Abstract
Purpose of review
Postoperative respiratory complications (PRCs) increase hospitalization time, 30-day mortality and costs by up to $35 000. These outcomes measures have gained prominence as bundled payments have become more common.
Recent findings
Results of recent quantitative effectiveness studies and clinical trials provide a framework that helps develop center-specific treatment guidelines, tailored to minimize the risk of PRCs. The implementation of those protocols should be guided by a local, respected, and visible facilitator who leads proper implementation while inviting center-specific input from surgeons, anesthesiologists, and other perioperative stakeholders.
Summary
Preoperatively, patients should be risk-stratified for PRCs to individualize intraoperative choices and postoperative pathways. Laparoscopic compared with open surgery improves respiratory outcomes. High-risk patients should be treated by experienced providers based on locally developed bundle-interventions to optimize intraoperative treatment and ICU bed utilization. Intraoperatively, lung-protective ventilation (procedure-specific positive end-expiratory pressure utilization, and low driving pressure) and moderately restrictive fluid therapy should be used. To achieve surgical relaxation, high-dose neuromuscular blocking agents (and reversal agents) as well as high-dose opioids should be avoided; inhaled anesthetics improve surgical conditions while protecting the lungs. Patients should be extubated in reverse Trendelenburg position. Postoperatively, continuous positive airway pressure helps prevent airway collapse and protocolized, early mobilization improves cognitive and respiratory function.
Keywords: lung-protective ventilation, postoperative respiratory complications, respiratory failure, score for prediction of postoperative respiratory complications (SPORC), upper airway, ventilator-induced lung injury
INTRODUCTION
Postoperative respiratory complications (PRCs) are common, with incidence estimates of 3–7.9% in general surgery [1,2] and higher rates reported in lung surgery [3]. The most important PRCs are reintubation, acute respiratory failure, pulmonary edema, pneumonia, and atelectasis. Measures of resource utilization have gained prominence as bundled payments have become more common [4]. PRCs increase hospitalization time, mortality, and costs [5▪,6▪,7,8]. For instance, in patients undergoing abdominal surgery, postoperative respiratory failure is associated with approximately 10-fold increased perioperative 30-day mortality [6▪]. Furthermore, postoperative reintubation, pulmonary edema, and atelectasis are predictors of adverse discharge disposition – defined as in-hospital mortality or discharge to a nursing home [5▪].
The pathological anatomy of respiratory complications can be categorized as respiratory muscle dysfunction or as disease of the airway itself. Functionally, we can subdivide PRCs into either upper airway-related [e.g., reintubation of an obstructive sleep apnea (OSA) patient, Fig. 1, upper pink box] or pulmonary (such as pulmonary edema, Fig. 1, lower blue box) [9,10].
FIGURE 1.
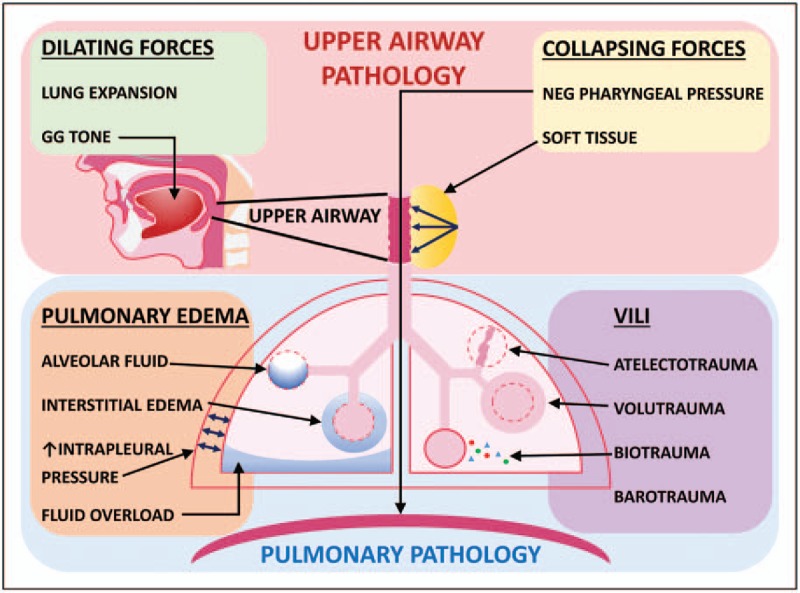
Upper airway and pulmonary disorders. Upper airway disorders are given in the pink box. Dilating forces (green box) include increased lung expansion and increased upper airway dilator muscle tone (genioglossus muscle shown). Collapsing forces (yellow box) include increased negative pharyngeal pressure generated by respiratory pump muscles (diaphragm shown), and increased soft tissue causing external mechanical load on the upper airway (yellow mass with arrows next to upper airway). Pulmonary disorders are given in the blue box. Pulmonary edema (orange box) with interstitial fluid (alveolus with surrounding fluid), alveolar fluid (blue alveolus), or both, can be caused by increased negative pulmonary pressure (blue arrows), fluid overload (blue base of lung), or multiple causes of interstitial edema. Ventilator-induced lung injury (purple box) can be due to barotrauma, atelectotrauma (deflated alveolus), biotrauma (multicolored dots), or volutrauma (distended alveolus). GG, genioglossus muscle; UA, upper airway; VILI, ventilator-induced lung injury.
The upper airway is an anatomical region between the soft palate, epiglottis, genioglossus muscle, and soft tissue anterior to the spinal column (Fig. 1), and its collapse leads to desaturation, atelectasis, and respiratory failure. Competing dilating and collapsing forces determine the patency of the upper airway [11,12] (Fig. 1, upper green and yellow boxes). Dilating forces include pharyngeal dilator muscles (e.g., the genioglossus and tensor palatini muscles) and caudal traction on the airway from expansion of the lungs [improved with increased positive end-expiratory pressure (PEEP) that increases end-expiratory lung volume]. Perioperatively, many factors such as sedatives, opioids, or hypoactive delirium can decrease airway dilator muscle tone. Dilating forces also decrease when caudal traction on the trachea is decreased (e.g., with atelectasis or supine position). Collapsing forces include external pressure from surrounding soft tissue (increased with pharyngeal airway edema, excess fat, hematoma, tumors, or supine position) and negative intraluminal pressure created by respiratory pump muscles (primarily the diaphragm and intercostal muscles). Significant postoperative pulmonary edema occurs in 1–2% of surgical patients undergoing general anesthesia [5▪]. Causes of noncardiogenic pulmonary edema include negative pressure pulmonary edema (NPPE; likely the most frequent postoperative mechanism), fluid maldistribution secondary to overload, and less frequently, neurogenic edema (associated with acute hypertension in brain trauma/surgery patients) and anaphylaxis.
Pulmonary edema can lead to unanticipated inpatient stays, the need for ICU admission, reintubation, and increased costs [9,10]. Disturbances of pulmonary fluid homeostasis can be induced by four pathways leading to increased interstitial fluid: increased hydrostatic pressure in the pulmonary capillary bed (or decreased pressure in the interstitium), decreased osmotic pressure of plasma, increased permeability of capillary membranes, or decreased return of fluid to the circulation via lymphatics. Acute upper airway collapse or laryngo/bronchospasm can cause NPPE secondary to high negative pulmonary pressures generated against a closed airway (Fig. 1, lower orange box). Atelectasis is the most frequent mechanism of perioperative desaturation and occurs minutes after induction of general anesthesia due to reduction of regional transpulmonary pressure in dependent areas [13]. Intraoperatively, this is accentuated by inflammation triggered by surgical incision and bacterial translocation, chest wall restriction, cephalad displacement of the diaphragm by surgical retractors, and supine position. Postoperatively, a restrictive lung pattern secondary to diaphragmatic dysfunction is observed, compromising respiratory mechanics and gas exchange.
The injurious effects of perioperative atelectasis are magnified by pain, high driving pressure, and inflammation. Accordingly, the anesthetic and surgical perioperative insults can create conditions of ‘multiple-hit’ lung injury, which can be further augmented by the tissue stress induced by intraoperative mechanical ventilation [14]. Even without excessive ventilator pressures (barotrauma), ventilator-induced lung injury has multiple causes (Fig. 1, lower purple box). Decreased compliance in derecruited areas causes overinflation of aerated lung tissue in nondependent areas with larger transpulmonary pressure (volutrauma). Cyclic lung derecruitment causes low-volume lung injury (atelectotrauma). Release of local proinflammatory mediators from biophysical forces and systemic inflammation triggered by surgical incision, tissue manipulation, bacterial translocation, and endotoxemia also contribute to lung injury (biotrauma) [14]. Thus, intraoperative optimization of ventilation with a maximally protective strategy is vital. We discuss below multilevel strategies that help maintain patency of the upper airway when it is unprotected and ventilation strategies that minimize lung trauma.
Preoperative screening and measures for prevention of postoperative respiratory complications
Preoperatively, patients should be screened to identify those at high risk for postoperative respiratory failure so that resources and management can be appropriately selected for these patients. Amongst surgical patients, many do not see an anesthesiologist prior to the day of surgery and instead are screened preoperatively via phone to generate or update the electronic healthcare record. Given the strong association between PRCs and various comorbid diseases, it may be fruitful to utilize readily available data on demographics and comorbidities to make predictions regarding respiratory complication risk. One such tool is the score for prediction of postoperative respiratory complications (SPORC), illustrated in Fig. 2[15].
FIGURE 2.
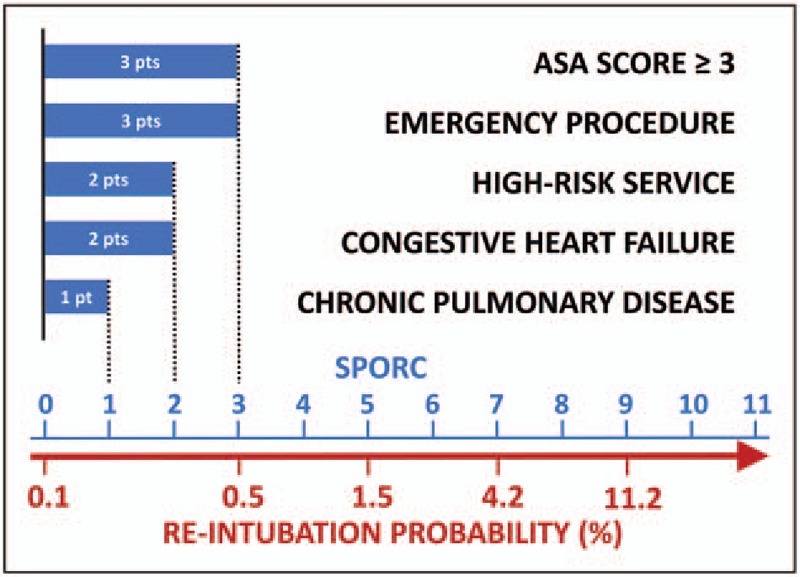
How to implement the SPORC. Point values (pts, shown as blue bars) are shown for the prediction factors: American Society of Anesthesiologists (ASA) score greater than or equal to three (three points), emergency procedure (three points), referring high-risk service (two points), history of congestive heart failure (two points), and chronic pulmonary disease (one point). The points for each risk factor are summed to reach a final SPORC score. The corresponding probability for reintubation is given on the red scale below the row of SPORC values. Reproduced with permission from [15]. SPORC, score for prediction of postoperative respiratory complications.
Please note that there are several risk factors not included in the SPORC, which are important but require a patient–provider interaction, such as wheezing, and clinical signs and symptoms of fluid overload. Current smokers have increased mortality risk following major surgery [16]. Smoking cessation at least 1 year prior to surgery abolishes the higher postoperative mortality risk and decreases the respiratory event rate [17].
Sarcopenia identified early after admission is a predictor of postoperative pulmonary complications and adverse discharge disposition [18▪,19▪▪]. Although preoperative lung rehabilitation improved the forced expiratory volume in one second in marginal patients undergoing lung cancer resection, there was no reduction in postoperative mortality, morbidity, or length of stay [20].
Box 1.
no caption available
Intraoperative
As OSA is an independent risk factor for difficult mask ventilation during induction of anesthesia [21], optimizing airway management with upright posture may be prudent in some patients in the preinduction stage to optimize mask ventilation, during anesthesia with an unprotected airway, postextubation, and immediately post partum. A bundle intervention for intraoperative care of patients with OSA has been recommended by Dr Shiroh Isono, Chiba, Japan and others [22▪].
After induction of general endotracheal anesthesia (GETA), lung-protective mechanical ventilation seeks to avoid derecruitment without overdistension of alveoli by keeping transpulmonary pressures such that the lung tissue stays in the linear part of its local pressure–volume curve. This strategy has gained widespread acceptance in ICUs following large studies revealing that it decreases morbidity and mortality in the setting of acute lung injury [23,24]. Clinicians should aim to achieve low transpulmonary pressures (typically expressed as driving pressures) and should choose procedure-specific PEEP. In patients undergoing GETA, protective ventilation was associated with decreased PRCs, with PEEP at least 5 cmH2O and a median plateau pressure of 16 cmH2O or less having the lowest risk of PRCs [25▪▪]. Protective effects of PEEP seem to be procedure-specific, as one study found that PEEP of at least 5 cmH2O in major abdominal surgery lowers odds of PRCs, while the same PEEP was not associated with significant effects on PRCs in craniotomy patients [26▪▪]. In addition, patients with poor chest wall compliance need higher levels of PEEP, and optimal aeration may require a recruitment maneuver [27▪].
Although high FiO2 may improve tissue oxygenation, it can impair pulmonary function. High FiO2 (mean of 0.79) compared with low FiO2 (mean of 0.31) was associated with significant, dose-dependent increases in rates of PRCs, and 30-day mortality [28▪].
Increased average minimum alveolar concentration of inhalational anesthetics (volatile anesthetics and nitrous oxide) is dose-dependently associated with a lower 30-day mortality, lower hospital cost, and lower risk of PRCs. This finding was robust to sensitivity analysis of multiple subgroups, but interestingly, was not found to be present in patients with the longest periods of hypotension (≥11 min with mean arterial pressure <55 mmHg) [29▪▪].
The use of intermediate-acting nondepolarizing neuromuscular blocking agents (NMBAs) is associated with an increased risk of postoperative desaturation to SaO2 less than 90% after extubation and reintubation requiring unplanned admission to an ICU [30]. These same outcomes are increased with the use of high-dose neostigmine, especially if neuromuscular transmission monitoring is not applied [30]. In another study, use of NMBAs and their reversal with neostigmine were also both found to dose-dependently increase the risk of respiratory complications. However, use of proper neostigmine reversal guided by neuromuscular transmission monitoring, defined as use of 60 μg/kg or less after recovery of train-of-four count of two, was associated with elimination of increased risk of PRCs [31▪▪]. Postoperative residual paralysis, defined as train-of-four T4/T1 less than 0.9, is associated with increased morbidity, delayed postanesthesia care unit (PACU) discharge by 60 min, and prolonged hospital length of stay [32–34]. Sugammadex administration decreases the incidence of postoperative residual paralysis compared with neostigmine [35▪]. However, even with sugammadex, residual neuromuscular blockade as high as 9.4% is possible when neuromuscular monitoring is not used [36].
Fluid administration impacts the course of patients. Both liberal and the most restrictive fluid resuscitation strategies have been associated with respiratory complications (pulmonary edema or increased deadspace during hypovolemia, respectively), whereas moderately restrictive fluid volumes are consistently associated with optimal postoperative outcomes [37▪▪,38,39▪]. Some data suggest that very high doses of opioids administered during surgery are associated with increased 30-day readmission rate [40].
Compared with anesthesia without an epidural or spinal, neuraxial blockade may reduce postoperative morbidity and mortality in disease-entity-based subcohorts [41]. Despite the effects of local anesthetics on motor function and sympathetic innervation, epidural anesthesia improves postoperative lung function and reduces PRCs [42]. A laparoscopic surgical approach may also have beneficial effects on postoperative respiratory outcomes compared with open surgery [43▪▪]. Different intraoperative factors that can be modulated to decrease PRCs are described in Table 1.
Table 1.
Strategies to minimize postoperative respiratory complications
| Intraoperative considerations | |||||
| Factor | Improved outcome | Favorable strategy | Respiratory complication | Study cohort | Reference |
| Ventilation | |||||
| Protective lung ventilation | Major PRC, hospital length of stay | PEEP ≥ 5 cmH2O, median tidal volume ≤10 ml/kg of predicted body weight, median plateau pressure ≤30 cmH2O | Pulmonary edema, respiratory failure, pneumonia, and reintubation | Noncardiac surgery with endotracheal intubation; major abdominal surgery | Ladha et al. [25▪▪], de Jong et al. [26▪▪] |
| Oxygen toxicity | Major PRC, mortality, and ICU admission | Low intraoperative inspiratory oxygen fraction (mean of 0.31) | Respiratory failure, reintubation, pulmonary edema, and pneumonia | Noncardiac surgery | Staehr-Rye et al. [28▪] |
| Recruitment maneuvers and PEEP titration | Lung volumes, respiratory system elastance, and oxygenation | A recruitment maneuver followed by end-expiratory pressure titration | – | Critically ill, mechanically ventilated, morbidly obese (BMI > 35) patients | Pirrone et al. [27▪] |
| Surgical factors | |||||
| Laparoscopic vs. open surgery | PRC | Laparoscopic surgical approach | Pleural effusion, respiratory insufficiency, ARDS, pulmonary infection, and pulmonary embolism | Major hepatectomy surgery | Fuks et al. [43▪▪] |
| Anesthetic factors | |||||
| Fluid administration | Length of stay, costs, postoperative ileus, pneumonia, major PRC, 30-day mortality and renal complications | Moderate/goal-directed fluid administration | Respiratory failure, reintubation, pulmonary edema, and pneumonia | In patients undergoing colon, rectal, hip, or knee surgery; 12 liberal fluid therapy RCTs; noncardiac surgery | Shin et al. [37▪▪]; Thacker et al. [39▪]; Corcoran et al. [38] |
| Dose of NMBAs and neostigmine | PRC | Low-dose use of NMBAs, proper neostigmine reversal (≤60 μg/kg after recovery of train-of-four count of 2) | Respiratory failure, reintubation, pulmonary edema, and pneumonia | Noncardiac surgery with NMBA use | McLean et al. [31▪▪] |
| Use of NMBA, and neostigmine | Oxygen desaturation and reintubation | No use of intermediate-acting NMBA and neostigmine | SpO2 < 90% with a decrease in oxygen saturation after extubation of >3%; reintubation | Noncardiac surgery | Grosse-Sundrup et al. [30] |
| Dose of inhalational anesthetics | Major PRC, mortality, hospital length of stay, costs | High-dose inhalational anesthetic | Respiratory failure, reintubation, pulmonary edema, and pneumonia | Noncardiac surgery with inhalational anesthetic use | Grabitz et al. [29▪▪] |
| Neuraxial anesthesia | Morbidity and mortality | Use of neuraxial blockade with epidural or spinal anesthesia | Pulmonary embolism, pneumonia, and respiratory depression | Randomized surgical cases with or without neuraxial anesthesia | Rodgers et al. [41] |
| Dose of opioids | 30-day readmission | Low-dose intraoperative opioid | Respiratory failure, reintubation, pulmonary edema, and pneumonia | Noncardiac surgery | Grabitz et al. [40] |
| Postoperative considerations | |||||
| Admission to ICU | Hospital length of stay, PRC, and costs | Optimal decision of postoperative ICU vs. ward admission | Respiratory failure, reintubation, pulmonary edema, and pneumonia | Noncardiac and nontransplant surgery | Thevathasan et al. [44▪] |
| Monitoring on surgical floor | Rescue events and transfers to ICU | Appropriate postoperative monitoring (e.g., pulse oximetry) | – | Orthopedic surgery | Taenzer et al. [45] |
| Postoperative analgesia | Opioid-induced respiratory depression | Opioid-sparing analgesia | Respiratory depression | Surgical patients with acute pain | Lee et al. [46▪▪] |
| CPAP | AHI, oxygen desaturations, mean oxygen saturation, and opioid-induced respiratory depression | CPAP treatment in postanesthesia care unit | Apnea–hypopnea index and oxygen desaturation | Bariatric surgery | Zaremba et al. [47▪▪] |
| Upright positioning | Pharyngeal collapsibility | Postural change from supine to sitting | – | Patients with OSA | Tagaito et al. [48] |
| Fowler's position | Apnea–hypopnea index, oxygen saturation <90% | Elevated body position | Apnea–hypopnea index, oxygen saturation <90% | OB, postdelivery | Zaremba et al. [49▪] |
| Avoid reintubation in surgical ICU patients | Reintubation | Avoid elevated blood urea nitrogen, low hemoglobin, and muscle weakness in SICU patients | Reintubation | Surgical ICU patients (noncardiac) | Piriyapatsom et al. [50▪▪], Farhan et al. [51] |
| Early mobilization in the ICU | Length of stay in the ICU, functional mobility at hospital discharge | Early, goal-directed mobilization using an interprofessional approach of closed-loop communication and SOMS algorithm | – | ICU patients, mechanically ventilated (<48 h; expected to require ≥24 h) | Schaller et al. [53▪▪] |
ARDS, acute respiratory distress syndrome; CPAP, continuous positive airway pressure; NMBA, neuromuscular blocking agent; PEEP, positive end-expiratory pressure; PRC, postoperative respiratory complication; RCT, randomized controlled trial.
Postoperative
In OSA, the upper airway is more collapsible [52], and supine positioning promotes upper airway collapse and doubles the AHI index compared with lateral position [54]. Likewise, in anesthetized, paralyzed patients with OSA, sitting position significantly improves the cross-sectional area of the retropalatal and retroglossal airways and decreases the closing pressure (indicating a more patent airway) compared with supine position [48]. In addition, neck extension in anesthetized patients with sleep-disordered breathing increases maximal oropharyngeal airway size and decreases closing pressures of the airway (indicating a more patent airway), whereas neck flexion with bite opening has the opposite effect [55]. Comparing sniffing position (neck flexion and upper cervical extension) with a neutral airway in anesthetized OSA patients, sniffing position increases airway cross-sectional area and decreases closing pressure (more open airway) [56].
Interestingly, sleep apnea is much more common in pregnant than nonpregnant women and persists into the early post-partum period. Post-partum airway obstruction is a major cause of anesthesia-related maternal death [57]. Consistent with findings in OSA, upper body elevation to 45 degrees increases the cross-sectional area of the upper airway as measured by acoustical pharyngometry and mitigates sleep apnea as measured by polysomnography in women 48 h after delivery [49▪]. It is prudent to extubate patients at risk of extubation failure in the reverse Trendelenburg position to decrease airway collapse after removal of the endotracheal tube [58].
Prolonged postoperative hypoxemia can occur during recovery from surgery [59▪]. Patients receiving opioids for postoperative analgesia are particularly at risk of experiencing desaturation due to opioid-induced respiratory depression [46▪▪]. A pharmacophysiologic interaction trial showed that continuous positive airway pressure compared with atmospheric pressure applied through an oronasal mask improved sleep-disordered breathing and ameliorated the respiratory-depressant effects of opioids postoperatively [47▪▪].
Furthermore, proper monitoring in the PACU can decrease the number of ICU transfers and improves patients’ outcomes by detecting early signs of respiratory complications [45]. Of note, it is important to use objective criteria to determine whether or not a patient needs to be admitted to an ICU. A recent study demonstrates that admission to the ICU rather than ward admission is associated with adverse outcomes in healthier patients [44▪]. Admission to the ICU strains the health system by increasing resource utilization and costs of hospital care. Furthermore, patients surviving an ICU stay subsequently demonstrate a higher risk of 5-year mortality [60].
Sarcopenia, frailty, and muscle strength are independent predictors of adverse discharge disposition and reintubation in surgical ICU patients [19▪▪,50▪▪,51]. Postoperatively, early mobilization improves functional independence. Our data demonstrate that early, goal-directed mobilization shortens patients’ length of stay in the SICU and improves functional mobility at hospital discharge [53▪▪]. Postoperative factors that can be modulated to decrease PRCs are described in Table 1.
Act locally: development and implementation of center-specific guidelines
Guideline-driven clinical decision pathways are efficiently encapsulated by algorithms. Those algorithms and performance improvement measures developed by multidisciplinary teams on a local hospital or departmental level are likely to be the most successful [61].
In general, the following steps are followed: plans are created to implement improvement throughout the system and effectiveness is continually monitored and changes are made as needed. As clinicians, there is often divergence between what we know from reading current literature and what we practice. This ‘knowing-doing gap’ is what implementation science seeks to overcome. We have recently shown that proper implementation of a clinical algorithm improves important patient outcomes. Of utter importance is the selection of a locally respected ‘facilitator’ who makes sure that all voices are heard and that plans made on the basis of algorithms are properly implemented [53▪▪]. In this way, a nuanced subject matter can be made into algorithmic, usable hospital bundles by a local multidisciplinary team (Fig. 3).
FIGURE 3.
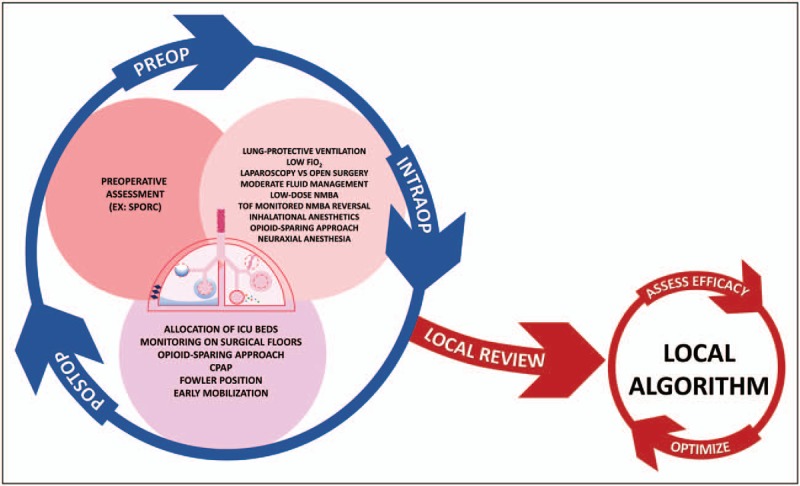
Review of literature and guidelines for creation of a locally implemented algorithm. Clinicians must think globally (blue circle with arrows) about the myriad preoperative (dark pink circle), intraoperative (light pink circle), and postoperative (light purple circle) factors that can potentially decrease postoperative respiratory complications. Review of this complex, global view by a local, respected, multidisciplinary team (red ‘local review’ arrow) can lead to the creation of a more easily and systematically implemented local algorithm that creates actionable hospital bundles (red circle). This local algorithm needs ongoing evaluation of efficacy, which should trigger optimization of the local algorithm (red arrows surrounding algorithm circle).
CONCLUSION
More than a 10-fold increase in mortality risk is incurred by severe PRCs leading to reintubation and unplanned ICU admission [5▪]. Thus, preoperative risk stratification to identify patients at increased risk of PRCs and subsequent tailoring of an anesthetic plan with respiratory optimization is warranted. Surgery type affects PRCs, and laparoscopic surgery improves respiratory outcomes. Intraoperative use of appropriately restrained fluid management, increased utilization of inhaled anesthetics (while not allowing hypotension), judicious and careful titration of NMBAs with appropriately monitored reversal, minimization of opioids, and lung-protective ventilation should be used. Postoperatively, patients at high risk of PRCs should be extubated in the reverse Trendelenburg position. Obese patients and those with OSA should be treated with an ‘OSA hospital bundle’ [22▪,52,58]. PACU discharge disposition should incorporate patient risk stratification for PRCs as a key element of respiratory safety. Local guidelines need to be developed to optimize patients’ triage. Early, goal-directed mobilization should be implemented. The creation of local algorithms and hospital bundles will facilitate implementation of these suggested evidence-based practices to improve patient outcomes.
Acknowledgements
We would like to thank Jeff and Judy Buzen for their support.
Financial support and sponsorship
The work was supported by an unrestricted grant from Jeff and Judy Buzen to Dr. Eikermann.
Conflicts of interest
Within the past 2 years, M.E. received investigator-initiated research funding from Merck and Jeff and Judy Buzen, Massachusetts, USA. He has equity stakes at Calabash Bioscience Inc. There are no other relationships or activities that could appear to have influenced the submitted work.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Mazo V, Sabaté S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology 2014; 121:219–231. [DOI] [PubMed] [Google Scholar]
- 2.Johnson RG, Arozullah AM, Neumayer L, et al. Multivariable predictors of postoperative respiratory failure after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg 2007; 204:1188–1198. [DOI] [PubMed] [Google Scholar]
- 3.Kim ES, Kim YT, Kang CH, et al. Prevalence of and risk factors for postoperative pulmonary complications after lung cancer surgery in patients with early-stage COPD. Int J Chron Obstruct Pulmon Dis 2016; 11:1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellimoottil C, Ryan AM, Hou H, et al. Medicare's new bundled payment for joint replacement may penalize hospitals that treat medically complex patients. Health Aff 2016; 35:1651–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5▪.Sonny A, Grabitz SD, Timm FP, et al. Impact of postoperative respiratory complications on discharge disposition, mortality, and re-admissions. ASA Abstr 2016. A5016. [Google Scholar]; Abstract evaluating the independent impact of postoperative reintubation, pneumonia, pulmonary edema, respiratory failure, postoperative desaturation, and atelectasis on patient-centered adverse outcomes.
- 6▪.Kim M, Brady JE, Li G. Interaction effects of acute kidney injury, acute respiratory failure, and sepsis on 30-day postoperative mortality in patients undergoing high-risk intraabdominal general surgical procedures. Anesth Analg 2015; 121:1536–1546. [DOI] [PubMed] [Google Scholar]; Observational cohort study highlighting the independent and additive association of AKI, sepsis, and acute respiratory failure on patient mortality.
- 7.Bailey JG, Davis PJB, Levy AR, et al. The impact of adverse events on healthcare costs for older adults undergoing nonelective abdominal surgery. Can J Surg 2016; 59:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg 2004; 199:531–537. [DOI] [PubMed] [Google Scholar]
- 9.Krodel DJ, Bittner EA, Abdulnour R, et al. Case scenario: acute postoperative negative pressure pulmonary edema. Anesthesiology 2010; 113:200–207. [DOI] [PubMed] [Google Scholar]
- 10.Krodel DJ, Bittner EA, et al. Negative pressure pulmonary edema following bronchospasm. Chest 2011; 140:1351–1354. [DOI] [PubMed] [Google Scholar]
- 11.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 2005; 172:1363–1370. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki N, Meyer MJ, Eikermann M. Postoperative respiratory muscle dysfunction pathophysiology and preventive strategies. Anesthesiology 2013; 118:961–978. [DOI] [PubMed] [Google Scholar]
- 13.Gunnarsson L, Tokics L, Gustavsson H, Hedenstierna G. Influence of age on atelectasis formation and gas exchange impairment during general anaesthesia. Br J Anaesth 1991; 66:423–432. [DOI] [PubMed] [Google Scholar]
- 14.Melo MFV, Eikermann M. Protect the lungs during abdominal surgery it may change the postoperative outcome. Anesthesiology 2013; 118:1254–1257. [DOI] [PubMed] [Google Scholar]
- 15.Brueckmann B, Villa-Uribe JL, Bateman BT, et al. Development and validation of a score for prediction of postoperative respiratory complications. Anesthesiology 2013; 118:1276–1285. [DOI] [PubMed] [Google Scholar]
- 16.Musallam KM, Rosendaal FR, Zaatari G, et al. Smoking and the risk of mortality and vascular and respiratory events in patients undergoing major surgery. JAMA Surg 2013; 148:755–762. [DOI] [PubMed] [Google Scholar]
- 17.Mason DP, Subramanian S, Nowicki ER, et al. Impact of smoking cessation before resection of lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database study. Ann Thorac Surg 2009; 88:362–370. Discussion 70–71. [DOI] [PubMed] [Google Scholar]
- 18▪.Makiura D, Ono R, Inoue J, et al. Preoperative sarcopenia is a predictor of postoperative pulmonary complications in esophageal cancer following esophagectomy: a retrospective cohort study. J Geriatr Oncol 2016; 7:430–436. [DOI] [PubMed] [Google Scholar]; Retrospective cohort study demonstrating the utility of sarcopenia assessment to predict postoperative respiratory complications (PRCs). Sarcopenia was assessed using metrics such as low muscle mass and low physical function.
- 19▪▪.Mueller N, Murthy S, Tainter CR, et al. Can sarcopenia quantified by ultrasound of the rectus femoris muscle predict adverse outcome of surgical intensive care unit patients as well as frailty? A prospective, observational cohort study. Ann Surg 2016; 264:1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]; A prospective, observational cohort study revealing that sarcopenia diagnosed by bedside ultrasound can predict adverse discharge disposition (discharge to a nursing home or in-house mortality) as well as frailty in the surgical ICU setting. As novice operators were able to accurately perform the measurements, this modality could be utilized as a novel predictive biomarker.
- 20.Hashmi A, Baciewicz FA, Jr, Soubani AO, Gadgeel SM. Preoperative pulmonary rehabilitation for marginal-function lung cancer patients. Asian Cardiovasc Thorac Ann 2017; 25:47–51. [DOI] [PubMed] [Google Scholar]
- 21.Kheterpal S, Martin L, Shanks AM, Tremper KK. Prediction and outcomes of impossible mask ventilation: a review of 50,000 anesthetics. Anesthesiology 2009; 110:891–897. [DOI] [PubMed] [Google Scholar]
- 22▪.Zaremba S, Mojica JE, Eikermann M. Perioperative sleep apnea: a real problem or did we invent a new disease? F1000Res 2016; 5: [DOI] [PMC free article] [PubMed] [Google Scholar]; Review that emphasizes implementation of a clinical algorithm to provide optimal care of surgical patients with obstructive sleep apnea.
- 23.Amato MBP, Barbas CSV, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998; 338:347–354. [DOI] [PubMed] [Google Scholar]
- 24.[No authors listed]. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000; 342:1301–1308. [DOI] [PubMed] [Google Scholar]
- 25▪▪.Ladha K, Vidal Melo MF, McLean DJ, et al. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. BMJ 2015; 351:h3646. [DOI] [PMC free article] [PubMed] [Google Scholar]; Prospectively designed observational outcomes study showing an association of decreased rates of pulmonary edema, respiratory failure, pneumonia, and reintubation with the use of lung protective mechanical ventilation [defined as a positive end-expiratory pressure (PEEP) of 5 cmH2O and plateau pressure of 16 cmH2O or less].
- 26▪▪.de Jong MAC, Ladha KS, Melo MFV, et al. Differential effects of intraoperative positive end-expiratory pressure (PEEP) on respiratory outcome in major abdominal surgery versus craniotomy. Ann Surg 2016; 264:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]; Observational study suggesting that PEEP at least 5 cmH2O should be used during major abdominal surgery as this range of settings decreased the risk of PRCs. In craniotomy patients, PEEP did not have the same protective benefit.
- 27▪.Pirrone M, Fisher D, Chipman D, et al. Recruitment maneuvers and positive end-expiratory pressure titration in morbidly obese ICU patients. Crit Care Med 2016; 44:300–307. [DOI] [PubMed] [Google Scholar]; Prospective, crossover, nonrandomized interventional study in medical and surgical ICUs showing that a recruitment maneuver followed by PEEP titration (in the range of ∼12 cmH2O) can improve respiratory mechanics in morbidly obese patients.
- 28▪.Staehr-Rye AK, Meyhoff CS, Rasmussen LS, et al. Does high intra-operative inspiratory oxygen fraction lead to postoperative respiratory complications? ASA Abstr 2015. A2059. [Google Scholar]; Abstract reporting that high intraoperative inspiratory oxygen fraction is dose-dependently associated with major respiratory complications and 30-day mortality.
- 29▪▪.Grabitz SD, Farhan HN, Ruscic KJ, et al. Dose-dependent protective effect of inhalational anesthetics against postoperative respiratory complications: a prospective analysis of data on file from three hospitals in New England. Crit Care Med 2017; 45:e30–e39. [DOI] [PubMed] [Google Scholar]; Prospectively designed outcomes study demonstrating the dose-dependent protective effect of inhalational anesthetics (volatile anesthetics and nitrous oxide) on development of PRCs.
- 30.Grosse-Sundrup M, Henneman JP, Sandberg WS, et al. Intermediate acting nondepolarizing neuromuscular blocking agents and risk of postoperative respiratory complications: prospective propensity score matched cohort study. BMJ 2012; 345:e6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31▪▪.McLean DJ, Diaz-Gil D, Farhan HN, et al. Dose-dependent association between intermediate-acting neuromuscular-blocking agents and postoperative respiratory complications. Anesthesiology 2015; 122:1201–1213. [DOI] [PubMed] [Google Scholar]; Prospectively designed observational outcomes study showing a dose-dependent increased risk of PRCs associated with the use of neuromuscular blocking agents as well as neostigmine, but suggests elimination of that risk with proper use of neostigmine reversal using neuromuscular transmission monitoring.
- 32.Berg H, Roed J, Viby-Mogensen J, et al. Residual neuromuscular block is a risk factor for postoperative pulmonary complications. A prospective, randomised, and blinded study of PRCs after atracurium, vecuronium and pancuronium. Acta Anaesthesiol Scand 1997; 41:1095–1103. [DOI] [PubMed] [Google Scholar]
- 33.Butterly A, Bittner EA, George E, et al. Postoperative residual curarization from intermediate-acting neuromuscular blocking agents delays recovery room discharge. Br J Anaesth 2010; 105:304–309. [DOI] [PubMed] [Google Scholar]
- 34.Staehr-Rye AK, Grabitz SD, Theathasan T, et al. Effects of residual paralysis on postoperative pulmonary function and hospital length of stay. ASA Abstr 2015. A3038. [Google Scholar]
- 35▪.Brueckmann B, Sasaki N, Grobara P, et al. Effects of sugammadex on incidence of postoperative residual neuromuscular blockade: a randomized, controlled study. Br J Anaesth 2015; 115:743–751. [DOI] [PubMed] [Google Scholar]; A randomized, controlled study showing that sugammadex reduces residual neuromuscular blockade and shortens the time from drug administration to operating room discharge.
- 36.Kotake Y, Ochiai R, Suzuki T, et al. Reversal with sugammadex in the absence of monitoring did not preclude residual neuromuscular block. Anesth Analg 2013; 117:345–351. [DOI] [PubMed] [Google Scholar]
- 37▪▪.Shin CH, Long DR, McLean D, et al. Effects of intraoperative fluid management on postoperative outcomes a hospital registry study. Ann Surg 2017; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; A hospital registry study demonstrating an association of intraoperative fluid dosing at the liberal and restrictive margins of observed practice and increased morbidity, mortality, cost, and length of stay.
- 38.Corcoran T, Rhodes JE, Clarke S, et al. Perioperative fluid management strategies in major surgery: a stratified meta-analysis. Anesth Analg 2012; 114:640–651. [DOI] [PubMed] [Google Scholar]
- 39▪.Thacker JK, Mountford WK, Ernst FR, et al. Perioperative fluid utilization variability and association with outcomes: considerations for enhanced recovery efforts in sample US surgical populations. Ann Surg 2016; 263:502–510. [DOI] [PubMed] [Google Scholar]; Outcomes study showing the association of increased hospital length of stay and total cost with high fluid volume given on the day of surgery.
- 40.Grabitz SD, Lihn AL, Burns S, et al. Effects of intraoperative administration of opioids on inpatient readmission: a prospective analysis of data on file. ASA Abstr 2016. A1220. [Google Scholar]
- 41.Rodgers A, Walker N, Schug S, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ 2000; 321:1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Groeben H. Epidural anesthesia and pulmonary function. J Anesth 2006; 20:290–299. [DOI] [PubMed] [Google Scholar]
- 43▪▪.Fuks D, Cauchy F, Fteriche S, et al. Laparoscopy decreases pulmonary complications in patients undergoing major liver resection: a propensity score analysis. Ann Surg 2016; 263:353–361. [DOI] [PubMed] [Google Scholar]; Retrospective, multi-institutional outcomes study showing that laparoscopic liver resection is associated with decreased postoperative pulmonary complications compared with open major hepatectomy.
- 44▪.Thevathasan T, Copeland CC, Lihn A-L, et al. Consequences of postoperative intensive care unit admission: a propensity score matched cohort study. ASA Abstr 2016. A1090. [Google Scholar]; Abstract presentation of an outcomes study showing that appropriate, well selected postoperative discharge to an ICU vs. floor admission improves multiple patient outcomes, including PRCs.
- 45.Taenzer AH, Pyke JB, McGrath SP, Blike GT. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology 2010; 112:282–287. [DOI] [PubMed] [Google Scholar]
- 46▪▪.Lee LA, Caplan RA, Stephens LS, et al. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology 2015; 122:659–665. [DOI] [PubMed] [Google Scholar]; Data from this closed-claims analysis demonstrate that opioid-related respiratory depression is multifactural and likely preventable by assessment, monitoring, and early intervention.
- 47▪▪.Zaremba S, Shin CH, Hutter MM, et al. Continuous positive airway pressure mitigates opioid-induced worsening of sleep-disordered breathing early after bariatric surgery. Anesthesiology 2016; 125:92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]; A randomized crossover trial showing that postoperative oxygenation and opioid-induced respiratory depression can be decreased by supervised continuous positive airway pressure treatment early after bariatric surgery.
- 48.Tagaito Y, Isono S, Tanaka A, et al. Sitting posture decreases collapsibility of the passive pharynx in anesthetized paralyzed patients with obstructive sleep apnea. Anesthesiology 2010; 113:812–818. [DOI] [PubMed] [Google Scholar]
- 49▪.Zaremba S, Mueller N, Heisig AM, et al. Elevated upper body position improves pregnancy-related OSA without impairing sleep quality or sleep architecture early after delivery. Chest 2015; 148:936–944. [DOI] [PubMed] [Google Scholar]; Randomized, crossover study demonstrating the beneficial effects of elevated upper body position on sleep-disordered breathing and upper airway cross-sectional area in early-post-partum women.
- 50▪▪.Piriyapatsom A, Williams EC, Waak K, et al. Prospective observational study of predictors of re-intubation following extubation in the surgical ICU. Respir Care 2016; 61:306–315. [DOI] [PubMed] [Google Scholar]; Prospective observational study in noncardiac, surgical ICU patients showing that elevated blood urea nitrogen, low hemoglobin, and muscle weakness are independent risk factors for reintubation.
- 51.Farhan H, Moreno-Duarte I, Latronico N. Acquired muscle weakness in the surgical care unit: nosology, epidemiology, diagnosis, and prevention. Anesthesiology 2016; 124:207–234. [DOI] [PubMed] [Google Scholar]
- 52.Isono S, Remmers JE, Tanaka A, et al. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol 1997; 82:1319–1326. [DOI] [PubMed] [Google Scholar]
- 53▪▪.Schaller SJ, Anstey M, Blobner M, et al. Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet 2016; 388:1377–1388. [DOI] [PubMed] [Google Scholar]; Multicentric, international, randomized controlled trial showing beneficial effects of early, goal-directed mobilization, and the importance of closed-loop communication in surgical ICU patients.
- 54.Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep 1984; 07:110–114. [DOI] [PubMed] [Google Scholar]
- 55.Isono S, Tanaka A, Tagaito Y, et al. Influences of head positions and bite opening on collapsibility of the passive pharynx. J Appl Physiol 2004; 97:339–346. [DOI] [PubMed] [Google Scholar]
- 56.Isono MDS, Tanaka MDA, Ishikawa MDT, et al. Sniffing position improves pharyngeal airway patency in anesthetized patients with obstructive sleep apnea. Anesthesiology 2005; 103:489–494. [DOI] [PubMed] [Google Scholar]
- 57.Berg CJ, Chang J, Callaghan WM, Whitehead SJ. Pregnancy-related mortality in the United States, 1991–1997. Obstet Gynecol 2003; 101:289–296. [DOI] [PubMed] [Google Scholar]
- 58.Zaremba S, Chamberlin NL, Eikermann M. Miller RD. Chapter 14: sleep medicine. Miller's anesthesia 8th ed.Philadelphia, PA: Saunders; 2014. 303–28e7. [Google Scholar]
- 59▪.Sun Z, Sessler DI, Dalton JE, et al. Postoperative hypoxemia is common and persistent: a prospective blinded observational study. Anesth Analg 2015; 121:709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study found that hypoxemia, including prolonged hypoxemia, was relatively common in postoperative, hospitalized patients. The degree of hypoxemia is often greatly underestimated by SpO2 recordings.
- 60.Lone NI, Gillies MA, Haddow C, et al. Five-year mortality and hospital costs associated with surviving intensive care. Am J Respir Crit Care Med 2016; 194:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmidt U, Eikermann M. Organizational aspects of difficult airway management: think globally, act locally. Anesthesiology 2011; 114:3–6. [DOI] [PubMed] [Google Scholar]


