Abstract
Background and Purpose:
Validation of laparoscopic total gastrectomy (LTG) for patients with gastric cancer has not been fully investigated. In particular, the technique for esophagojejunostomy remains controversial. We performed 103 cases of LTG for patients with gastric cancer between 2007 and 2013, in which all esophagojejunostomy reconstruction was performed with intracorporeal circular stapling esophagojejunostomy using the OrVil system except for the first 3 cases. The purpose of this study is to retrospectively analyze the clinical usefulness of LTG with intracorporeal circular stapling esophagojejunostomy using the OrVil system and oncological feasibility of LTG as compared with open total gastrectomy (OTG).
Patients and Method:
We retrospectively analyzed clinical course of consecutive 100 operations with LTG in comparison with consecutive 53 operations with OTG for patients with gastric cancer. As an estimation of short-term outcome, operative time, blood loss, postoperative hospital days and postoperative data of blood and drain examination were included. Moreover, relapse-free survival time and overall survival time stratified by each stage were calculated by log-rank test as an estimation of prognostic relevance.
Results:
Blood loss and postoperative hospital stay of LTG were significantly less than that of OTG. Postoperative complications were equivalent between the 2 groups and no patient died within 1 month post-LTG. Only 1 patient had recurrence and died for carcinomatosa peritonitis 50 months after LTG (median follow-up period: 44 mo).
Conclusions:
Our experience revealed that LTG with intracorporeal circular stapling esophagojejunostomy using the OrVil system could be performed safely and with acceptable oncological outcome for patients with gastric cancer.
Key Words: gastric cancer, laparoscopic total gastrectomy, intracorporeal circular stapling esophagojejunostomy using the OrVil system
Gastric cancer is the fourth-common malignant neoplasm that represents the second-greatest cause of cancer-related deaths, and frequently affects the populations of Laten America, Eastern Europe, China, Korea, and Japan.1,2 Laparoscopy-assisted distal gastrectomy (LADG) for early gastric cancer was first reported by Kitano et al3 and since then, LADG or laparoscopic distal gastrectomy (LDG) have been widely performed for patients with early gastric cancer in developed countries. Surgical benefit of LDG, such as reduced pain, shorter hospital stay, and early recovery of bowel movements over conventional open surgery had already been reported.4–7 The other studies have focused on its oncologic equivalency to open distal gastrectomy.8,9 However, laparoscopy-assisted total gastrectomy (LATG) has not been widespread because of the technical difficulty, particularly, alimentary tract reconstruction. In addition to reconstruction of esophagojejunostomy, paracardial lymph node dissection, vascular procedures along the greater curvature of the upper stomach, and vagotomy along the abdominal esophagus are more difficult compared with open total gastrectomy (OTG).10 There have been several reports on the safety and feasibility of LATG and outcomes of LATG versus OTG.11–14 On the other hands, these reports do not focus on the way of intracorporeal circular stapling esophagojejunostomy using the OrVil system.
The purpose of this present study is to clarify the clinical usefulness and oncological outcome of LATG with intracorporeal circular stapling esophagojejunostomy using the OrVil system in comparison with OTG.
PATIENTS AND METHODS
We analyzed gastric cancer database of consecutive 103 patients with gastric cancer who underwent LATG and 53 patients who underwent OTG at Jikei University Hospital between December 2005 and September 2013. Clinical and histopathologic data were classified according to the Japanese Classification of Gastric Carcinoma.15 Performance status of American Society of Anesthesiology 0-1, location of the tumor in the upper or middle third of the stomach without distant metastasis or invasion to adjacent organs were enrolled. Finally, 100 consecutive patients who underwent laparoscopic total gastrectomy (LTG) (median follow-up period: 44 mo) and 53 consecutive patients who underwent OTG (median follow-up period: 84.5 mo) were selected. The indication LTG was initially included depth of tumor invasion limited to mucosa and submucosa and absence of lymph node metastasis located in upper and middle of gastric portion in preoperative examinations. However, the indication of LTG was gradually extended to advanced gastric cancer. The clinical variables of all LTG and OTG patients were analyzed by retrospective database review.
A hundred of LTG was performed with intracorporeal circular stapling esophagojejunostomy using the OrVil system.
Operative Technique
OTG was performed in the usual manner through an approximately 20-cm long upper-middle incision. LTG was performed using 5 ports (two 5-mm bilateral costal arch ports: two 12-mm ports placed laterally to the rectus sheath, and one 12-mm camera port). An initial 12-mm trocar was carefully inserted through the umbilical incision for the laparoscopic scope using the open technique. Full examination of the abdominal cavity was performed to search for distant metastasis and direct invasion of adjacent organs. The CO2 pneumoperitoneum was maintained at 8 to 10 mm Hg during the operation. The dissection of lymph nodes was similar to the LDG, using the harmonic scalpel (Ethicon Endo Surgery, Cincinnati, OH) except for the pericardial area and distal pancreatic portion including splenic hilum. The trocar incision in the umbilical portion was extended 4 to 5 cm above; Roux-en-Y esophagojejunostomy and jejunojejunostomy reconstruction were performed through this incision. After amputating esophagus, a tube connected with OrVil is inserted from mouth through esophagus and then introduced through the stump of the esophagus. The jejunum was brought through the anterocolic route after dividing the jejunum 30 cm anal to the ligament of Treitz, and the shaft of the circular stapler was introduced into the distal segment of the jejunum followed by an end-to-side esophagojejunostomy. The access opening on the jejunal stump was then closed with a laparoscopic linear stapler. The side-to-side jejunojejunal anastomosis was performed using a 60 mm endoscopic linear stapler; the length between the esophagojejunostomy and the jejunojejunal anastomosis was approximately 40 cm. For OTG, end-to-side esophagojejunostomy was performed using a circular stapler (CDH, 25 mm; Ethicon Endo-Surgery or CEEA, 25 mm; Covidien).
The variables of short-term outcome included operative time, blood loss, duration of hospital days, and postoperative data of blood and drain discharge. Moreover, relapse-free survival time and overall survival time stratified by cancer stage were evaluated by log-rank test as an estimation of prognostic relevance.
Neoadjuvant and Adjuvant Treatments
Neoadjuvant chemotherapy was not used in the studied patients. Adjuvant chemotherapy was given in patients with pathologically identified stage II and III A. S-1 was administered orally twice a day according to body surface area (BSA) as follows: BSA<1.25 m2, 80 mg/d; 1.25 ≦BSA<1.5 m2, 100 mg/d; 1.5 m2 ≦BSA, 120 mg/d for 1 year.
Statistical Analysis
The significance of the data were determined using the Student t test or χ2 test. Survival curves of the patients were compared using the Kaplan-Meier method and analyzed by the log-rank test. A P-value<0.05 indicates significance. All analysis was performed by Excel Statistics 2012 (Social Survey Research Information Co. Ltd, Tokyo, Japan).
RESULTS
Short-term Outcome
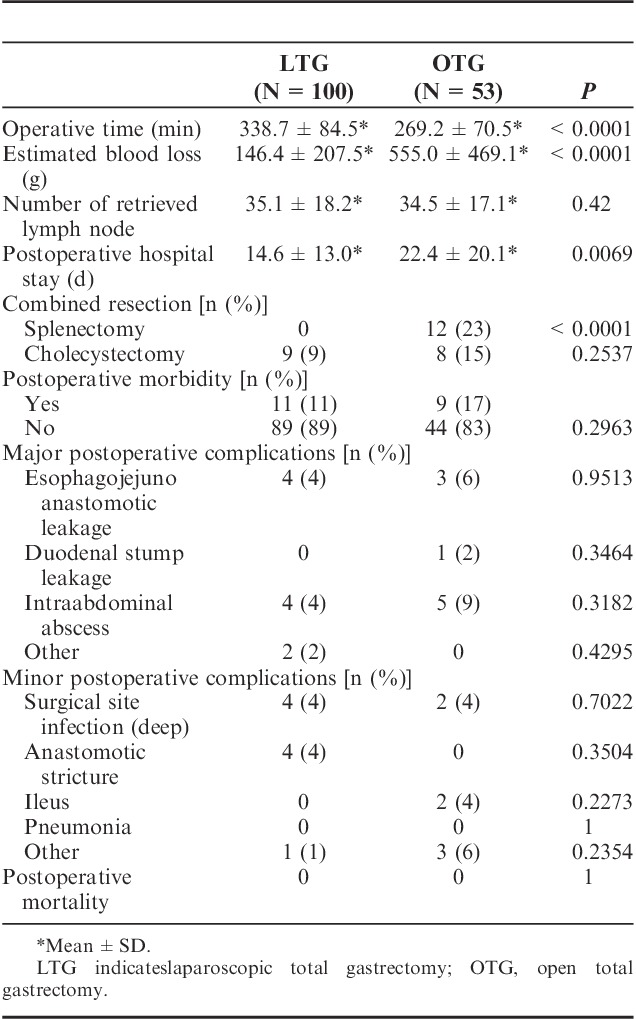
Clinical characteristics of the 2 groups are shown in Table 1. The patients’ body mass index between both groups had no statistical difference (23.6±3.1 vs. 23.2±3.6). As to pathologic characteristics, tumor size (37.1±25.1 vs. 49.7±32.7 mm), depth of tumor invasion (P=0.006), degree of lymph node metastasis (P=0.006), pathologic stage (P=0.0185) had statistical differences between the 2 groups. The average operating time was significantly longer in the LTG group as compared with OTG group (338.7±84.5 vs. 269.2±70.5 min). Estimated blood loss of LTG was less (146.4±207.5 vs. 555±469.1 g) and postoperative hospital stay of LTG was shorter (14.6±13 vs. 22.4±20.1 d) than those of OTG. Table 3 is demonstrating that LTG patients' serum total protein level on postoperative day 1 (5.16±0.47 vs. 4.95±0.59 g/dL) and day 3 (5.73±0.59 vs. 5.46±0.55 g/dL) were significantly higher than those of OTG group. Drain tube amylase (dAMY) level of LTG group on postoperative day 1 (796±1766 vs. 1904±4431 IU/L) and day 3 (258±396 vs. 1062±2029 IU/L) were also significantly low as compared with those of OTG. The number of retrieved lymph nodes between both groups had no statistical difference (35.1±18.2 vs. 34.5±17.1). Regarding as major complications after operation, occurrence rates of esophagojejuno anastomotic leakage (P=0.9513), duodenal stump leakage (P=0.3464), or intra-abdominal abscess (P=0.3182) did not have statistical differences between the 2 groups. There was no incidence of postoperative mortality in both groups (Table 2). In particular, we have to refer to the patients with Petersen’s hernia after LTG. We have experienced 4 patients which occurred >1 year after LTG and all these patients required additional operation. On the contrary, no Petersen’s hernia was observed in OTG group (Table 3).
TABLE 1.
Comparison of Clinicopathologic Characteristics Between Procedure of LTG and OTG
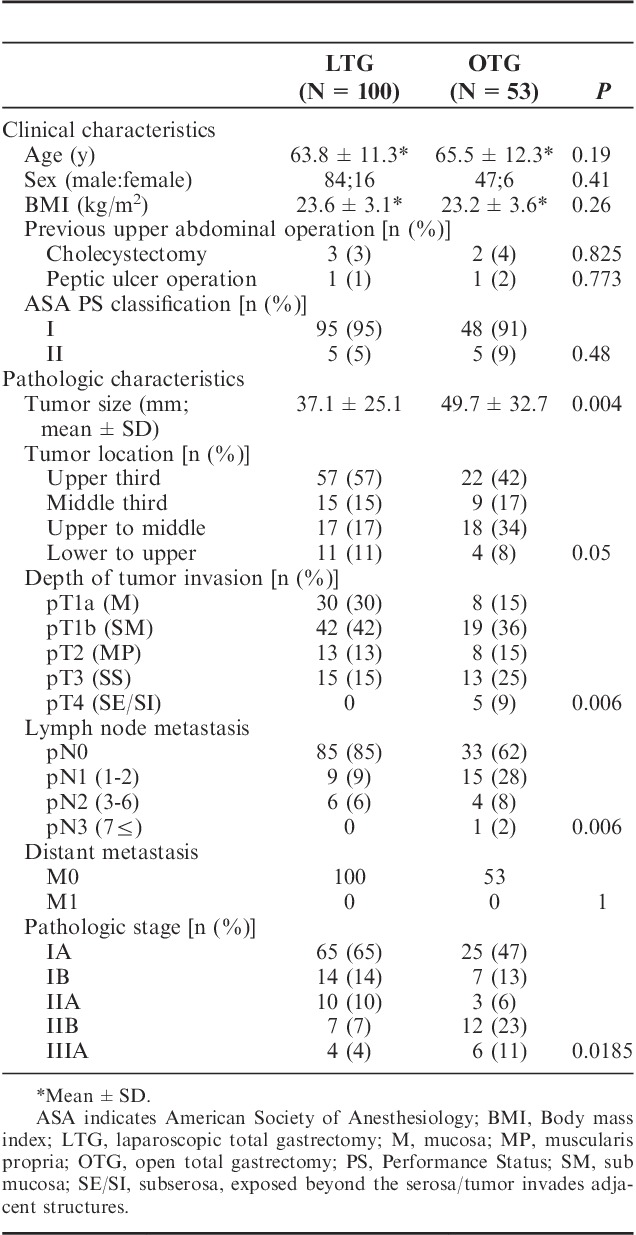
TABLE 3.
Comparison of Hematologic and Serum Chemical Profiles After LTG and OTG
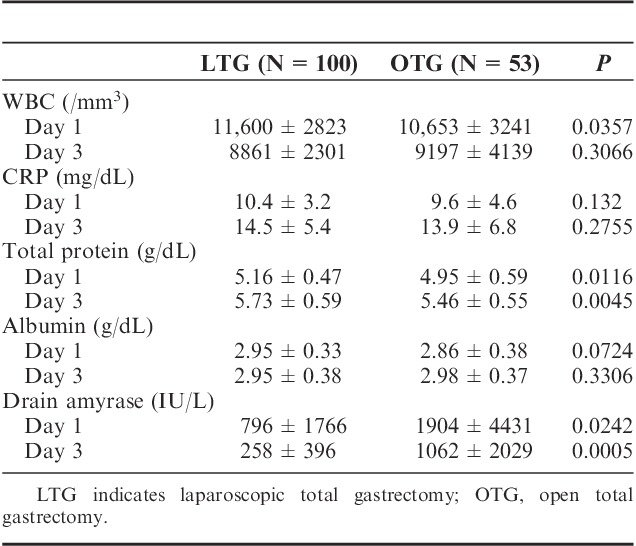
TABLE 2.
Comparison of Postoperative Outcomes Between LTG and OTG
Oncological Outcome
Median follow-up period was 44 months (range: 15 to 89 mo) in the LTG group and 84.5 months (range: 63 to 103 mo) in the OTG group. Figures 1–3 show relapse-free survival curves on each stages between LTG and OTG groups and there were no significant differences by log-rank test, respectively (P=0.5395, 0.1577, 0.5271). Only 1 patient had recurrence after LTG. Figures 4–6 show cumulative disease-specific survival curves on each stages between LTG and OTG groups, and there were also no significant differences by log-rank test (P=0.4359, 0.2671, 0.6547). Similar to the results of relapse-free survival time, the patients post-LTG group had better prognosis as compared with that of OTG, although the data did not reach statistically significant difference.
FIGURE 1.
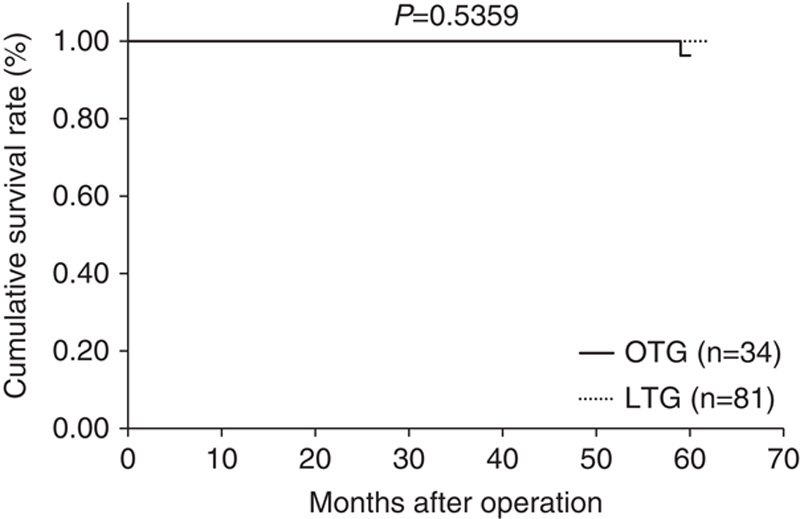
Relapse-free survival time between LTG and OTG (f-stage I). LTG indicates laparoscopic total gastrectomy; OTG, open total gastrectomy.
FIGURE 3.
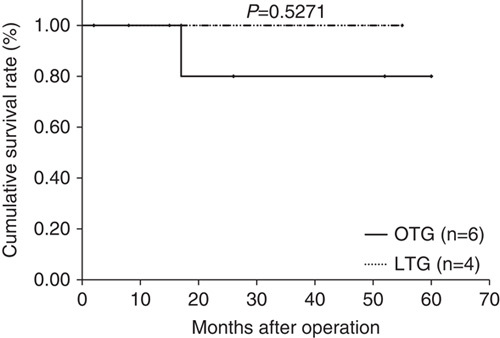
Relapse-free survival time between LTG and OTG (f-stage IIIA). LTG indicates laparoscopic total gastrectomy; OTG, open total gastrectomy.
FIGURE 4.
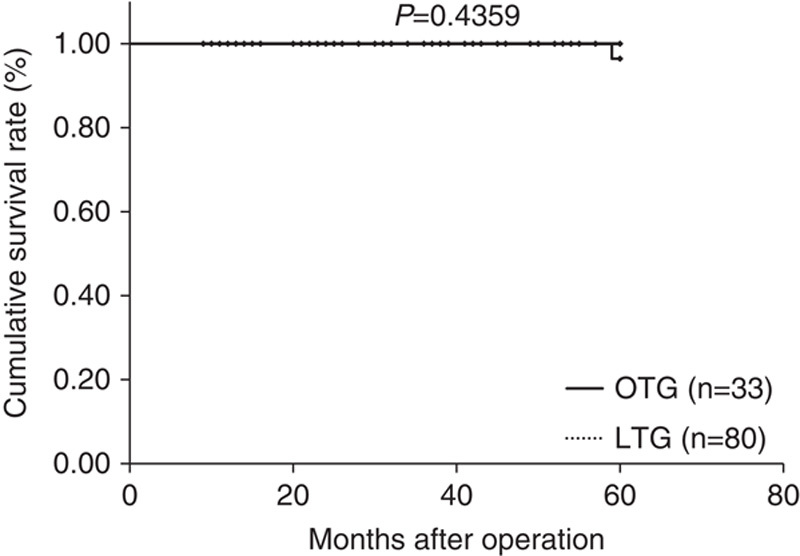
Disease specific survival time between LTG and OTG (f-stage I). LTG indicates laparoscopic total gastrectomy; OTG, open total gastrectomy.
FIGURE 6.
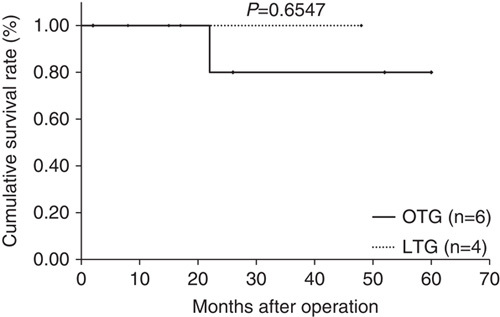
Disease specific survival time between LTG and OTG (f-stage IIIA). LTG indicates laparoscopic total gastrectomy; OTG, open total gastrectomy.
FIGURE 2.
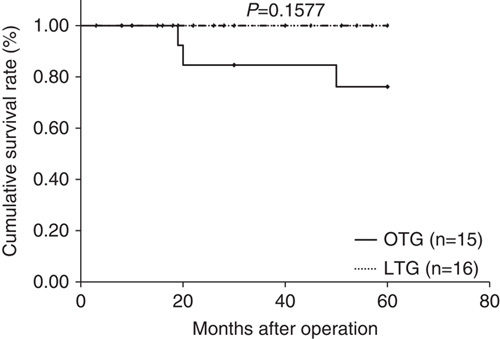
Relapse-free survival time between LTG and OTG (f-stage II). LTG indicates laparoscopic total gastrectomy; OTG, open total gastrectomy.
FIGURE 5.
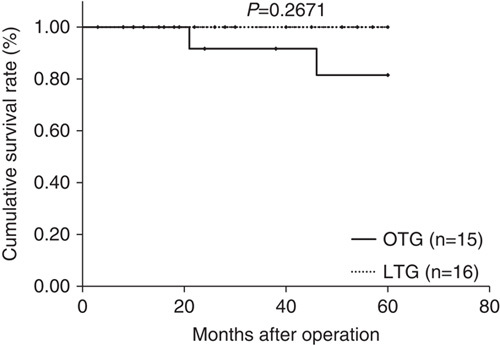
Disease specific survival time between LTG and OTG (f-stage II). LTG indicates laparoscopic total gastrectomy; OTG, open total gastrectomy.
DISCUSSION
LADG for early stage gastric cancer has gradually been spread and accepted in the world, due to short-term benefit such as less postoperative pain, faster recovery, and shorter postoperative hospital stays as well as its oncological equality as compared with open distal gastrectomy. In contrast, many surgeons hesitate to undertake LATG, because it is thought more difficult to perform it than LADG regarding dissection of lymph nodes at the splenic hilum or along the short gastric artery and particularly, the difficulty of esophagojejunostomy. The relative difficulty of LATG depends on the acquisition of a clear view of left upper abdomen around the gastric cardia. One method for resolving this problem is to use Nathanson Liver Retractor (Narberth, PA) which keep left lateral liver lobe out of the view during the operation. In OTG, Roux-en-Y anastomosis is usually performed after esophagojejunostomy, whereas Roux-en-Y anastomosis is initially performed in LTG. The proximal and distal sides of jejunum are laparoscopically marked with a dye 30 cm distal to the ligament of the Treitz. The jejunum is then delivered outside the abdominal wall and transected under direct vision through a minilapatomy. After side-to-side Roux-en-Y anastomosis and closure of the mesentery of the jejunum by a liner stapler, the intestine is replaced to the abdominal cavity. Esophagojejunostomy should be performed by avoiding twisting of the mesentery of the small intestine. Although various methods for reconstruction and anastomosis after LATG have been reported,16,17 we perform intracorporeal circular stapling esophagojejunostomy using the OrVil system (25 mm). We think that most important point at the time of this esophagojejunostomy is to obtain approximately 10 cm length of sacrifice portion of the jejunum. Through this procedure, we can easily stretch jejunum close to the edge of the esophagus, which enabled esophagojejunostomy of LTG by the anterocolic reconstruction route. The next important point is to amputate esophagus vertically against the esophageal axis with a stapler to avoid stricture of the anastomosis. We have not undergone anastomotic stricture since we started performing this procedure. Hence, we speculate an acute angle amputation of the esophagus could be one of the cause of anastomotic stricture. By then, we experienced 4 patients with anastomotic stricture after LTG, all of these patients completely recovered by endoscopic ballooning. Finally, closing Petersen’s defect to avoid Petersen’s hernia seems essential. Actually, we have experienced 4 cases of Petersen’s hernia after LTG and all of these did not undergo closure of Petersen’s defect at the time of LTG. Since 2013, we have changed to close the defect routinely and since then, we have not experienced Petersen’s hernia.
Our study revealed that the LTG group had longer operative time and lower blood loss than did the OTG group. The number of dissected lymph nodes did not differ between the groups. As for hematologic and blood chemical profiles, serum total protein level on postoperative day 1 and day 3 were significantly higher in the LTG group and drain amylase level on postoperative day 1 and day 3 were significantly low in the LTG group as compared with the OTG group. These results suggest that LTG is less invasive surgery. The incidences of postoperative complications did not differ between the 2 groups, similar to the results of previous studies.14,15 As to oncological outcome, although median follow-up period is not enough (44 mo), Only 1 patient had recurrence after LTG up to now. This result may represent the oncological feasibility of LTG for patients with gastric cancer. Our experience revealed that LTG with intracorporeal circular stapling esophagojejunostomy using the OrVil system could be performed safely and with acceptable oncological outcome feasibility in patients with gastric cancer. However, we need to practice a large numbered randomized controlled study comparing short-term and long-term outcomes between LATG and OTG for patients with gastric cancer.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. [DOI] [PubMed] [Google Scholar]
- 3.Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 4.Kitano S, Shiraishi N, Fujii K, et al. A randomized controlled trial comparing open vs. laparoscopy-assisted distal gastrectomy for treatment of early gastric cancer: an interim report. Surgery. 2002;131:306–311. [DOI] [PubMed] [Google Scholar]
- 5.Han HS, Kim YW, Yi NJ, et al. Laparoscopy-assisted D2 subtotal gastrectomy in early gastric cancer. Surg Laparosc Endosc Percutan Tech. 2003;13:361–365. [DOI] [PubMed] [Google Scholar]
- 6.Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs. laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168–173. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Yom CK, Han HS. Comparison of long-term outcomes of laparoscopy-assisted and open distal gastrectomy for early gastric cancer. Surg Endosc. 2009;23:1759–1763. [DOI] [PubMed] [Google Scholar]
- 8.An JY, Heo GU, Cheong JH, et al. Assessment of open versus laparoscopy-assisted gastrectomy in lymph node-positive early gastric cancer: a retrospective cohort analysis. J Surg Oncol. 2010;102:77–81. [DOI] [PubMed] [Google Scholar]
- 9.Kawamura H, Homma S, Yokota R, et al. Inspection of safety and accuracy of D2 lymph node dissection in laparoscopy-assisted distal gastrectomy. World J Surg. 2008;32:2366–2370. [DOI] [PubMed] [Google Scholar]
- 10.Sakuramoto S, Kikuchi S, Futawatari N, et al. Laparoscopy-assisted pancreas- and spleen preserving total gastrectomy for gastric cancer as compared with open total gastrectomy. Surg Endosc. 2009;23:2416–2423. [DOI] [PubMed] [Google Scholar]
- 11.Huscher CG, Mingoli A, Sgarzini G, et al. Totally laparoscopic total and subtotal gastrectomy with extended lymph node dissection for early and advanced gastric cancer: early and long term results of a 100-patient series. Am J Surg. 2007;194:839–844. [DOI] [PubMed] [Google Scholar]
- 12.Mochiki E, Toyomasu Y, Ogata K, et al. Laparoscopically assisted total gastrectomy with lymph node dissection for upper and middle gastric cancer. Surg Endosc. 2008;22:1997–2002. [DOI] [PubMed] [Google Scholar]
- 13.Topal B, Leys E, Ectors N, et al. Determinants of complications and adequacy of surgical resection in laparoscopic versus open total gastrectomy for adenocarcinoma. Surg Endosc. 2008;22:980–984. [DOI] [PubMed] [Google Scholar]
- 14.Lee MS, Lee JH, Park do J, et al. Comparison of short- and long-term outcomes of laparoscopic-assisted total gastrectomy and open total gastrectomy in gastric cancer patients. Surg Endosc. 2013;27:2598–2605. [DOI] [PubMed] [Google Scholar]
- 15.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. [DOI] [PubMed] [Google Scholar]
- 16.Ziqiang W, ZhiMin C, Jun C, et al. A modified method of laparoscopic side-to-side esophagojejunal anastomosis: report of 14 cases. Surg Endosc. 2008;22:2091–2094. [DOI] [PubMed] [Google Scholar]
- 17.Bo T, Peiwu Y, Feng Q, et al. Laparoscopy-assisted vs. open total gastrectomy for advanced gastric cancer: long-term outcomes and technical aspects of a case-control study. J Gastrointest Surg. 2013;17:1202–1208. [DOI] [PubMed] [Google Scholar]


