Summary
Lactation may protect women with previous gestational diabetes mellitus (GDM) from developing type 2 diabetes mellitus, but the results of existing studies are inconsistent, ranging from null to beneficial. We aimed to conduct a systematic review to gather available evidence. Databases MEDLINE, CINAHL, PubMed, and EMBASE were searched on December 15, 2015, without restriction of language or publication year. A manual search was also conducted. We included observational studies (cross‐sectional, case‐control, and cohort study) with information on lactation and type 2 diabetes mellitus incidence among women with previous GDM. We excluded case studies without control data. Data synthesis was conducted by random‐effect meta‐analysis. Fourteen reports of 9 studies were included. Overall risk of bias using RoBANS ranged from low to unclear. Longer lactation for more than 4 to 12 weeks postpartum had risk reduction of type 2 diabetes mellitus compared with shorter lactation (OR 0.77, 95% CI 0.01‐55.86; OR 0.56, 95% CI 0.35‐0.89; OR 0.22, 95% CI 0.13‐0.36; type 2 diabetes mellitus evaluation time < 2 y, 2‐5 y, and >5 y, respectively). Exclusive lactation for more than 6 to 9 weeks postpartum also had lower risk of type 2 diabetes mellitus compared with exclusive formula (OR 0.42, 95% CI 0.22‐0.81). The findings support the evidence that longer and exclusive lactation may be beneficial for type 2 diabetes mellitus prevention in women with previous GDM. However, the evidence relies only on observational studies. Therefore, further studies are required to address the true causal effect.
Keywords: gestational diabetes mellitus, lactation, meta‐analysis, prevention, systematic review, type 2 diabetes mellitus
1. INTRODUCTION
At present about 415 million adults suffer from diabetes, of which about 90% are type 2 diabetes mellitus.1 Diabetes is associated with life‐threatening morbidity, making the disease not only personal but also socioeconomic problem. In 2015, about 5 million people died because of diabetes.1
Gestational diabetes mellitus (GDM) is defined as “diabetes diagnosed in the second or third trimester of pregnancy that is not clearly overt diabetes.”2 The GDM occurs in nearly 14% of live births.1 Although hyperglycemia usually normalizes immediately after delivery, the risk of lifetime type 2 diabetes mellitus in women who had GDM is more than 7‐fold higher compared with in women with normoglycaemic pregnancies.3 Furthermore, up to 50% of women who had GDM developed type 2 diabetes mellitus within 5 years postpartum.4 Therefore, women with GDM are recognized to be at high risk of developing diabetes at younger ages and are therefore the target of preventive measures.
Intensive lifestyle modification is effective in preventing or delaying type 2 diabetes mellitus in women with previous GDM.5 However, postpartum women may face difficulties in adopting a healthy lifestyle mainly because of a lack of time.6, 7
Meanwhile, lactation is increasingly being recognized for its potential benefits on maternal glycemic metabolism. Childbearing itself is suggested to put women at risk for type 2 diabetes mellitus when compared with nulliparous women,8, 9 and breastfeeding may “reset” the burden10 and lower the risk of diabetes in dose‐response manner.11, 12 Although etiological evidence is yet to be established, several hypotheses for this beneficial effect have been proposed such as extra energy expenditure for milk production,13 visceral fat mobilization,10 and pancreatic beta‐cell rescue by prolactin14 and/or oxytocin.15
It is of great interest whether women with previous GDM, the high risk population for type 2 diabetes mellitus, benefit as well from breastfeeding practice. To date, several observational studies investigating the association between lactation and type 2 diabetes mellitus incidence after GDM pregnancy have been conducted with mixed results. Although there are several reviews written on this topic,16, 17 none was conducted systematically. Only few GDM guidelines recommend breastfeeding for maternal health with minimal evidence.18, 19, 20 To cover all the available evidence and to synthesize the data if available, we aimed to systematically review current findings on lactation for type 2 diabetes mellitus prevention in women with previous GDM.
2. METHODS
This systematic review was performed according to the MOOSE (Meta‐analysis Of Observational Studies in Epidemiology) guidelines21 and the Cochrane Handbook for Systematic Reviews for Intervention.22 The protocol was registered in advance on PROSPERO (CRD42016032699) and is accessible at http://www.crd.york.ac.uk/prospero/display_record.asp?ID=CRD42016032699.
2.1. Eligibility criteria and study selection
A study was considered eligible if (1) the participants were women with previous GDM, (2) it assessed the lactation intensity and/or duration of any lactation, and (3) it included the incidence of postpartum type 2 diabetes mellitus in the outcome. Observational studies (cross‐sectional, case‐control, and cohort study) were included. Studies with unclear number/rate of type 2 diabetes mellitus onset were excluded (eg, “high” incidence of diabetes, incidence rate of “dysglyceamia”).
After eliminating duplicate literatures in EndNote X7.1, 2 reviewers (K.T.N. and M.K.) independently selected potentially eligible reports with titles and abstracts. Full texts of reports that the 2 reviewers agreed on for inclusion were obtained for final selection and were reviewed separately. Any disagreement during the selection process was resolved through discussion with or consulting a third reviewer (E.O.).
2.2. Search strategy
Literature search was conducted by an information specialist on December 15, 2015, using databases MEDLINE, CINAHL, PubMed, and EMBASE. The search keywords included terms for “lactation” and “GDM.” The full search strategy for each database is provided in Table S1. No language or time restriction was applied. We also investigated the references lists of the retrieved papers for the search of additional relevant studies.
2.3. Data extraction and data synthesis
Information collected was as follows:
Study design, study period, and country where the study was conducted
Population number and characteristics (ie, age at delivery, nonpregnant body mass index [BMI], race/ethnicity)
Exclusion criteria
Lactation measures (ie, intention, initiation, intensity, and duration)
Diagnostic methods of GDM and type 2 diabetes mellitus
Type 2 diabetes mellitus evaluation time‐point, incidence rate, and hazard/risk ratio
Adjusted confounders for the analysis of breastfeeding and type 2 diabetes mellitus incidence
Conclusion on breastfeeding and type 2 diabetes mellitus incidence
One reviewer (K.T.N.) extracted data, and another reviewer (M.K.) checked for its integrity. We planned to contact authors or check original protocols for additional information if needed.
We conducted meta‐analysis for studies with comparable results using Review Manager software version 5.3 (RevMan5.3). The number of women with GDM and type 2 diabetes mellitus incidence in relation to breastfeeding measures were obtained from the reports or estimated through calculation by RevMan5.3. We used ruler to estimate the number/percentage in reports providing only graph without exact number.23 We combined odds ratio for dichotomous data using random‐effect models. All data were presented with 95% confidence intervals. We regarded heterogeneity as substantial when the I‐squared is greater than 60% and conducted subgroup analyses in such a case.
2.4. Risk of bias assessment and quality of evidence evaluation
Two researchers (K.T.N. and M.K.) independently assessed the methodological quality of each selected study. Again, any disagreement was resolved through discussion or consulting a third reviewer (E.O.). We used the Risk of Bias Assessment Tool for Nonrandomized Studies (RoBANS)24 for making judgments. We evaluated the quality of evidence with Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach using GRADEpro GDT.25
3. RESULTS
3.1. Study selection
A total of 1410 reports were identified through electronic search (Figure 1). Eight reports were added through hand search. Selection first with title and abstracts followed by full‐text screening yielded 14 reports for this review. The lists of excluded reports with reason for exclusion are shown in Table S2.
Figure 1.
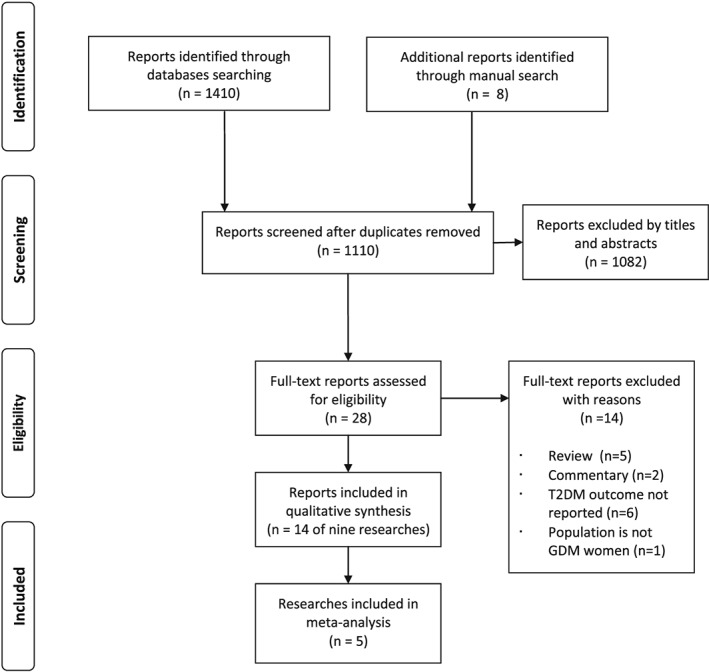
PRISMA flow diagram of search and selection
3.2. Characteristics of included studies
Fourteen reports included in this review were from 9 studies (4 reports for one study, and 2 reports each for 2 studies) involving more than 3600 women with GDM (Table 1).
Table 1.
Characteristics of included studies
| First author, year, country, study name, reference | GDM definition | Study period | GDM (n) | Population characteristics (age at delivery, nonpregnant BMI, race/ethnicity) | Major exclusion criteria | Lactation measure1 |
|---|---|---|---|---|---|---|
| Prospective cohort | ||||||
| Gunderson, 2015, USA, SWIFT,26, 27, 28, 29 | Carpenter‐Coustan criteria | 2 y | 1035 | Mean age (y): 33.9 (DM group), 33.3 (No DM group). Mean pre‐pregnancy BMI (kg/m2): 33.4 (DM group), 29.0 (No DM group). Race/ethnicity: mixed. | Pre‐existing DM, DM at 6‐9 wk postpartum, mixed or inconsistent feeding within 4‐6 wk postpartum. | ‐ Intensity at 6‐9 wk postpartum (exclusive lactation; mostly lactation; mostly formula and mixed or inconsistent lactation; exclusive formula) ‐ Duration |
| Gunderson, 2014, USA, CARDIA30 | Self‐report | 25 y | 154 | Age: NG. BMI: NG. Race/ethnicity: 50% white, 50% black. | Pre‐existing DM at baseline and/or DM before the first post‐baseline delivery. | Duration (lifetime) |
| Ziegler, 2012, Germany31 | German Diabetes Association criteria | 19 y | 304 | Median age (y): 31. BMI: NG. Race/ethnicity: presumably white | (Islet‐autoantibody positive) | ‐ Intensity (Full lactation with duration) ‐ Duration |
| Cross‐sectional | ||||||
| Buchanan, 1998, USA32, 33 | Recommendation of the third international workshop‐conference on GDM | NA | 122 | Mean age (y): 30.8 (NGT group), 32.3 (DM group). Mean pre‐pregnancy BMI (kg/m2): 30.4 (NGT group), 29.1 (DM group). Ethnicity: Latino. | ICA‐positive, on insulin therapy during pregnancy, not all FBG <7.2 mmol/L since the diagnosis of GDM | Status at 6 mo postpartum |
| Kim, 2011, Korea34 | Carpenter‐Coustan criteria | NA | 381 | Mean age (y): 33.6 (NGT group), 34.9 (DM group). Mean pre‐pregnancy BMI (kg/m2): 22.5 (NGT group), 24.9 (DM group). Race/ethnicity: presumably Asian | GADA‐positive. | Status at 6‐12 wk postpartum |
| Kjos, 1993, USA35 | NDDG criteria | NA | 809 | Mean age (y): 31.6 (Lactating group), 30.5 (Non‐lactating group). Mean BMI (kg/m2): 28.8 (Lactating group), 28.8 (Non‐lactating group). Ethnicity: 95% Latino. | Status at 4‐12 wk postpartum | |
| Urs, 2015, USA, NHANES36 | NG | NA | NG | Age, BMI, Race/ethnicity: NG. | Initiation | |
| Retrospective cohort | ||||||
| Kjos, 1998, USA23, 37 | NDDG criteria | 7.5 y | 809 (Non‐hormonal only; 443) | Mean age (y): 31.3, mean postpartum BMI (kg/m2): 29.6. Ethnicity: >97% Latino. | DM at 4‐16 wk postpartum. | Status at 4‐16 wk postpartum |
| Steube, 2005, USA, NHS II38 | Self‐report | 14 y | NG | Age, BMI, Race/ethnicity: NG. | Duration (lifetime) | |
Table 1.
(Continued)
| First author, year, study name, reference | T2DM definition | T2DM evaluation time | T2DM incidence among GDM women | Adjusted co‐variables for the analysis of lactation and T2DM incidence | Conclusion |
|---|---|---|---|---|---|
| Prospective cohort | |||||
| Gunderson, 2015, SWIFT,26, 27, 28, 29 | ADA criteria | OGTT at 1 and 2 y postpartum | 113 of 959 (11.8%) developed T2DM. Overall incidence rate was 5.64 cases per 1000 person‐mo (95% CI; 4.60‐6.68). | Age; race/ethnicity; education; pre‐pregnancy BMI; GDM treatment; sum of prenatal 3‐h, 100‐g OGTT Z score; gestational age at GDM diagnosis; subsequent birth during follow‐up; total PA, GI, animal fat intake; weight change from delivery to 1 y; LGA vs not LGA, newborn's hospital stay >3 d, NICU admission | The lactation intensity and duration of breastfeeding inversely associated with T2DM incidence in a graded manner (all P < .05). |
| Gunderson, 2014, CARDIA,30 | NG | Questionnaire at 7, 10, 15, 20, and/or 25 y after enrollment | 46 of 154 (29.9%) developed T2DM. Overall incidence rate was 17.9 per 1000 person‐years. | Pre‐pregnancy BMI; age; parity; family history; race; education. | Shorter lactation (0‐1 mo vs >9 mo) was associated with higher incidence of T2DM (Adjusted RH 3.0, 95% CI; 2.1‐13.3). |
| Ziegler, 2012,31 | ADA criteria | OGTT at 2 and 9 mo; 2, 5, 8, 11, 15, and 19 y postpartum | 147 of 304 (48.4%) developed T2DM. The 15‐year cumulative risk was 63.6% (95% CI 55.8‐71.4). | Age at delivery; insulin treatment during pregnancy; BMI at early pregnancy; smoking during pregnancy; parity status; recruitment year | Longer lactation (>3 mo vs no or <3 mo) was associated with 30% risk reduction in 15‐y DM incidence (P = .0002). Full lactation duration was inversely associated with DM incidence (P = .001). |
| Cross‐sectional | |||||
| Buchanan, 1998,32, 33 | NG | OGTT within 6 mo postpartum | 12 of 122 (9.8%) developed T2DM. | Not adjusted | Lactation rate 42% in DM, 49% in IGT and 71% in NGT group (P = .03) |
| Kim, 2011,34 | ADA criteria | OGTT at 6 to 12 wk postpartum | 30 of 573 (5.2%) developed T2DM. | Not adjusted | Lactation status did not affect postpartum glycemic status. |
| Kjos, 1993,35 | NDDG criteria | OGTT at 4 to 12 wk postpartum | 55 of 809 women (6.8%) developed T2DM | Not adjusted | T2DM incidence rate was 4.2% in lactating group and 9.4% in non‐lactating group (P = .01). |
| Urs, 2015, NHANES,36 | NG | NA | NG | Age; BMI; race/ethnicity; income; education; age at DM; number of live births | Adjusted OR for incident DM after GDM (vs no GDM) was 0.6 lower in women who breastfed compared to women who did not breastfeed. |
| Retrospective cohort | |||||
| Kjos, 1998,23, 37 | NDDG criteria | OGTT within 7.5 y | Average annual incidence rate was 8.7% (non‐hormonal group). | insulin treatment during index pregnancy; glucose AUC at initial postpartum OGTT; weight change from initial postpartum weight; completion of additional pregnancy; and prior use of OC | No significant difference in T2DM risk between women who were breastfeeding vs who were not breastfeeding. |
| Steube, 2005, NHS II,38 | Self‐report | Questionnaire up to 12 y | Incidence rate: 624 cases per 100 000 person‐years | parity, BMI at age 18 years, current BMI, dietary score quintile, PA, family history of DM, smoking status, birth weight of mother, and multivitamin use. | Lifetime lactation duration did not affect diabetes risk. |
Duration, evaluation of any lactation period; Initiation, evaluation of lactation experience; Intensity, evaluation of lactation or formula feeding exclusiveness; Status, evaluation of the lactation practice at the point of survey.
Abbreviations: ADA, American Diabetes Association; GI, glycemic index; IGT, impaired glucose tolerance; LGA, large for gestational age; NICU, neonatal intensive care unit; OC, oral contraceptive; NDDG, National Diabetes Data Group; OGTT, oral glucose tolerance test; OR, odds ratio; PA, physical activity; RH, relative hazards; T2DM, type 2 diabetes mellitus.
3.3. Study design, country
Three studies were prospective cohort study,26, 30, 31 2 were retrospective cohort study,23, 38 and 4 were cross‐sectional study.32, 34, 35, 36 There was no randomized control trial. Most studies were conducted in the United States, except for one in Germany31 and one in South Korea.34
3.4. Population
In each study 122 women32 to 1035 women26 with GDM were enrolled. Two studies did not provide the population number.36, 38
The GDM diagnostic criteria were available in 8 studies. Two studies used the Carpenter‐Coustan criteria,26, 34 2 studies used the National Diabetes Data Group criteria,23, 35 1 study used the recommendation of the Third International Workshop‐Conference on GDM32, 1 study used local criteria,31 and 2 were based on self‐report.30, 38
The mean/median age at delivery, mostly in the early 1930s, was provided in 6 studies,23, 26, 31, 32, 34, 35 mostly in early 1930s. The mean nonpregnant BMI was available in 5 studies,23, 26, 32, 34, 35 with all in overweight or obese range except in one study34 conducted in South Korea (<25 kg/m2). The race/ethnicity of the population was provided in 5 studies, 2 were multiracial,26, 30 and 3 consisted mainly of Hispanic populations.23, 32, 35
Exclusion of preexisting diabetes was clearly stated in 2 studies,26, 30 and presumably an additional 5 studies did do so because the diagnosis of GDM was based on glucose tolerance test and not on self‐report.23, 31, 32, 34, 35 Early postpartum DM was excluded in 2 studies.23, 26 Women with positive islet autoantibodies were excluded in 2 studies (one study with ICA‐positive32 and one study with GADA‐positive34). One study conducted subgroup analysis with positive islet autoantibody versus negative islet autoantibody results,31 and we used the data of the autoantibody‐negative population only.
3.5. Lactation measures
Four studies measured lactation by the duration of breastfeeding period,26, 30, 31, 38 2 of 4 measured the sum of lifetime lactation30, 38 and 2 studies measured index pregnancy.26, 31
Four studies assessed lactation status within the following: 6 months by Buchanan et al,32 6 to 12 weeks by Kim et al,34 4 to 12 weeks by Kjos et al,35 and 4 to 16 weeks by Kjos et al23 after delivery.
Lactation intensity was assessed in 2 studies with different methodology. One study evaluated the intensity at 6 to 9 weeks postpartum by measuring the amount of added formula milk to test the dose‐response effect for type 2 diabetes mellitus prevention and divided the participants into 4 groups: exclusive lactation, mostly lactation, mostly formula and mixed or inconsistent lactation, and exclusive formula.26 Another study assessed the full lactation period.31
3.6. T2DM evaluation, incidence
The diagnostic criteria for the evaluation of type 2 diabetes mellitus incidence were described in 6 studies; 3 studies applied the American Diabetes Association criteria,26, 31, 34 2 studies used the National Diabetes Data Group criteria,23, 35 and one was based on self‐report.38
Type 2 diabetes mellitus evaluation time ranged from 4 to 12 weeks to up to 19 years postpartum, and type 2 diabetes mellitus incidence rate increases in accordance with the evaluation time.
Covariables used to adjust for analyzing lactation measure and type 2 diabetes mellitus incidence varied by each study. The most frequently adjusted index was BMI, which was used in 5 studies.26, 30, 31, 36, 38 Age at delivery and parity status were used in 4 studies.26, 30, 31, 36 Race/ethnicity,26, 30, 36 education,26, 30, 36 weight/BMI change,23, 26, 38 and GDM treatment during pregnancy were used in 3 studies.23, 26, 31 Family history of DM,30, 38 smoking,31, 38 physical activity,26, 38 diet,26, 38 OGTT results,23, 26 and subsequent birth were adjusted in 2 studies.23, 26 Oral contraceptive use,23 multivitamin use,38 gestational age at diagnosis of GDM,26 income,36 birth weight of mother,38 enrollment year,31 and age at DM36 were used in one study.
As for the conclusions on type 2 diabetes mellitus incidence, 6 studies26, 30, 31, 32, 35, 36 reported results in favor of lactation, and 3 studies23, 34, 38 reported null results.
3.7. Risk of bias
The results of risk of bias assessment using RoBANS are summarized in Figure S1 and Table S3.
Selection biases that are caused by selection of participants were judged to be “low” for 7 studies23, 26, 30, 31, 32, 34, 35 and “unclear” for 2 studies36, 38 because the baseline diabetes statuses were not given. Selection biases that are caused by confounding variables were judged to be “high” in most studies,23, 30, 31, 32, 34, 35, 36 except 2 studies judged to be “low” because of adequate adjustment for covariables.26, 38 Performance biases indicating measurement of lactation were judged to be “low” for only one study26 in which trained interviewers measured lactation, “high” for 3 studies30, 31, 38 with self‐report, and “unclear” for 5 studies.23, 32, 34, 35, 36 All the studies were judged to be “low” for detection biases because type 2 diabetes mellitus incidence could not be influenced by the blinding methods for its assessment. Attrition biases were judged to be “unclear” in most studies except one31 that stated there was no difference in lactation rate regarding dropout status. Reporting biases were judged to be “unclear” in most studies except one study with the experimental protocol available.26
3.8. Synthesis of results
3.8.1. Lactation duration (longer lactation vs shorter lactation)
Five studies23, 26, 31, 34, 35 enrolling 3408 women were included in the meta‐analysis for longer (>4 to 12 wk postpartum) versus shorter (<4 to 12 wk postpartum) lactation of any intensity for preventing type 2 diabetes mellitus after GDM pregnancy (Figure 2). The remaining studies were not included because of different study design30, 38 and/or inadequate data.30, 32, 36 Publication bias was not assessed as the number of included studies was fewer than 10. The heterogeneity yielded was substantial (I 2 = 85%) that we conducted subgroup analysis. In a subgroup analysis comparing by T2DM evaluation time, <2 years versus 2 to 5 years versus >5 years, we found significant subgroup differences (P = .03). Meta‐analysis (random‐effect model) revealed significant risk reduction of T2DM incidence with longer lactation in subgroups with DM evaluation time longer than 2 years (OR 0.56, 95% CI 0.35‐0.89 for 2 to 5 y; OR 0.22, 95% CI 0.13‐0.36 for >5 y). The qualities of the evidence were judged to be “very low” with DM evaluation <2 years group and “low” with 2 to 5 years and >5 years group (Table S4).
Figure 2.
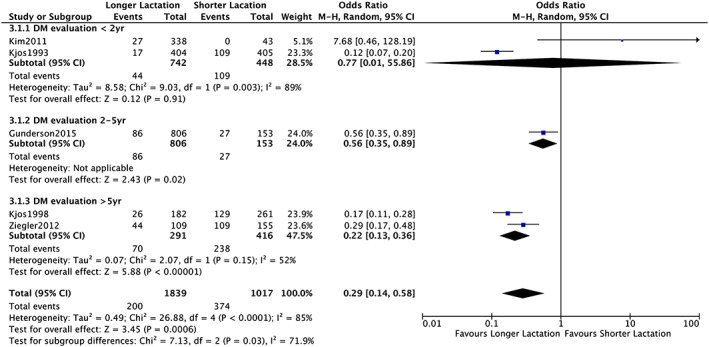
Forrest plot comparing “longer lactation” (>4‐12 wk) with “shorter lactation” (<4‐12 wk) with analysis of 3 subgroups on the basis of diabetes evaluation time; <2 y, 2‐5 y, and >5 y
3.8.2. Lactation intensity (exclusive lactation vs exclusive formula)
Two studies have assessed lactation intensity26, 31; however, only one of them compared the effect of exclusive lactation with exclusive formula for type 2 diabetes mellitus incidence.26 The risk of bias of this study was low (Table S3). The quality of the evidence was judged to be “moderate” graded up by its large (OR 0.42, 95% CI 0.22‐0.81) and dose‐response effect even with observational study design with small sample size (Table S5).
4. DISCUSSION
In this systematic review, we have shown that lactation of any intensity for more than 4 weeks to more than 12 weeks postpartum has statistically significant association with lower risk of type 2 diabetes mellitus in the long term (ie, >2 y). The effect of longer lactation was not obvious when diabetes was evaluated in early postpartum, but became more prominent with longer follow‐up (OR 0.77 95% CI 0.01‐55.86; OR 0.56 95% CI 0.35‐0.89; OR 0.22 95% CI 0.13‐0.36; <2 y, 2‐5 y, and >5 y, respectively). One likely explanation is that type 2 diabetes mellitus incidence after GDM pregnancy increases with time,4 and at least several years of follow‐up are required to judge the effect of exposure. Also, women developing type 2 diabetes mellitus in early postpartum (ie, 4‐12 wk postpartum) are definitely of the highest risk. The underlying etiology may be different from those who develop type 2 diabetes mellitus later. In fact, Ziegler et al reported that women with islet autoantibody developed diabetes much faster (median diabetes‐free duration, 4.5 mo) compared with women negative for the autoantibody, and no protective effect of lactation was observed in those women.31 All 3 studies in the subgroup evaluating type 2 diabetes mellitus at >3 years excluded early onset DM23, 26 or islet autoantibody‐positive population,31 suggesting a difference in population compared with the subgroup evaluating DM at <2 years (4‐12 wk and 6‐12 wk postpartum each).
Prolactin is one of the key factors for biochemical hypotheses of long‐term effect.39 Prolactin starts to elevate during pregnancy, peaks in term and stays above nonpregnant level with pulsatile secretion until weaning.40 Research on prolactin receptor knockout mice has clarified that prolactin plays a physiological role in pancreatic islet formation and function.41 Moderately elevated serum prolactin, which is the model for physiological elevation during pregnancy and postpartum, has also been shown to improve insulin secretion and insulin resistance in diabetic rats.42 However, full biological etiologies to explain the beneficial effect lasting long after weaning are lacking. Therefore, further studies on this topic are needed.
We have also found from a study with moderate evidence quality that exclusive lactation at 6 to 9 weeks postpartum was associated with lower risk of long‐term type 2 diabetes mellitus compared with exclusive formula (OR 0.42 95% CI 0.22‐0.81).26 The World Health Organization has recommended all mothers to exclusively breastfeed for the first 6 months followed by partial breastfeeding.43 However, only 37% of women were exclusively breastfeeding under 6 months in upper‐middle income countries.44 In addition, women with previous GDM are known for even lower breastfeeding rate compared with women with nondiabetic pregnancies.45, 46, 47 This may be due to increased risk of complications in both the mother and infant during the perinatal period,48 delayed lactogenesis,49 or the poor sucking pattern of infants.50 Therefore, exclusive breastfeeding for at least 6 to 9 weeks postpartum may be more achievable for women with GDM.
There are several limitations in this study. First, the evidence of this review relies only on observational studies in which we cannot confirm the causal relationship between lactation and type 2 diabetes mellitus. The effect of unknown confoundings or reverse causation cannot be ruled out even in well‐designed and adequately analyzed studies. This is because randomization of breastfeeding is infeasible both ethically and technically, although 2 randomized trials were conducted in the past when the benefits of breast milk were not proven.51, 52 Second, all the data used for the meta‐analysis were crude data without adjustment. Only few of the included studies adequately adjusted for covariables. Breastfeeding practices were reported to be influenced by multiple factors such as obesity,53 depression,54 insulin treatment during pregnancy,49 and how health conscious a mother is.55 These factors are likely to influence diabetes incidence, and they should be adjusted for. Third, analyses with stratification by the participants' characteristics such as ethnicity or BMI were not possible because of inadequate information. This limitation may deter us from drawing tailored conclusion for each woman in real practice. However, subgroup analysis showed fairly heterogeneous results for long‐term type 2 diabetes mellitus even in populations with diverse background, suggesting that the association remains.
5. CONCLUSION
In conclusion, the women with previous GDM lactating for more than 4 to 12 weeks postpartum have lower risk (moderate quality of evidence) of type 2 diabetes mellitus compared with women with shorter lactation period. Also, women with GDM exclusively lactating for more than 6 to 9 weeks postpartum have lower risk of type 2 diabetes mellitus compared with women with formula feeding. The etiology behind this potential long‐term beneficial effect of lactation remains poorly understood. The optimal support for women with GDM to breastfeed is not well studied. To investigate these unresolved issues between lactation and the prevention of type 2 diabetes mellitus, further studies are warranted in the future.
CONFLICTS OF INTEREST
None of the authors has conflict of interest related to this review.
Supporting information
Figure S1. Risk of bias graph of the included studies.
Table S1. Full search strategy.
Table S2. Excluded studies with reasons.
Table S3. Risk of bias assessment for the individual researches based on the Risk of Bias Assessment tool for Non‐randomized Studies (RoBANS).
Table S4. Summary of findings comparing “Longer Lactation” with “ Shorter Lactation” in 3 subgroups based on diabetes evaluation time; <2 years, 2‐5 years and >5 years.
Table S5. Summary of findings comparing “Exclusive Lactation” with “Exclusive Formula”.
ACKNOWLEDGEMENTS
The authors thank Ms Chiemi Kataoka and Ms Yuko Serizawa, Department of Health Policy, National Center for Child Health and Development, for conducting electronic search. The authors thank Ms Katsura Yasuda, Department of Health Policy, National Center for Child Health and Development, for helping prepare full texts needed for conducting systematic review. The authors thank Dr Julian Tang, Department of Education for Clinical Research, National Center for Child Health and Development, for professional English‐language editing.
Tanase‐Nakao K, Arata N, Kawasaki M, et al. Potential protective effect of lactation against incidence of type 2 diabetes mellitus in women with previous gestational diabetes mellitus: A systematic review and meta‐analysis. Diabetes Metab Res Rev. 2017;33:e2875 https://doi.org/10.1002/dmrr.2875.
REFERENCES
- 1. International Diabetes Federation . IDF diabetes atlas – 7th edition. Available: http://www.diabetesatlas.org/. Accessed May 15, 2016 [PubMed]
- 2. American Diabetes A . 2. Classification and diagnosis of diabetes. Diabetes Care. 2016;39(Suppl 1):S13–S22. [DOI] [PubMed] [Google Scholar]
- 3. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta‐analysis. Lancet. 2009;373:1773–1779. [DOI] [PubMed] [Google Scholar]
- 4. Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862–1868. [DOI] [PubMed] [Google Scholar]
- 5. Ratner RE, Christophi CA, Metzger BE, et al. Diabetes prevention program research G: prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93:4774–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Ryswyk E, Middleton P, Shute E, Hague W, Crowther C. Women's views and knowledge regarding healthcare seeking for gestational diabetes in the postpartum period: a systematic review of qualitative/survey studies. Diabetes Res Clin Pract. 2015;110:109–122. [DOI] [PubMed] [Google Scholar]
- 7. Peacock AS, Bogossian F, McIntyre HD, Wilkinson S. A review of interventions to prevent Type 2 diabetes after gestational diabetes. Women Birth. 2014;27:e7–e15. [DOI] [PubMed] [Google Scholar]
- 8. Liu B, Jorm L, Banks E. Parity, breastfeeding, and the subsequent risk of maternal type 2 diabetes. Diabetes Care. 2010;33:1239–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwarz EB, Brown JS, Creasman JM, et al. Am J Med. 2010;123:863 e861–863 e866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stuebe AM, Rich‐Edwards JW. The reset hypothesis: lactation and maternal metabolism. Am J Perinatol. 2009;26:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jager S, Jacobs S, Kroger J, et al. Breast‐feeding and maternal risk of type 2 diabetes: a prospective study and meta‐analysis. Diabetologia. 2014;57:1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aune D, Norat T, Romundstad P, Vatten LJ. Breastfeeding and the maternal risk of type 2 diabetes: a systematic review and dose‐response meta‐analysis of cohort studies. Nutr Metab Cardiovasc Dis. 2014;24:107–115. [DOI] [PubMed] [Google Scholar]
- 13. Butte NF, Wong WW, Hopkinson JM. Energy requirements of lactating women derived from doubly labeled water and milk energy output. J Nutr. 2001;131:53–58. [DOI] [PubMed] [Google Scholar]
- 14. Karnik SK, Chen H, McLean GW, et al. Menin controls growth of pancreatic beta‐cells in pregnant mice and promotes gestational diabetes mellitus. Science. 2007;318:806–809. [DOI] [PubMed] [Google Scholar]
- 15. Watanabe S, Wei FY, Matsunaga T, Matsunaga N, Kaitsuka T, Tomizawa K. Oxytocin protects against stress‐induced cell death in murine pancreatic beta‐cells. Sci Rep. 2016;6:25185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gunderson EP. The role of lactation in GDM women. Clin Obstet Gynecol. 2013;56:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Much D, Beyerlein A, Rossbauer M, Hummel S, Ziegler AG. Beneficial effects of breastfeeding in women with gestational diabetes mellitus. Mol Metab. 2014;3:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. American Diabetes A . 12. Management of diabetes in pregnancy. Diabetes Care. 2016;39(Suppl 1):S94–S98. [DOI] [PubMed] [Google Scholar]
- 19. Canadian Diabetes Association Clinical Practice Guidelines Expert C , Thompson D, Berger H, et al. Diabetes and pregnancy. Can J Diabetes. 2013;37(Suppl 1):S168–S183. [DOI] [PubMed] [Google Scholar]
- 20. Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4227–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. meta‐analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 22. The Cochrane Collaboration . Cochrane handbook for systematic reviews of interventions version 5.1.0. Available: http://handbook.cochrane.org/. Accessed May 15, 2016
- 23. Kjos SL, Peters RK, Xiang A, Thomas D, Schaefer U, Buchanan TA. Contraception and the risk of type 2 diabetes mellitus in latina women with prior gestational diabetes mellitus. JAMA. 1998;280:533–538. [DOI] [PubMed] [Google Scholar]
- 24. Kim SY, Park JE, Lee YJ, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66:408–414. [DOI] [PubMed] [Google Scholar]
- 25. GRADEpro GDT . Available at http://guidelinedevelopment.org. Accessed May 15, 2016
- 26. Gunderson EP, Hurston SR, Ning X, et al. Lactation and progression to type 2 diabetes mellitus after gestational diabetes mellitus: a prospective cohort study. Ann Intern Med. 2015;889‐898: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gunderson EP, Crites Y, Walton D, et al. Prediabetes and incident diabetes one year after GDM pregnancy in the swift longitudinal cohort. Diabetes. 2011;60:A347 [Google Scholar]
- 28. Gunderson EP, Hedderson MM, Chiang V, et al. Lactation intensity and postpartum maternal glucose tolerance and insulin resistance in women with recent GDM: the SWIFT cohort. Diabetes Care. 2012;35:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gunderson EP, Dewey K, Ning X, Quesenberry CP. Prospective study of lactation intensity at 6‐9 weeks postpartum and progression to incident diabetes based on annual OGTT screening after GDM pregnancy: swift. Diabetes. 2014;63:A94 [Google Scholar]
- 30. Gunderson EP, Lewis CE, Jacobs DR Jr, Gross M, Quesenberry CP Jr, Goff DC Jr. 25‐year prospective study of lactation duration and incidence of diabetes among cardia women screened before and after pregnancy. Diabetes. 2014;63:A336–A337. [Google Scholar]
- 31. Ziegler AG, Wallner M, Kaiser I, et al. Long‐term protective effect of lactation on the development of type 2 diabetes in women with recent gestational diabetes mellitus. Diabetes. 2012;61:3167–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buchanan TA, Xiang A, Kjos SL, et al. Gestational diabetes: antepartum characteristics that predict postpartum glucose intolerance and type 2 diabetes in Latino women. Diabetes. 1998;47:1302–1310. [DOI] [PubMed] [Google Scholar]
- 33. Buchanan TA, Xiang AH, Kjos SL, Trigo E, Lee WP, Peters RK. Antepartum predictors of the development of type 2 diabetes in Latino women 11‐26 months after pregnancies complicated by gestational diabetes. Diabetes. 1999;48:2430–2436. [DOI] [PubMed] [Google Scholar]
- 34. Kim S‐H, Kim M‐Y, Yang J‐H, et al. Nutritional risk factors of early development of postpartum prediabetes and diabetes in women with gestational diabetes mellitus. Nutrition. 2011;27:782–788. [DOI] [PubMed] [Google Scholar]
- 35. Kjos SL, Henry O, Lee RM, Buchanan TA, Mishell DR Jr. The effect of lactation on glucose and lipid metabolism in women with recent gestational diabetes. Obstet Gynecol. 1993;82:451–455. [PubMed] [Google Scholar]
- 36. Urs SS, Chandwani S. Benefits of breastfeeding on development of diabetes mellitus, in women with history of gestational diabetes mellitus using the national health and nutrition examiniation survey. Value Health. 2015;18:A70 [Google Scholar]
- 37. Kjos SL, Peters RK, Xiang A, Henry OA, Montoro M, Buchanan TA. Predicting future diabetes in Latino women with gestational diabetes. Utility of early postpartum glucose tolerance testing. Diabetes. 1995;44:586–591. [DOI] [PubMed] [Google Scholar]
- 38. Stuebe AM, Rich‐Edwards JW, Willett WC, Manson JE, Michels KB. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294:2601–2610. [DOI] [PubMed] [Google Scholar]
- 39. Retnakaran R, Ye C, Kramer CK, et al. Maternal serum prolactin and prediction of postpartum beta‐cell function and risk of prediabetes/diabetes. Diabetes Care. 2016; [DOI] [PubMed] [Google Scholar]
- 40. Ramos‐Romn MA. Prolactin and lactation as modifiers of diabetes risk in gestational diabetes. Horm Metab Res. 2011;43:593–600. [DOI] [PubMed] [Google Scholar]
- 41. Freemark M, Avril I, Fleenor D, et al. Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology. 2002;143:1378–1385. [DOI] [PubMed] [Google Scholar]
- 42. Park S. Kim da S, Daily JW, Kim SH: Serum prolactin concentrations determine whether they improve or impair beta‐cell function and insulin sensitivity in diabetic rats. Diabetes Metab Res Rev. 2011;27:564–574. [DOI] [PubMed] [Google Scholar]
- 43. Kramer MS, Kakuma R. The optimal duration of exclusive breastfeeding: a systematic review. Adv Exp Med Biol. 2004;554:63–77. [DOI] [PubMed] [Google Scholar]
- 44. Victora CG, Bahl R, Barros AJ, et al. Lancet Breastfeeding Series G: Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–490. [DOI] [PubMed] [Google Scholar]
- 45. Nommsen‐Rivers LA, Chantry CJ, Dewey KG. Early breastfeeding outcomes in gestational diabetic primiparas delivering term infants. FASEB J. 2010;24. [Google Scholar]
- 46. Doughty K, Reeves K, Ronnenberg A, Qian J, Sibeko L. Breastfeeding intentions and practices among women in the U.S. with gestational diabetes mellitus. FASEB J. 2015;29. [Google Scholar]
- 47. Finkelstein SA, Keely E, Feig DS, Tu X, Yasseen AS, Walker M. Breastfeeding in women with diabetes: lower rates despite greater rewards. A population‐based study. Diabet Med. 2013;30:1094–1101. [DOI] [PubMed] [Google Scholar]
- 48. Fallon A, Dunne F. Breastfeeding practices that support women with diabetes to breastfeed. Diabetes Res Clin Pract. 2015;110:10–17. [DOI] [PubMed] [Google Scholar]
- 49. Matias SL, Dewey KG, Quesenberry CP Jr, Gunderson EP. Maternal prepregnancy obesity and insulin treatment during pregnancy are independently associated with delayed lactogenesis in women with recent gestational diabetes mellitus. Am J Clin Nutr. 2014;99:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bromiker R, Rachamim A, Hammerman C, Schimmel M, Kaplan M, Medoff‐Cooper B. Immature sucking patterns in infants of mothers with diabetes. J Pediatr. 2006;149:640–643. [DOI] [PubMed] [Google Scholar]
- 51. Lucas A, Gore SM, Cole TJ, et al. Multicentre trial on feeding low birthweight infants: effects of diet on early growth. Arch Dis Child. 1984;59:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of breastfeeding intervention trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285:413–420. [DOI] [PubMed] [Google Scholar]
- 53. Turcksin R, Bel S, Galjaard S, Devlieger R. Maternal obesity and breastfeeding intention, initiation, intensity and duration: a systematic review. Matern Child Nutr. 2014;10:166–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dennis CL, McQueen K. Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Paediatr. 2007;96:590–594. [DOI] [PubMed] [Google Scholar]
- 55. Ninth annual national managed health care congress : Formulary decision criteria, sizing up lit‐based evidence, physician profiling are spotlighted. Formulary nn32:746 + 749‐752
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Risk of bias graph of the included studies.
Table S1. Full search strategy.
Table S2. Excluded studies with reasons.
Table S3. Risk of bias assessment for the individual researches based on the Risk of Bias Assessment tool for Non‐randomized Studies (RoBANS).
Table S4. Summary of findings comparing “Longer Lactation” with “ Shorter Lactation” in 3 subgroups based on diabetes evaluation time; <2 years, 2‐5 years and >5 years.
Table S5. Summary of findings comparing “Exclusive Lactation” with “Exclusive Formula”.


