Abstract
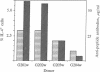
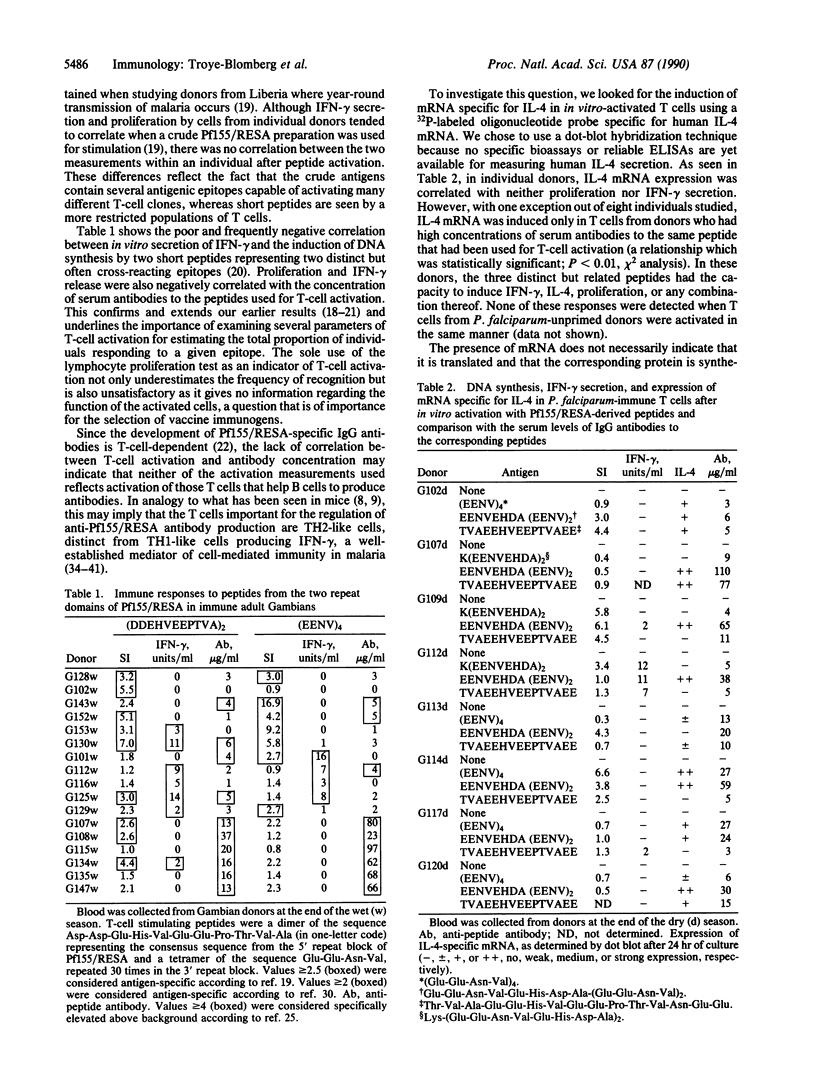
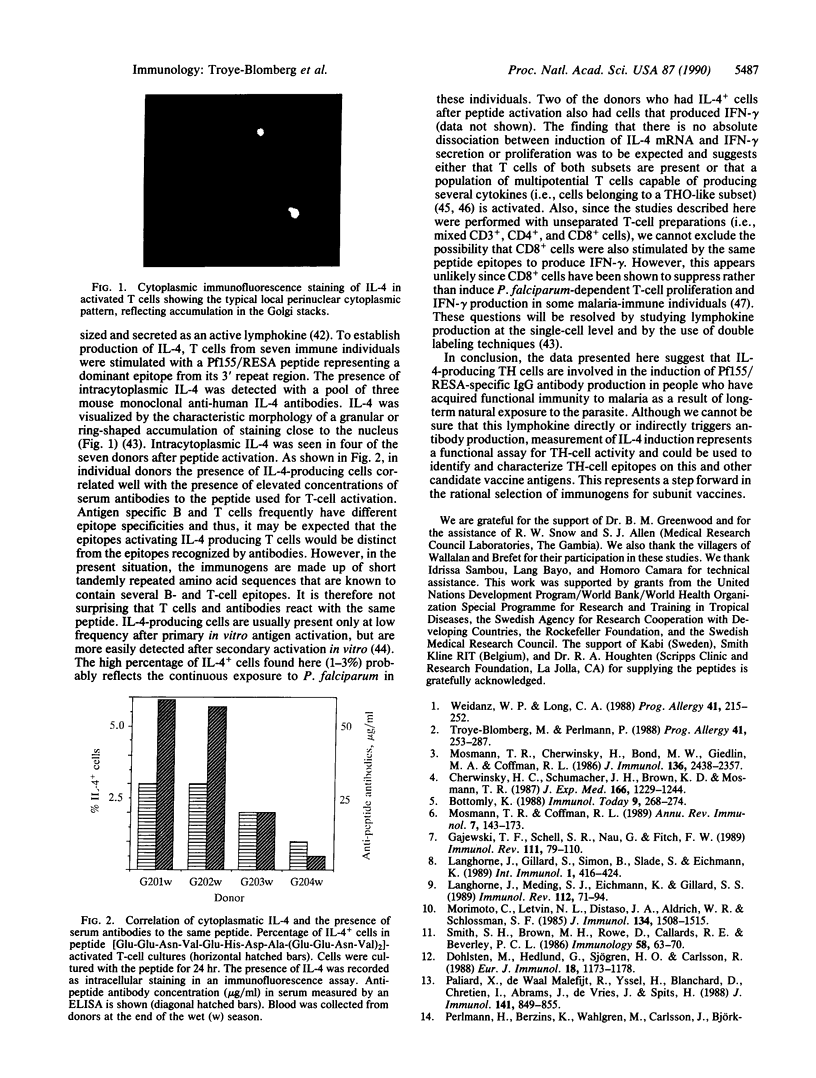
T cells play a crucial role in antibody-mediated and antibody-independent immunity against Plasmodium falciparum malaria. Therefore, a vaccine immunogen should include parasite-derived B- and T-cell epitopes capable of giving rise to protective responses in both systems. The P. falciparum antigen Pf155/ring-infected erythrocyte surface antigen (RESA), a vaccine candidate, contains immunodominant T- and B-cell epitopes located in the central (5') and C-terminal (3') invariant repeat regions of the molecule. To relate Pf155/RESA-peptide-specific responses of T cells to function, T cells from P. falciparum immune donors were activated with peptides corresponding to these immunodominant regions. Activation was measured as induction of interferon-gamma secretion, T-cell proliferation (DNA synthesis), or transcription and translation of interleukin 4 (IL-4) mRNA. Peptides from both regions were shown to induce interferon-gamma, IL-4, proliferation, or any combination. In individual donors, there was no correlation between these different activities. Rather, they were negatively correlated, demonstrating the importance of examining multiple parameters of T-cell activation when estimating the proportion of individuals responding to a given epitope. However, IL-4 mRNA and intracellular IL-4 could be induced in T cells of donors who had elevated concentrations of serum antibodies to the same peptide that was used for T-cell activation. These results suggest that a causal relationship exists between the activation of IL-4-producing T-cell subsets and production of the anti-Pf155/RESA-specific antibodies in individuals in which immunity has been induced by natural infection. This finding has implications that should be considered for the selection of immunogens to be included in a future P. falciparum subunit vaccine and for vaccine development in general.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson G., Ekre H. P., Alm G., Perlmann P. Monoclonal antibody two-site ELISA for human IFN-gamma. Adaptation for determinations in human serum or plasma. J Immunol Methods. 1989 Dec 20;125(1-2):89–96. doi: 10.1016/0022-1759(89)90081-1. [DOI] [PubMed] [Google Scholar]
- Andersson U., Sander B., Andersson J., Möller G. Concomitant production of different lymphokines in activated T cells. Eur J Immunol. 1988 Dec;18(12):2081–2084. doi: 10.1002/eji.1830181232. [DOI] [PubMed] [Google Scholar]
- Berzins T., Axelsson B., Hammarström M. L., Hammarström S., Perlmann P. Studies on the role of lymphocyte function-associated antigen 1 (LFA-1) in T cell activation. Scand J Immunol. 1988 Jan;27(1):7–16. doi: 10.1111/j.1365-3083.1988.tb02317.x. [DOI] [PubMed] [Google Scholar]
- Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1988 Sep;9(9):268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins W. E., Anders R. F., Pappaioanou M., Campbell G. H., Brown G. V., Kemp D. J., Coppel R. L., Skinner J. C., Andrysiak P. M., Favaloro J. M. Immunization of Aotus monkeys with recombinant proteins of an erythrocyte surface antigen of Plasmodium falciparum. Nature. 1986 Sep 18;323(6085):259–262. doi: 10.1038/323259a0. [DOI] [PubMed] [Google Scholar]
- Coppel R. L., Cowman A. F., Anders R. F., Bianco A. E., Saint R. B., Lingelbach K. R., Kemp D. J., Brown G. V. Immune sera recognize on erythrocytes Plasmodium falciparum antigen composed of repeated amino acid sequences. 1984 Aug 30-Sep 5Nature. 310(5980):789–792. doi: 10.1038/310789a0. [DOI] [PubMed] [Google Scholar]
- Dohlsten M., Hedlund G., Sjögren H. O., Carlsson R. Two subsets of human CD4+ T helper cells differing in kinetics and capacities to produce interleukin 2 and interferon-gamma can be defined by the Leu-18 and UCHL1 monoclonal antibodies. Eur J Immunol. 1988 Aug;18(8):1173–1178. doi: 10.1002/eji.1830180805. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Schofield L., Enea V., Schellekens H., van der Meide P., Collins W. E., Nussenzweig R. S., Nussenzweig V. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science. 1986 May 16;232(4752):881–884. doi: 10.1126/science.3085218. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Roeder W. D., Laxer J. A., Townsend K. S., Weaver C. T., Hom J. T., Linton J., Torbett B. E., Glasebrook A. L. A new murine CD4+ T cell subset with an unrestricted cytokine profile. J Immunol. 1989 Jul 15;143(2):518–525. [PubMed] [Google Scholar]
- Gajewski T. F., Schell S. R., Nau G., Fitch F. W. Regulation of T-cell activation: differences among T-cell subsets. Immunol Rev. 1989 Oct;111:79–110. doi: 10.1111/j.1600-065x.1989.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Greenwood B. M., Bradley A. K., Greenwood A. M., Byass P., Jammeh K., Marsh K., Tulloch S., Oldfield F. S., Hayes R. Mortality and morbidity from malaria among children in a rural area of The Gambia, West Africa. Trans R Soc Trop Med Hyg. 1987;81(3):478–486. doi: 10.1016/0035-9203(87)90170-2. [DOI] [PubMed] [Google Scholar]
- Hardy K. J., Sawada T. Human gamma interferon strongly upregulates its own gene expression in peripheral blood lymphocytes. J Exp Med. 1989 Sep 1;170(3):1021–1026. doi: 10.1084/jem.170.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabilan L., Troye-Blomberg M., Patarroyo M. E., Björkman A., Perlmann P. Regulation of the immune response in Plasmodium falciparum malaria: IV. T cell dependent production of immunoglobulin and anti-P. falciparum antibodies in vitro. Clin Exp Immunol. 1987 May;68(2):288–297. [PMC free article] [PubMed] [Google Scholar]
- Kabilan L., Troye-Blomberg M., Perlmann H., Andersson G., Högh B., Petersen E., Björkman A., Perlmann P. T-cell epitopes in Pf155/RESA, a major candidate for a Plasmodium falciparum malaria vaccine. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5659–5663. doi: 10.1073/pnas.85.15.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Miller L. H., Quakyi I. A., Keister D. B., Houghten R. A., Maloy W. L., Moss B., Berzofsky J. A., Good M. F. Cytotoxic T cells specific for the circumsporozoite protein of Plasmodium falciparum. Nature. 1988 Jul 21;334(6179):258–260. doi: 10.1038/334258a0. [DOI] [PubMed] [Google Scholar]
- Langhorne J., Gillard S., Simon B., Slade S., Eichmann K. Frequencies of CD4+ T cells reactive with Plasmodium chabaudi chabaudi: distinct response kinetics for cells with Th1 and Th2 characteristics during infection. Int Immunol. 1989;1(4):416–424. doi: 10.1093/intimm/1.4.416. [DOI] [PubMed] [Google Scholar]
- Langhorne J., Meding S. J., Eichmann K., Gillard S. S. The response of CD4+ T cells to Plasmodium chabaudi chabaudi. Immunol Rev. 1989 Dec;112:71–94. doi: 10.1111/j.1600-065x.1989.tb00553.x. [DOI] [PubMed] [Google Scholar]
- Maheshwari R. K., Czarniecki C. W., Dutta G. P., Puri S. K., Dhawan B. N., Friedman R. M. Recombinant human gamma interferon inhibits simian malaria. Infect Immun. 1986 Sep;53(3):628–630. doi: 10.1128/iai.53.3.628-630.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Paliard X., de Waal Malefijt R., Yssel H., Blanchard D., Chrétien I., Abrams J., de Vries J., Spits H. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J Immunol. 1988 Aug 1;141(3):849–855. [PubMed] [Google Scholar]
- Perlmann H., Berzins K., Wahlgren M., Carlsson J., Björkman A., Patarroyo M. E., Perlmann P. Antibodies in malarial sera to parasite antigens in the membrane of erythrocytes infected with early asexual stages of Plasmodium falciparum. J Exp Med. 1984 Jun 1;159(6):1686–1704. doi: 10.1084/jem.159.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann H., Perlmann P., Berzins K., Wåhlin B., Troye-Blomberg M., Hagstedt M., Andersson I., Högh B., Petersen E., Björkman A. Dissection of the human antibody response to the malaria antigen Pf155/RESA into epitope specific components. Immunol Rev. 1989 Dec;112:115–132. doi: 10.1111/j.1600-065x.1989.tb00555.x. [DOI] [PubMed] [Google Scholar]
- Playfair J. H., De Souza J. B. Recombinant gamma interferon is a potent adjuvant for a malaria vaccine in mice. Clin Exp Immunol. 1987 Jan;67(1):5–10. [PMC free article] [PubMed] [Google Scholar]
- Powers G. D., Abbas A. K., Miller R. A. Frequencies of IL-2- and IL-4-secreting T cells in naive and antigen-stimulated lymphocyte populations. J Immunol. 1988 May 15;140(10):3352–3357. [PubMed] [Google Scholar]
- Riley E. M., Jepsen S., Andersson G., Otoo L. N., Greenwood B. M. Cell-mediated immune responses to Plasmodium falciparum antigens in adult Gambians. Clin Exp Immunol. 1988 Mar;71(3):377–382. [PMC free article] [PubMed] [Google Scholar]
- Riley E. M., Jobe O., Whittle H. C. CD8+ T cells inhibit Plasmodium falciparum-induced lymphoproliferation and gamma interferon production in cell preparations from some malaria-immune individuals. Infect Immun. 1989 Apr;57(4):1281–1284. doi: 10.1128/iai.57.4.1281-1284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzepczyk C. M., Ramasamy R., Ho P. C., Mutch D. A., Anderson K. L., Duggleby R. G., Doran T. J., Murray B. J., Irving D. O., Woodrow G. C. Identification of T epitopes within a potential Plasmodium falciparum vaccine antigen. A study of human lymphocyte responses to repeat and nonrepeat regions of Pf155/RESA. J Immunol. 1988 Nov 1;141(9):3197–3202. [PubMed] [Google Scholar]
- Schofield L., Ferreira A., Altszuler R., Nussenzweig V., Nussenzweig R. S. Interferon-gamma inhibits the intrahepatocytic development of malaria parasites in vitro. J Immunol. 1987 Sep 15;139(6):2020–2025. [PubMed] [Google Scholar]
- Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987 Dec 17;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- Shear H. L., Srinivasan R., Nolan T., Ng C. Role of IFN-gamma in lethal and nonlethal malaria in susceptible and resistant murine hosts. J Immunol. 1989 Sep 15;143(6):2038–2044. [PubMed] [Google Scholar]
- Slade S. J., Langhorne J. Production of interferon-gamma during infection of mice with Plasmodium chabaudi chabaudi. Immunobiology. 1989 Oct;179(4-5):353–365. doi: 10.1016/S0171-2985(89)80041-5. [DOI] [PubMed] [Google Scholar]
- Smith S. H., Brown M. H., Rowe D., Callard R. E., Beverley P. C. Functional subsets of human helper-inducer cells defined by a new monoclonal antibody, UCHL1. Immunology. 1986 May;58(1):63–70. [PMC free article] [PubMed] [Google Scholar]
- Troye-Blomberg M., Andersson G., Stoczkowska M., Shabo R., Romero P., Patarroyo M. E., Wigzell H., Perlmann P. Production of IL 2 and IFN-gamma by T cells from malaria patients in response to Plasmodium falciparum or erythrocyte antigens in vitro. J Immunol. 1985 Nov;135(5):3498–3504. [PubMed] [Google Scholar]
- Troye-Blomberg M., Kabilan L., Riley E. M., Ortlund J., Andersson G., Perlmann H., Olerup O., Högh B., Petersen E., Snow R. W. T cell reactivity of defined peptides from a major Plasmodium falciparum vaccine candidate: the Pf155/RESA antigen. Immunol Lett. 1988 Nov;19(3):229–233. doi: 10.1016/0165-2478(88)90147-2. [DOI] [PubMed] [Google Scholar]
- Troye-Blomberg M., Perlmann P. T cell functions in Plasmodium falciparum and other malarias. Prog Allergy. 1988;41:253–287. doi: 10.1159/000415226. [DOI] [PubMed] [Google Scholar]
- Troye-Blomberg M., Riley E. M., Perlmann H., Andersson G., Larsson A., Snow R. W., Allen S. J., Houghten R. A., Olerup O., Greenwood B. M. T and B cell responses of Plasmodium falciparum malaria-immune individuals to synthetic peptides corresponding to sequences in different regions of the P. falciparum antigen Pf155/RESA. J Immunol. 1989 Nov 1;143(9):3043–3048. [PubMed] [Google Scholar]
- Weidanz W. P., Long C. A. The role of T cells in immunity to malaria. Prog Allergy. 1988;41:215–252. [PubMed] [Google Scholar]
- Yokoyama A., Evavold B., Dunn D. E., Quintans J. Production of IL-2 and IFN by TH2 clones. Immunol Lett. 1989 May;21(2):119–125. doi: 10.1016/0165-2478(89)90047-3. [DOI] [PubMed] [Google Scholar]