Abstract
Integration between implant and bone is an essential concept for osseous healing requiring hardware placement. A novel approach to hardware implantation, termed osseodensification, is described here as an effective alternative. 12 sheep averaging 65 kg had fixation devices installed in their C2, C3, and C4 vertebral bodies; each device measured 4 mm diameter×10 mm length. The left-sided vertebral body devices were implanted using regular surgical drilling (R) while the right-sided devices were implanted using osseodensification drilling (OD). The C2 and C4 vertebra provided the t=0 in vivo time point, while the C3 vertebra provided the t=3 and t=6 week time points, in vivo. Structural competence of hardware was measured using biomechanical testing of pullout strength, while the quality and degree of new bone formation and remodeling was assessed via histomorphometry. Pullout strength demonstrated osseodensification drilling to provide superior anchoring when compared to the control group collapsed over time with statistical significance (p < 0.01). On Wilcoxon rank signed test, C2 and C4 specimens demonstrated significance when comparing device pullout (p=0.031) for both, and C3 pullout tests at 3 and 6 weeks collapsed over time had significance as well (p=0.027). Percent bone-to-implant contact (%BIC) analysis as a function of drilling technique demonstrated an OD group with significantly higher values relative to the R group (p < 0.01). Similarly, percent bone-area-fraction-occupancy (BAFO) analysis presented with significantly higher values for the OD group compared to the R group (p=0.024). As a function of time, between 0 and 3 weeks, a decrease in BAFO was observed, a trend that reversed between 3 and 6 weeks, resulting in a BAFO value roughly equivalent to the t=0 percentage, which was attributed to an initial loss of bone fraction due to remodeling, followed by regaining of bone fraction via production of woven bone. Histomorphological data demonstrated autologous bone chips in the OD group with greater frequency relative to the control, which acted as nucleating surfaces promoting new bone formation around the implants, providing superior stability and greater bone density. This alternative approach to a critical component of hardware implantation encourages assessment of current surgical approaches to hardware implantation.
1. Introduction
Surgical fixation of implants into bone is a front-line approach to correcting skeletal deformities. A plethora of implants currently exist, differing by characteristics such as, but not limited to, alloy composition, porosity, and coating (Rao et al., 2014). Understanding currently available surgical endosteal implant fixations is based on the concept of osseointegration—the direct interaction between living bone and an implant—a principle that has been studied in reconstructive settings for over four decades (Albrektsson et al., 1981). Surgical applications of osseointegration emerged from Leventhal’s introduction of titanium as an adequate metal for fracture fixation (Leventhal, 1951), which was followed by Branemark’s work in both oral and appendicular bone studies (Albrektsson et al., 1981). Osseointegration of endosteal metallic devices has grown to encompass fields such as hand surgery (Möller et al., 2004), amputation prosthesis (Aschoff et al., 2010; Tillander et al., 2010; Van de Meent et al., 2013), and spine stabilization, to name a few.
Despite tremendous progress in the field of hardware fixation, surgical hardware and fixation failures in bone surgery have prompted the need to develop alternative solutions through improvements in materials, device design, and more recently, surgical instrumentation (Bròdano et al., 2014; Frosch et al., 2003; Khashan et al., 2013; Rihn et al., 2010). Material and device design explored together yielded a breakthrough advancement in the form of hydroxyapatite (HA) through a myriad of device designs beyond the scope of this manuscript (Barber et al., 1998; Facca et al., 2011; McAfee et al., 2003; Nepal et al., 2014; Salou et al., 2015; Sandén et al., 2002; Spivak and Hasharoni, 2001; Yerby et al., 1998; Yildirim et al., 2006; Zhou et al., 2014, 2015). The subject of improving osseointegration following initial surgical placement is paramount as it will ensure long lasting surgical hardware stability.
Traditionally, failure of implants to osseointegrate or maintain load bearing function were considered to be a consequence of insufficient initial contact with bone (Linder et al., 1988). The device’s primary stability describes the initial, tight mechanical interlocking between the implant and bone upon fixation (Coelho and Jimbo, 2014). This mechanical interlocking is essential for implant stability in settings of immediate load bearing, and is characterized by bone adjacent to implant as a consequence of interference due to instrumentation and device dimensional differences. However, when the strain exceeds bone elasticity, microcracks can form on bone surface. Though microcracks contribute to eventual bone remodeling and osseointegration achievement, excessive microcracking can induce widespread bone remodeling and surgical hardware instability and eventual failures (Coelho and Jimbo, 2014).
Substantial advances have been made with respect to surgical hardware hierarchical design (e.g. macrogeometry, surface texture, addition of growth factors) in an attempt to improve early fixture stability and osseointegration. However, the number of investigations concerning surgical instrumentation effects on early fixation and osseointegration is at least one order of magnitude smaller relative to other design parameters (Coelho and Jimbo, 2014). The limited body of literature addressing drilling technique and its effects on osseointegration suggests that implant stability can in fact be hastened by drilling protocol alterations in both maxillofacial and orthopedic settings (Galli et al., 2015a, 2015b; Giro et al., 2011, 2013; Sarendranath et al., 2015; Yeniyol et al., 2013). Specific to spine procedures, reviews in literature have primarily focused on the limited perspective of materials available to surgeons for clinical orthopedic applications (Rao et al., 2014).
Recently, a drilling approach termed osseodensification has been introduced for placement of endosteal fixtures (Huwais and Meyer, 2016). Osseodensification is performed in an attempt to develop a condensed autograft surrounding the implant, making it valuable in clinical settings where there is an anatomic paucity of bone (Lahens et al., 2016). Unlike traditional drilling protocols (which we refer to as subtractive drilling), osseodensification increases primary stability due to densification of the drilled osteotomy site walls centrifugally by means of non-subtractive drilling (Huwais and Meyer, 2016). The rationale is that compacted, autologous bone immediately in contact with an endosteal device will not only have higher degrees of primary stability due to physical interlocking between the bone and the device, but also facilitate osseointegration due to osteoblasts nucleating on instrumented bone in close proximity to the implant (Lahens et al., 2016). The objective of this study was to assess the biomechanical and histologic effects of osseodensification surgical instrumentation on a large animal, highly translational cervical spine model.
2. Materials and methods
2.1. Preclinical laboratory in vivo model
A highly translational large preclinical animal model was used in the present study. The sheep cervical spine model was selected due to its low-density bone configuration and its size, which allowed for the placement of all experimental groups nested within each subject so statistical power was maximized while minimizing the number of animals. The study was conducted according to ethical approval from the Institutional Animal Care and Use Committee. Twelve female sheep (each weighing approximately 65 kg and 2 years in age) were used in this study.
Prior to surgery, anesthesia was induced with sodium pentothal (15–20 mg/kg) in Normasol solution into the jugular vein and maintained with isoflurane (1.5–3%) in O2/N2O (50/50). Animal monitoring included ECG, end tidal CO2, SpO2 and body temperature, which was regulated by a circulating hot water blanket. Prior to surgery, the surgical site was shaved and iodine solution was applied to prepare the surgical site. An anterior access was performed by a 15 cm incision along the midline starting 5 cm below the cricoid cartilage. Access to the anterior flange of the vertebrae was accomplished by blunt dissection. Four as-machined (Emfils Colosso, Itu, Brazil) (4 mm diameter, 10 mm length) screw-type endosteal implants were placed bilaterally at the C3 vertebral body. The left side implants were inserted using regular surgical drilling (recommended by manufacturer) in a 3 step series of a 2.0 mm pilot, 3.2 mm and 3.8 mm twist drills (Emfils Colosso Drills, Itu, Brazil) while the right side were inserted using osseodensification (OD) drilling with Densah Bur (Versah, Jackson, MI, USA) 2.0 mm pilot, 2.8 mm, and 3.8 mm multi fluted tapered burs (Fig. 1). Osseodensification was performed at 1100 rpm in counterclockwise drilling (non-cutting) direction with saline irrigation. The insertion torque of all implants was recorded by a digital torque meter (Tonichi STC2-G, Tonishi, Japan). Layered closure with Vicryl 2-0 for muscle and 2-0 nylon for skin was performed. Cefazolin (500 mg) was administered intravenously pre-operatively and post-operatively. Postoperatively, food and water ad libitum was offered to the animals.
Fig. 1.
Geometric configuration of the (a) control and (b) experimental groups.
Six of the twelve sheep were sacrificed at three weeks post-surgery, and the remaining six were sacrificed after six weeks. Upon sacrifice, the C2, C3, and C4 vertebral bodies were collected. The C2 and C4 vertebrae were instrumented identically to the C3 vertebrae and only differed by being performed postmortem, providing the initial time point (t=0).
2.2. Preparation of samples: biomechanical testing and histomorphometry
The animals were sacrificed with an overdose of an anesthetic, and the implants and the surrounding bone tissue were removed en bloc. One implant from C2, C3, and C4 vertebrae (n=1/experimental group) (osseodensification and regular) were subjected to biomechanical testing to evaluate pullout strength, while the other implant (n=1/experimental group) was subjected to histology.
Mechanical testing (pullout strength) was performed using a universal testing machine (Instron Series 5560 Norwood, MA) with a cross-head speed of 0.02 mm/sec.
The remaining bone-implant blocks were gradually dehydrated in a series of alcohol solutions ranging from 70% to 100% ethanol and then embedded in a methyl methacrylate-based resin. Embedded blocks were then cut into sections using a diamond saw (Isomet, 2000, Buehler Ltd., Lake Bluff, IL, USA). The sections were ground on a grinding machine (Metaserv 3000, Buehler, Lake Bluff, IL, USA) under water irrigation with a series of SiC abrasive paper until they were approximately 100 μm thick, and the samples were then stained in Stevenel’s blue and Van Gieson to differentiate the soft and connective tissues (Del Cerro et al., 1980; Flotte et al., 1989; Jensen et al., 1991). Histology samples were evaluated histomorphometrically using image analysis software (ImageJ, NIH, Bethesda, MD). Bone-implant contact (BIC) and bone area fraction occupancy (BAFO) were quantified to evaluate the osteogenic parameters around the surface. BIC determines the degree of osseointegration by tabulating the percentage of bone contact over the entire relevant implant surface perimeter. BAFO measures bone growth within the implant threads as a percentage (Coelho et al., 2012; Leonard et al., 2009; Witek et al., 2013).
3. Statistical analysis
All biomechanical and histomorphometric testing data are presented as mean values with the corresponding 95% confidence interval values (mean ± CI). Insertion torque, pull-out strength, %BIC, and % BAFO data were analyzed using a linear mixed model with fixed factors of time in vivo (0, 3, and 6 weeks) and surgical drilling method -Regular (R), and Osseodensification (OD). All analyses were completed with IBM SPSS (v23, IBM Corp., Armonk, NY).
4. Results
No surgical site showed any signs of inflammation, infection, or failure of the implant, throughout the period of healing. Sharp dissection and clinical inspection demonstrated that all devices were integrated with bone and clinically stable.
4.1. Mechanical testing
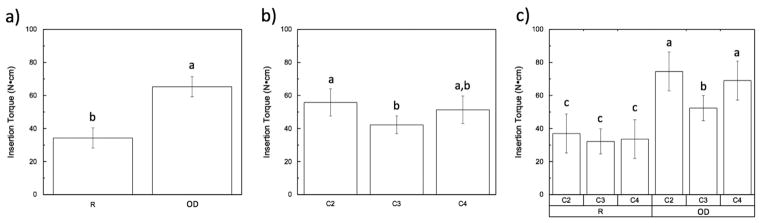
Statistical evaluation showed significantly higher levels of insertion torque for the OD (~65 N cm) group relative to the R (~35 N cm) group (p < 0.01) (Fig. 2A). Analyzing insertion torques as a function of implant location showed a significant difference (p=0.019) among the three different cervical vertebrae locations (Fig. 2B). Insertion torque as a function of cervical vertebrae location and drilling technique are shown in Fig. 2C (p=0.128). In the OD drilling technique there is a significantly lower value of insertion torque in vertebrae C3 (p < 0.05), while with the regular drilling technique there were no statistical differences observed.
Fig. 2.
(Insertion torque): (a) as a function of drilling technique (collapsed over time); (b) as a function of location; (c) as a function of location within each group (R vs. OD). The letters indicate statistically homogenous groups.
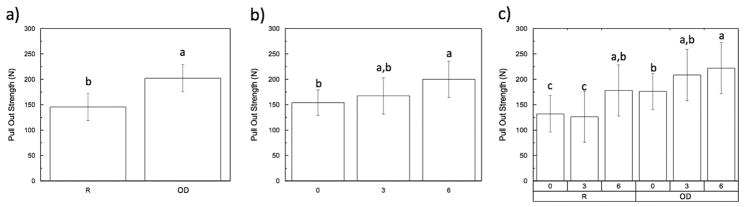
The pull-out strength followed a similar trend as initially observed with the insertion torque. When results were collapsed over time, regular drilling group (~150 N) was significantly lower (p < 0.01) in comparison to the approximately 225 N of the OD group (Fig. 3A). When analyzing the pull-out strength collapsed over group and as a function of time in vivo, a significant (p < 0.05) increase was observed between the 0 and 6-week groups, while the 3-week group presented intermediate values (Fig. 3B). The pull-out strength as a function of time in vivo and drilling technique are shown in Fig. 3C. For both R and OD drilling techniques, significant differences (p < 0.05) were detected between the 0 and 6-week samples with the same trend in the regular drilling group.
Fig. 3.
(Pull out strength): (a) as a function of drilling technique (collapsed over time); (b) as a function of time in vivo; (c) time points within each group (R vs. OD). The letters indicate statistically homogenous groups.
4.2. Histomorphometric analysis
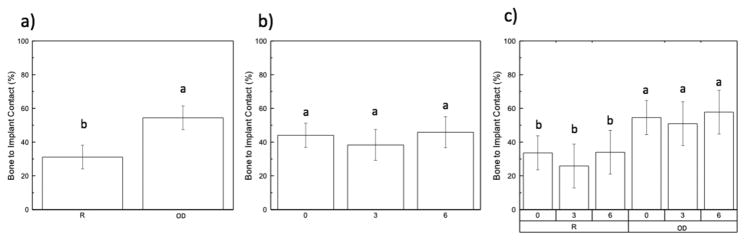
Statistical evaluation of the effect of drilling technique on BIC when collapsed over time in vivo is shown in Fig. 4A. When experimental data was collapsed over time in vivo, a significant difference between drilling techniques (p < 0.01) was observed. While analyzing BIC collapsed over group and as a function of time (Fig. 4B), there were no significant differences (p=0.252) between any of the times in vivo (0, 3, and 6-weeks). BIC values as a function of drilling technique and time in vivo are shown in Fig. 4C. There were no significant differences observed (p=0.930) at the three time points in vivo among the two different drilling techniques within groups. The OD experimental group presented significantly higher BIC values (p < 0.01) at all time points relative to the R drilling technique.
Fig. 4.
(Bone-implant contact, BIC): (a) as a function of drilling technique (collapsed over time); (b) as a function of time in vivo; (c) time points within each group (R vs. OD). The letters indicate statistically homogenous groups.
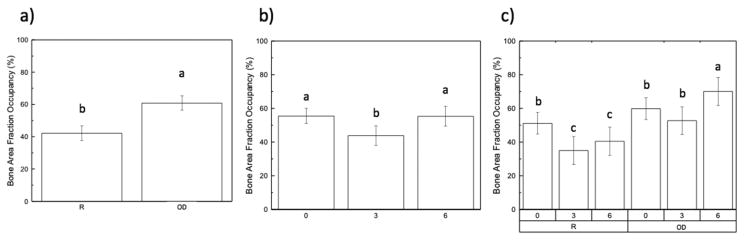
Statistical evaluation of the effect of drilling technique collapsed over time in vivo for BAFO is shown in Fig. 5A. A significant difference between drilling techniques was detected (p=0.00). Analyzing BAFO collapsed over both groups as a function of time demonstrated an initial decrease from 0-weeks to 3-weeks in vivo, with an increase observed from 3-weeks to 6-weeks. The 0 and 6-week time points showed no significant differences, while the 3-week group presented a significantly (p < 0.05) lower value comparison to two other time points (Fig. 5B). BAFO values as a function of drilling technique and time in vivo are shown in Fig. 5C. There were significant (p < 0.05) differences observed at the 6-week time point in vivo in the OD drilling technique, resulting in the highest value of approximately 70%. The regular drilling technique showed significantly (p < 0.05) lower values at the 3 and 6-week time points when compared to the OD drilling technique.
Fig. 5.
(Bone area fraction occupancy, BAFO): (a) as a function of drilling technique (collapsed over time); (b) as a function of time in vivo; (c) time points within each group (R vs. OD). The letters indicate statistically homogenous groups.
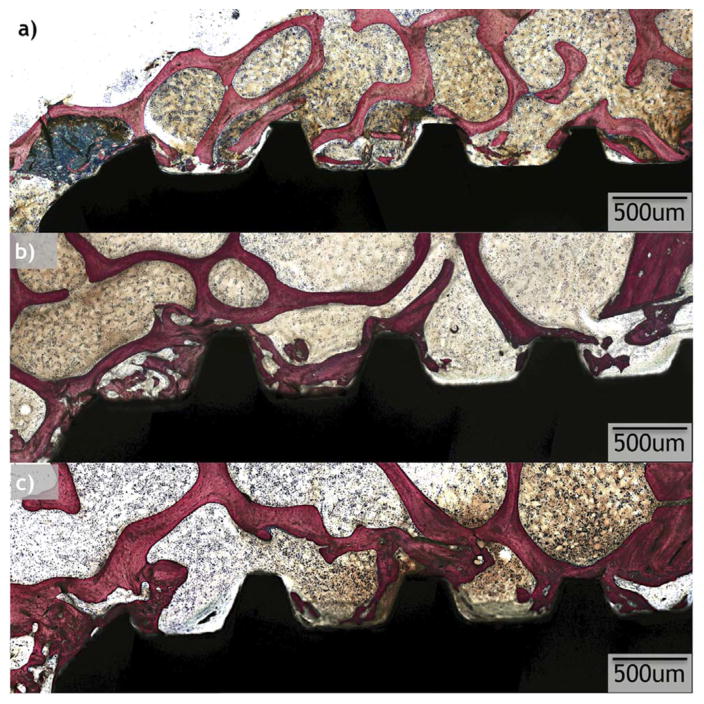
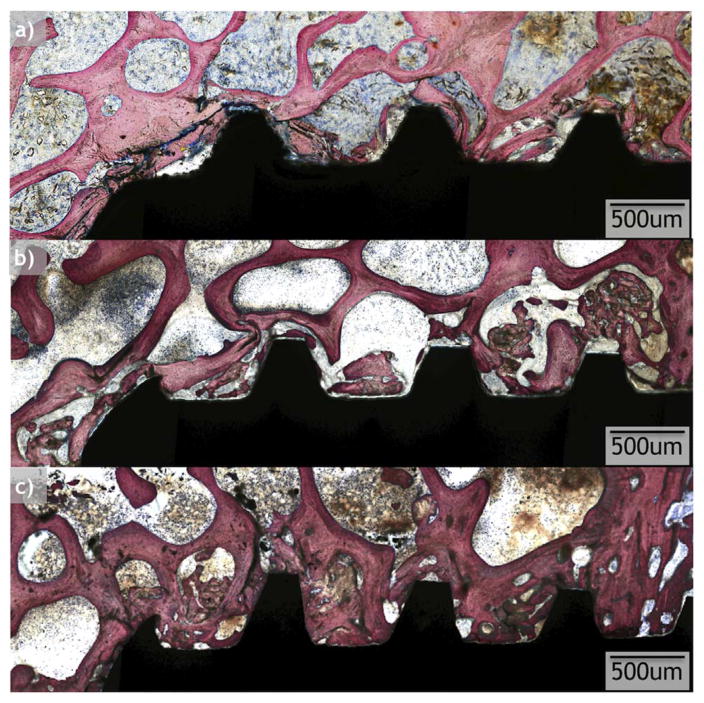
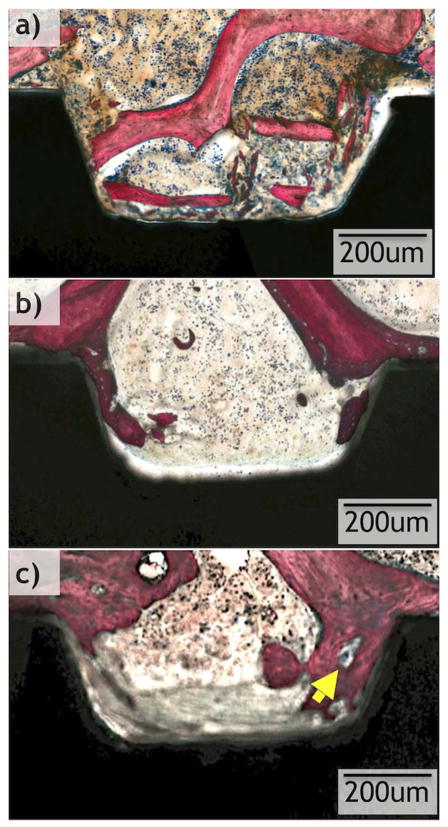
All implants considered for statistical analysis demonstrated osseointegration upon survey histologic evaluation (Figs. 6 and 7). R and OD drilling groups presented with different osseointegration patterns, and R demonstrated a lesser degree of osseointegration when compared to OD (Figs. 6 and 7). On optical micrograph at 0 weeks, the R group presented a bone lattice that is abruptly interrupted by subtractive drilling for hardware placement along with bone debris from drilling in proximity with the implant surface (Figs. 6a and 8a). In contrast, the OD group at 0 weeks presented a greater quantity of bone chips along with a distorted bone lattice suggestive of compression from the drilling technique (Figs. 7a and 9a). At 3 weeks, the R group micrographs depict initial new bone formation and remodeling sites at the bone-implant interface (Figs. 6b and 8b). The OD group at 3 weeks demonstrates that the bone chips and distorted lattice present at week 0 have acted as nucleating particles/surface for new bone formation, and bone growth is seen from implant towards bone as well as from bone towards the implant (Figs. 7b and 9b). At 6 weeks, the R group continues to demonstrate remodeling of bone (Figs. 6c and 8c), while the OD group shows a substantial increase in bone formation and bone chips from instrumentation embedded between old bone and the implant surface by a more extensive bone formation around the implant. Remodeling is extensive around the OD group at both newly formed bone as well as in bone chips (Figs. 7c and 9c).
Fig. 6.
Optical micrographs taken from samples at each time point (0, 3, and 6 weeks) from the regular (R) drilling group.
Fig. 7.
Optical micrographs taken from samples at each time point (0, 3, and 6 weeks) from the osseodensification (OD) drilling group.
Fig. 8.
Higher magnification optical micrographs taken from samples at each time point (0, 3, and 6 weeks) from the regular drilling group. Yellow arrows show remodeling sites. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 9.
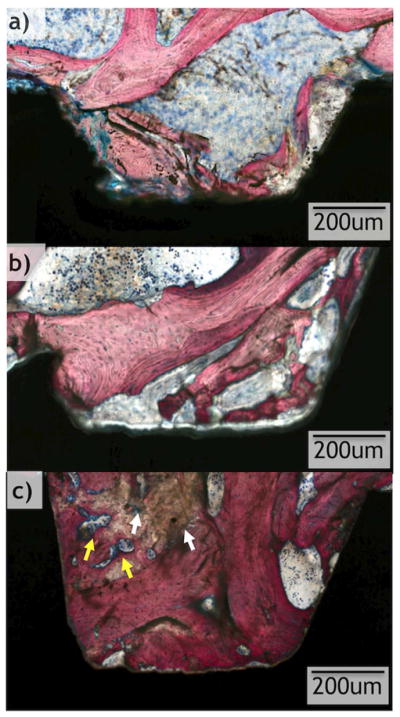
Higher magnification optical micrographs taken from samples at each time point (0, 3, and 6 weeks) from the osseodensification (OD) drilling group. White arrows show residual bone chips from surgical instrumentation, yellow arrows show remodeling sites. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
5. Discussion
The purpose of this study was to utilize a translational model to determine if the osseodensification drilling protocol would improve the osseointegration. In a recent study, osseodensification did prove effective at conserving bone in a low bone-density sheep hip model (Lahens et al., 2016), but to our knowledge, this is the first time osseodensification has been assessed in a spine model where hardware fixation is of utmost importance for the stability of rigid fixation constructs.
Failure to enhance primary implant stability in the spine has been the motivation to exploration of novel, superior fixation techniques. Although conventional orthopedic drilling protocols are the current standard of care for hardware fixation, drawbacks to this method have been observed at the bone-implant interface; for example, the friction between the drill and the osteotomy wall can cause thermal trauma which can induce bone necrosis (Albrektsson and Albrektsson, 1987; McCann et al., 2013; Pandey and Panda, 2013). The same issue is observed in techniques that involve reaming (McCann et al., 2013). Consequentially, osseointegration sometimes fails because the prerequisite, intimate contact between viable bone and implant prosthesis is not achieved.
Several forms of implant failure have been analyzed following the use of traditional drilling protocols. A recent review showed that approximately 50% of spinal implant failures were attributed to screw complications such as loosening (~16%) and fracture (~34%) (Eldin et al., 2014). In an attempt to prevent these type of failures, several studies have employed the use of hydroxyapatite (HA)-coated titanium alloy implants, since HA acts as a biomimetic material to promote osseointegration. Although widely used, it is documented that hydroxyapatite faces rapid wear, thus compromising implant stability (Sandén et al., 2002). Another contributing factor to some cases of spinal hardware fixation failure is the implant site. In patients with osteoporosis, the low-density bone of the spine increases the risk for implant loosening or pullout (Karami et al., 2015). In response to implant loosening, screw augmentation with various fixatives such as hydroxyapatite, polymethylmethacrylate, or tricalcium phosphate have been employed as potential solutions. However, a recent study showed that these augmented screws demonstrate non-significant increases in pull out strength relative to controls (Yi et al., 2015).
Other studies have explored autologous grafting in spinal surgery as a method to promote greater secondary stability (Bròdano et al., 2014; Frosch et al., 2003; Khashan et al., 2013; Rihn et al., 2010). Moving further from previous failures in implant placement and stability, a new surgical method was studied in a sheep spine in the present study. This new technique, osseodensification, is performed via gradual compaction of bone tissue, forming the walls of the osteotomy through densification, essentially acting as an auto-graft to supplement implant fixation (Huwais and Meyer, 2016). In a hip model where low bone density is present, osseodensification drilling was determined to have superior primary stability as measured by insertion torque, and demonstrated no impairment to osseointegration when compared to regular drilling, irrespective of implant macrogeometry (Lahens et al., 2016). The present study investigated if osseodensification would positively affect fixture stability and osseointegration degrees in the higher density bone found in vertebrae. From a theoretical standpoint, its higher density relative to hip bone may preclude significant effects of osseodensification in higher density bone.
Biomechanical competence is influenced by various factors, including implant macrogeometry, microgeometry, nanogeometry, and osteotomy technique (Coelho and Jimbo, 2014; Huwais and Meyer, 2016; Lahens et al., 2016). Primary stability was gauged via measurement of insertion torque (Fig. 1) and pull-out strength (Fig. 2) at t=0, where implants placed into osseodensified sites showed significantly higher values relative to control sites. Enhanced insertion torque is an indicator of stronger primary implant stability (Turkyilmaz et al., 2008). Nonetheless, our data for biomechanical pull-out strength contributes support to the claim that enhanced initial stability was established in our experimental samples due to osteo-compaction achieved via the osseodensification technique. It is likely that drill geometry contributed to these findings. The current approach to surgical instrumentation uses twist drills with positive rake angles to bore out a vacant osteotomy site. In contrast, osseodensification uses a tapered, four-flute bur drill that bores into bone at a negative rake angle to create an osteotomy in which the displaced bone is compacted and compressed circumferentially. Therefore, increase in biomechanical stability is likely due to the increased amount of interfacial bone for the osseodensification sites.
Overall, the only difference observed with respect to % bone-to-implant contact was found as a function of drilling technique; the experimental group demonstrated significantly higher values relative to the control. This is also likely due to the nature of the drilling protocols. i.e. the control utilizes a subtractive method whereas the osseodensification utilizes additive method. Within each respective group, there was no observable change over time.
As seen with BIC, the BAFO osseodensification group presented significantly higher values than the regular drilling group. As a function of time, between 0 and 3 weeks, a decrease in BAFO is observed. Between 3 and 6 weeks, BAFO increased to roughly the same percentage observed at t=0. This can be explained by the phenomena observed within the fixture threads. Surgical instrumentation promotes initial bone remodeling that takes place in tandem with new bone formation (Coelho and Jimbo, 2014; Eldin et al., 2014). the decrease in BAFO seen at 3 weeks indicates a loss of bone fraction due to remodeling, and the regain of bone fraction observed at 6 weeks can be attributed to the production of woven bone occupying the healing chambers. Nonetheless, BAFO values were higher for the osseodensification sites at all times evaluated.
The osseodensification effect on osseointegration can be explained by a further analysis of histology data. An overt difference with regard to implant stability (primary and secondary) and densification around the implants is seen in the samples drilled with the osseodensification drilling technique presented greater volume of woven bone as well as bone-implant contact relative to samples drilled with the subtractive technique. Non-vital bone chips were observed in greater frequency in the osseodensification group relative to the control. These chips are residual fragments that acted as nucleating surfaces, essentially performing the role of autologous grafts to promote new bone formation around the implants, hence the greater stability and density of bone observed in the OD group.
This study has shed light on osseodensification as seen in the sheep spine. Future studies comprising shorter and longer time points in vivo are suggested to elucidate how osseodensification drives the osseointegration pathway. The spine model used in this study was limited by its six-week end point, suggesting a need for longer follow-up and a greater sample size to obtain more established data on the long-term effects of osseodensification. Analysis of the osseodensification technique on a molecular level is also warranted, as it has yet to be explored. Further understanding and implementation of this technique has the potential to diminish hardware failure, offer successful orthopedic fixation at higher rates to patients suffering from compromised bone density/integrity, and drastically reduce time needed to heal from hardware implantation.
Limitations of the present investigation include the need to explore a pathologic bone state as an experimental group. While the spine is a site of low bone density, the bone conservation and enhanced stability osseodensification offers should be assessed in the setting of bone disease to gauge clinical viability. Also, no load was incurred on the implanted devices and studies that include loading of pedicle screws placed under regular and osseodensification drilling are under way. Despite these limitations, this technique can potentially improve the safety and success rates of bony drilling at all sites of low bone density and limited bone volume by using burred bone to facilitate stability.
Based on our data and analyses, successful osseointegration via osseodensification yielded significantly different biomechanical and histologic results relative to the regular drilling method. The results of this study garnered evidence to support the osseodensification method as a potential candidate for spinal surgical instrumentation for fixture placement.
Acknowledgments
The present study was partially funded by Brotech LLC, Jackson, MI and by a National Institute of Arthritis and Musculoskeletal and Skin Diseases award supplement 3R01AR068593-02S1.
References
- Albrektsson T, Albrektsson B. Osseointegration of bone implants: a review of an alternative mode of fixation. Acta Orthop Scand. 1987;58:567–577. doi: 10.3109/17453678709146401. [DOI] [PubMed] [Google Scholar]
- Albrektsson T, Brånemark PI, Hansson HA, Lindström J. Osseointegrated titanium implants: requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981;52:155–170. doi: 10.3109/17453678108991776. [DOI] [PubMed] [Google Scholar]
- Aschoff HH, Kennon RE, Keggi JM, Rubin LE. Transcutaneous, distal femoral, intramedullary attachment for above-the-knee prostheses: an endo-exo device. J Bone Jt Surg Am. 2010;92:180–186. doi: 10.2106/JBJS.J.00806. [DOI] [PubMed] [Google Scholar]
- Barber JW, Boden SD, Ganey T, Hutton WC. Biomechanical study of lumbar pedicle screws: does convergence affect axial pullout strength? Clin Spine Surg. 1998;11:215–220. [PubMed] [Google Scholar]
- Bròdano GB, Giavaresi G, Lolli F, Salamanna F, Parrilli A, Martini L, Griffoni C, Greggi T, Arcangeli E, Pressato D. Hydroxyapatite-based biomaterials versus autologous bone graft in spinal fusion: an in vivo animal study. Spine. 2014;39:E661–E668. doi: 10.1097/BRS.0000000000000311. [DOI] [PubMed] [Google Scholar]
- Coelho PG, Jimbo R. Osseointegration of metallic devices: current trends based on implant hardware design. Arch Biochem Biophys. 2014;561:99–108. doi: 10.1016/j.abb.2014.06.033. [DOI] [PubMed] [Google Scholar]
- Coelho PG, Marin C, Granato R, Giro G, Suzuki M, Bonfante EA. Biomechanical and histologic evaluation of non-washed resorbable blasting media and alumina-blasted/acid-etched surfaces. Clin Oral Implant Res. 2012;23:132–135. doi: 10.1111/j.1600-0501.2010.02147.x. [DOI] [PubMed] [Google Scholar]
- Del Cerro M, Cogen J, Del Cerro C. Stevenel’s blue, an excellent stain for optical microscopical study of plastic embedded tissues. Microsc Acta. 1980;83:117–121. [PubMed] [Google Scholar]
- Eldin M, Mohamed M, Ali AMA. Lumbar transpedicular implant failure: a clinical and surgical challenge and its radiological assessment. Asian Spine J. 2014;8:281–297. doi: 10.4184/asj.2014.8.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facca S, Lahiri D, Fioretti F, Messadeq N, Mainard D, Benkirane-Jessel N, Agarwal A. In vivo osseointegration of nano-designed composite coatings on titanium implants. ACS Nano. 2011;5:4790–4799. doi: 10.1021/nn200768c. [DOI] [PubMed] [Google Scholar]
- Flotte TJ, Seddon JM, Zhang Y, Glynn RJ, Egan KM, Gragoudas ES. A computerized image analysis method for measuring elastic tissue. J Investig Dermatol. 1989;93:358–362. [PubMed] [Google Scholar]
- Frosch KH, Sondergeld I, Dresing K, Rudy T, Lohmann CH, Rabba J, Schild D, Breme J, Stuermer KM. Autologous osteoblasts enhance osseointegration of porous titanium implants. J Orthop Res. 2003;21:213–223. doi: 10.1016/S0736-0266(02)00143-2. [DOI] [PubMed] [Google Scholar]
- Galli S, Jimbo R, Tovar N, Yoo DY, Anchieta RB, Yamaguchi S, Coelho PG. The effect of osteotomy dimension on osseointegration to resorbable media-treated implants: a study in the sheep. J Biomater Appl. 2015a;29:1068–1074. doi: 10.1177/0885328214553958. [DOI] [PubMed] [Google Scholar]
- Galli S, Naito Y, Karlsson J, He W, Andersson M, Wennerberg A, Jimbo R. Osteoconductive potential of mesoporous titania implant surfaces loaded with magnesium: an experimental study in the rabbit. Clin Implant Dent Relat Res. 2015b;17:1048–1059. doi: 10.1111/cid.12211. [DOI] [PubMed] [Google Scholar]
- Giro G, Marin C, Granato R, Bonfante EA, Suzuki M, Janal MN, Coelho PG. Effect of drilling technique on the early integration of plateau root form endosteal implants: an experimental study in dogs. J Oral Maxillofac Surg. 2011;69:2158–2163. doi: 10.1016/j.joms.2011.01.029. [DOI] [PubMed] [Google Scholar]
- Giro G, Tovar N, Marin C, Bonfante EA, Jimbo R, Suzuki M, Janal MN, Coelho PG. The effect of simplifying dental implant drilling sequence on osseointegration: an experimental study in dogs. Int J Biomater. 2013;2013:230310. doi: 10.1155/2013/230310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huwais S, Meyer E. Osseodensification: a novel approach in implant o preparation to increase primary stability, bone mineral density and bone to implant contact. Int J Oral Maxillofac Implant. 2016 doi: 10.11607/jomi.4817. [DOI] [PubMed] [Google Scholar]
- Jensen LN, Jensen JS, Gotfredsen K. A method for histological preparation of undecalcified bone sections containing acrylic bone cement. Biotech Histochem. 1991;66:82–86. doi: 10.3109/10520299109110555. [DOI] [PubMed] [Google Scholar]
- Karami KJ, Buckenmeyer LE, Kiapour AM, Kelkar PS, Goel VK, Demetropoulos CK, Soo TM. Biomechanical evaluation of the pedicle screw insertion depth effect on screw stability under cyclic loading and subsequent pullout. Clin Spine Surg. 2015;28:E133–E139. doi: 10.1097/BSD.0000000000000178. [DOI] [PubMed] [Google Scholar]
- Khashan M, Inoue S, Berven SH. Cell based therapies as compared to autologous bone grafts for spinal arthrodesis. Spine. 2013;38:1885–1891. doi: 10.1097/BRS.0b013e3182a3d7dc. [DOI] [PubMed] [Google Scholar]
- Lahens B, Neiva R, Tovar N, Alifarag AM, Jimbo R, Bonfante EA, Bowers MM, Cuppini M, Freitas H, Witek L. Biomechanical and histologic basis of osseodensification drilling for endosteal implant placement in low density bone. an experimental study in sheep. J Mech Behav Biomed Mater. 2016;63:56–65. doi: 10.1016/j.jmbbm.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Leonard G, Coelho P, Polyzois I, Stassen L, Claffey N. A study of the bone healing kinetics of plateau versus screw root design titanium dental implants. Clin Oral Implant Res. 2009;20:232–239. doi: 10.1111/j.1600-0501.2008.01640.x. [DOI] [PubMed] [Google Scholar]
- Leventhal GS. Titanium, a metal for surgery. J Bone Jt Surg Am. 1951;33:473–474. [PubMed] [Google Scholar]
- Linder L, Carlsson A, Marsal L, Bjursten LM, Branemark PI. Clinical aspects of osseointegration in joint replacement. A histological study of titanium implants. Bone Jt J. 1988;70:550–555. doi: 10.1302/0301-620X.70B4.3403596. [DOI] [PubMed] [Google Scholar]
- McAfee PC, Cunningham BW, Orbegoso CM, Sefter JC, Dmitriev AE, Fedder IL. Analysis of porous ingrowth in intervertebral disc prostheses: a nonhuman primate model. Spine. 2003;28:332–340. doi: 10.1097/01.BRS.0000048504.08086.42. [DOI] [PubMed] [Google Scholar]
- McCann PA, Sarangi PP, Baker RP, Blom AW, Amirfeyz R. Thermal damage during humeral reaming in total shoulder resurfacing. Int J Shoulder Surg. 2013;7:100. doi: 10.4103/0973-6042.118910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller K, Geijer M, Sollerman C, Lundborg G. Radiographic evaluation of osseointegration and loosening of titanium implants in the MCP and PIP joints. J Hand Surg. 2004;29:32–38. doi: 10.1016/j.jhsa.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Nepal M, Li L, Bae TS, Kim BI, Soh Y. Evaluation of osseointegration around tibial implants in rats by ibandronate-treated nanotubular ti-32nb-5zr alloy. Biomol Ther. 2014;22:563. doi: 10.4062/biomolther.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey RK, Panda SS. Drilling of bone: a comprehensive review. J Clin Orthop Trauma. 2013;4:15–30. doi: 10.1016/j.jcot.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PJ, Pelletier MH, Walsh WR, Mobbs RJ. Spine interbody implants: material selection and modification, functionalization and bioactivation of surfaces to improve osseointegration. Orthop Surg. 2014;6(81–89):1757–7861. doi: 10.1111/os.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihn JA, Kirkpatrick K, Albert TJ. Graft options in posterolateral and posterior interbody lumbar fusion. Spine. 2010;35:1629–1639. doi: 10.1097/BRS.0b013e3181d25803. [DOI] [PubMed] [Google Scholar]
- Salou L, Hoornaert A, Louarn G, Layrolle P. Enhanced osseointegration of titanium implants with nanostructured surfaces: an experimental study in rabbits. Acta Biomater. 2015;11:494–502. doi: 10.1016/j.actbio.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Sandén B, Olerud C, Petren-Mallmin M, Larsson S. Hydroxyapatite coating improves fixation of pedicle screws. Bone Jt J. 2002;84:387–391. doi: 10.1302/0301-620x.84b3.12388. [DOI] [PubMed] [Google Scholar]
- Sarendranath A, Khan R, Tovar N, Marin C, Yoo D, Redisch J, Jimbo R, Coelho PG. Effect of low speed drilling on osseointegration using simplified drilling procedures. Br J Oral Maxillofac Surg. 2015;53:550–556. doi: 10.1016/j.bjoms.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Spivak JM, Hasharoni A. Use of hydroxyapatite in spine surgery. Eur Spine J. 2001;10:S197–S204. doi: 10.1007/s005860100286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillander J, Hagberg K, Hagberg L, Brånemark R. Osseointegrated titanium implants for limb prostheses attachments: infectious complications. Clin Orthop Relat Res®. 2010;468:2781–2788. doi: 10.1007/s11999-010-1370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkyilmaz I, Aksoy U, McGlumphy EA. Two alternative surgical techniques for enhancing primary implant stability in the posterior maxilla: a clinical study including bone density, insertion torque, and resonance frequency analysis data. Clin Implant Dent Relat Res. 2008;10:231–237. doi: 10.1111/j.1708-8208.2008.00084.x. [DOI] [PubMed] [Google Scholar]
- Van de Meent H, Hopman MT, Frölke JP. Walking ability and quality of life in subjects with transfemoral amputation: a comparison of osseointegration with socket prostheses. Arch Phys Med Rehabil. 2013;94:2174–2178. doi: 10.1016/j.apmr.2013.05.020. [DOI] [PubMed] [Google Scholar]
- Witek L, Marin C, Granato R, Bonfante EA, Campos FEB, Gomes JB, Suzuki M, Coelho PG. Surface characterization, biomechanical, and histologic evaluation of alumina and bioactive resorbable blasting textured surfaces in titanium implant healing chambers: an experimental study in dogs. Int J Oral Maxillofac Implant. 2013;28:694–700. doi: 10.11607/jomi.2952. [DOI] [PubMed] [Google Scholar]
- Yeniyol S, Jimbo R, Marin C, Tovar N, Janal MN, Coelho PG. The effect of drilling speed on early bone healing to oral implants. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:550–555. doi: 10.1016/j.oooo.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Yerby SA, Toh E, McLain RF. Revision of failed pedicle screws using hydroxyapatite cement: a biomechanical analysis. Spine. 1998;23:1657–1661. doi: 10.1097/00007632-199808010-00008. [DOI] [PubMed] [Google Scholar]
- Yi S, Rim DC, Park SW, Murovic JA, Lim J, Park J. Biomechanical comparisons of pull out strengths after pedicle screw augmentation with hydroxyapatite, calcium phosphate, or polymethylmethacrylate in the cadaveric spine. World Neurosurg. 2015;83:976–981. doi: 10.1016/j.wneu.2015.01.056. [DOI] [PubMed] [Google Scholar]
- Yildirim OS, Aksakal B, Hanyaloglu SC, Erdogan F, Okur A. Hydroxyapatite dip coated and uncoated titanium poly-axial pedicle screws: an in vivo bovine model. Spine. 2006;31:E215–E220. doi: 10.1097/01.brs.0000210221.00778.c7. [DOI] [PubMed] [Google Scholar]
- Zhou R, Wei D, Cao J, Feng W, Cheng S, Du Q, Li B, Wang Y, Jia D, Zhou Y. Synergistic effects of surface chemistry and topologic structure from modified microarc oxidation coatings on ti implants for improving osseointegration. ACS Appl Mater Interfaces. 2015;7:8932–8941. doi: 10.1021/acsami.5b02226. [DOI] [PubMed] [Google Scholar]
- Zhou R, Wei D, Cheng S, Feng W, Du Q, Yang H, Li B, Wang Y, Jia D, Zhou Y. Structure, MC3T3-E1 cell response, and osseointegration of macroporous titanium implants covered by a bioactive microarc oxidation coating with microporous structure. ACS Appl Mater Interfaces. 2014;6:4797–4811. doi: 10.1021/am405680d. [DOI] [PubMed] [Google Scholar]