/12 ed. Rockville, MD: Report no.: AHRQ Publication no. 12(13)‐EHC132‐EF; 2012. [PubMed]
- 103. Drummond M. F., Sculpher M. J., Claxton K., Stoddart G. L., Torrance G. W. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2015. [Google Scholar]
- 104. Philips Z., Bojke L., Sculpher M., Claxton K., Golder S. Good practice guidelines for decision‐analytic modelling in health technology assessment: a review and consolidation of quality assessment. Pharmacoeconomics 2006; 24: 355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Search strategy.
Table S2 Exclusion criteria.
Table S3 List of high‐income countries available at: http://data.worldbank.org/about/country‐and‐lending‐groups
Table S4 EURONHEED checklist.


