Abstract
Aims
Heart failure (HF) treatment guided by physicians with access to real‐time pressure measurement from a wireless implantable pulmonary artery pressure (PAP) sensor (CardioMEMS), has previously been shown to reduce HF‐related hospital admissions in the CHAMPION trial. However, uncertainty remains regarding the value of CardioMEMS in European health systems where healthcare costs are significantly lower than in the USA.
Methods and results
A Markov model was developed to estimate the cost‐effectiveness of PAP‐guided treatment of HF using the CardioMEMS™ HF system compared with usual care. Cost‐effectiveness was measured as the incremental cost per quality‐adjusted life year (QALY) gained. In the base case analysis over a time horizon of 10 years, PAP‐guided HF therapy increased cost compared with usual care by £10 916 (€14 030). QALYs per patient for usual care and PAP‐guided patients were 2.57 and 3.14, respectively, reflecting an increase of 0.57 QALYs with PAP‐guided treatment. The resultant incremental cost‐effectiveness ratio (ICER) is £19 274 (€24 772) per QALY gained. The base case analysis did not include staff time, due to a lack of data concerning this variable. Running the model with estimated staff time included resulted in an increased ICER of between £22 342 and £25 464 per QALY gained (€28 709–32 721).
Conclusion
The analysis indicates that integrating wireless PAP monitoring into the management of UK HF patients is likely to be a cost‐effective addition to the HF treatment pathway for appropriate patients.
Keywords: Heart failure, Pulmonary artery pressure monitoring, Telemonitoring, Cost‐effectiveness
Introduction
In Europe, between 1% and 2% of the population have heart failure (HF).1 The syndrome is associated with reduced life expectancy2 and an impaired quality of life.3 In the UK, there were 160 000 hospitalizations for HF in 2013/14,4 with an in‐hospital mortality of 9.5% and a 1‐year mortality risk of 27%.5 One estimate from England's National Institute for Health and Care Excellence (NICE) suggests that the National Health Service (NHS) spends £2.3 billion on HF (∼2% of its total budget), with 70% of this expenditure due to hospitalization.6, 7 The situation is similar in many European countries.8 The primary symptom leading to hospitalization for worsening HF is dyspnoea, related to fluid retention and/or fluid redistribution, both of which are associated with an increase in pulmonary artery (PA) pressures (PAPs).9, 10, 11, 12
A novel implantable haemodynamic sensor (CardioMEMS™ HF system, St. Jude Medical Inc., Atlanta, GA, USA) has been developed to provide physicians with remote real‐time pressure measurements from the pulmonary artery. In a randomized controlled trial (RCT) of 550 NYHA class III HF patients with a previous HF hospitalization enrolled at 64 US hospitals, doctors that used these pressure measurements to guide their patients' medical therapy (treatment group) achieved a 33% reduction in HF‐related hospitalizations over an average study duration of 15 months13 compared with the control arm who had the device implanted but where the data were not used to guide management. The CardioMEMS sensor has been approved for use in the USA by the Food and Drug Administration (FDA), and in June 2016 the Heart Failure Association of the European Society of Cardiology (ESC) included the system in the ESC guidelines for the diagnosis and treatment of acute and chronic HF, indicating that the device may be considered for monitoring symptomatic patients with a previous HF hospitalizsation in order to reduce the risk of recurrent hospitalization (class IIb recommendation, level of evidence B).14 Unlike other implantable devices, the CardioMEMS pressure sensor does not require a battery so can therefore, in theory, continue to function indefinitely.
Modelling can be used to estimate the cost‐effectiveness of CardioMEMS by comparing its outcomes and costs with those of usual care. If these outcomes are estimated as quality‐adjusted life years (QALYs), then the incremental cost‐effectiveness ratio (ICER), or cost per QALY gained, can be calculated as the difference in costs divided by the difference in QALYs. This can aid decision‐makers in determining whether a new intervention is ‘value for money’ and should be funded or reimbursed. In England, NICE typically recommends in favour of funding interventions with an ICER < £20 000/QALY, but requires considerable clinical benefit to recommend funding interventions between £20 000 and £30 000/QALY, and typically recommends against funding interventions with an ICER > £30 000/QALY. The aim of this study is to estimate the likely incremental cost per QALY of CardioMEMS compared with usual care, from a European perspective, so as to determine if this technology is likely to be recommended according to typical NICE thresholds for cost‐effectiveness.
Methods
Scope of the model
A Markov model was developed in TreeAge 2015 (TreeAge Pro 2015, R1.0. TreeAge Software, Williamstown, MA, USA) to estimate the cost‐effectiveness of PAP‐guided treatment of HF using the CardioMEMS implantable pressure sensor compared with usual care strategies. The model was applied to a hypothetical population of 20 000 patients with HF.
The clinical effectiveness of PAP‐guided treatment was derived from the pivotal RCT15 on US patients which showed a significant decrease in hospitalizations related to HF, no effect on non‐HF hospitalizations, and a trend towards mortality benefit. Costs estimated in the model included costs of the implantation procedure including the device and related complications, costs of HF‐related hospitalizations, and costs of standard care.
Cost‐effectiveness results were estimated as incremental cost per QALY gained, using mean values of 1000 probabilistic sensitivity analysis (PSA) runs, with each PSA run using different estimates for the risks, hazard ratios (HRs), costs, and utilities to capture the uncertainty in these input parameters. In effect, the computer simulation runs each of these 1000 sets of sampled values for each modelled patient (20 000); the base case results below are the mean values of these 20 million (20 000 × 1000) simulations.
Model structure
The Markov model considered two health states: ‘stable heart failure’ and ‘dead’ (Figure 1). The patients were assigned a monthly probability of death based on their age and whether they had the intervention. Each month in the model, the patients who were alive were at risk of HF hospitalization or dying. HF hospitalization was not a health state but rather an event which patients might experience, an event which had costs associated with it. Patients who experienced HF hospitalization would then transition back to alive or dead at the end of that monthly cycle. Each patient then accrued QALYs and healthcare costs according to their hospitalization and treatment status. The model used a 10‐year time horizon; the costs and QALYs were estimated using a healthcare payer perspective and discounted at an annual discount rate of 3.5%. It was assumed that patients entering the model were aged 70, the typical age of HF patients who are remotely monitored in the developed world.16
Figure 1.
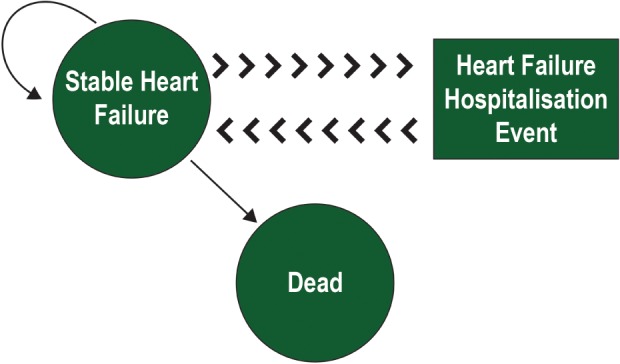
State transition diagram. The Markov model has two health states, stable heart failure (HF) and death. There is a monthly transition probability of moving from stable HF to dead state. Hospitalization is modelled as an event; there is a monthly risk of patients being hospitalized, which is used to estimate the costs/quality‐adjusted life years. The patients are assumed to revert back to stable HF after hospitalization.
Baseline mortality and hospitalization risk
The data for baseline risk of death were based on previous work by Griffiths et al. (in their 2014 model estimating cost‐effectiveness of ivabradine for the treatment of HF).17 They estimated mortality rates based on the CARE‐HF trial, an RCT conducted on NYHA class III and IV HF patients with a prior hospitalization event and adjusted using UK interim life tables and increases with age in 5‐yearly steps as shown in Table 1.
Table 1.
Summary of variables
| Variable group | Variable description | Distribution used in PSA | Mean value | Source |
|---|---|---|---|---|
| Model settings | Age of patients entering the model | 70 | ||
| Model time horizon | 10 | |||
| Costs | Cost of an implant complication | Fixed | £1090 | UK reference costs |
| Total cost of implant procedure including cost of equipment and device | Fixed | £12 000 | Estimate | |
| Monthly cost to deliver medical care | fixed | £36 | Griffiths et al. 17 | |
| Cost of a HF hospitalization | normal | £2038 | UK reference costs | |
| Hourly rate for Band 5 nurse | Not run in base case Sensitivity analysis only | £36 | PSSRU21 | |
| Hazard ratio | Hazard ratio reduction in HF hospitalization treatment cohort | Log normal | 0.67 | Abraham et al. 37 |
| Hazard ratio reduction in mortality treatment cohort | Log normal | 0.8 | Abraham et al. 37 | |
| Risk of implant complication | Fixed | 0.0272 | CHAMPION trial data | |
| Risks | Monthly risk of HF hospitalization | Beta | 0.035 | Klersy et al. 16 |
| Mortality risk age 45–50 | Beta | 0.00125 | Griffiths et al. 17 | |
| Age‐related mortality risk | Mortality risk age 50–55 | Beta | 0.00197 | Griffiths et al. 17 |
| Mortality risk age 55–60 | Beta | 0.00296 | Griffiths et al. 17 | |
| Mortality risk age 60–65 | Beta | 0.0046 | Griffiths et al. 17 | |
| Mortality risk age 65–70 | Beta | 0.00698 | Griffiths et al. 17 | |
| Mortality risk age 70–75 | Beta | 0.01044 | Griffiths et al. 17 | |
| Mortality risk age 75–80 | Beta | 0.01566 | Griffiths et al. 17 | |
| Mortality risk age 80–85 | Beta | 0.02136 | Griffiths et al. 17 | |
| Mortality risk age 85–90 | Beta | 0.02301 | Griffiths et al. 17 | |
| Mortality risk age 90+ | Beta | 0.01864 | Griffiths et al. 17 | |
| Utility values for CardioMEMS patients | Trial utility at 1 month for treatment group | Normal | 0.688 | CHAMPION trial data |
| Trial utility at 3 months for treatment group | Normal | 0.646 | CHAMPION trial data | |
| Trial utility at 6 months for treatment group | Normal | 0.617 | CHAMPION trial data | |
| Trial utility at 12 months for treatment group | Normal | 0.653 | CHAMPION trial data | |
| Utility values for usual care patients | Trial utility at 1 month for usual care | Normal | 0.645 | CHAMPION trial data |
| Trial utility at 3 months for usual care | Normal | 0.569 | CHAMPION trial data | |
| Trial utility at 6 months for usual care | Normal | 0.566 | CHAMPION trial data | |
| Trial utility at 12 months for usual care | Normal | 0.547 | CHAMPION trial data | |
| Utility value for stable HF patients | Utility for patients with chronic HF | Fixed | 0.57 | Matza et al. 19 |
| Disutility for hospitalization | Disutility for HF hospitalization after 5 years | Triangular | −0.1 | Klersy et al. 16 |
HF, heart failure; PSA, probabilistic sensitivity analysis; PSSRU, Personal Social Services Research Unit.
The baseline risk of hospitalization was based on a meta‐analysis of 21 HF telemonitoring studies of 5715 patients, median age 70.7 (range 45–78 years), with 54% of patients in NYHA class III.16 The average number of monthly HF‐related hospitalizations for patients in usual care is shown in Table 1.
Treatment effect
Hazard ratios associated with mortality and HF hospitalizations for CardioMEMS were taken from the CHAMPION trial, and were applied to the baseline monthly risk of mortality and HF hospitalization described above. The HRs used in the model are presented in Table 1. Due to the uncertainty regarding the mortality effect at this time, it was decided not to model the mortality effect beyond 5 years, after which the mortality risk is assumed to be the same for both cohorts within the model.
Prior to entering the Markov model, a small proportion (2.7%) of patients in the CardioMEMS cohort were assumed to experience an implant‐related complication, based on data from the CHAMPION trial.15
Health‐related quality of life
Health‐related quality of life (or utilities) for the patients was based on the European Quality of Life (EuroQoL) Five Dimensions, three level questionnaire (EQ‐5D‐3L) data, collected from the patients within the CHAMPION trial at 1, 3, 6, and 12 months. Mean utility values calculated over time for both cohorts (i.e. CardioMEMS and usual care) are presented in Table 1.
After 12 months, utility values were assumed to decrease at a rate of 0.008 per year, based on a longitudinal study in a population of Swedish HF patients.18 This assumption was also used in the recent Novartis submission to NICE for sacubitril–valsartan.7 Trial utilities were modelled out to 5 years, after which a utility of 0.57 was applied for chronic HF based on a recent study in the UK,19 which was again decreased at a rate of 0.008 per year thereafter.
After 5 years, when trial utilities ceased to be applied and in order to avoid double counting, a disutility for each HF hospitalization of –0.1 is applied to reflect the impact of hospitalizations on quality of life. This is similar to the disutility applied to hospitalizations from a previous cost‐utility analysis for HF technologies,20 Griffiths et al.,17 and, more recently, the Novartis submission to NICE for single technology appraisal of sacubitril–valsartan.7
Costs
As of autumn 2016, the cost of the device has not been released by the manufacturer as devices are not commercially available in Europe outside of use in clinical trials, and an implant procedure code is not available from the NHS. However, the authors have in this case assumed that the total cost of device acquisition and implant will be £12 000, based on the direct currency conversion from the average US MS‐DRG payment (DRG 264 accompanied by the ICD‐9‐CM procedure code of 38.26: insertion of implantable pressure sensor without lead for intracardiac or great vessel haemodynamic monitoring), currently set at US$17 750.
The cost of an implant complication is estimated as £1090, using a weighted average of the eight complications in the CHAMPION trial mapped to NHS reference costs.21 The cost of standard HF care (excluding hospitalizations) is £36.31 per month based on previous estimates,17 and is applied to stable HF patients in both cohorts. The cost of HF hospitalization is estimated from NHS reference costs as £2038, calculated as a weighted average of HF admissions.
Sensitivity analysis
One‐way sensitivity analysis was performed on the following parameters: HR for mortality reduction (0.55 to 1) from the lower bound of the 95% confidence interval (CI) from CHAMPION to 1 being no effect on mortality; HR for HF hospitalization (0.55 to 0.8) across the 95% CI from CHAMPION; cost of HF hospitalization (£1067 to £3884), from the lowest to highest costs of HF hospitalization in the NHS reference costs; and cost of implant procedure (±25%, £9000–£15 000).
A subgroup analysis of 119 patients in the CHAMPION trial with preserved EF (>40%) revealed that the treatment effect could be greater in these patients, reducing the hospitalization rate by 50%. This scenario was tested in the model.
The costs of HF hospitalization, implant complications, and monthly cost of treatment were acquired from several other European countries and the cohort model run using these inputs to simulate cost‐effectiveness in other countries.
Probabilistic sensitivity analysis was performed using 1000 runs employing different estimates for the risks, HRs, costs, and utilities.
Scenario analysis, including staff costs
A scenario analysis was also performed including the time spent on monitoring patients by a nurse (23–70 min per month). The lower bound of this range is from data collected by L.K. on CardioMEMS patients at his centre, and the upper bound is from a previous telemonitoring study using different technology.22 Physician time was included at 7 min per month, again from early real‐world data collected by L.K. at the University of California, San Francisco, Medical Centre. The costs for the time spent by nurses monitoring CardioMEMS patients remotely is based on an hourly rate of £36 for a Band 5 nurse, and for doctors £105/h for a medical consultant.21
Results
Base case analysis
In the base case analysis over a time horizon of 10 years, PAP‐guided HF therapy increased cost compared with usual care by £10 916 (i.e. from £6189 in usual care to £17 104 in PAP‐guided HF therapy).
The model estimated a mean survival of 4.79 years for patients being treated with usual care practices and 5.17 years for patients with treatment guided by PAP monitoring, an increase of 0.38 years (Figure 2).
Figure 2.
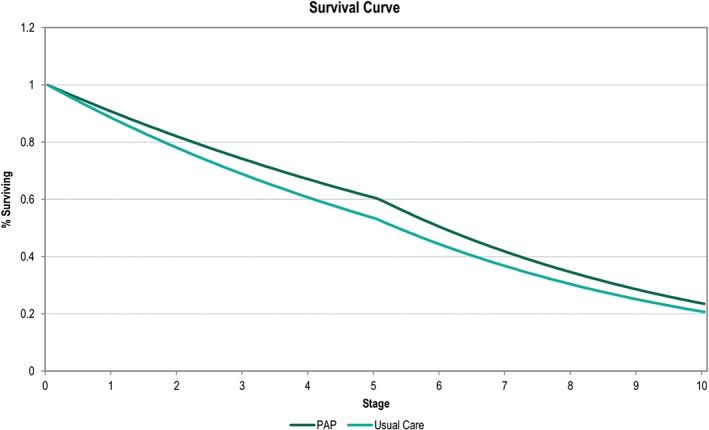
Survival curve showing the number of patients alive in the model over time. At 5 years, when the mortality effect from CHAMPION is removed from the model, the mortality rate increases. PAP, pulmonary artery pressure.
The QALYs per patient for usual care and PAP‐guided patients were 2.57 and 3.14, respectively, reflecting an increase of 0.57 QALYs with PAP‐guided treatment. The resultant ICER, the ratio between incremental costs and the QALYs, is estimated at £19 274/QALY.
Probabilistic sensitivity analysis
The results of the PSA displayed on a scatterplot (ellipse indicates 95% CI) show a tight cluster of results in the north‐east quadrant. A line indicating a willingness to pay (WTP) threshold of an ICER of £20 000 has been drawn for reference (Figure 3); this line represents the WTP threshold below which NICE typically recommends a new treatment be made available to NHS patients. Almost all of the points on the scatterplot are underneath the line, as the probability of PAP being cost‐effective at a WTP threshold of £20 000/QALY is 97.6%.
Figure 3.
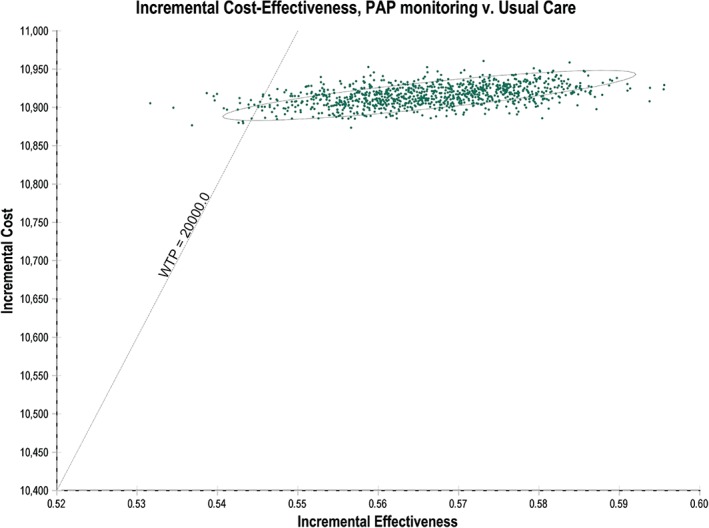
Cost‐effectiveness scatter plot. Each of the dots on the scatter plot is one of 1000 mean cost and utility results of 20 000 model runs each with different input values sampled from the input distributions. PAP, pulmonary artery pressure; WTP, willingness to pay. The WTP = £20 000/QALY (quality‐adjusted life year) is the typically accepted NICE (National Institute for Health and Care Excellence) threshold. Almost all of the samples (97.6%) fall under the WTP threshold, suggesting that CardioMEMS has a 97.6% probability of being cost‐effective at that threshold.
Sensitivity analysis
Sensitivity analyses performed do not substantially change the conclusions, as shown in Figure 4. Decreasing the mortality HR of PAP to 0.55 (from a base value of 0.8) resulted in an ICER of £11 205/QALY. This is due to a much greater difference in the expected QALYs between the PAP arm and the usual care arm when a lower HR was used. Similarly, increasing the mortality HR of PAP to 1 resulted in an ICER of £31 119/QALY. When the cost of the implant procedure was decreased to £9000 (from a base value of £12 000), the ICER went down to £14 106/QALY. Similarly, using a higher cost of implant procedure (£15 000) resulted in a higher ICER (£24 811/QALY). Decreasing the HF hospitalization HR of PAP to 0.55 (from a base value of 0.67) resulted in a lower ICER of £18 661/QALY. Again, this is due to a higher difference in the expected QALYs between the PAP arm and the usual care arm when a lower HR was used. Similarly, increasing the HF hospitalization HR of PAP to 0.8 resulted in a higher ICER of £21 191/QALY. When the cost of HF hospitalization was decreased to £1067 (from a base value of £2038), the ICER increased to £20 558/QALY. This is due to lower savings between the PAP arm and the usual care arm due to reduced HF hospitalizations when lower HF hospitalization costs were used. Similarly, the ICER decreased to £17 370/QALY when the cost of hospitalization was increased to £3884.
Figure 4.
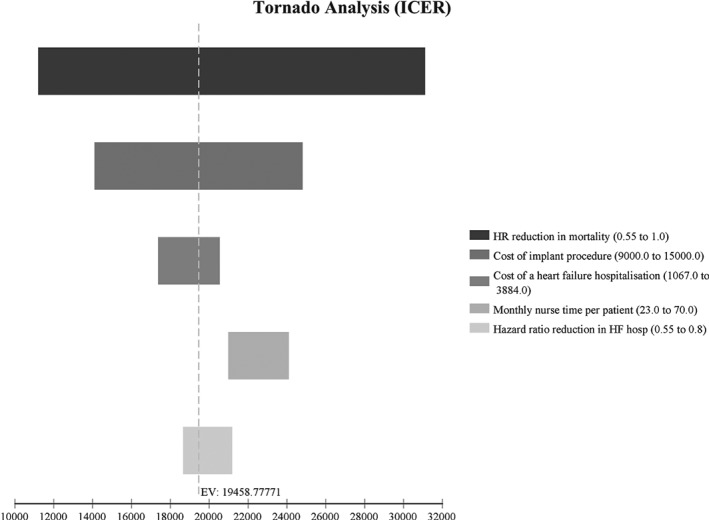
One‐way sensitivity analysis. The results of the sensitivity analysis can be compared in this tornado diagram; a larger bar indicates a greater impact on the incremental cost‐effectiveness ratio (ICER) or, in the case of mortality, indicates a wide range of uncertainty. The dotted line indicates the base case ICER. In the case of increased heart failure (HF) hospitalization costs the ICER is lower. HR, hazard ratio.
When the model was run using the increased treatment effect found in the preserved EF patients, costs for the treatment group were reduced to £16 518 and the resultant ICER was £18 169.
Comparison of model results in selected European countries
The model outputs were tested with cost inputs from four other European countries, The Netherlands, Belgium, Italy, and Germany. Comparative cost inputs and model outputs can be seen in Table 2. It should be noted that the clinical parameters were unchanged. These analyses suggest that CardioMEMS is likely to be cost‐effective in the countries selected.
Table 2.
Comparison of costs and cost‐effectiveness between countries
| UK | The Netherlands | Belgium | Italy | Germany | |
|---|---|---|---|---|---|
| Inputs | |||||
| Cost of an implant complication | £1090 | €2390 | €1630 | €3481 | €2668 |
| Cost of implant (includes device cost) | £12 000 | €15 000 | €15 000 | €15 000 | €15 000 |
| Monthly cost to deliver medical care | £36 | €99 | €27 | €148 | €231 |
| Cost of one hospital admission for HF | £2038 | €4545 | €2802 | €4500 | €4398 |
| Outputs | |||||
| Modelled cost of usual care | £6189 | €14 831 | €7187 | €17 556 | €22 121 |
| Modelled cost of PAP‐guided therapy | £17 104 | €27 472 | €20 582 | €30 483 | €35 468 |
| Incremental cost | £10 916 | €12 641 | €13 395 | €12 926 | €13 347 |
| ICER | £19 274 | €22 555 | €23 899 | € 23 064 | €23 814 |
HF, heart failure; ICER, incremental cost‐effectiveness ratio; PAP, pulmonary artery pressure.
Scenario analysis, including staff costs
Scenario analysis performed, including the costs for nurses and doctors managing the data from the pressure monitors, increased the ICERs. When it was assumed that the time spent by a nurse monitoring each PAP patient is 23 min/month and that doctors spent 7 min/month per patient, the ICER increased to £22 342/ QALY. If the time needed for nurses to manage these patients was assumed to be 70 min/month, the ICER increased to £25 464/QALY.
Discussion
The results of our cost‐utility analysis suggest that the CardioMEMS™ HF system could provide cost‐effective benefits to HF patients in the UK: the base case ICER of £19 274/QALY is below the thresholds of £20 000–£30 000 per QALY used by NICE.
The CardioMEMS device differs from previous telemonitoring technologies in that it is the first system to provide real‐time remote monitoring of PAP, with the goal of maintaining this pressure within a therapeutic range by adjusting medications in response to pressure trends. Previous remote monitoring systems have used non‐invasive measurements such as weight, heart rate, and blood pressure communicated to physicians via telephone or data links, or data from cardiac implantable electronic devices implanted for therapeutic reasons (such as an implantable cardioverter defibrillator or CRT device).
Meta‐analysis of several small studies of non‐invasive remote monitoring suggested that such approaches may reduce HF hospitalizations and perhaps mortality,23 but larger randomized trials have been neutral.24, 25, 26 A UK cost‐effectiveness analysis used modelling to compare structured telephone support where patients spoke to a human operator (usually a HF nurse), structured telephone support delivered by an automated machine, and remote monitoring of non‐invasive measurements. Remote monitoring was superior in terms of cost‐utility to either of the structured telephone support approaches, with an ICER of £9522/QALY gained over a lifetime horizon.20, 27
A recent meta‐analysis of remote monitoring of devices implanted for therapeutic reasons has suggested no change in mortality but a marked reduction in planned hospital visits, at the expense of an increase in unplanned hospital and emergency room visits.16 The CardioMEMS system directly measures PAP, and may provide an earlier indicator of decompensation,28 in addition targeting the haemodynamic congestion, rather than clinical congestion, targeted by traditional remote monitoring solutions. Besides being a new therapeutic target with drug therapies titrated to maintain PAP in a more normal range, the indicator showed improved efficacy under randomized conditions which is probaby due to the physical parameter being monitored, i.e. PAP, being better able to detect early signs of acute decompensation. Furthermore, the CHAMPION trial only included patients who were at least moderately symptomatic (NYHA class III), and perhaps with a greater potential to obtain benefit in terms of reduced hospitalization and improved quality of life than the other studies which tended also to recruit patients with milder symptoms.
Others have estimated the likely cost‐effectiveness of the CardioMEMS device in the American healthcare system. Sandhu and colleagues estimated an ICER of US$71 462 per QALY gained.29 The key differences, apart from the different costs of hospitalization in the different healthcare systems, are the assumptions relating to utilities. Sandhu et al. used the results of the disease‐specific Minnesota Living with Heart Failure (MLWHF) assessments in the CHAMPION trial, converted to EQ‐5D utilities using an algorithm, whereas the model presented here uses utility values derived directly from the EQ‐5D assessments collected in the CHAMPION trial because data quality (in terms of number of questionnaires completed) was much better for EQ‐5D. Both models extend the utility effects beyond the trial period, but in our model we decrement the effect after the initial 12‐month period, as previously described.17 Both models ascribe disutilities to hospitalization. The MLWHF results display a much lower health‐related quality of life in both the treatment and usual care cohorts and a reduced incremental effectiveness of treatment compared with that seen from the EQ‐5D responses. Sandhu et al. thereby report incremental QALYs of 0.28 over a lifetime analysis, whereas our model estimates the incremental QALYs to be 0.67 over a 10‐year time horizon.
Martinson et al. have also estimated the likely cost‐effectiveness of CardioMEMS from the US perspective over a 5‐year time horizon.30 They report a base case of US$29 593 per QALY using a payer perspective and using real‐world claims data for the cost of a HF hospitalization in the USA (US$16 770 for Medicare and US$30 100 for private insurance) rather than an assumed cost of US$12 832. They, like us, also did not map Minnesota scores across to health utility, but used the directly assessed EQ‐5D scores for health‐related quality of life.
The baseline mortality rate for HF in the usual care cohort was taken from previous work by Griffiths et al. in their model to estimate the cost‐effectiveness of ivabradine as part of a submission to NICE.17 They used the CARE‐HF trial31 to estimate age‐related mortality and we assumed that there were enough similarities between that population and the target population for CardioMEMS to use the figures as an appropriate baseline risk of death. Although CARE‐HF did not exclude NYHA class IV patients, the study population consisted of 94% NYHA class III patients. A total of 78% of CHAMPION patients had reduced EF at baseline, defined as <40%, compared with the CARE‐HF inclusion criteria of <35% and a mean EF in that trial of 26%.32 Baseline risk of hospitalization for the usual care cohort was from a meta‐analysis of randomized trials of telemonitoring of implanted devices by Klersy et al.;16 using this population probably underestimates the risk of hospitalizations and indeed the monthly risk of 3.5% used in the model is lower than the 5.8% calculated from the usual care cohort in the CHAMPION trial. We believe that this decision represents a conservative approach, and is likely to lead to an overestimate of the cost per QALY.
Recently, the first new disease‐modifying therapy for HF treatment has been approved for use and is available for doctors to prescribe in many European countries. Sacubitril–valsartan is indicated in the UK for use in patients with class II–IV symptoms and an EF of <35%. We did not attempt to model the treatment effect of this drug within this model because at this time it is at an early stage of adoption and it would be difficult to estimate the proportion of patients in our modelled population who might already be taking it or begin taking it within the modelled time period. Additionally, the effects in class III patients with a prior hospitalization (the modelled population) are not clear. In the PARADIGM trial, the primary endpoint was not significant in the subgroup of patients in NYHA class III (HR 0.93, 95% CI 0.79–1.09) nor did the drug reduce HF hospitalizations significantly in this subgroup (HR 1.08%, 95% CI 0.88–1.34).33 With these points in mind, we think that with the data available it would be difficult to model reliably how CardioMEMS and sacubitril–valsartan would interact. This lack of data is a limitation to the model at this time.
The time spent monitoring the patient also influences our estimates of cost‐effectiveness. Boyne et al. (TEHAF study)22 estimated that the telemonitoring system used in their trial required 70 min a month (an average of 2 min 20 s per day) of nurse time to triage data from patients and to take any appropriate actions; it is important to note that the technology used in the TEHAF study and the instructions on how to monitor patients were both very different from the CardioMEMS™ HF system. There are very specific recommendations for dealing with the data from the CardioMEMS™ HF system and acting upon it. The selection of the time estimate from TEHAF was made in order to demonstrate the resource impact of a labour‐intensive approach to monitoring. More recent experience over 18 months of using CardioMEMS in real‐world clinical practice at a single centre in the USA has indicated that HF nurses spend on average 23 min per patient, per month. This time includes the time spent by the nurse reviewing the tracings, calling the patient with medication changes, ordering and checking the blood tests required, and discussing data and actions with the physician. The medication changes are made using an algorithm derived from the CHAMPION study, emphasizing the judicious use of diuretics and vasodilators, as well as maximization of guideline‐based optimal medical therapy in patients with HF and reduced EF. The nurse spends an additional 10 min per month in documenting the medication changes in the electronic medical record. The physician spends on average 7 min per patient per month in reviewing the data with the nurses and setting up the treatment plan (Dr Liviu Klein, personal communication). We thought that a sensible approach to estimating the impact of introducing this technology in terms of human resources would be to use the data from Dr Klein's experience as the lower bound of a situational analysis and the figure from TEHAF as the upper bound. The ICER range of between £22 342 and £25 464/QALY indicates that the cost‐effectiveness of the technology is affected by the time that staff spend managing the data. However, this estimate is somewhat crude at this point in time because the actual impact on resources remains unknown; for this reason we did not include staff time in the base case analysis of this model; however, when more accurate data become available it must certainly be part of any future modelling. For instance, it is not yet clear whether there are associated reductions in other patient management activities (such as clinic visits or general practitioner visits) which have not been recognized. Due to the uncertainty we see in estimating this important aspect of the mode, we recommend that resource utilization be studied as part of any future registry or clinical trial in the European healthcare setting.
A subgroup analysis of patients with preserved EF indicated the possibility of greater effectiveness in this difficult to treat population.34 When the results from this analysis were used to inform the hospitalization rate in the model, the ICER decreased to £18 169. However, these results were based on only 22% of the enrolled CHAMPION population and should therefore be interpreted with caution.
All models and modelling analyses have to make assumptions and simplify reality in some way, which leads to limitations. The largest area of uncertainty in the single RCT on CardioMEMS to date is the effect on mortality. Although the 20% beneficial effect on mortality in the CHAMPION trial did not reach statistical significance, this trend is included in our base case. This approach is standard practice in economic modelling which recommends the use of point estimates even when effect differences lack conventional statistical significance.35, 36 Readers may note that the full range of the CI for mortality from the CHAMPION trial has not been modelled but rather the HR is varied from the lower bound of the CI (0.55) to 1. The values above one and the wide range of the CIs reflect that the study was underpowered to detect mortality. However, if we were to model a HR for mortality above one we would be testing the assumption that the device increases mortality and the model would produce a negative ICER, indicating that it costs money to reduce outcomes. There is of course no suggestion to date that the CardioMEMS system would increase mortality. Uncertainty around the effect on mortality using a one‐way sensitivity analysis shows that the ICER could be as high as £28 762 should the technology have no mortality benefit. Under this scenario, the higher ICER falls within the range of cost‐effectiveness where NICE would require evidence of considerable clinical benefit before making a recommendation that the technology be made available to patients in England. We have tried to make conservative choices when faced with decisions on structure and assumptions throughout the modelling process. These include selecting a baseline hospitalization rate which is likely to be below that of the population of interest and only modelling trial based utility effects to 5 years.
Cost inputs collected from other European countries show that while cost of care varies between countries, the technology remains cost‐effective within our model, with a range of €22 555–€23 899 per QALY. The new guidelines from the Heart Failure Association of the European Society of Cardiology14 recommend that monitoring using the CardioMEMS system may be considered for patients with symptomatic HF with a previous HF hospitalization. Our model suggests that such an approach is likely to be cost‐effective in the UK, at the currently considered threshold of value for money, and also in several other European countries.
Conclusion
This analysis suggests that the CardioMEMS™ HF system could provide a cost‐effective means for HF physicians to manage and treat patients outside of face‐to‐face clinic appointments, shifting care from hospital/clinic to home, reducing resource‐intensive hospitalizations, and improving the quality of life of patients suffering from HF.
Acknowledgements
We thank the CHAMPION investigators for access to the trial data.
Conflicts of interest: M.R.C. reports grants and personal fees from St. Jude Medical, during the conduct of the study; grants and personal fees from Boston Scientific and Bayer outside the submitted work; and personal fees from Medtronic, Novartis, Pfizer, Daiichi‐Sankyo, Bristol Meyers Squibb, and Servier outside the submitted work. L.K. reports grants and personal fees from St. Jude Medical outside the submitted work. M.S. is an employee of St. Jude Medical. P.T. reports personal fees from St. Jude Medical, during the conduct of the study; and personal fees from Novartis, Daiichi Sankyo, Pfizer and Vertex outside the submitted work
References
- 1. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007;93:1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jhund PS, Macintyre K, Simpson CR, Lewsey JD, Stewart S, Redpath A, Chalmers JW, Capewell S, McMurray JJ. Long‐term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population study of 5.1 million people. Circulation 2009;119:515–523. [DOI] [PubMed] [Google Scholar]
- 3. Jeon YH, Kraus SG, Jowsey T, Glasgow NJ. The experience of living with chronic heart failure: a narrative review of qualitative studies. BMC Health Serv Res 2010;10:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Townsend N, Bhatnagar P, Wilkins E, Wickramasinghe K, Rayner M. Cardiovascular Disease Statistics 2015. British Heart Foundation; 2015. [Google Scholar]
- 5. Mitchell P, Marle D, Akosua D, Shote A, McDonagh T, Hardman S, Dargie HJ, Cleland J. National Heart Failure Audit Report. NICOR; 2015. [Google Scholar]
- 6. National Institute for Health and Care Excellence . Chronic heart failure. Costing report https://www.nice.org.uk/guidance/cg108/resources/cg108-chronic-heart-failure-costing-report2.
- 7. Heart failure – sacubitril valsartan [ID822] | Guidance and guidelines: Committee Papers | NICE. https://www.nice.org.uk/guidance/indevelopment/gid-tag516/documents.
- 8. Braunschweig F, Cowie MR, Auricchio A. What are the costs of heart failure? Europace 2011;13 Suppl 2:ii13–ii17. [DOI] [PubMed] [Google Scholar]
- 9. Cotter G, Metra M, Milo‐Cotter O, Dittrich HC, Gheorghiade M. Fluid overload in acute heart failure—re‐distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail 2008;10:165–169. [DOI] [PubMed] [Google Scholar]
- 10. Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med 2006;119 ( 12 Suppl 1):S3–S10. [DOI] [PubMed] [Google Scholar]
- 11. Kato M, Stevenson LW, Palardy M, Campbell PM, May CW, Lakdawala NK, Stewart G, Nohria A, Rogers JG, Heywood JT, Gheorghiade M, Lewis EF, Mi X, Setoguchi S. The worst symptom as defined by patients during heart failure hospitalization: implications for response to therapy. J Card Fail 2012;18:524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schiff GD, Fung S, Speroff T, McNutt RA. Decompensated heart failure: symptoms, patterns of onset, and contributing factors. Am J Med 2003;114:625–630. [DOI] [PubMed] [Google Scholar]
- 13. Food and Drug Administration . CardioMEMS Champion HF Monitoring System PMA Amendment P100045. FDA and CardioMEMS Panel Package. Prepared for the October 9, 2013 Circulatory System Devices Panel Meeting. 2013; http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/CirculatorySystemDevicesPanel/UCM370692.pdf.
- 14. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 15. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS, CHAMPION Trial Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658–666. [DOI] [PubMed] [Google Scholar]
- 16. Klersy C, De Silvestri A, Gabutti G, Raisaro A, Curti M, Regoli F, Auricchio A. Economic impact of remote patient monitoring: an integrated economic model derived from a meta-analysis of randomized controlled trials in heart failure. Eur J Heart Fail 2011;13:450–459. [DOI] [PubMed] [Google Scholar]
- 17. Griffiths A, Paracha N, Davies A, Branscombe N, Cowie MR, Sculpher M. The cost effectiveness of ivabradine in the treatment of chronic heart failure from the UK National Health Service perspective. Heart 2014;100:1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berg J, Lindgren P, Mejhert M, Edner M, Dahlstrom U, Kahan T. Determinants of utility based on the EuroQol five‐dimensional questionnaire in patients with chronic heart failure and their change over time: results from the Swedish Heart Failure Registry. Value Health 2015;18:439–448. [DOI] [PubMed] [Google Scholar]
- 19. Matza LS, Stewart KD, Gandra SR, Delio PR, Fenster BE, Davies EW, Jordan JB, Lothgren M, Feeny DH. Acute and chronic impact of cardiovascular events on health state utilities. BMC Health Serv Res 2015;15:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thokala P, Baalbaki H, Brennan A, Pandor A, Stevens JW, Gomersall T, Wang J, Bakhai A, Al‐Mohammad A, Cleland J, Cowie MR, Wong R. Telemonitoring after discharge from hospital with heart failure: cost‐effectiveness modelling of alternative service designs. BMJ Open 2013;3:e003250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Personal Social Services Research Unit; 2015. [Google Scholar]
- 22. Boyne J, Vrijhoef HJ, Nieman FH, De Wit R, Kragten J, De Weerd GJ, Gorgels AP. Telemonitoring in patients with heart failure: results from a multicenter randomized controlled trial (the TEHAF study). J Am Coll Cardiol 2011;57:E389. [Google Scholar]
- 23. Inglis SC, Clark RA, Dierckx R, Prieto‐Merino D, Cleland JGF. Structured telephone support or non‐invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev 2015;10:CD007228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, Phillips CO, Hodshon BV, Cooper LS, Krumholz HM. Telemonitoring in patients with heart failure. N Engl J Med 2010;363:2301–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Bohm M, Boll H, Baumann G, Honold M, Koehler K, Gelbrich G, Kirwan BA, Anker SD. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation 2011;123:1873–1880. [DOI] [PubMed] [Google Scholar]
- 26. Ong MK, Romano PS, Edgington S, Aronow HU, Auerbach AD, Black JT, De Marco T, Escarce JJ, Evangelista LS, Hanna B, Ganiats TG, Greenberg BH, Greenfield S, Kaplan SH, Kimchi A, Liu H, Lombardo D, Mangione CM, Sadeghi B, Sadeghi B, Sarrafzadeh M, Tong K, Fonarow GC, Better Effectiveness After Transition–Heart Failure Research Group . Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the Better Effectiveness After Transition–Heart Failure (BEAT‐HF) Randomized Clinical Trial. JAMA Intern Med 2016;176:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pandor A, Thokala P, Gomersall T, Baalbaki H, Stevens JW, Wang J, Wong R, Brennan A, Fitzgerald P, Pandor A, Thokala P, Gomersall T, Baalbaki H, Stevens JW, Wang J, Wong R, Brennan A, Fitzgerald P. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess 2013;17:1–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adamson PB. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: new insights from continuous monitoring devices. Curr Heart Fail Rep 2009;6:287–292. [DOI] [PubMed] [Google Scholar]
- 29. Sandhu AT, Goldhaber‐Fiebert JD, Owens DK, Turakhia MP, Kaiser DW, Heidenreich PA. Cost‐effectiveness of implantable pulmonary artery pressure monitoring in chronic heart failure. JACC Heart Fail 2016;4:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martinson M, Bharmi R, Dalal N, Abraham WT, Adamson PB. Pulmonary artery pressure-guided heart failure management: US cost-effectiveness analyses using the results of the CHAMPION clinical trial. Eur J Heart Fail 2017;19:652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L, Cardiac Resynchronization‐Heart Failure (CARE-HF) Study Investigators . The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–1549. [DOI] [PubMed] [Google Scholar]
- 32. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Klein W, Tavazzi L, CARE‐HF study Steering Committee and Investigators . Baseline characteristics of patients recruited into the CARE‐HF study. Eur J Heart Fail 2005;7:205–214. [DOI] [PubMed] [Google Scholar]
- 33. McDowell TY, Smith K. Clinical Review NDA 207620 Entresto (Sacubitril/Valsartan). In: Products/ODEI DoCaR, editor. Online: FDA; 15 May 2015. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207620Orig1s000MedR.pdf
- 34. Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, Henderson J, Cowart P, Stevenson LW. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail 2014;7:935–944. [DOI] [PubMed] [Google Scholar]
- 35. Briggs A. Economic evaluation and clinical trials: size matters. BMJ 2000;321:1362–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Drummond MF, Sculpher MJ, Torrance GW, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. 3rd ed. Oxford: Oxford University Press; 2005. [Google Scholar]
- 37. Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB, CHAMPION Trial Study Group . Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow‐up results from the CHAMPION randomised trial. Lancet 2016;387:453–461. [DOI] [PubMed] [Google Scholar]


