Abstract
Background and aims
Alcohol use is a major contributor to injuries, mortality and the burden of disease. This review updates knowledge on risk relations between dimensions of alcohol use and health outcomes to be used in global and national Comparative Risk Assessments (CRAs).
Methods
Systematic review of reviews and meta‐analyses on alcohol consumption and health outcomes attributable to alcohol use.
For dimensions of exposure: volume of alcohol use, blood alcohol concentration and patterns of drinking, in particular heavy drinking occasions were studied. For liver cirrhosis, quality of alcohol was additionally considered. For all outcomes (mortality and/or morbidity): cause of death and disease/injury categories based on International Classification of Diseases (ICD) codes used in global CRAs; harm to others.
Results
In total, 255 reviews and meta‐analyses were identified. Alcohol use was found to be linked causally to many disease and injury categories, with more than 40 ICD‐10 three‐digit categories being fully attributable to alcohol. Most partially attributable disease categories showed monotonic relationships with volume of alcohol use: the more alcohol consumed, the higher the risk of disease or death. Exceptions were ischaemic diseases and diabetes, with curvilinear relationships, and with beneficial effects of light to moderate drinking in people without heavy irregular drinking occasions. Biological pathways suggest an impact of heavy drinking occasions on additional diseases; however, the lack of medical epidemiological studies measuring this dimension of alcohol use precluded an in‐depth analysis. For injuries, except suicide, blood alcohol concentration was the most important dimension of alcohol use. Alcohol use caused marked harm to others, which has not yet been researched sufficiently.
Conclusions
Research since 2010 confirms the importance of alcohol use as a risk factor for disease and injuries; for some health outcomes, more than one dimension of use needs to be considered. Epidemiological studies should include measurement of heavy drinking occasions in line with biological knowledge.
Keywords: Alcohol use, average volume, chronic disease, injury, patterns of drinking, risk‐relations, systematic review, unrecorded consumption
Introduction
Alcohol consumption has been identified as a major contributor to the burden of disease and mortality in all the global Comparative Risk Assessments (CRAs 1) conducted thus far as part of the Global Burden of Disease (GBD) studies 2, 3, 4, 5, 6, 7, and in the World Health Organization (WHO) Global Status Reports on Alcohol and Health and their predecessors 8, 9, 10. All CRAs restricted themselves to modifiable risk factors 11, where the modifications could be linked to reductions in the disease burden 12. As a consequence, they have become crucial for guiding health policy 13, not only in terms of primary prevention 14, 15, 16, but also in terms of secondary prevention and health systems management 17, 18, 19.
At the core of any CRA are the risk relations between different dimensions of exposure (in the present case, alcohol use) and particular diseases, disorders or injuries. Each of these relative risks is then combined with the extent of the respective exposure in a particular population to create alcohol‐attributable fractions (AAFs) for that population 20, 21. In most CRAs, including for alcohol, both the relative risk and the prevalence of exposure are continuous functions 22. Knowledge on and estimates of these risk relations have been evolving during the past 15 years (compare the overview from 2003 23, and especially since 2010 when the last overview on this topic in Addiction appeared 24, which the current review will update with the latest evidence. It will follow the structure of the previous reviews 23, 24: first, we will list disease and injury categories which are 100% alcohol‐attributable; secondly, we will address disease categories partly attributable to alcohol, and finally, injury categories which are partly attributable to alcohol will be discussed. In the discussion, we not only outline the limitations of our review, but also look to future research developments.
Methods
Search strategy
For this systematic review, we (a) searched the WHO International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD‐10) 2016 databank 25 for the term ‘alcohol*’ to identify disease and injury categories fully attributable to alcohol (see Table 1), and (b) updated all estimates of alcohol use–disease or injury relationships for partially attributable outcomes from the estimates in the most recent preceding publication 24, following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines 26, 27.
Table 1.
ICD‐10 categories with maximal one decimal with mention of alcohol or alcoholic.
| E24.4 | Alcohol‐induced pseudo‐Cushing's syndrome |
| F10 | Mental and behavioural disorders due to use of alcohol |
| F10.0 | Acute intoxication |
| F10.1 | Harmful use |
| F10.2 | Dependence syndrome |
| F10.3 | Withdrawal state |
| F10.4 | Withdrawal state with delirium |
| F10.5 | Psychotic disorder |
| F10.6 | Amnesic syndrome |
| F10.7 | Residual and late‐onset psychotic disorder |
| F10.8 | Other mental and behavioural disorders |
| F10.9 | Unspecified mental and behavioural disorder |
| G31.2 | Degeneration of nervous system due to alcohol |
| G62.1 | Alcoholic polyneuropathy |
| G72.1 | Alcoholic myopathy |
| I42.6 | Alcoholic cardiomyopathy |
| K29.2 | Alcoholic gastritis |
| K29.20 | Alcoholic gastritis, without mention of haemorrhage |
| K29.21 | Alcoholic gastritis, with haemorrhage |
| K70 | Alcoholic liver disease |
| K70.0 | Alcoholic fatty liver |
| K70.1 | Alcoholic hepatitis |
| K70.2 | Alcoholic fibrosis and sclerosis of liver |
| K70.3 | Alcoholic cirrhosis of liver |
| K70.4 | Alcoholic hepatic failure |
| K70.9 | Alcoholic liver disease, unspecified |
| K85.2 | Alcohol‐induced acute pancreatitis |
| K86.0 | Alcohol‐induced chronic pancreatitis |
| O35.4 | Maternal care for suspected damage to foetus from alcohol |
| P04.3 | Foetus and newborn affected by maternal use of alcohol |
| Q86.0 | Fetal alcohol syndrome (dysmorphic) |
| R78.0 | Finding of alcohol in blood |
| T51 | Toxic effect of alcohol |
| T51.0 | Ethanol |
| T51.1 | Methanol |
| T51.2 | Propanol |
| T51.3 | Fusel oil |
| T51.8 | Other alcohols |
| X45 | Accidental poisoning by and exposure to alcohol |
| X65 | Intentional self‐poisoning by and exposure to alcohol |
| Y15 | Poisoning by and exposure to alcohol, undetermined intent |
| Y90 | Evidence of alcohol involvement determined by blood alcohol level—different subcategories as defined by thresholds in mg/100 ml |
| Y91 | Evidence of alcohol involvement determined by level of intoxication |
| Y91.0 | Y91.0—Mild alcohol intoxication |
| Y91.1 | Y91.1—Moderate alcohol intoxication |
| Y91.2 | Y91.2—Severe alcohol intoxication |
| Y91.3 | Y91.3—Very severe alcohol intoxication |
| Y91.9 | Alcohol involvement, not otherwise specified |
| Z04.0 | Blood‐alcohol and blood‐drug test |
| Z50.2 | Alcohol rehabilitation |
| Z71.4 | Alcohol abuse counselling and surveillance for alcohol use disorder |
| Z72.1 | Alcohol use |
| Z81.1 | Family history of alcohol abuse |
We conducted a systematic literature search on AMED, CAB Abstracts, Embase, Health and Psychosocial Instruments, Healthstar, OVID Medline, PsycINFO, PubMed and Social Work Abstracts databases to identify systematic reviews and/or meta‐analyses. Key words were different alcohol categories and the respective outcome category, along with either ‘systematic review’ or ‘meta‐analysis’. All databases were searched from January 2008, the time limit of the last review of this series 24, to October 2016. Supporting information, Appendix S1 gives an overview on the exact search terms used and full results. To identify the appropriate studies from the search results, one author reviewed independently all titles and abstracts at the initial stage. The results were compared with previous searches and reviews conducted independently by other authors who were part of this overview for each health outcome category. Discrepancies between the authors after the title and abstract review were resolved by discussing the full text. No language or geographical restrictions were applied. In assessing and summarizing the results of the searches, our emphasis was on causality, pathophysiology and the key meta‐analyses.
Assessment of causality
We used the epidemiological definitions of causality, where alcohol had to be necessary, either alone or in combination with other antecedent conditions as a component cause 28. This translates into AAFs for partially attributable outcome categories, i.e. for outcome categories for which alcohol is a component cause. AAFs can be interpreted as the proportion of an outcome in a specific population, which would not occur if there had been no alcohol use 11, 29. In discussing the various conditions, we also refer to the Bradford Hill criteria 30, with most emphasis on pathophysiology.
Terminology
Unless specified otherwise, we will use the term ‘heavy drinking occasion’ for consuming quantities of 60+ g of pure alcohol on one occasion. Chronic heavy drinking indicates consumption on average per day of 60+ g of pure alcohol for men and 40+ g for women (for similar thresholds in alcohol exposure classifications, see 31, 32). Light to moderate drinking is used to refer to drinking patterns which, on average, entail fewer than 60 g of pure alcohol per day in men and fewer than 40 g in women.
Results
Disease and injury categories fully (100%) attributable to alcohol use
In the ICD‐10 25, alcohol is mentioned as part of several diseases and injuries, as well as in the chapter ‘Factors influencing health status and contact with health services’ (Z codes). Table 1 gives an overview of the over 40 codes in ICD which include ‘alcohol’ or ‘alcoholic’.
While there are more than 10 000 disease and injury codes, for only a small fraction (310) of the most frequent and important categories are there global data on cause of death or morbidity. All the 100% alcohol‐attributable categories in Table 1, except alcohol use disorders (F10), are too infrequent to be included in these 310 global cause of death or burden of disease statistical categories, either by the Institute for Health Metrics and Evaluation (IHME) 33 or the WHO 34. However, GBD CRA adds estimates for alcohol poisoning (X45) and fetal alcohol syndrome (Q86.0) to this label. The WHO Global Status Reports summarize F10 and X45 only under alcohol use disorders. The choice of broad categories in all global CRAs is based on the availability and quality of data. For most of the population world‐wide, affecting 38 million of 56 million annual deaths globally 35, there are no vital registries with cause of death information. For these deaths without vital registries, cause of death is estimated on the basis of verbal autopsies of subsamples and then scaled‐up 36. Verbal autopsy denotes a method of gathering health information concerning deceased individuals to determine their cause of death. Relevant health information and a description of symptoms and events preceding the death are determined based on interviews with next of kin, neighbours or friends of the deceased. This information is then analysed by trained health professionals or computer‐based algorithms to assign a probable cause of death. The resulting cause of death categories have to be broad, as it is impossible to determine a detailed cause of death via verbal autopsy 37. For any non‐fatal health categories, such as morbidity or disability, the data situation is worse than for mortality 38.
While almost all disease or injury categories 100% attributable to alcohol cannot be included in the global CRAs, they are often assessed in high‐income countries with national hospital records and vital registries and, thus, these categories should be included in national CRAs where possible. For example, alcoholic cardiomyopathy (I42.6) as a cause of death is available in approximately half of the countries as a cause of death 39, and thus could be included as part of alcohol attributable mortality in these countries.
Alcohol use disorders
For alcohol use disorders, as defined in the F10 category of ICD‐10, causality is clear by definition, as there would not be alcohol use disorders without alcohol use. The most important category of alcohol use disorders in terms of public health impact is alcohol dependence (F10.2), which is linked both to regular and irregular heavy drinking occasions (see the almost straight linear relationship between average level of drinking and number of symptoms for dependence 40). The link to irregular heavy drinking occasions is most evident in drinking cultures such as those in eastern Europe, where daily drinking is not common, not even among people with alcohol dependence 41. Alcohol dependence and other alcohol use disorders are usually assessed based on general population surveys as part of mental disorders (such as by the World Mental Health Survey 42). As such surveys are relatively infrequent or absent for many countries, for most CRAs to date the prevalence of alcohol use disorders had to be estimated, often using the level of per‐capita alcohol consumption or prevalence of heavy drinking predictors in the estimation 43, 44.
Accidental poisoning by and exposure to alcohol
Alcohol poisoning, which is the short term for the above‐specified injury category, is handled as part of alcohol use disorders in global CRAs. Alcohol poisoning is often assessed in hospitals for emergency room entries. Any blood alcohol concentration above 3 g/l should be considered as potentially life‐threatening, with increasing mortality risks associated with increasing blood alcohol concentrations 45; in many countries, cause of death from ‘alcohol poisoning’ may be given regularly for concentrations above 4 g/l. However, alcohol poisonings are underestimated markedly for two main reasons. First, alcohol use disorders in general are stigmatized, even over and above the general stigma of psychiatric disorders 46. As a consequence, death certificates may mention more neutral categories, such as heart disease categories, as the cause of death (47; see also the discussion on alcoholic liver cirrhosis below). The amount of misclassification can be substantial in some countries or regions. For example, Zaridze and colleagues 48 reported that in a series of more than 22 000 autopsies in a Russian city, 16% of decedents had more than 4 g/l and 8% had more than 5 g/l blood alcohol concentrations. Some of the deaths reported by Zaridze and colleagues 48 should have been coded as alcohol poisoning instead of the other codes given, often cardiovascular deaths. Similar misclassifications were found in other regions of Russia and surrounding countries 49. However, while this means that alcohol poisoning deaths have been under‐reported, this effect is too small to explain the positive association between heavy drinking and cardiovascular mortality in countries with irregular drinking of very large amounts of alcohol, such as the eastern European countries 50, 51. The second reason for the underestimation of alcohol poisoning are the rules applied to classify drug overdose deaths in ICD‐10 or earlier versions of the ICD 52, which give a priority for coding other substances than alcohol in case of involvement of multiple types of substance use in deaths (see also 53, 54). While polydrug use is common in drug overdose situations (e.g. 55), and alcohol is one of the substances often present with other illicit substances, alcohol is rarely recorded as the cause of death, even when it has been specified and reported as the most toxic component by the medico‐legal pathologist, and based on this should have been coded as the underlying cause of death 56.
Fetal alcohol spectrum disorders
Fetal alcohol spectrum disorders (FASD) are the leading known cause of preventable birth defects and developmental disabilities. FASD is an umbrella term that describes the full spectrum of deficits that can occur in prenatally alcohol‐exposed individuals. The most severe and important form of FASD in terms of public health, fetal alcohol syndrome (FAS), is characterized by clear morphological changes, functional deficits and high prevalence of comorbidities 57. FAS is the only expression of FASD in the ICD‐10 (see Table 1). While FASD is not yet in ICD, the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders included ‘Neurobehavioral disorder associated with prenatal alcohol exposure’ under ‘conditions for further study’ as the first step before including it as a formal diagnosis for clinical use (see Supporting information, Appendix, Section III 58). Studies by May and co‐workers 59, 60, 61 give some indication of the full spectrum of FASD.
While human research has not delineated, and perhaps cannot delineate fully, the pattern, amount and/or critical period of alcohol exposure necessary for structural and/or functional teratogenesis, animal models have shown that all stages of embryonic development are vulnerable to the teratogenic effects of ethanol, and that the type and severity of ethanol‐induced birth defects are dependent largely upon the pattern, dose and developmental stage of the embryo at the time of ethanol exposure 62, 63. Animal models demonstrate clearly that even low levels of prenatal alcohol exposure may lead to brain dysfunction which, in turn, contributes to behavioural abnormalities 64.
In human research, the link between heavy drinking occasions during pregnancy and the risk of FAS is well established 65, 66, 67, 68, 69, 70. For low amounts of alcohol (8–28 g per occasion), several studies have found that there is no increased risk of behavioural and/or developmental deficits in children 69, 71, 72, 73. However, there is some evidence that the consumption of 42–56 g per week during pregnancy may have adverse effects on neurodevelopment 70. To date, however, there are no longitudinal human studies that have followed alcohol‐exposed individuals over a sufficient amount of time and used FASD diagnostic criteria to establish the relationship between dose and/or pattern of alcohol intake during pregnancy and FASD.
For estimation of the prevalence of FAS and FASD, Popova and colleagues developed a methodology based on the prevalence of drinking during pregnancy, which will be used in future CRAs 74. However, disability weights 75 need to be established for both categories to estimate the burden of disease (currently only available for FAS 76).
Disease and injury categories partially attributable to alcohol use
In total, 255 unique reviews and meta‐analyses were identified (see Supporting information, Appendix S1). Table 2 gives an overview of global cause of death and outcome categories causally impacted by alcohol, as well as the most important meta‐analyses, including those used for the CRA of the upcoming WHO Global Status Report on Alcohol and Health (to be prepared in 2017; for graphs on the relationships between average level of alcohol use and disease, see Supporting information, Appendix S2).
Table 2.
Potentially alcohol‐attributable broad disease categories.
| Disease category | GBD 2015 Cause Name (Cause ID) 354 | ICD–10 codes for cause of deatha | Causality and reference to meta‐analyses/selected systematic reviews | Effect |
|---|---|---|---|---|
| Infectious diseases | ||||
| Tuberculosis | Tuberculosis 297 | A10‐A14, A15–A19.9, B90–B90.9, K67.3, K93.0, M49.0, P37.0 | Causality: Rehm et al., 2009 85 | Detrimental |
| Meta‐analyses: Lönnroth et al., 2008 86; Patra et al., 2014 355; Imtiaz et al., 2016 87 | ||||
| CRA calculations: Imtiaz et al., 2016 87 | ||||
| Human immunodeficiency virus/Acquired immune deficiency syndrome (HIV/AIDS) | HIV/AIDS 298 | B20‐B24.9 | Causality: Rehm et al., 2016 98; Williams et al., 2016 99 | Detrimental |
| Meta‐analyses: Shuper et al., 2009 112; Baliunas et al., 2010 102; Lan et al., 2016 100 | ||||
| CRA calculations: Rehm et al., 2016 98, for impact of alcohol on HIV incidence based on 113; Gmel et al., 2011 92, for the effect of alcohol use on mortality via medication non‐adherence | ||||
| Other sexually transmitted diseases | Sexually transmitted diseases excluding HIV (393) | A50–A58, A60–A60.9, A63–A63.8, B63, I98.0, K67.0–K67.2, M03.1, M73.0–M73.1, N70–N71.9, N73–N74.8 | Causality: Cook & Clark, 2005 121 | Detrimental |
| Meta‐analyses, CRA calculations: the behavioural causal pathway via alcohol's impact on decision making should be the same 98, 99, so we suggest the same AAFs as for HIV/AIDS, but without the effect of alcohol use on mortality via medication non‐adherence | ||||
| Lower respiratory infections: pneumonia | Lower respiratory infections 322 | A48.1, A70, J09–J15.8, J16–J16.9, J20–J21.9, P23.0–P23.4 | Causality: Samokhvalov et al., 2010 142; Traphagen et al., 2015 356, for heavy drinking und alcohol use disorders: Simet & Sisson, 2015 357 | Detrimental |
| Meta‐analysis and CRA calculations: Samokhvalov et al., 2010 142 | ||||
| Cancers | ||||
| Lip and oral cavity cancer | Lip and oral cavity cancer (444) | C0–C08.9, D00.00–D00.07, D10.0–D10.5, D11–D11.9, D37.01–D37.04, D37.09c | Causality: International Agency for Research on Cancer (IARC), 2010; 2012 145, 146: sufficient evidence for carcinogenicity in humansb | Detrimental |
| Meta‐analysis: Corrao et al., 2004 170; Bagnardi et al., 2015 169 | ||||
| CRA calculations: Bagnardi et al., 2015 169 | ||||
| Nasopharynx cancer | Nasopharynx cancer (447) | C11–C11.9, D00.08, D10.6, D37.05c | Causality: IARC, 2010; 2012 145, 146: sufficient evidence for carcinogenicity in humansb | Detrimental |
| Meta‐analysis: Corrao et al., 2004 170; Bagnardi et al., 2015 169 | ||||
| CRA calculations: Bagnardi et al., 2015 169 | ||||
| Other pharynx cancer | Other pharynx cancer (450) | C09–C10.9, C12–C13.9, D10.7c | Causality: IARC, 2010; 2012 145, 146: sufficient evidence for carcinogenicity in humans b | Detrimental |
| Meta‐analysis: Corrao et al., 2004 170; Bagnardi et al., 2015 169 | ||||
| CRA calculations: Bagnardi et al., 2015 169 | ||||
| Oesophagus cancer | Oesophageal cancer (411) | C15–C15.9, D00.1, D13.0c | Causality: IARC, 2010; 2012 145, 146: sufficient evidence for carcinogenicity in humansb | Detrimental |
| Meta‐analysis: Corrao et al., 2004 170; Bagnardi et al., 2015 169 | ||||
| CRA calculations: Bagnardi et al., 2015 169 | ||||
| Stomach cancer | Stomach cancer (414) | C16–C16.9, D00.2, D13.1, D37.1c | Causality: IARC, 2012 146: probably carcinogenic in humansb | Detrimental |
| Meta‐analysis: Bagnardi et al., 2015 169 | ||||
| CRA calculations: Bagnardi et al., 2015 169; stomach cancer may be included in CRA calculations where the threshold is set to include ‘probably carcinogenic’ | ||||
| Colon and rectum cancer | Colon and rectum cancer (441) | C18–C21.9, D01.0‐D01.3, D12‐D12.9, D37.3–D37.5c | Causality: IARC, 2010; 2012 145, 146: sufficient evidence for carcinogenicity in humansb | Detrimental |
| Meta‐analysis: Corrao et al., 2004 170; Bagnardi et al., 2015 169 | ||||
| CRA calculations: Bagnardi et al., 2015 169 | ||||
| Liver cancer | Liver cancer (417) | C22–C22.9, D13.4c | Causality: IARC, 2010; 2012 145, 146: sufficient evidence for carcinogenicity in humansb | Detrimental |
| Meta‐analysis: Corrao et al., 2004 170; Bagnardi et al., 2015 169 | ||||
| CRA calculations: Bagnardi et al., 2015 169 | ||||
| Pancreatic cancer | Pancreatic cancer (456) | C25–C25.9, D13.6–D13.7c | Causality: IARC, 2012 146: probably carcinogenic in humansb | Detrimental |
| Meta‐analysis: Bagnardi et al., 2015 169 | ||||
| CRA calculations: Bagnardi et al., 2015 169; pancreatic cancer has been included in some CRA calculations where the threshold was set to include ‘probably carcinogenic’ | ||||
| Larynx cancer | Larynx cancer (423) | C32–C32.9, D02.0, D14.1, D38.0c | Causality: IARC, 2010; 2012 145, 146: sufficient evidence for carcinogenicity in humansb | Detrimental |
| Meta‐analysis: Corrao et al., 2004 170; Bagnardi et al., 2015 169 | ||||
| CRA calculations: Bagnardi et al., 2015 169 | ||||
| Trachea, bronchus and lung cancer | Tracheal, bronchus, and lung cancer (426) | C33–C34.92, D02.1–D02.3, D14.2–D14.32, D38.1c | Causality: IARC, 2010; 2012 145, 146: neither sufficient evidence nor probably carcinogenic in humansb | Detrimental |
| Meta‐analysis: Bagnardi et al., 2015 169 | ||||
| CRA calculations: not relevant, as not yet established as causal pathway | ||||
| Female breast cancer | Breast cancer (429) | C50–C50.929, D05–D05.92, D24–D24.9, D48.6–D48.62, D49.3, N60–N60.99c | Causality: IARC, 2010; 2012 145, 146: sufficient evidence for carcinogenicity in humansb | Detrimental |
| Meta‐analyses: many meta‐analyses with similar results (for an overview see Shield et al., 2016 151) | ||||
| CRA calculations: Bagnardi et al., 2015 169 | ||||
| Other neoplasms | Other neoplasms (488) | C17–C17.9, C3–C31.9, C37–C38.8, C4–C41.9, C47–C5, C51–C52.9, C57–C57.8, C58–C58.0, C60–C60.9, C63–C63.8, C66–C66.9, C68.0–C68.8, C69–C7, C74–C75.8, D07.4, D09.2–D09.22, D13.2–D13.39, D14.0, D15–D16.9, D28.0–D28.1, D28.7, D29.0, D30.2–D30.22, D30.4–D30.8, D31–D33.9, D35–D36, D36.1–D36.7, D37.2, D38.2–D38.5, D39.2, D39.8, D41.2–D41.3, D42–D43.9, D44.1–D44.8, D45–D45.9, D47–D47.0, D47.2–D47.9, D48.0–D48.4, D49.6, D49.81, K31.7, K62.0–K62.1, K63.5, N84.0–N84.1 | Too diverse a category to establish any causal pathways from alcohol as a whole or to quantify any risk‐relations; thus, this category will not be quantified as a cause of death or morbidity category causally impacted by alcohol. | Detrimental |
| Diabetes mellitus | ||||
| Diabetes mellitus | Diabetes mellitus (587) | E10–E10.11, E10.3–E11.1, E11.3–E12.1, E12.3–E13.11, E13.3–E14.1, E14.3–E14.9, P70.0–P70.2, R73–R73.9 | Causality: Howard et al., 2004 188 | Beneficial or detrimental, depending on patterns of drinking and populations |
| Meta‐analyses: Baliunas et al., 2009 191; Knott et al., 2015 192; Li et al., 2016 193; in addition there were intervention studies with mixed results 194, 195 | ||||
| CRA calculations: Baliunas et al., 2009 191; currently in revision | ||||
| Neuropsychiatric disorders | ||||
| Alzheimer's disease and other dementias | Alzheimer disease and other dementias (543) | F00–F03.91, G30–G31.1, G31.8–G31.9 | Causality: Collins et al., 2009 212 for potential pathways of protective effects of light to moderate use; Ridley et al., 2013 210; Daulatzai, 2015 211, for mechanism of detrimental effects of heavy use | Detrimental; potential beneficial effect for light to moderate drinking |
| Meta‐analyses: Beydoun et al., 2014 207 | ||||
| CRA calculations: not yet included in CRA | ||||
| Unipolar depressive disorders | Major depressive disorder (568) | Has not been modelled in GBD as cause of death | Causality: Rehm et al., 2004 5; Boden & Fergusson, 2011 219; | Detrimental |
| Meta‐analyses: Boden & Fergusson, 2011 219; Foulds et al., 2015 358 | ||||
| CRA calculations: suggested to use Fergusson et al., 2009 221 to be conservative, based on prevalence of alcohol use disorders | ||||
| Epilepsy | Epilepsy / Epilepsy impairment envelope (545) | G40–G41.9 | Causality: Bartolomei, 2006 359; Barclay et al., 2008 236; Leach et al., 2012 237 | Detrimental |
| Meta‐analysis and CRA calculations: Samokhvalov et al., 2010 230 | ||||
| Cardiovascular diseases | ||||
| Hypertensive heart disease | Hypertensive heart disease (498) | I11–I11.9 | Causality: Puddey & Beilin, 2006 360; O'Keefe et al., 2014 239; in addition we have good evidence that interventions leading to reductions of alcohol use subsequently lead to reductions in blood pressure and hypertension 361, 362 | Detrimental, may depend on patterns of drinking for low volume in women |
| Meta‐analyses: Chen et al., 2008 363; Taylor et al., 2009 241; Briasoulis et al., 2012 242 | ||||
| CRA calculations: Taylor et al., 2009 241; new meta‐analyses in preparation | ||||
| Ischaemic heart disease | Ischaemic heart disease (493) | I20–I25.9 | Causality: Mukamal & Rimm, 2001 364; Collins et al., 2009 212; Roerecke & Rehm, 2014 248 | Beneficial or detrimental, dependent on level and patterns of drinking |
| Meta‐analyses: Ronksley et al., 2011 256; Roerecke & Rehm, 2011 365; Roerecke & Rehm, 2012; 2014 248, 257 | ||||
| CRA calculations: Rehm et al., 2016 268 | ||||
| Cardiomyopathy | Cardiomyopathy and myocarditis (499) | A39.52, B33.2–B33.24, D86.85, I40–I43.9, I51.4–I51.5 | Causality: Iacovoni et al., 2010 244; George & Figueredo, 2011 366; Rehm et al., 2017 39 | Detrimental |
| No meta‐analyses found. There is a separate category for alcoholic cardiomyopathy, which is responsible for 3–40% of all cardiomyopathies 244. Rehm and colleagues recently introduced a method to estimate AAFs for this condition 367 | ||||
| CRA calculations: Manthey et al., 2017 367 | ||||
| Atrial fibrillation and flutter | Atrial fibrillation and flutter (500) | I48–I48.92 | Causality: Rosenqvist, 1998 368; Rosenberg & Mukamal, 2012 369 | Detrimental |
| Meta‐analyses: Samokhvalov et al., 2010 370; Kodama et al., 2011 245; Larsson et al., 2014 371 | ||||
| CRA calculations: Samokhvalov et al., 2010 370 | ||||
| Heart failure | No GBD category; ICD codes are redistributed to other GBD categories, mainly to ischaemic heart disease | I50, I11.0, I13.0, I13.2 | Although there are many reviews about alcohol use and heart failure, including meta‐analyses (Supporting information, Appendix S1), this does not affect CRAs, as the category of ‘heart failure’, since the first GBD study, has been redistributed to other GBD cardiovascular categories, mainly to ischaemic heart disease 372 | Beneficial or detrimental, dependent on level and patterns of drinking |
| Ischaemic stroke | Ischaemic stroke (495) | G45–G46.8, I63–I63.9, I65–I66.9, I67.2–I67.3, I67.5–I67.6, I69.3–I69.398 | Causality: Puddey et al., 1999 255; Mazzaglia et al., 2001 373; Collins et al., 2009 212 | Beneficial or detrimental, dependent on level and patterns of drinking (similar to IHD) |
| Meta‐analyses: Reynolds et al., 2003 374; Patra et al., 2010 375; Zhang et al., 2014 376 | ||||
| CRA calculations: Patra et al., 2010 375; Rehm et al., 2016 268 | ||||
| Haemorrhagic and other non‐ischaemic stroke | Haemorrhagic stroke (496) | I60–I61.9, I62.0–I62.03, I67.0–I67.1, I68.1–I68.2, I69.0–I69.298 | Causality: Puddey et al., 1999 255; Mazzaglia et al., 2001 373; | Mainly detrimental, except for low doses |
| Meta‐analyses: Reynolds et al., 2003 374; Patra et al., 2010 375; Zhang et al., 2014 376 | ||||
| CRA calculations: Patra et al., 2010 375 | ||||
| Oesophageal varices | No GBD category | I85 | No meta‐analyses found | Detrimental |
| Global CRA calculations: not applicable, as category is too small. National CRA calculations: should be done with relative risk of liver cirrhosis | ||||
| Gastrointestinal diseases | ||||
| Cirrhosis of the liver | Cirrhosis and other chronic liver diseases (521) | B18–B18.9, I85–I85.9, I98.2, K70–K70.9, K71.3–K71.51, K71.7, K72.1–K74.69, K74.9, K75.8–K76.0, K76.6–K76.7, K76.9 | Causality: a causal impact of alcohol is by definition as for many liver diseases there are alcoholic subcategories in the ICD (see Table 1); pathogenesis: Gao & Bataller, 2011 279 | Detrimental |
| Meta‐analyses and CRA calculations: Rehm et al., 2010 280 | ||||
| Gall bladder and bile duct disease | Gallbladder and biliary diseases (534) | K80–K83.9 | Causality: not clear for the overall category (for gallstones see 377) | Potentially beneficial, but no relation to alcohol use in the only meta‐analyses for gallstones |
| Meta‐analyses: Shabanzadeh et al., 2016 378 | ||||
| CRA calculations: not relevant, as causality is not clear and the only meta‐analyses showed no association between alcohol use and gallstones | ||||
| Pancreatitis | Pancreatitis (535) | K85–K86.9 | Causality: not necessary, as there are two conditions of pancreatitis which are 100% alcohol attributable (see Table 1); for pathogenesis: Braganza et al., 2011 299; Yadav et al., 2013 300; Lankisch et al., 2015 301; Majumder & Chari, 2016 302 | Detrimental |
| Meta‐analyses: Irving et al., 2009 308; Sankaran et al., 2015 303; Samokhvalov et al., 2015 309 | ||||
| CRA calculations: Samokhvalov et al., 2015 309 | ||||
| Other digestive diseases | Other digestive diseases (541) | I84–I84.9, K20–K24, K31.0, K31.81–K31.819, K38–K38.2, K57–K62, K62.2–K62.6, K62.8–K62.9, K64–K64.9, K66.8, K67, K68–K68.9, K75.2–K75.4, K76.1–K76.5, K76.8–K76.89, K77–K77.8, K90–K90.9, K92.8–K92.89 | Too broad a category for quantifying the impact of alcohol use; there are no studies on the impact of alcohol use on this specific group of diseases | Mainly detrimental |
| Other disease categories considered | ||||
| Psoriasis | Psoriasis (655) | Not a cause of death in GBD | Causality: Farkas & Kemény, 2010 379; Brenaut et al., 2013 380; Richard et al., 2013 381; even though alcohol use has been shown to affect the immune system in general, the conclusion has been that causality for psoriasis has not yet been fully established (see also 382). Most studies are not about alcohol use as a risk factor for psoriasis, but about comorbidity of psoriasis and alcohol use disorders and increased risk of mortality 383. A large cohort study found high excess mortality of people with psoriasis mainly with alcohol‐attributable cause of deaths 384 | Detrimental |
| Meta‐analysis: Zhu et al., 2012 382 | ||||
| CRA calculations: not relevant, as causality has not been established | ||||
| Abortion | Maternal abortion, miscarriage, and ectopic pregnancy 371 | N96, O00‐O07.9 | While there are a number of reviews, no quantitative meta‐analyses have been carried out on this category (see Supporting information, Appendix S1 for details) | Detrimental |
| Preterm birth complications | Neonatal preterm birth complications 381 | P01.0‐P01.1, P07‐P07.39, P22‐P22.9, P25‐P28.9, P61.2, P77‐P77.9 | The only meta‐analyses on preterm birth complications covered low birth weight, preterm birth and small for gestational age 385, and the relative risk for preterm birth was not significant | Detrimental for some complications |
| CRA calculations: not relevant, as relative risk is not significant | ||||
ICD codes for non‐fatal disease outcomes are slightly different in the Global Burden of Disease (GBD), but for this overview table we did not want to introduce this distinction (for the respective ICD codes by the GBD, see 386;
for definitions, see 148.
The relationships between alcohol use and the respective cancer sites are based on studies with ICD‐10 C codes; the D codes were listed only, as we wanted to show compatibility with the GBD;
Shaded rows indicate a causal impact of alcohol, whether or not the relationship could be quantified. CRA = Comparative Risk Assessment; HIV = human immunodeficiency virus; AAF = alcohol‐attributable fractions.
In the following sections, we discuss the underlying reasons and pathways for major disease, injury and cause of death categories where causality has been established. An important consideration for each disease and mortality outcome are the questions of (a) which dimension of alcohol use is causally related; (b) if there are dose–response relationships within the respective dimension; and (c) whether there are gender differences (see also Supporting information, Appendix S2 for gender specific formulas). The overall results on modelled and biological relationships are summarized in Table 3.
Table 3.
Biological pathway and Comparative Risk Assessment (CRA) modelling of alcohol use and health outcomes.
| Statistical model | Biological pathway | |||
|---|---|---|---|---|
| Disease category | General regression of alcohol use on logarithmized RR | Irregular HD | HD | Irregular HD |
| Infectious diseases | ||||
| Tuberculosis | Linear | − | + | + |
| Human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) | Modelled indirectly via sexual decision making and impact on medication adherence | + | + | + |
| Other sexually transmitted diseases | Modelled indirectly via sexual decision‐making | + | + | + |
| Lower respiratory infections: pneumonia | linear | − | + | ? |
| Cancers | ||||
| Lip and oral cavity cancer | Almost linear | − | − | − |
| Nasopharynx cancer | Almost linear | − | − | − |
| Other pharynx cancer | Almost linear | − | − | − |
| Oesophagus cancer | Almost linear | − | − | − |
| Colon and rectum cancer | Almost linear | − | − | − |
| Liver cancer | Accelerated | − | − | − |
| Larynx cancer | Almost linear | − | − | − |
| Female breast cancer | Slightly accelerated | − | Some indications | − |
| Diabetes mellitus | ||||
| Diabetes mellitus | Curvilinear | + | + | ? |
| Neuropsychiatric disorders | ||||
| Alzheimer's disease and other dementias | Not clear; indications for curvilinear | − | + | − |
| Unipolar depressive disorders | Threshold | − | + | ? |
| Epilepsy | Linear | − | + | ? |
| Cardiovascular diseases | ||||
| Hypertensive heart disease | Accelerated | − | + | ? |
| Ischaemic heart disease | Curvilinear | + | + | + |
| Cardiomyopathy | Modelled indirectly via the proportion of alcoholic cardiomyopathy to cardiomyopathy in the countries with data | + | + | + |
| Atrial fibrillation and flutter | Linear | − | + | + |
| Ischaemic stroke | Curvilinear | + | + | + |
| Haemorrhagic and other non‐ischaemic stroke | Linear for women; accelerated for men | − | + | + |
| Gastrointestinal diseases | ||||
| Cirrhosis of the liver | Accelerated | − | + | − |
| Pancreatitis | Curvilinear for women; linear for men | − | + | + |
| Injuries | ||||
| Unintentional injuries | Modelled mainly via drinking level in the situation | + | + (tolerance) | + |
| Violence | Modelled mainly via drinking level in the situation | + | ? | + |
| Suicide | Modelled based on both volume of drinking and drinking in the situation | + | + | + |
RR: relative risk;
HD: chronic heavy drinking;
irregular HD: irregular heavy drinking.
Infectious diseases
Alcohol's effects on the immune system
Alcohol impacts the innate and the acquired immune system and, thus, increases vulnerability to infectious disease 77, 78. Alcohol exposure impairs the functioning of phagocytes such as polymorphonuclear leucocytes (especially neutrophils) and macrophages 79. These cells are responsible for the ingestion of dead cells and can be considered the immune system's first responders to inflammation 80. Alcohol exposure has a suppressive effect on the release of cytokines responsible for cell signalling and critical for regulation of the host defence 80, 81. This includes chemotactic signals that trigger the migration of polymorphonuclear leucocytes into the infected area. The effects of chronic alcohol use on the immune response are probably also to increase the risk of infectious disease 82, 83. Overall, the biological pathways suggest a more pronounced effect of heavy drinking occasions and, thus, more exponential pathways and a specifically high risk for alcohol use disorders.
Tuberculosis
Alcohol's impact on the immune system described above is immediately relevant to infection with tuberculosis (TB), as approximately one‐third of people in the world have been infected with Mycobacterium tuberculosis but are not yet ill and cannot transmit the disease (latent TB 84). However, only 10% of those infected develop active TB; for the rest, the immune system will be able to fight off the infection. Accordingly, a weakened immune system is critical for increasing susceptibility to TB infection, or for reactivation of latent TB, and alcohol plays a prominent role here 85. As a second important pathway, alcohol use may lead to a presence in social environments that facilitate the spread of tuberculosis infection 85. As a consequence, alcohol is one of the major risk factors for TB, especially in countries with high population densities and high infection rates of M. tuberculosis, with poverty being linked to both. Regarding for average level of consumption, there is clearly a dose–response relationship, with some indication that, for lower levels of consumption, the increase is less steep than for higher levels 86, 87.
Given the aetiology, one may suspect an impact of patterns of drinking, especially of irregular heavy drinking occasions, but the empirical evidence is scarce 88. In addition, the higher relative risks for alcohol use disorders or alcohol problems may serve as an indirect indicator 86, 87, as both are usually linked to heavy drinking occasions 40, 89, 90.
HIV/AIDS
The status of alcohol use as a cause for HIV infection, separate from its general impact on the immune system (see above), and of the effects of alcohol use on the course of HIV/AIDS, separate from non‐adherence to anti‐retroviral medications 91, 92, have been discussed in recent years 93, 94, 95, 96. Indeed, the evidence on both mechanisms was found to be non‐conclusive in most publications, and also at a meeting to discuss the causal role of alcohol use in HIV/AIDS organized by the WHO and the South African Medical Research Council in 2008 97. However, since 2008, considerable new scientific evidence has emerged which supports a causal role of alcohol. Systematic reviews and meta‐analyses are now available to allow the quantification of the impact of alcohol use on HIV/AIDS. In the following, we try to summarize recent developments (following closely 98; see also 99), and suggest an operationalization to quantify the causal impact of alcohol use on HIV/AIDS.
Alcohol use was found to be associated with HIV incidence and prevalence in systematic reviews and meta‐analyses 100, 101, 102, 103, 104, 105, 106. This association may have resulted, in part, from the causal impact of acute alcohol use on sexual decision‐making 107, resulting in condomless sex 105, 108, 109, 110, 111, 112, 113, 114. Alternatively, other variables could be causally responsible for the associations between alcohol use and HIV/AIDS, especially the effect of risk‐taking behaviours and other personality traits 96, 115.
To exclude such alternative explanations and corroborate the causal role of alcohol on HIV incidence via impacts on decision‐making concerning safer sex practices, a number of experiments have been conducted. Alcohol use was manipulated experimentally to assess its impact on condomless sex intentions. Systematic reviews and meta‐analyses of the results of these experimental trials clearly indicated the causal impact of acute alcohol use (clearly shown for a blood alcohol concentration of 0.07 g/dl or more, but possibly even below) use on decisions/intentions about condomless sex, above and beyond the influence of expectations about alcohol and of underlying risk‐relevant personality traits 113, 114. It should be noted that these experiments have been conducted in a number of key populations, including HIV‐positive people 116.
Clearly, any experimental studies on alcohol use and HIV can only use surrogate end‐points, i.e. intention for unsafe (condomless) sex rather than condomless sex itself or HIV infection. However, the results of the experimental studies corroborate the results of epidemiological cohort and cross‐sectional studies with condomless sex 105, 108, 109, 110, 111, 112, 117, 118, 119, 120, sexually transmitted diseases 121, 122 or HIV incidence 102 as end‐points. Moreover, there are meta‐analyses that show a clear link between intentions for condomless sex and actual sexual risk behaviour 123, 124, as well as between condomless sexual practices and HIV seroconversion 125, 126, 127.
Besides this pathway of sexual decision‐making, there are findings of biological effects of alcohol use on HIV transmission and disease progression (128 gives an overview; see also 129, 130, 131). These include clear evidence that heavy drinking or alcohol use disorders are associated with viral load increases and/or CD4 count declines, mediated partly by treatment adherence and partly by the pharmacological interactions with anti‐retroviral and other medications to treat comorbidities (for mechanisms see 99, 128, 130, 132, 133, 134; for pharmacological interactions see 135, 136). It should be noted, however, that delineation and quantification of causality in these biological pathways is difficult, as many factors interact 128, 134, 136, 137.
The above considerations allow only a conservative operationalization of the causal impact of alcohol use on HIV/AIDS based on its causal effect on decision making, assuming that there is a threshold for alcohol's effect on decision‐making of four drinks for women and five drinks for men (approximately 48+/60+ g on one occasion). A further causal impact is the effect of alcohol on impeding adherence to anti‐retroviral medications 92. The estimation of relative risk based on these two mechanisms is conservative in its assumptions, and the resulting AAFs are markedly lower than those from modelling exposure with relative risk for incidence 102 using the usual methodology for CRAs (see 98 for a comparison; for usual modelling strategies see 11).
Sexually transmitted diseases excluding HIV
Other sexually transmitted diseases have been found to be associated with alcohol use, especially with heavy drinking occasions 121. While some specific biological pathways may vary, the general impact of alcohol use on the immune system (see above) is also relevant for the incidence of these diseases. Moreover, the behavioural causal pathway of alcohol's impact on decision‐making should be the same 98, 99, so we suggest the same AAFs as for HIV/AIDS (excluding the AAF for the effect of alcohol use on mortality due to medication non‐adherence). The latter effect was specific for HIV/AIDS, as missing anti‐retroviral medications was shown to have marked effects on mortality 92, an effect not applying to medications for other sexually transmitted diseases. Moreover, the interactions between medications for HIV/AIDS and alcohol are not observed for medications for other sexually transmitted diseases and alcohol.
Lower respiratory infections: pneumonia
The constant exchange with the environment presents a specific challenge to the immune defences of the lower respiratory tract. Apart from the general immunosuppressive effects explained above, chronic alcohol exposure specifically impairs the immune defences and functioning of the lower respiratory tract, increasing the risk of both viral and bacterial pneumonia. Chronic alcohol exposure decreases saliva output, which leads to an increased colonization of bacteria in the oropharynx 138. Ciliary movement that is responsible for the transportation of trapped airborne particles and microorganisms can be impaired by heavy alcohol use, and the normal cough reflex can be weakened, increasing the risk of aspiration of oropharyngeal bacteria 80. Finally, chronic alcohol use severely impairs alveolar macrophages that constitute the first line of the cellular immune defence of the lungs 79, 138, 139. For an overview of the physiological mechanisms, see 138 and 140.
While the effect of alcohol use on pneumonia has been recognized since the 18th century 141, there has been a scarcity of systematic reviews and meta‐analyses quantifying the relative risk associated with different levels of alcohol use. The work of Samokhvalov and colleagues still seems to be the best review and quantitative summary 142. In line with what would be expected, based on the physiological effects, heavy and prolonged alcohol use and alcohol use disorders have been linked specifically to a high risk, while evidence of the effects of lower levels of use is less clear.
Cancers
The carcinogenic effects of ethanol (the main carcinogenic compound in alcoholic beverages 143) and its metabolites have been acknowledged by the International Agency for Research on Cancer (IARC) in three monographs 144, 145, 146, as well as by the Continuous Update Project of the World Cancer Research Fund and the American Institute for Cancer Research 147. Specifically, the biological, animal and epidemiological evidence has resulted in alcohol being classified as a group 1 carcinogenic agent for humans (i.e. the highest level of evidence of carcinogenicity; for guidelines and evaluation criteria see 148). Furthermore, the most recent IARC monographs found sufficient animal and epidemiological evidence to conclude that alcohol consumption plays a causal role in oral cavity, pharyngeal, laryngeal, oesophageal (limited to squamous cell carcinoma (SCC), liver, colon, rectal and female breast cancers 149, as well as some evidence for a probable relationship between alcohol consumption and stomach and pancreatic cancers 146. Lastly, there is limited epidemiological evidence of a relationship between alcohol consumption and kidney, thyroid, prostate, ovarian and endometrial cancers and Hodgkin's and non‐Hodgkin's lymphoma 149. Thus, the causal role of alcohol in the development of these cancers is uncertain.
There are various biological pathways by which the use of alcohol increases (and possibly decreases) the risk of cancer; the exact pathways are often unknown and likely to vary by cancer site. Based on current evidence, the main pathway by which alcohol use is hypothesized to increase the risk of cancer is through the metabolism of ethanol into its carcinogenic metabolite acetaldehyde, which forms DNA adducts leading to the development of cancer (see review in 143). There are at least four other pathways by which alcohol use may increase the risk of cancer. First, alcohol may alter the one carbon metabolism by inhibiting folate absorption, leading to increased homocysteine concentrations 150, 151, and by inhibiting folate cycle enzyme methionine synthase and the trans‐methylation enzymes methionine adenosyltransferase and DNA methyltransferase 150, 152. Secondly, alcohol may affect serum levels of hormones and related signalling pathways, leading to an increased risk of breast cancer, and possibly of prostate, ovarian and endometrial cancers 153, 154, 155. Thirdly, alcohol consumption may lead to alterations in serum levels of insulin‐like growth factor (IGF); however, this relationship is complex, with moderate chronic alcohol consumption increasing serum levels of IGF, and acute alcohol consumption leading to a decrease in IGF levels 156. Lastly, alcohol also has a strong interaction with tobacco smoking, particularly in terms of its carcinogenic effects on the oral cavity and oesophagus (SCC). Specifically, alcohol acts as a solvent for tobacco carcinogens 157, 158.
Conversely, alcohol may prevent the development of cancer through two biological pathways. First, by increasing insulin sensitivity, alcohol may decrease the risk of kidney cancer 159, 160; in contrast, insulin resistance has been observed to be a risk factor for cancer independent of other risk factors such as obesity 161, 162. Furthermore, the World Cancer Research Fund has found that there is strong evidence to suggest that alcohol consumption below 30 g per day on average is related causally to a decrease in the risk of developing kidney cancer 163. Secondly, resveratrol (the ‘red wine chemical’) has gained attention for its protective effects on the development of cancer 164, 165, 166 through its ability to inhibit nuclear factor kappa B (NF‐κB) (thus creating an anti‐inflammatory effect) and activator protein‐1 (AP‐1) transcription (thus inhibiting the conversion of procarcinogens into carcinogens 167). However, the effect of resveratrol in decreasing the risk of cancer is minimal, at best. To exhibit a protective effect against cancer (i.e. reduce the incidence of certain cancers of colon, liver and female breast) a certain minimum daily dose of resveratrol is required, and below this dose there will be no possible protective effect. The amount of resveratrol in wine is approximately a factor of 100 000 or more below this minimal effective daily dose and, thus, no protective effect is to be expected from such a low dosage (this would be similar to ingesting 1/100000 of an aspirin tablet 168).
The increase in the risk of developing cancer (stratified by cancer site) for increasing average daily amounts of alcohol consumed (measured in grams of pure alcohol consumed per day) has been observed to be linear on an exponentiated scale; however, the magnitude of these risk increases varies by cancer site 169, 170, 171. Furthermore, as with other diseases related causally to alcohol consumption, the relative risks for cancer are dependent upon the systematic search strategy, inclusion and exclusion criteria, reference group (and if this includes former drinkers) of the underlying studies 172, 173, 174, use of case–control and/or cohort studies 175 and use of categorical or continuous estimates for alcohol consumption 169 (for relative risk graphs see 176 and Supporting information, Appendix S2).
No threshold for the effects of alcohol use on the risk of cancer has been detected; however, especially for breast cancer, there is ample evidence of alcohol's effects even at low levels of average consumption 177, 178, 179. This results in a large breast cancer burden from relatively low doses (< 21 g per day) of alcohol 179. Furthermore, there is currently not enough epidemiological evidence to assess if the pattern of alcohol consumption modifies the risk of breast cancer 151. The main biological pathway seems to be through overall tissue exposure to acetaldehyde, which may not be affected by drinking patterns; however, through modifications of insulin‐like growth factor (IGF) serum levels, drinking patterns may have an effect on the risk of developing breast cancer (as well as other cancers, where modifications to IGF serum levels play a role 180).
The risk relationship between alcohol consumption and the development of cancer has been shown to be modified by genetic variations in the carbon metabolism pathway and the ethanol–acetaldehyde metabolic pathways 181, 182. Specifically, genetic variations in the aldehyde dehydrogenase 2 gene have been shown to affect the risk relationship between alcohol consumption and oral cavity and oesophageal cancer 175, 181, 183. As the prevalence of these genetic variations differs in different national populations, cancer is the first alcohol‐attributable disease category where genetic considerations play a role in modelling the effect of alcohol use in global CRAs of the GBD and the WHO (for a first such attempt, see 184).
Overall, the alcohol‐attributable cancer disease and mortality burden is high 8, 178. However, current estimates of the number of cancer cases and cancer deaths caused by alcohol are limited due to the inability to incorporate biological latency which, for many cancer sites, can be 20 years or more 185, 186. Future CRA studies will need to take into account this latency and the competing risks from alcohol‐related and ‐unrelated deaths 187.
Diabetes mellitus
There seems to be a beneficial effect of alcohol use on diabetes mellitus type 2 incidence 188, as evidenced in meta‐analyses and in systematic reviews 189, 190, 191, 192, 193. However, this seemingly unambiguous picture must be qualified by different results by gender and ethnicity. For instance, stratification of available data in the latest and most comprehensive meta‐analyses by Knott and colleagues 192 revealed that reductions in risk may apply to women only and may be absent in studies sampled in the Asian region. In addition, Knott 192 found that some beneficial effects disappeared when compared to life‐time abstainers, a problem not unique to diabetes (174; see below and discussion in 173). Also, intervention studies about the effects of reductions in the consumption of alcohol on glucose and insulin biomarkers in people with and without diabetes showed mixed results 194, 195. Irregular heavy drinking occasions may play a role in explaining the differences between studies and in the reviews (e.g. 196, 197), but there are not enough epidemiological studies on diabetes including this dimension of alcohol exposure to settle this question.
Whether a beneficial effect of alcohol on diabetes should be modelled in future CRAs will be a discussion in the respective technical advisory committees. This decision has important public health relevance (see 198 for additional considerations), as the effect is fairly large, given the prevalence of diabetes mellitus world‐wide 199, 200 and the relatively high effect size found in epidemiological studies on alcohol use and the incidence of type 2 diabetes mellitus 191, 192.
Neuropsychiatric disorders
Alzheimer's disease, other dementias and cognitive decline
The relationships of alcohol use to Alzheimer's disease, other forms of dementia and cognitive decline seem to be complex. On one hand, there is a possible protective effect of light to moderate drinking 201, 202, 203. On the other hand, systemic reviews revealed inconsistent results about a potential protective effect of alcohol use 204, 205. Several subtypes of dementia are clearly related detrimentally and causally to heavy drinking 206, and the most comprehensive review exhibited a J‐ or U‐shaped relationship between the intensity of alcohol use and the direction of the effect 207. A recent review also found evidence that heavy alcohol use predicts conversion from any type of mild cognitive impairment to dementia, and inconsistent evidence about whether moderate alcohol use predicts risk of dementia 208. In addition, a Mendelian randomization study did not provide any evidence of a causal impact of alcohol use on cognitive performance, although admittedly this is a more general concept than the disease categories discussed above 209.
Overall, while the negative impact of heavy drinking on dementia and cognitive functioning seems indisputable, with identified biological pathways 210, 211, a protective effect of light to moderate drinking has some biological plausibility 212, but evidence on this is inconsistent. This is due partly to the multitude of methodological problems which every review describes (e.g. see discussion in 213), such as inconsistent measurement of exposure and outcomes, inconsistent control of potential confounders and lack of consideration of sample attrition due to mortality.
Major depressive disorders
Most mental disorders, including major depressive disorders, have consistent associations with alcohol use, and especially with heavy drinking and alcohol use disorders 79, 80, 81, 214, 215, 216, 217. In addition to these associations, both the Diagnostic and Statistical Manual of Mental Disorders, 5th edition 58 and the ICD‐10 (25; see also 218) list alcohol‐induced mental disorders, including alcohol‐induced depressive disorders, thus building causality into the disorder category. However, these codes are not used in most countries (an exception is the United States, where it is a billable code for medical services), so we need to establish estimates of the causal impact of alcohol use on major depressive episodes in other ways.
There are three possible descriptions of the potential causal pathways that underlie the association between heavy alcohol use and alcohol use disorders and major depressive disorders 5, 219: (a) heavy drinking/alcohol use disorders cause depressive disorders; (b) depressive disorders increase alcohol use and cause alcohol use disorders (often discussed under the heading of a ‘self‐medication’ hypothesis 220); and (c) a reciprocal causal relationship or causation by another mechanism such as genetic vulnerability. Two reviews on this topic came to the same conclusion: that all three mechanisms are possible and probably existing, but the first mechanism—that alcohol use (especially heavy use and alcohol use disorders) causes depression—is stronger and more prevalent than the other pathways (5, 219; see also 221, 222).
How to estimate the causal impact of alcohol use on major depressive disorders remains in question. Given the current scarcity of meta‐analyses on alcohol use as a risk factor for major depressive disorders, this probably has to be performed indirectly from the risk relationships of alcohol use disorders and depressive disorders 219. To be conservative, these risk relationships should be applied only to depressive disorders with later onset than alcohol use disorders. Alternatively, the confounder‐controlled risks from Fergusson and colleagues 221 could be used [odds ratio (OR) = 1.66, 95% confidence interval (CI) = 1.08–2.55]. Both suggested solutions are conservative, as it has been demonstrated that alcohol use levels below heavy drinking are associated with higher risks than abstention 223.
In addition to its role in the aetiology of depressive disorders, alcohol use has been associated with worsening the depression course, and worse outcomes such as suicide/death risk, social functioning and health care utilization (214; specifically for suicide, see section on injury below). However, the literature on this is not detailed enough to derive reliable quantitative risk relationships.
Unprovoked seizures and epilepsy
The association between alcohol use and seizures has been known since ancient times, with alcohol withdrawal seizures being the best studied and described aspect 224, 225. However, in terms of public health, the effect of alcohol use on the development of epilepsy and seizures not resulting directly from alcohol withdrawal is more important (224, 225, 226, 227, 228; for an exact definition see 229). A meta‐analysis of the data on unprovoked seizures from six available studies showed an overall association between alcohol use and the risk of epilepsy with a pooled relative risk (RR) of 2.19 (95% CI = 1.83–2.63). In addition, there was a dose–response relationship, with RRs of 1.81 (95% CI = 1.59–2.07), 2.04 (95% CI = 2.00–2.97) and 3.27 (95% CI 2.52–4.26) for consuming 48, 72 and 96 g pure alcohol per day, respectively 224, 230. Alcohol use also fulfilled other Bradford Hill criteria, such as temporality and biological plausibility 225, 228. The time for developing epilepsy or repetitive unprovoked seizures in heavy drinkers is 10 or more years 228. The most plausible biological pathway is described by the ‘kindling effect’, which postulates that repeated withdrawals, even subclinical, may lead to gradual lowering of the seizure threshold and eventually to the development of epilepsy, or unprovoked seizures that occur even in those who no longer consume alcohol 231, 232. Other theories postulate cerebral atrophy, cerebrovascular infarctions, lesions, traumas, neuroplasticity and chronic electrolyte imbalances as leading to the onset of seizures 233, 234, 235. In addition, alcohol use may affect the clinical course of pre‐existing epilepsy either by changes in anti‐epileptic drug pharmacokinetics or by non‐compliance with prescribed medication 236, 237.
Cardiovascular diseases
The relationship between alcohol use and cardiovascular disease outcomes is complex, as different dimensions play a role for different outcomes 238, 239, 240. Clearly, chronic heavy drinking is detrimental (for blood pressure/hypertension 241, 242; ischaemic heart disease 243; cardiomyopathy 244; atrial fibrillation and flutter 245; all types of stroke 246), but there is also evidence for an increased risk associated with irregular heavy drinking, even in people who are on average light to moderate drinkers (ischaemic heart disease 247, 248, 249; ischaemic stroke 250; all types of stroke 251; different cardiovascular outcomes 252). For the effects of irregular heavy drinking occasions on cardiovascular disease, there are potentially four main mechanisms 253. First, irregular heavy drinking increases the risk of coronary artery disease via unfavourable impacts on blood lipids. Secondly, there are effects on clotting, increasing the risk of thrombosis. Thirdly, irregular heavy drinking affects the conducting system, leading to a greater risk of arrhythmias 254. Finally, any heavy drinking increases blood pressure, leading to acute or sustained hypertension 255.
With respect to non‐heavy drinking, there are beneficial and detrimental effects. Beneficial effects are seen mainly for ischaemic diseases, i.e. ischaemic heart disease and ischaemic stroke 256, 257. While these beneficial effects have been put into question for different reasons (e.g. 174, 258, 259), and while they may be overestimated using standard epidemiological methodology because of biased comparison groups 260, biological pathways corroborate some protective effect. The basic biological pathways for beneficial effects on ischaemic diseases are favourable changes in several surrogate biomarkers for cardiovascular risk, such as higher levels of high density lipoprotein cholesterol and adiponectin and lower levels of fibrinogen 255, 261, 262. However, the situation may be more complex, as there are indications that the beneficial effect on ischaemic outcomes cannot be found in certain countries such as India 263, 264. It remains to be seen if this reflects different drinking patterns among those who are, on average, light to moderate drinkers, or if there are genetic influences on the biological pathways leading to cardioprotection of light to moderate alcohol use (see also 249).
As different dimensions of alcohol use impact upon cardiovascular outcomes, instrumental variable approaches such as Mendelian randomization cannot answer questions of causality easily, as they assume linear relations with one dimension (for Mendelian randomizations studies see 259, 265; for a discussion of different dimensions of alcohol use with divergent predictions see 266).As a result, modelling of alcohol use on cardiovascular disease outcomes also has to take different dimensions of exposure into account. In the most recent CRAs, this was solved as follows 22, 267:
For hypertensive heart disease, ischaemic heart disease and both stroke types, the risk relations are specified for fatal and non‐fatal outcomes. Moreover, for ischaemic diseases, we used age‐specific risk relations 268.
For countries in eastern Europe (Russia and surrounding countries with similar drinking patterns), different relative risk estimates were used (269, based on 270). In particular, no beneficial effect was modelled because of detrimental drinking patterns and higher relative risk per heavy drinking occasion, as the average quantity per heavy drinking occasion in these countries is higher (see 41, 271, 272, 273 as background).
For all countries, for ischaemic heart disease and ischaemic stroke, we used risk relations which changed the risk function below 60 g of pure alcohol per day based on the presence or absence of heavy drinking occasions 268.
Modelling the impact of alcohol use this way for all countries in the WHO European Region between 1990 and 2014 revealed that alcohol‐attributable cardiovascular mortality was key to understanding the trends in alcohol‐attributable mortality as a whole 178, 274. For most countries in the region, alcohol‐attributable cardiovascular mortality was close to zero, as the detrimental effects on hypertensive heart disease, atrial fibrillation and haemorrhagic stroke more or less balanced the beneficial effects on ischaemic heart disease and ischaemic stroke 178. However, for countries with more heavy drinking occasions in the eastern part of the region, there was considerable alcohol‐attributable cardiovascular mortality; in some countries such as Russia, this even constituted the highest category of alcohol‐attributable mortality (178; see also 275).
Gastrointestinal diseases
Liver cirrhosis
Liver cirrhosis and the wider GBD category with other liver diseases is a major cause of death globally 200, even though it has not been included into the WHO targets for non‐communicable disease 276. Liver disease is linked clearly to alcohol 277, evidenced by several ICD codes for alcoholic liver diseases (Table 1), including simple alcoholic steatosis, hepatitis, fibrosis and cirrhosis and superimposed hepatocellular carcinoma, which is part of alcohol‐attributable cancers (see above). Globally, approximately half of all liver cirrhosis deaths and disability‐adjusted life years were estimated to be attributable to alcohol in 2012 8.
The pathogenesis of specific forms of alcoholic liver disease can be summarized as follows 278, 279. Alcohol use, especially heavy drinking occasions, induces changes in lipid metabolism (increases lipogenesis and mobilization of lipids and simultaneously decreases hepatic lipid catabolism), resulting in the accumulation of lipids in hepatocytes called fatty liver. Alcohol use can also cause an inflammatory response known as alcoholic hepatitis, or steatohepatitis if it is accompanied by hepatic lipid deposition. Although hepatic steatosis does not normally cause irreversible hepatic changes, persistence and severity of alcoholic hepatitis or steatohepatitis leads eventually to fibrosis and sclerotic changes in the liver that result in insidious replacement of hepatocytes with connective tissue (liver cirrhosis) and subsequent liver failure.
The dose–response relationship between average volume of alcohol use and liver cirrhosis is exponential, with the curve more pronounced for mortality than for non‐fatal morbidity 280. The more accelerated dose–response curve for mortality is due to the fact that liver damage can have different aetiologies (most prominently, hepatitis B or C 281), but if the liver is damaged continuation of alcohol use, even at relatively low quantities, can lead to death. Most research about the relationship between alcohol use and liver disease examined the overall tissue exposure (i.e. overall volume of alcohol consumption) following the tradition of Lelbach 282. However, there are also indications that patterns of drinking matter 283. More specifically, given the same amount of overall alcohol exposure, days without any alcohol consumption (‘liver holidays’) have been shown to be associated with a lower risk than daily drinking 284, 285.
Another dimension of alcohol use has been discussed specifically for liver cirrhosis: the quality of the alcoholic beverage, and particularly potential problems with hepatotoxic ingredients in unrecorded consumption (e.g. 286. Unrecorded consumption denotes all alcohol that is not registered and thus not controlled by routine state activities, such as home‐made, illegally produced or smuggled alcohol (for a definition see 287). While there have been some instances where ingredients of unrecorded alcohol have been found which could cause liver problems over and above the impact of ethanol 288, 289 these instances are limited, and the overall conclusion of relevant reviews has been that there is not sufficient evidence to link a sizable portion of liver cirrhosis mortality to unrecorded alcohol (290, 291; see also 292).
Another issue is the fact that alcoholic liver disease cannot be measured reliably via usual death registries or via verbal autopsies, as the assessment of whether a liver disease is due to alcohol use or other risk factors is impacted highly by socio‐cultural factors, in particular by stigma 46. In their seminal study in 12 cities in 10 countries, Puffer & Griffith 293 found that after triangulating data on death certificates with data from hospital records and interviews of attending physicians or family members, the number of deaths with alcoholic liver cirrhosis more than doubled, with the majority of new cases being recoded from categories of cirrhosis which do not mention alcohol. This under‐reporting of alcoholic liver cirrhosis has persisted in later studies 294, 295, 296; this seems to be the case for all disease categories fully attributable to alcohol use 296, 297 including, but not limited to, the disclosure of alcohol use disorders. As a consequence, in national CRAs based on death registries, estimations of alcohol‐attributable liver diseases should not be based on routine data from these registries, but estimated indirectly via measures which have no or less bias (such as attributable fractions of liver cirrhosis or liver disease in general). Exceptions should be made only for countries where there had been empirical studies on the validity of alcoholic liver disease as a cause of death.
Pancreatitis
As is the case for liver diseases (see above), there are ICD‐10 codes for alcoholic pancreatitis (see Table 1). The pathogenesis is different for acute and chronic pancreatitis, but alcohol use has a significant impact on the pathophysiology of both 298, 299, 300, 301, 302 and in the transition from acute to chronic pancreatitis (see 303 ). Specifically, in chronic pancreatitis, metabolism of alcohol leads to production of reactive oxygen species 304 and fatty acid ethyl esters 305, 306 that activate stellate cells and damage acinar cells of the pancreas. This process is mediated by sustained elevation of the cytosolic Ca2+ levels 307 and results ultimately in releasing pancreatic enzymes into the interstitium and in chronic inflammation 299. In acute pancreatitis a similar cascade of intra‐ and extracellular reactions leads to fatty acid ethyl esters (FAEE)‐induced increase of the Ca2+ release which results in massive necrosis of pancreatic acinar cells 307 and acute inflammation.
Regarding epidemiological results, the dose–response relationship seems to be accelerated for higher doses 308, 309, more pronounced in women, and in acute pancreatitis. There were not enough data to evaluate the impact of irregular heavy drinking occasions in those who are on average light to moderate drinkers, however.
Injuries
Alcohol use has long been identified as a major contributor to injuries of all kinds, with established causal links (for details see previous reviews 23, 24). Blood alcohol concentration is the most important dimension to impair vision, psychomotor skills/abilities and reaction‐time; all these processes and others in the central nervous system can be affected negatively, starting at as low as 0.03% blood alcohol concentration by volume 310. In addition, as already mentioned above, judgement about risk‐taking and other behavioural actions is impacted by alcohol use, again dose‐dependent. The dose–response relationship between acute alcohol use, measured through the blood alcohol concentration and injury, seem exponential for all injury types, albeit varying slightly by type of injury 311, 312, 313.
However, there is also interindividual heterogeneity, based in part on usual drinking habits. For instance, Krüger and colleagues found that for any given blood alcohol concentration, the risk for traffic injury would be lower for a driver who is a regular heavy drinker than for a light drinker 314. In other words, average volume of alcohol use also plays a role, even though this complexity of an interaction between acute and typical alcohol use is not modelled in current CRAs 315 or in other modelling of alcohol‐attributable injury harm 316.
The impact of alcohol use on suicide may be different from other types of injury, as it seems to be determined more by long‐term drinking patterns, such as heavy drinking or alcohol use disorders (e.g. 317, 318, even though there are also acute effects of alcohol use, e.g. on judgement 319, 320. Thus, it should be considered to model suicide in future CRAs differently from other types of injury, with more emphasis on chronic patterns of drinking, in particular heavy drinking.
Current modelling of alcohol‐attributable injuries in CRAs takes into account the number of drinking occasions of different sizes and the relative risks associated with these different exposures (for the most comprehensive analyses on risk relations see 311; for others see 312, 313; for the exact methodologies see 321, 322). The last estimation, as part of the larger study for the WHO European Region estimating alcohol‐attributable mortality in more than 50 countries for 25 years, revealed 178 that alcohol‐attributable injury rates did not decrease in the time‐period in the same way as injuries in general 323.
The final consideration about alcohol‐attributable injury is the estimation of harm to others than the drinker from injuries, which is described below.
Overview on biological pathways and CRA modelling strategies for each cause of death
Table 3 gives an overview of biological reasoning and CRA modelling for all partially attributable disease and injury categories. To explain further how to interpret this Table, let us give one example: haemorrhagic and other non‐ischaemic stroke. As indicated, the current statistical model is based on average volume of alcohol consumption only 375; see also the graphs in Supporting information, Appendix S2). However, the biological pathways (see above and Table 2) would clearly indicate an additional role for irregular heavy drinking occasions which could not be included to date into the model due to lack of data.
As can be seen, for several disease categories biological pathways would suggest more complex statistical models, which cannot be realized via the usual meta‐analytical procedures because of lack of data from underlying medical epidemiological studies.
Overview on different dimensions of alcohol use and disease and injury outcomes
Figure 1 tries to summarize our knowledge about the strength of the relationships between volume of alcohol consumption, on one hand, and specific heavy drinking occasions, on the other hand, and major disease categories. On one end of the spectrum are cancers, which all show a more or less linear relationship between alcohol use and risk of cancer as expressed in logarithmized relative risk compared to life‐time abstention: the higher the (average) volume of alcohol use, the higher the risk for cancer. The use of logarithmic scales for risk relations is customary for the statistical techniques used, meaning that a linear relationship in logarithmized relative risks actually translates into exponential risk relations in the real scales.
Figure 1.
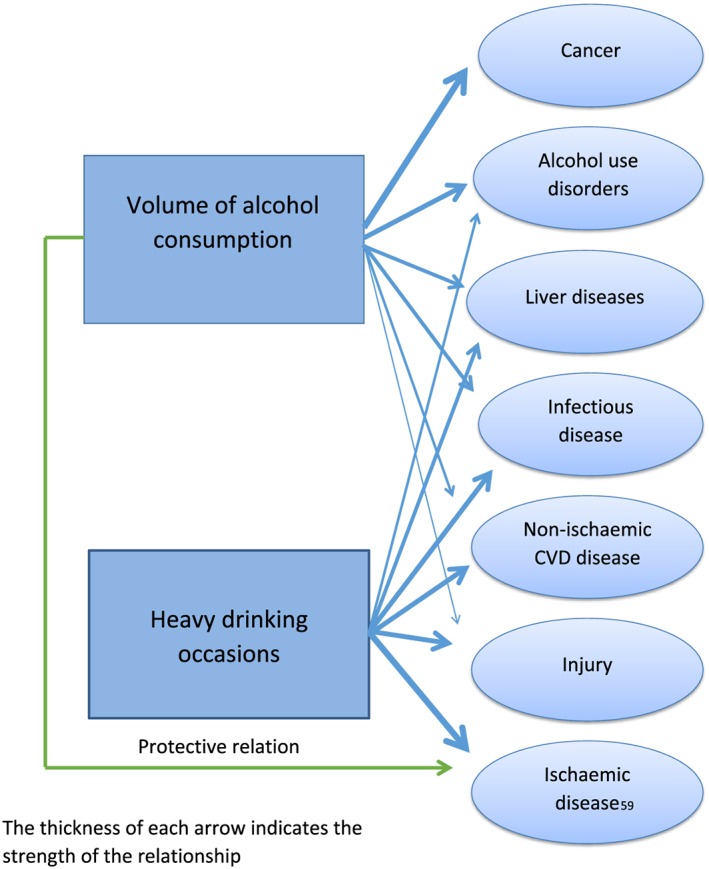
The impact of volume of alcohol use and heavy drinking upon major attributable disease outcomes.
At the other end of the spectrum are ischaemic diseases (i.e. ischaemic heart disease and ischaemic stroke), where there is a curvilinear relationship between average volume of alcohol use and risk, which is modified by heavy drinking occasions. Heavy drinking occasions seem primarily to determine the adverse risk and subsequent harm. In societies where most of the alcohol is consumed in non‐heavy drinking occasions, we expect an overall beneficial relationship of alcohol use on ischaemic diseases and an overall very small net impact of alcohol use on cardiovascular disease and mortality; in societies where most of the alcohol is consumed via heavy drinking occasions, the overall relationship should be detrimental for ischaemic disease, and even more so for cardiovascular disease and death. This hypothesis was also corroborated by the recent 25‐year trend analyses on alcohol‐attributable mortality in 52 countries of the WHO European Region 178. While such a hypothesis is based on individual‐level studies, it could not always be confirmed in ecological analyses such as time–series analyses (for confirmation see 5, 324; for essentially no relations in a number of countries in the European Union, see 325; for a result contrary to the hypothesis, see 326). However, ecological analysis may be impacted by other factors which cannot be controlled 327. For the disease categories in between, the ranking from top to bottom may be interpreted as deviation from a straight line (linear relationship) between alcohol use and relative risk of the respective disease category: the higher the impact of heavy drinking occasions, the more accelerated is the curve.
Summary of changes since the last review
Table 4 gives an overview of changes for partially attributable disease categories since the 2010 review 24. Fewer changes can be seen for injury, although there have been new meta‐analyses (see above) which are to be included in the planned new Global Status Report. Alcohol epidemiology is clearly a fast‐moving field, and our knowledge about alcohol's impact upon disease and mortality has increased. Clearly, as there have been no major updates in the ICD during the time from the last review, these categories have been stable.
Table 4.
Changes to the last review in partially attributable disease categories.
| Disease category | Causality | Risk relations | Comments (changes suggested for GSRAH 2017 versus 2014) |
|---|---|---|---|
| Infectious diseases | |||
| Tuberculosis | As in 2010 | New meta‐analysis | New risk relations suggested to be included for GSRAH 2017 |
| Human immunodeficiency virus/Acquired immune deficiency syndrome (HIV/AIDS) | New data on establishing causality for incidence | New methodology | Incidence suggested to be additionally included for GSRAH 2017 |
| Other sexually transmitted diseases | New data on establishing causality for incidence | New methodology | New disease category suggested to be included for GSRAH 2017 |
| Lower respiratory infections: pneumonia | As in 2010 | As in 2010 | As in GSRAH 2014 |
| Cancers | |||
| All cancer categories cancer | No change in cancer categories with sufficient evidence for carcinogenicity in humans; two new categories where evidence indicates probably relationships (stomach, pancreatic cancer) | New meta‐analyses | Discussion whether newly established categories where alcohol has been judged as probably carcinogenic in humans should be included; new risk relations suggested for GSRAH 2017 |
| Diabetes mellitus | |||
| Diabetes mellitus | Discussion based on new reviews and meta‐analyses | New meta‐analyses | Currently in revision to evaluate the new evidence; probably too late for GSRAH 2017 |
| Neuropsychiatric disorders | |||
| Alzheimer's disease and other dementias | Discussion based on new reviews and meta‐analyses | New meta‐analyses | Currently in revision to evaluate the new evidence; probably too late for GSRAH 2017 |
| Unipolar depressive disorders | New reviews | New meta‐analyses | New disease category suggested to be included for GSRAH 2017 |
| Epilepsy | New review (conducted in 2010 but not included in 24) | New meta‐analysis (conducted in 2010 but not included in 24 | As in GSRAH 2014 |
| Cardiovascular diseases | |||
| Hypertensive heart disease | New reviews | As in 2010 | As in GSRAH 2014 |
| Ischaemic heart disease | New reviews | New meta‐analyses | New risk relations suggested to be included for GSRAH 2017 |
| Cardiomyopathy | New reviews | New meta‐analyses | New disease category suggested to be included for GSRAH 2017 |
| Atrial fibrillation and flutter | As in 2010 | As in 2010 | As in GSRAH 2014 |
| Heart failure | As in 2010 | As in 2010 | Not included, as cases are distributed to clearer disease and cause of death categories |
| Ischaemic stroke | New reviews | New meta‐analyses | New risk relations suggested to be included for GSRAH 2017 |
| Haemorrhagic and other non‐ischaemic stroke | As in 2010 | New meta‐analyses | As in GSRAH 2014 |
| Gastrointestinal diseases | |||
| Cirrhosis of the liver | As in 2010 | As in 2010 | As in GSRAH 2014 |
| Gall bladder and bile duct disease | Not included as in 2010 | Not included as in 2010 | Neither in GSRAH 2014 nor suggested for GSRAH 2017 |
| Pancreatitis | As in 2010 | New meta‐analysis | New risk relations suggested to be included for GSRAH 2017 |
| Other disease categories considered | |||
| Psoriasis | New reviews but causality not yet established | Not relevant | Neither in GSRAH 2014 nor suggested for GSRAH 2017 |
| Abortion | New reviews | No meta‐analyses | Neither in GSRAH 2014 nor suggested for GSRAH 2017 |
| Preterm birth complications | New review | New meta‐analysis | No significant effect of alcohol –> should not be included |
The last review in this series was published in 2010 24. GSRAH = World Health Organization Global Status Reports on Alcohol and Health.
Health harm to others
Like tobacco, alcohol has a marked impact upon the health of others than the drinker 328, 329, 330, 331. Drinking of others as an external cause is usually not measured in health system classifications 332, so these impacts have to be estimated otherwise. In terms of CRAs, minimally three categories need estimation:
The impact of alcohol use during pregnancy on the health of the child: this can be captured mainly via FASD and FAS, as described above, and new algorithms for estimating incidence and prevalence of these conditions based on mother's drinking during pregnancy have been developed 74. Prevalence can then be multiplied with disability weights to derive burden (see above). Regarding fatal outcomes of FAS: while a recent study has found a life expectancy of 34 years 333, the overwhelming majority of these deaths are coded as resulting from comorbidities 57, and are not coded to FAS as a cause of death.
Alcohol use of others can have marked impact on all unintentional injuries. For instance, drinking by a parental care‐giver increases the chances of unintentional injury to a toddler 334, and parental alcohol misuse is a powerful predictor of a child's traumatic brain injury 335. Although others' drinking can impact upon a wide variety of unintentional injuries, it has been studied most fully in the context of driving and other traffic participation under the influence of alcohol (e.g. 329, 336). The burden in traffic injuries and fatalities, at least, can now be estimated more accurately, as there are global statistics by sex of driver and average number of passengers in each car 337.
The impact of alcohol on aggression and violence to others has been well established 23, 338, 339. However, its quantification becomes extremely complicated, as drinking of the victim 311, 340, 341 and drinking of the perpetrator seem to impact upon the risk and severity of violent acts 340, 342, the latter possibly in a curvilinear fashion 340. Moreover, the impact of alcohol use on violence is mediated by other variables 342, 343, including by culture 344. While all these mediating and moderating variables complicate estimation (for a first try within the framework of the CRAs see 345), the estimates found so far seem to indicate large effect sizes: thus, English and colleagues estimated that approximately half the hospitalizations due to assault were attributable to alcohol 31, and male homicide deaths in the Soviet Union dropped by 40% when per capita consumption dropped by 25% 346.
Discussion
This systematic review has shown that many disease and mortality outcomes are impacted causally by alcohol, most often in an accelerated dose–response fashion. Since the last review 24, many new reviews and meta‐analyses have appeared (see Table 4 and Supporting information, Appendix S1 for a complete listing), but while new alcohol‐attributable disease categories have been added, the general picture of alcohol use being a major contributor to the burden of mortality and disease has not changed.
Any systematic review is limited by the underlying literature. While the depth and quality of the literature varies by disease and mortality category, it is unfortunately still true that exposure measurement in many epidemiological studies is restricted to one measure of average volume of consumption, e.g. from a food frequency questionnaire or from simple quantity–frequency measures (for an explanation of these measures and their strengths see 347). Even though in recent years there have been more attempts to quantify other dimensions such as irregular heavy drinking occasions, these changes have come slowly, and for many outcomes meta‐analyses on patterns of drinking are not possible. Moreover, many studies measure alcohol use only once at baseline, and no changes of use over time can be incorporated into the models. Finally, the comparison group still is a problem 174: while using last‐year abstention may bias results by introducing sick‐quitters 348, life‐time abstention may be the theoretically preferred measure but has been proven to be unreliable 173, and in many high‐income countries life‐time abstainers are special groups which also differ on other outcome‐relevant measures. In summary, very little has changed since 2000, when these points had been already listed as barriers for improving knowledge on alcohol use and mortality outcomes 349. Mendelian randomization studies were added to our methodological arsenal 224, 259, but their assumptions are problematic if two dimensions are to be analysed simultaneously with one instrumental variable, as in the analyses on the impact of alcohol use on ischaemic heart disease (266; see also the discussion in the British Medical Journal 259).Improving measurement of alcohol exposure (including but not limited to measurement of chronic and irregular heavy drinking), as described in the limitations above, should be one of the research priorities. Other research priorities (see also 1) include:
Improving incorporating time lags 186 into future CRAs: this applies not only to effects of alcohol use, but also to all risk factors, as CRAs need to be comparative.
Improving our knowledge about risk relations: as indicated above, for most countries with the exception of Russia and surrounding countries 269, we assume that risk relations taken from the most comprehensive meta‐analysis are applicable. Given the genetic and environmental differences, we would expect some differences in risk relations between alcohol use and disease/mortality outcomes in different regions (see the example of genetically based varying cancer risks described above, which had marked implications for the population‐level burden of oesophagus cancer in Japan 175; see also some indications that alcohol use has different risk for cardiovascular events in Asians versus non‐Asians 263, 350). The biggest difference in risk relations will probably be found in injury outcomes, as these depend more upon environment than disease 311, 344. However, for any regional differences in risk, it has to be checked if these cannot be ascribed to differences in drinking patterns first, before they are applied to CRAs.
Improving our knowledge on health harm to others: currently, only a few studies exist on harm to others which can be translated into a CRA framework, and this should be a priority for future research. In particular, efforts to improve the recording of alcohol's involvement in injuries in hospital or emergency service records (e.g. 351, 352) should include attention to the involvement of others' drinking in the occurrence of the injury.
We would like to finish this review with a reminder that while the alcohol‐attributable burden of disease and mortality is large, it is only part of the harm of alcohol use. Social harm outside of health harm is impacted by similar dimensions of alcohol use (e.g. 90, 353), and should be included in any considerations of the overall impact of alcohol use in our societies.
Declaration of interests
None.
Supporting information
Appendix S1 Results of the systematic searches.
Appendix S2 Dose Response‐relationships between average volume of alcohol use and relative risk for mortality for partially alcohol‐attributable disease categories.
Acknowledgements
The current review was supported in part by the WHO Collaboration Centre on Mental Health and Addiction as part of the monitoring effort on alcohol use and health. We would like to thank Dr Vincenzo Bagnardi for allowing us to use and publish the formulas on the dose–response relationship between level of alcohol use and cancer based on 169. Finally, we would like to thank contributors for sending more than 100 mails and requests regarding the previous review, which helped to shape the current version.
Rehm, J. , Gmel, G. E. Sr , Gmel, G. , Hasan, O. S. M. , Imtiaz, S. , Popova, S. , Probst, C. , Roerecke, M. , Room, R. , Samokhvalov, A. V. , Shield, K. D. , and Shuper, P. A. (2017) The relationship between different dimensions of alcohol use and the burden of disease—an update. Addiction, 112: 968–1001. doi: 10.1111/add.13757.
References
- 1. Rehm J., Imtiaz S. A narrative review of alcohol consumption as a risk factor for global burden of disease. Subst Abuse Treat Prev Policy 2016; 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global Burden of Disease (GBD) 2015. Risk Factors Collaborators Global, regional, and national comparative risk assessment of 79 behavioral, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Forouzanfar M. H., Alexander L., Anderson H. R., Bachman V. F., Biryukov S., Brauer M. et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386: 2287–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lim S. S., Vos T., Flaxman A. D., Danaei G., Shibuya K., Adair‐Rohani H. et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rehm J., Room R., Monteiro M., Gmel G., Graham K., Rehn N. et al. Alcohol use In: Ezzati M., Lopez A. D., Rodgers A., Murray C. J. L., editors. Comparative Quantification of Health Risks: Global and Regional Burden Of Disease Attributable To Selected Major Risk Factors. Geneva, Switzerland: World Health Organization; 2004, pp. 959–1109. [Google Scholar]
- 6. Ezzati M., Lopez A. D., Rodgers A. D., Vander Horn S., Murray C. J. L., Comparative Risk Assessment Collaborating Group Selected major risk factors and global and regional burden of disease. Lancet 2002; 360: 1347–1360. [DOI] [PubMed] [Google Scholar]
- 7. Murray C. J. L., Lopez A. Global mortality, disability, and the contribution of risk factors: global burden of disease study. Lancet 1997; 349: 1436–1442. [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization . Global Status Report on Alcohol and Health. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 9. World Health Organization . Global Status Report on Alcohol and Health. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 10. Rehm J., Mathers C., Popova S., Thavorncharoensap M., Teerawattananon Y., Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol use disorders. Lancet 2009; 373: 2223–2233. [DOI] [PubMed] [Google Scholar]
- 11. Murray C. J. L., Ezzati M., Lopez A. D., Rodgers A., Vander Hoorn S. Comparative quantification of health risks: conceptual framework and methodological issues In: Ezzati M., Lopez A. D., Rodgers A., Murray C. J. L., editors. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Geneva, Switzerland: World Health Organization; 2004, pp. 1–38. [Google Scholar]
- 12. Rehm J., Taylor B., Patra J., Gmel G. Avoidable burden of disease: conceptual and methodological issues in substance abuse epidemiology. Int J Methods Psychiatr Res 2006; 15: 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Disease Control Priorities . About Disease Control Priorities, 3rd edn. University of Washington; 2016. Available at: http://dcp‐3.org/disease‐control‐priorities‐third‐edition/Web (accessed 11 October 2016) (Archived at http://www.webcitation.org/6n7sGJLT0).
- 14. Anderson P., Braddick F., Conrod P., Gual A., Hellman M., Matrai S. et al. The New Governance of Addictive Substances and Behaviours. Oxford, UK: Oxford University Press; 2017. [Google Scholar]
- 15. Chisholm D., Rehm J., van Ommeren M., Monteiro M. Reducing the global burden of hazardous alcohol use: a comparative cost‐effectiveness analysis. J Stud Alcohol 2004; 65: 782–793. [DOI] [PubMed] [Google Scholar]
- 16. Chisholm D., Doran C., Shibuya K., Rehm J. Comparative cost‐effectiveness of policy instruments for reducing the global burden of alcohol, tobacco and illicit drug use. Drug Alcohol Rev 2006; 25: 553–565. [DOI] [PubMed] [Google Scholar]
- 17. Medina‐Mora M. E., Monteiro M., Room R., Rehm J., Jernigan D., Sanchez‐Moreno D. et al. Alcohol Use and Alcohol Use Disorders In: Patel V., Chisholm D., Dua T., Laxminarayan R., Medina‐Mora M. E., editors. Mental, Neurological, and Substance Use Disorders. Washington, DC: The World Bank; 2015, pp. 127–144. [Google Scholar]
- 18. Petersen I., Evans‐Lacko S., Semrau M., Barry M., Chisholm D., Gronholm P. et al. Population and community platform interventions In: Patel V., Chisholm D., Dua T., Laxminarayan R., Medina‐Mora M. E., editors. Mental, Neurological, and Substance Use Disorders. Washington, DC: The World Bank; 2015, pp. 183–200. [PubMed] [Google Scholar]
- 19. Shidhaye R., Lund C., Chisholm D. Health care platform interventions In: Patel V., Chisholm D., Dua T., Laxminarayan R., Medina‐Mora M. E., editors. Mental, Neurological, and Substance Use Disorders. Washington, DC: The World Bank; 2015, pp. 201–218. [Google Scholar]
- 20. Walter S. D. The estimation and interpretation of attributable risk in health research. Biometrics 1976; 32: 829–849. [PubMed] [Google Scholar]
- 21. Walter S. D. Prevention of multifactorial disease. Am J Epidemiol 1980; 112: 409–416. [DOI] [PubMed] [Google Scholar]
- 22. Rehm J., Kehoe T., Gmel G., Stinson F., Grant B., Gmel G. Statistical modeling of volume of alcohol exposure for epidemiological studies of population health: the example of the US. Popul Health Metrics 2010; 8: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rehm J., Room R., Graham K., Monteiro M., Gmel G., Sempos C. The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease—an overview. Addiction 2003; 98: 1209–1228. [DOI] [PubMed] [Google Scholar]
- 24. Rehm J., Baliunas D., Borges G. L., Graham K., Irving H. M., Kehoe T. et al. The relation between different dimensions of alcohol consumption and burden of disease—an overview. Addiction 2010; 105: 817–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization . International Statistical Classification of Diseases and Related Health Problems 10th Revision [internet]; 2016. Available at: http://apps.who.int/classifications/icd10/browse/2016/en/Web (accessed 12 October 2016) (Archived at http://www.webcitation.org/6n7rylGgR).
- 26. Moher D., Liberati A., Tetzlaff J., Altman D. G., The Prisma Group Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: the PRISMA Statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M. et al. Preferred Reporting Items for Systematic Review and Meta‐Analysis Protocols (PRISMA‐P) 2015 statement. Syst Rev 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rothman K. J., Greenland S., Lash T. L. Modern Epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 29. Rehm J., Monteiro M., Room R., Gmel G., Jernigan D., Frick U. et al. Steps towards constructing a global comparative risk analysis for alcohol consumption: determining indicators and empirical weights for patterns of drinking, deciding about theoretical minimum, and dealing with different consequences. Eur Addict Res 2001; 7: 138–147. [DOI] [PubMed] [Google Scholar]
- 30. Hill A. B. The environment and disease: association or causation? Proc R Soc Med 1965; 58: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. English D., Holman C., Milne E., Winter M., Hulse G., Codde G., et al. The Quantification of Drug Caused Morbidity and Mortality in Australia 1995. Canberra, Australia: Commonwealth Department of Human Services and Health; 1995. [Google Scholar]
- 32. World Health Organization . International Guide for Monitoring Alcohol Consumption and Related Harm. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 33. Institute for Health Metrics and Evaluation . Global Burden of Disease (GBD) [internet]; 2013. Available at: http://www.healthdata.org/gbd/Web (accessed 19 July 2016) (Archived at http://www.webcitation.org/6n7rq1J9N).
- 34. World Health Organization . Global Health Estimates (GHE) [internet]; 2016. Available at: http://www.who.int/healthinfo/global_burden_disease/en/Web (accessed 19 July 2016) (Archived at http://www.webcitation.org/6n7rhyHx1).
- 35. World Health Organization . Civil registration: why counting births and deaths is important [Internet]; 2014. Available at: http://www.who.int/mediacentre/factsheets/fs324/en/Web (accessed 19 July 2016) (Archived at http://www.webcitation.org/6n7rXjl8y).
- 36. Murray C. J., Lozano R., Flaxman A. D., Serina P., Phillips D., Stewart A. et al. Using verbal autopsy to measure causes of death: the comparative performance of existing methods. BMC Med 2014; 12: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rehm J., Mathers C. Global comparative risk assessment—what level of detail can be achieved? Lancet 2009; 374: 477.19625077 [Google Scholar]
- 38. Goerdt A., Koplan J., Robine J., Thuriaux M., van Ginneken J. Non‐fatal health outcomes: Concepts, instruments and indicators In: Murray C., Lopez A., editors. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries and Risk Factors in 1990 and Projected to 2020. Boston, MA: Harvard School of Public Health on behalf of the World Health Organization and the World Bank; 1996, pp. 99–117. [Google Scholar]
- 39. Rehm J., Hasan O. S. M., Imtiaz S., Neufield M. Quantifying the contribution of alcohol to cardiomyopathy: a systematic review. Alcohol 2017; in press. [DOI] [PubMed] [Google Scholar]
- 40. Rehm J., Anderson P., Gual A., Kraus L., Marmet S., Room R. et al. The tangible common denominator of substance use disorders: a reply to commentaries to Rehm et al. (2013). Alcohol Alcohol 2014; 49: 118–122. [DOI] [PubMed] [Google Scholar]
- 41. Zaridze D., Lewington S., Boroda A., Scélo G., Karpov R., Lazarev A. et al. Alcohol and mortality in Russia: prospective observational study of 151,000 adults. Lancet 2014; 383: 1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kessler R. C., Haro J. M., Heeringa S. G., Pennell B. E., Ustun T. B. The World Health Organization World Mental Health Survey Initiative. Epidemiol Psychiatr Soc 2006; 15: 161–166. [DOI] [PubMed] [Google Scholar]
- 43. Rehm J. How should prevalence of alcohol use disorders be assessed globally? Int J Methods Psychiatr Res 2016; 25: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Flaxman A. D., Vos T., Murray C. J. L. An Integrative Metaregression Framework for Descriptive Epidemiology. Washington, DC: University of Washington Press; 2015. [Google Scholar]
- 45. National Institute on Alcohol Abuse and Alcoholism . Alcohol overdose: the dangers of drinking too much [internet]; 2015. Available at: http://pubs.niaaa.nih.gov/publications/AlcoholOverdoseFactsheet/Overdosefact.htm/Web (accessed 7 November 2016) (Archived at http://www.webcitation.org/6n7r72mxL).
- 46. Schomerus G., Lucht M., Holzinger A., Matschinger H., Carta M. G., Angermeyer M. C. The stigma of alcohol dependence compared with other mental disorders: a review of population studies. Alcohol Alcohol 2011; 46: 105–112. [DOI] [PubMed] [Google Scholar]
- 47. Hudson P. The medical examiner looks at drinking In: Ewing J. A., Rouse B. A.,. e., editors. Drinking: Alcohol in American Society—Issues and Current Research. Chicago, IL: Nelson‐Hall; 1978, pp. 71–92. [Google Scholar]
- 48. Zaridze D., Maximovitch D., Lazarev A., Igitov V., Boroda A., Boreham J. et al. Alcohol poisoning is a main determinant of recent mortality trends in Russia: evidence from a detailed analysis of mortality statistics and autopsies. Int J Epidemiol 2009; 38: 143–153. [DOI] [PubMed] [Google Scholar]
- 49. Rahu K., Palo E., Rahu M. Diminishing trend in alcohol poisoning mortality in Estonia: reality or coding peculiarity? Alcohol Alcohol 2011; 46: 485–489. [DOI] [PubMed] [Google Scholar]
- 50. Leon D. A., Shkolnikov V. M., McKee M., Kiryanov N., Andreev E. Alcohol increases circulatory disease mortality in Russia: acute and chronic effects or misattribution of cause? Int J Epidemiol 2010; 39: 1279–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sidorenkov O., Nilssen O., Nieboer E., Kleshchinov N., Grijibovski A. M. Premature cardiovascular mortality and alcohol consumption before death in Arkhangelsk, Russia: an analysis of a consecutive series of forensic autopsies. Int J Epidemiol 2011; 40: 1519–1529. [DOI] [PubMed] [Google Scholar]
- 52. World Health Organization International Statistical Classification of Diseases and Related Health Problems, 10th revision, vol. 2. Instruction Manual. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 53. Lahti R. A., Vuori E. Fatal alcohol poisoning: medico‐legal practices and mortality statistics. Forensic Sci Int 2002; 126: 203–209. [DOI] [PubMed] [Google Scholar]
- 54. Landen M., Castle S., Nolte K., Gonzales M., Escobedo L., Chatterjee B. et al. Methodological issues in the surveillance of poisoning, illicit drug overdose, and heroin overdose deaths in New Mexico. Am J Epidemiol 2003; 157: 273–278. [DOI] [PubMed] [Google Scholar]
- 55. Darke S. Heroin overdose. Addiction 2016; 111: 2060–2063. [DOI] [PubMed] [Google Scholar]
- 56. Lahti R. A., Sajantila A., Korpi H., Poikolainen K., Vuori E. Under‐recording of ethanol intoxication and poisoning in cause‐of‐death data: causes and consequences. Forensic Sci Int 2011; 212: 121–125. [DOI] [PubMed] [Google Scholar]
- 57. Popova S., Lange S., Shield K. D., Mihic A., Chudley A. E., Mukherjee R. A. S. et al. Co‐morbidity of Fetal Alcohol Spectrum Disorder: a systematic literature review and metaanalysis. Lancet 2016; 387: 978–987. [DOI] [PubMed] [Google Scholar]
- 58. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, 5th edn. Philadelphia, PA: American Psychiatric Association; 2013. [Google Scholar]
- 59. May P. A., Blankenship J., Marais A.‐S., Gossage J. P., Kalberg W. O., Barnard R. et al. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population‐based study. Alcohol Clin Exp Res 2013; 37: 818–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. May P. A., de Vries M., Marais A.‐S., Buckley D., Kalberg W. O., Adnams C. M. et al. The continuum of fetal alcohol spectrum disorders in four rural communities in South Africa: prevalence and characteristics. Drug Alcohol Depend 2016; 159: 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. May P. A., Marais A.‐S., de Vries M. M., Kalberg W. O., Buckley D., Hasken J. M. et al. The continuum of fetal alcohol spectrum disorders in a community in South Africa: prevalence and characteristics in a fifth sample. Drug Alcohol Depend 2016; 168: 274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. O'Leary‐Moore S. K., Parnell S. E., Lipinski R. J., Sulik K. K. Magnetic resonance‐based imaging in animal models of fetal alcohol spectrum disorder. Neuropsychol Rev 2011; 21: 167–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sulik K. K. Fetal alcohol spectrum disorder: pathogenesis and mechanisms. Handb Clin Neurol 2014; 125: 463–475. [DOI] [PubMed] [Google Scholar]
- 64. Hamilton D. A., Barto D., Rodriguez C. I., Magcalas C. M., Fink B. C., Rice J. P. et al. Effects of moderate prenatal alcohol exposure and age on social behavior, spatial response perseveration errors and motor behavior. Behav Brain Res 2014; 269: 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Abel E. L. Fetal Alcohol Abuse Syndrome. New York: Plenum Press; 1998. [Google Scholar]
- 66. Alvik A., Torgersen A. M., Aalen O. O., Lindermann R. Binge alcohol exposure once a week in early pregnancy predicts temperament and sleeping problems in the infant. Early Hum Dev 2011; 87: 827–833. [DOI] [PubMed] [Google Scholar]
- 67. Jacobson J. L., Jacobson S. W. Drinking moderately and pregnancy. Effects on child development. Alcohol Res Health 1999; 23: 25–30. [PMC free article] [PubMed] [Google Scholar]
- 68. Jones K. L., Smith D. W., Ulleland C. N., Streissguth A. P. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet 1973; 1: 1267–1271. [DOI] [PubMed] [Google Scholar]
- 69. Kelly Y., Sacker A., Gray R., Kelly J., Wolke D., Quigley M. A. Light drinking in pregnancy, a risk for behavioural problems and cognitive deficits at 3 years of age? Int J Epidemiol 2009; 38: 129–140. [DOI] [PubMed] [Google Scholar]
- 70. Sood B., Delaney‐Black V., Covington C., Nordstrom‐Klee B., Ager J., Templin T. et al. Prenatal alcohol exposure and childhood behavior at age 6 to 7 years: I. Dose–response effect. Pediatrics 2001; 108: 1–9. [DOI] [PubMed] [Google Scholar]
- 71. Gray R., Henderson J. Review of the Fetal Effects of Prenatal Alcohol Exposure. Oxford, UK: University of Oxford; 2006. [Google Scholar]
- 72. Linnet K. M., Dalsgaard S., Obel C., Wisborg K., Henriksen T. B., Rodriguez A. et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry 2003; 160: 1028–1040. [DOI] [PubMed] [Google Scholar]
- 73. Testa M., Quigley B. M., Eiden R. D. The effects of prenatal alcohol exposure on infant mental development: a metaanalytical review. Alcohol Alcohol 2003; 38: 295–304. [DOI] [PubMed] [Google Scholar]
- 74. Popova S., Lange S., Probst C., Gmel G., Rehm J. Estimation of national, regional and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: a systematic review and meta‐analysis. Lancet Glob Health 2017; DOI: 10.1016/S2214-109X(17)30021-9. [DOI] [PubMed] [Google Scholar]
- 75. Rehm J., Frick U. Valuation of health states in the U.S. study to establish disability weights: lessons from the literature. Int J Methods Psychiatr Res 2010; 19: 18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Salomon J. A., Haagsma J. A., Davis A., de Noordhout C. M., Polinder S., Havelaar A. H. et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health 2015; 3: e712–e723. [DOI] [PubMed] [Google Scholar]
- 77. Szabo G., Saha B. Alcohol's effect on host defense. Alcohol Res 2015; 37: 159–170. [PMC free article] [PubMed] [Google Scholar]
- 78. Cook R. T. Alcohol abuse, alcoholism, and damage to the immune system—a review. Alcohol Clin Exp Res 1998; 22: 1927–1942. [PubMed] [Google Scholar]
- 79. Zhang P., Bagby G. J., Happel K. I., Raasch C. E., Nelson S. Alcohol abuse, immunosuppression, and pulmonary infection. Curr Drug Abuse Rev 2008; 1: 56–67. [DOI] [PubMed] [Google Scholar]
- 80. Moss M. Epidemiology of sepsis: race, sex, and chronic alcohol abuse. Clin Infect Dis 2005; 41: S490–S497. [DOI] [PubMed] [Google Scholar]
- 81. Boe D. M., Nelson S., Zhang P., Bagby G. J. Acute ethanol intoxication suppresses lung chemokine production following infection with Streptococcus pneumoniae . J Infect Dis 2001; 184: 1134–1142. [DOI] [PubMed] [Google Scholar]
- 82. Szabo G., Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcohol Clin Exp Res 2009; 33: 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Goral J., Karavitis J., Kovacs E. J. Exposure‐dependent effects of ethanol on the innate immune system. Alcohol 2008; 42: 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. World Health Organization . Tuberculosis—fact sheet [internet]; 2016. Available at: http://www.who.int/mediacentre/factsheets/fs104/en/Web (accessed 13 October 2016) (Archived at http://www.webcitation.org/6n7r72mxL).
- 85. Rehm J., Samokhvalov A. V., Neuman M. G., Room R., Parry C. D., Lönnroth K. et al. The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health 2009; 9: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lönnroth K., Williams B., Stadlin S., Jaramillo E., Dye C. Alcohol use as a risk factor for tuberculosis—a systematic review. BMC Public Health 2008; 8: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Imtiaz S., Roerecke M., Shield K. D., Samokhvalov A. V., Lönnroth K., Rehm J. Alcohol use, alcohol use dose and alcohol‐related problem as risk factors for tuberculosis: meta‐analyses and burden of disease. Toronto, ON: Centre for Addiction and Mental Health; 2016. [Google Scholar]
- 88. Coker R., McKee M., Atun R., Dimitrova B., Dodonova E., Kuznetsov S. et al. Risk factors for pulmonary tuberculosis in Russia: case–control study. BMJ 2006; 332: 85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rehm J., Marmet S., Anderson P., Gual A., Kraus L., Nutt D. J. et al. Defining substance use disorders: do we really need more than heavy use? Alcohol Alcohol 2013; 48: 633–640. [DOI] [PubMed] [Google Scholar]
- 90. Rehm J., Gmel G. Patterns of alcohol consumption and social consequences. Results from an eight‐year follow‐up study in Switzerland. Addiction 1999; 94: 899–912. [DOI] [PubMed] [Google Scholar]
- 91. Hendershot C. S., Stoner S. A., Pantalone D. W., Simoni J. M. Alcohol use and antiretroviral adherence: review and meta‐analysis. J Acquir Immune Defic Syndr 2009; 52: 180–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gmel G., Shield K., Rehm J. Developing a methodology to derive alcohol‐attributable fractions for HIV/AIDS mortality based on alcohol's impact on adherence to antiretroviral medication. Popul Health Metrics 2011; 9: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hahn J. A., Woolf‐King S. E., Muyindike W. Adding fuel to the fire: alcohol's effect on the HIV epidemic in sub‐Saharan Africa. Curr HIV/AIDS Rep 2011; 8: 172–180. [DOI] [PubMed] [Google Scholar]
- 94. Fritz K., Morojele N., Kalichman S. Alcohol: the forgotten drug in HIV/AIDS. Lancet 2010; 376: 398–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rehm J., Parry C. D. Alcohol consumption and infectious diseases in South Africa. Lancet 2009; 374: 2053. [DOI] [PubMed] [Google Scholar]
- 96. Shuper P. A., Neuman M., Kanteres F., Baliunas D., Joharchi N., Rehm J. Causal considerations on alcohol and HIV/AIDS—a systematic review. Alcohol Alcohol 2010; 45: 159–166. [DOI] [PubMed] [Google Scholar]
- 97. Parry C. D. H., Rehm J. R., Poznyak V., Room R. Alcohol and infectious diseases: are there causal linkages? Addiction 2009; 104: 331–332. [DOI] [PubMed] [Google Scholar]
- 98. Rehm J., Probst C., Shield K. D., Shuper P. A. Does Alcohol Use Have a Causal Effect on HIV Incidence and Disease Progression? A Review of the Literature and a Modelling Strategy for Quantifying the Effect. Toronto, ON: Centre for Addiction and Mental Health; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Williams E. C., Hahn J. A., Saitz R., Bryant K., Lira M. C., Samet J. H. Alcohol use and Human Immunodeficiency Virus (HIV) infection: Current knowledge, implications, and future directions. Alcohol Clin Exp Res 2016; 40: 2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lan C. W., Scott‐Sheldon L. A., Carey K. B., Johnson B. T., Carey M. P. Prevalence of alcohol use, sexual risk behavior, and HIV among Russians in high‐risk settings: a systematic review and meta‐analysis. Int J Behav Med 2016; DOI: 10.1007/s12529-016-9596-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Schensul J. J., Singh S. K., Gupta K., Bryant K., Verma R. Alcohol and HIV in India: a review of current research and intervention. AIDS Behav 2010; 14: S1–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Baliunas D., Rehm J., Irving H., Shuper P. Alcohol consumption and risk of incident human immunodeficiency virus infection: a meta‐analysis. Int J Public Health 2010; 55: 159–166. [DOI] [PubMed] [Google Scholar]
- 103. Pithey A., Parry C. Descriptive systematic review of sub‐Saharan African studies on the association between alcohol use and HIV infection. SAHARA J 2009; 6: 155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Van H., Koblin B. A. HIV, alcohol, and noninjection drug use. Curr Opin HIV AIDS 2009; 4: 314–318. [DOI] [PubMed] [Google Scholar]
- 105. Woolf S. E., Maisto S. A. Alcohol use and risk of HIV infection among men who have sex with men. AIDS Behav 2009; 13: 757–782. [DOI] [PubMed] [Google Scholar]
- 106. Fisher J. C., Bang H., Kapiga S. H. The association between HIV infection and alcohol use: a systematic review and meta‐analysis of African studies. Sex Transm Dis 2007; 34: 856–863. [DOI] [PubMed] [Google Scholar]
- 107. Steele C., Josephs R. Alcohol myopia: its prized and dangerous effects. Am Psychol 1990; 45: 921–933. [DOI] [PubMed] [Google Scholar]
- 108. Scott‐Sheldon L. A., Walstrom P., Carey K. B., Johnson B. T., Carey M. P. Alcohol use and sexual risk behaviors among individuals infected with HIV: a systematic review and meta‐analysis 2012 to early 2013. Curr HIV/AIDS Rep 2013; 10: 314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Woolf‐King S. E., Maisto S. A. Alcohol use and high‐risk sexual behavior in sub‐Saharan Africa: a narrative review. Arch Sex Behav 2011; 40: 17–42. [DOI] [PubMed] [Google Scholar]
- 110. Sales J. M., Brown J. L., Vissman A. T., DiClemente R. J. The association between alcohol use and sexual risk behaviors among African American women across three developmental periods: a review. Curr Drug Abuse Rev 2012; 5: 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kalichman S. C., Simbayi L. C., Kaufman M., Cain D., Jooste S. Alcohol use and sexual risks for HIV/AIDS in sub‐Saharan Africa: systematic review of empirical findings. Prev Sci 2007; 8: 141–151. [DOI] [PubMed] [Google Scholar]
- 112. Shuper P. A., Joharchi N., Irving H., Rehm J. Alcohol as a correlate of unprotected sexual behavior among people living with HIV/AIDS: review and meta‐analysis. AIDS Behav 2009; 13: 1021–1036. [DOI] [PubMed] [Google Scholar]
- 113. Scott‐Sheldon L. A., Carey K. B., Cunningham K., Johnson B. T., Carey M. P. Alcohol use predicts sexual decision‐making: a systematic review and meta‐analysis of the experimental literature. AIDS Behav 2016; 20 Suppl 1: S19–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Rehm J., Shield K. D., Joharchi N., Shuper P. Alcohol consumption and the intention to engage in unprotected sex: systematic review and meta‐analysis of experimental studies. Addiction 2012; 107: 51–59. [DOI] [PubMed] [Google Scholar]
- 115. Shuper P. A., Joharchi N., Rehm J. Personality as a predictor of unprotected sexual behavior among people living with HIV/AIDS: a systematic review. AIDS Behav 2014; 18: 398–410. [DOI] [PubMed] [Google Scholar]
- 116. Shuper P. A., Joharchi N., Rehm J. Protocol for a controlled experiment to identify the causal role of acute alcohol consumption in condomless sex among HIV‐positive MSM: study procedures, ethical considerations, and implications for HIV prevention. AIDS Behav 2015; 20: 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cooper M. L. Alcohol use and risky sexual behavior among college students and youth. J Stud Alcohol 2002; 14: 101–117. [DOI] [PubMed] [Google Scholar]
- 118. Halpern‐Felsher B. L., Millstein S. G., Ellen J. M. Relationship of alcohol use and risky sexual behavior: a review and analysis of findings. J Adolesc Health 1996; 19: 331–336. [DOI] [PubMed] [Google Scholar]
- 119. Heath J., Lanoye A., Maisto S. A. The role of alcohol and substance use in risky sexual behavior among older men who have sex with men: a review and critique of the current literature. AIDS Behav 2011; 16: 578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Leigh B. C., Stall R. Substance use and risky sexual behavior for exposure to HIV. Issues in methodology, interpretation, and prevention. Am Psychol 1993; 48: 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Cook R. L., Clark D. B. Is there an association between alcohol consumption and sexually transmitted diseases? A systematic review. Sex Transm Dis 2005; 32: 156–164. [DOI] [PubMed] [Google Scholar]
- 122. Li Q., Stanton B. Alcohol use and sexual risk behaviors and outcomes in China: a literature review. AIDS Behav 2010; 14: 1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sheeran P., Abraham C., Orbell S. Psychosocial correlates of heterosexual condom use: a meta‐analysis. Psychol Bull 1999; 125: 90–132. [DOI] [PubMed] [Google Scholar]
- 124. Sheeran P., Orbell S. Do intentions predict condom use? Meta‐analysis and examination of six moderator variables. Br J Soc Psychol 1998; 37: 231–250. [DOI] [PubMed] [Google Scholar]
- 125. Detels R., English P., Visscher B. R., Jacobson L., Kingsley L. A., Chmiel J. S. et al. Seroconversion, sexual activity, and condom use among 2915 HIV seronegative men followed for up to 2 years. J Acquir Immune Defic Syndr 1989; 2: 77–83. [PubMed] [Google Scholar]
- 126. Pinkerton S. D., Abramson P. R. Effectiveness of condoms in preventing HIV transmission. Soc Sci Med 1997; 44: 1303–1312. [DOI] [PubMed] [Google Scholar]
- 127. Smith D. K., Herbst J. H., Zhang X., Rose C. E. Condom effectiveness for HIV prevention by consistency of use among men who have sex with men in the United States. J Acquir Immune Defic Syndr 2015; 68: 337–344. [DOI] [PubMed] [Google Scholar]
- 128. Pandrea I., Happel K. I., Amedee A., Bagby G. J., Nelson S. Alcohol's role in HIV transmission and disease progression. Alcohol Res Health 2010; 33: 203–218. [PMC free article] [PubMed] [Google Scholar]
- 129. Amedee A. M., Nichols W. A., Robichaux S., Bagby G. J., Nelson S. Chronic alcohol abuse and HIV disease progression: studies with the non‐human primate model. Curr HIV Res 2014; 12: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hahn J. A., Samet J. H. Alcohol and HIV disease progression: weighing the evidence. Curr HIV/AIDS Rep 2010; 7: 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Justice A. C., McGinnis K. A., Tate J. P., Braithwaite R. S., Bryant K. J., Cook R. L. et al. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend 2016; 161: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bagby G. J., Amedee A. M., Siggins R. W., Molina P. E., Nelson S., Veazey R. S. Alcohol and HIV effects on the immune system. Alcohol Res 2015; 37: 287–297. [PMC free article] [PubMed] [Google Scholar]
- 133. Kumar S., Jin M., Ande A., Sinha N., Silverstein P. S., Kumar A. Alcohol consumption effect on antiretroviral therapy and HIV‐1 pathogenesis: role of cytochrome P450 isozymes. Expert Opin Drug Metab Toxicol 2012; 8: 1363–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Molina P. E., Amedee A. M., Winsauer P., Nelson S., Bagby G., Simon L. Behavioral, metabolic, and immune consequences of chronic alcohol or cannabinoids on HIV/AIDs: studies in the non‐human primate SIV model. J Neuroimmune Pharmacol 2015; 10: 217–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Neuman M. G., Monteiro M., Rehm J. Drug interactions between psychoactive substances and antiretroviral therapy in individuals infected with human immunodeficiency and hepatitis viruses. Subst Use Misuse 2006; 41: 1395–1463. [DOI] [PubMed] [Google Scholar]
- 136. Neuman M. G., Schneider M., Nanau R. M., Parry C. HIV‐antiretroviral therapy induced liver, gastrointestinal, and pancreatic injury. Int J Hepatol 2012; 2012: 760706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Schneider M., Neuman M., Chersich M., Parry C. Alcohol and antiretroviral therapy—a lethal cocktail. J AIDS Clin Res 2011; S1: 005. [Google Scholar]
- 138. Mehta A. J., Guidot D. M. Alcohol abuse, the alveolar macrophage and pneumonia. Am J Med Sci 2012; 343: 244–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Siggins R. W., Melvan J. N., Welsh D. A., Bagby G. J., Nelson S., Zhang P. Alcohol suppresses the granulopoietic response to pulmonary Streptococcus pneumoniae infection with enhancement of STAT3 signaling. J Immunol 2011; 186: 4306–4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Bhatty M., Pruett S. B., Swiatlo E., Nanduri B. Alcohol abuse and Streptococcus pneumoniae infections: consideration of virulence factors and impaired immune responses. Alcohol 2011; 45: 523–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Rush B. An Inquiry Into the Effects of Ardent Spirits Upon the Human Body and Mind: With an Account of the Means of Preventing, and of the Remedies for Curing Them, 8th edn. Reprint. Exeter: NH: Richardson; 1785. [Google Scholar]
- 142. Samokhvalov A. V., Irving H. M., Rehm J. Alcohol consumption as a risk factor for pneumonia: systematic review and meta‐analysis. Epidemiol Infect; 138: 1789–1795. [DOI] [PubMed] [Google Scholar]
- 143. Pflaum T., Hausler T., Baumung C., Ackermann S., Kuballa T., Rehm J. et al. Carcinogenic compounds in alcoholic beverages: an update. Arch Toxicol 2016; 90: 2349–2367. [DOI] [PubMed] [Google Scholar]
- 144. International Agency for Research on Cancer . Alcohol Drinking. Lyon, France: International Agency for Research on Cancer, World Health Organization; 1988. [Google Scholar]
- 145. International Agency for Research on Cancer . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Alcohol Consumption and Ethyl Carbamate. Lyon, France: International Agency For Research On Cancer; 2010. [PMC free article] [PubMed] [Google Scholar]
- 146. International Agency for Research on Cancer . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 100E: Personal Habits and Indoor Combustions. Lyon, France: International Agency for Research on Cancer; 2012. [PMC free article] [PubMed] [Google Scholar]
- 147. World Cancer Research Fund International Continuous Update Project (CUP), WCRF International . Available at: http://www.wcrf.org/int/research‐we‐fund/continuous‐update‐project‐cup/Web (accessed 21 October 2016) (Archived at http://www.webcitation.org/6n7qgzug3).
- 148. International Agency for Research on Cancer . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans [internet]; 2016. Available at: http://monographs.iarc.fr/ENG/Preamble/currentb6evalrationale0706.php/Web (accessed 21 October 2016) (Archived at http://www.webcitation.org/6n7qT2PxS).
- 149. Baan R., Straif K., Grosse Y., Secretan B., El Ghissassi F., Bouvard V. et al. Carcinogenicity of alcoholic beverages. Lancet Oncol 2007; 8: 292–293. [DOI] [PubMed] [Google Scholar]
- 150. Varela‐Rey M., Woodhoo A., Martinez‐Chantar M. L., Mato J. M., Lu S. C. Alcohol, DNA methylation, and cancer. Alcohol Res 2013; 35: 25. [PMC free article] [PubMed] [Google Scholar]
- 151. Shield K. D., Soerjomataram I., Rehm J. Alcohol use and breast cancer: a critical review. Alcohol Clin Exp Res 2016; 40: 1166–1181. [DOI] [PubMed] [Google Scholar]
- 152. Choi S. W., Frisco S. Interactions between folate and aging for carcinogenesis. Clin Chem Lab Med 2005; 43: 1151–1157. [DOI] [PubMed] [Google Scholar]
- 153. Cauley J. A., Lucas F. L., Kuller L. H., Stone K., Browner W., Cummings S. R. Elevated serum estradiol and testosterone concentrations are associated with a high risk for breast cancer. Ann Intern Med 1999; 130: 270–277. [DOI] [PubMed] [Google Scholar]
- 154. Key T. J., Appleby P. N., Reeves G. K., Travis R. C., Alberg A. J., Barricarte A. et al. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol 2013; 14: 1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Hankinson S. E., Eliassen A. H. Circulating sex steroids and breast cancer risk in premenopausal women. Horm Cancer 2010; 1: 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Yu H., Berkel H. Do insulin‐like growth factors mediate the effect of alcohol on breast cancer risk? Med Hypotheses 1999; 52: 491–496. [DOI] [PubMed] [Google Scholar]
- 157. International Agency for Research on Cancer . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Volume 96—Alcohol Consumption and Ethyl Carbamate. Lyon, France: International Agency for Research on Cancer; 2007. [Google Scholar]
- 158. Talamini R., Bosetti C., La Vecchia C., Dal Maso L., Levi F., Bidoli E. et al. Combined effect of tobacco and alcohol on laryngeal cancer risk: a case–control study. Cancer Causes Control 2002; 13: 957–964. [DOI] [PubMed] [Google Scholar]
- 159. Park S. M., Lim M. K., Shin S. A., Yun Y. H. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. J Clin Oncol 2006; 24: 5017–5024. [DOI] [PubMed] [Google Scholar]
- 160. Lee J. E., Hunter D. J., Spiegelman D., Adami H. O., Albanes D., Bernstein L. et al. Alcohol intake and renal cell cancer in a pooled analysis of 12 prospective studies. J Natl Cancer Inst 2007; 99: 801–810. [DOI] [PubMed] [Google Scholar]
- 161. Chow W. H., Dong L. M., Devesa S. S. Epidemiology and risk factors for kidney cancer. Nat Rev Urol 2010; 7: 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Zhang G. M., Zhu Y., Ye D. W. Metabolic syndrome and renal cell carcinoma. World J Surg Oncol 2014; 12: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. World Cancer Research Fund (WRCF). International, American Institute for Cancer Research . Continuous Update Project report: Food, Nutrition, Physical Activity, and Kidney Cancer. London, UK: WRCF International; 2015.
- 164. Langcake P., Pryce R. J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol Plant Path 1976; 9: 77–86. [Google Scholar]
- 165. Baur J. A., Mai A. Revelations into resveratrol's mechanism. Nat Med 2012; 18: 500–501. [Google Scholar]
- 166. Siemann E. H., Creasy L. L. Concentration of the phytoalexin resveratrol in wine. Am J Enol Vitic 1992; 43: 49–52. [Google Scholar]
- 167. Kraft T. E., Parisotto D., Schempp C., Efferth T. Fighting cancer with red wine? Molecular mechanisms of resveratrol. Crit Rev Food Sci Nutr 2009; 49: 782–799. [DOI] [PubMed] [Google Scholar]
- 168. Lachenmeier D. W., Godelmann R., Witt B., Riedel K., Rehm J. Can resveratrol in wine protect against the carcinogenicity of ethanol? A probabilistic dose–response assessment. Int J Cancer 2014; 134: 144–153. [DOI] [PubMed] [Google Scholar]
- 169. Bagnardi V., Rota M., Botteri E., Tramacere I., Islami F., Fedirko V. et al. Alcohol consumption and site‐specific cancer risk: a comprehensive dose–response meta‐analysis. Br J Cancer 2015; 112: 580–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Corrao G., Bagnardi V., Zambon A., La Vecchia C. A meta‐analysis of alcohol consumption and the risk of 15 diseases. Prev Med 2004; 38: 613–619. [DOI] [PubMed] [Google Scholar]
- 171. Scoccianti C., Straif K., Romieu I. Recent evidence on alcohol and cancer epidemiology. Future Oncol 2013; 9: 1315–1322. [DOI] [PubMed] [Google Scholar]
- 172. Fillmore K. M., Kerr W. C., Stockwell T., Chikritzhs T., Bostrom A. Moderate alcohol use and reduced mortality risk: systematic error in prospective studies. Addict Res Theory 2006; 14: 101–132. [DOI] [PubMed] [Google Scholar]
- 173. Rehm J., Irving H., Ye Y., Kerr W. C., Bond J., Greenfield T. K. Are lifetime abstainers the best control group in alcohol epidemiology? On the stability and validity of reported lifetime abstention. Am J Epidemiol 2008; 168: 866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Zeisser C., Stockwell T. R., Chikritzhs T. Methodological biases in estimating the relationship between alcohol consumption and breast cancer: the role of drinker misclassification errors in meta‐analytic results. Alcohol Clin Exp Res 2014; 38: 2297–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Roerecke M., Shield K. D., Higuchi S., Yoshimura A., Larsen E., Rehm M. X. et al. Estimates of alcohol‐related oesophageal cancer burden in Japan: systematic review and meta‐analyses. Bull World Health Organ 2015; 93: 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Shield K. D., Parry C., Rehm J. Chronic diseases and conditions related to alcohol use. Alcohol Res 2013; 35: 155–171. [PMC free article] [PubMed] [Google Scholar]
- 177. Rehm J. Light or moderate drinking is linked to alcohol related cancers, including breast cancer. BMJ 2015; 351: h4400. [DOI] [PubMed] [Google Scholar]
- 178. Shield K. D., Rylett M., Rehm J. Public health successes and missed opportunities In: Trends in Alcohol Consumption and Attributable Mortality in the WHO European Region, 1990–2014. Copenhagen, Denmark: WHO European Region; 2016. [Google Scholar]
- 179. Bagnardi V., Rota M., Botteri E., Tramacere I., Islami F., Fedirko V. et al. Light alcohol drinking and cancer: a meta‐analysis. Ann Oncol 2013; 24: 301–308. [DOI] [PubMed] [Google Scholar]
- 180. Chen W. Y., Rosner B., Hankinson S. E., Colditz G. A., Willett W. C. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA 2011; 306: 1884–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Brooks P. J., Enoch M. A., Goldman D., Li T. K., Yokoyama A. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLOS Med 2009; 6: 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Boffetta P., Hashibe M. Alcohol and cancer. Lancet Oncol 2006; 7: 149–156. [DOI] [PubMed] [Google Scholar]
- 183. Yokoyama A., Omori T. Genetic polymorphisms of alcohol and aldehyde dehydrogenases and risk for esophageal and head and neck cancers. Alcohol 2005; 35: 175–185. [DOI] [PubMed] [Google Scholar]
- 184. Praud D., Rota M., Rehm J., Shield K., Zatonski W., Hashibe M. et al. Cancer incidence and mortality attributable to alcohol consumption. Int J Cancer 2016; 138: 1380–1387. [DOI] [PubMed] [Google Scholar]
- 185. Holmes J., Meier P. S., Booth A., Guo Y., Brennan A. The temporal relationship between per capita alcohol consumption and harm: a systematic review of time lag specifications in aggregate time series analyses. Drug Alcohol Depend 2012; 123: 7–14. [DOI] [PubMed] [Google Scholar]
- 186. Rehm J., Patra J., Popova L. Alcohol drinking cessation and its effect on oesophageal and head and neck cancers: a pooled analysis. Int J Cancer 2007; 121: 1132–1137. [DOI] [PubMed] [Google Scholar]
- 187. Kontis V., Mathers C. D., Rehm J., Stevens G. A., Shield K. D., Bonita R. et al. Contribution of six risk factors to achieving the ‘25 × 25’ NCD mortality reduction target. Lancet 2014; 384: 427–437. [DOI] [PubMed] [Google Scholar]
- 188. Howard A. A., Arnsten J. H., Gourevitch M. N. Effect of alcohol consumption on diabetes mellitus: a systematic review. Ann Intern Med 2004; 140: 211–219. [DOI] [PubMed] [Google Scholar]
- 189. Koppes L. L. J., Bouter L. M., Dekker J. M., Heine R. J., Hendricks H. F. J. Moderate alcohol consumption lowers the risk of type 2 diabetes. A meta‐analysis of prospective observational studies. Diabetes Care 2005; 28: 719–725. [DOI] [PubMed] [Google Scholar]
- 190. Carlsson S., Hammar N., Grill V. Alcohol consumption and type 2 diabetes meta‐analysis of epidemiological studies indicates a U‐shaped relationship. Diabetologia 2005; 48: 1051–1054. [DOI] [PubMed] [Google Scholar]
- 191. Baliunas D., Taylor B., Irving H., Roerecke M., Patra J., Mohapatra S. et al. Alcohol as a risk factor for type 2 diabetes—a systematic review and meta‐analysis. Diabetes Care 2009; 32: 2123–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Knott C., Bell S., Britton A. Alcohol consumption and the risk of type 2 diabetes: a systematic review and dose–response meta‐analysis of more than 1.9 million individuals from 38 observational studies. Diabetes Care 2015; 38: 1804–1812. [DOI] [PubMed] [Google Scholar]
- 193. Li X. H., Yu F. F., Zhou Y. H., He J. Association between alcohol consumption and the risk of incident type 2 diabetes: a systematic review and dose–response meta‐analysis. Am J Clin Nutr 2016; 103: 818–829. [DOI] [PubMed] [Google Scholar]
- 194. Schrieks I. C., Heil A. L., Hendriks H. F., Mukamal K. J., Beulens J. W. The effect of alcohol consumption on insulin sensitivity and glycemic status: a systematic review and meta‐analysis of intervention studies. Diabetes Care 2015; 38: 723–732. [DOI] [PubMed] [Google Scholar]
- 195. Hirst J. A., Aronson J. K., Feakins B. G., Ma C., Farmer A. J., Stevens R. J. Short‐ and medium‐term effects of light to moderate alcohol intake on glycaemic control in diabetes mellitus: a systematic review and meta‐analysis of randomized trials. Diabet Med 2016; DOI: 10.1111/dme.13259. [DOI] [PubMed] [Google Scholar]
- 196. Heianza Y., Arase Y., Saito K., Tsuji H., Fujihara K., Hsieh S. D. et al. Role of alcohol drinking pattern in type 2 diabetes in Japanese men: the Toranomon Hospital Health Management Center Study 11 (TOPICS 11). Am J Clin Nutr 2013; 97: 561–568. [DOI] [PubMed] [Google Scholar]
- 197. Vaeth P. A., Caetano R., Durazo E. M. Ethnicity and alcohol consumption among US adults with diabetes. Ann Epidemiol 2014; 24: 720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Babor T., Rehm J., Jernigan D., Vaeth P., Monteiro M., Lehman H. Alcohol, diabetes and public health interventions in the Americas. Rev Panam Salud Publ/Pan Am J Public Health 2012; 32: 151–155. [DOI] [PubMed] [Google Scholar]
- 199. Global Burden of Disease (GBD) DALYs and HALE Collaborators (2016). Global, regional, and national disability‐adjusted life‐years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2015; 388: 1603–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Global Burden of Disease (GBD) Mortality and Causes of Death Collaborators (2016). Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2015; 388: 1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Letenneur L. Moderate alcohol consumption and risk of developing dementia in the elderly: the contribution of propsective studies. Ann Epidemiol 2007; 17: S43–5. [Google Scholar]
- 202. Peters R., Peters J., Warner J., Beckett N., Bulpitt C. Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age Ageing 2008; 37: 505–512. [DOI] [PubMed] [Google Scholar]
- 203. Anstey K. J., Mack H. A., Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta‐analysis of prospective studies. Am J Geriatr Psychiatry 2009; 17: 542–555. [DOI] [PubMed] [Google Scholar]
- 204. Di Marco L. Y., Marzo A., Muñoz‐Ruiz M., Ikram M. A., Kivipelto M., Ruefenacht D. et al. Modifiable lifestyle factors in dementia: a systematic review of longitudinal observational cohort studies. J Alzheimers Dis 2014; 42: 119–135. [DOI] [PubMed] [Google Scholar]
- 205. Lafortune L., Martin S., Kelly S., Kuhn I., Remes O., Cowan A. et al. Behavioural risk factors in mid‐life associated with successful ageing, disability, dementia and frailty in later life: a rapid systematic review. PLoS One 2016; 11: e0144405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Gupta S., Warner J. Alcohol‐related dementia: a 21st‐century silent epidemic? Br J Psychiatry 2008; 193: 351–353. [DOI] [PubMed] [Google Scholar]
- 207. Beydoun M. A., Beydoun H. A., Gamaldo A. A., Teel A., Zonderman A. B., Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta‐analysis. BMC Public Health 2014; 24: 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Cooper C., Sommerlad A., Lyketsos C. G., Livingston G. Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta‐analysis. Am J Psychiatry 2015; 172: 323–334. [DOI] [PubMed] [Google Scholar]
- 209. Kumari M., Holmes M. V., Dale C. E., Hubacek J. A., Palmer T. M., Pikhart H. et al. Alcohol consumption and cognitive performance: a Mendelian randomization study. Addiction 2014; 109: 1462–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Ridley N. J., Draper B., Withall A. Alcohol‐related dementia: an update of the evidence. Alzheimers Res Ther 2013; 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Daulatzai M. A. ‘Boomerang neuropathology’ of late‐onset Alzheimer's disease is shrouded in harmful ‘BDDS’: breathing, diet, drinking, and sleep during aging. Neurotox Res 2015; 28: 55–93. [DOI] [PubMed] [Google Scholar]
- 212. Collins M. A., Neafsey E. J., Mukamal K. J., Gray M. O., Parks D. A., Das D. K. et al. Alcohol in moderation, cardioprotection, and neuroprotection: epidemiological considerations and mechanistic studies. Alcohol Clin Exp Res 2009; 33: 206–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Piazza‐Gardner A. K., Faffud T. J., Barry A. E. The impact of alcohol on Alzheimer's disease: a systematic review. Aging Ment Health 2013; 17: 133–146. [DOI] [PubMed] [Google Scholar]
- 214. Sullivan L. E., Fiellin D. A., O'Connor P. G. The prevalence and impact of alcohol problems in major depression: a systematic review. Am J Med 2005; 118: 330–341. [DOI] [PubMed] [Google Scholar]
- 215. Kessler R. C., Crum R. M., Warner L. A., Nelson C. B., Schulenberg J., Anthony J. C. Lifetime co‐occurrence of DSM‐III‐R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry 1997; 54: 313–321. [DOI] [PubMed] [Google Scholar]
- 216. Regier D., Farmer M., Rae D., Locke B., Keith S., Judd L. et al. Comorbidity of mental disorders with alcohol and other drug abuse: results from the Epidemiological Catchment Area (ECA) Study. JAMA 1990; 264: 2511–2518. [PubMed] [Google Scholar]
- 217. Helzer J. E., Pryzbeck T. R. The co‐occurrence of alcoholism with other psychiatric disorders in the general population and its impact on treatment. J Stud Alcohol 1988; 49: 219–224. [DOI] [PubMed] [Google Scholar]
- 218. World Health Organization . Alcohol dependence with alcohol‐induced mood disorder ‐ F10.24 [internet]; 2016. Available at: http://icd10coded.com/cm/ch5/F10‐F19/F10/F10.24/Web (accessed 31 October 2016) (Archived at http://www.webcitation.org/6n7pFo5Vx).
- 219. Boden J. M., Fergusson D. M. Alcohol and depression. Addiction 2011; 106: 906–914. [DOI] [PubMed] [Google Scholar]
- 220. Bolton J. M., Robinson J., Sareen J. Self‐medication of mood disorders with alcohol and drugs in the National Epidemiologic Survey on Alcohol and Related Conditions. J Affect Disord 2009; 115: 367–375. [DOI] [PubMed] [Google Scholar]
- 221. Fergusson D. M., Boden J. M., Horwood L. J. Tests of causal links between alcohol abuse or dependence and major depression. Arch Gen Psychiatry 2009; 66: 260–266. [DOI] [PubMed] [Google Scholar]
- 222. Falk D. E., Yi H. Y., Hilton M. E. Age of onset and temporal sequencing of lifetime DSM‐IV alcohol use disorders relative to comorbid mood and anxiety disorders. Drug Alcohol Depend 2008; 94: 234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Haynes J. C., Farrell M., Singleton N., Meltzer H., Araya R., Lewis G. et al. Alcohol consumption as a risk factor for anxiety and depression. Br J Psychiatry 2005; 187: 544–551. [DOI] [PubMed] [Google Scholar]
- 224. Samokhvalov A. V., Farid A. K., Selby P., Rehm J. Alcohol‐related seizure disorders In: Reuber M., Schachter S. C., editors. Borderland of Epilepsy Revisited. New York, NY: Oxford University Press; 2012, pp. 144–154. [Google Scholar]
- 225. Bartolomei F., Suchet L., Barrie M., Gastaut J. L. Alcoholic epilepsy: a unified and dynamic classification. Eur Neurol 1997; 37: 13–17. [DOI] [PubMed] [Google Scholar]
- 226. Hattemer K., Knake S., Oertel W. H., Hamer H. M., Rosenow F. Recurrent alcohol‐induced seizures in a patient with chronic alcohol abuse. Epileptic Disord 2008; 10: 162–164. [DOI] [PubMed] [Google Scholar]
- 227. Hillbom M., Pieninkeroinen I., Leone M. Seizures in alcohol‐dependent patients: epidemiology, pathophysiology and management. CNS Drugs 2003; 17: 1013–1030. [DOI] [PubMed] [Google Scholar]
- 228. Devetag F., Mandich G., Zaiotti G., Toffolo G. G. Alcoholic epilepsy: review of series and and proposed classification and etiopathogenesis. Ital J Neurol Sci 1983; 4: 275–284. [DOI] [PubMed] [Google Scholar]
- 229. Fisher R. S., van Emde Boas W., Blume W., Elger C., Genton P., Lee P. et al. Epileptic seizures and epilepsy: definitions proposed by the international league against epilepsy (ILAE) and the international bureau for epilepsy (IBE). Epilepsia 2005; 46: 470–472. [DOI] [PubMed] [Google Scholar]
- 230. Samokhvalov A. V., Irving H., Mohapatra S., Rehm J. Alcohol consumption, unprovoked seizures and epilepsy: a systematic review and meta‐analysis. Epilepsia 2010; 51: 1177–1184. [DOI] [PubMed] [Google Scholar]
- 231. Ballenger J. C., Post R. M. Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry 1978; 133: 1–14. [DOI] [PubMed] [Google Scholar]
- 232. Gonzalez L. P., Veatch L. M., Ticku M. K., Becker H. C. Alcohol withdrawal kindling: mechanisms and implications for treatment. Alcohol Clin Exp Res 2001; 25: 197s–201s. [DOI] [PubMed] [Google Scholar]
- 233. Dam A. M., Fuglsang‐Frederikse A., Svarre‐Olsen U., Dam M. Late‐onset epilepsy: etiologies, types of seizure, and value of clinical investigation, EEG, and computerized tomography scan. Epilepsia 1985; 26: 227–231. [DOI] [PubMed] [Google Scholar]
- 234. Freedland E. S., McMicken D. B. Alcohol‐related seizures, part I: pathophysiology, differencial diagnosis and evaluation. J Emerg Med 1993; 11: 463–473. [DOI] [PubMed] [Google Scholar]
- 235. Rathlev N. K., Ulrich A. S., Delanty N., D'Onofrio D., D'Onofrio G. Alcohol‐related seizures. J Emerg Med 2006; 31: 157–163. [DOI] [PubMed] [Google Scholar]
- 236. Barclay G. A., Barvour J., Stewart S., Day C. P., Gilvarry E. Adverse physical effects of alcohol misuse. Adv Psychiatr Treat 2008; 14: 139–151. [Google Scholar]
- 237. Leach J. P., Mohanraj R., Borland W. Alcohol and drugs in epilepsy: pathophysiology, presentation, possibilities, and prevention. Epilepsia 2012; 53: 48–57. [DOI] [PubMed] [Google Scholar]
- 238. Roerecke M., Rehm J. Alcohol intake revisited: risks and benefits. Curr Atheroscler Rep 2012; 14: 556–562. [DOI] [PubMed] [Google Scholar]
- 239. O'Keefe J. H., Bhatti S. K., Bajwa A., DiNicolantonio J. J., Lavie C. J. Alcohol and cardiovascular health: the dose makes the poison... or the remedy. Mayo Clin Proc 2014; 89: 382–393. [DOI] [PubMed] [Google Scholar]
- 240. Klatsky A. L. Alcohol and cardiovascular diseases: where do we stand today? J Intern Med 2015; 278: 238–250. [DOI] [PubMed] [Google Scholar]
- 241. Taylor B., Irving H. M., Baliunas D., Roerecke M., Patra J., Mohapatra S. et al. Alcohol and hypertension: gender differences in dose–response relationships determined through systematic review and meta‐analysis. Addiction 2009; 104: 1981–1990. [DOI] [PubMed] [Google Scholar]
- 242. Briasoulis A., Agarwal V., Messerli F. H. Alcohol consumption and the risk of hypertension in men and women: a systematic review and meta‐analysis. J Clin Hypertension 2012; 14: 792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Roerecke M., Rehm J. Chronic heavy drinking and ischaemic heart disease: a systematic review and meta‐analysis. Open Heart 2014; 1: e000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Iacovoni A., De Maria R., Gavazzi A. Alcoholic cardiomyopathy. J Cardiovasc Med 2010; 11: 884–892. [DOI] [PubMed] [Google Scholar]
- 245. Kodama S., Saito K., Tanaka S., Horikawa C., Saito A., Heianza Y. et al. Alcohol consumption and risk of atrial fibrillation: a meta‐analysis. J Am Coll Cardiol 2011; 57: 427–436. [DOI] [PubMed] [Google Scholar]
- 246. Patra J., Taylor B., Irving H., Roerecke M., Baliunas D., Mohapatra S. et al. Alcohol consumption and the risk of morbidity and mortality from different stroke types—a systematic review and meta‐analysis. BMC Public Health 2010; 10: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Roerecke M., Rehm J. Irregular heavy drinking occasions and risk of ischemic heart disease: a systematic review and meta‐analysis. Am J Epidemiol 2010; 171: 633–644. [DOI] [PubMed] [Google Scholar]
- 248. Roerecke M., Rehm J. Alcohol consumption, drinking patterns, and ischemic heart disease: a narrative review of meta‐analyses and a systematic review and meta‐analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med 2014; 12: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Leong D. P., Smyth A., Teo K. K., McKee M., Rangarajan S., Pais P. et al. Patterns of alcohol consumption and myocardial infarction risk: observations from 52 countries in the INTERHEART case–control study. Circulation 2014; 130: 390–398. [DOI] [PubMed] [Google Scholar]
- 250. Guiraud V., Amor M. B., Mas J. L., Touzé E. Triggers of ischemic stroke: a systematic review. Stroke 2010; 41: 2669–2677. [DOI] [PubMed] [Google Scholar]
- 251. O'Donnell M. J., Chin S. L., Rangarajan S., Xavier D., Liu L., Zhang H. et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case–control study. Lancet 2016; 388: 761–775. [DOI] [PubMed] [Google Scholar]
- 252. Mostofsky E., Chahal H. S., Mukamal K. J., Rimm E. B., Mittleman M. A. Alcohol and immediate risk of cardiovascular events: a systematic review and dose–response meta‐analysis. Circulation 2016; 133: 979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. McKee M., Britton A. The positive relationship between alcohol and heart disease in Eastern Europe: potential physiological mechanisms. J R Soc Med 1998; 91: 402–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Tonelo D., Providência R., Gonçalves L. Holiday heart syndrome revisited after 34 years. Arq Bras Cardiol 2013; 101: 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Puddey I. B., Rakic V., Dimmitt S. B., Beilin L. J. Influence of pattern of drinking on cardiovascular disease and cardiovascular risk factors—a review. Addiction 1999; 94: 649–663. [DOI] [PubMed] [Google Scholar]
- 256. Ronksley P. E., Brien S. E., Turner B. J., Mukamal K. J., Ghali W. A. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta‐analysis. BMJ 2011; 342: d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Roerecke M., Rehm J. The cardioprotective association of average alcohol consumption and ischaemic heart disease: a systematic review and meta‐analysis. Addiction 2012; 107: 1246–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Stockwell T., Greer A., Fillmore K., Chikritzhs T., Zeisser C. How good is the science. BMJ 2012; 344: e2276. [DOI] [PubMed] [Google Scholar]
- 259. Holmes M. V., Dale C. E., Zuccolo L., Silverwood R. J., Guo Y., Ye Z. et al. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ 2014; 349: g4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Emberson J. R., Bennett D. A. Effect of alcohol on risk of coronary heart disease and stroke: causality, bias, or a bit of both? Vasc Health Risk Manag 2006; 2: 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Rehm J., Sempos C., Trevisan M. Average volume of alcohol consumption, patterns of drinking and risk of coronary heart disease—a review. J Cardiovasc Risk 2003; 10: 15–20. [DOI] [PubMed] [Google Scholar]
- 262. Brien S. E., Ronksley P. E., Turner B. J., Mukamal K. J., Ghali W. A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta‐analysis of interventional studies. BMJ 2011; 342: d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Yusuf S., Hawken S., Ounpuu S., Dans T., Avezum A., Lanas F. et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study. Lancet 2004; 364: 937–952. [DOI] [PubMed] [Google Scholar]
- 264. Roy A., Prabhakaran D., Jeemon P., Thankappan K. R., Mohan V., Ramakrishnan L. et al. Impact of alcohol on coronary heart disease in Indian men. Atherosclerosis 2010; 210: 531–535. [DOI] [PubMed] [Google Scholar]
- 265. Tolstrup J. S., Wium‐Andersen M. K., Ørsted D. D., Nordestaard B. G. Alcohol consumption and risk of atrial fibrillation: Observational and genetic estimates of association. Eur J Prev Cardiol 2016; 23: 1514–1523. [DOI] [PubMed] [Google Scholar]
- 266. Frick U., Rehm J. Can we establish causality with statistical analyses? The example of epidemiology In: Wiedermann W., von Eye A., editors. Statistics and Causality: Methods for Applied Empirical Research. Hoboken, NJ: Wiley; 2016, pp. 407–432. [Google Scholar]
- 267. Kehoe T., Gmel G. Jr., Shield K., Gmel G. Sr., Rehm J. Determining the best population‐level alcohol consumption model and its impact on estimates of alcohol‐attributable harms. Popul Health Metrics 2012; 10: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Rehm J., Shield K. D., Roerecke M., Gmel G. Modelling the impact of alcohol consumption on cardiovascular disease mortality for comparative risk assessments: an overview. BMC Public Health 2016; 16: 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Shield K., Rehm J. Russia‐specific relative risks and their effects on the estimated alcohol‐attributable burden of disease. BMC Public Health 2015; 15: 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Zaridze D., Brennan P., Boreham J., Boroda A., Karpov R., Lazarev A. et al. Alcohol and cause‐specific mortality in Russia: a retrospective case–control study of 48,557 adult deaths. Lancet 2009; 373: 2201–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Malyutina S., Bobak M., Kurilovitch S., Ryizova E., Nikitin Y., Marmot M. Alcohol consumption and binge drinking in Novosibirsk, Russia, 1985–95. Addiction 2001; 96: 987–995. [DOI] [PubMed] [Google Scholar]
- 272. Bobak M., Room R., Pikhart H., Kubinova S., Malyutina S., Pajak A. et al. Contribution of drinking patterns to differences in rates of alcohol related problems between three urban populations. J Epidemiol Community Health 2004; 58: 238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Rehm J. What can we learn from Russia about alcohol epidemiology and alcohol policy? Lancet 2014; 383: 1440–1442. [DOI] [PubMed] [Google Scholar]
- 274. Shield K. D., Rylett M., Rehm J. Public health gains and missed opportunities. Trends in alcohol consumption and attributable mortality in the WHO European Region, 1990–2014: a report to the WHO European Region, Toronto, Canada: Centre for Addiction and Mental Health; 2016.
- 275. Britton A., McKee M. The relation between alcohol and cardiovascular disease in Eastern Europe: explaining the paradox. J Epidemiol Community Health 2000; 54: 328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Lopez A. D., Williams T. N., Levin A., Tonelli M., Singh J. A., Burney P. G. J. et al. Remembering the forgotten non‐communicable diseases. BMC Med 2014; 12: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Rehm J., Samokhvalov A. V., Shield K. D. Global burden of alcoholic liver diseases. J Hepatol 2013; 59: 160–168. [DOI] [PubMed] [Google Scholar]
- 278. European Association for the Study of the Liver (EASL) . EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol 2012; 57: j399–j420. [DOI] [PubMed] [Google Scholar]
- 279. Gao B., Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 2011; 141: 1572–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Rehm J., Taylor B., Mohapatra S., Irving H., Baliunas D., Patra J. et al. Alcohol as a risk factor for liver cirrhosis ‐ a systematic review and meta‐analysis. Drug Alcohol Rev 2010; 29: 437–445. [DOI] [PubMed] [Google Scholar]
- 281. Mokdad A. A., Lopez A. D., Shahraz S., Lozano R., Mokdad A. H., Stanaway J. et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med 2014; 12: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Lelbach W. K. Epidemiology of alcoholic liver disease. Prog Liver Dis 1976; 5: 515. [PubMed] [Google Scholar]
- 283. Rehm J., Roerecke M. Patterns of drinking and liver cirrhosis—what do we know and where do we go? J Hepatol 2015; 62: 1061–1067. [DOI] [PubMed] [Google Scholar]
- 284. Marugame T., Yamamoto S., Yoshimi I., Sobue T., Inoue M., Tsugane S. Patterns of alcohol drinking and all‐cause mortality: results from a large‐scale population‐based cohort study in Japan. Am J Epidemiol 2007; 165: 1039–1046. [DOI] [PubMed] [Google Scholar]
- 285. Askgaard G., Grønbæk M., Kjaer M. S., Tjønneland A., Tolstrup J. S. Alcohol drinking pattern and risk of alcoholic liver cirrhosis: a prospective cohort study. J Hepatol 2015; 62: 106–107.current issue [DOI] [PubMed] [Google Scholar]
- 286. Szücs S., Sarvary A., McKee M., Adany R. Could the high level of cirrhosis in central and eastern Europe be due partly to the quality of alcohol consumed? An exploratory investigation. Addiction 2005; 100: 536–542. [DOI] [PubMed] [Google Scholar]
- 287. Lachenmeier D. W., Gmel G., Rehm J. Unrecorded alcohol consumption In: Boyle P., Boffetta P., Lowenfels A. B. et al., editors. Alcohol: Science, Policy, and Public Health. Oxford, UK: Oxford University Press; 2013, pp. 132–142. [Google Scholar]
- 288. Leitz J., Kuballa T., Rehm J., Lachenmeier D. W. Chemical analysis and risk assessment of Diethyl Phthalate in alcoholic beverages with special regard to unrecorded alcohol. PLoS One 2009; 4: e8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Solodun Y. V., Monakhova Y. B., Kuballa T., Samokhvalov A. V., Rehm J., Lachenmeier D. W. Unrecorded alcohol consumption in Russia: toxic denaturants and disinfectants pose additional risks. Interdiscip Toxicol 2011; 4: 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Rehm J., Kanteres F., Lachenmeier D. W. Unrecorded consumption, quality of alcohol and health consequences. Drug Alcohol Rev 2010; 29: 426–436. [DOI] [PubMed] [Google Scholar]
- 291. Lachenmeier D. W., Monakhova Y. B., Rehm J. Influence of unrecorded alcohol consumption on liver cirrhosis mortality. World J Gastroenterol 2014; 20: 7217–7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Rehm J., Kailasapillai S., Larsen E., Rehm M. X., Samokhvalov A. V., Shield K. D. et al. A systematic review of the epidemiology of unrecorded alcohol consumption and the chemical composition of unrecorded alcohol. Addiction 2014; 109: 880–893. [DOI] [PubMed] [Google Scholar]
- 293. Puffer R. R., Griffith G. W. Patterns of Urban Mortality: Report of the Inter‐American Investigation of Mortality. Washington, DC: Pan American Health Organization; 1967. [Google Scholar]
- 294. Prytz H., Anderson H. Underreporting of alcohol‐related mortality from cirrhosis is declining in Sweden and Denmark. Scand J Gastroenterol 1988; 23: 1035–1043. [DOI] [PubMed] [Google Scholar]
- 295. Haberman P. W., Weinbaum D. F. Liver cirrhosis with and without mention of alcohol as cause of death. Br J Addict 1990; 85: 217–222. [DOI] [PubMed] [Google Scholar]
- 296. Tuusov J., Lang K., Väli M., Pärna K., Tõnisson M., Ringmets I. et al. Prevalence of alcohol‐related pathologies at autopsy: Estonian forensic study of alcohol and premature death. Addiction 2014; 109: 2018–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Pollock D. A., Boyle C. A., DeStefano F., Moyer L. A., Kirk M. L. Underreporting of alcohol‐related mortality on death certificates of young U.S. army veterans. JAMA 1987; 258: 345–348. [PubMed] [Google Scholar]
- 298. Yadav D., Hawes R. H., Brand R. E., Anderson M. A., Money M. E., Banks P. A. et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med 2009; 169: 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Braganza J., Lee S., McCloy R., McMahon M. Chronic pancreatitis. Lancet 2011; 377: 1184–1197. [DOI] [PubMed] [Google Scholar]
- 300. Yadav D., Lowenfels A. B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013; 144: 1252–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Lankisch P., Apte M., Banks P. Acute pancreatitis. Lancet 2015; 386: 85–96. [DOI] [PubMed] [Google Scholar]
- 302. Majumder S., Chari S. T. Chronic pancreatitis. Lancet 2016; 387: 1957–1966. [DOI] [PubMed] [Google Scholar]
- 303. Sankaran S. J., Xiao A. Y., Wu L. M., Windsor J. A., Forsmark C. E., Petrov M. S. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta‐analysis. Gastroenterology 2015; 149: 1490–1500. [DOI] [PubMed] [Google Scholar]
- 304. Gonzalez F. J. Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutat Res 2005; 569: 101–110. [DOI] [PubMed] [Google Scholar]
- 305. Pandol S. J., Raraty M. Pathobiology of alcoholic pancreatitis. Pancreatology 2007; 7: 105–114. [DOI] [PubMed] [Google Scholar]
- 306. Witt H., Apte M. V., Keim V., Wilson J. S. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology 2007; 132: 1557–1573. [DOI] [PubMed] [Google Scholar]
- 307. Gerasimenko J. V., Gerasimenko O. V., Petersen O. H. The role of Ca(2+) in the pathophysiology of pancreatitis. J Physiol 2014; 592: 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Irving H. M., Samokhvalov A., Rehm J. Alcohol as a risk factor for pancreatitis. A systematic review and meta‐analysis. J Pancreas 2009; 10: 387–392. [PMC free article] [PubMed] [Google Scholar]
- 309. Samokhvalov A. V., Rehm J., Roerecke M. Alcohol consumption as a risk factor for acute and chronic pancreatitis: a systematic review and a series of meta‐analyses. EBioMedicine 2015; 2: 1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Eckardt M., File S., Gessa G., Grant K., Guerri C., Hoffman P. et al. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res 1998; 22: 998–1040. [DOI] [PubMed] [Google Scholar]
- 311. Cherpitel C., Ye Y., Bond J., Borges G., Autonoma M., Monteiro M. et al. Alcohol attributable fraction for injury morbidity from the dose–response relationship of acute alcohol consumption: emergency department data from 18 countries. Addiction 2015; 110: 1724–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Taylor B., Irving H. M., Kanteres F., Room R., Borges G., Cherpitel C. et al. The more you drink, the harder you fall: a systematic review and meta‐analysis of how acute alcohol consumption and injury or collision risk increase together. Drug Alcohol Depend 2010; 110: 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Taylor B., Rehm J. The relationship between alcohol consumption and fatal motor vehicle injury: high risk at low alcohol levels. Alcohol Clin Exp Res 2012; 36: 1827–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Krüger H. P., Kazenwadel J., Vollrath M. Grand Rapids effects revisited: accidents, alcohol and risk In: Kloeden C. N., McLean A. J., editors. Alcohol, Drugs, and Traffic Safety: Proceedings of the 13th International Conference on Alcohol, Drugs and Traffic Safety, Adelaide. Adelaide: NHMRC Road Accident Research Unit, University of Adelaide; 1995, pp. 222–230. [Google Scholar]
- 315. Rehm J. Commentary on Cherpitel et al. (2015): improving global estimates of alcohol‐attributable injury. Addiction 2015; 110: 1733–1734. [DOI] [PubMed] [Google Scholar]
- 316. Rafia R., Brennan A. Modelling methods to estimate the potential impact of lowering the blood alcohol concentration limit from 80 mg/100 ml to 50 mg/100 ml in England and Wales: Report to the National Institute for Health and Clinical Excellence [Internet]. The University of Sheffield; 2010. Available at: https://www.nice.org.uk/media/default/About/what‐we‐do/NICE‐guidance/NICE‐guidelines/Public‐health‐guidelines/Additional‐publications/Blood‐alcohol‐content‐road‐traffic‐modelling.pdf/Web (accessed 3 November 2016) (Archived at http://www.webcitation.org/6n7oz91nt).
- 317. Darvishi N., Farhadi M., Haghtalab T., Poorolajal J. Alcohol‐related risk of suicidal ideation, suicide attempt, and completed suicide: a meta‐analysis. PLoS One 2015; 10: e0126870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Norström T., Rossow I. Alcohol consumption as a risk factor for suicidal behavior: a systematic review of associations at the individual and at the population level. Arch Suicide Res 2016; 20: 489–506. [DOI] [PubMed] [Google Scholar]
- 319. Cherpitel C. J., Borges G. L., Wilcox H. C. Acute alcohol use and suicidal behavior: a review of the literature. Alcohol Clin Exp Res 2004; 28: 18S–28S. [DOI] [PubMed] [Google Scholar]
- 320. Borges G., Loera C. R. Alcohol and drug use in suicidal behaviour. Curr Opin Psychiatry 2010; 23: 195–204. [DOI] [PubMed] [Google Scholar]
- 321. Taylor B., Shield K., Rehm J. Combining best evidence: a novel method to calculate the alcohol‐attributable fraction and its variance for injury mortality. BMC Public Health 2011; 11: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Shield K. D., Gmel G. Jr., Patra J., Rehm J. Global burden of injuries attributable to alcohol consumption in 2004: a novel way of calculating the burden of injuries attributable to alcohol consumption. Popul Health Metrics 2012; 10: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Haagsma J. A., Graetz N., Bolliger I., Naghavi M., Higashi H., Mullany E. C. et al. The global burden of injury: incidence, mortality, disability‐adjusted life years and time trends from the Global Burden of Disease study 2013. Inj Prev 2016; 22: 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Gmel G., Rehm J., Frick U. Trinkmuster, Pro‐Kopf‐Konsum von Alkohol und koronare Mortalitat [Drinking patterns, per capita consumption of alcohol and coronary mortality]. Sucht 2003; 49: 95–104. [Google Scholar]
- 325. Hemström Ö. Per capita alcohol consumption and ischaemic heart disease mortality. Addiction 2001; 96: S93–S112. [DOI] [PubMed] [Google Scholar]
- 326. Pun V. C., Lin H., Kim J. H., Yip B. H., Chung V. C., Wong M. C. et al. Impacts of alcohol duty reductions on cardiovascular mortality among elderly Chinese: a 10‐year timeseries analysis. J Epidemiol Community Health 2013; 67: 514–518. [DOI] [PubMed] [Google Scholar]
- 327. Morgenstern H. Ecological studies In: Rothman K., Greenland S., Lash T., editors. Modern Epidemiology, 3rd edn. Philadelphia, PA: Lippincott, Williams and Wilkins; 2008, pp. 511–531. [Google Scholar]
- 328. Gell L., Ally A., Buykx P., Hope A., Meier P. Alcohol's Harm to others: An Institute of Alcohol Studies report. London, UK: Institute of Alcohol Studies; 2015. [Google Scholar]
- 329. Laslett A. M., Catalano P., Chikritzhs T., Dale C., Doran C., Ferris J. et al. The Range and Magnitude of Alcohol's Harm to Others. Fitzroy, Australia: Turning Point Alcohol and Drug Centre; 2010. [Google Scholar]
- 330. Laslett A. M., Room R., Ferris J., Wilkinson C., Livingston M., Mugavin J. Surveying the range and magnitude of alcohol's harm to others in Australia. Addiction 2011; 106: 1603–1611. [DOI] [PubMed] [Google Scholar]
- 331. Navarro H. J., Doran C. M., Shakeshaft A. P. Measuring costs of alcohol harm to others: a review of the literature. Drug Alcohol Depend 2011; 114: 87–99. [DOI] [PubMed] [Google Scholar]
- 332. Laslett A., Waleewong O., Obot I., Benegal V., Hettige S., Florenzano R. et al. Scoping response system management of alcohol's harm to others in lower middle income countries. Nord Stud Alcohol Drugs 2016; 33: 515–536. [Google Scholar]
- 333. Thanh N. X., Jonsson E. Life expectancy of people with fetal alcohol syndrome. J Popul Ther Clin Pharmacol 2016; 23: e53–9. [PubMed] [Google Scholar]
- 334. Damashek A., Williams N. A., Sher K., Peterson L. Relation of caregiver alcohol use to unintentional childhood injury. J Pediatr Psychol 2009; 34: 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Winqvist S., Jokelainen J., Luukinen H., Hillbom M. Parental alcohol misuse is a powerful predictor for the risk of traumatic brain injury in childhood. Brain Inj 2007; 21: 1079–1085. [DOI] [PubMed] [Google Scholar]
- 336. Quinlan K., Shults R. A., Rudd R. A. Child passenger deaths involving alcohol‐impaired drivers. Pediatrics 2014; 133: 966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. World Health Organization . Global Status Report on Road Safety 2015. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 338. Bushman B., Cooper H. Effects of alcohol on human aggression: an integrative research review. Psychol Bull 1990; 107: 341–354. [DOI] [PubMed] [Google Scholar]
- 339. Graham K., West P. Alcohol and Crime In: Heather N., Peters T. J., Stockwell T., editors. International Handbook of Alcohol Dependence and Problems. London: John Wiley & Sons; 2001, pp. 439–470. [Google Scholar]
- 340. Abbey A., Clinton‐Sherrod A. M., McAuslan P., Zawacki T., Buck P. O. The relationship between the quantity of alcohol consumed and the severity of sexual assaults committed by college men. J Interpers Violence 2003; 18: 813–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Clausen T., Martinez P., Towers A., Greenfield T., Kowal P. Alcohol consumption at any level increases risk of injury caused by others: data from the study on Global Ageing and adult health. Subst Abuse Res Treat 2016; 9: 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Hull J. G., Bond C. F. J. Social and behavioral consequences of alcohol consumption and expectancy: a meta‐analysis. Psychol Bull 1986; 99: 347–360. [PubMed] [Google Scholar]
- 343. Ito T. A., Miller N., Pollock V. E. Alcohol and aggression: a meta‐analysis on the moderating effects of inhibitory cues, triggering events, and self‐focused attention. Psychol Bull 1996; 120: 60–82. [DOI] [PubMed] [Google Scholar]
- 344. Room R., Rossow I. The share of violence attributable to drinking. J Subst Use 2001; 6: 218–228. [Google Scholar]
- 345. Shield K. D., Rylett M. J., Gmel G., Rehm J. Part 1. Trends in alcohol consumption and alcohol‐attributable mortality in the EU in 2010 In: World Health Organization Regional Office for Europe , editor. Status Report on Alcohol and Health in 35 European Countries 2013. Copenhagen: WHO Regional Office for Europe; 2013, pp. 3–14. [Google Scholar]
- 346. Shkolnikov V. M., Nemtsov A. V. The anti‐alcohol campaign and variations in Russian mortality In: Bobadilla J. L., Costello C. A., Mitchell F., editors. Premature Death in the New Independent States. Washington, DC: National Academy Press; 1997, pp. 239–261. [PubMed] [Google Scholar]
- 347. Rehm J. Measuring quantity, frequency and volume of drinking. Alcohol Clin Exp Res 1998; 22: 4S–14S. [DOI] [PubMed] [Google Scholar]
- 348. Shaper A., Wannamethee G., Walker M. Alcohol and mortality in British men: explaining the U‐shaped curve. Lancet 1988; 2: 1267–1273. [DOI] [PubMed] [Google Scholar]
- 349. Rehm J. Alcohol consumption and mortality. What do we know and where should we go? Addiction 2000; 95: 989–995. [DOI] [PubMed] [Google Scholar]
- 350. Chen X., Zhou L., Zhang Y., Yi D., Liu L., Rao W. et al. Risk factors of stroke in Western and Asian countries: a systematic review and meta‐analysis of prospective cohort studies. BMC Public Health 2014; 14: 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. McKenzie K., Harrison J. E., McClure R. J. Identification of alcohol involvement in injury‐related hospitalisations using routine data compared to medical record review. Aust J Public Health 2010; 34: 146–152. [DOI] [PubMed] [Google Scholar]
- 352. Saunders J. B., Room R. Enhancing the ICD system in recording alcohol's involvement uin disease and injury. Alcohol Alcohol 2012; 47: 216–218. [DOI] [PubMed] [Google Scholar]
- 353. Klingemann H., Gmel G. Mapping Social Consequences of Alcohol Consumption. Dordrecht, the Netherlands: Kluwer Academic Publishers; 2001. [Google Scholar]
- 354. Institute of Health Metrics and Evaluation . GBD 2015 Codebook, Institute of Health Metrics and Evaluation. 2015. Available at: http://ghdx.healthdata.org/sites/default/files/ihme_query_tool/IHME_GBD_2015_CODEBOOK.zip/Web (accessed 25 October 2016) (Archived at http://www.webcitation.org/6n7o8JnWs).
- 355. Patra J., Jha P., Rehm J., Suraweera W. Tobacco smoking, alcohol drinking, diabetes, low body mass index and the risk of self‐reported symptoms of active tuberculosis: individual participant data (IPD) meta‐analyses of 72,684 individuals in 14 high tuberculosis burden countries. PLoS One 2014; 9: E96433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Traphagen N., Tian Z., Allen‐Gipson D. Chronic ethanol exposure: pathogenesis of pulmonary disease and dysfunction. Biomolecules 2015; 5: 2840–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Simet S. M., Sisson J. H. Alcohol's effects on lung health and immunity. Alcohol Res 2015; 37: 199–208. [PMC free article] [PubMed] [Google Scholar]
- 358. Foulds J. A., Adamson S. J., Boden J. M., Williman J. A., Mulder R. T. Depression in patients with alcohol use disorders: systematic review and meta‐analysis of outcomes for independent and substance‐induced disorders. J Affect Disord 2015; 1: 47–59. [DOI] [PubMed] [Google Scholar]
- 359. Bartolomei F. Epilepsy and alcohol. Epileptic Disord 2006; 8: S72–8. [Google Scholar]
- 360. Puddey I. B., Beilin L. J. Alcohol is bad for blood pressure. Clin Exp Pharmacol Physiol 2006; 33: 847–852. [DOI] [PubMed] [Google Scholar]
- 361. Xin X., He J., Frontini M. G., Ogden L. G., Motsamai O. J., Whelton P. K. Effects of alcohol reduction on blood pressure: a meta‐analysis of randomized controlled trials. Hypertension 2001; 38: 1112–1117. [DOI] [PubMed] [Google Scholar]
- 362. Roerecke M., Kaczorowski M., Tobe S. W., Gmel G., Hasan O. S. M., Rehm J. The effect of a reduction in alcohol consumption on blood pressure: a systematic review and meta‐analysis of trial data. Lancet Public Health 2017; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Chen L., Smith G. D., Harbord R. M., Lewis S. J. Alcohol intake and blood pressure: a systematic review implementing a Mendelian Randomization Approach. PLoS Med 2008; 5: 461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Mukamal K. J., Rimm E. B. Alcohol's effects on the risk for coronary heart disease. Alcohol Res Health 2001; 25: 255–261. [PMC free article] [PubMed] [Google Scholar]
- 365. Roerecke M., Rehm J. Ischemic heart disease mortality and morbidity in former drinkers: a meta‐analysis. Am J Epidemiol 2011; 173: 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. George A., Figueredo V. M. Alcoholic cardiomyopathy: a review. J Card Fail 2011; 17: 844–849. [DOI] [PubMed] [Google Scholar]
- 367. Manthey J., Imtiaz S., Neufeld J., Rylett M., Rehm J. Quantifying the Global Contribution of Alcohol Consumption to Cardiomyopathy. Toronto, ON, Canada: 2017. [DOI] [PMC free article] [PubMed]
- 368. Rosenqvist M. Alcohol and cardiac arrhythmias. Alcohol Clin Exp Res 1998; 22: 318s–322s. [DOI] [PubMed] [Google Scholar]
- 369. Rosenberg M. A., Mukamal K. J. The estimated risk of atrial fibrillation related to alcohol consumption. J Atr Fibrillation 2012; 5: 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Samokhvalov A. V., Irving H. M., Rehm J. Alcohol as a risk factor for atrial fibrillation: a systematic review and meta‐analysis. Eur J Cardiovasc Prev Rehabil 2010; 17: 706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Larsson S. C., Drca N., Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose–response meta‐analysis. J Am Coll Cardiol 2014; 64: 281–289. [DOI] [PubMed] [Google Scholar]
- 372. Murray C. J. L., Lopez A. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997; 349: 1269–1276. [DOI] [PubMed] [Google Scholar]
- 373. Mazzaglia G., Britton R., Altmann D. R., Chenet L. Exploring the relationship between alcohol consumption and non‐fatal or fatal stroke: a systematic review. Addiction 2001; 96: 1743–1756. [DOI] [PubMed] [Google Scholar]
- 374. Reynolds K., Lewis B., Nolen J., Kinney G., Sathya B., He J. Alcohol consumption and risk of stroke: a meta‐analysis. JAMA 2003; 289: 579–588. [DOI] [PubMed] [Google Scholar]
- 375. Patra J., Taylor B., Irving H., Roerecke M., Baliunas D., Mohapatra S. et al. Alcohol consumption and the risk of morbidity and mortality for different stroke types—a systematic review and meta‐analysis. BMC Public Health 2010; 10: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Zhang C., Qin Y. Y., Chen Q., Jiang H., Chen X. Z., Xu C. L. et al. Alcohol intake and risk of stroke: a dose–response meta‐analysis of prospective studies. Int J Cardiol 2014; 174: 669–677. [DOI] [PubMed] [Google Scholar]
- 377. Tseng M., Everhart J. E., Sandler R. S. Dietary intake and gallbladder disease: a review. Public Health Nutr 1999; 2: 161–172. [DOI] [PubMed] [Google Scholar]
- 378. Shabanzadeh D. M., Sørensen L. T., Jørgensen T. Determinants for gallstone formation—a new data cohort study and a systematic review with meta‐analysis. Scand J Gastroenterol 2016; 51: 1239–1248. [DOI] [PubMed] [Google Scholar]
- 379. Farkas A., Kemény L. Psoriasis and alcohol: is cutaneous ethanol one of the missing links? Br J Dermatol 2010; 162: 771–716. [DOI] [PubMed] [Google Scholar]
- 380. Brenaut E., Horreau C., Pouplard C., Barnetche T., Paul C., Richard M. A. et al. Alcohol consumption and psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol 2013; 27: 30–35. [DOI] [PubMed] [Google Scholar]
- 381. Richard M. A., Barnetche T., Horreau C., Brenaut E., Pouplard C., Aractingi S. et al. Psoriasis, cardiovascular events, cancer risk and alcohol use: evidence‐based recommendations based on systematic review and expert opinion. J Eur Acad Dermatol Venereol 2013; 27: 2–11. [DOI] [PubMed] [Google Scholar]
- 382. Zhu K. J., Zhu C. Y., Fan Y. M. Alcohol consumption and psoriatic risk: a meta‐analysis of case–control studies. J Dermatol 2012; 39: 770–773. [DOI] [PubMed] [Google Scholar]
- 383. Adamzik K., McAleer M. A., Kirby B. Alcohol and psoriasis: sobering thoughts. Clin Exp Dermatol 2013; 38: 819–822. [DOI] [PubMed] [Google Scholar]
- 384. Poikolainen K., Karvonen J., Pukkala E. Excess mortality related to alcohol and smoking among hospital‐treated patients with psoriasis. Arch Dermatol 1999; 135: 1490–1493. [DOI] [PubMed] [Google Scholar]
- 385. Patra J., Bakker R., Irving H., Jaddoe V. W. V., Malini S., Rehm J. Dose–response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA)—a systematic review and meta‐analyses. Int J Obstet Gynaecol 2011; 118: 1411–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Global Burden of Disease Study . Global Burden of Disease Study 2015. Causes of death and Nonfatal Causes Mapped to ICD codes. Seattle, WA: Institute for Health Metrics and Evaluation; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Results of the systematic searches.
Appendix S2 Dose Response‐relationships between average volume of alcohol use and relative risk for mortality for partially alcohol‐attributable disease categories.


