Abstract
BACKGROUND
Despite advances in therapy, the majority of adult patients diagnosed with acute myeloid leukemia (AML) develop disease recurrence and die of their disease. Early intensification of treatment for AML using timed sequential therapy (TST) has been proposed as a means of improving the survival outcome in children. The Children’s Cancer Group demonstrated that children with AML who were randomized to receive 2 courses of daunorubicin, cytarabine, thioguanine, etoposide, and dexamethasone (the DCTER regimen) given 10 days apart had an improved event-free survival (EFS) and disease-free survival (DFS) (42% ± 7% and 55% ± 9%, respectively, at 2 years). Reports have suggested an improved outcome in adult patients with AML using TST (at the cost of increased toxicity). The current study was conducted to evaluate the feasibility and effectiveness of the intensively timed DCTER regimen for non-core–binding factor AML in adult patients aged <50 years.
METHODS
Between February 2004 and August 2005, 61 patients received this timed sequential induction regimen. Their outcomes were compared with matched historical patients treated with the combination of idarubicin and cytarabine (IA).
RESULTS
The median follow-up for surviving patients was 67 months (range, 35-85 months). The timed sequential DCTER regimen had a lower complete remission (CR) rate when compared with the IA combination, (71% vs 80%, respectively), but this appeared to be counterbalanced by a higher long-term leukemia-free survival rate using the intensified regimen (48% vs 30%, respectively) in patients who achieved a CR (P = .06).
CONCLUSIONS
The intensively timed regimen of DCTER was found to induce durable remissions in adult patients with AML, including those patients with high-risk disease. The identification of patients who would potentially benefit from such an intensive regimen may justify the higher early risk of early treatment failure that was found to accompany the intensified DCTER regimen in selected patients.
Keywords: intensively timed chemotherapy, acute myeloid leukemia (AML), remission, outcome
Several strategies have been evaluated to improve the outcome of patients with acute myeloid leukemia (AML), including the addition of novel agents to standard chemotherapy regimens or their intensification by the administration of a second course of chemotherapy in induction before bone marrow recovery, or timed sequential therapy (TST).TST delivers a second course of chemotherapy on Day 10 of treatment, presumably when noncycling blasts are recruited to the cell cycle, to maximally kill residual leukemia cells.1,2 In children with de novo AML, TST has been shown to improve event-free survival (EFS) and overall survival (OS).3 In a large study by the Acute Leukemia French Association (ALFA), a different TST strategy using cytarabine and daunorubicin did not appear to improve EFS or OS in adult patients with AML. However, patients aged <35 years who were treated on the trial did have a significantly longer recurrence-free interval.4 Others have reported that although TST could induce disease remission in patients, it did not improve OS.5 Buchner et al administered a second course of chemotherapy at Day 21 of induction regardless of the patients’ white blood cell (WBC) count and concluded that such double induction, given before bone marrow recovery, provided effective treatment in adults with AML.6 Further support for induction TST comes from its use in patients with primary refractory AML and in patients with first disease recurrence, in whom TST may successfully induce remission.7-9 The main concern with TST in adults is excessive mucosal or myelosuppressive toxicity and consequent infection and death.7 Although the ALFA trial did not report such toxicity, other investigators have reported excess morbidity in adult patients receiving TST.4,10,11 Nonetheless, given the results noted with the combination of daunorubicin, cytarabine, thioguanine, etoposide, and prednisone (DCTER regimen), and before the ALFA trial was reported, we conducted a phase 2 trial of TST in adult patients aged ≤50 years with newly diagnosed AML.
MATERIALS AND METHODS
Patient Eligibility
Patients with newly diagnosed AML or high-risk myelodys-plastic syndrome (MDS) (using the World Health Organization criteria) who were ages 1 to 50 years were eligible. Patients with acute promyelocytic leukemia or patients with core-binding factor (CBF) leukemias (inv16, t[16;16] or t[8;21]) were ineligible. Patients with a rapidly rising WBC count could be enrolled and receive the first course of the DCTER regimen but could not continue to the Day 10 treatment if they were found to have any of the favorable cytogenetic abnormalities noted earlier.
There were no eligibility constraints for organ function, such as elevated creatinine or bilirubin, and there were no restrictions for performance status (PS) at the time of diagnosis. The study was approved by The University of Texas M. D. Anderson Cancer Center institutional review board, and informed consent was obtained for each patient entering the trial, following the guidelines of the Declaration of Helsinki.
Treatment Regimen
All patients were offered induction therapy in high efficiency particulate air (HEPA)-filtered rooms (protected environment). The regimen is summarized in Table 1. Treatment was comprised of 4 identical cycles of chemotherapy, with the first 2 cycles designated as Course 1 and the next 2 cycles designated as Course 2. Each cycle was comprised of daunorubicin at a dose of 20 mg/m2/day on Days 1 through 4, cytarabine at a dose of 200 mg/m2/day as a continuous infusion on Days 1 through 4, etoposide at a dose of 100 mg/m2/day as a continuous infusion on Days 1 through 4, 6-thioguanine at a dose of 100 mg/m2/day by mouth daily on Days 1 through 4, dexamethasone at a dose of 6 mg/m2/day by mouth daily on Days 1 through 4, and intrathecal cytarabine at a dose of 70 mg on Day 1. As in the pediatric trial, granulocyte–colony-stimulating factor (G-CSF) at a dose of 5 μg/kg subcutaneously was administered daily beginning on Day 7 and continued until the absolute neutrophil count (ANC) was >1000/μL for 2 consecutive days in an effort to shorten the duration of severe aplasia; the use of G-CSF was not intended to recruit malignant cells into the cell cycle. The second cycle of chemotherapy was scheduled to begin on Day 11. Only those patients with hypotension (systolic blood pressure <90 mm Hg) or documented colitis did not proceed to the second cycle on Day 11. Colitis was considered to be present if fever, abdominal tenderness, abnormal bowel sounds, and an abnormal computed tomography (CT) scan consistent with colitis were noted. Those patients with a delay in Cycle 2 of chemotherapy were restarted on treatment if their blood pressure stabilized or the colitis was resolving clinically before Day 21 of Course 1 or Course 2. Dose modifications in subsequent cycles for grade 3 to 4 nonhematologic toxicity or grade 3 to 4 prolonged myelosuppression were allowed at the discretion of the treating physician (grading determined according to the Common Toxicity Criteria for Adverse Effects v3.0 staging system). Patients remained as inpatients until Day 35 of therapy or until the ANC was >1000/μL, whichever occurred first. Prophylactic antibiotics were not initiated until Day 10 of therapy to avoid drug interactions. A bone marrow aspirate was obtained just before Course 1 of Cycle 2. Generally, if there was doubt regarding whether the bone marrow was recovering with blasts or with a high number of normal, immature precursors, a repeat bone marrow aspirate was performed before declaring the patient as responding or resistant to therapy. The bone marrow aspirate was then repeated between Days 28 and 42 to confirm disease remission.
Table 1.
The DCTER Regimen
| Induction |
| Daunorubicin, 20 mg/m2/d on D 1-4 and 11-14 |
| Cytarabine, 200 mg/m2/d on D 1-4 and 11-14 |
| Thioguanine, 100 mg/m2/d on D 1-4 and 11-14 |
| Etoposide, 100 mg/m2/d on D 1-4 and 11-14 |
| Dexamethasone, 6 mg/m2/d on D 1-4 and 11-14 |
| Intrathecal cytarabine, 70 mg on D 1 and 11 |
| Repeat above induction as above if patient enters remission |
| Consolidation |
| Cytarabine, 3 g/m2 every12 h on D 1 and 2 and D 8 and 9 |
| E. coli asparaginase, 6000 IU on D 2 and 9 |
DCTER indicates daunorubicin, cytarabine, thioguanine, etoposide, and prednisone; E. coli, Escherichia coli.
Patients in complete remission (CR) after the second course of chemotherapy who had recovered to an ANC >1000/μL were then given 2 courses of consolidation chemotherapy with cytarabine at a dose of 3 g/m2 administered intravenously over 3 hours twice daily on Days 1, 2, 8, and 9. L-asparaginase was given 6 hours after the final dose of cytarabine on Days 2 and 9 as described by Capizzi et al.12 The above consolidation course was repeated at the time of recovery of the ANC to 500/μL and the platelet count to 50 × 103/μL. The dose schedule for children ages 1 to 3 years was written in mg/kg, but no patients aged <14 years were enrolled.
Patients who did not achieve a CR at the end of the induction TST were considered to be resistant and were taken off the study.
Definitions
A CR was regarded as <5% blasts in the bone marrow aspirate with an ANC >1000/μL and a platelet count >100 × 103/μL with morphologically normal bone marrow and the absence of extramedullary disease. Induction deaths were those occurring after registration on study and before the initiation of the second course of chemotherapy. Postremission treatment-related deaths were defined as deaths in patients in first CR occurring after the completion of the first 2 intensively timed cycles of chemotherapy. Recurrence-free survival (RFS) was calculated as the time in remission from CR until disease recurrence. OS was defined as the time from first diagnosis until death from any cause.
Statistical Analysis
The primary endpoint of this phase 2 trial was to evaluate the RFS in adult patients with AML. The CR rate and the OS rate were secondary endpoints. Because of concerns over toxicity, safety monitoring with stopping rules for early death or ineffectiveness were instituted for both the intermediate-risk population and the population with a poor prognosis according to the baseline expectations for these groups. We defined intermediate risk as patients with normal cytogenetics or any karyo-type other than −5/−7 or complex, whereas poor prognosis was based on the presence of −5 or −7 or complex cytogenetics (≥3 abnormalities). Safety monitoring for each subgroup was performed after enrollment in increments of 5 patients. The criteria for stopping the study early were not met and the study accrual proceeded without interruption. RFS and OS curves were generated using the Kaplan-Meier method. These curves were then compared with historical survival curves for similarly aged patients with AML who were treated with idarubicin (12 mg/m2 daily × 3 days) plus cytarabine (1.5 g/m2 daily × 4 days) at The University of Texas M. D. Anderson Cancer Center using the log-rank test. CR rates were compared using the Fisher exact test.
RESULTS
Patient Characteristics
Table 2 details patient characteristics at the time of diagnosis. The trial began in February 2004 and enrollment ended in August 2005. Sixty-one patients were enrolled on the study, and the median follow-up for the surviving patients was 67 months (range, 35 months-85 months). The majority of patients had a PS of 0 to 1 (54 patients; 89%), and 7 (11%) patients with a PS of 2 to 3 were enrolled. The median age was 41 years (range, 14 years-49 years). The median presenting WBC count was 7.9 thousand/μL (range, 0.7-300.5/μL). The median platelet count was 54 thousand/μL (range, 4-295/μL). Thirteen (21%) patients were classified as having poor-risk disease because of the presence of −5/ −7 or complex abnormalities on cytogenetic evaluation, and 3 (5%) patients had insufficient metaphases at the time of diagnosis. Using the Medical Research Council criteria for standard-risk and poor-risk cytogenetics, there were 15 high-risk patients and 43 standard-risk patients.13 One patient did not undergo cytogenetic testing and 2 patients had insufficient metaphases. Fifteen (25%) patients had either refractory anemia with excess blasts (RAEB) or RAEB in transformation. One patient presented with central nervous system involvement manifested by the presence of leukemic cells in the spinal fluid. Three patients had AML not otherwise classified.
Table 2.
Patient Characteristics
| Patient Characteristics (n=61) | No. (%) |
|---|---|
| Male:female ratio | 33:28 |
| Median age (range), y | 41 (14-49) |
| Median WBC, ×109/L, (range) | 7.9 (0.7-300.5) |
| Median platelet count, ×109/L (range) | 54 (4-295) |
| ECOG Performance status | |
| 0-1 | 54 |
| 2-3 | 7 |
| Cytogenetics | |
| Diploid | 24 (39) |
| -5/-7 | 13 (21) |
| Miscellaneous | 21 (34) |
| Insufficient/not done | 3 (5) |
| FAB classification | |
| M0 | 3 (5) |
| M1 | 11 (18) |
| M2 | 10 (16) |
| M4 | 8 (13) |
| M5 | 4 (7) |
| M6 | 5 (8) |
| M7 | 2 (3) |
| RAEB | 7 (11) |
| RAEB-T | 8 (13) |
| Unclassified | 3 (5) |
WBC indicates white blood cell count; ECOG, Eastern Cooperative Oncology Group; FAB, French-American-British; RAEB, refractory anemia with excess blasts; RAEB-t, RAEB in transformation.
Response to Intensified Induction
The first 2 patients treated on the protocol did not receive the Day 11 therapy because of severe diarrhea. Two other patients did not receive full doses of thioguanine because of grade 3 diarrhea. Two patients died with pneumonia of an unidentified pathogen before Day 11 therapy. All subsequent patients proceeded to receive the full regimen, including Day 11 therapy. Overall, 43 (70%) patients achieved a CR. Five (8%) patients died during or shortly after the first 2 courses of chemotherapy at Days 2, 6, 66, 71, and 74, respectively, without achieving count recovery. Thirteen (21%) patients had no blast reduction in the bone marrow or had increased blasts in the bone marrow at Day 20 of therapy, and were regarded as having disease that was resistant to therapy. Four (7%) of the resistant patients underwent transplantation, and 1 patient was alive at the time of last follow-up. The median time to achieving a CR was 36 days (range, 27 days-98 days). The 5 induction deaths were attributed to infection; 3 were caused by Candida species (1 albicans, 1 krusei, and 1 glabrata) and 2 were caused by bacterial sepsis (Enterococcus and Pseudomonas). Among the 13 patients with poor-risk disease, 2 (15%) died during induction and 3 (23%) had disease that was refractory to therapy. Eight (62%) patients achieved a CR, 2 of whom were alive at the time of last follow-up. Three patients with poor-risk cytogenetics underwent transplantation in first CR, and 2 patients underwent transplantation with refractory disease.
Toxicity During Induction
The principal grade 3-4 toxicities that occurred during intensified induction are listed in Table 3. The most common nonhematologic, grade 3-4 toxicities were diarrhea and colitis, which were encountered in 23 (38%) and 17 (28%) patients, respectively. Hyperbilirubinemia was also relatively common, occurring in 13 (21%) patients.
Table 3.
Grade 3-4 Toxicities
| Toxicity | No. | % |
|---|---|---|
| Cellulitis | 1 | 2 |
| Colitis | 17 | 28 |
| Diarrhea | 23 | 38 |
| DVT | 1 | 2 |
| Edema | 2 | 3 |
| Fatigue | 2 | 3 |
| Hemorrhage (gastrointenstinal) | 1 | 2 |
| Hemorrhage (vaginal) | 1 | 2 |
| Hepatic | 13 | 21 |
| Hypoxia | 1 | 2 |
| Mental/hallucination | 1 | 2 |
| Mucositis | 1 | 2 |
| Nausea/vomiting | 10 | 16 |
| Pain (headache, rectal, abdominal) | 6 | 10 |
| Platelet reaction | 1 | 2 |
| Pleural effusion | 1 | 2 |
| Rash | 3 | 5 |
| Renal | 1 | 2 |
| Respiratory failure | 3 | 5 |
| Thrombosis | 1 | 2 |
DVT indicates deep vein thrombosis.
Postinduction Results
At the time of last follow-up, 21 patients had died after achieving a CR after 2 cycles of intensified induction; 18 patients developed disease recurrence and died of leukemia and 3 died in CR. In total, 36 patients who were enrolled died of either toxicity or disease recurrence. Of the remaining 25 (41%) patients, 3 developed disease recurrence and subsequently underwent transplantation. These transplanted patients were all free of disease at the time of last follow-up, after >4 years off of therapy.
Long-Term Outcome
Overall, 36 (59%) patients had died either of toxicity or disease recurrence at the time of last follow-up. At the time of last follow-up, all of the remaining 25 (41%) patients were at least 4 years beyond the time of initial therapy. Figure 1 shows the OS for patients treated on this regimen compared with a historical population matched for age, cytogenetic risk group (no low-risk patients were included), and performance status who were treated with idarubicin and high-dose cytarabine (IA). Because the protocols did not run concurrently, the treatment year could not be matched. Figure 2 shows the RFS for patients on the DCTER protocol versus those in the historical IA population. There was no statistically significant difference found between the OS or the RFS of the 2 groups (P ≥.5 for both comparisons). For potential cure, when considering all patients, the 95% confidence interval for the difference between the 2 treatments is -0.9 to 0.34.
Figure 1.
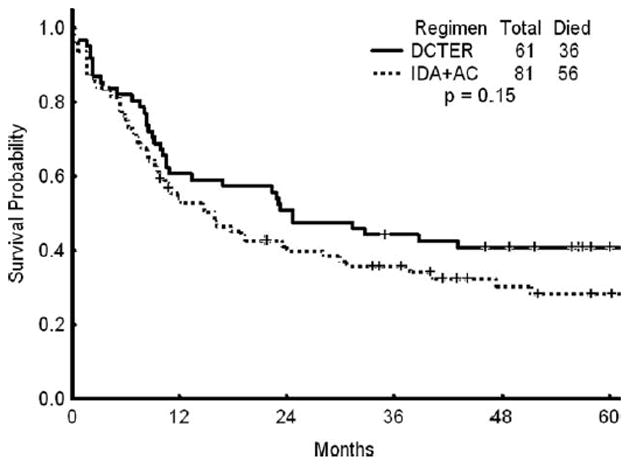
Overall survival is shown for patients treated with the combination of daunorubicin, cytarabine, thioguanine, etoposide, and prednisone (DCTER regimen) versus those treated with idarubicin (IDA) and cytarabine (AC).
Figure 2.
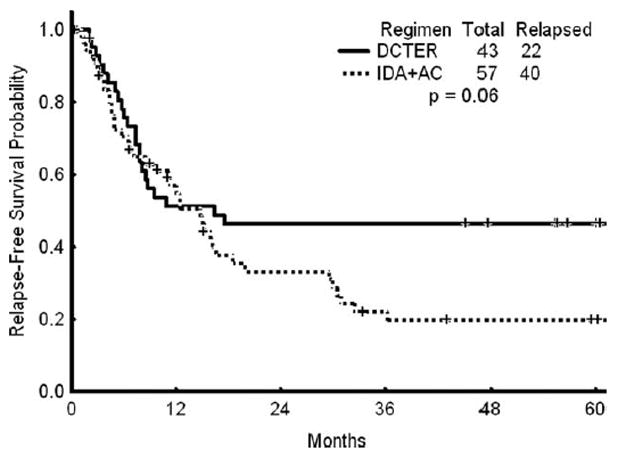
Recurrence-free survival in patients treated with the combination of daunorubicin, cytarabine, thioguanine, etoposide, and prednisone (DCTER regimen) versus those treated with idarubicin (IDA) and cytarabine (AC).
DISCUSSION
In this phase 2 study, we implemented a pediatric regimen of intensively TST for AML in adults with intermediate-risk and poor-risk AML and high-risk MDS who were aged <50 years. Concerns about toxicity in the group of AML patients with a relatively favorable prognosis with CBF-AML and AML-M3 precluded the inclusion of these patients in this trial. Overall, the DCTER regimen produced a RFS rate of 48%. This compares favorably with the outcome of a matched cohort of patients treated with IA. However, this is at the cost of a greater number of induction failures because of disease resistance and infectious deaths. This induction mortality occurred despite the use of reverse isolation inside a laminar air flow protective environment on a specialized adult leukemia ward. The majority of deaths and toxicities were related to gastrointestinal complications. We did not initiate infection prophylaxis until Day 10 of therapy because prior data had suggested that infectious death is unusual before Day 10. The earlier use of infection prophylaxis may have potentially decreased the frequency of gastrointestinal morbidity and mortality, and could be considered in future studies of patients undergoing intensively TST for AML. Nevertheless, only 2 deaths occurred before the initiation of infection prophylaxis; the delay does not appear to have been too detrimental to patient outcome.
For patients with poor-prognosis AML, intensively TST using the DCTER regimen was found to be effective in inducing durable remissions. The number of patients in the current study was too small to make a conclusive statement concerning the OS and RFS rates of the high-risk individuals. However, 2 of 8 high-risk patients who achieved CR remained alive and free of disease at the time of last follow-up, >4 years after therapy without transplantation. Durable remissions in high-risk AML patients using intensively TST have been previously reported by both Geller et al and Braess et al.14,15 This finding would suggest that intensively TST for AML may be an effective modality in selected high-risk patients. This is in contrast to the recently published data from the Eastern Cooperative Oncology Group (ECOG) that used a higher anthracycline dose in initial AML therapy and found that high-risk patients did not benefit from the intensified anthracy-cline.16 Information regarding FLT3 mutation status and nucleophosmin-1 (NPM-1) mutation status in these patients was not available at the time of the study. To the best of our knowledge, how adult patients with or without these mutations would fare when treated with intensively timed induction of the DCTER regimen is not known. Once patients achieve remission on intensively TST, the RFS appears to compare favorably to that of patients treated with IA. It is interesting to note that the IA regimen contains high-dose cytarabine, which in itself has been associated with an improved survival in younger patients with AML compared with standard regimens containing standard-dose cytarabine.17 This would suggest that if measures to combat early death could be used, intensively timed use of the DCTER regimen might be an acceptable option for a younger adult with newly diagnosed standard-risk or high-risk AML.
Finally, intensively TST using the DCTER regimen has perhaps reached a limit with regard to the intensity of conventional chemotherapy. It is clear that for certain patients with AML, intensification of the induction regimen may provide benefit. However, for other patients, particularly those with chromosome 5 and 7 abnormalities and complex cytogenetics, increasing the intensity of conventional chemotherapy agents appears to provide no additional benefit and is associated with increasing toxicity. We have previously demonstrated that patients with such cytogenetic abnormalities appear to have an improved outcome when hypomethylating agents are used for their initial treatment.18 Furthermore, the introduction of agents with specific activity against the molecular targets responsible for neoplastic transformation in the leukemic cells such as the FLT3 tyrosine kinase inhibitor and their incorporation into the currently available induction regimens may benefit specific subsets of patients with AML.19,20 The identification of those patients most likely to benefit from chemotherapy intensification and reserving such regimens for those patients, as well as strategies to minimize the initial toxicity associated with these regimens, is likely to be the most effective way of deriving benefit from such a strategy.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
References
- 1.Burke PJ, Karp JE, Braine HG, Vaughan WP. Timed sequential therapy of human leukemia based upon the response of leukemic cells to humoral growth factors. Cancer Res. 1977;37:2138–2146. [PubMed] [Google Scholar]
- 2.Karp JE, Donehower RC, Enterline JP, et al. In vivo cell growth and pharmacologic determinants of clinical response in acute myelogenous leukemia. Blood. 1989;73:24–30. [PubMed] [Google Scholar]
- 3.Woods WG, Kobrinsky N, Buckley JD, et al. Timed-sequential induction therapy improves postremission outcome in acute myeloid leukemia: a report from the Children’s Cancer Group. Blood. 1996;87:4979–4989. [PubMed] [Google Scholar]
- 4.Castaigne S, Chevret S, Archimbaud E, et al. Randomized comparison of double induction and timed-sequential induction to a “3 + 7” induction in adults with AML: long-term analysis of the Acute Leukemia French Association (ALFA) 9000 study. Blood. 2004;104:2467–2474. doi: 10.1182/blood-2003-10-3561. [DOI] [PubMed] [Google Scholar]
- 5.Kalaycio M, Advani A, Pohlman B, et al. Timed sequential induction chemotherapy and risk-adapted postremission therapy for acute myelogenous leukemia. Am J Hematol. 2008;83:831–834. doi: 10.1002/ajh.21260. [DOI] [PubMed] [Google Scholar]
- 6.Buchner T, Hiddemann W, Wormann B, et al. Double induction strategy for acute myeloid leukemia: the effect of high-dose cytarabine with mitoxantrone instead of standard-dose cytarabine with daunorubicin and 6-thioguanine: a randomized trial by the German AML Cooperative Group. Blood. 1999;93:4116–4124. [PubMed] [Google Scholar]
- 7.Martino R, Guardia R, Altes A, Sureda A, Brunet S, Sierra J. Time sequential chemotherapy for primary refractory or relapsed adult acute myeloid leukemia: results of the phase II Gemia protocol. Haematologica. 1999;84:226–230. [PubMed] [Google Scholar]
- 8.Revesz D, Chelghoum Y, Le QH, Elhamri M, Michallet M, Thomas X. Salvage by timed sequential chemotherapy in primary resistant acute myeloid leukemia: analysis of prognostic factors. Ann Hematol. 2003;82:684–690. doi: 10.1007/s00277-003-0730-1. [DOI] [PubMed] [Google Scholar]
- 9.Archimbaud E, Thomas X, Leblond V, et al. Timed sequential chemotherapy for previously treated patients with acute myeloid leukemia: long-term follow-up of the etoposide, mitoxantrone, and cytarabine-86 trial. J Clin Oncol. 1995;13:11–18. doi: 10.1200/JCO.1995.13.1.11. [DOI] [PubMed] [Google Scholar]
- 10.Petersdorf SH, Rankin C, Head DR, et al. Phase II evaluation of an intensified induction therapy with standard daunomycin and cytarabine followed by high dose cytarabine for adults with previously untreated acute myeloid leukemia: a Southwest Oncology Group study (SWOG-9500) Am J Hematol. 2007;82:1056–1062. doi: 10.1002/ajh.20994. [DOI] [PubMed] [Google Scholar]
- 11.Cassileth PA, Lee SJ, Litzow MR, et al. Intensified induction chemotherapy in adult acute myeloid leukemia followed by high-dose chemotherapy and autologous peripheral blood stem cell transplantation: an Eastern Cooperative Oncology Group trial (E4995) Leuk Lymphoma. 2005;46:55–61. doi: 10.1080/10428190412331283288. [DOI] [PubMed] [Google Scholar]
- 12.Capizzi RL, Davis R, Powell B, et al. Synergy between high-dose cytarabine and asparaginase in the treatment of adults with refractory and relapsed acute myelogenous leukemia-a Cancer and Leukemia Group B Study. J Clin Oncol. 1988;6:499–508. doi: 10.1200/JCO.1988.6.3.499. [DOI] [PubMed] [Google Scholar]
- 13.Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- 14.Geller R, Burke P, Karp J, et al. A 2-step timed sequential treatment for acute myelocytic leukemia. Blood. 1989;74:1499–1506. [PubMed] [Google Scholar]
- 15.Braess J, Spiekermann K, Staib P, et al. Dose-dense induction with sequential high-dose cytarabine and mitoxantrone (S-HAM)and pegfilgrastim results in a high efficacy and short duration of critical neutropenia in de novo acute myeloid leukemia: a pilot study of the AMLCG. Blood. 2009;113:3903–3910. doi: 10.1182/blood-2008-07-162842. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361:1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kern W, Estey EH. High-dose cytosine arabinoside in the treatment of acute myeloid leukemia: review of 3 randomized trials. Cancer. 2006;107:116–124. doi: 10.1002/cncr.21543. [DOI] [PubMed] [Google Scholar]
- 18.Ravandi F, Issa JP, Garcia-Manero G, et al. Superior outcome with hypomethylating therapy in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome and chromosome 5 and 7 abnormalities. Cancer. 2009;115:5746–5751. doi: 10.1002/cncr.24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stone RM, DeAngelo DJ, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 20.Ravandi F, Cortes J, Jones D, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol. 2010;28:1856–1862. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]


