Abstract
Exponentially rising CO 2 (currently ~400 μatm) is driving climate change and causing acidification of both marine and freshwater environments. Physiologists have long known that CO 2 directly affects acid–base and ion regulation, respiratory function and aerobic performance in aquatic animals. More recently, many studies have demonstrated that elevated CO 2 projected for end of this century (e.g. 800–1000 μatm) can also impact physiology, and have substantial effects on behaviours linked to sensory stimuli (smell, hearing and vision) both having negative implications for fitness and survival. In contrast, the aquaculture industry was farming aquatic animals at CO 2 levels that far exceed end‐of‐century climate change projections (sometimes >10 000 μatm) long before the term ‘ocean acidification’ was coined, with limited detrimental effects reported. It is therefore vital to understand the reasons behind this apparent discrepancy. Potential explanations include 1) the use of ‘control’ CO 2 levels in aquaculture studies that go beyond 2100 projections in an ocean acidification context; 2) the relatively benign environment in aquaculture (abundant food, disease protection, absence of predators) compared to the wild; 3) aquaculture species having been chosen due to their natural tolerance to the intensive conditions, including CO 2 levels; or 4) the breeding of species within intensive aquaculture having further selected traits that confer tolerance to elevated CO 2. We highlight this issue and outline the insights that climate change and aquaculture science can offer for both marine and freshwater settings. Integrating these two fields will stimulate discussion on the direction of future cross‐disciplinary research. In doing so, this article aimed to optimize future research efforts and elucidate effective mitigation strategies for managing the negative impacts of elevated CO 2 on future aquatic ecosystems and the sustainability of fish and shellfish aquaculture.
Keywords: aquatic carbonation, carbon dioxide, climate change, food security, ocean acidification, recirculating aquaculture system
Introduction – Climate change, high CO2 and global food security
In 2015, atmospheric CO2 concentrations had risen to an annual average higher than 400 μatm the first time in over 800 000 years (Lüthi et al., 2008; Dlugokencky & Pieter, 2016), as a result of anthropogenic CO2 emissions. The potential implications of this postindustrial rise in CO2 were predicted over 110 years ago (Krogh, 1904); yet, it was only recently that governments agreed to take action on this issue. Despite 196 nations taking an unprecedented stance on climate change last year by signing the COP21 agreement to curtail emissions, CO2 concentrations are still projected to approach 1000 μatm by 2100 (Pörtner et al., 2014). Around a quarter of anthropogenic CO2 emissions have been absorbed by the oceans (Pörtner et al., 2014). Whilst this results in a phenomenon commonly referred to as ocean acidification, elevated atmospheric CO2 is also driving a large elevation in the average aquatic CO2 in fresh and brackish water systems, regardless of diurnal and seasonal variation. What is more, seasonal oscillations of aquatic CO2 in the future are predicted to amplify over time which will likely result in CO2 levels that exceed 1000 μatm for several months each year well before 2100 (McNeil & Sasse, 2016). Occurring simultaneously with warming, pollution, habitat degradation, disease outbreaks and overfishing, this aquatic acidification is therefore threatening not only aquatic ecosystems but also global food security (FAO, 2014, Porter et al., 2014).
Anthropogenic CO2 emissions accelerate alongside growth of the global human population, which is projected to exceed 9.6 billion by 2100 (Gerland et al., 2014). This same growth has also resulted in at least 80% of world fish stocks being overexploited (FAO, 2014, Pauly & Zeller, 2016). Aquaculture is therefore crucial to ensure the continued provision of fish and shellfish protein for human consumption, particularly for developing countries and small island nations (Bennett et al., 2016). Indeed, aquaculture is one of the fastest growing food‐producing industries globally (8.8% annual growth for the last 30 years) (FAO, 2014), and it is the only foreseeable way of increasing seafood1 production in the face of this human population expansion. However, to ensure aquaculture is able to maximize its potential for addressing global food security, a number of challenges need to be resolved concerning water availability and quality, environmental impacts and vulnerability to changing climatic conditions. Recirculating aquaculture systems (RAS) address many of these issues (Martins et al., 2010) and enable the sustainable intensification of aquaculture. These systems significantly reduce water requirements, relocate production of aquatic organisms away from a natural environmental setting and minimize environmental impacts. They also enable a tighter control of pathogens and other environmental parameters, potentially improving animal welfare and biosecurity, but they create some additional problems, particularly associated with accumulation of CO2.
A common problem, two perspectives
Physiologists have known for decades that raising the CO2 partial pressure in water to well above atmospheric levels (e.g. 10 000 μatm) has a direct effect on aquatic organisms in terms of acid–base and ion regulation, respiratory function and aerobic performance (Cameron & Randall, 1972). More recently, climate change studies have shown that CO2 levels projected for end of this century (e.g. 800–1000 μatm) can negatively affect development, physiology and fitness‐related behaviours in aquatic animals (see below). Due to the very high stocking densities achieved in most aquaculture settings, as well as the methods employed to control pH and O2, CO2 often accumulates, particularly in RAS. However, despite recent evidence on the potential detrimental effects of CO2 exposure at a level projected for 2100 (1000 μatm), the aquaculture industry was intensively farming fish and shellfish successfully at much higher CO2 levels long before the term ‘ocean acidification’ was coined. The levels at which the effects of CO2 are perceived as problematic, therefore, appear to differ greatly between the connected yet traditionally disparate fields of climate change and aquaculture (Fig. 1).
Figure 1.
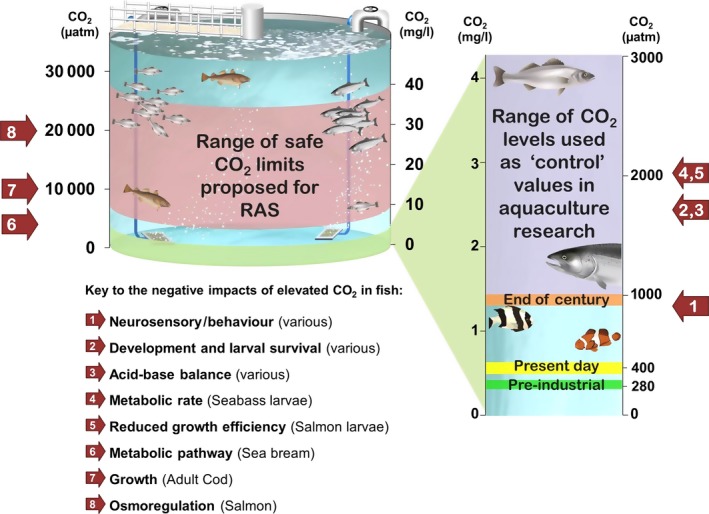
Diagrammatic representation of the levels at which elevated carbon dioxide is considered problematic within recirculating aquaculture systems (RAS) (caused by accumulation of excreted CO 2 due to high stocking densities) and under global aquatic acidification (marine and freshwater, caused by rising atmospheric CO 2). Numbered arrows, and corresponding key indicate the levels at which CO 2 is demonstrated to have significant impacts on fish development, physiology and behaviour. The expanded view on the right side highlights CO 2 levels in relation to climate change scenarios in greater detail (0–3000 μatm or 0–4 mg L−1). Conversion of CO 2 levels between μatm and mg L−1 in this diagram is based on 35 psu sea water at 15°C. Fish images Kovalevska and Kazakov maksim/shutterstock.com. References corresponding to numbered arrows indicate levels of CO 2 shown to have a significant impact of fish development, physiology or behaviour; 1) Hamilton et al. (2014), Jutfelt & Hedgärde (2013), Simpson et al. (2011), Nilsson et al. (2012); 2) Chambers et al. (2014), Frommel et al. (2012, 2014), Maneja et al. (2014), Tseng et al. (2013); 3) Esbaugh et al. (2016, 2012), Heuer et al. (2012); 4) Pope et al. (2014); 5) Ou et al. (2015); 6) Michaelidis et al. (2007); 7) Tirsgaard et al. (2015); & 8) Seidelin et al. (2001).
Current guidelines for intensive RAS propose safe CO2 levels ranging from 15 to 40 mg L−1 (Fivelstad et al., 1999, 2015; Blancheton, 2000; Petochi et al., 2011). These equate to an upper limit of CO2 ranging from >5000 to >30 000 μatm which are 12.5 to 75 times higher than current atmospheric levels, respectively. Furthermore, far from being an issue exclusively associated with RAS and finfish production, elevated CO2 levels appear synonymous with intensive aquaculture more generally. For example, over 40% of Norwegian salmon smolt hatcheries (flow‐through and RAS) report CO2 levels >5400 μatm (Noble et al., 2012), whereas Bangladeshi shrimp ponds are shown to experience CO2 levels averaging >17 000 μatm (Saksena et al., 2006; Sahu et al., 2013).
In stark contrast, recent studies emerging from aquatic acidification research have demonstrated that just 2.0‐ to 2.5‐fold increases in CO2 levels projected for the end of this century (e.g. 800–1000 μatm) can have dramatic and long‐lasting effects on the development, physiology and behaviour of both fish and invertebrates (Briffa et al., 2012; Schalkhausser et al., 2012; Heuer & Grosell, 2014; Watson et al., 2014; Welch et al., 2014). For example, exposure to 1000 μatm during early life cycle stages has been shown to result in reduced survival as well as a number of sublethal effects including tissue damage (e.g. Frommel et al., 2012, 2014; Chambers et al., 2014), altered calcification (e.g. Arnold et al., 2009; Maneja et al., 2013), reduced size (e.g. Talmage & Gobler, 2009; Maneja et al., 2014), reduced metabolic rate (e.g. Small et al., 2016), delayed development and altered gene expression (e.g. Tseng et al., 2013; Goncalves et al., 2016) in a range of different marine organisms. What is more, similar effects are also demonstrated in freshwater, with Ou et al. (2015) showing a significant effect of elevated CO2 (1000–2000 μatm) on the larval development of pink salmon Oncorhynchus gorbuscha. The authors reported a reduction in larval length, total wet and dry mass and reduced production efficiency (conversion of yolk into tissue growth).
In impacting a diverse array of aquatic organisms during early life stages, increased partial pressure of CO2 in aquatic environments above present‐day atmospheric levels is likely a bottleneck for organism production. This in turn would significantly impact aquaculture practices that depend upon a reliable source of larvae or juveniles. In 2007, these impacts were realized with the upwelling of elevated CO2, aragonite undersaturated sea water off the US west coast, significantly impacting oyster hatchery production as a direct result of changing climatic conditions (Barton et al., 2012). In addition to providing a case study in which to investigate the impact of ocean acidification on shellfish production globally, this event highlighted the significant advances achieved when climate change scientists and aquaculture practitioners work closely together. Unifying their research efforts to overcome this phenomenon, the climate change community and shellfish growers were able to successfully identify the root cause of this issue and put in place a number of mitigation strategies and monitoring protocols to minimize impacts in the future (Barton et al., 2015).
Far from being restricted to early life stages, a growing number of studies have also shown sublethal physiological impacts of elevated CO2 (range 1000–2000 μatm) in a number of species which include impacted respiratory gas transport, acid–base balance and gut carbonate excretion (e.g. Lannig et al., 2010; Esbaugh et al., 2012, 2016; Heuer et al., 2012; Wei et al., 2015). Rapid and efficient acid–base compensation has been demonstrated in a number of species at elevated CO2 concentrations (e.g. Melzner et al., 2009; Ern & Esbaugh, 2016; Lewis et al., 2016). However, such physiological responses incur energetic costs and could therefore have negative implications for production efficiency and body condition both in aquaculture and natural settings. Likewise, a wide range of behaviours are shown to be disrupted under elevated CO2, such as those linked to sensory stimuli (including smell, hearing and vision; e.g. Simpson et al., 2011; Nilsson et al., 2012; Roggatz et al., 2016) and cognitive‐related functions (such as lateralization, learning, bold‐shy phenotypes and escape behaviour; e.g. Schalkhausser et al., 2012; Jutfelt et al., 2013; Hamilton et al., 2014; Watson et al., 2014), which will have clear detrimental implications at the population level (Munday et al., 2009, 2010; Chivers et al., 2014). However, animals reared in many aquaculture settings are living in a relatively benign environment, being provided with abundant food, relatively constant environmental conditions, protection against disease and absence of a predation threat. Therefore, it is perhaps not surprising that the ecologically relevant physiological and behavioural disruptions caused by end‐of‐century CO2 levels in OA studies have not emerged from aquaculture studies. Equally it may be possible these behavioural effects have not been noted as they are not typically measured in aquaculture studies. Nevertheless, this does not mean that animals reared in an aquaculture setting are not facing problems associated with elevated CO2 that potentially influence their health and/or production efficiency.
Cross‐discipline interaction to improve understanding of CO2 consequences
Given these contrasting views, combining the knowledge that has arisen from climate change and aquaculture research is crucial to allow a more in‐depth understanding of the physiological and ecological responses of aquatic animals to elevated CO2. The opportunity to compare these two fields directly is appealing, and should enable a more accurate prediction of the consequences of changing climatic conditions for wild populations and intensive aquaculture practices alike. However, at present, such comparison is not straightforward. This is partly due to the different experimental measures and reporting protocols typically adopted by each of these scientific fields. To facilitate this process, it would be fruitful to develop a collective research agenda and implement standard operating procedures with respect to hypothesis development, experimental outcomes and data reporting.
The comparison is also complicated by rather different species often being used in aquaculture compared to OA research, with the former inevitably relying on species that are amenable to domestication, which may go hand in hand with greater environmental tolerance. Indeed, when considering contrasting results from aquatic acidification and aquaculture fields, it is worth noting that responses from even closely related species can often vary significantly. For example, Ferrari et al. (2011) demonstrated a striking and unexpected difference for the impact of CO2 on the antipredator response of closely related damselfish species. Similarly, Lefevre (2016) and Heuer & Grosell (2014) highlight heterogeneity in physiological responses to elevated CO2 that argues against a unifying physiological theory for defining CO2 tolerance, and which needs to be accounted for when modelling and predicting the impacts of climate change. Indeed explaining such interspecies variability with respect to CO2 tolerance may provide a mechanistic understanding of why species used in aquaculture may be relatively tolerant to the CO2 levels prevalent within intensive production. However, it is important to note that even cod reared under end‐of‐century CO2 levels (1000 μatm) exhibit avoidance behaviour towards these conditions when presented with a choice, indicating negligible habituation and suggesting these conditions are unfavourable (Jutfelt & Hedgärde, 2013). Furthermore, a growing body of evidence shows that levels of CO2 experienced in aquaculture may be more detrimental than traditionally perceived (Heuer & Grosell, 2014). For example, Tirsgaard et al. (2015) and Ou et al. (2015) demonstrated detrimental effects of elevated CO2 in cod and salmon, respectively, species traditionally grown successfully under aquaculture settings. Exposure to 9200 μatm resulted in longer meal processing time and less efficient digestion in cod (Tirsgaard et al., 2015), whilst exposure to 2000 μatm reduced growth and production efficiency in salmon larvae (Ou et al., 2015), end‐point measures that are of specific importance to aquaculture production. Thus, differences between these two fields in the perceived impact of elevated CO2 cannot be explained solely by variability in interspecific responses. Measuring the impact of elevated CO2 on a diverse array of physiological and behavioural endpoints, not just those traditionally perceived as important for aquaculture production, is thus vital. It is also crucial to measure these responses in as many species as possible, both finfish and shellfish, as well as those traditionally perceived as CO2 tolerant and CO2 sensitive. By doing so, it will be possible to optimize water quality parameters within aquaculture, based on a species‐specific suite of physiological and behavioural CO2 tolerance endpoints. Targeting these conditions has the potential to maximize growth efficiency and health of aquaculture species, enhancing the sustainability of seafood production. With that aim, it is critical to understand the practical considerations of reducing and maintaining environmental conditions, particularly CO2, in an aquaculture context. Targets should thus be set that optimize productivity and welfare of the aquaculture species, but which are equally achievable in a practical and economical context (Noble et al., 2012).
To optimize research efforts and ensure data are both scientifically robust and comparable, a unified protocol for selecting, manipulating, measuring and finally reporting carbonate chemistry parameters is also needed. This is of particular importance given the methods of carbonate chemistry manipulation employed within intensive aquaculture, for example the addition of a strong alkali to buffer changes in pH, such as sodium hydroxide (NaOH), sodium bicarbonate (NaHCO3), calcium hydroxide (Ca(OH)2) or calcium oxide (CaO). This is the most commonly used of all water chemistry quality management practices in aquaculture, being typically employed in a diverse array of aquaculture settings (Boyd et al., 2016). However, this method of pH compensation additionally elevates alkalinity, often significantly beyond any natural analogue (Ellis et al., in preparation), and depending on the alkali used can have dramatic indirect effects on additional water chemistry parameters, some of which are shown themselves to influence a number physiological processes in aquatic organisms (Boyd et al., 2016; Middlemiss et al., 2016). A further crucial issue is the selection of experimental controls representing present‐day CO2 levels (400 μatm), and we propose this should be a common reference point for both climate change and aquaculture researchers. Control levels employed within aquaculture research typically exceed 1000 μatm (range 1000–3000 μatm) (Fivelstad et al., 1999; Petochi et al., 2011) and thus surpass most of the ‘high CO2’ treatments used as end‐of‐century projections in climate change studies. To complicate matters further, reporting CO2 levels as mg L−1 in aquaculture studies overlooks the impact of temperature and salinity on the solubility of CO2 and the resulting impact these have on the partial pressure of this gas (Weiss, 1974; Dickson, 2011). For example, for the same mg L−1 concentration, the actual partial pressure of CO2 varies by more than threefold between cold freshwater and warm sea water (Fig. 2). This is critical because it is partial pressure (not the mg L−1 concentration) that determines the internal (blood) levels of CO2 and its impact on physiology, behaviour, growth, etc. At present, the scarcity of sufficient water chemistry parameters being presented, the lack of environmentally relevant controls and the prevalence of reporting CO2 levels as mg L−1 in aquaculture literature preclude an unambiguous comparison between data from these two fields.
Figure 2.
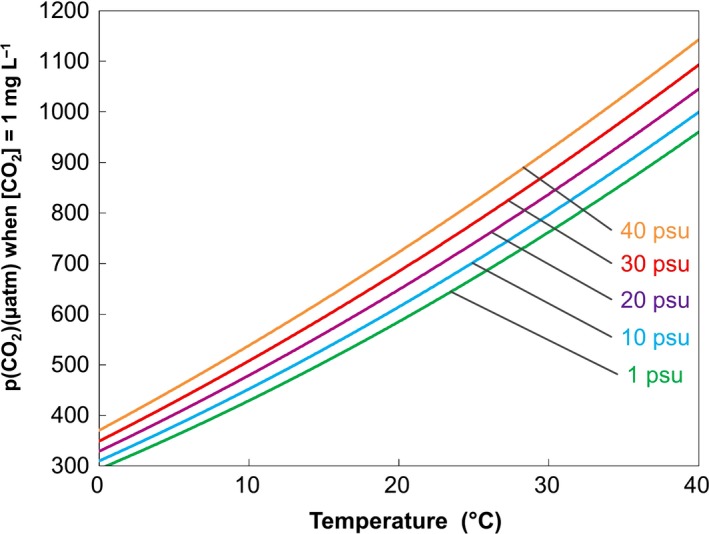
Schematic representation of the conversion of 1 mg L−1 dissolved CO 2 concentration into partial pressure (μatm) at a range of different temperatures and salinities. This shows the very large influence of temperature in particular (up to 3.2‐fold higher partial pressure at the warmest temperature compared to the coolest) but also salinity (up to 26% higher partial pressure at the highest salinity compared to freshwater) on the CO 2 partial pressure due to the impact these abiotic factors have on the solubility of CO 2 in water (Dickson, 2011; Weiss, 1974). Conversion of dissolved CO 2 in mg L−1 to partial pressure in μatm was undertaken using the CO2SYS programme (Pierrot et al., 2006), using dissociation constants from Mehrbach et al. (1973), refit by Dickson & Millero (1987), and KSO 4 using Dickson (1990), with values for CO 2 solubility at different temperatures and salinities checked against Weiss (1974).
Finally, understanding and reporting the provenance of the study species/population will be important to enable a more in‐depth assessment of CO2 tolerance, that is whether animals are wild‐caught, laboratory‐bred or reared within an aquaculture setting (potentially already at very high CO2 when considered in a climate change experimental context). It is fair to say that many (though not all) laboratory‐based climate change studies benefit from easy access to study species available from aquaculture. The systematic selection of traits of interest by the aquaculture industry, such as fast growth and resistance to pathogens, has inherently selected for good performance under intensive farming conditions. In that context, it is possible, and even likely, that additional nontarget traits have also been selected, potentially including those involved in CO2 tolerance. Indeed, enhanced CO2 tolerance has been demonstrated in selectively bred populations of the Sydney rock oyster, compared to its wild‐type congeners (Parker et al., 2011, 2015; Thompson et al., 2015). Furthermore, as demonstrated by Malvezzi et al. (2015), early life survival at elevated CO2 concentrations can have a significant additive genetic element (i.e. highly heritable), which under sufficient selection pressure could elicit a strong and rapid evolutionary response. It is highly likely therefore that aquaculture practices operating at elevated CO2 concentrations would elicit sufficient selection pressure to directly select for CO2 tolerance during early life stages, leading to the rapid evolution of the population in just a few generations. Thus, exploring the traits selected for in broodstock within intensive aquaculture offers a fascinating opportunity to investigate multigenerational adaptation to CO2 levels experienced under intensive production conditions in aquaculture species. In addition, it will be vital to undertake multigenerational studies in order to discern the transgenerational acclimation to elevated CO2 of different fish species with respect to different behavioural (e.g. Welch et al., 2014) and physiological (e.g. Miller et al., 2012) endpoints. Combining the understanding from these two fields will therefore help determine the physiological basis for CO2 tolerance, determine its true ecological consequence and determine its ecological impacts over relevant timescales.
Conclusions
The yield from wild capture fisheries has plateaud since the late 1980s and human consumption from aquaculture exceeded that from wild sources for the first time in 2014 (FAO, 2014). Furthermore, as stated previously, aquaculture is likely to be the only pathway for increasing seafood production in the future. Moving from a capture to a culture mentality requires a shift in attitude that will require time, a luxury that is ill‐afforded in the rapidly changing environment of the Anthropocene. Creating opportunities for the aquatic acidification community and the aquaculture industry to work together should help to speed up this process and enable the aquaculture industry to rapidly adapt by using better‐informed decisions to a) optimize the water chemistry conditions within intensive aquaculture to suit the species and/or b) select traits within the species to suit intensive aquaculture conditions. This will help address the environmental, economic and social impacts of this developing sector towards a sustainable intensification of production, enhancing food security and its resilience to climate change. Equally, this cross‐discipline interaction should also improve our capability to predict and mitigate the consequences of the changing chemistry for natural ecosystems in a future ‘high’ CO2 world.
Author contributions
R.W.W. won the funding for aquaculture and aquatic acidification projects that stimulated this article and produced Fig. 2. R.E led the formulation of the paper and produced Fig. 1. M.U compiled the initial draft. All authors contributed equally to discussions, figure development, editing and production of the final manuscript.
Competing financial interests
The authors declare no competing financial interests.
Acknowledgements
The authors wish to acknowledge the funding that has contributed to ideas within this manuscript. This includes a United Kingdom Ocean Acidification Research Program (UKOARP) Project (NE/H01750X/1 to R.W.W.) cofunded by the Natural Environment Research Council (NERC), the Department for Environment, Food and Rural Affairs (Defra) and the Department of Energy and Climate Change (DECC), together with various BBSRC‐funded projects (BB/J00913X/1, BB/N013344/1 and BB/M017583/1 to R.W.W.).
Note
Seafood in this context refers to all fish and shellfish species produced under fresh, brackish or marine conditions and intended for human consumption
Contributor Information
Robert P. Ellis, Email: R.P.Ellis@exeter.ac.uk
Mauricio A. Urbina, Email: mauriciourbina@udec.cl
Rod W. Wilson, Email: R.W.Wilson@exeter.ac.uk
References
- Arnold KE, Findlay HS, Spicer JI, Daniels CL, Boothroyd D (2009) Effect of CO2‐related acidification on aspects of the larval development of the European lobster, Homarus gammarus (L.). Biogeosciences, 6, 1747–1754. [Google Scholar]
- Barton A, Hales B, Waldbusser GG, Langdon C, Feely RA (2012) The Pacific oyster, Crassostrea gigas, shows negative correlation to naturally elevated carbon dioxide levels: implications for near‐term ocean acidification effects. Limnology and Oceanography, 57, 698–710. [Google Scholar]
- Barton A, Waldbusser GG, Feely RA, Hales B, Langdon CJ (2015) Impacts of coastal acidification on the Pacific Northwest shellfish industry and adaptation strategies implemented in response. Oceanography, 28, 146–159. [Google Scholar]
- Bennett NJ, Blythe J, Tyler S, Ban NC (2016) Communities and change in the anthropocene: understanding social‐ecological vulnerability and planning adaptations to multiple interacting exposures. Regional Environmental Change, 16, 907–926. [Google Scholar]
- Blancheton JP (2000) Developments in recirculation systems for Mediterranean fish species. Aquacultural Engineering, 22, 17–31. [Google Scholar]
- Boyd CE, Tucker CS, Somridhivej B (2016) Alkalinity and hardness: critical but elusive concepts in aquaculture. Journal of the World Aquaculture Society, 47, 6–41. [Google Scholar]
- Briffa M, de la Haye K, Munday PL (2012) High CO2 and marine animal behaviour: potential mechanisms and ecological consequences. Marine Pollution Bulletin, 64, 1519–1528. [DOI] [PubMed] [Google Scholar]
- Cameron JN, Randall DJ (1972) The effect of increased ambient CO2 on arterial CO2 tension, CO2 content and pH in rainbow trout. Journal of Experimental Biology, 57, 673–680. [DOI] [PubMed] [Google Scholar]
- Chambers RC, Candelmo AC, Habeck EA et al (2014) Effects of elevated CO2 in the early life stages of summer flounder, Paralichthys dentatus, and potential consequences of ocean acidification. Biogeosciences, 11, 1613–1626. [Google Scholar]
- Chivers DP, McCormick MI, Nilsson GE et al (2014) Impaired learning of predators and lower prey survival under elevated CO2: a consequence of neurotransmitter interference. Global Change Biology, 20, 515–522. [DOI] [PubMed] [Google Scholar]
- Dickson AG (1990) Standard potential of the reaction: and the standard acidity constant of the ion HSO4 ‐ in synthetic sea water from 273.15 to 318.15 K. The Journal of Chemical Thermodynamics, 22, 113–127. [Google Scholar]
- Dickson AG (2011) The carbon dioxide system in seawater: equilibrium chemistry and measurements In: Guide to Best Practices for Ocean Acidification Research and Data Reporting. (eds Riebesell U, Fabry VJ, Hansson L, Gattuso JP.), pp 17–40. Publications Office of the European Union, Luxembourg. [Google Scholar]
- Dickson AG, Millero FJ (1987) A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep‐Sea Research, 34, 1733–1743. [Google Scholar]
- Dlugokencky E, Pieter T (2016) Trends in Atmospheric Carbon Dioxide. (ed Division N‐E‐GM ).
- Ern R, Esbaugh AJ (2016) Hyperventilation and blood acid–base balance in hypercapnia exposed red drum (Sciaenops ocellatus). Journal of Comparative Physiology B, 186, 447–460. [DOI] [PubMed] [Google Scholar]
- Esbaugh AJ, Heuer R, Grosell M (2012) Impacts of ocean acidification on respiratory gas exchange and acid–base balance in a marine teleost, Opsanus beta . Journal of Comparative Physiology B, 182, 921–934. [DOI] [PubMed] [Google Scholar]
- Esbaugh AJ, Ern R, Nordi WM, Johnson AS (2016) Respiratory plasticity is insufficient to alleviate blood acid–base disturbances after acclimation to ocean acidification in the estuarine red drum, Sciaenops ocellatus . Journal of Comparative Physiology B, 186, 97–109. [DOI] [PubMed] [Google Scholar]
- FAO (2014) The State of World Fisheries and Aquaculture ‐ Opportunities and Challenges. Food and Agriculture Organization of the United Nations, Rome, Italy. [Google Scholar]
- Ferrari MCO, Dixson DL, Munday PL, McCormick MI, Meekan MG, Sih A, Chivers DP (2011) Intrageneric variation in antipredator responses of coral reef fishes affected by ocean acidification: implications for climate change projections on marine communities. Global Change Biology, 17, 2980–2986. [Google Scholar]
- Fivelstad S, Olsen AB, Kloften H, Ski H, Stefansson S (1999) Effects of carbon dioxide on Atlantic salmon (Salmo salar) smolts at constant pH in bicarbonate rich freshwater. Aquaculture, 178, 171–187. [Google Scholar]
- Fivelstad S, Kvamme K, Handeland S, Fivelstad M, Olsen AB, Hosfeld CD (2015) Growth and physiological models for Atlantic salmon (Salmo salar L.) parr exposed to elevated carbon dioxide concentrations at high temperature. Aquaculture, 436, 90–94. [Google Scholar]
- Frommel AY, Maneja R, Lowe D et al (2012) Severe tissue damage in Atlantic cod larvae under increasing ocean acidification. Nature Climate Change, 2, 42–46. [Google Scholar]
- Frommel AY, Maneja R, Lowe D et al (2014) Organ damage in Atlantic herring larvae as a result of ocean acidification. Ecological Applications, 24, 1131–1143. [DOI] [PubMed] [Google Scholar]
- Gerland P, Raftery AE, Ševčíková H et al (2014) World population stabilization unlikely this century. Science, 348, 234–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves P, Anderson K, Thompson EL, Melwani A, Parker L, Ross PM, Raftos DA (2016) Rapid transcriptional acclimation following transgenerational exposure of oysters to ocean acidification. Molecular Ecology, 25, 4836–4849. [DOI] [PubMed] [Google Scholar]
- Hamilton TJ, Holcombe A, Tresguerres M (2014) CO2‐induced ocean acidification increases anxiety in rockfish via alteration of GABAA receptor functioning. Proceedings of the Royal Society of London B: Biological Sciences, 281, 20132509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer RM, Grosell M (2014) Physiological impacts of elevated carbon dioxide and ocean acidification on fish. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 307, R1061–R1084. [DOI] [PubMed] [Google Scholar]
- Heuer RM, Esbaugh AJ, Grosell M (2012) Ocean acidification leads to counterproductive intestinal base loss in the Gulf Toadfish (Opsanus beta). Physiological and Biochemical Zoology: Ecological and Evolutionary Approaches, 85, 450–459. [DOI] [PubMed] [Google Scholar]
- Jutfelt F, Hedgärde M (2013) Atlantic cod actively avoid CO2 and predator odour, even after long‐term CO2 exposure. Frontiers in Zoology, 10, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutfelt F, Bresolin de Souza K, Vuylsteke A, Sturve J (2013) Behavioural disturbances in a temperate fish exposed to sustained high‐CO2 levels. PLoS ONE, 8, e65825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A (1904) On the tension of carbonic acid in natural waters and especially in the sea. Meddelelser om Grønland, 26, 231–342. [Google Scholar]
- Lannig G, Eilers S, Pörtner HO, Sokolva IM, Bock C (2010) Impact of ocean acidification on energy metabolism of oyster, Crassostrea gigas ‐ changes in metabolic pathways and thermal response. Marine Drugs, 8, 2318–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre S (2016) Are global warming and ocean acidification conspiring against marine ectotherms? A meta‐analysis of the respiratory effects of elevated temperature, high CO2 and their interaction Conservation Physiology, 4, cow009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C, Ellis RP, Vernon E, Elliot K, Newbatt S, Wilson RW (2016) Ocean acidification increases copper toxicity differentially in two key marine invertebrates with distinct acid‐base responses. Scientific Reports, 6, 21554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthi D, Le Floch M, Bereiter B et al (2008) High‐resolution carbon dioxide concentration record 650,000‐800,000 years before present. Nature, 453, 379–382. [DOI] [PubMed] [Google Scholar]
- Malvezzi AJ, Murray CS, Feldheim KA et al (2015) A quantitative genetic approach to assess the evolutionary potential of a coastal marine fish to ocean acidification. Evolutionary Applications, 8, 352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneja R, Frommel A, Geffen A, Folkvord A, Piatkowski U, Chang M, Clemmesen C (2013) Effects of ocean acidification on the calcification of otoliths of larval Atlantic cod Gadus morhua . Marine Ecology Progress Series, 477, 251–258. [Google Scholar]
- Maneja RH, Dineshram R, Thiyagarajan V et al (2014) The proteome of Atlantic herring (Clupea harengus L.) larvae is resistant to elevated pCO2 . Marine Pollution Bulletin, 86, 154–160. [DOI] [PubMed] [Google Scholar]
- Martins CIM, Eding EH, Verdegem MCJ et al (2010) New developments in recirculating aquaculture systems in Europe: a perspective on environmental sustainability. Aquacultural Engineering, 43, 83–93. [Google Scholar]
- McNeil BI, Sasse TP (2016) Future ocean hypercapnia driven by anthropogenic amplification of the natural CO2 cycle. Nature, 529, 383–386. [DOI] [PubMed] [Google Scholar]
- Mehrbach C, Culberson CH, Hawley JE, Pytkowicz RM (1973) Measurements of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnology and Oceanography, 18, 897–906. [Google Scholar]
- Melzner F, Göbel S, Langenbuch M, Gutowska MA, Pörtner H‐O, Lucassen M (2009) Swimming performance in Atlantic Cod (Gadus morhua) following long‐term (4–12 months) acclimation to elevated seawater. Aquatic Toxicology, 92, 30–37. [DOI] [PubMed] [Google Scholar]
- Michaelidis B, Spring A, Pörtner HO (2007) Effects of long‐term acclimation to environmental hypercapnia on extracellular acid–base status and metabolic capacity in Mediterranean fish Sparus aurata . Marine Biology, 150, 1417–1429. [Google Scholar]
- Middlemiss KL, Urbina MA, Wilson RW (2016) Effects of seawater alkalinity on calcium and acid–base regulation in juvenile European lobster (Homarus gammarus) during a moult cycle. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 193, 22–28. [DOI] [PubMed] [Google Scholar]
- Miller GM, Watson S‐A, Donelson JM, McCormick MI, Munday PL (2012) Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nature Climate Change, 2, 858–861. [Google Scholar]
- Munday PM, Dixson DL, Donelson JM, Jones GP, Pratchett MS, Devitsina GV, Døving KB (2009) Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proceedings of the National Academy of Science USA, 106, 1848–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday PL, Dixson DL, McCormick MI, Meekan M, Ferrari MCO, Chivers DP (2010) Replenishment of fish populations is threatened by ocean acidification. Proceedings of the National Academy of Science USA, 107, 12930–12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson GE, Dixson DL, Domenici P, McCormick MI, Sørensen C, Watson S‐A, Munday PL (2012) Near‐future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nature Climate Change, 2, 201–204. [Google Scholar]
- Noble C, Kankainen M, Setälä J, Berrill IK, Ruohonen K, Damsgård B, Toften H (2012) The bio‐economic costs and benefits of improving productivity and fish welfare in aquaculture: utilizing CO2 stripping technology in norwegian atlantic salmon smolt production. Aquaculture Economics & Management, 16, 414–428. [Google Scholar]
- Ou M, Hamilton TJ, Eom J et al (2015) Responses of pink salmon to CO2‐induced aquatic acidification. Nature Climate Change, 5, 950–955. [Google Scholar]
- Parker L, Ross PM, O'Connor WA (2011) Populations of the Sydney rock oyster, Saccostrea glomerata, vary in response to ocean acidification. Marine Biology, 158, 689–697. [Google Scholar]
- Parker LM, O'Connor WA, Raftos DA, Pörtner H‐O, Ross PM (2015) Persistence of positive carryover effects in the oyster, Saccostrea glomerata, following transgenerational exposure to ocean acidification. PLoS ONE, 10, e0132276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly D, Zeller D (2016) Catch reconstructions reveal that global marine fisheries catches are higher than reported and declining. Nature Communications, 7, 10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petochi T, Di Marco P, Priori A, Finoia MG, Mercatali I, Marino G (2011) Coping strategy and stress response of European sea bass Dicentrarchus labrax to acute and chronic environmental hypercapnia under hyperoxic conditions. Aquaculture, 315, 312–320. [Google Scholar]
- Pierrot EC, Lewis E, Wallace DWR (2006) CO2SYS Dos Program Developed for CO2 System Calculations. ORNL/CDIAC‐105. Carbon Dioxide Information Analysis Centre, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, TN. [Google Scholar]
- Pope EC, Ellis RP, Scolamacchia M et al (2014) European sea bass, Dicentrarchus labrax, in a changing ocean. Biogeosciences, 11, 2519–2530. [Google Scholar]
- Porter JR, Xie L, Challinor AJ et al (2014) Food security and food production systems In: Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. (eds Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, Maccracken S, Mastrandrea PR, White LL.), pp. 485–534. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA. [Google Scholar]
- Pörtner HO, Karl DM, Boyd PW et al (2014) Ocean systems In: Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. (eds Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, Maccracken S, Mastrandrea PR, White LL.), pp. 411–484. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA. [Google Scholar]
- Roggatz CC, Lorch M, Hardege JD, Benoit DM (2016) Ocean acidification affects marine chemical communication by changing structure and function of peptide signalling molecules. Global Change Biology, 22, 3914–3926. [DOI] [PubMed] [Google Scholar]
- Sahu BC, Adhikari S, Mahapatra AS, Dey L (2013) Carbon, nitrogen, and posphorous budget in scampi (Macrobrachium rosenbergii) culture ponds. Environmental Monitoring and Assessment, 185, 10157–10166. [DOI] [PubMed] [Google Scholar]
- Saksena DN, Gaidhane DM, Singh H (2006) Limnology of Kharland (saline) ponds of Ratnagiri, Maharashtra in relation to prawn culture potential. Journal of Environmental Biology, 27, 49–53. [PubMed] [Google Scholar]
- Schalkhausser B, Bock C, Stemmer K, Brey T, Pörtner H‐O, Lannig G (2012) Impact of ocean acidification on escape performance of the king scallop, Pecten maximus, from Norway. Marine Biology, 160, 1995–2006. [Google Scholar]
- Seidelin M, Brauner CJ, Jensen FB, Madsen SS (2001) Vacuolar‐Type H+‐ATPase and Na+, K+‐ATPase expression in gills of Atlantic Salmon (Salmo salar) during isolated and combined exposure to hyperoxia and hypercapnia in fresh water. Zoological Science, 18, 1199–1205. [DOI] [PubMed] [Google Scholar]
- Simpson SD, Munday PL, Wittenrich ML, Manassa R, Dixson DL, Gagliano M, Yan HY (2011) Ocean acidification erodes crucial auditory behaviour in a marine fish. Biology Letters, 7, 917–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DP, Calosi P, Boothroyd D, Widdicombe S, Spicer JI (2016) The sensitivity of the early benthic juvenile stage of the European lobster Homarus gammarus (L.) to elevated pCO2 and temperature. Marine Biology, 163, 1–12. [Google Scholar]
- Talmage SC, Gobler CJ (2009) The effects of elevated carbon dioxide concentrations on the metamorphosis, size, and survival of larval hard clams (Mercenaria mercenaria), bay scallops (Argopecten irradians), and Eastern oysters (Crassostrea virginica). Limnology and Oceanography, 54, 2072–2080. [Google Scholar]
- Thompson EL, O'Connor W, Parker L, Ross P, Raftos DA (2015) Differential proteomic responses of selectively bred and wild‐type Sydney rock oyster populations exposed to elevated CO2 . Molecular Ecology, 24, 1248–1262. [DOI] [PubMed] [Google Scholar]
- Tirsgaard B, Moran D, Steffensen JF (2015) Prolonged SDA and reduced digestive efficiency under elevated CO2 may explain reduced growth in Atlantic cod (Gadus morhua). Aquatic Toxicology, 158, 171–180. [DOI] [PubMed] [Google Scholar]
- Tseng Y‐C, Hu MY, Stumpp M, Lin L‐Y, Melzner F, Hwang P‐P (2013) CO2‐driven seawater acidification differentially affects development and molecular plasticity along life history of fish (Oryzias latipes). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 165, 119–130. [DOI] [PubMed] [Google Scholar]
- Watson S‐A, Lefevre S, McCormick MI, Domenici P, Nilsson GE, Munday PL (2014) Marine mollusc predator‐escape behaviour altered by near‐future carbon dioxide levels. Proceedings of the National Academy of Sciences B, 281, 20132377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Wang Q, Ning X et al (2015) Combined metabolome and proteome analysis of the mantle tissue from Pacific oyster Crassostrea gigas exposed to elevated pCO2 . Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 13, 16–23. [DOI] [PubMed] [Google Scholar]
- Weiss RF (1974) Carbon dioxide in water and seawater: the solubility of a non‐ideal gas. Marine Chemistry, 2, 203–215. [Google Scholar]
- Welch MJ, Watson S‐A, Welsh JQ, McCormick MI, Munday PL (2014) Effects of elevated CO2 on fish behaviour undiminished by transgenerational acclimation. Nature Climate Change, 4, 1086–1089. [Google Scholar]


