Abstract
Objective
To provide clinically relevant insights on the identification of the muscles and techniques involved in the safe and effective use of onabotulinumtoxinA for chronic migraine prophylaxis.
Background
Although guidance on the use of onabotulinumtoxinA for chronic migraine is available, based on the Phase III Research Evaluating Migraine Prophylaxis Therapy (PREEMPT) clinical program, clinical experience has shown that insufficient understanding of the anatomy and function of the head and neck muscles may lead to undesirable outcomes and suboptimal efficacy.
Design/Methods
Each muscle involved in the standardized PREEMPT injection paradigm is reviewed with a thorough description of each muscle's anatomy (ie, muscle description and location, innervation, vascular supply) and function. Key insights based on clinical experience are also provided to help improve outcomes.
Results
The identification of the muscles in the PREEMPT injection paradigm should be based on each patient's unique anatomy and injections should be administered using the advised techniques. A thorough examination of the patient prior to treatment is also critical to determine if any preexisting conditions may increase the risk for unwanted outcomes and appropriate expectations should be communicated.
Conclusions
Thorough knowledge of the functional anatomy of the muscles involved in the standardized PREEMPT injection paradigm is critical to achieve the efficacy and safety observed in clinical trials. In addition, it is important to assess a patient's baseline condition to anticipate the risk for unwanted outcomes that may result from treatment.
Keywords: chronic migraine, onabotulinumtoxinA, injection technique, clinical
INTRODUCTION
OnabotulinumtoxinA is the only treatment approved by the US Food and Drug Administration for the prevention of headaches in patients with chronic migraine (CM).1 The mechanism of action of onabotulinumtoxinA in treating pain is most likely related to the inhibition of nociceptive mediator release from afferent neurons, thereby attenuating peripheral pain signaling to the brain.2, 3 The efficacy of onabotulinumtoxinA as a prophylactic treatment for CM may be attributed to the notion that extracranial administration decreases the release of nociceptive mediators and decreases the sensitivity of meningeal receptors through downregulation of their activity.2, 4 The safety and efficacy of onabotulinumtoxinA for CM has been demonstrated in the Phase III Research Evaluating Migraine Prophylaxis Therapy (PREEMPT) clinical program,5, 6, 7, 8 which used a fixed‐site, fixed‐dose injection paradigm that was established based on data from earlier clinical trial experience.9, 10, 11 In the United States, the standardized PREEMPT injection paradigm specifies the usage of a total dose of 155 Units of onabotulinumtoxinA to be delivered to 7 specific muscle groups;12 however, a total dose of up to 195 Units is approved in select countries allowing for additional injections into the temporalis, occipitalis and/or trapezius muscles using a follow‐the‐pain method.13, 14 The 7 specific muscle groups of the standard paradigm align with the peripheral nerve distribution of the trigeminal, occipital, and cervical sensory nerves (Fig. 1). The indicated injection sites were found to be effective for CM headache prophylaxis.5, 6, 11
Figure 1.
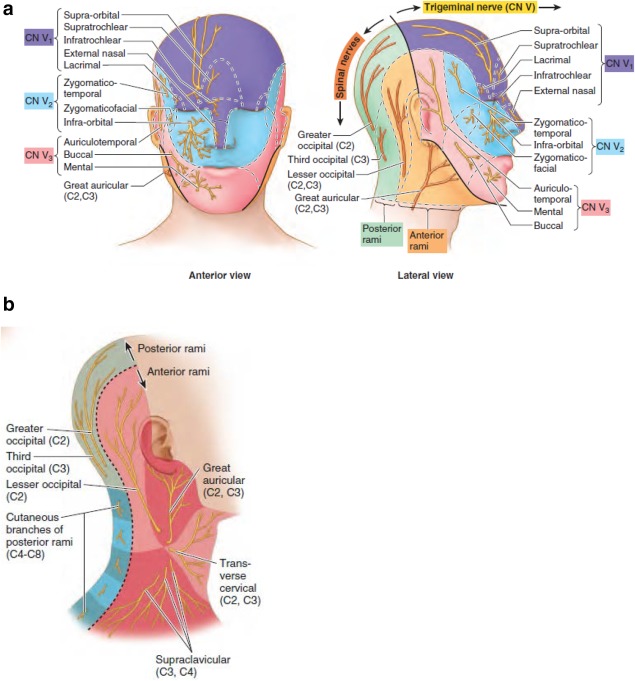
Distribution of peripheral nerves. (a) Anterior and lateral view of the trigeminal (CN V) and occipital (C2, C3) sensory nerves. (b) Cervical sensory nerves (C2, C3). Reproduced with permission.16
Data from the double‐blind phases of the PREEMPT clinical trial program showed neck pain (6.7%) and muscular weakness (5.5%) as the most commonly reported injection‐related adverse events; eyelid ptosis was also reported (3.3%).15 Ptosis can be due to different causes (Fig. 2). Preexisting eyelid ptosis can be due either to dehiscence or weakness of the levator palpebral muscle with aging. Alternatively it can be associated with lesions of Cranial Nerve III, also causing weakness of the levator palpebral superioris, or in cases where Mueller's muscle is weakened (ie, Horner's syndrome, where sympathetic innervation is affected).16 Brow ptosis is naturally caused by aging, associated with a “non‐prominent” orbital bone structure (ie, eye brow ptosis is seldom seen in those patients who have prominent or deep orbital bone structure). Weakening of the frontalis muscle both laterally and medially will also cause eyebrow ptosis. Usually a medial weakening of the lower one third of the frontalis muscle is more symptomatic than associated lateral weakening of the frontalis muscle. In the case of “pseudo ptosis,” characterized by excessive upper eyelid skin or blepharochalasis, which is corrected by surgical blepharoplasty, there is often associated compensatory activity in the frontalis muscle to elevate the eyelid.
Figure 2.
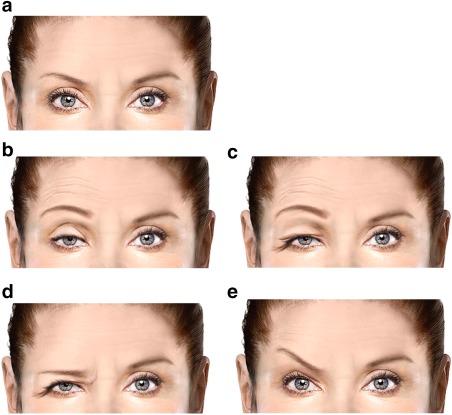
Various depictions of ptosis. (a) No ptosis. (b) Lid ptosis with compensatory frontal overactivity. (c) Pseudo ptosis. (d) Brow ptosis. (e) Medial brow ptosis with compensatory lateral brow elevation.
Based on clinical experience, it has been recognized that an incomplete understanding of the anatomy and muscle function of the head and neck can produce adverse events such as lid or brow ptosis or neck pain and weakness. The appearance of unwanted outcomes and adverse events may cause injectors to lower the onabotulinumtoxinA dose to decrease the likelihood of these events. Alternately, injectors may also completely omit treatment of certain muscles or areas of the head and neck where pain is present as patients may request avoidance of those areas impacted by the injection sites to reduce unwanted outcomes and adverse events. For optimal efficacy of onabotulinumtoxinA, injections should be administered every 12 weeks; however, if patients experience unwanted events, they may delay treatments waiting for these events to resolve. It must be cautioned that lowering doses, avoiding muscles, or delaying repeat treatments may lead to suboptimal efficacy.
Thus, there is a need for further guidance in identifying the correct muscle sites for injection and in applying optimal techniques to achieve the efficacy and safety observed in clinical trials. To do so, it is important to recognize that each patient's head and neck anatomy varies and that treatment should be administered considering an individual's unique muscle locations. It is also imperative to keep in mind that the point of injection differs from the actual point of delivery at the tip of the needle. In addition, a thorough and thoughtful assessment of any preexisting conditions, which may indicate a greater risk for unwanted outcomes or adverse events, is needed at each treatment. Furthermore, it is important to follow up with a patient to understand their outcomes and to adjust treatment approaches to muscle(s) accordingly rather than acting to avoid or delay injections.
The objective of this manuscript is to review the relevant anatomical features to help guide the injection of the mandatory muscles involved in the fixed‐dose, fixed‐site PREEMPT injection paradigm. A thorough understanding of these factors will help physicians to strengthen their injection technique and minimize potential adverse outcomes for their patients.
ANATOMY OF THE MUSCLES IN THE PREEMPT INJECTION PARADIGM
The standardized PREEMPT injection paradigm indicates a total dose of 155 Units of onabotulinumtoxinA to be administered to 31 injection sites distributed across the corrugator, procerus, frontalis, temporalis, occipitalis, cervical paraspinal, and trapezius muscle groups (Fig. 3).11
Figure 3.
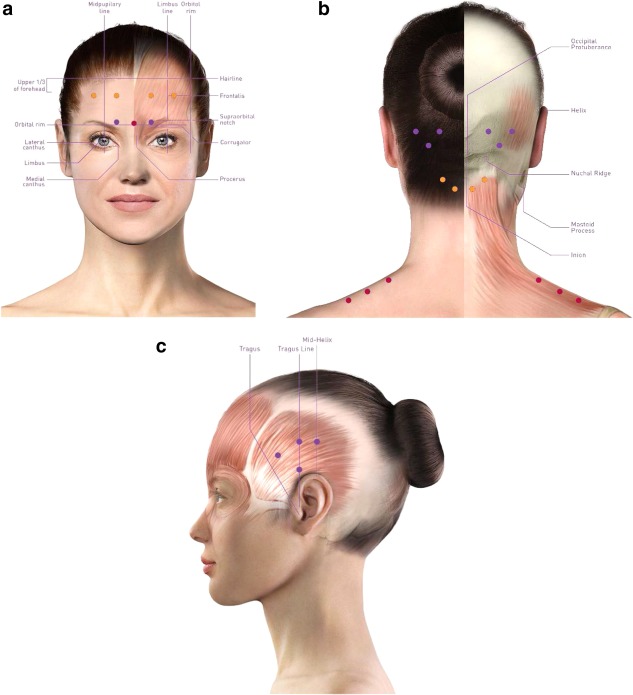
Fixed‐site, fixed‐dose PREEMPT injection site locations. (a) Corrugator, as depicted by purple dots; procerus, as depicted by the red dot; frontalis, as depicted by orange dots. (b) Occipitalis area, as depicted by purple dots; cervical paraspinal area, as depicted by orange dots; trapezius, as depicted by red dots. (c) Temporalis, as depicted by purple dots.
Corrugator
Ascending from the bone at the medial end of the superciliary arch is the corrugator muscle.16 The muscle fibers pass laterally and slightly upwards and insert into the skin in the middle of the supraorbital margin and superciliary arch.17 The corrugator muscle is partially blended with the orbicularis oculi and occipitofrontalis.17 The temporal branches of the facial nerves innervate the corrugator.17 Branches from adjacent arteries, mainly from the superficial temporal and ophthalmic arteries, provide the vascular supply.17 The supraorbital and supratrochlear nerves pass through the corrugator muscle.17 The corrugator muscle acts to pull the eyebrows down and medially, which mainly causes vertical wrinkle lines to occur in the skin between the brows.16, 17
According to the standardized PREEMPT injection paradigm, a total of 5 Units of onabotulinumtoxinA is injected into each corrugator muscle (Fig. 3a).11 The corrugator muscle is situated at the medial aspect of the orbital rim (bony landmark). To confirm the location, the patient is asked to furrow their brow to activate the corrugator and cause medial and inferior movement of the brow. Once the muscle has been located, the muscle should be palpated and pinched by holding it between the thumb and index finger. Five Units of onabotulinumtoxinA is injected at an approximate 90° angle, with the bevel of the needle pointing upward into the medial belly of the muscle. As the needle is inserted, there is skin resistance, which lessens when the muscle is penetrated. This decrease in resistance is termed a “muscle pop” and is the method used to determine the site of injection. Once the muscle pop occurs, inject into the superficial muscle; the needle is then in the deep portion of the medial corrugator, which is anatomically just over the supratrochlear foramen. However, the actual location of this muscle for injection should be based on the patient's individual anatomy. To avoid post‐injection headache, the injection should be administered while remaining just above the periosteum. If the injection is too far superior or above the corrugator muscle, brow ptosis can occur due to depression of the medial brow as the frontalis elevating function is lost and the corrugator depressing function remains unopposed. Weakening the corrugator muscle can cause mild elevation of the medial eyebrow.
Procerus
The procerus is a small inverted pyramidal‐shaped muscle that lies close to and often intermingles with the medial side of the anterior belly of the frontalis muscle as well as the corrugator.17 The muscle runs from the fascia aponeurosis covering the nasal bones and lateral nasal cartilages and inserts into the skin at the inferior forehead between the eyebrows.16 The procerus is innervated by the temporal and lower zygomatic branches of the facial nerve; the supratrochlear nerve passes through this muscle and medial corrugator.17 This muscle mainly receives its vascular supply by the branches of the facial artery.17 The medial portion of the eyebrow and the skin of the lower forehead are drawn down by the procerus muscle, producing transverse ridges or dynamic lines or wrinkling over the bridge of the nose and is also co‐activated by the corrugator muscle.16, 17
The PREEMPT injection paradigm indicates 1 injection of 5 Units of onabotulinumtoxinA to be administered to 1 site in the procerus muscle (Fig. 3a).11 The procerus injection will be based approximately midway between the 2 corrugator injections. To confirm the location of the procerus muscle, the patient is asked to furrow their brow, which will activate the belly of the muscle causing the medial furrowing to occur. Once identified, 5 Units of onabotulinumtoxinA should be injected superficially into the belly of the muscle at a 90° angle to ensure the injection is administered into the procerus rather than a nearby muscle. Injections placed too superiorly may inadvertently lead to penetration of the frontalis muscle. If injected too deep, hitting the periosteum, post‐injection headache can be triggered.
Frontalis
The frontalis muscle is adherent to the superficial fascia of the lower forehead, in particular the eyebrows, with no bony attachments.17 The muscle fibers blend with those of adjacent muscles (ie, procerus) and ascend to join the epicranial fronto‐occipital aponeurosis. Innervation of the frontalis occurs via the temporal branches of the facial nerve.17 Anatomically, there is usually found a bifurcation between the right and left sides of the frontalis muscle, leaving a fascial v‐shaped area over the central portion of the forehead that varies from patient to patient. The supratrochlear and supraorbital nerves pass through this muscle at the nerve exit points through the supratrochlear and supraorbital foramen; it then branches diffusely in a location that is superficial to the frontalis muscle, within the investing fascia, providing sensation to the forehead.16, 17 The frontalis muscle receives its vascular supply from branches of the superficial temporal and ophthalmic arteries.17 The action of the frontalis muscle involves elevation of the eyebrows to produce expressions such as surprise, and can cause deep transverse wrinkles on the forehead.16, 17 The antagonists for brow depression are the corrugators, procerus, and orbicularis oculi muscles.17
One injection of 5 Units of onabotulinumtoxinA into 4 sites (total 20 Units) is specified in the PREEMPT injection paradigm for the frontalis muscle (Fig. 3a).11 The injection points can be identified by first visually drawing a line up from the medial edge of the supraorbital rim. Patients will be injected into the muscle in the upper third of the forehead at least one to two fingerbreadths above the corrugator injection site. The lateral muscle injection areas are parallel and approximately 1 fingerbreadth lateral to the medial injection site, which is roughly in line with either the mid‐pupillary line or the lateral edge of the cornea, which is the limbus line. However, individual anatomy must be considered to avoid lower than desired injections since all forehead shapes and sizes differ.
Since the frontalis is an elevator muscle, weakening can cause brow ptosis or exacerbate preexisting brow ptosis. To reduce the risk of these unwanted effects, injections should be administered in the upper third of the forehead only and not too far laterally. In addition, the needle should be inserted at a 45° angle superiorly and in the superficial aspect to avoid reaching the periosteum, which may trigger post‐injection headache/migraine, since the frontalis muscle is thin. Injectors should always keep in mind that the injection point within the subcutaneous plane differs from the actual medication delivery point.
In the scenario that lateral brow elevation does occur, it is usually compensatory due to weakness of the medial frontalis muscle and is typically indicative of suboptimal positioning of frontalis and/or corrugator injections. Lateral brow elevation may be avoided by injecting the frontalis optimally into the upper third of the forehead as described above, and ensuring that the corrugator injection site is correctly positioned. If there is preexisting frontalis compensation as a result of lid ptosis or pseudo‐ptosis, conditions that should be sought during the pre‐injection examination, particular care is needed in identifying the injection site for the frontalis injections to avoid compensatory lateral brow elevation.
Temporalis
The temporalis muscle is a large, flat, triangular muscle that attaches proximally to the floor of the temporal fossa and deep surface of the temporal fascia.17 There is a narrow, distal attachment to the tip and medial surface of the coronoid process and anterior border of the ramus of the mandible.17 The temporalis is innervated by the motor division of the trigeminal nerve; the auriculotemporal and zygomatico‐temporal cutaneous nerve branches of the trigeminal nerve pass through this muscle.16, 17 The superficial temporal arteries, which arise from the external carotid artery, provide the vascular supply.17 After protrusion, the posterior, horizontal fibers of the temporalis retract the mandible, while the anterior, vertical fibers elevate the mandible and close the mouth and are involved in clenching the teeth.16, 17
Based on the PREEMPT injection paradigm, 1 injection of 5 Units are to be administered to each of 4 sites on either side for a total of 40 Units divided across 8 sites (Fig. 3b).11 The first injection site is identified by first locating the tragus of the ear and imagining a vertical line extending up the side of the head; the first injection is on this vertical line (Tragus line) and at least 2 fingerbreadths above the tragus. The second site can be located by moving approximately 1‐2 fingerbreadths up from the first injection site, while still keeping in line with the tragus. The third site is approximately 1 fingerbreadth anterior and in between the first 2 injections, still within the hairline. The fourth site is approximately 1 fingerbreadth back from the second injection site and in line with the mid‐portion (helix) of the ear. A finger can be placed on the middle of the helix of the ear to guide the injection. To confirm the locations, the patient may be asked to clench their teeth while palpating to identify muscle impulse of the temporalis contraction. All injections are administered at a 45° angle.
This set of injections is prone to bleeding due to the many blood vessels (veins) that ascend anterior to the tragus and end in the scalp in this region. The superficial temporal artery runs anterior to the tragus but is not as easily penetrated with the needle due to the thick perivascular sheath that covers it, providing resistance. To minimize bleeding, draw back on the needle to assess for a blood flush and inspect the patient to ensure any bleeding is managed post‐injection. Due to the risk of bleeding events, safety should be discussed with patients taking Coumadin or other anticoagulants. Patients may report hearing a crinkling/crackling sound during the injection as a result of the needle penetrating the fibrous fascia that covers this muscle. In addition, it is important to be aware that repeated injections may lead to temporalis muscle atrophy with the development of an hour‐glass appearance of the head.18 If this occurs, the injections are performed sufficiently behind the hairline.
Occipitalis
Arising by tendinous fibers from the lateral two‐thirds of the highest nuchal line of the occipital bone and adjacent region of the mastoid part of the temporal bone is the occipitalis muscle.17 The occipitalis muscle extends upwards and laterally to join the aponeurotic fascia and is innervated by the posterior auricular branch of the facial nerve.17 The greater occipital nerve lies medial to this muscle, and the lesser occipital nerve lies on its lateral aspect.17 The occipitalis is a small muscle that receives its vascular supply from branches of the posterior auricular and occipital arteries.17 Working together with the frontalis muscle, the occipitalis acts to pull the scalp backwards.16, 17 The frontalis and occipitalis muscles should be considered a functional muscle complex linked by fascia. The occipitalis is part of the fronto‐occipital aponeurotic fascial system and is activated when frowning or elevating the eyebrows.16, 17
The occipitalis muscle region receives 5 Units of onabotulinumtoxinA to 3 sites on each side of the head for a total of 30 Units divided across 6 sites, as stated by the PREEMPT injection paradigm (Fig. 3c).11 To identify the first site, the occipital protuberance is palpated and the most posterior point in the midline (ie, inion) is located. The nuchal ridge is then palpated and the tip of the mastoid process behind the ear is located. With the location of these two points, the thumb should be placed on the midpoint of the occipital protuberance (ie, inion) and index finger on the tip of the mastoid process. The space between the thumb and the index finger should then be divided in half. The injection should be administered just above the nuchal ridge at this midpoint. The second injection site is located approximately a diagonal fingerbreadth up and out toward the helix of the ear for injection at the 10 o'clock position on the left (2 o'clock on the right). The third site can be located by measuring approximately a diagonal fingerbreadth up and medial for injection at the 2 o'clock position on the left (10 o'clock on the right). The injections should be administered at a 45° angle to the superficial aspect of the muscle (ie, just below the dermis). The needle should be angled upward away from the neck and the injection should be administered above the nuchal ridge.
Patients may experience neck pain and/or weakness if injections are administered too low (ie, below the nuchal ridge); the injector must ensure that these injections do not involve the suboccipital region. Since the injection sites are in close proximity to the greater and lesser occipital nerves, patients should be made aware that the injections may cause some pain.
Cervical Paraspinal Muscle Group
The cervical paraspinal muscle group is made up of multiple muscles including the trapezius, splenius capitis and cervicis, and semispinalis capitis. The splenius capitis ascends from the inferior half of the nuchal ligament and spinous processes of the superior six thoracic vertebrae.17 The inferior, anterior attachment of the splenius capitis occurs at the lateral aspect of the mastoid process and lateral third of the superior nuchal line.16, 17 The semispinalis arises from approximately half of the vertebral column and is divided into three parts according to their superior attachments (ie, semispinalis capitis, semispinalis thoracis, and semispinalis cervicis).16, 17 The semispinalis capitis forms the longitudinal bulge in the back of the neck near the median plane and originates from the transverse processes of the C4‐T12 vertebrae.16, 17 The cervical paraspinal muscle group is innervated by the posterior rami of the spinal nerves; the third occipital nerve traverses this group of muscles near the midline.16, 17 Laterally the greater and lesser occipital nerves emerge through these muscles.16 This muscle group receives its vascular supply from branches of the occipital artery.16, 17 This group of muscles helps the support and stabilization of the neck in addition to the rotation and/or extension of the head.16, 17
According to the PREEMPT injection paradigm, 5 Units of onabotulinumtoxinA is to be administered to 2 sites on each side for a total dose of 20 Units across 4 sites in the cervical paraspinal muscle group near the midline (Fig. 3c).11 The first injection site is approximately 1 cm left of the midline of the cervical spine and approximately 3 cm (2 fingerbreadths) inferior to the occipital protuberance. The second site is measured approximately 1 fingerbreadth diagonally up at a 45° angle toward the helix of the ear from the first injection. The injections should be administered in the most superficial aspect of the muscle, angling the needle 45° and superiorly. To aid in the placement of the injections, the patient should be positioned upright with the head in a neutral position. If the neck is flexed too far forward, injections may be too deep. Penetrating the fascia, which is variable, should be sufficient to avoid an injection that is too deep. Injections that are too low or too deep in this muscle group can lead to muscle weakness and neck pain. Injectors can consider the cervical paraspinals as suboccipital muscles to ensure that the injection sites are not too low. In addition, a horizontal line can be visualized across the neck, approximately 2 fingerbreadths down from the occipital protuberance, to make certain the injections remain above the line and are not administered too low in the neck. In general, injections should occur higher and just inferior to the nuchal ridge, where there is a thick fascial condensation and which will minimize the potential for exacerbated neck weakness, keeping in mind that these injections occur in the hairline.
It is important to be aware that some CM patients may have preexisting neck pain and/or weakness; therefore, patients should be assessed at baseline to properly set expectations of possible adverse events.
Trapezius
The trapezius muscle is a large, flat, triangular, superficial muscle.17 It attaches proximally in the medial third of the superior nuchal line, external occipital protuberance, nuchal ligament, and spinous processes of the C7‐T12 vertebrae.16, 17 Distal attachment of the trapezius occurs at the lateral third of the clavicle and acromion and spine of the scapula.16, 17 The trapezius is innervated by the spinal accessory nerve (ie, CN XI, motor fibers) and C3 and C4 spinal nerves (ie, pain and proprioceptive fibers); the sensory rami of C2, C3 and C4 run across this muscle.16, 17 The muscle is supplied by the transverse cervical artery.17 The action of the muscle includes stabilization and movement of the scapula and support for the arm.16, 17
One injection of 5 Units of onabotulinumtoxinA to each of 3 sites on either side of the trapezius, for a total of 30 Units divided across 6 sites, is denoted in the PREEMPT injection paradigm (Fig. 3c).11 The first injection site can be identified by visually dividing the upper portion of the trapezius muscle in half, from the inflection point of the neck (ie, necklace line) to the acromion (acromio‐clavicular joint); the midpoint of this location is where the injection should be administered. The second injection is located at the midpoint of the first injection site and the acromion. The third injection should be administered at the midpoint between the first injection site and the necklace line. Injections should occur in the supraclavicular portion of the muscle, lateral to the neckline, and medial to the deltoid and the acromio‐clavicular joint. The injections into the trapezius should be administered horizontally and superficially to avoid injecting too deep. However, in select situations where the skin or subcutaneous tissue is thick, a deeper penetration may be required to be in the superficial tissue. Injecting too high or too deep may lead to neck and/or shoulder weakness, as well as neck pain due to compensatory muscle activity. Injections that are administered too far laterally will be in the deltoid muscle, resulting in shoulder weakness.
Prior to treatment administration, each patient should be assessed for possible preexisting neck and/or shoulder weakness. In particular, patients with small frames may be predisposed to weakness following injection in this muscle area. These initial observations will help to set expectations for possible adverse events.
Follow‐the‐Pain Injection Sites
The PREEMPT studies used a fixed‐site, fixed‐dose injection paradigm where intramuscular injections of 155 Units of onabotulinumtoxinA were administered across 7 head and neck muscles and allowed physician discretion to inject an additional 40 Units of onabotulinumtoxinA across 3 muscle groups (the follow‐the‐pain approach).5, 6, 7, 8 Therefore, in the PREEMPT studies, a total of 195 Units of onabotulinumtoxinA across 39 sites using the follow‐the‐pain approach was permitted.11 The fixed‐dose, fixed‐site injection paradigm of the PREEMPT clinical trial program was used as the basis for US regulatory approval of onabotulinumtoxinA for chronic migraine. In other countries, a total dose of onabotulinumtoxinA of up to 195 Units is approved allowing for additional injections into the temporalis, occipitalis, and/or trapezius muscles using the follow‐the‐pain method.13, 14
Any additional injections administered under the follow‐the‐pain strategy should follow the general guidance outlined in this paper. If additional onabotulinumtoxinA is required into the temporalis muscle, it is suggested that the additional drug is administered into up to 2 additional sites into the temporalis muscle on each side, as required, but not into the standard injection sites. Similarly, should it be determined that additional injections into the occipitalis muscle are beneficial, the additional onabotulinumtoxinA may be administered as 2 injections into either the right or left side or 1 injection into the occipitalis muscle on each side, depending on the area identified as having maximal tenderness. In our experience, neck weakness and neck pain are associated with high doses of onabotulinumtoxinA into the trapezius muscle. Therefore, additional injections into the trapezius muscle should generally be avoided where possible, even under a follow‐the‐pain strategy.
DISCUSSION
The PREEMPT injection paradigm has been published and is available.11 This manuscript emphasizes the importance of understanding the anatomy behind each injection site to optimize efficacy and minimize unwanted outcomes and adverse events. Based on the PREEMPT clinical trial program data, the most commonly reported injection‐related AEs were neck pain, muscular weakness, ptosis and headache.5, 6, 7, 8, 11, 15 These may be minimized not only by identifying the correct injection sites and implementing the advised injection techniques but also by thoroughly assessing the patient before treatment.
Prior to the first injection of onabotulinumtoxinA, it is critical to first assess the patient's head and neck anatomy, since variation in anatomy will lead to variations in target muscle locations. The standard PREEMPT fingerbreadth measurements should be used as a general guidance only, while actual injection sites should be located based on the patient's unique anatomy. Considerable variability exists between patients with regard to muscle mass, brow width and height, and preexisting weakness. These factors will impact the location of the injection sites. Therefore, it is essential to be familiar with anatomical landmarks to aid in identifying the location of the PREEMPT injection sites.
Determination of a patient's preexisting conditions at baseline is also essential prior to treatment administration, as it will help to set appropriate expectations. In particular, each patient should be examined for preexisting eyelid or eyebrow ptosis, pseudoptosis of the eyelids, neck pain, and neck weakness. Patients with CM may have preexisting eyelid ptosis with frontalis compensatory activity (ie, a Babinski‐2 or Reverse Babinski sign associated with hemifacial spasm).19, 20, 21 This involves an up‐going eyebrow due to compensation of the frontalis muscle for eyelid closure. The presence of any soft tissue under the orbital ridge and resting on the eyelid may contribute to pseudoptosis. Pseudoptosis requires frontalis compensatory activity to raise the redundant tissue off the eyelid by elevating the brow. If the brow is weakened by placing onabotulinumtoxinA injections into the mid‐section of the frontalis, only the lateral frontalis remains to provide this compensatory activity, resulting in lateral eyebrow elevation. Injections that weaken the lateral frontalis may worsen the pseudoptosis. In addition, the patient's neck stability, posture, torsion, symmetry, or other possible abnormalities should be assessed to determine whether they may be at increased risk for adverse events.
The physician can often predict the side effects that may be more likely to appear based on examination of the patient prior to injection. For example, a patient with ptosis or pseudoptosis at baseline may experience exacerbation of these unwanted outcomes upon injection of the frontalis muscles. In these cases, particular care in placement of the injection into the lateral frontalis and corrugator muscles is required.
On the other hand, a patient with preexisting neck pain and/or weakness may be at higher risk for exacerbation of their condition upon injection of the areas associated with the occipitalis, cervical paraspinal, or trapezius muscle groups. Further, patients with smaller frames may be at higher risk for neck weakness. Indicated injection sites can still be injected with minimal side effects and unwanted outcomes as long as correct injection sites are targeted and treatments are administered using the advised techniques. Treatment expectations, including risk of unwanted and/or adverse events, should be discussed with the patient prior to treatment. This will help to avoid omission of injection sites based on concerns.
CONCLUSIONS
OnabotulinumtoxinA is a safe and effective prophylactic treatment option for patients with CM. Accurate target muscle localization and injection angles and depths are required to achieve optimal outcomes and to minimize adverse events. Firm knowledge of the functional anatomy of the head and neck muscles is critical for identification of the standardized PREEMPT injection site locations. This, combined with a thorough assessment of a patient's baseline condition, should help to achieve the outcomes observed in clinical trials.
STATEMENT OF AUTHORSHIP
Category 1
(a) Conception and Design
Andrew M. Blumenfeld, Stephen D. Silberstein, David W. Dodick, Sheena K. Aurora, Mitchell F. Brin, William J. Binder
(b) Acquisition of Data
NA
(c) Analysis and Interpretation of Data
NA
Category 2
(a) Drafting the Manuscript
Andrew M. Blumenfeld, Stephen D. Silberstein, David W. Dodick, Sheena K. Aurora, Mitchell F. Brin, William J. Binder
(b) Revising It for Intellectual Content
Andrew M. Blumenfeld, Stephen D. Silberstein, David W. Dodick, Sheena K. Aurora, Mitchell F. Brin, William J. Binder
Category 3
(a) Final Approval of the Completed Manuscript
Andrew M. Blumenfeld, Stephen D. Silberstein, David W. Dodick, Sheena K. Aurora, Mitchell F. Brin, William J. Binder
Conflict of Interest: Blumenfeld: Consulting agreements with Allergan, including intellectual property and promotional activities. In the past 12 months, Dr. Blumenfeld has provided promotional activities and consulted for: Avanir, Teva, Pernix, Supernus, Depomed, Dentex, Zosano Pharma, GLG, Guidepoint, and Autodigest. Silberstein: As a consultant and/or advisory panel member, Dr. Silberstein receives honoraria from Alder Biopharmaceuticals, Allergan plc, Amgen, Avanir Pharmaceuticals, Inc., Curelator, Inc., Depomed; Dr. Reddy's Laboratories, eNeura, Inc., electroCore Medical, LLC, Lilly USA, LLC, Supernus Pharmaceuticals, Inc., Teva Pharmaceuticals, and Trigemina, Inc. Dodick: In the past 12 months, he has served on advisory boards and has consulted for Allergan, Amgen, Alder, Merck, eNeura, Eli Lilly & Company, Autonomic Technologies, Teva, Tonix, and Novartis. Within the past 12 months, he has received royalties, funding for travel, speaking, or editorial activities from the following: Haymarket Media Group, Ltd., SAGE Publishing, Synergy, Allergan, Lippincott Williams & Wilkins, Oxford University Press, and Cambridge University Press; he serves as Editor‐in‐Chief of Cephalalgia and on the editorial boards of The Neurologist, Lancet Neurology, and Postgraduate Medicine. He receives publishing royalties for Wolff's Headache, 8th edition (Oxford University Press, 2009) and Handbook of Headache (Cambridge University Press, 2010).
Aurora: Consultant to Allergan, Dr. Reddy's Laboratory, and Teva. Research support from Amgen, Gamma core, Labryus, Eli Lilly, and Teva. Full‐time employee of Eli Lilly.
Brin: Employee of Allergan plc and receives stock.
Binder: Minority shareholder of Miotox, LLC, an intellectual property company that receives royalties from Allergan. Consultant to Implantech Associates, Inc., a medical device company.
REFERENCES
- 1. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: Breaking down barriers to optimal treatment and prevention. Headache. 2015;55(Suppl. 2):103‐122. quiz 123‐126. [DOI] [PubMed] [Google Scholar]
- 2. Burstein R, Zhang X, Levy D, Aoki KR, Brin MF. Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: Therapeutic implications for migraine and other pains. Cephalalgia. 2014;34:853‐869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ramachandran R, Yaksh TL. Therapeutic use of botulinum toxin in migraine: Mechanisms of action. Br J Pharmacol. 2014;171:4177‐4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang X, Strassman AM, Novack V, Brin MF, Burstein R. Extracranial injections of botulinum neurotoxin type A inhibit intracranial meningeal nociceptors' responses to stimulation of TRPV1 and TRPA1 channels: Are we getting closer to solving this puzzle? Cephalalgia. 2016;36:875‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aurora SK, Dodick DW, Turkel CC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double‐blind, randomized, placebo‐controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793‐803. [DOI] [PubMed] [Google Scholar]
- 6. Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double‐blind, randomized, placebo‐controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30:804‐814. [DOI] [PubMed] [Google Scholar]
- 7. Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56‐week PREEMPT clinical program. Headache. 2011;51:1358‐1373. [DOI] [PubMed] [Google Scholar]
- 8. Aurora SK, Dodick DW, Diener HC, et al. OnabotulinumtoxinA for chronic migraine: Efficacy, safety, and tolerability in patients who received all five treatment cycles in the PREEMPT clinical program. Acta Neurol Scand. 2014;129:61‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Binder WJ, Brin MF, Blitzer A, Schoenrock LD, Pogoda JM. Botulinum toxin type A (BOTOX) for treatment of migraine headaches: An open‐label study. Otolaryngol Head Neck Surg. 2000;123:669‐676. [DOI] [PubMed] [Google Scholar]
- 10. Blumenfeld AM, Binder W, Silberstein SD, Blitzer A. Procedures for administering botulinum toxin type A for migraine and tension‐type headache. Headache. 2003;43:884‐891. [DOI] [PubMed] [Google Scholar]
- 11. Blumenfeld A, Silberstein SD, Dodick DW, Aurora SK, Turkel CC, Binder WJ. Method of injection of onabotulinumtoxinA for chronic migraine: A safe, well‐tolerated, and effective treatment paradigm based on the PREEMPT clinical program. Headache. 2010;50:1406‐1418. [DOI] [PubMed] [Google Scholar]
- 12.BOTOX® (OnabotulinumtoxinA) for Injection, for Intramuscular, Intradetrusor, or Intradermal Use Irvine, CA: Allergan; 2016.
- 13.BOTOX. ([OnabotulinumtoxinA] for Injection, Intramuscular, Intradetrusor, or Intradermal Use) Summary of Product Characteristics. Marlow, Bucks, UK: Allergan Ltd.; 2015.
- 14.BOTOX. ([OnabotulinumtoxinA] for Injection, Intramuscular, Intradetrusor, or Intradermal Use) Product Monograph. Markham, Ontario, Canada: Allergan, Inc.; 2014.
- 15. Dodick DW, Turkel CC, DeGryse RE, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled results from the double‐blind, randomized, placebo‐controlled phases of the PREEMPT clinical program. Headache. 2010;50:921‐936. [DOI] [PubMed] [Google Scholar]
- 16. Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy (7th ed.). Philadelphia, PA: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 17. Standring S. Gray's Anatomy: The Anatomical Basis of Clinical Practice (40th ed.). Edinburgh: Churchill Livingstone/Elsevier; 2008. [Google Scholar]
- 18. Guyuron B, Rose K, Kriegler JS, Tucker T. Hourglass deformity after botulinum toxin type A injection. Headache. 2004;44:262‐264. [DOI] [PubMed] [Google Scholar]
- 19. Valencia C, Cuadrado ML, Barahona‐Hernando R, et al. [Migraine‐triggered hemifacial spasm: Another case study]. Neurologia. 2014;29:61‐62. [DOI] [PubMed] [Google Scholar]
- 20. Barahona‐Hernando R, Cuadrado ML, Garcia‐Ptacek S, et al. Migraine‐triggered hemifacial spasm: Three new cases. Cephalalgia. 2012;32:346‐349. [DOI] [PubMed] [Google Scholar]
- 21. Pawlowski M, Gess B, Evers S. The Babinski‐2 sign in hemifacial spasm. Mov Disord. 2013;28:1298‐1300. [DOI] [PubMed] [Google Scholar]


