Abstract
Objective
Interferon‐γ (IFNγ) is implicated in the pathogenesis of discoid lupus erythematosus (DLE). This study sought to evaluate a single dose of AMG 811, an anti‐IFNγ antibody, in patients with DLE.
Methods
The study was designed as a phase I randomized, double‐blind, placebo‐controlled crossover study of the pharmacodynamics, safety, and clinical efficacy of AMG 811 in patients with DLE. Patients received a single subcutaneous dose of AMG 811 (180 mg) or placebo. The patients in sequence 1 received AMG 811 followed by placebo, while those in sequence 2 received placebo followed by AMG 811. Pharmacodynamic end points included global transcriptional analyses of lesional and nonlesional skin, IFNγ blockade signature (IGBS) transcriptional scores in the skin and blood, keratinocyte IFNγ RNA scores, and serum levels of CXCL10 protein. Additional end points were efficacy outcome measures, including the Cutaneous Lupus Erythematosus Disease Area and Severity Index, and safety outcome measures.
Results
Sixteen patients with DLE were enrolled in the study (9 in sequence 1 and 7 in sequence 2). AMG 811 treatment reduced the IGBS score (which was elevated in DLE patients at baseline) in both the blood and lesional skin. The keratinocyte IFNγ RNA score was not affected by administration of AMG 811. Serum CXCL10 protein levels (which were elevated in the blood of DLE patients) were reduced with AMG 811 treatment. The AMG 811 treatment was well tolerated but did not lead to statistically significant improvements in any of the efficacy outcome measures.
Conclusion
AMG 811 treatment led to changes in IFNγ‐associated biomarkers and was well tolerated, but no significant clinical benefit was observed in patients with DLE.
Skin involvement (cutaneous lupus) occurs in 70–85% of patients with systemic lupus erythematosus (SLE) and is classified according to the morphologic characteristics of the lesions and the histopathologic appearance of the skin 1. Discoid lesions, the most common manifestation of chronic cutaneous lupus erythematosus, are well‐defined lesions, characterized by disk‐shaped, erythematous, scaly patches that can cause atrophy and scarring. Biopsy samples of lesional skin from patients with discoid lupus erythematosus (DLE) show deposition of immunoglobulins, complement, and mononuclear cell infiltrates, indicating that this disease may have an antigen‐driven immunologic pathogenesis 2.
Members of the interferon (IFN) family have been implicated in the pathogenesis of DLE 3, and analyses of skin samples have shown strong evidence of activation of IFN signaling. Elevated levels of IFNγ messenger RNA have been described in DLE skin specimens relative to normal skin, and immunohistochemical analyses have shown a selective staining of IFNγ receptors in DLE skin samples compared to normal skin samples 4. IFNγ modulates the function of several populations of immune system cells, including B cells, T cells, and macrophages. In skin, IFNγ is an inhibitor of keratinocyte growth 5. Elevated levels of IFNγ‐inducible 10‐kd protein (CXCL10) and infiltrates of CXCR3‐expressing T cells have also been found in DLE skin biopsy specimens 6.
AMG 811 is a human anti‐IFNγ antibody (IgG1 isotype) that selectively targets human IFNγ 7. Previous trials of AMG 811 in SLE patients with and those without nephritis showed an acceptable safety and tolerability profile 8, 9, 10. The objective of the present study was to evaluate the safety, tolerability, pharmacodynamics, and efficacy of a single dose of AMG 811 in patients with DLE.
SUBJECTS AND METHODS
Study design
This study was a phase I multicenter, randomized, double‐blind, placebo‐controlled, 2‐period crossover study that was designed to evaluate a single dose of AMG 811 in comparison to placebo. The treatment was administered in 1 of 2 sequences: in sequence 1, patients received AMG 811 followed by placebo, while in sequence 2, patients received placebo followed by AMG 811. Twenty patients with DLE (with or without SLE) were planned for the study (12 for sequence 1 and 8 for sequence 2).
A single dose of AMG 811 (180 mg) or placebo was administered subcutaneously on day 1 (start of period 1) and on day 85 (start of period 2). Biopsy specimens of lesional and nonlesional skin were collected at baseline, and biopsy specimens of lesional skin were collected on days 15 and 57 postdosing. The presence of lesions was predetermined by each of the investigators before dosing was initiated. In most patients, the lesions were identified in a similar anatomic region. Nonlesional skin specimens were obtained from regions with similar light exposure as selected lesional sites. Blood samples for pharmacodynamic biomarker testing were collected at the following time points: in period 1, at baseline, predose, 6 hours postdose, and days 2, 15, 29, and 57; in period 2, predose, 6 hours postdose, and days 99, 113, 141, and 197 (end of study). Efficacy outcomes were evaluated at screening, baseline, and days 15, 29, and 57 (period 1) and days 85, 99, 113, 141, 169, and 197 (period 2). This study was conducted in accordance with the Declaration of Helsinki.
Patients
Eligible patients were ages 18–70 years at randomization, had a body mass index of 18–40 kg/m2, and were diagnosed as having DLE with or without SLE based on the Gilliam and Sontheimer classification 1. All eligible patients had a skin biopsy sample that exhibited features consistent with the diagnosis of DLE, and all had previously demonstrated intolerance to antimalarial therapy or had received ≥3 months of antimalarial therapy and had continued to display residual disease activity. Moreover, all eligible patients had stable disease for 4 weeks prior to screening, had received a stable dose of topical steroids no stronger than medium potency (class I, II, or III) for ≥2 weeks and/or systemic immunosuppressive therapy at a stable dose for ≥8 weeks before randomization, had received oral prednisone at a dosage of ≤20 mg/day (or equivalent), and had a current vaccination history. Exclusion criteria included a history of malignancy, signs or symptoms of viral, bacterial, or fungal infection within 30 days of randomization or recent history of repeated infections, an underlying significant medical condition other than SLE, a history of receiving immunosuppressant drugs that predispose to infection, or having received a live vaccine within 3 months of randomization.
Study outcome measures
Gene expression profiling in the skin and blood
Transcriptional differences between lesional and nonlesional skin were assessed using microarray analysis. Frozen skin samples were disrupted with a Multi‐Sample Bio Pulverizer (Research Products International) and homogenized with a TissueRuptor (Qiagen). Total RNA was isolated using the mirVana Micro‐RNA Isolation kit (Applied Biosystems), modified to include on‐column DNase treatment with RNase‐free DNase (Qiagen). The integrity of the RNA samples was assessed using a Bioanalyzer 2100 (Bio‐Rad). The total RNA concentration was measured on an ND‐1000 Spectrophotometer (NanoDrop). Labeled complementary DNA was generated using a NuGEN Ovation kit (NuGEN Technologies) and hybridized to GeneChip HT_HG‐U133+PM microarrays using a GeneTitan instrument (Affymetrix). Analyses were conducted with log2‐transformed intensities that had been normalized at the array level to have comparable intensity spectrum. Only samples with an RNA integrity number (RIN) of >7 were used. Whole blood PAXgene samples were processed and assayed by microarray as previously described 8, except that the samples were Cy3‐labeled and hybridized once to a custom 180k Agilent array with the content of the Human Gene Expression 44K (AMADID #026822; Agilent Technologies), replicated 4 times.
Pharmacodynamic outcome measures
Pharmacodynamic outcome measures included changes in the IFNγ blockade signature (IGBS) RNA score in the serum and skin, changes in the keratinocyte IFNγ RNA score in the skin, and changes in the levels of CXCL10 protein in the serum. The IGBS was derived from 2 microarray experiments. First, whole blood from healthy volunteers was stimulated in vitro with IFNγ and used to identify genes that were up‐regulated by IFNγ. Second, whole blood from patients in a phase Ia study 8 was used to identify genes that showed reduced expression following AMG 811 treatment. The IGBS score is a weighted average of the top 10 genes identified in those experiments, as previously described 8.
The keratinocyte IFNγ RNA score is based on a set of genes that are preferentially expressed in keratinocytes stimulated with IFNγ, but not those stimulated with interleukin‐17A (IL‐17A), IL‐22, or tumor necrosis factor 11. For reference, the keratinocyte IFNγ RNA score was measured in a set of 30 skin samples from healthy volunteers, as was the covariance of the expression of each gene. The numeric score for each disease skin sample from DLE patients was the Mahalanobis skin, which is similar to a multidimensional Z score and increases with changes in the expression of each gene in the signature set. The scores for each gene's contribution in the inverse covariance matrix were adjusted for the gene set in the reference normal samples.
Serum CXCL10 protein concentrations were determined using a commercially available enzyme‐linked immunosorbent assay in accordance with the manufacturer's instructions (R&D Systems). Whole blood from healthy volunteers was obtained from the Amgen Research Blood Donor program.
Clinical outcome measures
The study clinical outcome measures included the Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) score for disease activity (CLASI‐A; score range 0–70) 12. Additional exploratory clinical outcome measures included the CLASI score for damage (CLASI‐D; score range 0–56), the physician's assessment of skin disease, the patient's self‐assessment of skin disease, and the SLE Disease Activity Index 2000 13 (for patients with underlying SLE). In period 1 (baseline to day 84), baseline was defined as day 1 predose, while in period 2 (day 85 to day 197), baseline was defined as day 85 predose. The sole placebo group was the sequence 2, period 1 group (having first received placebo during period 1), since the sequence 1, period 2 group may have had potential confounding by exposure to AMG 811 (having received AMG 811 first).
Statistical analysis
The primary goal of CLASI‐A and keratinocyte IFNγ RNA analyses was to provide estimates of effect size (with uncertainty), and statistical significance, although the study lacked sufficient power to achieve statistical significance. Percentage changes from baseline in CLASI‐A scores did not follow normal distribution; therefore, Hodges‐Lehmann estimates of the median values with associated 90% confidence intervals (90% CIs) were provided. Corresponding P values were based on exact Wilcoxon–Mann‐Whitney tests.
Global transcriptional analyses were performed on log2‐transformed and normalized fluorescence intensity values of gene expression. Differentially expressed genes and gene signatures were identified by determining the contrast in mean baseline expression values within lesional skin compared to nonlesional skin, which was estimated from a linear model that included factors for skin diagnosis (lesional or nonlesional), RIN, and patient.
IGBS scores were analyzed in a mixed‐effects linear model that included time on active treatment as a fixed effect and patient as a random factor. IGBS score plots are shown with simultaneous 95% CIs, using pooled estimates of standard error corrected for small sample sizes.
Serum CXCL10 protein levels were analyzed using a mixed‐effects regression model, including factors for treatment, serum CXCL10 protein concentrations before treatment, time after treatment, and a treatment × time interaction term. Time and treatment were fixed factors, and patient was a random factor.
RESULTS
Characteristics of the patients
Of the 20 patients planned for the study, only 16 were enrolled, because enrollment was slow. Of the 16 enrolled patients, 9 were assigned to sequence 1 and 7 were assigned to sequence 2. Two patients (1 from each sequence) discontinued treatment during period 1 (1 withdrew consent and 1 discontinued for other reasons). Fifteen patients received treatment with AMG 811 (9 in period 1 and 6 in period 2). These patients were therefore included in the AMG 811 analysis group, and 7 patients were included in the placebo group. The mean age was 49.2 years, and most of the patients (75%) were female. The majority of patients (88%) had comorbid SLE (Table 1).
Table 1.
Demographic and clinical characteristics of the study subjects at baselinea
|
Sequence 1 (n = 9) |
Sequence 2 (n = 7) |
All patients (n = 16) |
|
|---|---|---|---|
| Sex, no. (%) female | 8 (89) | 4 (57) | 12 (75) |
| Age, mean ± SD years | 47.1 ± 10.7 | 51.9 ± 7.5 | 49.2 ± 9.4 |
| Race, no. (%) | |||
| White | 6 (67) | 4 (57) | 10 (63) |
| Black/African American | 2 (22) | 3 (43) | 5 (31) |
| Asian | 1 (11) | 0 | 1 (6) |
| Comorbid SLE, no. (%) | 8 (89) | 6 (86) | 14 (88) |
| Receiving prednisone, no. (%) | 3 (33) | 4 (57) | 7 (44) |
| Prednisone dose, mean ± SD mg/day | 9.67 ± 8.96 | 6.88 ± 3.75 | 8.07 ± 6.00 |
| Receiving antimalarial drug, no. (%) | 6 (67) | 5 (71) | 11 (69) |
| CLASI‐A score, mean ± SD | 19.00 ± 6.18 | 21.86 ± 10.16 | 20.25 ± 7.99 |
| CLASI‐D score, mean ± SD | 14.56 ± 9.13 | 19.14 ± 3.34 | 16.56 ± 7.38 |
SLE = systemic lupus erythematosus; CLASI‐A = Cutaneous Lupus Erythematosus Disease Area and Severity Index for disease activity; CLASI‐D = Cutaneous Lupus Erythematosus Disease Area and Severity Index for damage.
Changes in pharmacodynamic outcome measures
The IGBS score was elevated in lesional skin compared to nonlesional skin. In lesional skin, treatment with AMG 811 significantly reduced the mean IGBS score on day 57 compared to the mean value at baseline (P < 0.05) (Figure 1A), although the mean IGBS score in lesional skin was not reduced to the level observed at baseline in nonlesional skin, and was significantly higher than the baseline nonlesional skin IGBS score (P < 0.05) (Figure 1A). A significant reduction in the IGBS score was also observed in the peripheral blood (data not shown).
Figure 1.
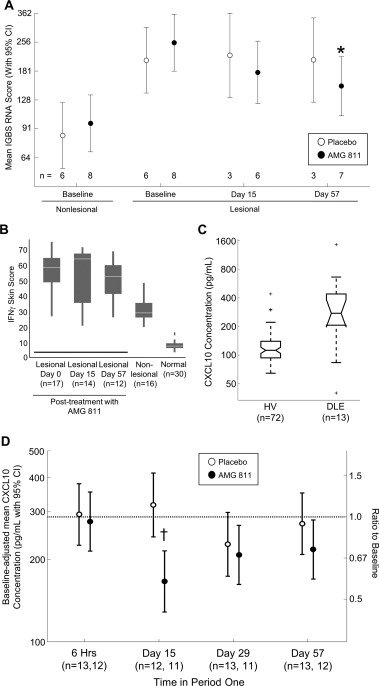
Changes in biomarker expression with AMG 811 treatment. A, Interferon‐γ (IFNγ) blockade signature (IGBS) scores in nonlesional skin at baseline and in lesional skin of patients with discoid lupus erythematosus (DLE) at baseline and on days 15 and 57 after treatment with AMG 811 or placebo (in period 1). ∗ = P = 0.0063 versus AMG 811–treated lesional samples at baseline. B, Keratinocyte IFNγ RNA score in lesional skin of DLE patients at baseline and on days 15 and 57 after AMG 811 treatment compared with nonlesional DLE skin and normal healthy control skin. Results are shown as box plots, where the horizontal line within the boxes represents the median, the boxes represent the first and third quartiles, and the bars outside the boxes represent the minimum and maximum values. C, Serum CXCL10 protein concentrations in healthy volunteers (HV) and untreated patients with DLE. Results are shown as irregular box plots, where the horizontal line within the boxes represents the median, the notches represent an estimate of the uncertainty about the median, the boxes represent the first and third quartiles, and the dashed bars represent the lower and upper ends of the farthest observed data point within 1.5 times the interquartile range; the plus signs represent outliers. D, Baseline‐adjusted mean serum CXCL10 protein concentrations in DLE patients treated with AMG 811 or placebo. The dashed horizontal line represents the mean baseline value. † = P adjusted = 0.0038 for the intraday (day 15) comparison between AMG 811 and placebo. 95% CI = 95% confidence interval.
The keratinocyte IFNγ RNA score was increased at baseline in both the lesional and nonlesional skin of patients with DLE compared to the normal skin of reference healthy patients. In DLE patients, the keratinocyte IFNγ score was higher in lesional skin than in nonlesional skin (Figure 1B). In lesional skin, treatment with AMG 811 did not have a significant impact on the keratinocyte IFNγ RNA score on days 15 or 57 postdosing as compared to that at baseline (Figure 1B).
The baseline levels of serum CXCL10 protein were elevated in patients with DLE compared to the levels in reference healthy volunteers (Figure 1C). The serum CXCL10 protein levels were significantly reduced in patients treated with AMG 811 compared to those receiving placebo (P < 0.05), with a maximal reduction of −1.7‐fold (95% CI −2.2, −1.3) from baseline to day 15 (Figure 1D).
Safety outcomes
Fourteen (93.3%) of 15 patients in the AMG 811 group and 4 (57.1%) of 7 patients in the placebo group had a treatment‐emergent adverse event (AE). Arthralgia, headache, hypertriglyceridemia, and upper respiratory tract infections were the only AEs reported in >1 patient (n = 2 patients each [13.3%]) in the AMG 811 group. Two of these events were also reported in the placebo group: hypertriglyceridemia in 1 patient (14.3%) and headache in 2 patients (28.6%). No AEs of grade ≥2 occurred in more than 1 patient in either group. No deaths or withdrawals due to AEs were reported.
Three patients (20.0%) in the AMG 811 group reported experiencing a serious AE (SAE) during the study, whereas no SAEs were reported in the placebo group. SAEs reported within the first 12 weeks following AMG 811 administration included 1 case of Legionella pneumonia (deemed to be treatment related), while another patient experienced 4 different SAEs, including gastroenteritis, migraine (deemed to be treatment related), splenic infarction, and viral gastroenteritis. The SAE of Legionella pneumonia occurred on study day 47; the patient received levofloxacin and the pneumonia resolved 16 days later. One patient reported the development of a subdural hematoma (deemed to be unrelated to treatment) that occurred ∼182 days after the patient had received AMG 811; the event resolved in ∼2 weeks.
Changes in clinical outcome measures
Overall, the median changes in the CLASI‐A score were not statistically significantly different between patients who received AMG 811 and those who received placebo over the first 85 days (Figures 2A and B). No significant clinical benefits were seen in patients with DLE after AMG 811 treatment in terms of improvements in the CLASI‐D score, physician's assessment of skin disease, or patient's self‐assessed skin disease (data not shown).
Figure 2.
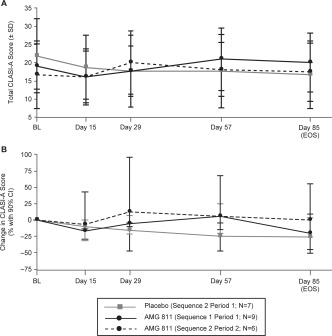
Clinical efficacy as measured by the Cutaneous Lupus Erythematosus Disease Area and Severity Index disease activity (CLASI‐A) score. The mean total CLASI‐A scores (A) and median percentage change in CLASI‐A scores (B) at baseline (BL) predose and on postdose days 15, 29, 57, and 85 (end of study [EOS]) are shown for patients who received placebo followed by AMG 811 (sequence 2) in period 1, patients who received AMG 811 followed by placebo (sequence 1) in period 1, and patients who received AMG 811 followed by placebo (sequence 2) in period 2. 90% CI = 90% confidence interval.
DISCUSSION
Cutaneous lupus, including DLE, is the result of aberrant autoimmune activation in the skin 14. Patients with DLE have an elevated IFN signature in the blood, suggesting that the pathogenic mechanisms are similar in patients with SLE and those with cutaneous lupus 15. AMG 811 is a monoclonal antibody that blocks IFNγ. In this study of patients with DLE, treatment with AMG 811 demonstrated clear pharmacologic activity in both the blood and lesional skin, as measured using the IGBS score. Notably, after treatment, the IGBS score did not reach the levels seen in nonlesional skin, suggesting that either incomplete blockade of the pathway or partial activation by other cytokines may occur.
When IFNγ activity was measured using the keratinocyte IFNγ RNA score, DLE lesional skin, DLE nonlesional skin, and normal skin from healthy volunteers were shown to have correspondingly varying levels of IFNγ activity, with the scores in DLE nonlesional skin being intermediate to those in lesional and normal skin. Treatment with AMG 811 did not significantly impact the keratinocyte IFNγ RNA score in the skin.
Given the pharmacologic responses to AMG 811 in both the blood and skin as measured by the IGBS and keratinocyte IFNγ RNA scores, the single dose of AMG 811 administered to patients with DLE possibly did not provide sufficient coverage to elicit a clinical response that could be measured by the CLASI‐A. The 180‐mg subcutaneous dose of AMG 811 covered the target adequately as assessed on the basis of changes in the serum CXCL10 levels over ≥56 days—a duration of treatment consistent with the measurement of efficacy in other DLE studies 8, 16, 17—but the dose level of AMG 811 required to penetrate the skin and overcome local production of IFNγ may be higher. Alternatively, despite coverage of the IFNγ pathway, possibly other pathways, including the type I IFN pathways, may need to be inhibited concurrently to impact clinical disease. Furthermore, the heterogeneity of this population and the role of several interdependent and independent pathways may preclude the use of blockade of a single IFN pathway as an effective treatment for the majority of patients with SLE and DLE. Overall, these results do not provide a clear answer to the role of IFNγ in the pathogenesis of DLE. While differences in gene expression profiles in the blood and skin indicate dysregulation of the IFNγ pathway, conclusions about causality cannot be drawn given the possible incomplete blockade of the pathway in the tissue and the lack of discernible clinical impact.
Conduct of proof‐of‐concept SLE trials is challenging in terms of enrollment and trial design. Given the large number of patients required, duration of treatment, and the complex composite disease outcome measures needed, small clinical trials (particularly organ‐specific trials) that maximize data collection and provide evidence of pharmacodynamic and clinical impact are valuable in the early development of novel agents for SLE. The crossover design utilized in this study allowed us to obtain both placebo‐ and treatment‐related data in the overall analysis and helped us to minimize patient‐to‐patient variability. This design addressed one of the concerns postulated by patients who are considering participation in a clinical trial, that of potentially receiving placebo treatment. Enrollment of patients with DLE, most of whom also had SLE, enabled the collection of data from the skin in addition to blood and serum, which collectively enabled a rich set of data from which to conduct robust pharmacodynamic analyses. Despite the unclear results, we would encourage the consideration of such a study design, when appropriate, in the future development of agents for SLE.
In conclusion, treatment with AMG 811 led to changes in biomarkers associated with IFNγ in the blood and skin of patients with DLE. The treatment was well tolerated, but there was no significant clinical improvement observed with the single dose (180 mg) of AMG 811 administered in this study.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Chung had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Werth, Fiorentino, Boedigheimer, Chiu, Wang, Welcher, Russell, Martin, Chung.
Acquisition of data
Werth, Fiorentino, Sullivan, Chiu, Wang, Damore, Bigler, Welcher, Russell, Martin, Chung.
Analysis and interpretation of data
Werth, Fiorentino, Sullivan, Boedigheimer, Chiu, Wang, Arnold, Welcher, Russell, Martin, Chung.
ROLE OF THE STUDY SPONSOR
Amgen Inc., facilitated the study design, provided writing assistance for the manuscript, and reviewed and approved the manuscript prior to submission. The authors independently collected the data, interpreted the results, and had the final decision to submit the manuscript for publication. Publication of this article was not contingent upon approval by Amgen Inc.
ACKNOWLEDGMENTS
We thank Dikran Toroser (Amgen Inc.) and Julia R. Gage (on behalf of Amgen Inc.) for assistance with the writing of the manuscript.
ClinicalTrials.gov identifier: NCT01164917.
Supported by Amgen Inc. Dr. Werth's work was supported by the Department of Veterans Affairs Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development.
Dr. Werth holds a patent for the Cutaneous Lupus Erythematosus Disease Area and Severity Index (owned by the University of Pennsylvania) used in this study. Drs. Sullivan, Boedigheimer, Arnold, and Chung and Ms Wang own stock or stock options in Amgen Inc. Drs. Welcher, Russell, Chiu, Damore, Bigler, and Martin are former employees of Amgen Inc. and own stock or stock options in Amgen Inc.
REFERENCES
- 1. Gilliam JN, Sontheimer RD. Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J Am Acad Dermatol 1981;4:471–5. [DOI] [PubMed] [Google Scholar]
- 2. Fabbri P, Cardinali C, Giomi B, Caproni M. Cutaneous lupus erythematosus: diagnosis and management. Am J Clin Dermatol 2003;4:449–65. [DOI] [PubMed] [Google Scholar]
- 3. Achtman JC, Werth VP. Pathophysiology of cutaneous lupus erythematosus. Arthritis Res Ther 2015;17:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Toro JR, Finlay D, Dou X, Zheng SC, LeBoit PE, Connolly MK. Detection of type 1 cytokines in discoid lupus erythematosus. Arch Dermatol 2000;136:1497–501. [DOI] [PubMed] [Google Scholar]
- 5. Shirakata Y. Regulation of epidermal keratinocytes by growth factors. J Dermatol Sci 2010;59:73–80. [DOI] [PubMed] [Google Scholar]
- 6. Wenzel J, Uerlich M, Worrenkamper E, Freutel S, Bieber T, Tuting T. Scarring skin lesions of discoid lupus erythematosus are characterized by high numbers of skin‐homing cytotoxic lymphocytes associated with strong expression of the type I interferon‐induced protein MxA. Br J Dermatol 2005;153:1011–5. [DOI] [PubMed] [Google Scholar]
- 7. Chen P, Vu T, Narayanan A, Sohn W, Wang J, Boedigheimer M, et al. Pharmacokinetic and pharmacodynamic relationship of AMG 811, an anti‐IFN‐γ IgG1 monoclonal antibody, in patients with systemic lupus erythematosus. Pharm Res 2015;32:640–53. [DOI] [PubMed] [Google Scholar]
- 8. Welcher AA, Boedigheimer M, Kivitz AJ, Amoura Z, Buyon J, Rudinskaya A, et al. Blockade of interferon‐γ normalizes interferon‐regulated gene expression and serum CXCL10 levels in patients with systemic lupus erythematosus. Arthritis Rheumatol 2015;67:2713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Werth VP, Fiorentino D, Cohen SB, Fivenson D, Hansen C, Zoog S, et al. A phase I single‐dose crossover study to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics, and clinical efficacy of AMG 811 (anti‐IFN‐gamma) in patients with discoid lupus erythematosus [abstract]. Arthritis Rheum 2013;65 Suppl:S682. [Google Scholar]
- 10. Martin DA, Amoura Z, Romero‐Diaz J, Chong YB, Sanchez‐Guerrero J, Chan T, et al. A multiple dose study of AMG 811 (anti‐IFN‐gamma) in patients with systemic lupus erythematosus and active nephtiris [abstract]. Ann Rheum Dis 2015;74:337. [Google Scholar]
- 11. Nograles KE, Zaba LC, Guttman‐Yassky E, Fuentes‐Duculan J, Suarez‐Farinas M, Cardinale I, et al. Th17 cytokines interleukin (IL)–17 and IL‐22 modulate distinct inflammatory and keratinocyte‐response pathways. Br J Dermatol 2008;159:1092–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. J Albrecht, L Taylor, JA Berlin, S Dulay, G Ang, S Fakharzadeh, et al. The CLASI (Cutaneous Lupus Erythematosus Disease Area and Severity Index): an outcome instrument for cutaneous lupus erythematosus. J Invest Dermatol 2005;125:889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gladman DD, Ibanez D, Urowitz MB. Systemic Lupus Erythematosus Disease Activity Index 2000. J Rheumatol 2002;29:288–91. [PubMed] [Google Scholar]
- 14. Dey‐Rao R, Sinha AA. Genome‐wide transcriptional profiling of chronic cutaneous lupus erythematosus (CCLE) peripheral blood identifies systemic alterations relevant to the skin manifestation. Genomics 2015;105:90–100. [DOI] [PubMed] [Google Scholar]
- 15. Braunstein I, Klein R, Okawa J, Werth VP. The interferon‐regulated gene signature is elevated in subacute cutaneous lupus erythematosus and discoid lupus erythematosus and correlates with the cutaneous lupus area and severity index score. Br J Dermatol 2012;166:971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kreuter A, Tomi NS, Weiner SM, Huger M, Altmeyer P, Gambichler T. Mycophenolate sodium for subacute cutaneous lupus erythematosus resistant to standard therapy. Br J Dermatol 2007;156:1321–7. [DOI] [PubMed] [Google Scholar]
- 17. Shah A, Albrecht J, Bonilla‐Martinez Z, Okawa J, Rose M, Rosenbach M, et al. Lenalidomide for the treatment of resistant discoid lupus erythematosus. Arch Dermatol 2009;145:303–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


