Abstract
Importance
Physicians often must decide whether to treat acute stroke patients locally, or refer them to a more distant Primary Stroke Center (PSC). There is little evidence on how much the increased risk of prolonged travel time offsets benefits of specialized PSC care.
Objective
To examine the association of case-fatality with receiving care in PSCs for stroke patients, and to identify whether prolonged travel time offsets the effect of PSCs.
Design
Retrospective cohort study of stroke patients (2010–2013). Drive times were calculated based on ZIP centroids, and street-level road network data. We used an instrumental variable (differential travel time to PSC) analysis to control for unmeasured confounding.
Setting
100% sample of Medicare fee-for-service claims
Participants
Medicare stroke patients admitted in 2010–2013
Exposure
Admission to PSC
Main outcomes
7-day, and 30-day post-admission case-fatality rate
Results
Among 865,184 stroke patients (mean age 78.9 years, 55.5% females), 53.9% were treated in PSCs. We found that admission to PSCs was associated with 1.8% lower 7-day (95% CI, −2.1% to −1.4%), and 1.8% lower 30-day case-fatality (95% CI, −2.3% to −1.4%); or 56 stroke patients needed to be treated in PSCs to save one life at 30 days. Receiving treatment in PSCs was associated with a 30-day survival benefit for patients traveling less than 90 minutes, but traveling more than 90 minutes offset any benefit of PSC care.
Conclusions and Relevance
Hospitalization of stroke patients in PSCs was associated with decreased 7- and 30-day case-fatality in comparison to non-certified hospitals. Travelling for more than 90 minutes to receive care offset the 30-day survival benefit of PSC admission.
Keywords: primary stroke center, case fatality rate, travel times, Medicare, stroke, resource utilization, instrumental variable
INTRODUCTION
Stroke is one of the leading causes of death and long-term disability in the United States.1,2 In an effort to maximize positive outcomes, referral centers have been established to ensure adherence to guidelines and efficient delivery of disease-specific care.3 The backbone of this effort is the certification of Primary Stroke Centers (PSCs) by The Joint Commission (TJC). Several groups have demonstrated a small case-fatality benefit from stroke center hospitalization for hemorrhagic and ischemic stroke patients.4–6 However, previous studies were either based on regional centers of excellence (not certified by a national agency),6 or did not adjust for unmeasured confounders.
Positive outcomes for stroke patients depend on the timely administration of thrombolytics, evaluation for endovascular treatment, neurosurgical consultation in cases of hemorrhage, and targeted neurocritical care. The implementation of regionalization incentives (directing all stroke patients to PSCs), similar to other areas of medicine, 7–10 can have a significant impact on travel times and outcomes. This is of particular importance when considering the well-recognized access disparities for PSCs across states.11–14 Thus the potential benefit of an admission to a PSC needs to be weighed against the impact of longer travel times. Previous literature15 has not addressed this question, leaving a critical knowledge gap for the emergency systems involved in the care of stroke patients.
We used a national cohort of Medicare patients in order to identify how much the increased risk of longer travel time offsets potential benefits of specialized PSC care. We used real US road network information for travel time calculations, and evaluated the association of 7- and 30-day case-fatality rates with receiving care in a PSC, using an instrumental variable analysis based on the differential travel time to a PSC versus a non-PSC hospital.
METHODS
Cohort creation
Our study was approved by the Dartmouth Committee for Protection of Human Subjects. We used data from a 100% sample of Medicare beneficiaries enrolled in fee-for-service programs or non-risk bearing health maintenance organizations from 2010–2013 to identify stroke cases, classified as primary inpatient code 430.xx, 431.xx, 433.xx, or 434.xx of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) in inpatient Medicare Claims. Exclusion criteria can be found in Figure S1.
Data Sources
A PSC certification is awarded by TJC, based on guidelines from the Brain Attack Coalition and the American Heart/Stroke Association. The list of TJC-certified PSCs is publicly available, and was accessed via http://www.jointcommission.org for the year 2010. Admission to a PSC was determined by the certification status of the first hospital to which the patient was admitted, and not by subsequent transfers.
Although we had no information on the patient’s location at the time of the stroke, the Framingham study16 has demonstrated that most strokes happen at home. Population-weighted ZIP Code centroids (points) were used to represent patient origins (2010 data; Maponics, White River Junction, VT).
Latitude and longitude coordinates of hospitals, using the 2010 AHA hospital file, were utilized as possible destinations. PSC locations were matched to AHA hospital locations. All centroids were referenced to the WGS84 datum.
Outcome and covariates
Our primary outcomes were 7-day and 30-day post-admission case-fatality. The date of death was determined based on the Medicare Denominator File. Age categories (65–69, 70–74, 75–79, 80–84, 85–99), ethnicity and race categories (white, black, Asian, and other, based on self-reporting), as well as stroke type (ischemic, intracerebral hemorrhage (ICH), and subarachnoid hemorrhage (SAH)) were created. We created quintiles of ZIP code income based on a five-year panel of the American Community Survey (2007–2011). Poverty rate (based on ACS data) was also included to reflect the differing distribution of income within the zip code.
Comorbidities, for which outcomes were adjusted (eTable 1), included: myocardial infarction, arrhythmia, congestive heart failure, hyperlipidemia, coagulopathy, hypertension, peripheral vascular disease, tobacco use, diabetes, and chronic renal failure. Comorbidies were determined based on the immediate prior 6-month look-up period. Hierarchical condition categories (HCCs) during the 6 months prior to admission were created based on the SAS code provided by the Centers for Medicare and Medicaid Services (CMS). While the purpose of HCCs was to create a risk-adjustment approach for expenditures, they are also a highly predictive measure of case-fatality.17 The HCCs were divided into quintiles for the analysis. We used ICD-9 codes to identify use of thrombolytics (99.10) and mechanical thrombectomy (39.74).
Assessment of ground travel times
Street-level network data, from ESRI’s StreetMap North America v10.2, were used to calculate the optimal travel time routes. Travel time paths and their distances between origin and destination points were calculated to find the optimal routes using ESRI’s ArcGIS software with the Network Analyst extension. Total travel time calculations were adjusted for population density.12 (Supplemental Methods)
To comply with CMS reporting requirements, a minimum of 11 stroke patients per ZIP code was required for maps showing geographic location of stroke patients. The patterns are very similar to those with no minimum cell size.
Statistical analysis
The primary analysis examined the association of receiving treatment in a PSC with the 7-day and 30-day case-fatality rate, with all analysis done at the individual patient level. Classic observational techniques are limited by nonrandom selection of patients to hospitals. For example if patients with more unobserved confounding factors are more likely to be transferred to PSCs, the estimated benefit of PSCs will be biased downward. We attempted to address this unmeasured confounding using an instrumental variable approach, which has been employed in multiple prior studies of comparative effectiveness research.18–20
Given the likelihood that stroke patients will be taken to the nearest hospital, we use the differential travel time of the patient to the closest PSC versus the closest non-PSC hospital as a “natural randomization” to assign patients to a PSC (treatment) or non-PSC (control) hospital. It is calculated by subtracting the travel time of the patient’s location to the closest PSC hospital from the travel time of the patient’s location to the closest non-PSC institution. Differential distance is the most widely accepted instrument used in the literature, and is a strong predictor of hospital admission.18,21,22 The standard rule23 for a strong instrument is that the F-statistic for the association of the instrument and exposure exceeds 10. In our case, it exceeded 1600 for all analyses.
The second key assumption is that our instrument is not associated with unmeasured health status (exclusion restriction criterion). We consider the plausibility of this assumption by testing whether those living relatively closer to PSCs have similar underlying measured illness compared to those living further from PSCs. For this purpose, we used our full set of risk-adjusters to estimate predicted mortality based on factors such as age, HCC scores, type of stroke, and other factors. We then compare our mortality risk “index” between the half of the sample living closest to PSCs and the half furthest away, clustered at the regional (hospital referral region) level.
Before controlling for unmeasured confounders we investigated the association of PSC admission and mortality using a Probit regression, controlling for all known confounders in our data. Subsequently, our instrumental variable analysis model was based on a two-stage approach with a Probit function in the second stage to account for the binary dependent variable. Probit is similar to a logistic regression, but allows an estimate of the differential probability of the outcome (rather than an odds ratio) by calculating the marginal effects (partial derivative) after adjusting for all independent variables.24 For sensitivity analysis, we also considered an instrumental variable Poisson model to estimate risk ratios. In all these analyses, we controlled for the sociodemographic and comorbidity variables mentioned previously, including type of stroke.
To investigate whether the impact of longer travel time offset the benefit of treatment in a PSC, we stratified the analysis above with respect to 5 pre-specified categories of patient travel time: < 20 minutes, 20–40 minutes, 40–60 minutes, 60 to 90 minutes and over 90 minutes. Additional sensitivity analysis stratified our baseline models for separate regions of the US (Northeast, Northwest, West, and South), for older (over 75 years old) or younger (65–74 years old) patients, and for urban or rural residence. In post-hoc sensitivity analysis we repeated our analyses for the subgroups of ischemic stroke, SAH, and ICH, although our study was not individually powered for these subcategories; we also considered an analysis that excluded hospital transfers. We did not adjust for multiple comparisons.
Mean Imputation for patients with missing urbanicity and income data (6.5% of the sample) did not affect the results and so these patients were excluded. Numbers needed to treat were calculated as the inverse of the absolute risk reduction as appropriate. All probability values were the result of two-sided tests, and the significance level was set at 0.05. SAS version 10 (SAS Institute, Cary, NC) and STATA version 14 (StataCorp, College Station, TX) were used for statistical analysis.
RESULTS
Cohort characteristics
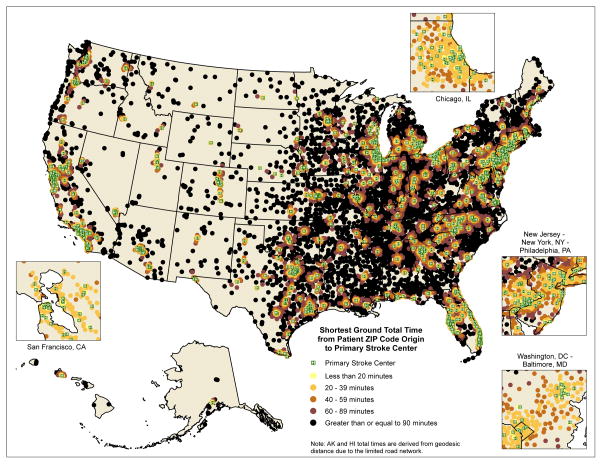
Figure S1 describes the creation of our cohort. During the study period, 865,184 elderly Medicare fee-for-service beneficiaries (mean age 78.9 years, 55.5% females) presented with a stroke. There was a total of 976 PSCs across the United States, with 466,334 patients (53.9%) from our cohort being treated in PSCs. Nearly one-quarter (24%) of the cohort resided closer to a PSC than a non-PSC institution. The distribution of patient characteristics stratified by whether they received treatment in PSCs can be seen in Table 1. There was significant regional variation in access to PSCs as demonstrated in Figure 1.
Table 1.
Patient characteristics
| Total | Treated in Primary Stroke Centers | Treated in non-certified institutions§ | |
|---|---|---|---|
| Variables | |||
| Age (SD), years | 78.9 (7.9) | 78.6 (7.8) | 79.1 (7.9) |
| Male | 207,417 (43.5%) | 108,606 (44.4%) | 99,348 (42.6%) |
| Poverty* | 49,589 (10.4%) | 23,482 (9.6%) | 26,120 (11.2%) |
| Race | |||
| White | 417,695 (87.6%) | 213,544 (87.3%) | 205,227 (88%) |
| Black | 43,391 (9.1%) | 22,993 (9.4%) | 20,523 (8.8%) |
| Asian | 6,199 (1.3%) | 3,669 (1.5%) | 2,565 (1.1%) |
| Other | 9,536 (2.0%) | 4,403 (1.8%) | 4,897 (2.1%) |
| Urbanicity | |||
| Urban | 239,840 (50.3%) | 147,988 (60.5%) | 92,352 (39.6%) |
| Suburban | 158,305 (33.2%) | 81,699 (33.4%) | 72,727 (32.9%) |
| Rural | 78,676 (16.5%) | 14,921 (6.1%) | 68,133 (27.5%) |
| Income (SD)*, $ | 45,000 (17,000) | 47,000 (18,000) | 43,000 (16,000) |
| HCC (SD) | 2.5 (1.4) | 2.5 (1.5) | 2.4 (1.4) |
| Comorbidities¶ | |||
| Myocardial infarction | 144,000 (30.2%) | 74,361 (30.4%) | 69,730 (29.9%) |
| Cardiac arrhythmia | 13,017 (27.3%) | 67,512 (27.6%) | 63,200 (27.1%) |
| Congestive heart failure | 61,987 (13.0%) | 31,065 (12.7%) | 31,250 (13.4%) |
| Hyperlipidemia | 174,040 (36.5%) | 94,174 (38.5%) | 80,225 (34.4%) |
| Coagulopathy | 9,060 (1.9%) | 5,137 (2.1%) | 3,731 (1.6%) |
| Hypertension | 344,742 (72.3%) | 177,097 (72.4%) | 168,612 (72.3%) |
| Peripheral vascular disease | 41,960 (8.8%) | 22,259 (9.1%) | 19,823 (8.5%) |
| Tobacco use | 54,358 (11.4%) | 29,598 (12.1%) | 24,954 (10.7%) |
| Diabetes Mellitus | 120,636 (25.3%) | 60,174 (24.6%) | 60,653 (26.0%) |
| Chronic renal failure | 46,728 (9.8%) | 23,972 (9.8%) | 22,855 (9.8%) |
SD: Standard Deviation; HCC: Hierarchical Comorbidity Categories
Output represents crude numbers and percentages in parentheses for categorical variables, and mean and standard deviation for continuous variables
This was based on a five-year panel of the American Community Survey (2007–2011)
Based on 12-month look-back before the date of the procedure
Differences between groups are statistically significant (P<0.05) unless otherwise indicated
Figure 1.
Map of the United States demonstrating the shortest ground travel time to a Primary Stroke Center (PSC) of Medicare stroke patients, using road network data. Travel times for AK and HI have been calculated based on geodesic distances. Green dots represent PSCs, while all other dots represent ZIP code centroids of various total travel times to the closest PSC.
Differences in interventions and hospitalization characteristics between patients admitted in PSCs and those admitted in non-PSC institutions can be seen in eTable 2. Patients admitted to a PSC were more likely to receive IV-tPA (6.0% versus 2.8%), or undergo mechanical thrombectomy (1.0% versus 0.2%) for ischemic stroke, in comparison to their counterparts in non-PSC institutions. Final disposition of stroke patients is described in eTable 3.
Case-fatality and treatment in a PSC
In the first 7 days after admission for acute stroke, there were 40,143 (8.6%) deaths for patients hospitalized in PSCs, and 31,097 (7.8%) deaths for patients hospitalized in non-PSCs. The corresponding 30-day deaths were 75,151 (16.1%) in PSCs, and 61,397 (15.4%) in non-PSCs.
In multivariable regression, controlling for all health status and socio-demographic factors, admission to a PSC was associated with 0.7% higher 7-day case-fatality (95% CI, 0.6% to 0.8%), and 0.6% higher 30-day case-fatality (95% CI, 0.5% to 0.7%). However, this estimate is potentially biased because it doesn’t control for unmeasured confounding.
To address this limitation of unmeasured confounding we employed differential distance as an instrument (Table 2, S4). The latter was a strong instrument for PSC admission. When the PSC was at least one hour closer than the nearest non-PSC hospital, 87.5% of patients were admitted to a PSC. When the PSC was one hour further from the non-PSC, only 38.8% of patients were admitted to a PSC. We did not find evidence that those who lived nearest to a PSC were sicker than those living far from a PSC; predicted mortality in the “near to PSC” group was 15.81%, while the “far from PSC” mortality was 15.74% (P = 0.573).
Table 2.
Association of PSC admission with outcome measures
| Models | ||||
|---|---|---|---|---|
| 7-day mortality | 30-day mortality | |||
| Adjusted difference (95% CI) | P-value | Adjusted difference (95% CI) | P-value | |
| Probit regression* | 0.7% (0.6% to 0.8%) | <0.01 | 0.6% (0.5% to 0.7%) | <0.01 |
| IV analysis¶ | −1.8% (−2.1% to −1.4%) | <0.01 | −1.8% (−2.3% to −1.4%) | <0.01 |
| Midwest | ||||
| IV analysis¶ | −2.6% (−3.5% to −1.8%) | <0.01 | −2.3% (−3.4% to −1.2%) | <0.01 |
| Northeast | ||||
| IV analysis¶ | −1.8% (−2.4% to −1.2%) | <0.01 | −1.7% (−2.4% to −0.9%) | <0.01 |
| West | ||||
| IV analysis¶ | −1.8% (−3.2% to −0.5%) | <0.01 | −3.3% (−5.1% to −1.6%) | <0.01 |
| South | ||||
| IV analysis¶ | −0.8% (−1.3% to −0.3%) | <0.01 | −1.0% (−1.6% to −0.4%) | <0.01 |
| Patients older than 75 years | ||||
| IV analysis¶ | −1.9% (−2.3% to −1.4%) | <0.01 | −1.8% (−2.4% to −1.2%) | <0.01 |
| Patients 65 to 64 years old | ||||
| IV analysis¶ | −1.4% (−1.9% to −0.9%) | <0.01 | −1.6% (−2.2% to −0.9%) | <0.01 |
| Urban residence | ||||
| IV analysis¶ | −1.1% (−1.6% to −0.5%) | <0.01 | −0.7% (−1.4% to 0.1%) | 0.069 |
| Non-urban residence | ||||
| IV analysis¶ | −2.1% (−2.5% to −1.7%) | <0.01 | −2.4% (−3.0% to −1.8%) | <0.01 |
95% CI: 95% Confidence Interval; IV: instrumental variable
Numbers represent probability differences
Controlled for all sociodemographic and comorbidity variables
Two-stage approach with a Probit function in the second stage, using differential travel time of the patient to a PSC versus a non-PSC hospital as an instrument
Our analysis suggests (Table 2) that PSC admission was associated with 1.8% lower 7-day case-fatality (95% CI, −2.1% to −1.4%). Similarly, PSC admission was associated with 1.8% lower 30-day case-fatality (95% CI, −2.3% to −1.4%). This corresponded to 56 patients needed to be treated (NNT) in a PSC to save one life at 30 days post-admission for acute stroke.
PSC survival benefit and travel time
Receiving treatment in a PSC was associated with 30-day survival benefit for patients traveling less than 20 minutes (adjusted difference, 2.7%; 95% CI, 1.5% to 3.9; NNT=37), 20–39 minutes (adjusted difference, 1.8%; 95% CI, 1.3% to 2.2%; NNT=56), 40–59 minutes (adjusted difference, 2.6%; 95% CI, 0.7% to 2.8%; NNT=38), and 60–89 minutes (adjusted difference, 1.7%; 95% CI, 0.2% to 2.4%; NNT=59). Traveling for more than 90 minutes to receive care yielded no net benefit of PSC admission (adjusted difference, 0.1%; 95% CI −3.1% to 3.3%). Similar associations were observed for 7-day outcomes, with travel time offsetting the effect of PSC admission at 60 minutes (Table 3).
Table 3.
Survival benefit of PSC admission stratified on travel time
| Travel time | ||||
|---|---|---|---|---|
| 7-day survival benefit* | 30-day survival benefit* | |||
| Adjusted difference (95% CI) | P-value | Adjusted difference (95% CI) | P-value | |
| Less than 20 minutes | 2.0% (1.2% to 2.9%) | <0.01 | 2.7% (1.5% to 3.9) | <0.01 |
| 20 to 40 minutes | 1.6% (1.2% to 1.9%) | <0.01 | 1.8% (1.3% to 2.2%) | <0.01 |
| 40 to 60 minutes | 1.9% (2.5% to 1.3%) | <0.01 | 2.6% (0.7% to 2.8%) | <0.01 |
| 60 to 90 minutes | 0.7% (−0.1% to 1.5%) | 0.10 | 1.7% (0.2% to 2.4%) | <0.01 |
| Over 90 minutes | 1.3% (−1.2% to 3.9%) | 0.31 | 0.1% (−3.1% to 3.3%) | 0.95 |
Numbers represent probability differences in a two-stage approach with a Probit function in the second stage, using differential travel time of the patient to a PSC versus a non-PSC hospital as an instrument.
Sensitivity Analysis
We considered relative risk estimates for 7-day and 30-day case-fatality using an instrumental variable Poisson regression with the same covariates (eTable 2). The risk ratio for admission to a PSC was 0.82 (95% C.I., 0.76 to 0.88) for 30-day case-fatality, and 0.70 (95% C.I., 0.64 to 0.78) for 7-day case-fatality. These imply roughly similar absolute differences in case-fatality as those in the primary analysis. We stratified the instrumental variable analysis for case-fatality along several dimensions (Table 2). We observed regional variation for the 4 regions of the US. Estimates stratified by age or urban residence were similar to baseline.
In post-hoc sensitivity analyses we repeated our main instrumental variable analyses in subgroups stratified on stroke type, recognizing that our study was not specifically powered to address this question (eTable 5). For patients with ischemic stroke PSC admission was associated with 1.7% lower 7-day case-fatality (95% CI, −2.0% to −1.4%). PSC admission was associated with 2.8% lower 7-day case-fatality for patients with SAH (95% CI, −9.5% to 3.9%) and 1.3% lower 7-day case-fatality for patients with ICH (95% CI, −3.9% to 1.4%), although the latter two associations were not significant. Lastly, excluding transfers did not change our results (eTable 5).
DISCUSSION
Among Medicare patients, treatment in a PSC was associated with decreased 7- and 30-day post-admission case-fatality, in comparison to non-certified institutions. Travelling for more than 90 minutes to receive care offsets the 30-day survival benefit of PSCs (60 minutes for the 7-day survival benefit). These results are statistically significant, and are also clinically significant, implying one life saved for every 56 treated in a PSC. With the current distribution of PSCs, 16.4% of patients are located over 90 minutes by ground transportation from the nearest PSC.
Prior studies have investigated the association of hospitalization in PSCs with stroke outcomes. Lichtman et al,5 in a national cohort of ischemic stroke patients, demonstrated that hospitalization in PSCs was associated with slightly lower 30-day case-fatality in comparison to non-certified hospitals, although the difference was not statistically significant. Additionally, in a separate study4 they demonstrated that patients with hemorrhagic stroke receiving care in PSCs had significantly improved 30-day case-fatality, in comparison to their counterparts admitted to non-PSCs. The authors recognized that the major limitation of their results is the presence of unmeasured confounding because of selection bias.18,19 Proximity, severity of disease, and insurance coverage can be some of the factors that might affect patient disposition.
In order to address these limitations and account for such confounders, we used an instrumental variable analysis, using differential travel time as an instrument. Differential distance has been used before in similar observational studies of comparative effectiveness.18,19 In a regional cohort, Xian et al6 utilized an instrumental variable analysis to demonstrate superior outcomes for ischemic stroke patients hospitalized in local centers of excellence. This analysis focused on locally certified hospitals (different from the PSCs, which are certified by The Joint Commission), and is specific to New York State.
Given the potential for improved stroke outcomes with PSC admissions, identifying the optimal time frame to receive care in these institutions is of central importance. This question has not been addressed before in the literature. Our time calculations built on the work of Albright et al12 and others,13,14 who investigated the access of all US residents (regardless of age, and whether they had a stroke) to PSCs. The advantage of our analysis lies in using a large comprehensive cohort of stroke patients. In addition, contrary to prior work12–14 utilizing straight-line distance calculations of ground travel time, we employed real world road network data for the conterminous U.S., contemporary to the study years. This simulates closely the ground path, through which the patient could reach a PSC, taking into account the impact of natural obstacles, like mountains or rivers.
Travel times longer than 90 minutes appear to negate 30-day mortality gains arising from admission to a PSC. As suggested by our finding of higher thrombolytic and mechanical thrombectomy rates in PSCs, superior outcomes in PSCs likely reflect organized, disease-specific, efficient care, timely-administration of optimal treatments, and efficient blood pressure optimization. Among those living between 60–90 minutes from a PSC, the finding that PSC benefits arise only after 30 days (but not at 7 days) could reflect additional post-acute services available through PSCs.
The access map (Figure 1) of the United States demonstrates a significant proportion of stroke patients are outside of this 90-minute window. These access disparities have stimulated discussions about more thoughtful creation of stroke centers within the confines of a single state,25 or nationally.12 The establishment or certification of new centers can be prohibitive from a cost perspective. Building on the experience of trauma care, optimal utilization of air services, with the existing PSC locations, could expand access within this time frame for almost all stroke patients. This is just one approach from a plethora of available options to address disparities in access, and follow the recommendations of the Institute of Medicine26,27 to maximize the use of local referral centers. Other potential solutions include expanding telemedicine applications, enhancing smaller hospitals into Acute Stroke Ready Hospitals, and creating broader hospital networks.28,29 Further investigations are necessary to identify the best combination of approaches to treat stroke patients.
The present study has several limitations. First, coding inaccuracies can affect our estimates, although several reports have demonstrated that coding for stroke has good association with medical record review.30,31 Second, residual confounding can bias our results, for example because of differences in time from stroke onset, and stroke severity unmeasured in the Medicare claims data. We attempted to minimize such bias in an instrumental variable analysis, which simulates randomization by balancing the treatment and control groups in terms of unmeasured confounders. That our predicted mortality index was so similar for the group living near a PSC (most likely admitted to a PSC), and the control group far from a PSC (and least likely to be admitted to a PSC), is reassuring.
A third limitation is that we cannot necessarily identify what it is about PSCs that reduce mortality rates; these could include factors such as emergency room delays, availability of telestroke, timing of interventions, withdrawal of care, rehabilitation during hospitalization, and the use of emergency medical transportation. Fourth, our data is based on the Medicare population, with potentially different results for the commercially insured. However, three quarter of all strokes happen in patients over 65 years of age, the overwhelming majority of whom are covered by Medicare.1
Fifth, we underestimate the potential risks of longer travel time in ambulances, since patients who die while in the ambulance may not appear in the Medicare claims data. In a recent study of urban stroke patients treated in ambulances, the incidence of any death in the ambulance was just 0.2% (12 of 7098 patients), suggesting that the incremental effects of longer ambulance rides would not reverse our findings.32 Sixth, assigning populations to ZIP code centroids may give falsely low travel times for some patients, while overestimating travel times in others. To adjust for this we integrated in our travel time calculations previously validated indicators of average traffic delays, based on the urbanicity of the patient’s residence. Seventh, we recognize that our estimate of no benefit for patients traveling over 90 minutes carries with it a relatively wide confidence interval. Eighth, the scope of this analysis included only PSCs certified by The Joint Commission, and excluded state certified hospitals, or those participating in national quality improvement programs. Ninth, we had no information on the neurologic status of our patients at the time of discharge, and therefore we could not analyze the differences between PSC and non-certified institutions for these outcomes. Finally, causality cannot easily be established based on ecologic data, despite the use of an instrumental variable analysis.
Conclusions
Among Medicare patients, treatment in a PSC was associated with decreased 7-day, and 30-day post-admission case fatality rates. Travelling for more than 90 minutes to receive care offset the 30-day survival benefit of PSCs. Further investigations are necessary to identify the best combination of approaches to improve access to centers of excellence and stroke outcomes.
Supplementary Material
Acknowledgments
Funding. National Institute on Aging (P01-AG19783), and National Institutes of Health Common Fund (U01-AG046830-01). The funders had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and the preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Jon Skinner and Kimon Bekelis had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Centers for Disease Control and Prevention (CDC) Prevalence of Stroke — United States, 2006–2010. MMWR Morb Mortal Wkly Rep. 2012;61(20):379–382. [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.Schellinger PD, Köhrmann M. 4.5-hour time window for intravenous thrombolysis with recombinant tissue-type plasminogen activator is established firmly. Stroke. 2014;45(3):912–913. doi: 10.1161/STROKEAHA.113.002700. [DOI] [PubMed] [Google Scholar]
- 4.Lichtman JH, Jones SB, Leifheit-Limson EC, Wang Y, Goldstein LB. 30-day mortality and readmission after hemorrhagic stroke among Medicare beneficiaries in Joint Commission primary stroke center-certified and noncertified hospitals. Stroke. 2011;42(12):3387–3391. doi: 10.1161/STROKEAHA.111.622613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lichtman JH, Jones SB, Wang Y, Watanabe E, Leifheit-Limson E, Goldstein LB. Outcomes after ischemic stroke for hospitals with and without Joint Commission-certified primary stroke centers. Neurology. 2011;76(23):1976–1982. doi: 10.1212/WNL.0b013e31821e54f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xian Y, Holloway RG, Chan PS, et al. Association between stroke center hospitalization for acute ischemic stroke and mortality. JAMA. 2011;305(4):373–380. doi: 10.1001/jama.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birkmeyer JD, Siewers AE, Marth NJ, Goodman DC. Regionalization of high-risk surgery and implications for patient travel times. JAMA. 2003;290(20):2703–2708. doi: 10.1001/jama.290.20.2703. [DOI] [PubMed] [Google Scholar]
- 8.Epstein AM. Volume and outcome--it is time to move ahead. N Engl J Med. 2002;346(15):1161–1164. doi: 10.1056/NEJM200204113461512. [DOI] [PubMed] [Google Scholar]
- 9.Birkmeyer JD. Raising the bar for pancreaticoduodenectomy. Ann Surg Oncol. 2002;2002(9):9. doi: 10.1007/BF02557516. [DOI] [PubMed] [Google Scholar]
- 10.Daley J. Invited commentary: quality of care and the volume-outcome relationship--what’s next for surgery? Surgery. 2002;131(1):16–18. doi: 10.1067/msy.2002.120237. [DOI] [PubMed] [Google Scholar]
- 11.Schwamm LH, Reeves MJ, Pan W, et al. Race/ethnicity, quality of care, and outcomes in ischemic stroke. Circulation. 2010;121(13):1492–1501. doi: 10.1161/CIRCULATIONAHA.109.881490. [DOI] [PubMed] [Google Scholar]
- 12.Albright KC, Branas CC, Meyer BC, et al. ACCESS: acute cerebrovascular care in emergency stroke systems. Arch Neurol. 2010;67(10):1210–1218. doi: 10.1001/archneurol.2010.250. [DOI] [PubMed] [Google Scholar]
- 13.Mullen MT, Wiebe DJ, Bowman A, et al. Disparities in accessibility of certified primary stroke centers. Stroke. 2014;45(11):3381–3388. doi: 10.1161/STROKEAHA.114.006021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adeoye O, Albright KC, Carr BG, et al. Geographic access to acute stroke care in the United States. Stroke. 2014;45(10):3019–3024. doi: 10.1161/STROKEAHA.114.006293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higashida R, Alberts MJ, Alexander DN, et al. Interactions within stroke systems of care: a policy statement from the American Heart Association/American Stroke Association. Stroke. 2013;44(10):2961–2984. doi: 10.1161/STR.0b013e3182a6d2b2. [DOI] [PubMed] [Google Scholar]
- 16.Kelly-Hayes M, Wolf PA, Kase CS, Brand FN, McGuirk JM, D’Agostino RB. Temporal patterns of stroke onset. The Framingham Study. Stroke. 1995;26(8):1343–1347. doi: 10.1161/01.str.26.8.1343. [DOI] [PubMed] [Google Scholar]
- 17.Song Y, Skinner J, Bynum J, Sutherland J, Wennberg JE, Fisher ES. Regional variations in diagnostic practices. N Engl J Med. 2010;363(1):45–53. doi: 10.1056/NEJMsa0910881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neuman MD, Rosenbaum PR, Ludwig JM, Zubizarreta JR, Silber JH. Anesthesia technique, mortality, and length of stay after hip fracture surgery. JAMA. 2014;311(24):2508–2517. doi: 10.1001/jama.2014.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan HJ, Norton EC, Ye Z, Hafez KS, Gore JL, Miller DC. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA. 2012;307(15):1629–1635. doi: 10.1001/jama.2012.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA. 2007;297(3):278–285. doi: 10.1001/jama.297.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClellan M, McNeil BJ, Newhouse JP. Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. JAMA. 1994;272(11):859–866. [PubMed] [Google Scholar]
- 22.Valley TS, Sjoding MW, Ryan AM, Iwashyna TJ, Cooke CR. Association of Intensive Care Unit Admission With Mortality Among Older Patients With Pneumonia. JAMA. 2015;314(12):1272–1279. doi: 10.1001/jama.2015.11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staiger D, Stock JH. Instrumental Variables Regression with Weak Instruments. Econometrica. 1997;65(3):557–586. [Google Scholar]
- 24.Garabedian LF, Chu P, Toh S, Zaslavsky AM, Soumerai SB. Potential bias of instrumental variable analyses for observational comparative effectiveness research. Ann Intern Med. 2014;161(2):131–138. doi: 10.7326/M13-1887. [DOI] [PubMed] [Google Scholar]
- 25.Leira EC, Fairchild G, Segre AM, Rushton G, Froehler MT, Polgreen PM. Primary stroke centers should be located using maximal coverage models for optimal access. Stroke. 2012;43(9):2417–2422. doi: 10.1161/STROKEAHA.112.653394. [DOI] [PubMed] [Google Scholar]
- 26.Committee on Quality of Health Care in America; Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: 2001. [Google Scholar]
- 27.Committee on the Future of Emergency Care in the United States Health System Board on Health Care Services Institute of Medicine. The Future of Emergency Care: Hospital-Based Emergency Care: At the Breaking Point. Washington, DC: 2007. [Google Scholar]
- 28.Alberts MJ, Wechsler LR, Jensen ME, et al. Formation and function of acute stroke-ready hospitals within a stroke system of care recommendations from the brain attack coalition. Stroke. 2013;44(12):3382–3393. doi: 10.1161/STROKEAHA.113.002285. [DOI] [PubMed] [Google Scholar]
- 29.Kulcsar M, Gilchrist S, George MG. Improving stroke outcomes in rural areas through telestroke programs: an examination of barriers, facilitators, and state policies. Telemed J E Health. 2014;20(1):3–10. doi: 10.1089/tmj.2013.0048. [DOI] [PubMed] [Google Scholar]
- 30.Tirschwell DL, Longstreth WTJ. Validating administrative data in stroke research. Stroke. 2002;33(10):2465–2470. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 31.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43(5):480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 32.Ebinger M, Winter B, Wendt M, et al. Effect of the use of ambulance-based thrombolysis on time to thrombolysis in acute ischemic stroke: a randomized clinical trial. JAMA. 2014;311(16):1622–1631. doi: 10.1001/jama.2014.2850. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.