Abstract
The human microbiome has been shown to influence a number of chronic conditions associated with impaired bone mass and bone quality including obesity, diabetes and inflammatory bowel disease. The connection between the microbiome and bone health, however, has not been well studied. The few studies available demonstrate that the microbiome can have a large effect on bone remodeling and bone mass. The gut microbiome is the largest reservoir of microbial organisms in the body and consists of over a thousand different species interacting with one another in a stable dynamic equilibrium. How the microbiome can affect organs distant from the gut is not well understood, but is believed to occur through regulation of nutrition, regulation of the immune system and/or translocation of bacterial products across the gut endothelial barrier. Here we review each of these mechanisms and discuss their potential effect on bone remodeling and bone mass. We review how preclinical studies of bone-microbiome interactions are challenging because the microbiome is sensitive to genetic background, housing environment, and vendor source. Additionally, although the microbiome exhibits a robust response to external stimuli, it rapidly returns to its original steady state after a disturbance, making it difficult to sustain controlled changes in the microbiome over time periods required to detect alterations in bone remodeling, mass or structure. Despite these challenges, an understanding of the mechanisms by which the gut microbiome affects bone has the potential to provide insights into the dissociation between fracture risk and bone mineral density in patients including those with obesity, diabetes or inflammatory bowel disease. In addition, alteration of the gut microbiome has the potential to serve as a biomarker of bone metabolic activity as well as a target for therapies to improve bone structure and quality using pharmaceutical agents or pre- or probiotics.
Keywords: microbiome, inflammation, osteoporosis, fracture, osteoimmunology
I. Introduction
The human microbiome consists of the microbial species that inhabit the human body and their secreted products (1,2). Each individual hosts trillions of microbes, a population that vastly outnumber native mammalian cells. Microbiota confer benefits to the host that include vitamin production (3), nutrient and energy extraction from diet (4), metabolic function (5), regulation of innate and adaptive immunity (6,7), and protection from pathogenic organisms (8). Alterations in the microbiome have been associated with a number of chronic conditions in humans including inflammatory bowel diseases (9), obesity (10), metabolic disease (11), malnutrition (12), neurological disorders (13), cancer (14), and cardiovascular disease (15).
The human microbiome is established soon after birth, usually by colonization by microbial flora present in the birth canal (16). The microbiota is shaped subsequently by diet and environmental exposure, and reaches a steady state at about three years of age (16). The great majority of the human microbiome is located within the gastrointestinal system. The human gut microbiota consists of over 1000 distinct microbial species, many of them not yet well characterized. Roughly two-thirds of the microbial species composition is unique to each individual (17). The human gut microbiota is dominated by organisms from the Bacteroidetes and Firmictues phyla (18). Once established in an individual, the contents of the microbial community in the gut enter a dynamic equilibrium as the hundreds of different species compete and interact with one another and the host immune system in complex networks of interdependence. The relative abundance of species within the gut flora fluctuates from day to day based on changes in diet (18,19), but in general retains its basal constitutive state despite these transient disruptions. For example, after a stimulus such as a course of antibiotics or short gastrointestinal infection, the contents of the gut microbiota mostly return to their initial state, although the resulting gut microbial community may be less stable than it was prior to treatment (20) and small changes in content may occur (e.g. species with similar function may replace each other (19)). Hence, while the gut microbiome is relatively stable it can be changed by long periods of sustained stimuli or factors that produce large perturbations in the gut flora. Factors that have been shown to alter the steady state of the gut flora include aging (16), diet (19), environment (21), physiologic state, and chronic treatment with oral antibiotics (22).
Alterations in the composition of the gut microbiome have been implicated either directly or indirectly in the de-regulated bone remodeling associated with obesity, diabetes, inflammatory bowel disease and rheumatoid arthritis (Table 1). In this review we explore the potential effects of the gut microbiome on bone, first by discussing the potential mechanisms that explain how changes in microbial populations in the gut can have effects at distant organs, and second by reviewing preclinical findings linking changes in the gut microbiome to alterations in bone mass. Lastly we discuss the challenges in the study of bone-microbiome interactions.
Table 1.
Alterations in the gut microbiota have been associated with many of the factors that cause osteoporosis and/or fragility fractures.
| Contributor to Osteoporosis | Reported Alterations in Gut Microbiota |
|---|---|
| Poor acquisition of bone mass during growth leading to low BMD in adulthood | Absence of gut microbiota associated with altered bone mass in mice (57,59). |
| Alterations in circulating sex hormones | Chemically induced estrogen depletion does not result in bone loss in germ-free animals (58) Probiotic treatment reduces ovariectomy associated bone loss(58,70). |
| Diet/Nutrition | Gut microbiota regulate production/absorption of vitamins (6) |
| Aging | Gut microbiota composition is correlated with indices of frailty in the elderly (Barthel Index, Functional Independence Measures) (52,96). |
| Obesity/Diabetes | Gut microbiota influence caloric intake and the development of obesity (10,65). |
| Gastrointestinal Disease | Inflammatory Bowel Disease is related to the microbiome and leads to osteopenia independent of its effects on nutrition (10,83,97). |
II. How the Gut Microbiome Affects Distant Organs
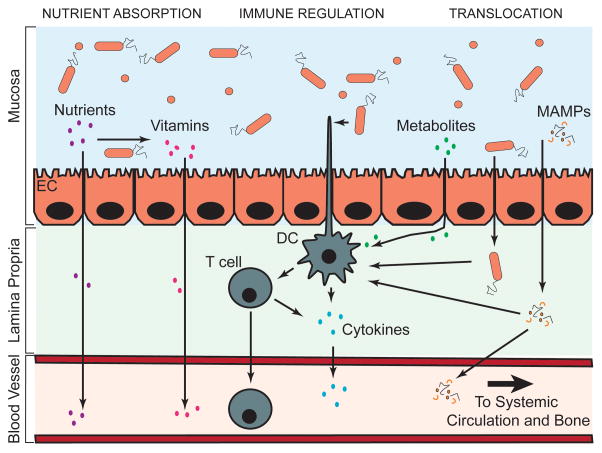
Although there are a number of studies demonstrating that the gut microbiome can influence the natural history of many clinical disorders, the field has been limited in terms of mechanistic explanations (23), especially with regard to the effect of the microbiome on organs distant from the gut. Figure 1 illustrates the three potential mechanisms by which the gut microbiota can influence bone tissues: regulation of nutrient absorption at the gut epithelium, regulation of the mucosal and systemic immune system, and translocation of microbial contents across the gut endothelial barrier.
Figure 1.
The gut microbiome can influence remote organs through regulation of nutrient absorption, regulation of the immune system or translocation of bacterial products. Molecular products and activated T cells enter the systemic circulation where they can migrate to distant organs including bone. Abbreviations: DC – Dendritic Cell, EC – Endothelial Cell, MAMPs – Microbial Associated Molecular Patterns.
It is well established that alterations in the gut microbiome can alter nutrient absorption, including the ability of the host to absorb calories from food. For example, low dose antibiotics commonly used in livestock feed promote increased animal growth and bone size by altering the gut flora and increasing caloric absorption from food (24–26). In addition to influencing caloric absorption, the intestinal microbiota aids in host and microbial metabolism through the biosynthesis of vitamins, including cobalamin (B12), biotin (B7), folate, thiamine (B1), pyridoxal phosphate, pantothenic acid (B5), niacin (B3), vitamin K and tetrahydrofolate (6,16). These vitamins are absorbed at the gut lining and distributed throughout the body through the systemic circulation along with nutrients. Vitamins metabolized in the gut have functions throughout the body, including regulation of the metabolism of proteins, aiding in the formation of red blood cells, maintenance of the central nervous system, metabolism of carbohydrates and fat, regulation of cell division and repair, ensuring proper cardiac function, regulation of blood clotting, and maintenance of bone mass(16).
The gut microbiome is also known to influence the development and function of the host immune system. The immune system is stimulated at the gut endothelial barrier by metabolites released by the gut flora as well as by direct contact between microorganisms and immune cells (27). The interactions between the gut flora and the immune system are reciprocal; the immune system regulates commensal composition and localization, while the interactions with the commensal flora are crucial for the development and function of an effective immune system (27). Immune cells, including T cells and dendritic cells interact with the microbial flora at the gut lining and migrate to lymph nodes to activate either pro- or anti-inflammatory immune responses. These cells also may release soluble pro- or anti-inflammatory mediators or cytokines into the circulation and by this mechanism modulate systemic bone remodeling. Additionally, activated immune cells can migrate to the bone tissues where they can directly regulate bone remodeling by the release of products, including the potent osteoclast-inducing factor, receptor activator of NF-κβ ligand (RANKL), or other bone active molecules (28,29). Bacteria-derived short chain fatty acids are well-known regulators of immune cells. They are synthesized by bacterial fermentation of carbohydrates in the colon where they can act as an energy source for epithelial cells in the colon, but can also promote the induction and activity of regulatory T cells and thereby inhibit immune cell responses (27,30–32). The commensal flora also compete with invading organisms for nutrients and produce antimicrobial molecules and metabolites that hinder pathogen survival and promote tighter junctions between epithelial cells to prevent translocation of pathogens into the systemic circulation. Lastly, the gut microbiome plays a crucial role in immune system development and control by regulating and suppressing inflammatory responses to food products that can serve as ingested antigens (27,33–36).
The gut microbiome can also influence distant organs by introducing microbial associated molecular patterns (MAMPs) into the systemic circulation (37). MAMPs such as lipopolysaccharide, peptidoglycan, flagellin and cell free DNA secreted by bacteria or which are retained after cell death are sufficiently small enough to be transported across the gut endothelial barrier and enter into the systemic circulation. Once distributed to remote organs such as bone, MAMPs can activate innate or adaptive immune responses to produce local inflammation. In bone, MAMPs are known to have a direct effect on bone remodeling through stimulation of innate immune receptors on bone cells, including toll-like receptor 2 (TLR2) (which responds to peptidoglycan) (38), TLR4 (which responds to lipopolysaccharide) (39,40), and TLR5 (which responds to flagellin) (41–43). In addition to the translocation of MAMPs, viable bacteria can cross the gut endothelial barrier through a process known as bacterial translocation. Once considered a controversial topic, bacterial translocation is now a well-recognized phenomenon (44,45). In individuals with a normal immune system, bacterial translocation is rare, but in disease states, gut inflammation can increase intestinal permeability allowing more bacteria to cross the endothelial barrier (46). Translocation of gastrointestinal bacteria has been detected in patients with bowel cancer, bowel obstruction, Crohn’s disease, ulcerative colitis, hemorrhagic shock, and trauma (46). Translocated bacteria are usually killed rapidly by immune cells, but even after induction of cell death, small amounts of MAMPs may be released into the systemic circulation (37). Lastly, some bacteria that cross the gut endothelial barrier can penetrate and survive inside native cells where they avoid an immune response (37,47). It is unclear if translocated bacteria are able to migrate to distant organs (presumably while occupying a host cell), although evidence that orthopaedic implant infection can start from “hematogenous seeding” demonstrates that bacteria may travel to bone through the circulatory system (48).
III. Evidence of Bone-Microbiome Interactions
There is no single cause of osteoporosis and multiple mechanisms are involved in the pathogenesis of osteopenia, including for example, poor acquisition of bone mass during skeletal growth, limited physical activity, poor nutritional history, alterations in sex steroid hormone levels and genetic background. Additionally, osteopenia can be secondary to other conditions, including inflammatory disorders such as inflammatory bowel disease or pharmacological treatments such as glucocorticoids (49,50). To date there is relatively little direct evidence relating osteoporosis or osteopenia to the state of the gut microbiome, although there is substantial indirect evidence. For example, gut microbial diversity changes with age (51) and is negatively correlated with clinical indices of frailty in the elderly (52). Additionally, many of the risk factors associated with the development of osteoporosis and osteopenia are also associated with alterations in the gut microbiome (Table 1).
Bone Growth
Recent investigations in animal models show that the presence and contents of the gut microbiota influence the accumulation of bone mass during growth. One of the most useful tools for studying the gut microbiome are germ-free mice. Germ-free animals are raised in a sterile incubator and are never exposed to detectable microorganisms and therefore do not have a microbiome (germ-free incubators are not to be confused with specific pathogen free facilities) (2,53,54). Additionally, animals raised in a germ-free environment fail to develop a mature immune system and display altered physiology and organ morphology (55), which is a recognized limitation of their use, but at the same time they provide one of the best means of studying the microbiome (56). There are conflicting data regarding the effects of a germ-free state on bone. An early report showed that germ free 7–9 week old female C57Bl/6 mice had increased bone mineral density, 39% greater femoral metaphyseal trabecular bone volume fraction, reduced osteoclast surface and increased mineralizing surface compared to conventionally raised animals (57). The bone phenotype in germ-free mice was shown to be reversible by reconstituting the gut microbiota with flora from a conventionally raised animal. Partially confirming this finding, a recent study reported that female germ-free C57Bl/6 mice had greater femoral cortical volume and cortical thickness than conventionally raised mice at 20 weeks of age, although there was no significant change in trabecular bone volume fraction (58). In contrast, a study of 8 week old male BALB/c mice reported that germ-free animals had reduced femoral length, cortical thickness and bone mineral density compared to animals raised in conventional housing (59). It is unclear if the contradictory findings among these studies are a result of animal sex, age, or differences between mouse strain (C57Bl/6 v. BALB/c, Table 2, (59)).
Table 2.
A summary of the effects of disruption or absence of the gut flora on bone mass and structure in mice.
| Source | Mouse Strain | Mouse Age (weeks) | Mouse Sex | Treatment | Bone Measurement | Results |
|---|---|---|---|---|---|---|
| (57) | C57Bl/6J | 7–9 | Female | Germ-free | Micro-CT, pQCT, histomorphometry | Germ-free mice showed substantial increases in trabecular and cortical bone volume |
| (58) | C57Bl/6J | 20 | Female | Germ-free | Micro-CT | Germ-free mice showed increased cortical thickness, no change in trabecular BV/TV |
| (59) | BALB/c | 7 | Male | Germ-free | Micro-CT | Germ-free animals showed reduced cortical and trabecular bone |
| (66) | C57Bl/6J | 7, 11 | Female | Low dose Antibiotics | DXA | BMD increased at 7 weeks, no difference at 11 weeks |
| (67) | C57Bl/6J | 20 | Female | Low dose Antibiotic | DXA | BMD increased |
| (67) | C57Bl/6J | 20 | Male | Low dose Antibiotic | DXA | No Change in BMD |
| (68) | C57Bl/6J | 3–20 | Female | Pulsed Oral Antibiotics | DXA | BMC increased at 7 weeks of age no difference at later ages |
Genetic Background
Inbred mouse strains are commonly used for studying the effect of genetic background on bone phenotype (60). Differences in the gut microbiota have been observed among inbred mouse strains (61), raising the possibility that the microbiome may contribute to differences in bone phenotype among some of these mouse strains. The Toll-like receptor 5 (TLR5) deficient mouse provides an example of how genetic background can alter the microbiome and organs distant from the gut. TLR5 is the innate immune receptor for bacterial flagellin and has no known endogenous ligand. Hence, changes in phenotype in the TLR5 deficient mouse are entirely dependent on host-microbe interactions. In the TLR5 deficient mouse, the inability to respond to flagellin leads to alterations in the gut microbiota, including reduced stability of the microbial community and increased expression of flagellin by commensal flora. Increased flagellin expression leads to increased bacterial motility and increased translocation of bacteria across the endothelial barrier where the bacteria can trigger an immune response leading to inflammation in the gut epithelium (62). As a result of increased gut inflammation, the TLR5 deficient mouse develops mild insulin resistance, low grade systemic inflammation and mild increases in adiposity mimicking the condition of metabolic syndrome in humans (63). Of interest, TLR5 deficient mice do not develop the metabolic syndrome-like traits if they are raised in a germ-free environment or have their gut flora decimated by chronic oral antibiotic treatment. While the bone phenotype of the TLR5 deficient mouse has not been characterized, it is clear that loss of TLR5 can lead to alterations in the microbiome that have effects on distant organs, supporting the idea that some mice may have a microbiome-dependent bone phenotype.
Differential regulation of the microbiome among mouse strains may explain why germ-free BALB/c mice show reduced bone mass as compared to conventionally raised animals (59) while C57Bl/6 mice have increased bone mass when raised in a germ-free environment (57). The potential role of the gut microbiome on bone is illustrated in the model depicted in Figure 2. Consider a hypothetical mouse strain in which the genetic background leads to the development of a gut microbial community that results in reduced inflammation and promotes increased bone mass. Mice from this inbred strain, when raised in a germ-free environment, would display reduced bone mass as compared to conventionally raised animals from the same strain. Alternatively, another mouse strain could develop a microbiome that enhances pro-inflammatory responses in the gut endothelium, leading to a reduced bone mass. Germ-free mice from this second strain would show increased bone mass compared to conventionally raised animals. The capacity of the gut microbiome to influence bone remodeling therefore has the potential to confound the interpretation of results in studies that use inbred mouse strains to understand the effect of genetic background on bone. For example, the bone phenotype in a mouse strain may be sensitive to factors that influence the microbiome (vendor, environment, etc.); and second, it suggests that genetic characterization of phenotypes through Quantitative Trait Locus (QTL) or related analyses (60) may identify bone phenotypes with genes related to regulation of the microbiome rather than genes that directly regulate bone physiology. In adult humans, bone mineral density is 50–80% heritable (64), an association believed to be dominated by genetic background as compared to environmental factors. The gut microbiome is also heritable (65), and it is unclear to how much heritability of BMD in humans is a result of heritability of gut flora.
Figure 2.
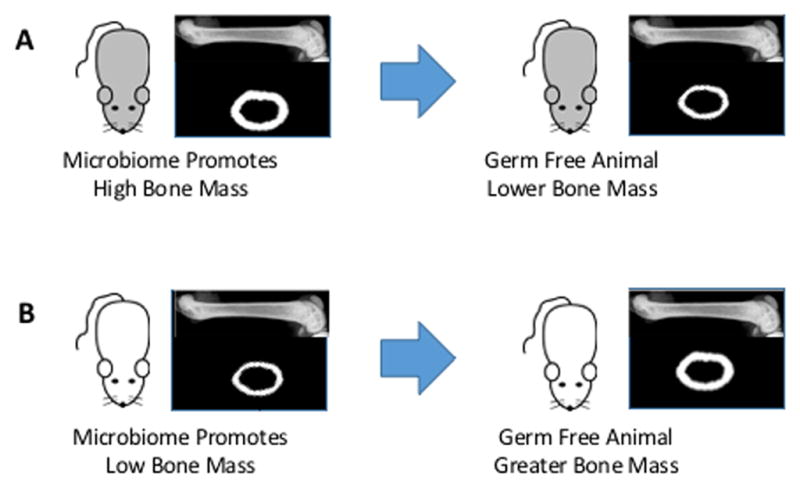
Absence of gut flora may not have the same effect on bone in all experimental mouse models. (A) A mouse with a microbiome that supports a high bone mass phenotype. This mouse shows reduced bone strength when raised in a germ-free environment. (B) A mouse with a microbiome that promotes a low bone mass phenotype. This mouse shows increased bone mass when raised in a germ-free environment.
Antibiotic Treatment History
Administration of oral antibiotics represents an environmental factor that can greatly influence the gut microbiome. Recent studies have reported that antibiotic treatment can influence skeletal growth and bone mineral content in mice, as measured by whole body DXA, and that the effects of antibiotics are influenced by sex and age. For example, low dose antibiotics starting at weaning resulted in increased whole body growth rates early in life (66,67). Some changes in DXA-derived bone mineral density were observed, but changes in BMD varied based on animal age and sex (66,67) (Table 2). In another study, pulsed antibiotic treatment (mimicking isolated rounds of treatment in children) followed by a high fat diet resulted in increases in bone growth and whole body bone mineral content (68). In all cases, antibiotic treatment was associated with noticeable reductions in gut microbial diversity. These studies and others illustrate the important role of the gut microbiome in the regulation of bone homeostasis, particularly during the period of skeletal growth, and indicate the need for further investigation examining the relationship between the gut microbiome, genetic background and bone.
Nutrition
The microbiome can have a profound effect on nutrient absorption and caloric uptake, which can have direct or indirect effects on bone metabolism. Chronic under nutrition has been shown to modify the gut microbiome and is associated with impaired bone growth during adolescence. In a recent study, microbiota from healthy and undernourished children (6–18 months of age) were transplanted into young germ-free mice. Five weeks after microbiota transplantation, mice receiving gut flora from healthy children saw more rapid increases in body weight and lean mass than those receiving microbiota from undernourished individuals (69). Paradoxically, animals receiving gut flora from undernourished donors showed increased femoral cortical bone volume and bone mineral density compared to animals receiving microbiota from healthy donors. In a related study, using a different experimental design, animals raised on a nutrient depleted diet showed impaired bone growth (reduced bone length) and the effect on bone growth was shown to be ameliorated to some degree by monocolonization with specific strains of Lactobacillus or complete reconstitution of the gut flora (59). While neither of these studies provided information on bone microstructure or biomechanics, they both clearly demonstrate that in cases of nutritional deficiency, changes in the microbiota contribute independently to bone growth and development.
Sex Hormones
Alterations in sex hormones are a primary stimulus for bone loss in humans and recent investigations show that changes in the gut microbiome are correlated with alterations in hormone status and bone loss. Germ-free mice demonstrate resistance to bone loss following pharmacologically-induced estrogen depletion (58). These effects were attributed to failure to up-regulate the production of pro-osteoclastogenic cytokines RANKL, tumor necrosis factor (TNF) and interleukin-17 that occurred in the estrogen-depleted mice grown under standard conditions. They then showed that treatment of mice grown under standard conditions with Lactobacillus or commercially available probiotic supplement were completely protected against bone loss associated with estrogen depletion (58). Similarly, there are two reports that probiotic treatment prevented ovariectomy-induced bone loss in mice (70,71). Two studies in rats suggest that treatment with antibiotics can ameliorate ovariectomy-induced bone loss (72,73). Interestingly, inflammatory phenotypes associated with altered gut flora can differ between males and females (63), suggesting that circulating sex hormones may influence microbiome-dependent phenotypes. In humans, the microbiome is altered during pregnancy (74), providing further evidence that hormonal status influences the microbiome. Studies explaining sexual dimorphism in microbiome-dependent bone phenotypes have not been reported.
Probiotics and Other Clinical Conditions
In addition to preventing estrogen-depletion induced bone loss, oral dosing with Lactobacillus probiotics has been associated within increased bone density in broader populations. Mature male mice (14 week old, C57Bl/6) treated with Lactobacillus probiotics for 4 weeks showed a 45% increase in femoral and vertebral trabecular bone volume fraction, increased bone formation, and a reduction in circulating pro-inflammatory cytokine expression, but no changes in cortical bone were observed (75). In contrast, females treated with the same probiotics exhibited no significant changes in bone phenotype or alterations in circulating inflammatory markers. The increases in bone volume fraction were attributed to alterations in calcium/and or nutrient absorption in the gut rather than alterations in systemic inflammation. Additionally, Lactobacillus probiotics were recently shown to prevent bone loss and alterations in adiposity and bone marrow fat in a model of type I diabetes (streptozotocin) (76), an effect attributed to maintenance of Wnt10b expression after induction of type I diabetes. A series of studies in rats suggest that prebiotics (molecules that promote growth of beneficial microbes) can mediate bone loss following ovariectomy (see (77,78) for reviews) and can influence bone acquisition (79,80).
Alterations in the gut microbiome have been observed in other clinical conditions in which osteopenia develops. For example, inflammatory bowel disease is associated with large changes in the gut microbiota (81) and patients with inflammatory bowel disease are also at risk for osteopenia, osteoporosis and associated fragility fractures (82,83). Osteopenia associated with inflammatory bowel disease has been attributed to impaired absorption of calcium, reduced circulating levels of vitamin D and vitamin K or bone loss following treatment with glucocorticoids (84), but recent studies have indicated that inflammation in the gut and systemically are associated with enhanced production of potent osteoclastogenic cytokines, which are key contributors to bone loss, independent of absorption of calcium and other nutrients (83,85). Multiple animal models of colitis, including dextran sodium sulfate dosing, HLA-B27 transgenic rats and IL10 −/− knockout mice display reduced bone mass, bone volume fraction and bone strength (86–89) and the effects cannot be explained solely by impaired nutritional absorption (84). Dextran sodium sulfate induced colitis is enhanced in mice deficient in vitamin D receptor or an enzyme related to vitamin D hydroxylation (Cyp27B1) (90) and the gut microbiota is also changed, but whether these change in gut microbiota contribute to impaired bone mass or are simply correlated with alterations in vitamin D metabolism is not known.
Although existing evidence in mice clearly demonstrates that the microbiome can influence bone mass and structure, the specific mechanisms behind these changes are not well understood. Existing data are often conflicting, most likely due to differences in study design, including animal age and genetic background, as well as the imaging modalities employed (mouse DXA and/or inconsistent microcomputed tomography resolution). Many of the characteristics of bone phenotype that are well understood in mice and other animal models are not well understood in the context of the microbiome. For example, investigation of how the microbiome influences bone growth and development is limited to a few studies using mouse DXA. Relatively few of the studies reported to date have described the relative abundance of the commensal flora or reported correlations between the contents of the gut microbiota and bone phenotype (59,69,70). None of the studies reported to date have described the effect of the gut microbiome on bone strength or tissue material properties.
IV. Future Directions
Although the microbiome has been a topic of study since the advent of antibiotics, the development of high-throughput sequencing technologies over the last decade has allowed for rapid advancements in the field. These studies have established the importance of the microbiome in mammalian physiology, but the vast majority of the studies fail to provide insights into the mechanistic pathways responsible for these effects (23).
While the challenges of studying the effects of the microbiome on the major organ systems have been described (91), the study of the effects of the microbiome on bone physiology presents special challenges. First, the relatively slow rate of change of bone presents a challenge because it is difficult to experimentally create a sustained change in gut flora. For example, a common approach for manipulating the gut flora is to transfer the microbiota from a donor into a germ-free animal. When exposed to the new host environment, the contents of the transferred microbiota change over time (92) but the composition of the microbiota is rarely sustained long enough for a detectable change in skeletal phenotype (a month or more in mice). Second, preclinical studies relevant to osteoporosis concentrate on the adult skeletal phenotype, which requires older animals. Transfer of gut microbiota is not as effective in older animals (93,94) and the adult phenotype can be quite sensitive to the timing of microbial exposure (95), making it difficult to study changes in gut flora after skeletal maturity. Lastly, methods of manipulating the gut flora as a form of treatment remain poorly understood. Methods of altering an established microbiome, or even replacing an “unhealthy” microbiome with a “healthy” microbiome are still under development. Despite these challenges, there is substantial evidence that the microbiome has a significant effect on bone mass and bone physiology and further studies are needed to not only define the mechanisms by which the microbiome modulates skeletal phenotype, but also to develop approaches for manipulating the microbiome to maintain bone homeostasis and function.
We see two major challenges to advancing our understanding the links between the microbiome and bone. First, there have been few clinical reports linking the constituents of the gut microbiome to osteoporosis or other bone diseases. Correlations between bone mineral density and the gut microbiota have not yet been reported, but given the non-invasive nature of assessment, are feasible and could provide considerable insight. Second, given the conflicting effects of the gut microbiome on bone in mouse models, it is likely that the microbiome may be influencing bone phenotype and physiology in many well-established models. It may be necessary to repeat many well established studies examining bone growth, mass, structure, strength, and fracture healing under conditions of altered or disrupted gut microbiota to understand the effects of the gut flora on bone physiology. In some cases what we now consider to be an established effect of genetic background or a drug treatment may actually be secondary to regulation of the gut microbiota.
Acknowledgments
This publication was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (U.S) under Award Number AR068061 and by the Office of the Assistant Secretary of Defense for Health Affairs through the office of the Congressionally Directed Medical Research Programs (CDMRP) under Award No. W81XWH-15-1-0239. The content of the work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Defense.
Author Responsibilities: All authors contributed to original drafting of the manuscript and review and approval of the manuscript.
REFERENCES CITED
- 1.Claesson MJ, O’Sullivan O, Wang Q, Nikkila J, Marchesi JR, Smidt H, de Vos WM, Ross RP, O’Toole PW. Comparative analysis of pyrosequencing and a phylogenetic microarray for exploring microbial community structures in the human distal intestine. PLoS One. 2009;4(8):e6669. doi: 10.1371/journal.pone.0006669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon JI. Honor thy gut symbionts redux. Science. 2012;336(6086):1251–3. doi: 10.1126/science.1224686. [DOI] [PubMed] [Google Scholar]
- 3.LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. 2013;24(2):160–168. doi: 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15(13):1546–58. doi: 10.2174/138161209788168164. [DOI] [PubMed] [Google Scholar]
- 5.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–9. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 6.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327–36. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slack E, Hapfelmeier S, Stecher B, Velykoredko Y, Stoel M, Lawson MAE, Geuking MB, Beutler B, Tedder TF, Hardt W-D, Bercik P, Verdu EF, McCoy KD, Macpherson AJ. Innate and Adaptive Immunity Cooperate Flexibly to Maintain Host-Microbiota Mutualism. Science. 2009;325(5940):617–620. doi: 10.1126/science.1172747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamada N, Chen GY, Inohara N, Nunez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013;14(7):685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kostic AD, Xavier RJ, Gevers D. The Microbiome in Inflammatory Bowel Disease: Current Status and the Future Ahead. Gastroenterology. 2014;146(6):1489–1499. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 11.Chassaing B, Aitken JD, Gewirtz AT, Vijay-Kumar M. Gut microbiota drives metabolic disease in immunologically altered mice. Adv Immunol. 2012;116:93–112. doi: 10.1016/B978-0-12-394300-2.00003-X. [DOI] [PubMed] [Google Scholar]
- 12.Kane AV, Dinh DM, Ward HD. Childhood Malnutrition and the Intestinal Microbiome Malnutrition and the microbiome. Pediatr Res. 2015;77(0):256–262. doi: 10.1038/pr.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014;38:1–12. doi: 10.1016/j.bbi.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13(11):800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang WHW, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest. 2014;124(10):4204–4211. doi: 10.1172/JCI72331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–7. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagpal R, Yadav H, Marotta F. Gut Microbiota: The Next-Gen Frontier in Preventive and Therapeutic Medicine? Front Med (Lausanne) 2014;1:15. doi: 10.3389/fmed.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–30. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.David LA, Materna AC, Friedman J, Campos-Baptista MI, Blackburn MC, Perrotta A, Erdman SE, Alm EJ. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014;15(7):R89. doi: 10.1186/gb-2014-15-7-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4554–61. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips ML. Gut Reaction: Environmental Effects on the Human Microbiota. Environ Health Perspect. 2009;117(5):A198–A205. doi: 10.1289/ehp.117-a198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jernberg C, Lofmark S, Edlund C, Jansson JK. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology-Sgm. 2010;156:3216–3223. doi: 10.1099/mic.0.040618-0. [DOI] [PubMed] [Google Scholar]
- 23.Huttenhower C, Knight R, Brown CT, Caporaso JG, Clemente JC, Gevers D, Franzosa EA, Kelley ST, Knights D, Ley RE, Mahurkar A, Ravel J, White O Scientists for Advancement of Microbiome R. Advancing the microbiome research community. Cell. 2014;159(2):227–30. doi: 10.1016/j.cell.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jukes TH, Williams WL. Nutritional effects of antibiotics. Pharmacol Rev. 1953;5(4):381–420. [PubMed] [Google Scholar]
- 25.Ross E, Yacowitz H. Effect of Penicillin on Growth and Bone Ash of Chicks Fed Different Levels of Vitamin-D and Phosphorus. Poultry Sci. 1954;33(2):262–265. [Google Scholar]
- 26.Rusoff LL, Fussell JM, Hyde CE, Crown RM, Gall LS. Parenteral Administration of Aureomycin to Young Calves with a Note on Mode of Action. J Dairy Sci. 1954;37(5):488–497. [Google Scholar]
- 27.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–41. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Toraldo G, Li A, Yang X, Zhang H, Qian WP, Weitzmann MN. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109(9):3839–48. doi: 10.1182/blood-2006-07-037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pacifici R. T cells, osteoblasts, and osteocytes: interacting lineages key for the bone anabolic and catabolic activities of parathyroid hormone. Ann N Y Acad Sci. 2015 doi: 10.1111/nyas.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–5. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–50. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 32.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–73. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiyono H, McGhee JR, Wannemuehler MJ, Michalek SM. Lack of oral tolerance in C3H/HeJ mice. J Exp Med. 1982;155(2):605–10. doi: 10.1084/jem.155.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wannemuehler MJ, Kiyono H, Babb JL, Michalek SM, McGhee JR. Lipopolysaccharide (LPS) regulation of the immune response: LPS converts germfree mice to sensitivity to oral tolerance induction. J Immunol. 1982;129(3):959–65. [PubMed] [Google Scholar]
- 35.Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997;159(4):1739–45. [PubMed] [Google Scholar]
- 36.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011;241(1):241–59. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Potgieter M, Bester J, Kell DB, Pretorius E. The dormant blood microbiome in chronic, inflammatory diseases. FEMS Microbiol Rev. 2015;39(4):567–91. doi: 10.1093/femsre/fuv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takami M, Kim N, Rho J, Choi Y. Stimulation by toll-like receptors inhibits osteoclast differentiation. J Immunol. 2002;169(3):1516–23. doi: 10.4049/jimmunol.169.3.1516. [DOI] [PubMed] [Google Scholar]
- 39.Zou W, Bar-Shavit Z. Dual modulation of osteoclast differentiation by lipopolysaccharide. J Bone Miner Res. 2002;17(7):1211–8. doi: 10.1359/jbmr.2002.17.7.1211. [DOI] [PubMed] [Google Scholar]
- 40.Itoh K, Udagawa N, Kobayashi K, Suda K, Li X, Takami M, Okahashi N, Nishihara T, Takahashi N. Lipopolysaccharide promotes the survival of osteoclasts via Toll-like receptor 4, but cytokine production of osteoclasts in response to lipopolysaccharide is different from that of macrophages. J Immunol. 2003;170(7):3688–95. doi: 10.4049/jimmunol.170.7.3688. [DOI] [PubMed] [Google Scholar]
- 41.Chamberlain ND, Vila OM, Volin MV, Volkov S, Pope RM, Swedler W, Mandelin AM, 2nd, Shahrara S. TLR5, a novel and unidentified inflammatory mediator in rheumatoid arthritis that correlates with disease activity score and joint TNF-alpha levels. J Immunol. 2012;189(1):475–83. doi: 10.4049/jimmunol.1102977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim SJ, Chen Z, Chamberlain ND, Essani AB, Volin MV, Amin MA, Volkov S, Gravallese EM, Arami S, Swedler W, Lane NE, Mehta A, Sweiss N, Shahrara S. Ligation of TLR5 promotes myeloid cell infiltration and differentiation into mature osteoclasts in rheumatoid arthritis and experimental arthritis. J Immunol. 2014;193(8):3902–13. doi: 10.4049/jimmunol.1302998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kassem A, Henning P, Kindlund B, Lindholm C, Lerner UH. TLR5, a novel mediator of innate immunity-induced osteoclastogenesis and bone loss. FASEB J. 2015;29(11):4449–60. doi: 10.1096/fj.15-272559. [DOI] [PubMed] [Google Scholar]
- 44.O’Boyle CJ, MacFie J, Mitchell CJ, Johnstone D, Sagar PM, Sedman PC. Microbiology of bacterial translocation in humans. Gut. 1998;42(1):29–35. doi: 10.1136/gut.42.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsujimoto H, Ono S, Mochizuki H. Role of translocation of pathogen-associated molecular patterns in sepsis. Dig Surg. 2009;26(2):100–9. doi: 10.1159/000206143. [DOI] [PubMed] [Google Scholar]
- 46.Berg RD. Bacterial translocation from the gastrointestinal tract. Adv Exp Med Biol. 1999;473:11–30. doi: 10.1007/978-1-4615-4143-1_2. [DOI] [PubMed] [Google Scholar]
- 47.Fung TC, Bessman NJ, Hepworth MR, Kumar N, Shibata N, Kobuley D, Wang K, Ziegler CG, Goc J, Shima T, Umesaki Y, Sartor RB, Sullivan KV, Lawley TD, Kunisawa J, Kiyono H, Sonnenberg GF. Lymphoid-Tissue-Resident Commensal Bacteria Promote Members of the IL-10 Cytokine Family to Establish Mutualism. Immunity. 2016;44(3):634–46. doi: 10.1016/j.immuni.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016;387(10016):386–94. doi: 10.1016/S0140-6736(14)61798-0. [DOI] [PubMed] [Google Scholar]
- 49.Marcus R, Dempster DW, Bouxsein ML. The nature of osteoporosis. In: Marcus R, Feldman D, Dempster DW, Luckey M, Cauley JA, editors. Osteoporosis. 4. Vol. 1. Academic Press; Waltham, MA, USA: 2013. pp. 21–30. [Google Scholar]
- 50.Nordin BEC. Reflections on osteoporosis Osteoporosis. 4. Vol. 1. Academic Press; Waltham, MA, USA: 2013. pp. 31–50. [Google Scholar]
- 51.Mariat D, Firmesse O, Levenez F, Guimaraes V, Sokol H, Dore J, Corthier G, Furet JP. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jackson M, Jeffery IB, Beaumont M, Bell JT, Clark AG, Ley RE, O’Toole PW, Spector TD, Steves CJ. Signatures of early frailty in the gut microbiota. Genome Med. 2016;8(1):8. doi: 10.1186/s13073-016-0262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fitzgerald RJ. Gnotobiotic contribution to oral microbiology. J Dent Res. 1963;2:549–52. doi: 10.1177/00220345630420016601. [DOI] [PubMed] [Google Scholar]
- 54.Bibiloni R. Rodent models to study the relationships between mammals and their bacterial inhabitants. Gut Microbes. 2012;3(6):536–43. doi: 10.4161/gmic.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Falk PG, Hooper LV, Midtvedt T, Gordon JI. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol Rev. 1998;62(4):1157–70. doi: 10.1128/mmbr.62.4.1157-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams SC. Gnotobiotics. Proc Natl Acad Sci U S A. 2014;111(5):1661. doi: 10.1073/pnas.1324049111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sjogren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Backhed F, Ohlsson C. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27(6):1357–67. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li JY, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, Darby TM, Weitzmann MN, Mulle JG, Gewirtz AT, Jones RM, Pacifici R. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016 doi: 10.1172/JCI86062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwarzer M, Makki K, Storelli G, Machuca-Gayet I, Srutkova D, Hermanova P, Martino ME, Balmand S, Hudcovic T, Heddi A, Rieusset J, Kozakova H, Vidal H, Leulier F. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016;351(6275):854–7. doi: 10.1126/science.aad8588. [DOI] [PubMed] [Google Scholar]
- 60.Jepsen KJ. Functional interactions among morphologic and tissue quality traits define bone quality. Clin Orthop Relat Res. 2011;469(8):2150–9. doi: 10.1007/s11999-010-1706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Connor A, Quizon PM, Albright JE, Lin FT, Bennett BJ. Responsiveness of cardiometabolic-related microbiota to diet is influenced by host genetics. Mamm Genome. 2014;25(11–12):583–99. doi: 10.1007/s00335-014-9540-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cullender TC, Chassaing B, Janzon A, Kumar K, Muller CE, Werner JJ, Angenent LT, Bell ME, Hay AG, Peterson DA, Walter J, Vijay-Kumar M, Gewirtz AT, Ley RE. Innate and adaptive immunity interact to quench microbiome flagellar motility in the gut. Cell Host Microbe. 2013;14(5):571–81. doi: 10.1016/j.chom.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328(5975):228–31. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uitterlinden AG, Zillikens MC, Rivadeneira F. Genetic determinants of osteoporosis. In: Marcus R, Feldman D, Dempster DW, Luckey M, Cauley JA, editors. Osteoporosis. 4. Vol. 1. Elsevier; 2014. pp. 563–604. [Google Scholar]
- 65.Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, Spector TD, Clark AG, Ley RE. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–99. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, Li H, Alekseyenko AV, Blaser MJ. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621–6. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, Zarate Rodriguez JG, Rogers AB, Robine N, Loke P, Blaser MJ. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705–21. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nobel YR, Cox LM, Kirigin FF, Bokulich NA, Yamanishi S, Teitler I, Chung J, Sohn J, Barber CM, Goldfarb DS, Raju K, Abubucker S, Zhou Y, Ruiz VE, Li H, Mitreva M, Alekseyenko AV, Weinstock GM, Sodergren E, Blaser MJ. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat Commun. 2015;6:7486. doi: 10.1038/ncomms8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, Subramanian S, Manary MJ, Trehan I, Jorgensen JM, Fan YM, Henrissat B, Leyn SA, Rodionov DA, Osterman AL, Maleta KM, Newgard CB, Ashorn P, Dewey KG, Gordon JI. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science. 2016;351(6275) doi: 10.1126/science.aad3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Britton RA, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, Parameswaran N, McCabe LR. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol. 2014;229(11):1822–30. doi: 10.1002/jcp.24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohlsson C, Engdahl C, Fak F, Andersson A, Windahl SH, Farman HH, Moverare-Skrtic S, Islander U, Sjogren K. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One. 2014;9(3):e92368. doi: 10.1371/journal.pone.0092368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pytlik M, Folwarczna J, Janiec W. Effects of doxycycline on mechanical properties of bones in rats with ovariectomy-induced osteopenia. Calcif Tissue Int. 2004;75(3):225–30. doi: 10.1007/s00223-004-0097-x. [DOI] [PubMed] [Google Scholar]
- 73.Williams S, Wakisaka A, Zeng QQ, Barnes J, Martin G, Wechter WJ, Liang CT. Minocycline prevents the decrease in bone mineral density and trabecular bone in ovariectomized aged rats. Bone. 1996;19(6):637–44. doi: 10.1016/s8756-3282(96)00302-x. [DOI] [PubMed] [Google Scholar]
- 74.Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Backhed HK, Gonzalez A, Werner JJ, Angenent LT, Knight R, Backhed F, Isolauri E, Salminen S, Ley RE. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150(3):470–80. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McCabe LR, Irwin R, Schaefer L, Britton RA. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol. 2013;228(8):1793–8. doi: 10.1002/jcp.24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang J, Motyl KJ, Irwin R, MacDougald OA, Britton RA, McCabe LR. Loss of Bone and Wnt10b Expression in Male Type 1 Diabetic Mice Is Blocked by the Probiotic Lactobacillus reuteri. Endocrinology. 2015;156(9):3169–82. doi: 10.1210/EN.2015-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scholz-Ahrens KE, Schaafsma G, van den Heuvel EG, Schrezenmeir J. Effects of prebiotics on mineral metabolism. Am J Clin Nutr. 2001;73(2 Suppl):459S–464S. doi: 10.1093/ajcn/73.2.459s. [DOI] [PubMed] [Google Scholar]
- 78.Scholz-Ahrens KE, Ade P, Marten B, Weber P, Timm W, Acil Y, Gluer CC, Schrezenmeir J. Prebiotics, probiotics, and synbiotics affect mineral absorption, bone mineral content, and bone structure. The Journal of nutrition. 2007;137(3 Suppl 2):838S–46S. doi: 10.1093/jn/137.3.838S. [DOI] [PubMed] [Google Scholar]
- 79.Roberfroid MB, Cumps J, Devogelaer JP. Dietary chicory inulin increases whole-body bone mineral density in growing male rats. J Nutr. 2002;132(12):3599–602. doi: 10.1093/jn/132.12.3599. [DOI] [PubMed] [Google Scholar]
- 80.Takahara S, Morohashi T, Sano T, Ohta A, Yamada S, Sasa R. Fructooligosaccharide consumption enhances femoral bone volume and mineral concentrations in rats. J Nutr. 2000;130(7):1792–5. doi: 10.1093/jn/130.7.1792. [DOI] [PubMed] [Google Scholar]
- 81.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104(34):13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bikle DD. Osteoporosis in gasterointestinal, pancreatic and hepatic diseases. In: Marcus R, Feldman D, Nelson DA, Rosen CJ, editors. Osteoporosis. 3. Vol. 2. Elsevier Academic Press; San Diego, CA, USA: 2008. pp. 1203–1226. [Google Scholar]
- 83.Ali T, Lam D, Bronze MS, Humphrey MB. Osteoporosis in inflammatory bowel disease. Am J Med. 2009;122(7):599–604. doi: 10.1016/j.amjmed.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ghishan FK, Kiela PR. Advances in the understanding of mineral and bone metabolism in inflammatory bowel diseases. Am J Physiol Gastrointest Liver Physiol. 2011;300(2):G191–201. doi: 10.1152/ajpgi.00496.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ciucci T, Ibanez L, Boucoiran A, Birgy-Barelli E, Pene J, Abou-Ezzi G, Arab N, Rouleau M, Hebuterne X, Yssel H, Blin-Wakkach C, Wakkach A. Bone marrow Th17 TNFalpha cells induce osteoclast differentiation, and link bone destruction to IBD. Gut. 2015;64(7):1072–81. doi: 10.1136/gutjnl-2014-306947. [DOI] [PubMed] [Google Scholar]
- 86.Dresner-Pollak R, Gelb N, Rachmilewitz D, Karmeli F, Weinreb M. Interleukin 10-deficient mice develop osteopenia, decreased bone formation, and mechanical fragility of long bones. Gastroenterology. 2004;127(3):792–801. doi: 10.1053/j.gastro.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 87.Akhter MP, Jung LK. Decreased bone strength in HLA-B27 transgenic rat model of spondyloarthropathy. Rheumatology. 2007;46(8):1258–62. doi: 10.1093/rheumatology/kem104. [DOI] [PubMed] [Google Scholar]
- 88.Hamdani G, Gabet Y, Rachmilewitz D, Karmeli F, Bab I, Dresner-Pollak R. Dextran sodium sulfate-induced colitis causes rapid bone loss in mice. Bone. 2008;43(5):945–50. doi: 10.1016/j.bone.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 89.Harris L, Senagore P, Young VB, McCabe LR. Inflammatory bowel disease causes reversible suppression of osteoblast and chondrocyte function in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296(5):G1020–9. doi: 10.1152/ajpgi.90696.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ooi JH, Li Y, Rogers CJ, Cantorna MT. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J Nutr. 2013;143(10):1679–86. doi: 10.3945/jn.113.180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goodrich JK, Di Rienzi SC, Poole AC, Koren O, Walters WA, Caporaso JG, Knight R, Ley RE. Conducting a microbiome study. Cell. 2014;158(2):250–62. doi: 10.1016/j.cell.2014.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lundberg R, Toft MF, August B, Hansen AK, Hansen CH. Antibiotic-treated versus germ-free rodents for microbiota transplantation studies. Gut Microbes. 2016;7(1):68–74. doi: 10.1080/19490976.2015.1127463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hansen CH, Nielsen DS, Kverka M, Zakostelska Z, Klimesova K, Hudcovic T, Tlaskalova-Hogenova H, Hansen AK. Patterns of early gut colonization shape future immune responses of the host. PLoS One. 2012;7(3):e34043. doi: 10.1371/journal.pone.0034043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hansen CH, Metzdorff SB, Hansen AK. Customizing laboratory mice by modifying gut microbiota and host immunity in an early “window of opportunity”. Gut Microbes. 2013;4(3):241–5. doi: 10.4161/gmic.23999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352(6285):539–44. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O’Sullivan O, Fitzgerald GF, Deane J, O’Connor M, Harnedy N, O’Connor K, O’Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP, O’Toole PW. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–84. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 97.Byrne FR, Morony S, Warmington K, Geng Z, Brown HL, Flores SA, Fiorino M, Yin SL, Hill D, Porkess V, Duryea D, Pretorius JK, Adamu S, Manoukian R, Danilenko DM, Sarosi I, Lacey DL, Kostenuik PJ, Senaldi G. CD4+CD45RBHi T cell transfer induced colitis in mice is accompanied by osteopenia which is treatable with recombinant human osteoprotegerin. Gut. 2005;54(1):78–86. doi: 10.1136/gut.2003.035113. [DOI] [PMC free article] [PubMed] [Google Scholar]