Abstract
Aims
Haemodynamic‐guided heart failure (HF) management effectively reduces decompensation events and need for hospitalizations. The economic benefit of clinical improvement requires further study.
Methods and results
An estimate of the cost‐effectiveness of haemodynamic‐guided HF management was made based on observations published in the randomized, prospective single‐blinded CHAMPION trial. A comprehensive analysis was performed including healthcare utilization event rates, survival, and quality of life demonstrated in the randomized portion of the trial (18 months). Markov modelling with Monte Carlo simulation was used to approximate comprehensive costs and quality‐adjusted life years (QALYs) from a payer perspective. Unit costs were estimated using the Truven Health MarketScan database from April 2008 to March 2013. Over a 5‐year horizon, patients in the Treatment group had average QALYs of 2.56 with a total cost of US$56 974; patients in the Control group had QALYs of 2.16 with a total cost of US$52 149. The incremental cost‐effectiveness ratio (ICER) was US$12 262 per QALY. Using comprehensive cost modelling, including all anticipated costs of HF and non‐HF hospitalizations, physician visits, prescription drugs, long‐term care, and outpatient hospital visits over 5 years, the Treatment group had a total cost of US$212 004 and the Control group had a total cost of US$200 360. The ICER was US$29 593 per QALY.
Conclusions
Standard economic modelling suggests that pulmonary artery pressure‐guided management of HF using the CardioMEMS™ HF System is cost‐effective from the US‐payer perspective. This analysis provides the background for further modelling in specific country healthcare systems and cost structures.
Keywords: Economic analysis, Congestive heart failure, Haemodynamic monitoring, Heart failure hospitalization
Introduction
Heart failure (HF) is a complex clinical syndrome and global public health problem affecting an estimated 26 million people worldwide.1 Despite increased utilization of pharmacological2, 3, 4, 5, 6, 7, 8, 9 and device therapy options10, 11, 12, 13 that improve clinical outcomes in randomized controlled trials, morbidity and mortality in HF remain a major burden to patients, their caregivers, and national healthcare systems. Patients with HF frequently experience worsening symptoms related to accumulation of excess intravascular volume and congestion, requiring hospitalization to provide intravenous medical support to restore normal volume.14, 15, 16, 17, 18, 19 Heart failure is cited as the most frequent cause of hospitalization in the US Medicare population, resulting in >1 million admissions per year (accounting for 1–2% of all hospitalizations).20, 21 The economic impact of HF in the USA is profound, with the total costs of HF estimated to increase from US$31 billion in 2012 to US$70 billion in 2030 secondary to an ageing population.22 Cost‐effective HF management strategies are required to address this growing problem.
Elevated cardiac filling pressures are associated with higher rates of re‐hospitalization and mortality in patients with HF.23, 24 Independent of LVEF, rises in cardiac filling pressures can often be detected several weeks prior to patients experiencing symptoms of HF decompensation that require hospitalization.25, 26 Remote monitoring of intracardiac and pulmonary artery pressures (PAPs) in patients with HF using implantable haemodynamic monitoring devices can provide physicians with access to actionable pathophysiological information and help improve the HF management decision‐making process necessary to prevent HF hospitalizations.27, 28, 29, 30 A novel wireless PAP measurement system (CardioMEMS™ HF System, St. Jude Medical, Inc., Atlanta, GA, USA) was evaluated in the CardioMEMS™ Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association (NYHA) functional Class III Heart Failure Patients (CHAMPION) trial.30, 31 The CHAMPION trial was a prospective, multicentre, randomized, single‐blind clinical study in 550 patients that tested the incremental impact of PAP‐guided HF management on clinical outcomes compared with HF management based on current American College of Cardiology Foundation/American Heart Association practice guidelines only.
The PAP‐guided HF management group in the CHAMPION trial experienced a significant reduction in HF hospitalization rates, a greater reduction in PAPs, fewer patients hospitalized for HF, and more days alive and outside of the hospital for HF, and exhibited an improvement in quality of life when compared with guideline‐directed standard of care HF management only (Control group).30 These long‐term benefits were seen in patients with HF and preserved EF,32 secondary pulmonary hypertension,33 and co‐morbid chronic obstructive CAD.34 Hospitalization reductions were seen after an average of 18 months of randomized follow‐up, with additional long‐term benefits noted in the 13 months of ‘open‐access’, which immediately followed the end of the randomized follow‐up in the trial.35 Based on these data, the CardioMEMS™ HF System was approved by the US Food and Drug Administration for HF management in NYHA class III HF patients with a HF hospitalization within the last 12 months. This study is a comprehensive analysis of the cost‐effectiveness of this treatment strategy in the context of the US medical system.
Methods
A Markov model was utilized to approximate the course of management observed in the CHAMPION trial for the Treatment and Control groups using Monte Carlo simulation. The objective was to estimate the costs and cost‐effectiveness over a time horizon extended beyond the study follow‐up period. Healthcare utilization event rates, survival, and quality of life were based on study data. We took the perspective of the payer, and focused on the Medicare and private insurance patient populations as they represent the vast majority of patients eligible for the CardioMEMS™ HF System in the USA, and cost data were available for these two patient populations to conduct our analyses. Model endpoints included cost of treatment and preference‐weighted survival, which were then used to calculate incremental cost per life year gained and cost per quality‐adjusted life year (QALY) gained for the treatment compared with control.
Model structure
The Markov model employed four states: ‘stable HF’, ‘hospitalized for HF’, ‘hospitalized for other cause’, and ‘death’ (Figure 1). Each simulated patient transitioned through the states in cycles of 1 month, incurring costs and accumulating effects associated with each state. The total time horizon of the simulation for the base case was 5 years (or 60 cycles). Costs and effects were discounted at 3% per year.36 A total of 100 000 patients were simulated in each group.
Figure 1.
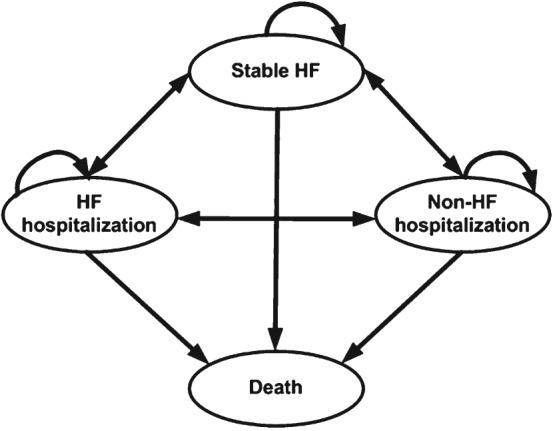
Markov model used to approximate the course of management observed in the CHAMPION trial for the Treatment and Control groups. HF, heart failure.
A patient in the ‘stable HF’ state was characterized as a patient that received typical care for HF including physician visits, prescription drugs, long‐term care, and outpatient hospital visits. A patient in the ‘hospitalized for HF’ state was characterized as a patient undergoing inpatient hospitalization related to a primary diagnosis of HF. Since the average length of stay for a HF hospitalization in the USA is 5.2 days for all patients according to the Healthcare cost and utilization project (HCUP) National statistics (2012),37 we assumed that in this state, a patient incurred the same expense as in the ‘stable HF’ state in addition to the cost of HF hospitalization. In the model simulation, patients in the Control group started in the ‘stable HF’ state, and the Treatment group started the simulation with an implant‐related hospitalization.
A patient in the ‘hospitalized for other cause’ state was characterized as a patient undergoing inpatient hospitalization for any reason other than a primary diagnosis of HF. Since the average length of stay for a hospitalization for any cause in the USA is 4.5 days for all patients according to the HCUP National statistics (2012),37 we assumed that in this state, a patient incurred the same expense as in the ‘stable HF’ state in addition to the cost of other‐cause hospitalization. ‘Death’ was an absorbing state.
As part of the prospective CHAMPION trial design, all patients initially underwent implantation of the PAP sensor prior to randomization. All patients then remained in their randomized study group until the last patient to be enrolled completed at least 6 months of study follow‐up (Randomized Access Period). At the conclusion of the Randomized Access Period, all active patients transitioned to a follow‐up period where study physicians then had access to PAP information for all patients (Open Access Period). For this cost‐effectiveness analysis (CEA), estimates of the transition probabilities among states, mortality, and EQ‐5D preference weight utilities in each state came from the complete Randomized Access Period of the CHAMPION trial (Table 1). All hospitalizations were adjudicated by a Clinical Event Classification (CEC) committee as part of the CHAMPION trial and classified in one of two ways: inpatient hospitalization associated with HF or inpatient hospitalization not associated with HF.
Table 1.
Long‐term clinical outcomes from CHAMPION trial Randomized Access Period35
| Treatment group (n = 270) | Control group (n = 280) | ||
|---|---|---|---|
| Heart failure hospitalizations, n (events/patient‐year) | 182 (0.46) | 279 (0.68) | Diff 97 (0.23), NNT 4, HR 0.67 (33% RRR) (95% CI 0.55–0.80), P < 0.0001a |
| Non heart failure hospitalizations, n (events/patient‐year) | 372 (0.93) | 393 (0.96) | Diff 21 (0.03), HR 0.97 (3% RRR) (95% CI 0.84–1.12), P = 0.6790a |
| Death, n (%) | 50 (18.5%) | 64 (22.9%) | Non‐significant, HR 0.80 (95% CI 0.55–1.15), P = 0.23b |
| EQ‐5D‐3 L utilities (US preference weights) | |||
| Baseline | 0.711 | ||
| 6 months | 0.719 | 0.681 | P = 0.056c |
| 12 months | 0.739 | 0.660 | P = 0.003c |
NNT, number needed to treat; RRR, relative risk reduction.
Hazard ratio (HR), 95% confidence interval (CI), and P‐value from the Andersen–Gill model.
HR and 95% CI from the Cox proportional hazards model, P‐value from log‐rank test.
P‐value from two‐sided Wilcoxon test.
Health‐related quality of life (HRQoL) was assessed via the EQ‐5D‐3 L38 questionnaire at baseline, 6 months, and 12 months in the CHAMPION trial. For the model, HRQoL at 12 months was carried forward for the 60‐month model. We utilized US population‐based EQ‐5D‐3 L preference weights for this economic analysis.39 QALYs were accumulated based on the assumption that the preference weight was constant between measurement intervals.
Cost of healthcare utilization
Currently, the USA has no single national system of health insurance. Health insurance is purchased in the private marketplace or provided by the government to certain groups (e.g. Medicare insurance to the elderly and disabled population). Because of this, we used a payer mix based on the age distribution in the CHAMPION study cohort. Patients less than 65 years old at implant were assumed to be paid through private insurance, and those 65 years or older at implant were assumed to be paid by Medicare.
In the USA, the implant of the CardioMEMS™ HF System is associated with the MS‐DRG (Medicare Severity Diagnosis Related Groups) payment for 264 accompanied by the ICD‐9‐CM (International Classification of Diseases, Ninth Revision, Clinical Modification) procedure code of 38.26: insertion of an implantable pressure sensor without a lead for intracardiac or great vessel haemodynamic monitoring. In the base case, the cost of system implantation in the Treatment group was US$17 75040 and we made the assumption that all implants occurred on a unique scheduled day for each patient, and did not occur during a pre‐existing HF hospitalization.
Payer costs for hospitalizations post‐implant (which are reimbursements) were determined from the Truven Health MarketScan® April 2008 to March 2013 Commercial Claims and Encounters and Medicare Supplemental and Coordination of Benefits Database. This MarketScan® database represents the de‐identified health services of employees, dependents, and retirees in the USA with primary or Medicare supplemental coverage through privately insured fee‐for‐service, point‐of‐service, or capitated health plans. Overall, it includes ∼20% of the US population across all 50 states. All enrolment records and inpatient, outpatient, ancillary, and drug claims were tabulated.
We identified a HF cohort from this claims database by ascertaining patients with an inpatient hospitalization with primary diagnosis of HF (ICD‐9‐CM diagnosis code of 428.X). The HF cohort consisted of 200 471 patients that had Medicare advantage insurance and private insurance. The Medicare patients consisted of 50% male patients with an average age of 80 ± 8 years. The private insurance patients consisted of 60% male patients with an average age of 57 ± 9 years. The distribution of geographic locations for all patients were: Northeast (20%), North Central (33%), South (31%), and West (14%). In this HF cohort, we identified payments for three types of healthcare utilization: average cost of inpatient hospitalization with primary diagnosis code of HF, average cost of inpatient hospitalization not related to HF, and annual costs for outpatient healthcare utilization of any type for these patients (Table 2). These payments corresponded to the states of ‘hospitalized for HF’, ‘hospitalized for other cause’, and ‘stable HF’ in the model. The payments included facility cost and professional fees associated with the event, adjusted to 2014 US dollars based on the consumer price index (CPI) inflation from the Bureau of Labor Statistics.
Table 2.
Post‐implant healthcare utilization from MarketScan®
| Medicare | Private insurance | |
|---|---|---|
| Treatment reimbursement from cohort of 200 471 HF patients (MarketScan®) | ||
| Average reimbursement per HF hospitalization (US$/event) | US$16 770 | US$30 100 |
| Average reimbursement per non‐HF hospitalization (US$/event) | US$20 290 | US$32 400 |
| Annual outpatient healthcare utilization (US$/year) | US$17 288 | US$23 067 |
HF, heart failure.
The costs related to ‘stable HF’ included the majority of expenses a non‐hospitalized HF patient incurs receiving medical care; this included physician visits, prescription drugs, long‐term care, and outpatient hospital visits. In addition to this, a patient implanted with the CardioMEMS™ HF System was assumed to incur a monthly cost of US$45 associated with the professional and technical components of reimbursement for remote physiological monitoring. Since remote physiological monitoring is conducted for various reasons for patients that have not been implanted with the CardioMEMS™ HF System, we assumed that 25% of the standard of care patients also incur this monthly cost.
The CHAMPION trial reported eight device‐ and system‐related complications (DSRCs) during 575 implant attempts.3 All of these events occurred within the first 30 days of implant. Using the details from hospital admission and discharge records for each one of the eight DSRCs, we ascertained an MS‐DRG for each such hospitalization, and then determined an average cost of DSRCs based on the 2014 reimbursement for the MS‐DRGs. No further device‐ or system‐related complications or sensor failures were reported in an average of 31 months following implant.36
Cost‐effectiveness outcome measures
The primary efficacy endpoint of the CHAMPION trial was the rate of HF‐related hospitalizations. Thus, our primary effectiveness endpoint was the incremental cost‐effectiveness ratio (ICER) comparing the costs and QALYs of HF hospitalization outcomes in the PAP Treatment and Control groups. The model was used to extrapolate this endpoint to 5 years.
One‐way sensitivity analyses were performed to assess the impact of varying selected model parameters while holding other variables fixed at their base case values. These parameters included HF hospitalization rates, mortality rates, cost of HF hospitalization, monitor implant cost, and payer mix. The goal was to understand how robust the base case results were to uncertainty about the values used. In addition, sensitivity analyses were conducted on the costs included in the analysis: HF hospitalizations only, all‐cause hospitalizations, and all chronic HF management costs including all‐cause hospitalizations. Two probabilistic sensitivity analyses (PSAs) were performed with 100 000 resamples of the model parameters to examine the impact of their combined uncertainty. Tabulation of distributions used for the parameters in the probabilistic sensitivity analyses are provided in the Supplementary material online, Table S1. An assumption in this model is that the outpatient costs increase due to an improved survival benefit (not due to other changes).
Results
Primary cost‐effectiveness analysis: comparison of heart failure hospitalization outcomes
The purpose of PAP monitoring via CardioMEMS was to reduce HF hospitalizations, hence the primary CEA focused on comparing HF hospitalization outcomes. The costs associated with HF hospitalizations, device‐ and system‐related complications, and remote physiological monitoring were accumulated. Over a 5‐year time horizon, patients in the Treatment group had average QALYs of 2.56 (discounted at 3%) with a total cost of US$56 974; patients in the Control group had QALYs of 2.16 with a total cost of US$52 149. The ICER was US$12 262 per QALY (Table 3).
Table 3.
Cost‐effectiveness analysis base‐case costs and survival over a 5‐year time horizon
| Primary CEA endpoint: HF hospitalization outcomes | All‐cause hospitalization outcomes | Comprehensive patient management outcomes | ||||
|---|---|---|---|---|---|---|
| Treatment group | Control group | Treatment group | Control group | Treatment group | Control group | |
| Cumulative average cost | US$56 974 | US$ 52 149 | US$140 966 | US$133 681 | US$212 004 | US$200 360 |
| Cumulative QALYs | 2.56 | 2.16 | 2.56 | 2.16 | 2.56 | 2.16 |
| Cumulative average years survival | 3.70 | 3.47 | 3.70 | 3.47 | 3.70 | 3.47 |
| Incremental cost effectiveness ratio (US$/QALY) | US$12 262 | US$18 515 | US$29592 | |||
| Cost reduction for each patient under treatment post implant (US$/year)a | US$4443 | US$5261 | US$5296 | |||
CEA, cost‐effectiveness analysis; HF, heart failure; QALY, quality adjusted life year.
Cost saving per life year for the treatment group.
Sensitivity analyses of cost sources
Cost‐effectiveness analysis: comparing all‐cause hospitalization outcomes
Heart failure management is expensive, and, historically, studies related to CRT management of HF patients have compared all‐cause hospitalization outcomes. Although the CardioMEMS™ HF System did not have a significant impact on non‐HF‐related hospitalizations, we compared all hospitalizations for the two groups to enable a comparison with historical studies. The costs associated with all‐cause hospitalizations, device‐ and system‐related complications, and remote physiological monitoring were accumulated. Over a 5‐year time horizon, patients in the Treatment group had average QALYs of 2.56 (discounted at 3%) with a total cost of US$140 966; patients in the Control group had QALYs of 2.16 with a total cost of US$133 681. The ICER was US18 515 per QALY (Table 3).
Cost‐effectiveness analysis: comparing comprehensive management
The total cost of patient management is the sum of various components for both HF and non‐HF management care. The costs associated with hospitalizations for any reason, device‐ and system‐related complications, and remote physiological monitoring were accumulated. To these were added the costs of patient management, which include HF and non‐HF hospitalizations, physician visits, prescription drugs, long‐term care, and outpatient hospital visits. Over the 5‐year time period, patients in the Treatment group had a total cost of US$212 004 and patients in the Control group had a total cost of US$200 360. The ICER was US$29 593 per QALY (Table 3).
One‐way sensitivity analyses
The ICER was sensitive to the time horizon (Figure 2). Compared with the base case ICER of US$12 262/QALY at 5 years, the ICERs at 4 years and 7 years were US$34 909/QALY and US$5412/QALY, respectively. The ICERs decreased over time; this is a common observation when a high‐cost procedure occurs at the beginning of the time horizon, indicating that the therapy is cost‐effective primarily resulting from averted hospitalizations.
Figure 2.
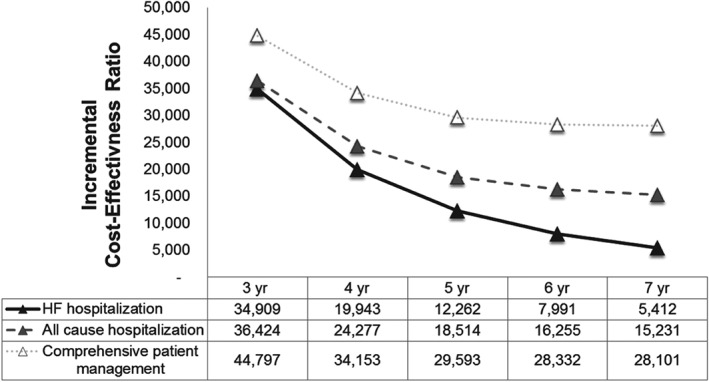
Time horizon analysis: incremental cost‐effectiveness ratios vs. time horizon (3–7 years). HF, heart failure; yr, year.
The ICERs were also assessed when HF hospitalization rates, HF hospitalization costs, and mortality rates were varied. These are reported in the tornado plot on Figure 3. The ICERs varied between US$10 960/QALY and US$14 311/QALY when HF hospitalization rate varied between the 95% confidence interval of the hospitalization rates from the CHAMPION trial. The sensitivity around HF hospitalization cost showed the ICERs to vary between –US$6172/QALY and US$30 696/QALY. When the cost of HF hospitalization was 50% higher than the base case, the ICER at a 5‐year time horizon of –US$6172 resulted from the treatment arm accumulating lower costs compared with the control arm. The ICERs varied between US$8456/QALY and US$16 854/QALY when the mortality rates varied between the 95% confidence bounds of the CHAMPION trial mortality rates.
Figure 3.
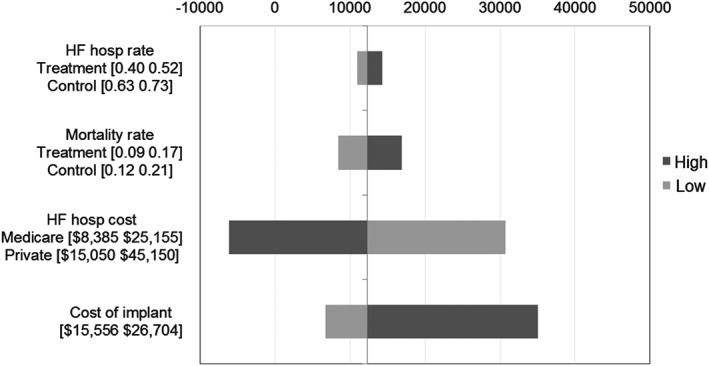
Sensitivity analysis. HF, heart failure.
The ICER was sensitive to the implant cost, and for an implant cost range of US$15 556 to US$26 704, the ICER ranged from US$6686/QALY and US$35 018/QALY. The implant costs—low and high ranges—were determined using the national DRG payment for MS‐DRG code 264 with and without the new technology add‐on payment (NTAP) in the case of the inpatient hospitalizations, and the APC (Ambulatory Payment Classification) code 0319 with and without the Pass‐Through payment.
According to the HCUP NIS (Nationwide inpatient sample) data set from 2012,37 amongst all inpatient hospitalizations associated with a primary or secondary diagnosis of HF identified via ICD‐9‐CM code 428.X, there were 5 021 800 discharges for HF with the payer as Medicare, and 674 680 discharges for HF in patients that had private insurance. Using these two payer categories (Medicare and private payer), 88% of HF hospitalizations occurred in Medicare beneficiaries. Using this 88% (Medicare) and 12% (private insurance) distribution to form the assumption of patient mix, the ICER was US$20 734/QALY.
Probabilistic sensitivity analyses
For the primary CEA (using HF hospitalization costs only), the PSA showed that at the willingness‐to‐pay threshold of US$25 000, >85% of the simulations were cost‐effective; >99% were cost‐effective at the US$50 000 threshold. Further, for the CEA using comprehensive patient management costs, the PSA showed that at the willingness‐to‐pay threshold of US$50 000, 87% of the simulations were cost‐effective; >99% were so at the US$100 000 threshold. The cost‐effectiveness acceptability is shown in the Supplementary material online, Figures S1–S4.
Discussion
This study demonstrated that haemodynamic‐guided HF management is a cost‐effective strategy to improve outcomes in outpatient management of patients with chronic HF. Cost‐effectiveness calculations determined that this strategy was well below the most commonly used threshold of US$50 000 per QALY. This threshold has become an accepted benchmark for cost‐effectiveness in the USA and is often attributed to the US decision to mandate Medicare coverage for patients with end‐stage renal disease in the 1970s.41 Some economists as well as the World Health Organization (WHO) have argued, on the basis of plausible assumptions about people's values and attitudes toward risk, for a threshold of 2–3 times the per capita annual income, which would imply a US threshold of US$110 000 to US$160 000 per QALY.42 This, indeed, would be a contemporary estimate of the costs of treating end‐stage renal disease with haemodialysis.
Various factors result in a therapy being cost‐effective, and in the case of PAP‐guided HF care, the cost‐effectiveness is attributable to the reduction in HF hospitalization rates, reduction in mortality, and improvement in quality of life. For the base case, as well as all‐cost sensitivity analyses, the ICERs were shown to be consistently below the US$50 000/QALY maximum acceptable threshold. An important determinant of cost‐effectiveness is the reimbursement for the system implantation. CardioMEMS™ is a new technology and in early stages of the reimbursement cycle. The uncertainty around the implant reimbursement was handled in our model by a sensitivity analysis around the implant cost range of US$15 556 and US$26 704. For this wide range, the therapy was shown to be consistently cost‐effective.
Management of HF with pharmacological therapies and implantable device therapy has been studied extensively to prove their clinical effectiveness.43 The ICERs for medical device therapy for HF patients are between US$10 900 and US$303 000/QALY, depending on the intervention considered. Cost‐effectiveness of pharmacological therapies such as ACE inhibitors, ARBs, and beta‐blockers has been assessed in the USA and Europe, and they have been shown to be cost‐effective, with ICERs over a lifetime lower than US$17 900/QALY. Implantable cardioverter defibrillators (ICDs), CRT‐P pacemakers, and CRT‐D defibrillations have been evaluated in the HF population for which these devices are known to have clinical benefit. Cost‐effectiveness studies of ICDs are sensitive to the patient population selected in each individual trial, and ICERs vary from US$34 000 to > US$70 000 per QALY gained over a lifetime.43 ICERs across countries are variable due to variations in healthcare practices and prices. As an illustration, Feldman et al. showed that the ICER for CRT‐P vs. optimal medical therapy is US$22 900/QALY in the USA; an ICER of US$10 900–US$29 700 in the UK; an ICER of US$37 200/QALY in Spain, and an ICER of US$14 600/QALY in Belgium.44
The COMPANION trial, for example, conducted a comparison of CRT‐P vs. optimal medical therapy and CRT‐D vs. optimal medical therapy. Implant costs for a CRT‐D were assumed to be US$29 500 and for a CRT‐P were US$20 500. Inclusion criteria included NYHA functional class III or IV plus a HF treatment in the preceding 12 months over and above other criteria. The ICERs for CRT‐P and CRT‐D vs. optimal medical therapy were US$19 600 and US$43 000, respectively, over a 7‐year time horizon.44 Using the CPI for medical care inflation of an average of 4.03%, the ICER for CRT‐P vs. optimal medical therapy and CRT‐D vs. optimal medical therapy would be US$27 513/QALY and US$60 360/QALY, respectively. Modelling the CHAMPION trial cost‐effectiveness over a 7‐year time horizon and using all‐cause hospitalization costs, which is similar to the methods used in COMPANION, compares favourably with this at US$15 231/QALY (US$ 2014).
An independent CEA was developed by Sandhu et al. 45 that concluded that the CardioMEMS device is cost‐effective with an ICER of US$82 301 in patients with reduced EF and US$47 768 in those with preserved EF. Our analysis produced different estimates of the ICERs for several reasons: first, Sandhu et al. used a societal perspective and modelled lifetime costs and effects. Our model used a payer perspective and modelled costs and effects over 5 years. Secondly, the parameter estimates in the Sandhu model differed from those in our model. For example, Sandhu and colleagues assumed the cost of a HF hospitalization in the USA to be US$12 832. Our analysis used real‐world claims data from 200 471 HF patients, which demonstrated an average payer cost of US$16 770 for Medicare and US$30 100 for private insurance patients. Thirdly, Sandhu and colleagues mapped MLHFQ (Minnesota Living with Heart Failure Questionnaire) scores into the EQ‐5D scores based on an existing algorithm, which is an acceptable method, but inferior to direct measurement of utilities. Our analysis used the EQ‐5D utilities which were measured at baseline and several times during follow‐up in the CHAMPION trial. Finally, Sandhu et al. used mortality rates based on the relative risk of death associated with hospitalization; our analysis used mortality rates observed in the CHAMPION trial. Methodological differences in the model perspective, time horizon, and parameters resulted in different estimates of the ICER. However, both our analysis and the report from Sandhu et al. conclude that using the CardioMEMS HF System to manage HF patients is cost‐effective in the US setting.
In spite of introduction of effective HF medical and device therapies, HF hospitalizations continue to rise and population‐based HF mortality remains high. Over half the significant US cost of HF management arises due to hospitalization. Average annual medical expenditures per Medicare HF beneficiary are estimated to be US$33 247,46 with total annual Medicare costs estimated at US$40 billion. Reductions in hospitalizations seen in the CHAMPION trial are very encouraging and suggest that haemodynamic monitoring of HF patients will provide a much‐needed tool to assist outpatient management of high‐risk patients. Hospitalizations due to all causes were also reduced in the CHAMPION Treatment group. Detailed information about guideline‐directed medical therapy use at baseline and changes during the 6 months of haemodynamic‐guided care in the CHAMPION trial was recently published by Costanzo et al. 47 Both the control and treatment groups started the trial with high prevalence of guideline‐directed medical therapies at baseline at target doses. Both groups were receiving significantly higher doses of loop diuretics at the end of the 6‐month efficacy endpoint; however, more increases and decreases in diuretics were seen in the treatment group compared with the controls. Additionally, treatment group patients had significant increases in neurohormonal intervention and vasodilator therapies, which were not seen in the control group. The trial demonstrated that active personalization of HF management, guided by frequent haemodynamic assessment, was associated with less need for hospitalization and improvement in delivery of disease‐modifying medications.
Within the US healthcare system, management of HF using a PAP sensor is expected to impact further readmissions within 30 days of discharge. The Centers for Medicare and Medicaid Services (CMS) has instituted financial penalties for hospitals with a higher than expected HF readmission rate for Medicare patients. The potential impact of the CardioMEMS HF System on HF 30‐day readmissions was not studied in this model. Adoption of this treatment strategy at hospitals struggling with HF hospitalization and 30‐day readmissions could potentially help address an unmet need within the US healthcare system, and may be addressed in separate analyses.
Heart failure is a global burden; while it is reasonable to expect that the clinical outcomes in Europe would be similar to those found in the current US‐based study, a direct cost comparison using simple currency translation is not an accurate method to assess the economic impact in Europe. Hence, we present here a CEA study from the US payer perspective and expect that this will pave the way for future studies in individual countries wherein the unique measures that are relevant to each healthcare system and cost structure for each country are appropriately dealt with. The largest costs in our primary CEA are the implant hospitalization cost and the HF hospitalization cost. This study includes a sensitivity analysis using the cost of HF hospitalization at US$8358–US$25 155. The costs for HF hospitalizations in many European countries are quite variable and sometimes much lower; for example at £2515 in the UK and €2400 in Germany. For the lower of the two, assuming the same cost of implant as the US model, the ICER would be US$45 002/QALY.
Conclusions
The model suggests that PAP‐guided management of HF is cost‐effective. The ICERs, when considered for HF management or comprehensive management, were well below the conventional US acceptability threshold of US$50 000.
Heart failure remains an increasing global problem.22, 48 Coupled with the ageing population and thus increasing numbers of HF patients, the pressure on healthcare payers to reduce hospitalizations will continue unabated. Strategies such as CardioMEMS, which decrease the rate of hospitalization, are likely to be only more cost‐effective in future.
Conflict of interest: M.MM. and W.T.A. are Consultants to St. Jude Medical; R.B., N.D., and P.B.A. are employees of St. Jude Medical.
Supporting information
Table S1. Distributions used for parameters in the probabilistic sensitivity analysis.
Figure S1. Full model incremental cost‐effectiveness scatter plot.
Figure S2. Full model cost‐effectiveness acceptability curve.
Figure S3. HF hospitalization model incremental cost‐effectiveness scatter plot.
Figure S4. HF hospitalization model cost‐effectiveness acceptability curve.
The copyright line for this article was changed on 3 March 2017 after original online publication.
References
- 1. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CS, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014;63:1123–1133. [DOI] [PubMed] [Google Scholar]
- 2. The SOLVD Investigators . Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 3. MERIT‐HF Study Group . Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT‐HF). Lancet 1999;353:2001–2007. [PubMed] [Google Scholar]
- 4. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 5. Packer M, Poole‐Wilson PA, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Rydén L, Thygesen K, Uretsky BF. Comparative effects of low and high doses of the angiotensin‐converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation 1999;100:2312–2318. [DOI] [PubMed] [Google Scholar]
- 6. Castaigne A, Roecker EB, Schultz MK, DeMets DL; Carvedilol Prospective Randomized Cumulative Survival Study Group . Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651–1658. [DOI] [PubMed] [Google Scholar]
- 7. Cohn JN, Tognoni G. A randomized trial of the angiotensin‐receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667–1675. [DOI] [PubMed] [Google Scholar]
- 8. Maggioni AP, Anand I, Gottlieb SO, Latini R, Tognoni G, Cohn JN. Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin‐converting enzyme inhibitors. J Am Coll Cardiol 2002;40:1414–1421. [DOI] [PubMed] [Google Scholar]
- 9. Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S; CHARM Investigators and Committees . Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM‐Overall programme. Lancet 2003;362:759–766. [DOI] [PubMed] [Google Scholar]
- 10. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML; Multicenter Automatic Defibrillator Implantation Trial II Investigators . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 11. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L for the Cardiac Resynchronization‐Heart Failure (CARE‐HF) Study Investigators . The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–1549. [DOI] [PubMed] [Google Scholar]
- 12. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM for the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators . Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004;350:2140–2150. [DOI] [PubMed] [Google Scholar]
- 13. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp‐Channing N, Davidson‐Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH; Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT) Investigators. Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 14. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Wilson Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 15. Kato M, Stevenson LW, Palardy M, Campbell PM, May CW, Lakdawala NK, Stewart G, Nohria A, Rogers JG, Heywood JT, Gheorghiade M, Lewis EF, Mi X, Setoguchi S. The worst symptom as defined by patients during heart failure hospitalization: implications for response to therapy. J Card Fail 2012;18:524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med 2006;119 (12 Suppl 1):S3–S10. [DOI] [PubMed] [Google Scholar]
- 17. Schiff GD, Fung S, Speroff T, McNutt RA. Decompensated heart failure: symptoms, patterns of onset, and contributing factors. Am J Med 2003;114:625–630. [DOI] [PubMed] [Google Scholar]
- 18. Cotter G, Metra M, Milo‐Cotter O, Dittrich HC, Gheorghiade M. Fluid overload in acute heart failure—re‐distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail 2008;10:165–169. [DOI] [PubMed] [Google Scholar]
- 19. Metra M, Dei CL, Bristow MR. The pathophysiology of acute heart failure—it is a lot about fluid accumulation. Am Heart J 2008;155:1–5. [DOI] [PubMed] [Google Scholar]
- 20. Centers for Medicare & Medicaid Services . 100% MEDPAR Inpatient Hospital National Data for Fiscal Year 2011 http://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/MedicareFeeforSvcPartsAB/Downloads/DRG11.pdf.
- 21. Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure‐associated hospitalizations in the United States. J Am Coll Cardiol 2013;61:1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Piña IL, Trogdon JG. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Drazner MH, Rame JE, Stevenson LW, Dries DL. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. N Engl J Med 2001;345:574–581. [DOI] [PubMed] [Google Scholar]
- 24. Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J 2007;154:260–266. [DOI] [PubMed] [Google Scholar]
- 25. Zile MR, Bennett TD, St John SM, Cho YK, Adamson PB, Aaron MF, Aranda JM Jr, Abraham WT, Smart FW, Stevenson LW, Kueffer FJ, Bourge RC. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 2008;118:1433–1441. [DOI] [PubMed] [Google Scholar]
- 26. Adamson PB. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: new insights from continuous monitoring devices. Curr Heart Fail Rep 2009;6:287–292. [DOI] [PubMed] [Google Scholar]
- 27. Adamson PB, Magalski A, Braunschweig F, Böhm M, Reynolds D, Steinhaus D, Luby A, Linde C, Ryden L, Cremers B, Takle T, Bennett T. Ongoing right ventricular hemodynamics in heart failure: clinical value of measurements derived from an implantable monitoring system. J Am Coll Cardiol 2003;41:565–571. [DOI] [PubMed] [Google Scholar]
- 28. Bourge RC, Abraham WT, Adamson PB, Aaron MF, Aranda JM Jr, Magalski A, Zile MR, Smith AL, Smart FW, O'Shaughnessy MA, Jessup ML, Sparks B, Naftel DL, Stevenson LW; COMPASS‐HF Study Group . Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS‐HF study. J Am Coll Cardiol 2008;51:1073–1079. [DOI] [PubMed] [Google Scholar]
- 29. Abraham WT, Adamson PB, Hasan A, Bourge RC, Pamboukian SV, Aaron MF, Raval NY. Safety and accuracy of a wireless pulmonary artery pressure monitoring system in patients with heart failure. Am Heart J 2011;161:558–566. [DOI] [PubMed] [Google Scholar]
- 30. Abraham WT, Adamson PB, Hasan A, Bourge RC, Aaron MF, Costanzo MR, Stevenson MW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS; CHAMPION Trial Study Group . Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658–666. [DOI] [PubMed] [Google Scholar]
- 31. Adamson PB, Abraham WT, Aaron M, Aranda JM Jr, Bourge RC, Smith A, Stevenson LW, Bauman JG, Yadav JS. CHAMPION trial rationale and design: the long‐term safety and clinical efficacy of a wireless pulmonary artery pressure monitoring system. J Card Fail 2011;17:3–10. [DOI] [PubMed] [Google Scholar]
- 32. Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, Henderson J, Cowart P, Stevenson LW. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail 2014;7:935–944. [DOI] [PubMed] [Google Scholar]
- 33. Benza RL, Raina A, Abraham WT, Adamson PB, Lindenfeld J, Miller AB, Bourge RC, Bauman J, Yadav J. Pulmonary hypertension related to left heart disease: insight from a wireless implantable hemodynamic monitor. J Heart Lung Transplant 2015;34:329–337. [DOI] [PubMed] [Google Scholar]
- 34. Krahnke JS, Abraham WT, Adamson PB, Bourge RC, Bauman J, Ginn G, Martinez FJ, Criner GJ; Champion Trial Study Group . Heart failure and respiratory hospitalizations are reduced in patients with heart failure and chronic obstructive pulmonary disease with the use of an implantable pulmonary artery pressure monitoring device. J Card Fail 2015;21:240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abraham WT, Stevenson LW, Bourge RC, Lindenfeld J, Bauman J, Adamson PB. Sustained efficacy of pulmonary artery pressure to guide adjustments of chronic heart failure therapy: complete follow‐up results from the CHAMPION Randomised Trial. Lancet 2016;387:453–461. [DOI] [PubMed] [Google Scholar]
- 36. Miller G, Randolph S, Forkner E, Smith B, Galbreath AD. Long‐term cost‐effectiveness of disease management in systolic heart failure. Med Decis Making 2009;29:325–333. [DOI] [PubMed] [Google Scholar]
- 37. Agency for Healthcare Research and Quality . National statistics on all stays. http://hcupnet.ahrq.gov/. [DOI] [PubMed] [Google Scholar]
- 38. Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ‐5D health states: development and testing of the D1 valuation model. Med Care 2005;43:203–220. [DOI] [PubMed] [Google Scholar]
- 39. Dolan P, Gudex C. Time preference, duration and health state valuations. Health Econ 1995;44:289–299. [DOI] [PubMed] [Google Scholar]
- 40. Federal Register August 17, 2015;80:49440–49441. http://www.gpo.gov/fdsys/pkg/FR‐2015‐08‐17/pdf/2015‐19049.pdf.
- 41. Neumann PJ, Cohen JT, Weinstein MC. Updating cost‐effectiveness—the curious resilience of the $50,000‐per‐QALY threshold. N Engl J Med 2014;371:796–797. [DOI] [PubMed] [Google Scholar]
- 42. Weinstein MC. How much are Americans willing to pay for a quality‐adjusted life year? Med Care 2008l;46:343–345. [DOI] [PubMed] [Google Scholar]
- 43. Rohde LE, Bertoldi EG, Goldraich L, Polanczyk CA. Cost‐effectiveness of heart failure therapies. Nat Rev Cardiol 2013;10:338–354. [DOI] [PubMed] [Google Scholar]
- 44. Feldman AM, de LG, Bristow MR, Saxon LA, De Marco T, Kass DA, Boehmer J, Singh S, Whellan DJ, Carson P, Boscoe A, Baker TM, Gunderman MR. Cost effectiveness of cardiac resynchronization therapy in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial. J Am Coll Cardiol 2005;46:2311–2321. [DOI] [PubMed] [Google Scholar]
- 45. Sandhu AT, Goldhaber‐Fiebert JD, Owens DK, Turakhia MP, Kaiser DW, Heidenreich PA. Cost‐effectiveness of implantable pulmonary artery pressure monitoring in chronic heart failure. JACC Heart Fail 2016;4:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dall TM, Blanchard TD, Gallo PD, Semilla AP. The economic impact of Medicare Part D on congestive heart failure. Am J Manag Care 2013;19(6 Suppl):s97–s100. [PubMed] [Google Scholar]
- 47. Costanzo MR, Stevenson LW, Adamson PB, Desai AS, Heywood JT, Bourge RC, Bauman J, Abraham WT. Interventions linked to decreased heart failure hospitalizations during ambulatory pulmonary artery pressure monitoring. JACC Heart Fail 2016;4:333–344. [DOI] [PubMed] [Google Scholar]
- 48. Guha K, McDonagh T. Heart failure epidemiology: European perspective. Curr Cardiol Rev 2013;9:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Distributions used for parameters in the probabilistic sensitivity analysis.
Figure S1. Full model incremental cost‐effectiveness scatter plot.
Figure S2. Full model cost‐effectiveness acceptability curve.
Figure S3. HF hospitalization model incremental cost‐effectiveness scatter plot.
Figure S4. HF hospitalization model cost‐effectiveness acceptability curve.


