Abstract
Background—
Although multiple approaches have been used to create biological pacemakers in animal models, induced pluripotent stem cell–derived cardiomyocytes (iPSC-CMs) have not been investigated for this purpose. We now report pacemaker function of iPSC-CMs in a canine model.
Methods and Results—
Embryoid bodies were derived from human keratinocytes, their action potential characteristics determined, and their gene expression profiles and markers of differentiation identified. Atrioventricular blocked dogs were immunosuppressed, instrumented with VVI pacemakers, and injected subepicardially into the anterobasal left ventricle with 40 to 75 rhythmically contracting embryoid bodies (totaling 1.3–2×106 cells). ECG and 24-hour Holter monitoring were performed biweekly. After 4 to 13 weeks, epinephrine (1 μg kg−1 min−1) was infused, and the heart removed for histological or electrophysiological study. iPSC-CMs largely lost the markers of pluripotency, became positive for cardiac-specific markers. and manifested If-dependent automaticity. Epicardial pacing of the injection site identified matching beats arising from that site by week 1 after implantation. By week 4, 20% of beats were electronically paced, 60% to 80% of beats were matching, and mean and maximal biological pacemaker rates were 45 and 75 beats per minute. Maximum night and day rates of matching beats were 53±6.9 and 69±10.4 beats per minute, respectively, at 4 weeks. Epinephrine increased rate of matching beats from 35±4.3 to 65±4.0 beats per minute. Incubation of embryoid bodies with the vital dye, Dil, revealed the persistence of injected cells at the site of administration.
Conclusions—
iPSC-CMs can integrate into host myocardium and create a biological pacemaker. Although this is a promising development, rate and rhythm of the iPSC-CMs pacemakers remain to be optimized.
Keywords: action potentials, atrioventricular block, dogs, embryoid bodies, myocardium
WHAT IS KNOWN
For those arrhythmias that require a pacemaker, electronic devices are state-of-the-art therapy.
Biological pacemakers would have the advantage of requiring no batteries or leads while also incorporating autonomic responsiveness.
Biological pacemakers have been created/delivered using viruses carrying receptors, channels, or transcription factors.
They have also been created by delivering embryonic stem cell–derived cardiomyocytes, mesenchymal stem cells carrying the HCN2 pacemaker gene, or by using autologous sinus node myocytes
WHAT THE STUDY ADDS
This study creates a biological pacemaker using cardiac myocytes derived from human induced pluripotent stem cells for the first time.
The study used dogs as the experimental animals and so the xenograft involved immunosuppression. However, if developed for therapeutic use, the patient’s own cells can be used to generate the induced pluripotent stem cell–derived cardiomyocytes, eliminating the requirement for immunosuppression.
The study demonstrated good pacemaker function for ≤13 weeks at which time the immune response was too great, and the animal was euthanized.
Although electronic pacemakers are the current treatment for symptomatic atrioventricular block or sinoatrial node dysfunction, the maintenance they require and the risk of complications have motivated research on biological alternatives.1–3 The result, over the past 15 years, has been the exploration of gene- and cell-based approaches to initiate automaticity in atria and ventricles. Major gene therapy strategies, summarized recently,2 have included enhancement of depolarizing inward ion currents and reduction of hyperpolarizing outward currents during diastole, enhancement of excitability, and overexpression of transcription factors to transform native myocytes into pacemaker-like cells. In these settings, either naked plasmids or viral gene therapy has been the approach. A second hybrid route has been the implantation of human mesenchymal stem cells (MSCs) carrying pacemaker genes into the heart or the use of cell fusion to join cells carrying pacemaker genes with native myocytes.4,5 Finally, human embryonic stem cells have been differentiated into a cardiogenic lineage to provide phenotypic and functional pacemakers in the hearts of immunosuppressed pigs.6 As shall be detailed in the Discussion, although some of these approaches have provided reasonable pacemaker function in large animals in complete heart block and with backup electronic pacemakers, each approach also has important limitations: gene therapies are dependent on the duration of function of the vectors carrying the genes and raise questions about insertion site into the genome of the cell; MSC-based therapies are limited by the tendency of the stem cells to migrate from their implantation site; and embryonic stem cells (ESCs) carry the need for lifelong immunosuppression plus the risk of tumor formation.
See Editorial by Liang et al
Given these limitations, the more recent availability of techniques to create induced pluripotent stem cells (iPSCs) from adult cells7,8 and to differentiate these into a cardiogenic lineage9–11 has raised the possibility of an autologous approach to biological pacing. We have described a method to generate iPSCs from human hair follicle keratinocytes11 and demonstrated that appropriate transcription factors can transform these into spontaneously beating cells that are responsive to autonomic agonists and antagonists. They exhibit beat rate variability and power law behavior similar to that of human sinus node cells.12 We hypothesized that the embryoid bodies (EBs) composed of spontaneously beating iPSC-derived cardiomyocytes (iPSC-CMs) can integrate with ventricular myocardium in vivo to pace the hearts of dogs in complete heart block. The major advantage of the method is the potential to generate spontaneously beating cardiomyocytes from a patient’s own tissue, thus eliminating the critical need for immunosuppression.
Methods
The experimental animal protocol complied with the Guide for the Care and use of Laboratory Animals published by the US National Institutes of Health (publication 85-23, revised 1996) and was approved by the Stony Brook University Animal Care and Use Committee.
iPSC Culture, EB Induction, and Differentiation Into iPSC-CMs
Human hairs were plucked from healthy volunteers who signed consent forms (approval 3116, Rambam Health Care Campus, Haifa, Israel) per the Helsinki Committee for Experiments on Human Subjects. iPSCs were generated from human hair follicle keratinocytes using the STEMCCA Cassette (Cre-Excisable Constitutive Polycistronic Lentivirus Reprogramming Kit; a single lentiviral vector containing the 4 factors: oct4, sox2, klf4, and c-myc) as previously described.11,12 iPSCs generated from these keratinocytes were grown on mitomycin C–inactivated mouse embryonic fibroblasts, with knockout ESC/iPSC medium (Invitrogen, Carlsbad, CA) containing 80% DMEM (Dulbecco's Modified Eagle Medium)/F-12, 20% knockout serum replacement, 0.1 mmol/L mercaptoethanol, 1% penicillin–streptomycin, and 1% NEAA (non-essential amino acid), supplemented with 4 ng/mL of basic fibroblast growth factor to keep the cells in an undifferentiated state. The media were replaced every other day, and the cells were passaged once a week.
iPSCs were spontaneously differentiated toward EBs and cardiomyocytes as previously described.9–12 The iPSCs were detached using 1 mg/mL type IV collagenase (Invitrogen) and transferred to ultra-low attachment surface plates (Corning). Resultant EBs were grown in DMEM (Sigma) supplemented with 20% defined fetal bovine serum (HyClone), GlutaMAX, penicillin/streptomycin, and nonessential amino acids (all from Invitrogen). The EBs were cultured in suspension for 10 days and plated on 0.1% gelatin-coated (Sigma) plates. Spontaneously contracting areas were marked under the microscope and were cut using beveled pipet tips.
Affymetrix GeneChip Expression Analysis
RNA was extracted from iPSCs and from beating EBs using the RNeasy kit (Qiagen, Germantown, MD), and analysis of gene expression in both iPSCs and the EBs was performed on Affymetrix Human Genome U133 Plus 2.0 array (Affymetrix, Santa Clara, CA) according to the manufacturer’s recommendations. Raw microarray data were processed with the Affymetrix MAS 5.0 algorithm. We compared the iPSC gene expression profile to that of EBs.
Intact Canine Studies
EBs were prepared as above. Under sterile conditions, 13 adult mongrel dogs weighing 22 to 28 kg were anesthetized with propofol (6–8 mg/kg, IV), then intubated, and artificially ventilated with isoflurane (2%–3%) and oxygen. A pacemaker lead (Dextrus, a generous gift from Guidant, Minneapolis, MN) was introduced into the right ventricular apex via a jugular venous approach. An electronic pacemaker (Adapta, a generous gift from Medtronic, Minneapolis, MN) set at 60 beats per minute was placed in a subcutaneous pocket and was reprogrammed to VVI 35 beats per minute after a 2- to 6-day blanking period during which dogs recovered from surgery. After pacemaker implantation, the left femoral vein was catheterized, and complete atrioventricular block was induced via radiofrequency ablation.
A thoracotomy was then performed via the fifth left intercostal space, and the pericardium was incised. A purse-string suture was made in the anterobasal left ventricle, and 40 to 75 rhythmically contracting EBs (incorporating 1.3–2×106cells) derived from human keratinocytes (see above) were injected ≈3 to 4 mm deep to the epicardium. Cells were administered in a total volume of 0.4 to 0.7 mL phosphate-buffered saline solution via a 16-gauge needle. Once the injection was completed, the needle was withdrawn, and the purse-string suture was tied to prevent reflux of the cell suspension. We paced the site of injection via an epicardial electrode to identify ECG morphology of beats arising from the injection site (pace-mapped matching beats).
Immunosuppression Protocol
To prevent rejection of human EBs, we used the following oral immunosuppression protocol: cyclosporine A, 10 mg/kg 2× daily, started 2 days before surgery; mycophenolate mofetil (10 mg/kg 1× daily), started 48 hours post-surgery and continued for 4 days, after which the dose was increased to 2× daily; and prednisolone, 1 mg/kg, started on the day of surgery and tapered by 0.2 mg/kg every 2 weeks.
Animal Monitoring Procedures
ECG, 24-hour Holter monitoring, and overdrive pacing were performed twice a week after the 2- to 6-day postsurgical recovery period. In dogs with spontaneous rates faster than 30 beats per minute and a functional electronic pacemaker, endogenous pacemaker activity was suppressed by 30 s of electronic pacing at 80 beats per minute.
Twenty-four–hour monitoring was performed via Holter ECG (Rozinn; Scottcare, Glendale, NY). We calculated maximal beating rates from 30-s strips of a stable rhythm at maximal rate. We performed detailed analysis of the percentages of matching and nonmatching beats (using pace-mapped beats at the time of epicardial pacing as a reference) and electronically paced beats and the 24-hour average beating rate of pace-mapped matching rhythms. To analyze circadian variation, we compared the mean and maximal rates of matching beats and dependence on electronic backup during sleep (02:00–04:00 am) versus physical activity (08:00–10:00 am) as reported previously.13
β-Adrenergic Responsiveness
On the day of terminal study, animals were anesthetized as above. To evaluate β-adrenergic responsiveness, epinephrine (1 μg kg−1 min−1) was infused for 10 minutes. The chest was then opened, and the heart was removed and immersed in Tyrode solution equilibrated with 95% O2–5% CO2 containing (in mmol/L) 131 NaCl, 18 NaHCO3, 4 KCl, 1.8 CaCl2, 0.5 MgCl2, 1.8 NaH2PO4, and 5.5 dextrose. The left ventricle was opened, and a transmural portion of the myocardium, including the injection area, was then removed for immunohistochemical and histological or microelectrode studies.
Measurements of Transmembrane Action Potentials
Induced Pluripotent Stem Cell–Derived Cardiomyocytes
Action potentials were recorded from spontaneously contracting small cell clusters or isolated cells generated by dissociating iPSC-CMs after plating on top of gelatin or fibronectin-coated glass coverslips.14 Specifically, the EBs generated by our differentiation protocol were inspected under microscope to identify contracting areas. In general, ≈25% of the EBs demonstrated spontaneous contractions. To obtain cardiomyocytes for the electrophysiological experiments, we surgically isolated small contracting areas from the whole contracting EB. These areas were then enzymatically treated to yield spontaneously contracting small clusters (containing ≤5 cells) and single cardiomyocytes, used for action potential recordings. The pacemaker current If was recorded only from single cardiomyocytes. The patch pipette solution contained (mmol/L) 120 KCl, 1 MgCl2, 3 Mg-ATP, 10 HEPES, and 10 EGTA (pH, 7.3). The bath solution contained (mmol/L) 140 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose (pH, 7.4; all materials were purchased from Sigma-Aldrich, St. Louis, MO). Axopatch 200B, Digidata 1322, and pClamp10 (Molecular Devices, Sunnyvale, CA) were used for data amplification, acquisition, and analysis.
Canine Ventricular Myocardium
transmural ventricular strips (≈1.5×1.0×0.1 cm) were filleted with parallel surgical blades perpendicular to the surface of the anterobasal left ventricular free wall. The strip included the region of EBs injection. Preparations were placed in a 4-mL tissue bath and superfused with Tyrode solution (37°C; pH, 7.35±0.05) at a rate of 12 mL/min and were permitted to beat spontaneously. Conventional microelectrode techniques were used to record transmembrane potentials from the area of EBs injection (2–4 mm under the suture subepicardially).
Histology and Immunohistochemistry
To study iPSCs and evolving EBs before injection, formalin-fixed, paraffin-embedded cell blocks were sectioned to 4 μm and mounted on charged glass slides (Superfrost Plus; Fisher Scientific, Pittsburgh, PA) and baked overnight at 60°C. The slides were deparaffinized in xylene and rehydrated through graded alcohols. Endogenous peroxidase activity was blocked by 5-minute treatment with 3.0% hydrogen peroxide. Antigen retrieval was performed in a Decloaking Chamber (Biocare Medical, Walnut Creek, CA) by heating the slides to 12°C for 10 minutes in a 20 mmol/L concentration of citrate buffer (pH, 6.0). All slides were incubated with sox2 polyclonal antibody, diluted 1:100 (Cell Signaling Technology, Danvers, MA), and CTNT monoclonal antibody, diluted 1:1000 (Abcam, Cambridge, MA) at room temperature for 60 minutes. Staining was visualized by an indirect avidin/biotin-based immunoperoxidase method following the manufacturer’s protocol (Vector Laboratories, Burlingame, CA). Subsequently, the sections were developed using 3,3′-diaminobenzidine (DakoCytomation, Carpentaria, CA) and 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium substrate (Vector Laboratories, Burlingame, CA). The sections were dehydrated in graded alcohols, put into histo-clear, and mounted with vector mount cover slipping media (Vector Laboratories). Negative controls were performed on all sections using subclass-matched rabbit and mouse IgGs that were generated against unrelated antigens.
To allow identification of EBs in vivo, we studied 1 additional dog for which the EBs were first stained with the vital dye Dil (V-22885, Invitrogen) for 20 minutes at 37°C and then washed with medium and suspended in phosphate-buffered saline before injection into the heart. After 9 days, the animal was euthanized, and a section of the myocardium surrounding the injection site was removed and placed in fixative. Serial sections 10 μm thick were taken and stained with DAPI or Cx43 (Connexin 43), as previously described.15 Polyclonal antibodies raised against Cx43 (Zymed Laboratories Inc) were used. For any 3 serial sections, one was stained with DAPI, another for Cx43, and the third was used to monitor Dil fluorescence
Affymetrix GeneChip Expression Analysis
To characterize the differentiation of human iPSCs into functional cardiomyocytes, we investigated gene expression in undifferentiated cells and in beating EBs using the Affymetrix GeneChip Human Genome U133 Plus 2.0 Array. Analysis of gene expression showed that after iPSC differentiation, the markers of pluripotency had disappeared, whereas cardiac myocyte-specific factors dramatically increased (Table). This outcome was consistent with a loss of pluripotency and cardiac maturation of the iPSC-CMs. These results are concordant with previously reported differentiation of iPSCs into cardiac myocytes after formation of EBs.3 Figure 1 shows a representative stain for troponin T (denoting cardiomyocyte lineage) and sox2 (denoting multipotency) in a set of EBs shortly before injection into a canine heart. Note that some cells in the mature EBs still stain brown, indicating multipotency and a few cells in the sox2-positive EB stain blue, indicating the beginning evolution to a more mature cell.
Figure 1.

SOX2 (brown) and troponin T (blue) immunostaining identify the presence of multipotent cells and differentiated cardiac cells, respectively. Arrows indicate cells with a dual positive immunostaining suggestive of cells in a differentiating or dedifferentiating state.
Table.
After Differentiation of iPSCs to Beating EBs, Markers of Pluripotency Diminish, Whereas Cardiac Phenotype Markers Increase

Duration of Follow-Up and Data Analysis
Ten of the 13 animals prepared were followed for the duration of the study. One animal had an inadequate injection of cells and no biological pacemaker function. Two animals with no electronic pacemaker backup and poor biological pacemaker function (resulting in unacceptably low heart rates) were euthanized before week 4. Data from these 3 animals revealed idioventricular rates with nonmatching beats of 29±1.2 beats per minute on day 5 and 34±4.3 beats per minute at week 3. Follow-up for the 10 remaining dogs ranged from 4 to 13 weeks depending on clinical tolerance of immunosuppressive therapy, duration and function of biological pacemakers, and presence of a functional electronic pacemaker.
Statistical Analysis
Normally distributed data are presented as mean±SEM. The effects of Ivabradine on mean heart beat rate were analyzed using a 1-way ANOVA followed by the Holm–Sidak method. P<0.05 per Holm–Sidak method was considered statistically significant. In analyzing the percentage of matching beats for in vivo experiments, statistical significance was examined by a 1-way ANOVA for repeated measures. Post hoc analysis was performed with the Bonferroni correction. Offline data analysis and statistical evaluations were performed with SigmaPlot (Systat Software Inc, San Jose, CA) and Prism (GraphPad Software, San Diego, CA). In all tests, P<0.05 was considered significant.
The authors had full access to and take responsibility for the integrity of the data. All authors have read and agree to the article as written.
Results
Affymetrix Gene Chip Expression Analysis
To characterize the differentiation of human iPSCs into functional cardiomyocytes, we investigated gene expression in undifferentiated cells and in beating EBs using the Affymetrix GeneChip Human Genome U133 Plus 2.0 Array. Analysis of gene expression showed that after iPSC differentiation, the markers of pluripotency had disappeared, whereas cardiac myocyte-specific factors dramatically increased (Table). This outcome was consistent with a loss of pluripotency and cardiac maturation of the iPSC-CMs. These results are concordant with previously reported differentiation of iPSCs into cardiac myocytes after formation of EBs.9 Figure 1 shows a representative stain for troponin T (denoting cardiomyocyte lineage) and Sox2 (denoting multipotency) in a set of EBs shortly before injection into a canine heart. Note that some cells in the mature EBs still stain brown, indicating multipotency and a few cells in the SOX2-positive EB stain blue, indicating the beginning of evolution to a more mature cell.
Involvement of If in iPSC-CM Automaticity
To determine whether the EBs contain cardiomyocytes resembling SA node cells capable of pacing the canine heart, we investigated in iPSC-CMs the effects of the If blocker ivabradine on automaticity and on If. Figure 2A illustrates a representative experiment in which ivabradine attenuated automaticity in a concentration-dependent manner, until complete block at 10 μmol/L. In 3 of 12 experiments in which the effect of ivabradine on automaticity was investigated, 10 μmol/L eliminated automaticity. In the other 9 experiments, ivabradine markedly attenuated automaticity, but complete block did not occur. The effect of ivabradine on automaticity in all 12 experiments is summarized in Figure 2B.
Figure 2.
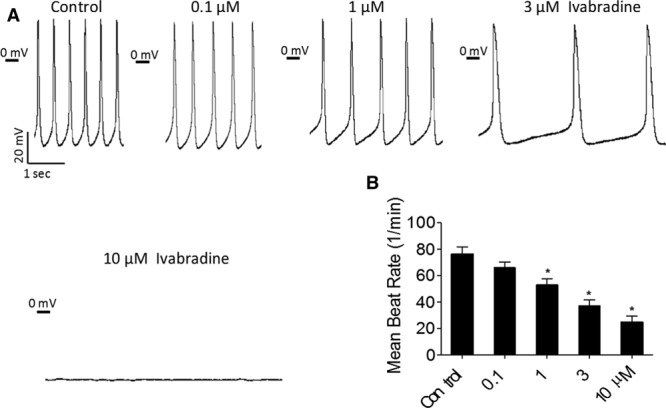
The effect of ivabradine on automaticity of induced pluripotent stem cell–derived cardiomyocytes (iPSC-CMs). A, Representative experiment showing action potentials recorded in absence (control panel) and presence of 0.1, 1, 3, and 10 μmol/L ivabradine. B, Summary of mean beat rate in absence and presence of 0.1, 1, 3, and 10 µmol/L ivabradine. Ivabradine induced a dose-dependent decrease in the mean beat rate (n=12 cells). In B, One-way ANOVA was performed followed by the Holm–Sidak method. *P<0.05 vs control. P <0.05 per Holm–Sidak method was considered statistically significant. All experiments were performed at 36°C.
In a complementary set of experiments, BaCl2, 500 µmol/L, was included in the Tyrode perfusing the bath to block Ik1. Because of the different experimental conditions, the effects of ivabradine on automaticity were not included in Figure 2B. In this set of experiments, action potentials and If were recorded sequentially (switching from current- to voltage-clamp mode) from the same cell in the absence and presence of ivabradine. Here, ivabradine 10 µmol/L completely eliminated automaticity (eg, Figure 3A). We found that the iPSC-CMs express a prominent If which is concentration dependently attenuated by ivabradine (representative experiment from cell in Figure 3A shown in Figure 3B and 3C; summary of 7 experiments, Figure 3D). Collectively, these results demonstrate that the automaticity of cardiomyocytes used to pace the canine heart has a major If dependence.
Figure 3.

The effect of ivabradine on If in induced pluripotent stem cell–derived cardiomyocytes (iPSC-CMs). Combined current- and voltage-clamp recordings were performed in the same cell. A, Spontaneous action potentials recorded in control Tyrode solution (left). Ivabradine at 10 μmol/L blocked automaticity (right). B, A representative experiment illustrating the dose-dependent attenuation of If by ivabradine. C, In the voltage-clamp protocol, the membrane was clamped from a holding potential of −40 to −120 mV in 10 mV steps for 2 s pulse duration. Interpulse interval: 15 s. D, Current-voltage relations of If (n=7 cells) illustrating the dose-dependent If attenuation by ivabradine. BaCl2 (500 μmol/L) was included in the extracellular solution to inhibit IK1. All experiments were performed at 36°C.
Intact Animal Studies
Pace-mapped matching beats became evident by the end of week 1 of implantation, and the biological pacemaker beating rate increased through week 2 and remained stable through weeks 4 to 5 (Figure 4A). Two dogs that had no electronic pacemaker lead were followed for 9 and 13 weeks; they had 60% to 80% of matching beats (Figure 4A). In the remaining 8 animals, dependence on electronic backup pacing decreased from week 1 to week 2 to 3 and then stabilized (Figure 4B). In these 8 animals, escape times were stable for the duration of the study (4.9±0.9 s on week 1 versus 4.3±0.6 s on week 2–3 and 3.5±0.7 s on week 4–5, all P>0.05). In the animals that displayed pace-mapped rhythms on 24-hour Holter recordings, mean and maximum rate of the matching rhythms increased from week 1 to week 2 to 3 and then remained stable until the end of the study (Figure 4C).
Figure 4.
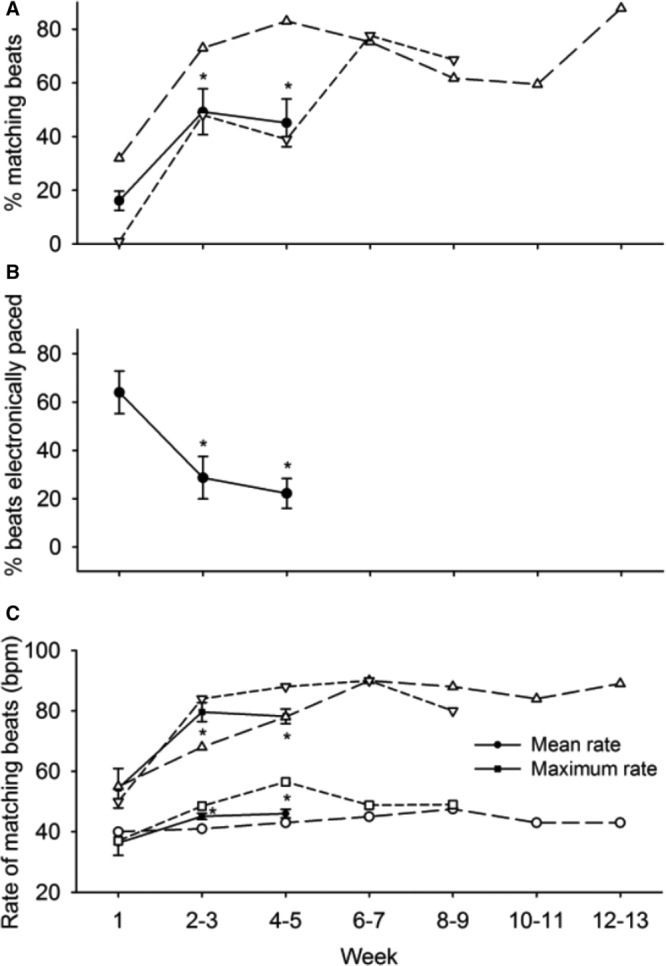
A, Percentage of beats matched to the pacing site in 10 dogs during 4- to 5-wk follow-up period. B, Percentage of electronically paced beats in 8 dogs during 4- to 5-wk follow-up period. Two dogs with longer follow-up periods shown in (A) and (C) were not included because they had no electronic pacemaker. C, Mean and maximum pace-mapped beating rates during 4- to 5-wk follow-up period. Number of dogs that displayed matching rhythm was 9 at wk 1 and 4 to 5, and 10 at wk 2 to 3. *P<0.05 vs wk 1 (ANOVA and Bonferroni correction). In addition, (A) and (C) are plotted individual data of the 2 animals that were followed through wk 8 to 9 and 12 to 13, respectively.
An example of 1 animal that had an optimal response is shown in Figure 5. On day 5, the resting rate of the matching beats was 40 beats per minute and increased to 49 beats per minute on day 19 (Figure 5A). Also shown in Figure 5A is the occasional occurrence of a nonmatching idioventricular rhythm, at a slower rate on day 5. Figure 5B shows the percent matching beats and maximum rate achieved for the same dog, and Figure 5C shows the automatic rhythm recorded from tissue removed from the site of the biological pacemaker implant during the terminal study. Hence, in an animal showing good pacemaker function, a stable automatic rhythm was demonstrated in isolated tissue slices. In contrast, in animals in which there was no significant biological pacemaker function, no such automaticity was seen (data not shown).
Figure 5.
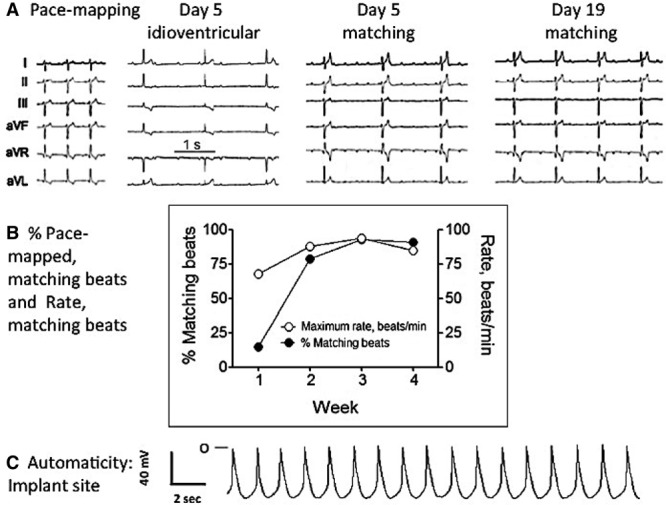
A, Representative ECGs recorded during pace mapping at time of implant and 5 and 19 d after embryoid bodies (EBs) implantation. B, Time course of the percent and maximum rate of matching beats recorded from the same dog during 4 wk of follow-up. C, Automatic rhythm recorded from a ventricular slab removed from the site of EB injection on the day of the terminal study.
Autonomic Modulation
To investigate whether biological pacemaker function was autonomically modulated, we compared the rates of pace-mapped rhythms during 2 hours of sleep (2:00–4:00 am) with 2 hours of feeding and activity (8:00–10:00 am) during steady-state expression of the biological pacemaker (week 4). As expected based on a normal circadian rhythm, mean and maximum rates of the pace-mapped rhythm were faster during the morning compared with the night. That is, mean night and day rates were 43±4.0 and 50±8.4 beats per minute, respectively (P<0.05; n=9), and maximum night and day rates were 53±6.9 and 69±10.4 beats per minute, respectively (P<0.05; n=9).
On the final day of the study, we infused epinephrine (1 μg kg−1 min−1×10 minutes) to 10 isoflurane-anesthetized dogs. In the control period before epinephrine infusion, matching beats were seen in 5 of 10 dogs. With epinephrine infusion, 9 of the 10 dogs displayed a rhythm pace mapped to the injection site. The maximum rate of the matching rhythm during epinephrine infusion in these 9 dogs was 65±4.0 beats per minute, compared with the baseline rate (whether matching or not) of 35±4.3 beats per minute (P<0.05).
To test whether cells we had injected were still residing at the injection site, we studied 1 additional animal for which we had incubated EBs for 20 minutes with the vital dye Dil before injection of the EBs into the heart (Methods). At 9 days, the resting rate of the matching beats was 24 beats per minute, increasing to 70 beats per minute after epinephrine injection. The animal was euthanized, and a section of the myocardium surrounding the injection site was removed and studied. As demonstrated in Figure 6, cells containing Dil in the cytoplasm were found along the injection track in close proximity to myocytes. The 2 arrows in the merged image (Figure 6 lower) of Figure 6 indicate Cx43 staining for the area occupied by iPSCs.
Figure 6.

Induced pluripotent stem cells (iPSCs) were stained with the vital dye Dil, and serial sections were stained as indicated in Methods. Upper, from left to right, Dil, Cx43, and DAPI staining. The merged image (lower) is from an area containing Dil-stained cells intercalated between myocytes. Green=Cx43 (Connexin 43) staining, Blue=DAPI, Red–Orange=Dil. Arrows indicate Cx43 staining within the region occupied by Dil-stained cells. Red background is because of leaching of the Dil from the delivered iPSCs.
Discussion
We designed this study to test whether spontaneously beating iPSC-CMs can integrate with myocardium in vivo to pace the heart. We found evidence for this at several levels of testing: histological studies demonstrated the presence of iPSC-CMs in the injected region. Microelectrode recordings from EBs before injection and from the injected region (Figures 2 and 3, and 5, respectively) showed automaticity with maximum diastolic potential and phase 4 depolarization similar to those of normal sinoatrial node cells. Studies in the intact dog in the setting of complete atrioventricular block demonstrated that iPSC-CMs can pace the heart for ≤3 months (with termination of the study determined not by failure of function but by the need to stop immunosuppression). The pacemaker function demonstrated was similar to those in many other studies.1–3 However, beating rates of pace-mapped rhythms were 40 to 50 beats per minute, and the rhythms originating from the injection sites were present for 50% of the time, a result not as good as those seen with viral or MSC delivery of various HCN gene variants1–5 or with the combined HCN2/AC1,16 HCN2/SKM1,17 or dominant negative Kir2.1/HCN218 gene variants or the transcription factor TBX18.19,20 Moreover, the escape times of the pace-mapped rhythms (3.5–4.9 s) are inadequate. At best with viral approaches or using adult human MSCs as a carrier for the pacemaker gene, we and others have seen escape times consistently <2 s.4,16–20 Hence, as shall be discussed below, much has to be done to optimize the function of iPSC-CMs if they are to be considered for eventual clinical use.
Another important issue for any biological pacemaker is its response to the autonomic nervous system. Ideally, pacemakers should function seamlessly in the complex interface between autonomic regulation and physical activity.12,21 Previous in vitro studies have demonstrated that human iPSC-CMs beat spontaneously and respond to autonomic agonists and antagonists.9,12,22 In this study, mean and maximum beating rates of the pace-mapped rhythm were significantly faster during periods of activity/feeding than during rest. These findings are concordant with those expected of a normal response to circadian modulation. In addition, epinephrine infusion to anesthetized dogs during the terminal study uncovered and induced a significant increase in rate of the matching rhythm. These data demonstrate that an iPSC-based biological pacemaker can function in a physiological, rate-responsive manner.
The contribution of If to automaticity of iPSC-CMs was demonstrated by the following findings: (1) the If blocker ivabradine23,24 attenuated and frequently terminated automaticity in a concentration-dependent manner; (2) the presence of prominent If; and (3) in agreement with the effects on automaticity, ivabradine markedly inhibited If. These findings are consistent with previous reports. For example, Sartiani et al25 and Ma et al26 demonstrated the presence of If in human ESC-derived cardiomyocytes and in iPSC-CMs, respectively. Further, we recently reported that the If blocker zatebradine (10 μmol/L) attenuated the spontaneous firing rate and If in human ESC-CMs.27 Similarly, Sartiani et al25 reported that zatebradine decreased the slope of the diastolic depolarization and the spontaneous rate of human ESC-derived cardiomyocytes.
Although even the lowest number of EBs used in the study contained a sufficient mass of cells to induce pacemaker function, this observation highlights a critical flaw in the use of the EB. That is, in injecting EBs one is inserting into the heart a roughly spherical structure whose cells have pacemaker properties. However, as a sphere, only those cells at or near (within 2 space constants) the surface will interact with the surrounding myocardium, and, as such, the inward current needed for phase 4 depolarization will, in effect, be diluted by the large IK1 of surrounding ventricular myocytes. What is needed to optimize function is that the full depolarizing current available from the iPSC-derived cells be distributed predictably to the myocardium.
A first step in achieving this would be by administering a cell suspension rather than a suspension of macroscopic EBs. Our previous studies using human MSCs as a carrier for the HCN2 gene have demonstrated dispersion of the human MSCs within a relatively small area.4,5 The flaw in this approach was the tendency of the cells to migrate elsewhere after about 6 to 8 weeks. Use of a cell suspension in our iPSCs pacemaker experiments also will permit cell administration through a finer needle, and with this, the ability to use catheter delivery rather than the transepicardial approach through a thoracotomy used in this study.
A second step required in going forward is to identify the type of cell to be delivered as an iPSC-derived pacemaker. During evolution from iPSCs to iPSC-CMs in EBs, markers of pluripotency were dramatically reduced, whereas those of cardiogenesis increased (Table). This is the direction of change one would hope for in inducing maturation of the iPSCs, but it also raises the possibility that iPSC-derived cardiac pacemakers might lose their pacing function if they mature further as they engraft in vivo. Moreover, although our data suggest that pluripotency may not be a major concern (as the EBs injected manifest some markers of multipotency—Figure 1—but not pluripotency), even a small residual population of multipotent cells may create a problem. The solution we see here (and also for selecting cells of a particular lineage) is the use of molecular beacons as markers and of fluorescence-activated cell sorting to ensure a uniform population of the desired cell type that can be delivered.28
Hence, although the iPSC-derived pacemaker does function in the heart, much has to be done to optimize that function. A major aspect of this is determining precisely what cell type is incorporated in the EB (and when moving on to cells in suspension, in the suspension itself). Initial publications on iPSCs forced into cardiogenic lineages stressed the notion that 3 populations of cells are present from the outset: so-called nodal cells, atrial cells, and ventricular cells.26 These were all identified via their action potential phenotypes. More recent data stress that although the 3 phenotypes can be identified, their quantitative presence varies with time after onset of culture: the nodal phenotype appears first, this is later accompanied by the atrial phenotype, and then, as the atrial phenotype diminishes, the ventricular phenotype appears.29 Very importantly, the atrial phenotype shows automatic rates higher than both the nodal and the ventricular phenotypes. If we extrapolate this information to the EBs injected into the canine heart in our (or any) study, it is clear that we do not know which type of cell is dominating in the EB and what rate it is generating. Hence, a major effort must be put into determining how each of the cell types as a pure population contributes to generation of biological pacemaker function, and whether 1 phenotype is optimal for all cardiac chambers, or if the best phenotype for 1 chamber may not function as well in another chamber. If either the nodal or the atrial phenotype is shown to be desirable (assuming the ventricular evolves ultimately to have no pacemaker activity) then means to isolate this as a pure population and to ensure its maintenance as that population having pacemaker properties going forward in time must be assured.29
Although the need for immunosuppression has been a major issue in the use of ESC-derived pacemakers (and was required in this study in which we administered human iPSC-derived cells as a xenotransplant to dogs), this would not be a concern for any clinical application of iPSC-derived pacemakers. We state this because the pacemakers in this setting would be derived from autologous cells. Although this does raise the issue of the age and health of the individual providing the cells (and needing a pacemaker), advances to date provide hope that concerns about age will not prevent good cell function.
Given the impressive challenges to development of an iPSC-derived biological pacemaker, one might ask, why bother. The justification for bothering derives from 2 sets of observations: first, given the excellence of electronic pacing, there is not a time constraint on developing the ideal biological pacemaker. We have the time to get it right before attempting to advance to the clinic, and what we fear most is the overhasty approach that results in a poor outcome that might have been avoided. When and if the biological pacemaker is good enough to be tested, it should be tested—and not before. The second reason is that for the most part, the biological pacemakers we and others have developed over the past 15 years are good enough for proof of concept, but still raise questions for clinical application.1–3 Although all have created a basis for some optimism, even the most promising functional results obtained with gene-based constructs have shown insufficient duration of function to warrant clinical translation.19,20,25 In addition, cell-based biological pacemakers, which have provided longer-term pacemaker function, have been hampered by the need for immunosuppression and the risk of cell migration.4,6 Given these outcomes, although the field remains a promising one, new approaches are still to be sought, and we think the autologous iPSC-derived pacemaker, despite the challenges of perfecting it, does warrant the effort.
Appendix
From the Department of Physiology and Biophysics (S.C., E.P.A., Y.-P.J., T.R., X.Q., I.A.P., S.V.D., P.R.B., I.S.C., M.R.R.) and Department of Pathology (S.B.), Stony Brook University, New York, NY; Department of Physiology, Biophysics and Systems Biology, The Rappaport Institute for Research in the Medical Sciences, Ruth and Bruce Rappaport Faculty of Medicine, Technion, Haifa, Israel (M.B.-A., S.N., O.B.); and Departments of Pharmacology (P.D., M.R.R.) and Pediatrics (M.R.R.), Columbia University, New York, NY.
Sources of Funding
This work was supported by National Heart Lung and Blood Institute grant HL111401 (Cohen); The Israeli Science Foundation (Binah); The Israeli Ministry of Science and Technology (Binah); The Rappaport Institute (Binah); The Moxie Foundation (Binah); The University of Michigan—Israel Partnership for Research (Binah).
Disclosures
None.
Footnotes
Drs Chauveau, Anyukhovsky, M. Ben-Ari, and Dr Naor contributed equally as first authors.
References
- 1.Cho HC, Marbán E. Biological therapies for cardiac arrhythmias: can genes and cells replace drugs and devices? Circ Res. 2010;106:674–685. doi: 10.1161/CIRCRESAHA.109.212936. doi: 10.1161/CIRCRESAHA.109.212936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen MR. Gene therapy and biological pacing. N Engl J Med. 2014;371:1158–1159. doi: 10.1056/NEJMcibr1408897. doi: 10.1056/NEJMcibr1408897. [DOI] [PubMed] [Google Scholar]
- 3.Rosen MR, Robinson RB, Brink PR, Cohen IS. The road to biological pacing. Nat Rev Cardiol. 2011;8:656–666. doi: 10.1038/nrcardio.2011.120. doi: 10.1038/nrcardio.2011.120. [DOI] [PubMed] [Google Scholar]
- 4.Plotnikov AN, Shlapakova I, Szabolcs MJ, Danilo P, Jr, Lorell BH, Potapova IA, Lu Z, Rosen AB, Mathias RT, Brink PR, Robinson RB, Cohen IS, Rosen MR. Xenografted adult human mesenchymal stem cells provide a platform for sustained biological pacemaker function in canine heart. Circulation. 2007;116:706–713. doi: 10.1161/CIRCULATIONAHA.107.703231. doi: 10.1161/CIRCULATIONAHA.107.703231. [DOI] [PubMed] [Google Scholar]
- 5.Potapova I, Plotnikov A, Lu Z, Danilo P, Jr, Valiunas V, Qu J, Doronin S, Zuckerman J, Shlapakova IN, Gao J, Pan Z, Herron AJ, Robinson RB, Brink PR, Rosen MR, Cohen IS. Human mesenchymal stem cells as a gene delivery system to create cardiac pacemakers. Circ Res. 2004;94:952–959. doi: 10.1161/01.RES.0000123827.60210.72. doi: 10.1161/01.RES.0000123827.60210.72. [DOI] [PubMed] [Google Scholar]
- 6.Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, Huber I, Satin J, Itskovitz-Eldor J, Gepstein L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–1289. doi: 10.1038/nbt1014. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Germanguz I, Sedan O, Zeevi-Levin N, Shtrichman R, Barak E, Ziskind A, Eliyahu S, Meiry G, Amit M, Itskovitz-Eldor J, Binah O. Molecular characterization and functional properties of cardiomyocytes derived from human inducible pluripotent stem cells. J Cell Mol Med. 2011;15:38–51. doi: 10.1111/j.1582-4934.2009.00996.x. doi: 10.1111/j.1582-4934.2009.00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novak A, Barad L, Zeevi-Levin N, Shick R, Shtrichman R, Lorber A, Itskovitz-Eldor J, Binah O. Cardiomyocytes generated from CPVTD307H patients are arrhythmogenic in response to β-adrenergic stimulation. J Cell Mol Med. 2012;16:468–482. doi: 10.1111/j.1582-4934.2011.01476.x. doi: 10.1111/j.1582-4934.2011.01476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novak A, Shtrichman R, Germanguz I, Segev H, Zeevi-Levin N, Fishman B, Mandel YE, Barad L, Domev H, Kotton D, Mostoslavsky G, Binah O, Itskovitz-Eldor J. Enhanced reprogramming and cardiac differentiation of human keratinocytes derived from plucked hair follicles, using a single excisable lentivirus. Cell Reprogram. 2010;12:665–678. doi: 10.1089/cell.2010.0027. doi: 10.1089/cell.2010.0027. [DOI] [PubMed] [Google Scholar]
- 12.Mandel Y, Weissman A, Schick R, Barad L, Novak A, Meiry G, Goldberg S, Lorber A, Rosen MR, Itskovitz-Eldor J, Binah O. Human embryonic and induced pluripotent stem cell-derived cardiomyocytes exhibit beat rate variability and power-law behavior. Circulation. 2012;125:883–893. doi: 10.1161/CIRCULATIONAHA.111.045146. doi: 10.1161/CIRCULATIONAHA.111.045146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shlapakova IN, Nearing BD, Lau DH, Boink GJ, Danilo P, Jr, Kryukova Y, Robinson RB, Cohen IS, Rosen MR, Verrier RL. Biological pacemakers in canines exhibit positive chronotropic response to emotional arousal. Heart Rhythm. 2010;7:1835–1840. doi: 10.1016/j.hrthm.2010.08.004. doi: 10.1016/j.hrthm.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Ari M, Schick R, Barad L, Novak A, Ben-Ari E, Lorber A, Itskovitz-Eldor J, Rosen MR, Weissman A, Binah O. From beat rate variability in induced pluripotent stem cell-derived pacemaker cells to heart rate variability in human subjects. Heart Rhythm. 2014;11:1808–1818. doi: 10.1016/j.hrthm.2014.05.037. doi: 10.1016/j.hrthm.2014.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valiunas V, Doronin S, Valiuniene L, Potapova I, Zuckerman J, Walcott B, Robinson RB, Rosen MR, Brink PR, Cohen IS. Human mesenchymal stem cells make cardiac connexins and form functional gap junctions. J Physiol. 2004;555(pt 3):617–626. doi: 10.1113/jphysiol.2003.058719. doi: 10.1113/jphysiol.2003.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boink GJ, Nearing BD, Shlapakova IN, Duan L, Kryukova Y, Bobkov Y, Tan HL, Cohen IS, Danilo P, Jr, Robinson RB, Verrier RL, Rosen MR. Ca(2+)-stimulated adenylyl cyclase AC1 generates efficient biological pacing as single gene therapy and in combination with HCN2. Circulation. 2012;126:528–536. doi: 10.1161/CIRCULATIONAHA.111.083584. doi: 10.1161/CIRCULATIONAHA.111.083584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boink GJ, Duan L, Nearing BD, Shlapakova IN, Sosunov EA, Anyukhovsky EP, Bobkov E, Kryukova Y, Ozgen N, Danilo P, Jr, Cohen IS, Verrier RL, Robinson RB, Rosen MR. HCN2/SkM1 gene transfer into canine left bundle branch induces stable, autonomically responsive biological pacing at physiological heart rates. J Am Coll Cardiol. 2013;61:1192–1201. doi: 10.1016/j.jacc.2012.12.031. doi: 10.1016/j.jacc.2012.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cingolani E, Yee K, Shehata M, Chugh SS, Marbán E, Cho HC. Biological pacemaker created by percutaneous gene delivery via venous catheters in a porcine model of complete heart block. Heart Rhythm. 2012;9:1310–1318. doi: 10.1016/j.hrthm.2012.04.020. doi: 10.1016/j.hrthm.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Hu YF, Dawkins JF, Cho HC, Marbán E, Cingolani E. Biological pacemaker created by minimally invasive somatic reprogramming in pigs with complete heart block. Sci Transl Med. 2014;6:245ra94. doi: 10.1126/scitranslmed.3008681. doi: 10.1126/scitranslmed.3008681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapoor N, Liang W, Marbán E, Cho HC. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nat Biotechnol. 2013;31:54–62. doi: 10.1038/nbt.2465. doi: 10.1038/nbt.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brubaker PH, Kitzman DW. Chronotropic incompetence: causes, consequences, and management. Circulation. 2011;123:1010–1020. doi: 10.1161/CIRCULATIONAHA.110.940577. doi: 10.1161/CIRCULATIONAHA.110.940577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bucchi A, Barbuti A, Baruscotti M, DiFrancesco D. Heart rate reduction via selective ‘funny’ channel blockers. Curr Opin Pharmacol. 2007;7:208–213. doi: 10.1016/j.coph.2006.09.005. doi: 10.1016/j.coph.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 24.DiFrancesco D, Borer JS. The funny current: cellular basis for the control of heart rate. Drugs. 2007;67(suppl 2):15–24. doi: 10.2165/00003495-200767002-00003. [DOI] [PubMed] [Google Scholar]
- 25.Sartiani L, Bettiol E, Stillitano F, Mugelli A, Cerbai E, Jaconi ME. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cells. 2007;25:1136–1144. doi: 10.1634/stemcells.2006-0466. doi: 10.1634/stemcells.2006-0466. [DOI] [PubMed] [Google Scholar]
- 26.Ma J, Guo L, Fiene SJ, Anson BD, Thomson JA, Kamp TJ, Kolaja KL, Swanson BJ, January CT. High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am J Physiol Heart Circ Physiol. 2011;301:H2006–H2017. doi: 10.1152/ajpheart.00694.2011. doi: 10.1152/ajpheart.00694.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weisbrod D, Peretz A, Ziskind A, Menaker N, Oz S, Barad L, Eliyahu S, Itskovitz-Eldor J, Dascal N, Khananshvili D, Binah O, Attali B. SK4 Ca2+ activated K+ channel is a critical player in cardiac pacemaker derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2013;110:E1685–E1694. doi: 10.1073/pnas.1221022110. doi: 10.1073/pnas.1221022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ben-Ari M, Naor S, Zeevi-Levin N, Schick R, Jehuda RB, Reiter I, Raveh A, Grijnevitch I, Barak O, Rosen MR, Weissman A, Binah O. Developmental changes in electrophysiological characteristics of human-induced pluripotent stem-cell derived cardiomyocytes. Heart Rhythm. 2016;13:2379–2387. doi: 10.1016/j.hrthm.2016.08.045. doi: 10.1016/j.hrthm.2016.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Y, Lu Z, Cohen IS, Scarlata S. Development of a universal RNA beacon for exogenous gene detection. Stem Cells Transl Med. 2015;4:476–482. doi: 10.5966/sctm.2014-0166. doi: 10.5966/sctm.2014-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]


