Abstract
The diagnosis of chronic pancreatitis remains challenging in early stages of the disease. This report defines the diagnostic criteria useful in the assessment of patients with suspected and established chronic pancreatitis. All current diagnostic procedures are reviewed and evidence based statements are provided about their utility and limitations. Diagnostic criteria for chronic pancreatitis are classified as definitive, probable or insufficient evidence. A diagnostic (STEP-wise; S-survey, T-tomography, E-endoscopy and P-pancreas function testing) algorithm is proposed that proceeds from a non-invasive to a more invasive approach. This algorithm maximizes specificity (low false positive rate) in subjects with chronic abdominal pain and equivocal imaging changes. Futhermore, a nomenclature is suggested to further characterize patients with established chronic pancreatitis based on TIGAR-O (T-toxic, I-idiopathic, G-genetic, A- autoimmune, R-recurrent and O-obstructive) etiology, gland morphology (Cambridge criteria) and physiologic state (exocrine, endocrine function) for uniformity across future multi-center research collaborations. This guideline will serve as a baseline manuscript that will be modified as new evidence becomes available and our knowledge of chronic pancreatitis improves.
Keywords: chronic pancreatitis, diagnosis, guidelines, evidence-based
Introduction
At the 2011 meeting of the American Pancreatic Association, a Chronic Pancreatitis Conference was held to review the medical literature to develop the first United States practice guideline for chronic pancreatitis. The APA Practice Guidelines in Chronic Pancreatitis is a three part evidence-based document that reviews the current literature on the diagnosis (Part 1), treatment (Part 2) and management of complications (Part 3) of chronic pancreatitis. The objective of this report is to provide an initial platform on which to build further evidence-based recommendations for the management of chronic pancreatitis as new evidence becomes available.
Chronic pancreatitis (CP) is characterized by chronic, progressive pancreatic inflammation and scarring, irreversibly damaging the pancreas, and resulting in loss of exocrine and endocrine function. It was first described in the medical literature in 1788 by Sir Thomas Cawley, however, a review in the New England Journal of Medicine review stated, despite the “thousands of reports dealing with this disease; it remains an enigmatic process of uncertain pathogenesis, unpredictable clinical course and uncertain treatment.” (1) Fortunately, since that landmark publication there have been major advances in our understanding of CP pathogenesis and pathophysiology, including pancreatic fibrosis, etiologic risk factors, natural history, as well as associated genetic and epigenetic changes (2–6). Clinical diagnostic tools have also seen considerable improvement with advances in endoscopic and radiologic imaging techniques, and the development of endoscopy assisted pancreas function testing has widened its clinical use (7–10). Most importantly, multicenter prospective randomized trials are underway using clearly defined outcomes, including validated pain scales, cost analysis and disease-specific quality of life measures, to clarify the roles of endoscopy and surgery in the management of chronic pancreatitis and its complications (11–18).
The clinical manifestations of chronic pancreatitis can include abdominal pain, steatorrhea and diabetes, as well as numerous acute and chronic complications (16, 18, 19). Current treatments can only provide temporary pain relief and manage complications, but are unable to arrest or slow the progression of this often debilitating illness (13, 19, 20). Additionally, a subset of chronic pancreatitis patients develops pancreatic adenocarcinoma, which is generally advanced at the time of diagnosis due to the marked morphologic changes in the gland that can “mask” tumors, preventing early detection (21, 22).
Both basic scientists and clinical researchers are actively investigating the pathogenesis of chronic pancreatitis (23–25). The resulting advances will certainly improve diagnosis, management and treatment of chronic pancreatitis in the future, but until then evidence-based current practice strategies are needed. Where there is no clear evidence, expert opinion from experienced basic scientists, gastroenterologists, therapeutic endoscopists and surgeons, who specialize in pancreatology, must prevail. Unlike acute pancreatitis, there is a paucity of practice guidelines for chronic pancreatitis in the medical literature. Italian, German and Spanish Consensus Guideline for Chronic Pancreatitis have recently been published and both provide valuable insight into the numerous challenges facing diagnosis and management of this complex disorder.
Methods
The primary aim of this report is to provide current practice guidelines for chronic pancreatitis from an evidence-based review of the medical literature and expert opinion. The development of the APA Practice Guidelines in Chronic Pancreatitis involved the following steps:
A 1-day multidisciplinary symposium, led by leaders in the field of chronic pancreatitis, to review the literature on chronic pancreatitis as it pertains to diagnosis, treatment and management of complications. The organization committee (DC, SV, and CF) invited participation from speakers, moderators and thought leaders specializing in gastroenterology, surgery and therapeutic endoscopy, from numerous academic centers throughout the United States.
The final program was approved by the American Pancreatic Association and organized into three sessions: 1) Diagnostic Guidelines, 2) Treatment Guidelines and 3) Complications and Long Term Management.
Experts reviewed the medical literature, and presented evidence pertaining to chronic pancreatitis, epidemiology, diagnosis, treatment and management of its complications.
Each of the three sessions concluded with commentary from thought leaders summarizing the major challenges facing physicians treating patients with chronic pancreatitis.
Open discussions and break-out meetings were organized after each session to allow for all 2011 APA conference attendees to participate in debate and discussion of controversial areas, and to assist in the development of future practice guidelines.
The conference lectures were transcribed and edited by the speakers, then reviewed and collated by session chairpersons and moderators into a working draft for each of the three sessions.
For recommendations, APA used the classification by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) with slight changes. Strength of recommendation is either strong or conditional (weak). Factors influencing strong recommendation include the quality of evidence, presumed patient-important outcomes, and cost. Conditional recommendation denotes the variability in preferences and values, or more uncertainty, implying less certainty, higher cost, or resource consumption. Quality of evidence is high, A (further research unlikely to change confidence in the estimate of the clinical effect), moderate, B (further research may change confidence), low, C (further research very likely to impact confidence) and very low, D (an estimate of the effect is uncertain). However, in the GRADE approach, high quality evidence may not translate into strong recommendation and weak evidence may result in a strong recommendation. (26)
The conference organization committee reviewed and edited the session drafts, compiling a working draft of the APA Chronic Pancreatitis Practice Guidelines.
The working draft was submitted to independent reviewers, experts in each major session area, for critique and commentary.
A final draft was submitted to the APA Governing Board for editorial review.
After further editorial revisions, the official APA Practice Guideline for Chronic Pancreatitis was finalized and submitted for peer-reviewed publication.
Evidence-Based Report on Diagnostic Guidelines
Chronic pancreatitis should be in the differential diagnosis of a patient with typical features of epigastric pain with radiation to the back, steatorrhea, weight loss, or recurrent acute pancreatitis. Patients generally have known risk factors for chronic pancreatitis such as moderate to heavy alcohol or tobacco exposure. Because the disease is irreversible and carries a social stigma, the diagnosis needs to be certain (i.e., definitive, probable) before labeling a patient with this chronic illness. Furthermore, clinical evaluation is warranted in patients with a high suspicion of disease to rule out pancreas carcinoma that can mimic both its clinical and imaging presentation.
In general, the radiologic and endoscopic evaluation of a patient with suspected chronic pancreatitis should progress from a least invasive to more invasive approach to establish a diagnosis. A CT scan of the pancreas is usually the initial imaging modality of choice. Patients with equivocal or mild CT imaging findings or refractory symptoms may be referred to specialized centers for additional studies such as magnetic resonance imaging (MRI)/secretin-enhanced magnetic resonance cholangiopancreatography (sMRCP) or endoscopic procedures such as endoscopic ultrasound (EUS), endoscopic retrograde cholangiopancreatography (ERCP), and pancreas function testing.
The post-test probability of chronic pancreatic disease is markedly influenced by the clinical history, physical examination, etiologic risk factor profile, and results of radiologic and endoscopic imaging tests. Despite exhaustive attempts at diagnosis, up to 5 to 10% of patients cannot be clearly diagnosed with certainty because of disagreement or discordance between imaging and endoscopic findings.
This manuscript is the first of a three part guideline proposed by the American Pancreatic Association to assist physicians caring for individuals with suspected chronic pancreatitis. We review the current epidemiology, pathology, radiology, endoscopy and physiologic testing methods and procedures that are currently utilized in the evaluation of suspected chronic pancreatitis. We present evidence to support a definitive diagnosis and propose a nomenclature that can be used at academic centers to standardize clinical research reporting of data.
It is our hope that this initial document will serve as an initial platform on which to build further evidence-based recommendations as more knowledge becomes available and as our understanding of chronic pancreatitis pathophysiology improves.
Topic 1. Epidemiology and Risk Factors
Dhiraj Yadav, MD – University of Pittsburgh Medical Center; Pittsburgh, PA (Lead discussant)
Evidence-Based Medicine Statements
Data on population-based estimates of chronic pancreatitis is emerging
A small fraction of patients progress from acute to chronic pancreatitis
-
Alcohol and smoking are independent risk factors for chronic pancreatitis
both are associated with disease progression and their risks are likely multiplicative
The spectrum of risk factors for chronic pancreatitis have broadened
-
Genetic discoveries are rapidly uncovering new susceptibility factors
knowledge of gene, gene-environment interactions may translate into new diagnostic and treatment paradigms
Level of evidence
Conditional recommendation, level of evidence - low
Conditional recommendation, level of evidence - moderate
Strong recommendation, level of evidence - high
Conditional recommendation, level of evidence - low
Strong recommendation, level of evidence – moderate
In the past 50 years we have learned a lot about the epidemiology and risk factors associated with chronic pancreatitis (CP). Most epidemiologic data for CP comes from large case series and cross sectional studies. Population-based data on CP is scarce due to its low incidence and prevalence; difficulty in establishing an early diagnosis; and, variable time course for progression from acute (AP) to CP. The overall incidence of CP (Table 1) ranges from 4.4 to 11.9 per 100,000 per year(26–33). The incidence is higher in men than women by a factor of 1.5 to 3. Data on prevalence of CP are even scarcer and range from 36.9 – 41.8 per 100,000 persons(28, 29).
Table 1.
Incidence and Prevalence of chronic pancreatitis in population-based studies
| Design | Population | Year(s) | Incidence (per 100,000) | ||
|---|---|---|---|---|---|
| All | Males | Females | |||
| Incidence | |||||
| Chart review | Luneberg County, Germany | 1988–95 | 6.4 | 8.2 | 1.9 |
| Moravia, Czech Republic | 1999 | 7.9 | NA | NA | |
| Olmsted County, USA | 1997–2006 | 4.4 | 5.2 | 3.8 | |
| Survey | Japan | 2007 | 11.9 | NA | NA |
| Administrative data | Britain | 1999–2000 | 8.6 | 12.4 | 4.8 |
| USA | 1988–2004 | 8.1 | 4.1 | 4.0 | |
| Netherlands | 2004 | 8.4 | 11.4 | 5.7 | |
| Allegheny County, USA | 1996–2005 | 7.8 | 8.3 | 7.2 | |
| Prevalence | |||||
| Survey | Japan | 1994 | 36.9 | 53.2 | 21.2 |
| Chart review | Olmsted County, USA | 2006 | 41.8 | 51.5 | 33.9 |
The etiology of CP has traditionally been classified as alcohol, hereditary, obstructive, hyperlipidemia and idiopathic (Table 2). The TIGAR-O risk factor classification has been proposed(10) as follows: Toxic-Metabolic, Idiopathic, Genetic, Autoimmune, Recurrent and severe AP associated CP and Obstructive etiologic factors. This classification system was developed with the premise that the risk of developing CP in an individual is determined by the presence of one or more risk factors. A similar MANNHEIM classification has been proposed that also recognizes the role of multiple etiologic factors in disease development(34).
Table 2.
Classification system for etiology and risk factors for chronic pancreatitis
| Classification for Chronic Pancreatitis Etiology |
|
|
| Traditional |
| Alcohol, Idiopathic, Hereditary, Obstructive, Hyperlipidemia |
|
|
| TIGAR-O |
| Toxic-Metabolic: Alcohol, tobacco smoking, hypercalcemia, hyperlipidemia, chronic renal failure, medications, toxins |
| Idiopathic: Early onset, late onset, Tropical |
| Genetic mutations: PRSS1, CFTR, SPINK1, other |
| Autoimmune: Isolated, syndromic |
| Recurrent and Severe Acute Pancreatitis Associated Chronic Pancreatitis: Postnecrotic (severe acute pancreatitis), vascular disease/ischemic, post-irradiation |
| Obstructive: Pancreas divisum, sphincter of Oddi disorders, duct obstruction (eg. Tumor), post-traumatic pancreatic duct scars |
|
|
| M-ANNHEIM |
| M – multiple risk factors including: |
| Alcohol consumption: Excessive (>80 g/d), increased (20–80 g/d), moderate (<20 g/d) |
| Nicotine consumption |
| Nutritional factors: High calorie proportion of fat and protein, hyperlipidemia |
| Hereditary factors: Hereditary, familial, idiopathic (early-onset, late-onset), tropical |
| Efferent duct factors: Pancreas divisum, annular pancreas and other congenital abnormalities of the pancreas, pancreatic duct obstruction (e.g. tumors), post-traumatic pancreatic duct scars, sphincter of Oddi dysfunction |
| Immunological factors: Autoimmune pancreatitis |
| Miscellaneous and rare metabolic disorders: Hypercalcemia, hyperparathyroidism, chronic renal failure, drugs, toxins |
Alcohol and smoking contribute greatly to the development of CP. Alcohol is the single most common etiologic factor and accounts for 44 to 65% cases in population and non-population based studies(35–45). Compared to the general population, the spectrum of alcohol consumption in patients with CP is clearly shifted to the right. The prevalence of pancreatitis in the setting of alcoholism is 3–6 times higher when compared with non drinkers(46), and the absolute risk of pancreatitis among heavy drinkers is 2.5 to 3%(46, 47). There appears to be a threshold of 4–5 drinks/day of alcohol consumption that clearly increases the risk of developing CP(12, 47). Even at lower levels of consumption, alcohol likely plays a disease-modifying role. Experimental data generated using hyper-stimulation models have demonstrated that heavy alcohol exposure increases the susceptibility of the pancreas to other injury(48–50); and after the initiation of pancreatic injury, alcohol modifies the immune response(51).
The association between smoking and CP is more uniform across epidemiologic studies. Smoking is a dose-dependent co-factor for causation of CP(12, 52–58). The risk of developing CP in subjects smoking less than 1 pack of cigarettes per day is 2.4 [0.9–6.6] and increases to 3.3 [1.4–7.9] in those smoking more than 1 pack per day. Overall, smokers are on average three times more likely to develop CP compared to non-smokers (pooled risk estimate 2.8 [1.7–4.8]). More importantly, smoking cessation reduces the risk ratio estimate for CP by about 50% from 2.4 [1.8–4.2] in current smokers to 1.4 [1.1–1.9] in former smokers(59). Similarly, early smoking cessation following clinical onset of CP has been shown to reduce the risk of developing calcifications, while continued smoking is associated with increased risk of disease progression(60). The risk associated with smoking and alcohol together is important and likely multiplicative(12). While alcohol and smoking increase the risk of progression individually, disease progression is more likely in the setting of continued alcohol and smoking(61).
Several genetic variations have been associated with pancreatitis including PRSS1, PRSS2, SPINK1, CTRC, CASR and CFTR(62–72). The role of these gene mutations in CP is becoming increasingly recognized and better understood. It is important to note that these genetic mutations are all primarily linked to the trypsin pathway; the proportion of CP with known genetic mutations is small; and the association of these genetic variations is much stronger in the non-alcoholic etiologies compared to alcoholic pancreatitis (Table 3). Furthermore, specific mutations have been associated with varying degrees of genetic penetrance (80% for PRSS1), and varying risk of idiopathic pancreatitis (CFTR), tropical pancreatitis (CTRC) and non-alcoholic chronic pancreatitis (SPINK1).
Table 3.
Prevalence and consequence from known genetic mutations in chronic pancreatitis
| Gene | Gene Name | Consequence | Prevalence (%) | Comments | |
|---|---|---|---|---|---|
| General Population | CP | ||||
| PRSS1 | Cationic Trypsinogen | Increased trypsinogen activation, decreased trypsin degradation | <0.01 | 2–3 | Penetrance for AP (80%), CP (40%) |
| SPINK1 | Pancreatic Secretory Trypsin Inhibitor (PSTI) | Failure of trypsin degradation | 3–8 | 8 | *Acute phase protein *Risk related to one/two mutations, another risk factor *Risk much higher in non-alcoholic CP |
| CTRC | Chymotrypsin C | Failure of trypsin degradation | ≤1 | 3–4 | Risk higher in Tropical/Idiopathic CP |
| CASR | Calcium Sensing Receptor | Increased extracellular ionized calcium; increased trypsin activation and failed trypsin degradation | 10 | 18 | May have role in alcohol-related pancreatitis, or hyperparathyroidism-induced pancreatitis |
| CFTR | Cystic Fibrosis Transmembrane Conductance receptor | Impaired flushing of pancreatic ducts leading to trypsin activation | 2–3 (ΔF508) 5 (other) |
6 8–9 |
*Risk in pancreas sufficient patients: ~25% *About one third idiopathic CP are compound heterozygotes *Increased recognition of the role of “Atypical/Benign” mutations (e.g. R75Q, etc.) |
The pathogenesis of CP can likely be summarized by a two-hit hypothesis model. In the setting of Pre-existing Acute Pancreatitis Risk Factors (genetic, metabolic, environmental) an initial first Acute Pancreatitis hit initiates or activates the immune system, followed by Post-Acute Pancreatitis followed by complete recovery or progression to CP(73).
In summary, population based data on the epidemiology of CP is emerging and improving our understanding of the epidemiology, risk factors and pathogenesis of the disease. The spectrum of risk factors for CP has broadened. Alcohol and smoking are independent risk factors for CP and interact in a multiplicative manner. Continuous alcohol and smoking abuse are associated with disease progression. Calculating attributable risk for individual risk factors is difficult due to the complex nature of the disease and the variability of genetic and epigenetic factors. However, it is likely that the attributable risk of alcohol for CP is approximately 40% and that of smoking approaches 25%. Only a small fraction of patients with AP progress to CP21,38,51,52. Genetic discoveries are rapidly unraveling new susceptibility factors and the knowledge of gene and gene-environment interactions will likely translate into targeted diagnostic and treatment strategies.
Topic 2. Pathologic Definitions
Daniel S. Longnecker, MD – Dartmouth Medical Center; Hanover, NH (Lead discussant)
Anatomic Pathology-Based statements
Chronic pancreatitis is characterized by atrophy and fibrosis of the exocrine tissue with or without chronic inflammation
Scarring of the parenchyma may be focal, patchy, or diffuse
Progressive fibrosis and atrophy may lead to exocrine insufficiency (steatorrhea) followed by endocrine insufficiency (diabetes)
Autoimmune pancreatitis can mimick pancreas carcinoma
Level of evidence
The usual level-of-evidence statements are generally not used in anatomic pathology.
The pathology of chronic pancreatitis (CP) varies with etiology, but there are changes that are present in all types. Typical changes seen at the gross level include reduction in size of the pancreas, dilatation of ducts, loss of lobular pattern in areas of scarring, and the presence of stones in the ducts (Figure 1). Several of these features are variable and may not be seen in all cases.
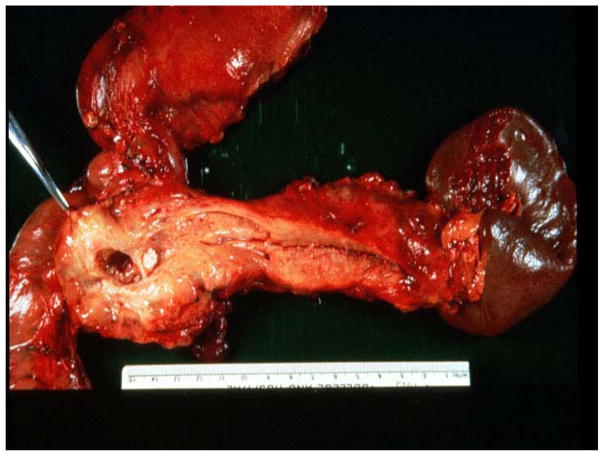
Fig. 1. Pancreas with chronic pancreatitis (CP).
The stomach is at the top, the duodenum at the lower left, and the spleen on the right with the pancreas extending from the spleen to the duodenum. The pancreas is small, probably because of atrophy. The massively dilated main duct with a white stone is seen in the head and body of the pancreas. These are all characteristics of CP. Photo courtesy of Edward Bradley.
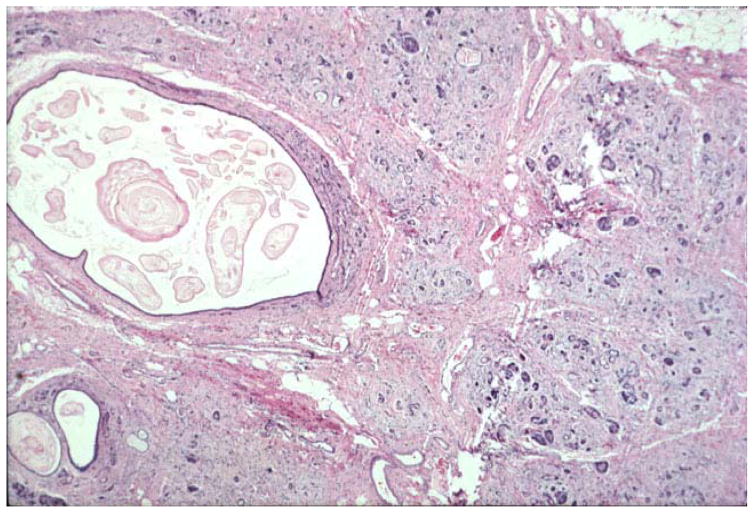
Histologically, the two most common features of CP are loss of acinar tissue (atrophy) and fibrosis. The fibrosis may surround the lobules (perilobular or interlobular fibrosis), or extend into the lobules of acinar tissue (intralobular fibrosis) (Figures 2 and 3). A chronic inflammatory infiltrate may be present (Fig. 2), but this feature is highly variable and disappears late in the course of CP (Figure 3). A diagnosis of CP may be made on the basis of atrophy and fibrosis in the absence of other changes. Islets often survive until late in the course of the disease—becoming more closely spaced as acinar tissue is lost (Figure 4). In cystic fibrosis which may be regarded as a special form of CP, there is dilation of ducts resulting from the abnormal secretions with progressive fibrosis and loss of acinar tissue (Figure 5).
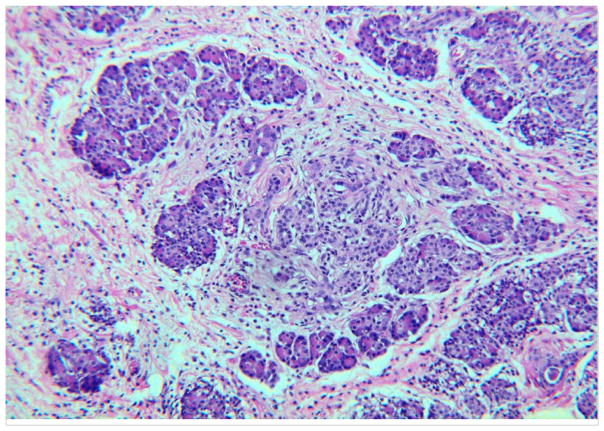
Fig. 2. Pancreas with CP.
Note the fibrosis between and surrounding the lobules of acinar tissue. This is perilobular or interlobular fibrosis. The fibrosis extends into the lobule at the center and that is referred to as intralobular fibrosis. There is a chronic inflammatory infiltrate in this pancreas, best seen in the fibrous tissue. Hematoxylin and eosin stain (H&E).
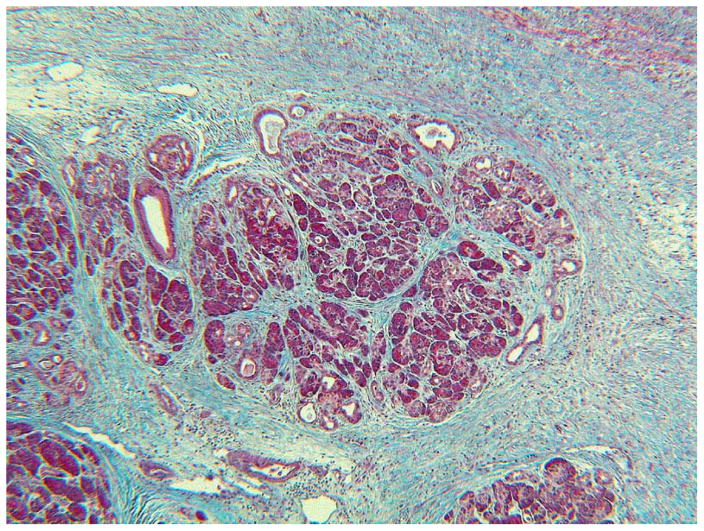
Fig. 3. Perilobular and Intralobular Fibrosis.
The collagen is blue. You can see the massive amounts of collagen around the lobule and extending into the lobule. The acini are atrophic. Small ducts along the top margin of the lobule are somewhat dilated. These are all characteristics of CP. There is less inflammatory infiltrate here than in Fig. 2. Connective tissue stain.
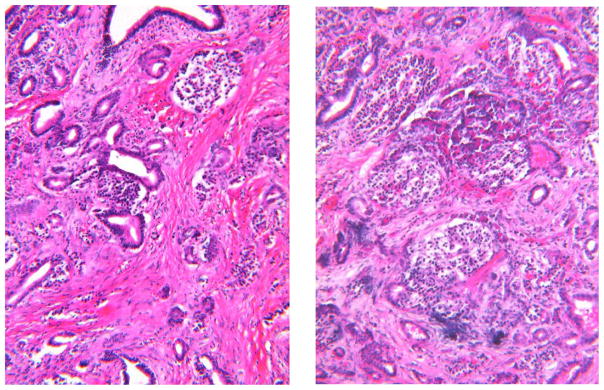
Fig. 4. Atrophy and Fibrosis (alcoholic CP).
The amount of collagen (pink) is increased, especially in the left-hand panel, Note the slight dilation of ducts and the loss of acinar tissue. On the right there are 5 islets located close together because of the loss of acinar tissue. Foci of calcification are blue. H&E
Fig. 5. Pancreas in cystic fibrosis.
Note the diffuse fibrosis, loss of acinar tissue, persistence of ducts and some islets. The ducts are dilated and contain mucus. This is an advanced stage of cystic fibrosis in which inflammation has largely “burned out”. H&E
Chronic pancreatitis can be a patchy or localized process with regional involvement (Figure 6). This is best understood by considering the mechanisms of pathogenesis, in particular the necrosis-fibrosis hypothesis which posits that CP develops as a result of multiple episodes of acute pancreatitis with necrosis and scarring. This process may be patchy at first, progressing to a diffuse pattern following multiple episodes. This is commonly considered to be the mechanism in alcoholic CP, paraduodenal CP and likely hereditary pancreatitis. On the other hand, duct obstruction can lead to progressive fibrosis and loss of acinar tissue that may be localized or segmental, as in the presence of an obstructing neoplasm, or may be diffuse as is characteristic of cystic fibrosis.
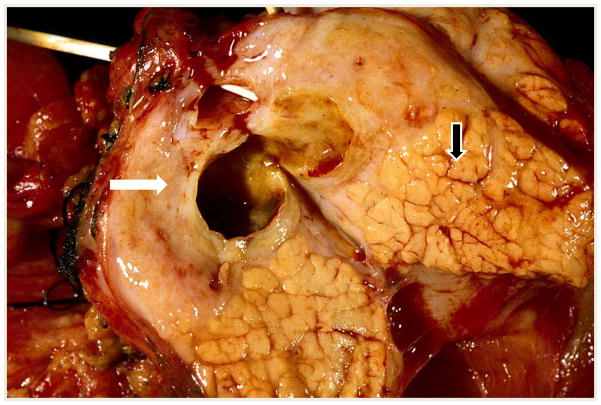
Fig. 6. CP can be a patchy or localized process.
In the upper left portion of this transected pancreas there is a dilated duct surrounded by scar tissue (fibrous tissue) with virtually complete loss of acinar lobules (WHITE ARROW). In the lower right portion of the same trans-section, there is relatively good preservation of the acinar lobules illustrating that CP can have quite localized involvement(BLACK ARROW).
In a review focused on alcoholic pancreatitis, we find the statement that “both alcoholic and non-alcoholic chronic pancreatitis result in indistinguishable pancreatic damage” (74). A recently reported international comparison study provides evidence that the participating pathologists did not distinguish between alcoholic and obstructive CP, although they did become proficient at recognizing autoimmune pancreatitis (AIP) and its subtypes (75). AIP is probably the most histologically distinct type of CP (Figure 7). However, some expert pathologists do observe characteristics which distinguish different CP etiologies, thereby providing a basis for subclassification. Klöppel provides a histologic classification that includes alcoholic, hereditary, autoimmune, paraduodenal, and obstructive CP based on differences in the prevalence of various criteria such as necrosis, pseudocyst, fibrosis, duct lumen, duct contents, and duct epithelium (76). For example, necrosis and pseudocyst are much more likely to be seen in alcoholic CP than in the other forms. However, there is clearly an overlap of most histologic features among all types. A universal histologic grading and scoring system is needed to correlate clinical features with surgical pathologic findings.
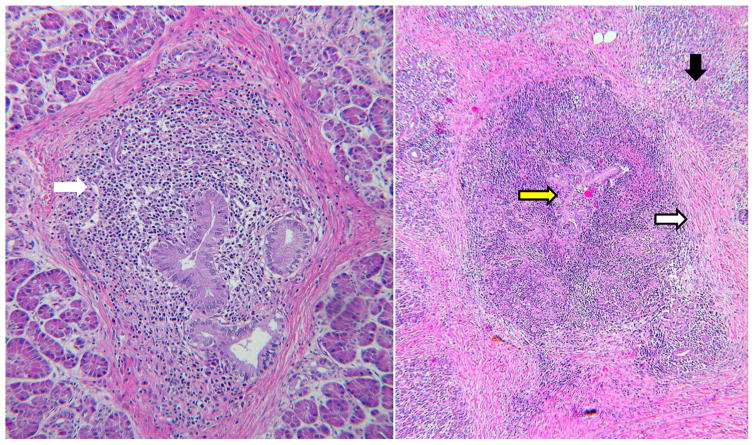
Fig. 7. Inflammation of pancreatic duct walls is the most distinctive feature of AIP.
Although lobules can be involved, inflammation in the duct wall is more conspicuous. On the left there is inflammation in the duct wall (WHITE ARROW) and fibrosis around the duct. On the right there is severe ductitis (YELLOW ARROW) with paraductal and interlobular fibrosis (WHITE ARROW)and atrophy of acinar tissue(BLACK ARROW). H&E
In summary, atrophy and fibrosis of the exocrine tissue are the key features seen in CP. There may or may not be evidence of chronic inflammation, but it is quite appropriate to refer to CP as a fibroinflammatory disease. The scarring can be focal initially and may progress to become diffuse. The loss of acinar tissue may result in exocrine insufficiency and ultimately loss of islet tissue with diabetes. Acute phase pseudocysts may persist in CP that has developed via the necrosis-fibrosis pathway.
Topic 3. CHRONIC PANCREATITIS: ULTRASOUND AND COMPUTED TOMOGRAPHY
Frank H. Miller, MD - Northwestern University; Chicago, IL (Lead discussant)
Evidence-Based Medicine Statements
Ultrasound and CT are best for the late findings of chronic pancreatitis but are limited in the diagnosis of early or mild pancreatitis.
Intraductal pancreatic calcifications are the most specific and reliable sonographic and CT signs of chronic pancreatitis.
CT is helpful for the diagnosis of complications of chronic pancreatitis.
CT is helpful for diagnosis of other conditions that can mimic chronic pancreatitis.
Level of evidence
Conditional recommendation, level of evidence – moderate
Strong recommendation, level of evidence - moderate
Strong recommendation, level of evidence – moderate
Conditional recommendation, level of evidence - low
The clinical diagnosis of chronic pancreatitis, especially in its early stages, can be difficult and frustrating for patients and treating physicians. Diagnosis of CP by Ultrasound and CT imaging relies on changes in morphology of the pancreas, easily detected in the setting of advanced disease, but challenging in early CP. The frequent lack of histopathologic confirmation of the diagnosis further complicates evaluation. The key findings are parenchymal loss (glandular atrophy), chronic inflammation, and fibrosis of the pancreas. Important ductal findings include beading, dilated side branch radicals, enlargement of the main pancreatic duct as well as dystrophic intraductal calcifications.
Transabdominal ultrasound has been used for many years to evaluate the pancreas. While there have been improvements in hardware and high resolution transducers, there have not been any recent studies to evaluate the diagnosis of CP using ultrasound. Ultrasound is a noninvasive, inexpensive and rapid method of evaluating morphological changes in the pancreas, however, considerable limitations reduce its diagnostic utility. Initially, bowel gas and the patient’s body habitus may obscure the pancreas. Assuming adequate visualization, the majority of transabdominal ultrasound findings are neither specific nor sensitive in the diagnosis of CP. The classic sonographic findings of CP are pancreatic calcifications (Figure 8). These are seen as multiple echogenic foci in as many as 40% of patients.(77) These foci may or may not shadow based on their size and may show color Doppler twinkling artifact.(78) There is no correlation between exocrine function and the number of calcifications.(79, 80) Late findings seen on ultrasound include alterations of the size and echogenicity of the gland, pancreatic calcifications, pancreatic duct dilatation and irregularity, and biliary dilatation. Ultrasound can be used to visualize pseudocysts and complications of chronic pancreatitis, including biliary dilatation and splenic vein thrombosis. Pancreatic parenchymal echogenicity may be normal or decreased in CP, making it an unreliable diagnostic feature.(77, 81, 82)
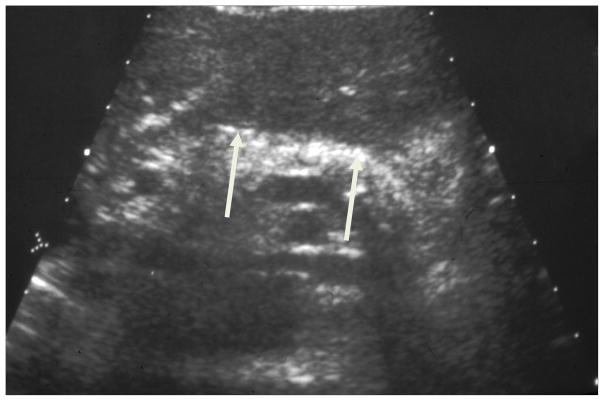
Figure 8. Ultrasound in Chronic Pancreatitis.
Transabdominal ultrasound demonstrating calcifications (white arrow) in the pancreas due to chronic pancreatitis.
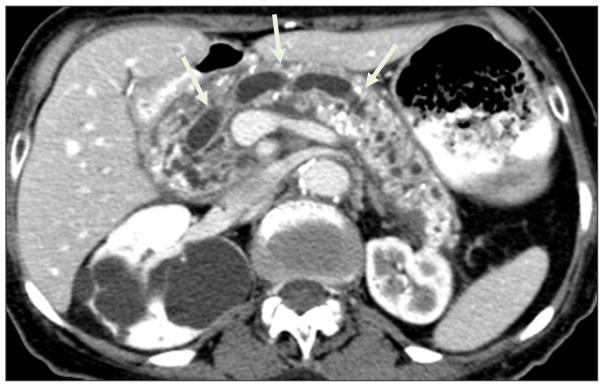
CT is considered by many to be the best initial imaging test for CP.(82–85) It is widely available and allows for comprehensive detailed evaluation of the pancreas. Despite marked improvements in CT, including the development of MDCT, there have not been any recent studies evaluating the sensitivity or specificity for CP. The classical CT findings in chronic pancreatitis are dilatation of the pancreatic duct, pancreatic calcifications, and parenchymal atrophy.(82–85) A retrospective study from the Mayo Clinic published in 1989 studied 56 patients with documented chronic pancreatitis(Figure 9).(86) A dilated pancreatic duct and secondary radicals was the most common findings seen in 68% of patients (Figure 8).(86) Intraductal calcifications were seen in as many as 50% of patients (Figure 10).(86) These were scattered or clustered, focal or diffuse. Calcifications develop from deposition of calcium carbonate in inspissated intraductal protein plugs. Calcifications, however, are associated with advanced or severe CP (Figure 11).(87) Other findings include pancreatic parenchymal atrophy (54%), although this is variable and the pancreas can even be enlarged (30%) or normal in appearance (7%) making the diagnosis difficult (Figure 12) .(86) Unfortunately, parenchymal atrophy is neither sensitive nor specific and can be seen as a normal aging process. Many patients may have a normal-appearing pancreas on CT despite severe exocrine dysfunction. Other CT findings include main pancreatic duct dilatation and dilated secondary radicals. The main pancreatic duct is classically beaded and irregular; however, the main duct may also be regularly contoured. CT is especially helpful in identifying complications of CP, including pseudocysts, portosplenic venous thrombosis, collaterals and arterial pseudoaneurysms, and pancreatico-pleural fistulas.(82–85) CT is especially helpful because it can exclude other causes of abdominal pain or weight loss besides CP. In the literature, studies evaluating the CT features of chronic pancreatitis are from the 1980s. Since then vast improvements in CT technology, including multidetector CT, multiple phase imaging following contrast enhancement and thinner collimation, have undoubtedly improved the sensitivity of the diagnosis of chronic pancreatitis. Reevaluation of chronic pancreatitis using CT is long overdue.(82)
Figure 9.
CT Findings in Chronic Pancreatitis
Figure 10. Ductal changes in Chronic Pancreatitis.
Dilated, beaded main pancreatic duct (white arrow) and parenchymal atrophy and calcifications.
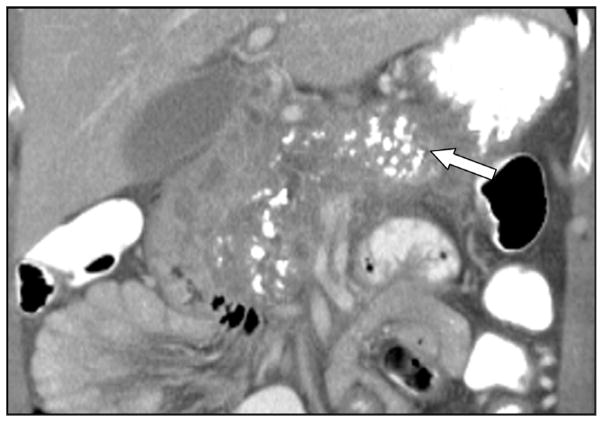
Figure 11. Calcifications in Chronic Pancreatitis.
Intraductal and parenchymal calcifications (white arrow) in chronic pancreatitis.
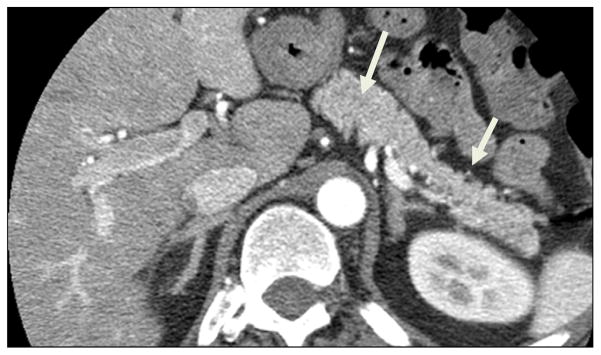
Figure 12. CT can appear normal in CP.
CT image shows typical pancreatic appearance without calcifications or radiologic evidence to suggest chronic pancreatitis. Follow-up MRI/MRCP showed markedly abnormal main pancreatic duct and side branches.
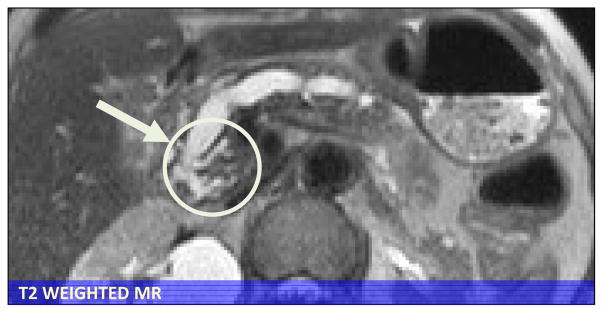
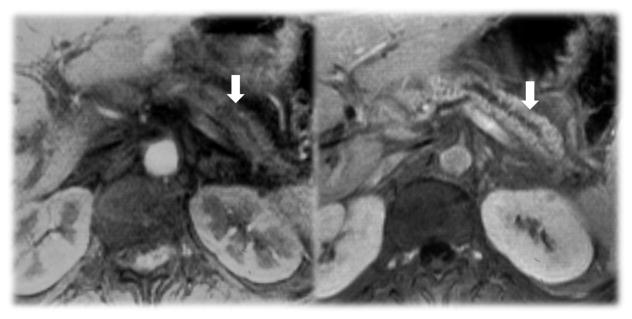
It is important to distinguish chronic pancreatitis from cancer using imaging studies, however this is not always possible.(88–90) Features that favor CP is intraductal or parenchymal calcifications, lack of obstructing mass, irregular dilatation of the pancreatic duct, and relatively limited atrophy of the gland. The presence of a “duct-penetrating” sign, that is a dilated duct or branches which penetrate an apparent mass, favors chronic pancreatitis(Figure 13).(88) Features favoring cancer include pancreatic duct dilatation with associated mass at the site of obstruction with associated atrophy of the pancreas, vascular invasion and metastases.
Figure 13. Duct penetrating sign in “mass-forming” CP.
Distinguishing CP from cancer can be difficult in mass forming chronic pancreatitis. Visualization of the pancreas duct as it penetrates the mass (white arrow and circle) on MRI favors a diagnosis of CP. ERCP and biopsy suggested adenocarcinoma but surgical pathology Whipple specimen confirmed chronic pancreatitis.
In summary, CT is useful as an initial radiologic test; helpful to visualize calcifications and duct abnormalities, and exclude other non-CP etiologies for pain or weight loss. In addition, ultrasound and CT are best for the late findings of CP but have limitations especially in the early diagnosis of mild to chronic pancreatitis. Imaging is quite helpful for the diagnosis of complications of CP.
Topic 4. MRI Imaging
Koenraad J. Mortele, MD - Beth Israel Deaconess Medical Center; Boston, MA (Lead discussant)
Evidence-Based Medicine Statements
Compared to US and CT, MRI is a more sensitive imaging tool for the diagnosis of chronic pancreatitis.
Ductal abnormalities are very specific and reliable MRI signs of chronic pancreatitis.
Signal intensity changes in the pancreas, seen on MRI, may precede ductal abnormalities and suggest early chronic pancreatitis.
Stimulation of the pancreas using IV secretin may improve the diagnostic accuracy in the detection of ductal and parenchymal abnormalities seen in chronic pancreatitis.
Level of evidence
Conditional recommendation, level of evidence – moderate
Conditional recommendation, level of evidence – low
Conditional recommendation, level of evidence – low
Conditional recommendation, level of evidence – low
Chronic pancreatitis (CP) leads to irreversible parenchymal and ductal changes in the pancreas. Magnetic resonance imaging (MRI) may be able to provide an early diagnosis of CP so patients can be treated early on, or patients can be applied treatment options that may prevent progression. The diagnosis of early CP typically relies on the presence of clinical symptoms, pancreatic exocrine function testing (the “gold” standard) and imaging. MRI is highly sensitive and specific to make the diagnosis of CP by evaluating both parenchymal and ductal changes, especially in patients with more advanced CP. However, MRI can also help in the diagnosis of early CP by evaluating the exocrine response of both the ducts and parenchyma after hormonal stimulation of the gland using intravenous secretin.
When applying a standard MRI/MRCP protocol, radiologists should look, from a ductal perspective, for changes that are induced by the periductal fibrosis, the resultant duct ectasia, and obstructed outflow. These changes of side-branch abnormalities, main duct dilation, and strictures, or presence of intraductal stone and intraparenchymal cyst formation can be graded using the Cambridge Classification (Figure 14). In addition to evaluating the ductal changes, MRI is also very sensitive to detect parenchymal abnormalities; what radiologists specifically should look for is subtle signal intensity decreases within the gland, especially on fat-suppressed T1-weighted images. It is very important to realize that these parenchymal abnormalities may precede the ductal abnormalities. Therefore, CT or ultrasound may be falsely negative in the detection of early CP because of their lack of detecting subtle ductal abnormalities. These tests fail to depict these parenchymal changes that are only detected with MRI. Another important conventional MRI feature of CP is the delayed and diminished enhancement of the gland following gadolinium chelates administration. Therefore, in summary for conventional MRI/MRCP, the parenchymal features of CP include, in addition to atrophy (Figure 15), abnormal decreased signal intensity on the fat-suppressed T1-weighted images (Figure 16) and delayed and limited enhancement following contrast administration (Figure 17).
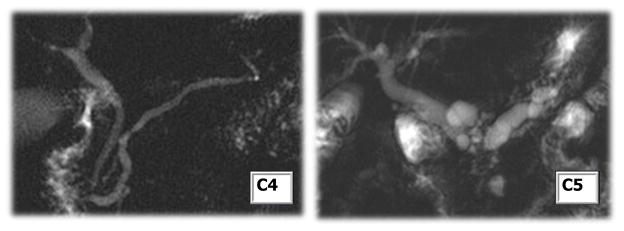
Figure 14. Classic CP ductal changes.
Cambridge 4 (minor main pancreas ductal abnormalities with side branch changes, left) and Cambridge 5 (major main pancreas ductal changes, right) in CP
Figure 15. Glandular atrophy.
Sequential (right to left) contrast enhanced MRI showing glandular atrophy and dilation of main pancreas duct in CP
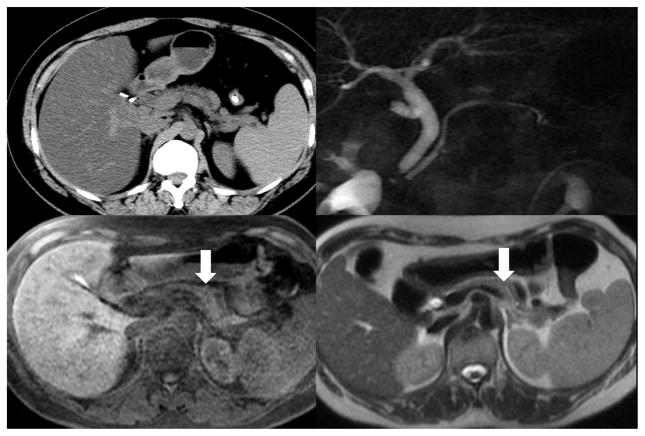
Figure 16. Low T1 Signal.
MRI displaying decreased pancreas signal intensity on T1-weighted images (lower right and left). No major CP changes observed on CT (upper left) or MRCP (Upper right)
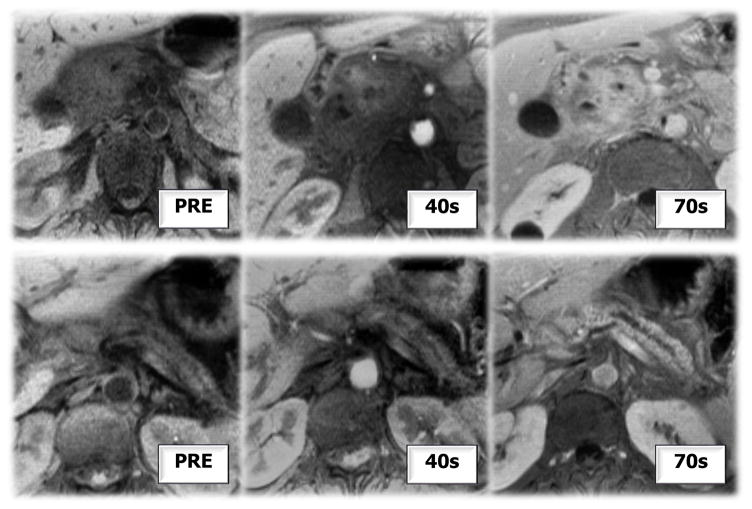
Figure 17. Delayed Contrast Enhancement.
Pre and post contrast MRI showing delayed contrast enhancement of pancreas
As mentioned above, one could currently explore the MRI appearances of early CP following hormonal stimulation of the pancreas, using IV secretin, and look for new or improved detection of ductal and parenchymal abnormalities (Figure 18). One’s primary assessment in evaluating the pancreas with secretin-enhanced MRCP should focus on the pancreatic duct compliance (PDC) which defines a normal distention (of approximately 1 mm) of the ducts following secretin stimulation and then a recovery of the duct diameter to baseline (10 minutes after IV secretin injection). Several investigators have evaluated the time to peak main pancreatic duct dilation after IV secretin stimulation to differentiate a normal pancreas from a pancreas in patients who have CP. Patients with early CP may have completely normal conventional MRCP/MRI studies and only the secretin stimulation will depict the mild abnormal pancreatic ductal compliance. Furthermore, one should also evaluate the IV secretin-augmented MRCP images for increased number of side branches or new recognition of side branches, and evaluate the exocrine pancreatic function by assessing the production and excretion of bicarbonate and fluid by the gland. The latter can be evaluated both quantitatively and semi-qualitatively. Semi-qualitatively, one can apply a rudimentary grading system that evaluates the filling of the duodenal and jejunal loops of bowel following stimulation. A much more accurate way is to actually measure the exact amount of fluid that gets produced by the gland using a multislice fast T2-weighted sequence and a very simple mathematical model. Measuring the signal intensity of the fluid and the 3-D area of fluid before and after the stimulation of the pancreas with IV secretin allows measuring how much fluid is being produced by the gland over time and can provide a flow rate chart. This quantitative model has been used successfully to evaluate the exocrine fluid flow rate in patients with CP pre and post secretin administration.
Figure 18. Secretin MRCP.
Serial sequential images of pancreas displaying ductal and duodenal filling changes after secretin administration
Lastly, some future MRI applications are on the horizon that may have immediate impact on the way we image patients with CP. The more universal use of high field strength magnets now allows for increased signal-to-noise in the pancreas, and using those high field strength magnets, we can depict more subtle abnormalities in the pancreas compared with lower field strength magnets. Perfusion MRI explores that fact that the pancreas in patients with CP has decreased and delayed enhancement and aims to detect subtle alterations in pancreatic parenchymal perfusion. Diffusion-weighted MR imaging measures the restriction of free Brownian motion (diffusion) of water molecules in the gland. The more fibrosis there is the more likely there will be less diffusion of water molecules (the latter is measured as ADC – apparent diffusion coefficient); therefore, ADC values will be lower in patients with CP than in normal patients. Exploiting this idea, one can actually evaluate the gland using diffusion MRI after IV secretin stimulation and enhance the sensitivity to depict subtle abnormalities in diffusion restriction, and separate normal patients from those with early CP.
In summary, MRI is a great tool to detect ductal and parenchymal changes in CP patients; both at “baseline” or after stimulation with IV secretin. The complexity of the disease, however, remains challenging and limits the accuracy of MRI. Ductal changes may be preceded by parenchymal changes and vice versa. Additional findings can only be detected if one stimulates the gland with IV secretin. There probably is a need for a “EUS like” MRI based staging system, which doesn’t exist at this point, combining the ductal changes with the parenchymal changes, and with the findings following IV secretin stimulation.
TOPIC 5. ENDOSCOPIC ULTRASOUND
Michael J. Levy, MD - Mayo Clinic Rochester; Rochester, MN (Lead discussant)
Evidence-Based Medicine Statements
The ideal threshold number of EUS criteria necessary to diagnose CP has not been firmly established, but presence of ≥5 and ≤2 strongly suggests or refutes the diagnosis of CP.
EUS features of CP are not necessarily pathologic and may occur as a normal aging, as a normal variant, or due to non-pathologic asymptomatic fibrosis in the absence of endocrine or exocrine dysfunction.
The relatively poor interobserver agreement (IOA) for EUS CP features limits the diagnostic accuracy and overall utility of EUS for diagnosing CP.
Level of evidence
Strong recommendation, level of evidence – low
Strong recommendation, level of evidence – low
Strong recommendation, level of evidence – moderate
EUS is an often used diagnostic tool for evaluating chronic pancreatitis due to the ability to visualize subtle alterations in pancreatic structure before other imaging modalities and functional tests are abnormal. While the EUS sensitivity is enhanced as one lowers the necessary number of criteria for diagnosis, the already poor specificity further decreases, often leading to unnecessary and often risky interventions. Some have even performed total pancreatectomy and auto-islet cell transplant for pain secondary to presumed CP, only to find normal histology in the resected specimen.
The EUS diagnosis is based on ductal and parenchymal criteria described by the International Working Group using minimum standard terminology (MST) and the criteria have been linked to distinct pathologic correlates identified on histological examination. (Figure 19) (91–93) While the MST system classically lists 9 potentially abnormal features, others also consider two additional features including an enlarged pancreas and narrowing of the pancreatic duct. The number of EUS criteria needed to diagnose CP varies among endosonographers and institutions.
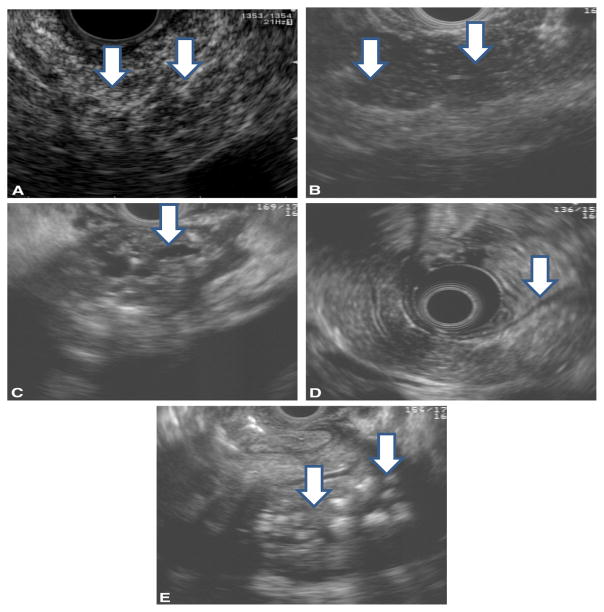
Figure 19. Selected CP EUS criteria.
EUS morphologic features of chronic pancreatitis. (adapted from reference 9)
A. Hyperechoic foci and strands.
B. Lobularity.
C. Dilated, irregular pancreatic duct
D. Hyperechoic duct margin
E. Calcified, shadowing stones.
The ideal threshold number of EUS criteria necessary to diagnose CP has not been firmly established. For patients with no pancreatic pathology and those with severe CP, EUS provides reliable and accurate diagnosis. However, the diagnosis is seldom in doubt for such patients. EUS offers the greatest potential to impact patient care in persons with early or mild CP, however it is in this subset of patients that results are most uncertain and unreliable. Previously there was broad agreement that the presence of 5 or more features reliably establishes the diagnosis of CP and that absence of all features reliably excludes CP. (91, 94)
However, given that some EUS features are actually physiologic and others pathologic, the 5 criteria threshold may not be appropriate. It has been suggested that the presence of 1–2 EUS features should be regarded as a normal gland and that the presence of 3–4 criteria may indicate early or mild CP. Diagnosing CP based solely on minimal EUS criteria, with otherwise negative or inconclusive findings, is strongly discouraged. It is important to correlate the EUS findings with clinical, structural, and functional analyses in this group of patients with possible early or indeterminate disease.
Pancreatic injury is a progressive process that leads to increasing structural abnormalities and functional deficits over time. The degree of injury necessary to designate CP is not clearly defined. While exocrine pancreatic insufficiency in the form of steatorrhea does not typically occur until 70–90% or more of functional capacity is lost, the occurrence, timing and severity of disease manifestations greatly vary. Our goal is to accurately diagnose early CP to allow effective intervention to positively influence the natural disease course. However, there is a paucity of data confirming the impact of any currently available therapies or interventions. Efforts to enhance early diagnosis are typically associated with over-diagnosis and decreased diagnostic specificity. Given the limited impact of early interventions and risk of delivering unnecessary and risky care, most set test parameters to favor a greater positive predictive value at the cost of a somewhat delayed diagnosis.
Many have questioned the predictive value of individual EUS criteria and suggested their respective predictive values may differ significantly. Rajan et al evaluated 120 patients without evidence of pancreatic disease who underwent EUS for a non-pancreaticobiliary indication. (95) They identified at least one parenchymal and/or ductal EUS abnormality in 28% of patients, with a trend toward increasing numbers of abnormal features with age: <40 years (23%), 40 to 60 years (25%), and >60 years (39%); (p = 0.13). Hyperechoic stranding (n = 22) was the most common finding in all age groups. They concluded that ductal or parenchymal calculi, duct narrowing, duct dilatation, or the presence of more than 3 features appeared to be the most specific features for diagnosing CP regardless of age. Others have reported similar findings. (93, 96)
The Rosemont classification was developed from a consensus conference that included 32 internationally-recognized endosonographers. (97) Participants defined each EUS CP criterion and applied these features in a manner that was felt to optimize the diagnostic accuracy. Major and minor criteria were developed and subdivided into major A and major B because of a perceived difference in predictive accuracy. Parenchymal major A criteria included (1) hyperechoic foci with shadowing and (2) well-circumscribed lobularity and the only ductal major A criteria was main pancreatic duct (MPD) calculi. The only parenchymal major B criteria was lobularity with honeycombing. Minor parenchymal criteria were cysts, stranding, non-shadowing hyperechoic foci, and lobularity with noncontiguous lobules. Minor ductal criteria were dilated ducts (body ≥3.5 mm and tail ≥1.5 mm), irregular MPD contour, dilated side branches ≥1 mm, hyperechoic duct margin. The diagnosis of CP based on the “Rosemont Classification” was “consistent” if one of the following was present (A) 1 major A feature (+) ≥3 minor features (B) 1 major A feature (+) major B feature (C) 2 major A features. All other combinations of features were categorized as “suggestive”, “indeterminate” or “normal.” While this classification system represents an important step forward due to the use of weighted criteria, their findings were based solely on expert opinion.
It is uncertain which EUS features are pathologic, normal age-related findings, normal anatomic variants, or due to non-diagnostic asymptomatic fibrosis in the absence of endocrine or exocrine dysfunction. (95) Such “asymptomatic” fibrosis has been reported in alcoholism, advanced age, male gender, obesity, and cigarette smoking. These features have been associated with abnormal pancreatic imaging and histology in patients without evidence of CP, and are reported in as many as 60% of alcoholics without clinical evidence of CP. While some of these patients may have mild disease destined to increase in severity over time, the lifetime risk of CP in alcoholics is only 2–5%, far less than the presence of fibrosis alone. EUS findings must be interpreted with caution due to the inability to distinguish this clinically and metabolically benign “asymptomatic” fibrosis from true CP.
One of the greatest limitations in the use of EUS for evaluating CP is the relatively poor interobserver agreement (IOA). Wallace et al studied the interpretations from 11 expert endosonographers, blinded to clinical information, who evaluated videotaped examinations for the presence of 9 CP criteria among 33 patients with CP. (92) While agreement was good in 2 features, duct dilatation (kappa = 0.6) and lobularity (kappa = 0.51), agreement was poor for the other 7 features (kappa < 0.4). There was moderate overall agreement for the final diagnosis of CP (kappa = 0.45). Topazian et al assessed IOA for interpretation of EUS in persons at high risk for pancreatic cancer using recorded video clips and found similar results. (98)
In summary, while EUS is an important diagnostic tool for evaluating many gastrointestinal and non-gastrointestinal disorders, the same may not be true for suspected CP. Despite advances in EUS technology and training, there has been no meaningful progress in EUS-based diagnosis since initial reports. The limitations of EUS are greatest for patients within the early/ indeterminate CP and definite CP diagnostic range [>2, <5]. Definitive diagnosis in this vulnerable patient population must include pancreas function testing and advanced radiologic imaging such as MRI and S-MRCP. Further study is needed to refine the definitions of EUS features of CP, to determine the relative predictive value of each feature, to establish an ideal threshold number of criteria and to consider the composite results in a manner that optimizes their diagnostic and prognostic utility. We must also improve our understanding of the impact of factors such as aging, obesity, and smoking on imaging. Any meaningful advance in the use of EUS will mandate broader reproducibility in image interpretation and improved interobserver agreement. If we are to error in the use of EUS, we should do so in a manner that favors more stringent diagnostic criteria to avoid over-diagnosis and delivery of unnecessary and potentially risky therapies. This approach will lead to failed diagnosis in some patients, but is favored given the current lack of reliably effective therapies.
Topic 6. ERCP
Richard Kwon, MD, MS - University of Michigan Medical Center; Ann Arbor, MI (Lead discussant)
Evidence-Based Medicine Statements
Endoscopic retrograde pancreatogram (ERP) is rarely used for diagnostic purposes.
The correlation between the Cambridge Criteria and histology is highest in advanced chronic pancreatitis.
Multiple confounders limit the interpretation of ductal changes by Cambridge Criteria.
Level of evidence
Strong recommendation, level of evidence - moderate
Strong recommendation, level of evidence - moderate
Strong recommendation, level of evidence - low
With the widespread availability of cross-sectional imaging (CT and MRI) and endoscopic ultrasound (EUS), endoscopic retrograde pancreatograms (ERP) are rarely, if ever, used to diagnose chronic pancreatitis and instead, have largely been relegated to a therapeutic procedure. ERP had been utilized to identify ductal abnormalities or obstructions, to clarify ductal anatomy prior to surgical intervention, and to confirm the patency of post-surgical anastomoses, including pancreaticojejunostomies(99). Now recent guidelines, published by the ASGE in 2006, recommend that ERP should be reserved for patients in whom the diagnosis still remains unclear after pancreatic function testing or other noninvasive (CT or MRI) or less invasive imaging studies (EUS) have been performed(100).
The diagnosis of chronic pancreatitis by ERP is made on the basis of changes in the main pancreatic duct and side branches, requiring a quality pancreatogram to most accurately delineate the ductal anatomy (101). Contrast should be injected through the length of the pancreatic duct to the tail, as well as into the secondary branches while avoiding acinarization of contrast (101). It is also important to avoid intraductal air bubbles and movement artifact, which will contribute to misinterpretation of the pancreatogram.
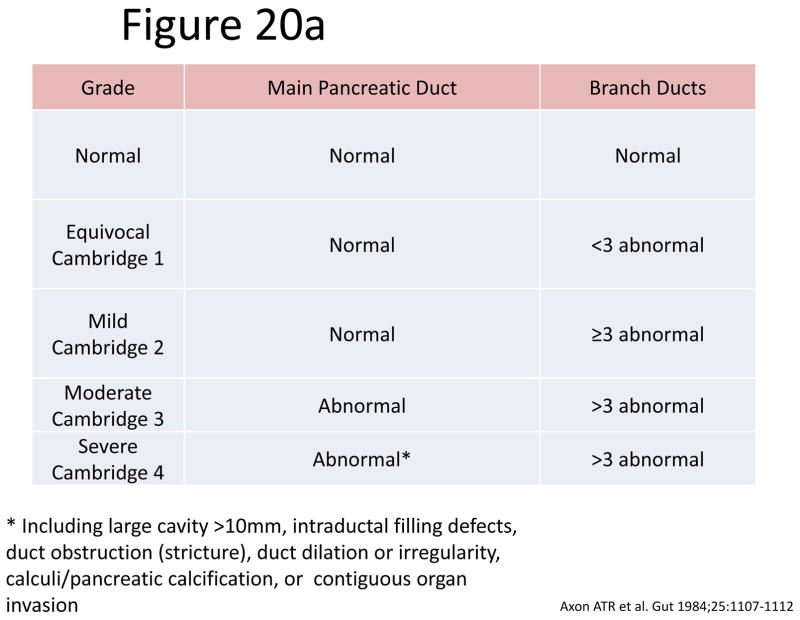
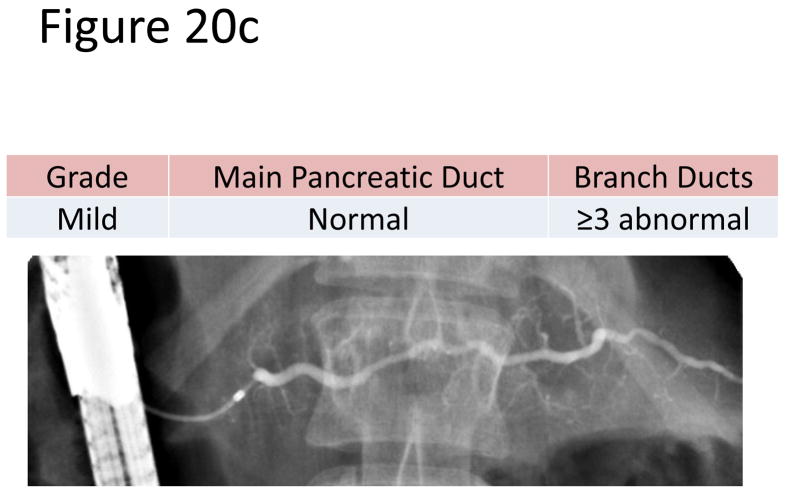
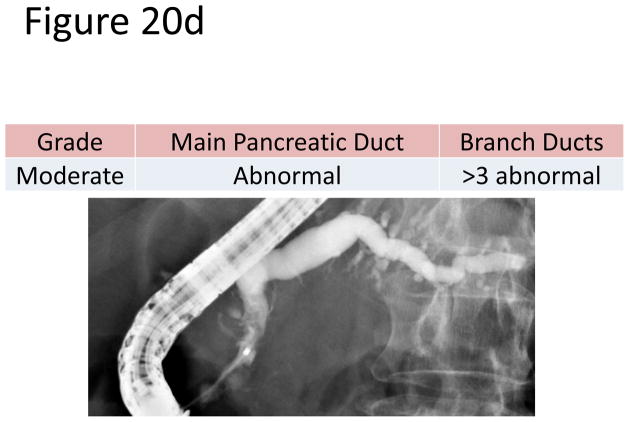
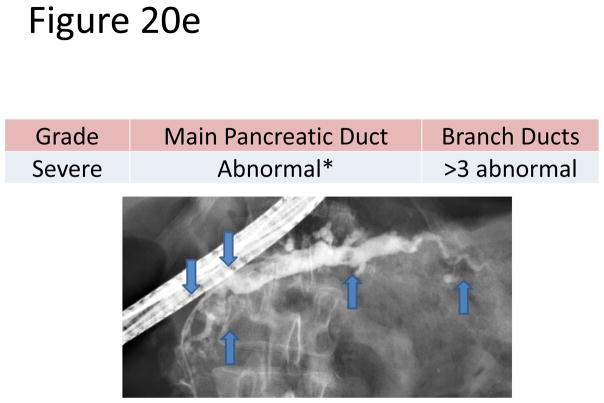
The most accepted criteria for scoring ductal changes on pancreatograms are the Cambridge Criteria (101) (Figure 20a) which grade pancreatograms from normal or equivocal to mild, moderate or severe chronic pancreatitis based on main duct and side branch abnormalities (Figure 20b–e). Main duct abnormalities include dilation, narrowing, strictures, filling defects and leaks or cavities. Identification of these abnormalities can be subjective as their definitions are not clear. Average normal main duct diameters were 3.6, 2.7 and 1.6 mm in the head, body and tail respectively(101), though the upper limited normal may be as high as 6.5, 5 and 3 mm in the head, body and tail, respectively(101–103). Main duct dilation is either general (involving more than 2/3 of the main duct) or local (< 2/3 of the ducts). Severe dilation was defined as being > 1 cm. Side branch abnormalities include decreased number, shortened length and dilated or narrowed caliber, however, the criteria to distinguish normal from abnormal are not clearly defined.
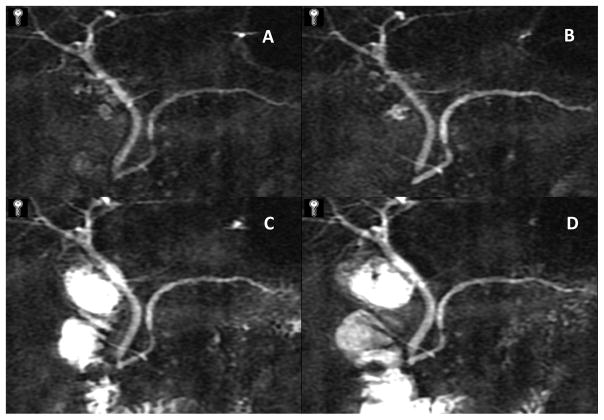
Figure 20. Figures 20a-3 Cambridge Criteria 1–4.
Retrograde pancreatograms exhibiting various stages of change in pancreatic duct for advancing Cambridge criteria 1–4. (adapted from reference 3)
There are several limitations which account for the decline in the use of ERP for diagnostic purposes. The ERP procedure is invasive and carries a risk of post-procedure complications, including acute pancreatitis. ERP can be operator dependent and the interpretation of pancreatograms is subject to interobserver variability(104). Moreover, pancreatograms allow visualization of the ductal anatomy only, without any parenchymal imaging. Other potential confounders to interpretation of pancreatograms include age-related ductal changes(101, 105–107), post-acute pancreatitis ductal changes(108, 109), as well as PanIN-related branch duct changes(110, 111), which are indistinguishable from ductal changes related to CP.
The experts at the Cambridge conference admitted that pancreatography “does not imply coincidence with the severity of disease pathology or functional status”(101). A recent study of 31 patients who underwent both ERCP and pancreatic resection showed a correlation between the Cambridge criteria and histology in 74% (n=23)(112). This correlation between pancreatogram and histology was higher in patients with moderate and marked disease (77%) by ERP than those with normal, equivocal or mild changes (67%)(112). In a post-mortem study of 69 patients without chronic pancreatitis, pancreatic duct abnormalities on pancreatograms led to a diagnosis of chronic pancreatitis in 81% of the patients (minimal 37%, moderate 33%, severe 11%). Therefore, considering ductal abnormalities alone can lead to a misdiagnosis of chronic pancreatitis (107). In terms of correlation with other tests, there is good correlation between ERP and MRCP(113, 114). The correlation with pancreatic function testing may be modest, particular with early disease(115, 116) and is the subject of a later discussion.
In summary, ERP can yield useful diagnostic information though it is rarely used as a diagnostic modality in chonic pancreatitis. The correlation of abnormalities on pancreatogram with histology and function is higher in advanced disease than early disease.
Topic 7. Indirect Pancreatic Function Testing
John G. Lieb, II, MD - University of Pennsylvania; Philadelphia, PA (Lead discussant)
Evidence-Based Medicine Statements
Indirect pancreatic function tests generally are sensitive for steatorrhea, and useful in quantifying the degree of exocrine insufficiency.
Indirect pancreatic function tests are moderately sensitive and specific for diagnosing advanced chronic pancreatitis, but are less so for diagnosing early chronic pancreatitis.
The fecal elastase assay, the polyclonal assay more than the monoclonal, can be limited in specificity, especially if the stool sample is watery and/or in the presence of small bowel disease.
Fecal chymotrypsin may be useful in detecting compliance with exogenous pancreatic enzyme supplementation.
Fecal fat assays are sensitive for steatorrhea but are of limited utility due to the cumbersome nature of patient collection and lab handling of samples. In addition, strict adherence to dietary recommendations for several days is required.
Level of Evidence
Conditional recommendation, level of evidence - low
Conditional recommendation, level of evidence - strong
Conditional recommendation, level of evidence - low
Conditional recommendation, level of evidence - low
Conditional recommendation, level of evidence - moderate
Indirect pancreatic function tests, such as serum trypsinogen, fecal elastase and fecal fat measurements, do not require direct hormonal stimulation of the pancreas.(117) Typically, indirect pancreatic function tests are sensitive only for late chronic pancreatitis (when steatorrhea is already present).(118) Thus they are best used to quantify the degree of insufficiency in established chronic pancreatitis, rather than diagnosing chronic pancreatitis.(119) For example, indirect pancreatic function tests are modestly sensitive and specific even for advanced chronic pancreatitis.(120–122) In general, indirect tests of pancreatic function should always be accompanied by cross sectional CT or MRI imaging studies to rule out malignancy.
In rare circumstances when direct pancreatic function testing may not be available or tolerated, indirect pancreatic function tests may be used to assist in the diagnosis of chronic pancreatitis. In this setting, it is crucial to understand the characteristics of the type of assay being used (serum trypsin by RIA vs ELISA and monoclonal vs polyclonal fecal elastase assays) and whether the patient has complied with pretesting preparations (high fat diet for fecal fat measurement, etc). Laboratory factors also play a role; for example, a fecal elastase test should be performed on a solid stool sample to avoid falsely positive results.(123) Serum amylase and lipase measurement should not be entirely discounted as a potentially useful indirect test of pancreatic function especially if they have a low value.(124–127)
Typically when performing indirect tests to diagnose chronic pancreatitis, the reference values should likely meet a more stringent threshold than when performed in patients with established/obvious chronic pancreatitis. In this setting, at least 2 indirect pancreatic function tests, sampling different substrates (blood, stool), should be performed. For example, a serum trypsin measured by RIA of less than 20pg/dL (128) and a fecal elastase <50μg/dL stool by monoclonal antibody in the same patient are probably diagnostic of chronic pancreatitis, though further study is needed in this area. Classic false-positives fecal elastase measurements (low levels) occur in small bowel bacterial overgrowth and watery stool.(129, 130) Conversely, classic false-negative serum trypsin measurements (normal/high levels) occur when performed in the setting of acute pancreatic inflammation.(131) In the absence of other supporting evidence, intermediate indirect pancreatic function test results (monoclonal fecal elastase 50–200μg/dL, or trypsin 20–29pg/dL by RIA) should be interpreted with caution when used to diagnose chronic pancreatitis. A polyclonal fecal elastase may be a better test,(132) however, the monoclonal assay is currently better standardized, yet some large US commercial reference labs reflexively use the polyclonal assay. Newer methods such as 13C-MTG triglyceride breath testing, while shown to be sensitive and specific in select tertiary referral centers,(133, 134) have not yet been universally adopted in the United States.
Fecal fat measurement alone is highly nonspecific and in isolation cannot diagnose chronic pancreatitis. Exocrine pancreatic insufficiency (steatorrhea) in the setting of established chronic pancreatitis is currently defined as more than 7 grams of fat per 24 hours measured by a 72 hour fecal fat test.(135) This reference value is valid only in the appropriate clinical context: small bowel bacterial overgrowth and celiac sprue/short gut and inflammatory bowel disease have been excluded. A positive qualitative fecal fat (more than 6 droplets per high power field), which is a fairly insensitive test for steatorrhea,(136, 137) or monoclonal fecal elastase (<50μg/dL) under similar circumstances would also support the diagnosis of exocrine insufficiency. Fecal chymotrypsin is currently only available through one company in the U.S., however, because it detects porcine pancreatic elastase, it may be useful to evaluate patient compliance with exogenous pancreatic enzyme supplementation.(138)
The role of tests of endocrine function such as a serum pancreatic polypeptide level, other than traditional definitions of diabetes such as a hemoglobin A1C, a fasting glucose or glucose tolerance test, has not been well characterized in the assessment of chronic pancreatitis and these endocrine tests are probably neither sensitive nor specific for CP but may serve as outcome measures worthy of further investigation. (139, 140)
Topic 8: Direct Pancreatic Function Tests
Linda S. Lee, MD – Brigham and Women’s Hospital; Boston, MA (Lead discussant)
Evidence-Based Statements
Direct pancreatic function tests have high sensitivity for detecting late chronic pancreatitis, but lower sensitivity (70–75%) for early chronic pancreatitis.
The traditional secretin and CCK PFTs performed with the oro-duodenal tube pancreas fluid collection are highly accurate, but require fluoroscopy for confirmation of tube placement and are not widely utilized.
The endoscopic PFT (ePFT) has good correlation with the traditional Dreiling PFT.
Level of evidence
Strong recommendation, level of evidence - low
Strong recommendation, level of evidence - moderate
Strong recommendation, level of evidence - moderate
Direct pancreatic function tests (PFT) have a long history, from traditional testing dating back to the early 1900s to newer techniques, including the endoscopically assisted and the purely endoscopic methods. Direct PFT may be limited by, among other factors, false positives for at least several months following a bout of acute pancreatitis and false negatives in some patients who have early chronic pancreatitis. Direct PFT is capable of detecting secretory dysfunction in patients with at least 30% pancreas damage and therefore has a high sensitivity for late chronic pancreatitis, but certainly will miss patients with less severe pancreatic damage. Sensitivity of the traditional Dreiling tube test for early chronic pancreatitis decreases to 70–75%. (1,2,3)
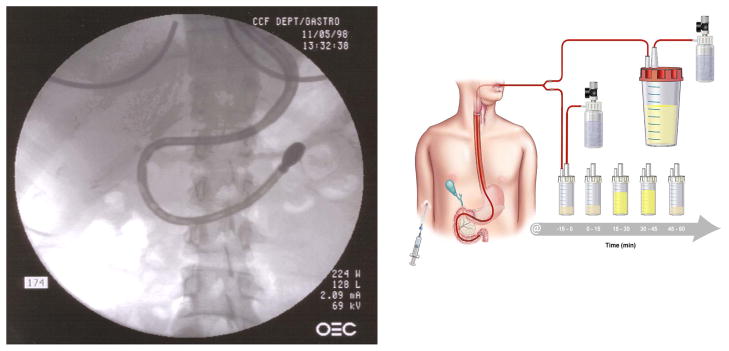
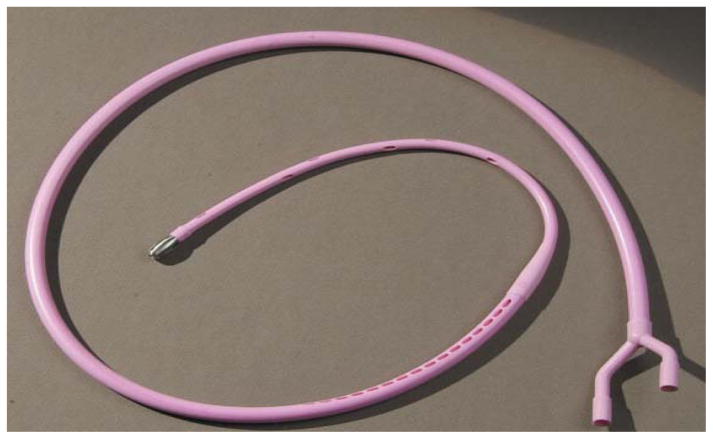
The original secretin stimulated direct PFT was performed with the Dreiling tube and popularized at the University of Florida (Figure 21). This test is highly accurate and measures duct cell function. This double lumen, 26 French oro-duodenal tube with both gastric and duodenal ports is introduced using fluoroscopy guidance. The patient’s oropharynx is medicated with topical anesthesia. Under fluoroscopic guidance, the weighted tip of the Dreiling tube is swallowed and advanced close to the ligament of Treitz and the tapered radiopaque portion of the tube is positioned at the pylorus. Low, constant suction is applied to collect duodenal fluid at 0, 15, 30, 45, and 60 minutes following an intravenous bolus injection of secretin, and the fluid is analyzed for pH, volume, and bicarbonate concentration. Drawbacks of this procedure may include patient discomfort, need for fluoroscopy, and limited availability of the traditional Dreiling tubes. Patients with nausea, gastroparesis, and pyloric stenosis pose a challenge to performing this procedure.
Figure 21.
Traditional secretin PFT with Dreiling tube
Another direct PFT is the CCK PFT developed at the Mayo Clinic, which is a dual tube technique using two double lumen tubes for perfusion and aspiration of both gastric and duodenal contents. This test measures acinar cell function. This test incorporates a marker perfusion system to account for the amount of gastric and duodenal fluid that is “lost” or escapes aspiration into the distal small bowel lumen to accurately calculate the total pancreatic enzyme secretory output. Disadvantages of this procedure include limited availability of the special “dual tube” system, need for fluoroscopy, utilization of a PEG/mannitol marker and need for continuous intravenous CCK infusion during the duration of the test. (4) Combining secretin and CCK injection does not appear to yield improved results and, in fact, peak bicarbonate concentration over 1 hour is the best correlate to histologic findings.
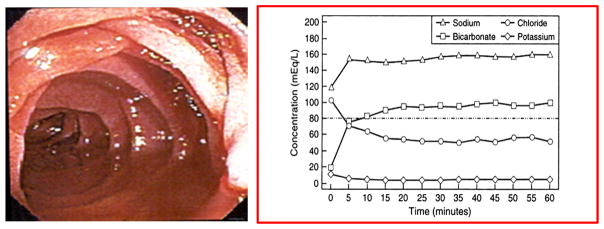
Several endoscopic variations of pancreas function testing have been developed. (5–11) The purely, endoscopic pancreatic function test (ePFT) developed at The Cleveland Clinic allows the patient to be moderately sedated, typically with a long acting benzodiazepine and narcotic, for the upper endoscopy while pancreas fluid is suctioned via the upper EGD or EUS endoscope. (6) Following intravenous bolus injection of secretin (0.2mcg/kg), duodenal fluid is aspirated from the duodenal bulb. When performing ePFT, it is important to completely aspirate gastric secretions to avoid contamination of duodenal fluid by the acidic gastric contents. Placing the patient at 30 degrees or in Trendelenburg prevents distal migration and loss of duodenal fluid. It is also very important to aspirate and discard approximately 2–4 mL of duodenal fluid before each collection to rinse residual gastric fluid from the suction channel. For the full one-hour test, normal peak bicarbonate level is greater than or equal to 80 mEq/L. The shortened screening test uses a peak bicarbonate cutpoint of 75 meq/L. Advantages of this test include its simplicity, universal availability of upper endoscopy, and lack of need for fluoroscopy. While modest sedation does not affect ePFT results, the impact of heavy sedation and/or general anesthesia on ePFT results is unclear and may confound function test result interpretation.(7) There is good correlation between the traditional Dreiling test and the ePFT test. (Figure 22)
Figure 22.
Endoscopic Pancreatic Function Test (ePFT)
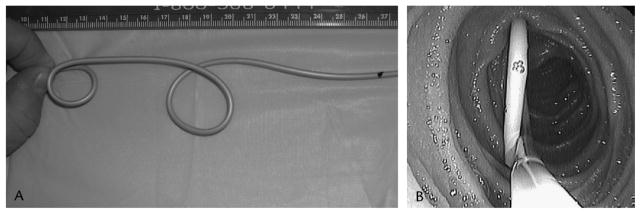
A newer disposable, latex-free version of the Dreiling tube can also be endoscopically placed over a guidewire with fluoroscopic assistance (Figure 23) while the Liguory tube (Frank Burton method) can be advanced over a wire during upper endoscopy without fluoroscopy. (10) The disposable Liguory tube has a pigtail tip with side ports at the end (Figure 24). Following placement of the tube, the patient is transferred to the recovery area where secretin is administered and pancreatic function test performed. The average time for endoscopic Dreiling tube placement is approximately 10 minutes in combination with 2 minutes of fluoroscopy time. Intraductal PFT performed during ERCP requires cannulation of the pancreatic duct followed by injection of secretin, and aspiration of pancreatic secretions from within the pancreatic duct for approximately 15 minutes. Following secretin injection, the bicarbonate level in pancreas fluid continues to rise and does not plateau for about 15 to 30 minutes (see Figure 22). Therefore, timing of aspirations is critical for accurate functional assessment. A recent study comparing intraductal PFT during ERCP to the standard Dreiling test reported a 20% specificity and 80% sensitivity for the intraductal test. (11)
Figure 23.
New Dreiling Tube
Figure 24.
Liguory Drainage Tube (Burton Method)
In summary, the one-hour ePFT and Dreiling tube collection methods are the non-histologic gold standards for diagnosis of early chronic pancreatitis. A shortened 30–45 minute ePFT test may be used to screen for chronic pancreatitis. In practice, patients referred with chronic abdominal pain undergoing evaluation for early CP may undergo screening for CP using EUS combined with the shortened 45-minute ePFT to assess for both structural and functional changes of CP. Because the 45-minute test has slightly lower specificity than the full 1-hour test, patients with borderline results should undergo a full 1-hour PFT with the endoscopic or Dreiling tube method.
TOPIC 9. CORRELATION OF IMAGING AND FUNCTION WITH HISTOLOGY
Tyler Stevens, MD - Cleveland Clinic; Cleveland, Ohio (Lead discussant)
Evidence-Based Medicine Statements
As structural severity worsens in CP, exocrine function declines
Both EUS and PFT results correlate with fibrosis in CP
A combined approach (e.g. EUS/ePFT) could improve detection of minimal change chronic pancreatitis (MCCP)
Level of evidence
Strong recommendation, level of evidence - moderate
Conditional recommendation, level of evidence - low
Conditional recommendation, level of evidence - low
Mild exocrine insufficiency is frequently present in mild and severe CP, and progressive structural fibrosis is reflected in exocrine function decline. However, this relationship is not linear, nor are imaging and PFT results perfectly concordant. Multiple studies have shown suboptimal concordance of ERCP and PFT results, with the greatest discrepancy in mild disease. In one example, the concordance rate for secretin PFT and ERCP in non-calcific CP was only 47% (141). EUS is now favored over ERCP for the endoscopic diagnosis of CP diagnosis due to increased safety and the ability to evaluate both the ductal and parenchymal architecture. As with ERCP, similar discordance has been seen between EUS criteria and PFT results. The overall concordance rates between EUS and secretin PFT range from 48 to 70% in 3 past studies comparing EUS and secretin PFT (142–144). In 302 patients undergoing a combined EUS and secretin endoscopic PFT, a moderate correlation was observed between peak bicarbonate concentration and EUS score (r= −0.59) (144).
Only a few recent studies evaluating ERCP, EUS, and PFT have incorporated histological reference standards. Two studies compared secretin and cholecystokinin PFT results with histological fibrosis. Twenty-five patients underwent a PFT prior to surgery for painful CP (145). A discriminative analysis revealed moderate correlations of certain PFT parameters (e.g. peak bicarbonate and trypsin concentrations) with specific histological features (e.g. atrophy, small duct dilation). Weaker correlations were observed between other parameters. Another study compared PFT results with pancreatic histology in 108 patients undergoing a variety of abdominal surgeries (146). Most patients had surgery done for non-pancreatic indications (e.g. cholecystectomy) that included performance of a wedge biopsy of the pancreas. A significant correlation (r=0.59) was observed between the overall PFT and histological rating scales.
Three recent studies (147–149) have compared preoperative EUS criteria with histology in patients undergoing pancreatic resections or operative biopsy, using Ammann’s pancreatic fibrosis score (150). These studies differ in the patient populations (CP vs. pancreatic masses) disease severity (minimal change vs. calcific), and the fibrosis score cutpoint used to diagnose CP. The overall correlation coefficients for EUS vs. fibrosis scores range from 0.40 to 0.85, suggesting a ‘moderate’ to ‘very good’ correlation. The sensitivity and specificity estimates for EUS compared with the histological reference standard range from 83–91% and 80–100%, respectively. Another study reported preoperative EUS results in 50 patients undergoing total pancreatectomy with autologous islet cell transplant (151). One to 3 wedge biopsies were obtained for histological examination prior to enzymatic digestion of the gland for islet cell isolation. The histological findings of fibrosis, atrophy, or inflammation were considered individually diagnostic of CP. This study was less encouraging for EUS as a diagnostic test. Compared with histology, the area under the receiver operating characteristics curve was only 0.593, indicating poor discrimination. Furthermore, the negative and positive predictive values were poor even when the EUS cut point was optimized for each.
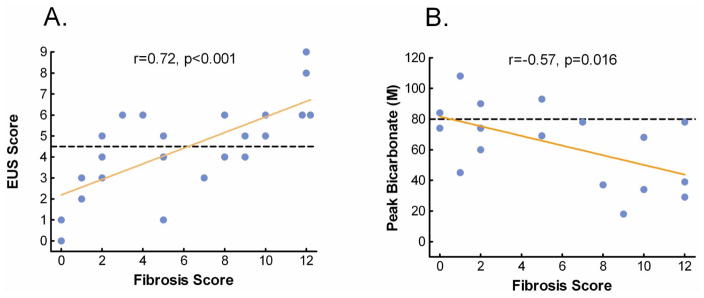
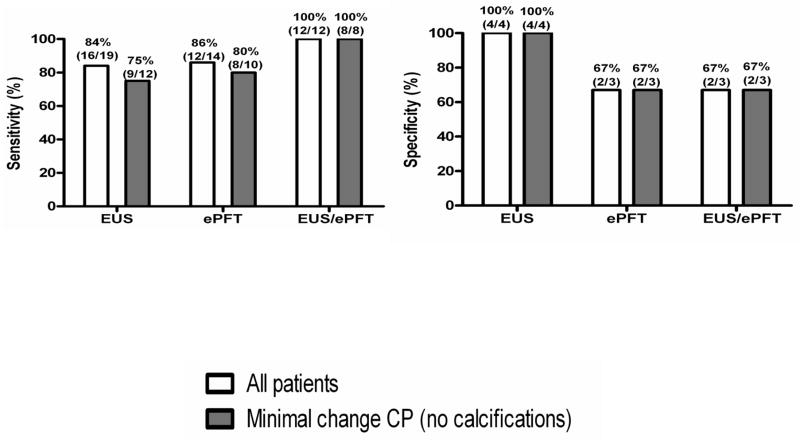
It is controversial whether EUS or PFT is most sensitive for detecting early CP. It is clear that some patients with significant structural changes have preserved exocrine function; while others have minimal or no EUS features and might still have an abnormal PFT indicating early CP. In a recent study, EUS and/or a secretin endoscopic PFT were done in 25 patients within one year of resection or open biopsy (149). Twelve patients had both tests done and had histological fibrosis indicating CP. Correlations for EUS and ePFT were 0.72 and 0.57 respectively (Figure 25). Of these, all 25 had either an abnormal EUS or ePFT, indicating 100% sensitivity of the combined approach (Figure 26). Although the sample size is small, these results imply that combining structural with function testing may optimize the detection of minimal change CP.
Figure 25. Correlation of EUS and PFT with Pancreas Fibrosis.
There is moderate correlation of EUS and ePFT to pancreatic fibrosis
Figure 26. Diagnostic Accuracy of EUS, ePFT or Combined EUS/ePFT.
A combined EUS/ePFT may be the most accurate means of accessing for early chronic pancreatitis
In summary, the overall correlation of EUS and PFT with histology appears to be in the moderate to very good range. The studies comparing EUS and PFT with histology have significant limitations. Most were done using a highly selected sample of patients undergoing planned pancreatic surgery. The studies are heavily weighted with severe disease, which may falsely elevate sensitivity (i.e. spectrum bias). Also, not all patients undergoing the EUS or PFT go on to receive a histological diagnosis, which produces verification bias. The use of wedge pancreatic biopsies in some studies may lead to sampling bias. A combined EUS/ePFT approach may be most useful for the evaluation of patients with chronic abdominal pain and suspected early or “minimal change” chronic pancreatitis.
Summary of Diagnostic Guidelines in Chronic Pancreatitis
Darwin L. Conwell, MD, MS – Ohio State University Wexner Medical Center; Columbus, Ohio (Lead discussant)
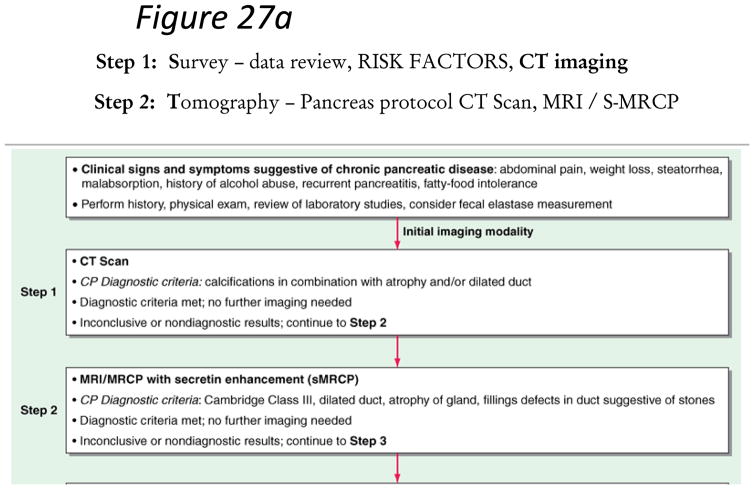
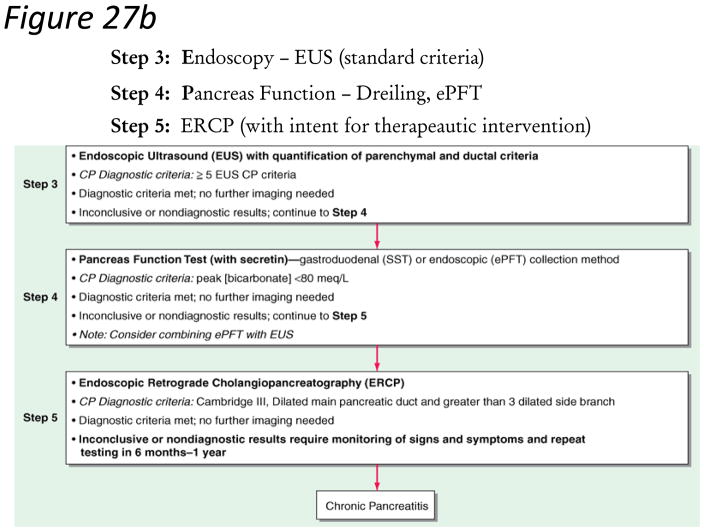
Confirming a diagnosis of chronic pancreatitis is clear in highly suspicious patients (recurrent pancreatitis, alcohol or smoking abuse) with steatorrhea, weight loss and marked/moderate morphologic changes in the gland. The diagnosis of chronic pancreatitis still remains challenging in early stages (equivocal and mild morphologic or physiologic changes) of the disease. These patients should not be classified as having CP until definitive diagnostic features are evident. Our guidelines propose a diagnostic algorithm that proceeds from a non-invasive to more invasive diagnostic approach (Figure 27a–b). This algorithm maximizes specificity (low false positive rate) in subjects with chronic abdominal pain and equivocal imaging changes. The diagnosis of chronic pancreatitis is made based on definitive criteria. All patients with suspected chronic pancreatitis should have a dedicated pancreatic protocol CT scan or MRI/MRCP to rule out pancreas carcinoma.
Figure 27. Figure 27a–b. STEP-wise Algorithm Approach to Diagnosis of Chronic Pancreatitis.
Step 1: Survey – data review, risk factors, CT imaging. Step 2: Tomography – Pancreas protocol CT Scan, MRI/S-MRCP. Step 3: Endoscopy – EUS (standard criteria). Step 4 – Pancreas function – Dreiling, ePFT. Step 5: ERCP (with intent for therapeutic intervention)
Our guidelines define the diagnostic evidence for chronic pancreatitis as definitive, probable and insufficient based on current knowledge of the natural history of the disease (Table 5). Without sufficient evidence, patients should not be mislabeled as having chronic pancreatitis, when in fact they may have chronic abdominal pain syndrome and a remote history of ERCP-induced pancreatitis or ductal changes. Given there is no current therapy to alter disease progression, it is better to error on the side of not labeling the patient with chronic pancreatitis. In most cases, longitudinal follow-up is recommended with serial imaging and physiologic testing in equivocal/mild cases with insufficient evidence of chronic pancreatitis until definitive evidence is apparent.
Table 5.
Diagnostic Evidence for Chronic Pancreatitis Diagnosis
| Evidence | Criteria |
|---|---|
| Definitive Evidence (any of the following) | Moderate/marked pancreas imaging morphology
Pancreatic calcifications Histologic confirmation |
| Probable Evidence: (abnormal imaging/suggestive history + abnormal physiology) | Mild pancreas imaging morphology or Recurrent pseudocyst/pancreatitis plus Abnormal pancreas physiology
|
| Insufficient Evidence | Equivocal pancreas imaging morphology or Abdominal pain with any of the following: No history of pancreatitis ( lipase < 3x ULN) Normal imaging Family history of pancreatitis Prior ERCP with pancreatic duct stenting Presence of TIGAR-O risk factors
|
Evidence should be interpreted in a patient with history and physical examination suspicious for chronic pancreatitis such as abdominal pain, history of acute pancreatitis, steatorrhea responsive to pancreatic enzyme replacement therapy, heavy alcohol or smoking history, genetic risk factors of family history
Once a diagnosis of chronic pancreatitis is confirmed, our guidelines recommend a comprehensive characterization of chronic pancreatitis in regards to etiology (TIGAR-O; see Yadav, D Epidemiology section Table 2), morphology (modified from MANNHEIM group, Table 6) and physiologic state (Table 7). In an attempt to standardize research studies across centers, a nomenclature is proposed (Table 8). To conclude, case studies are presented to emphasize salient features of the proposed diagnostic evaluation (Table 9).
Table 6.
Pancreas Morphology Imaging Grade (I – IV)
| Equivocal (I) | Mild (II) | Moderate (III) | Marked (IV) | |
|---|---|---|---|---|
| Ultrasound | Same as CT | Same as CT | Same as CT | Same as CT |
| CT/MRI Scan | 1 of the following: main duct enlarged (2–4 mm), slight gland enlargement, heterogenous parenchyma, small cavities(<10mm), irregular ducts, focal pancreatitis, increased echogenicity of main duct wall, irregular head/body contour | >=2 of the following: enlarged main duct (2–4mm), gland enlargement, heterogenous parenchyma, small cavities (<10mm), irregular ducts, focal acute pancreatitis, increased echogenecity of main duct wall, irregular head/body contour | Cannot distinguish from mild | moderate changes plus >=1 of the following: large cavities (>10mm), gland enlargement, intraductal filling defects/calculi, duct obstruction, stricture or gross irregularity |
| EUS (0–9 criteria standard criteria) | 0–2 | 3– 4 | >= 5 | |
| MRCP/ERCP | <3 abnormal side branch changes | >=3 abnormal side branch changes | abnormal main duct; >3 abnormal side branches | moderate changes plus 1 of the following: obstruction, filling defects, severe irregularity/dilation of main duct |
Reference: 34
Table 7.
Pancreas Physiology Stage
| Stage | Physiologic Definition |
|---|---|
| A | Normal secretory, exocrine and endocrine function |
| B | Secretory dysfunction
|
| C | Exocrine insufficiency
|
| D | Endocrine insufficiency
|
| E | Both C and D |
| X | not classified/unknown |
Table 8.
APA Chronic Pancreatitis Nomenclature
| In an attempt to combine the latest diagnostic criteria into a working nomenclature that incorporates etiology, morphology and physiologic status we propose the following nomenclature for chronic pancreatitis once probable or definitive evidence is present: Chronic [TIGARO Etiology – induced] Pancreatitis + MANNHEIM/Cambridge Imaging Grade [Normal (0) – Marked (IV)] + Physiology Stage (A, B, C, D, E, X) |
| Examples: |
| Nomenclature example 1: Chronic Toxic (Alcohol, smoking)-induced Pancreatitis, Imaging Grade III, Physiology Stage C – alcohol and smoking risk factors, ductal abnormalities on imaging with exocrine insufficiency as measured by fecal elastase, fecal fat or serum trypsin |
| Nomenclature Example 2: Chronic Genetic (PRSS1 mutation)-induced Pancreatitis, Imaging Grade II, Physiology Stage E- genetic risk factors, mild imaging changes with both exocrine (as above) and endocrine insufficiency as measured by glycohemoglobin, fasting glucose or glucose tolerance test |
| Nomenclature Example 3: Chronic Severe Acute (pancreatic necrosis)-induced Pancreatitis, Imaging Grade II, Physiology Stage A – sequelae of necrotizing pancreatitis with mild ductal changes and preserved glandular function |
Table 9.
Chronic Pancreatitis Case Studies
| Diagnosis Case 1: Insufficient Evidence. 35 year old female with chronic narcotic requiring abdominal pain. A CT scan is normal without evidence of a pancreas mass. MRI/s-MRCP is normal. Pain is similar to ERCP induced pancreatitis pain. No history of pancreatitis prior to or after resolution of ERCP-induced acute pancreatitis. Stool fecal elastase is > 500 ug/g on a solid sample. Tertiary referral center EUS has 3 criteria and ePFT has a peak bicarbonate of 83 meq/L. |
| Diagnosis: Chronic Abdominal Pain Syndrome |
| Diagnosis Case 2: Probable Evidence. 56 year old male smoker with history of alcohol abuse 10 years earlier who presents with intermittent abdominal pain and mild lipase enzyme elevations to 2x ULN. CT scan is negative for pancreas mass. Fecal elastase is 225 UG/G on a solid stool sample. MRI shows decreased T1 signal and s-MRCP reveals a few prominent side branches. EUS shows 4 criteria and ePFT has a peak bicarbonate of 69 meq/L. |
| Diagnosis: Chronic Alcohol-induced Pancreatitis, Imaging Grade II, Physiology Stage B |
| Diagnosis Case 3: Definitive Evidence. 33 year old female with acute onset of severe acute pancreatitis. No prior history or use of alcohol or drugs. CT scan consistent with interstitial pancreatitis without a pancreas mass. Father had a pancreaticojejunostomy when he was 25 years old for recurrent pancreatitis. Further testing reveal mildly elevated glucose to 150 and glycohemogin in 7.5 mg/dL. Genetic testing revealed PRSS1 mutation. Patient was followed for several months and continued to have recurrent pancreatitis and non-narcotic requiring abdominal pain. MRI had a low T1 signal and s-MRCP revealed an mildly irregular main pancreas duct with multiple side branche dilations. Total pancreatectomy with autologous islet transplant performed. Final pathology reveled inflammation, fibrosis and atrophy consistent with chronic pancreatitis. |
| Diagnosis (prior to TPIAT): Chronic Genetic (PRSS1)-induced Pancreatitis, Imaging Grade III, Physiology Stage D |
| Diagnosis Case 4: Definitive Evidence. 18 year old female college freshman presents to ED with acute onset of abdominal pain, nausea and vomiting. Amylase and lipase both > 3x ULN, LFTs are normal. CT imaging suggests focal inflammation and fullness of pancreas. MRI scan is “suggestive of autoimmune pancreatitis” with focal dilation of pancreatic tail in region of parenchymal inflammation. EUS confirms findings on cross sectional imaging; no evidence for a mass. Peri-ampullary biopsies and staining for IgG4 are negative. Serum IgG4 is normal. Fecal elastase > 500 UG/G. Patient abdominal pain requiring narcotics to control, intractable to 5 weeks of steroids and 1 week hospitalization for pancreatic rest (NPO). Repeat MRI and EUS confirm previous findings with persistent focal dilation of distal duct, FNA biopsy non-diagnostic. Distal pancreatectomy recommended for intractable pain and concern of focal duct dilation. Pathology revealed duct-centric lymphopasmacytic infiltration suggestive of autoimmune pancreatitis; IgG4 staining is negative. |
| Diagnosis: Chronic Autoimmune Pancreatitis, Imaging Grade IV, Physiology Stage A |
It is our intent that this manuscript serve as a baseline “working document” that will be modified as new evidence becomes available and our knowledge of chronic pancreatitis improves.
Table 4.
EUS Criteria for Chronic Pancreatitis and Histological Correlates
| EUS Criteria for Chronic Pancreatitis and Histological Correlates | |
|---|---|
| EUS Criteria | Histological Correlate |
| Parenchymal Features | |
| Hyperechoic foci | Focal fibrosis |
| Hyperechoic strands | Bridging fibrosis |
| Lobular contour | Interlobular fibrosis |
| Cysts | Cyst/Pseudocyst |
| Ductal Features | |
| Main duct dilatation (mm) | >3 head, >2 body, >1 tail |
| Duct irregularity | Focal dilatation/narrowing |
| Hyperechoic margins | Periductal fibrosis |
| Visible side branches | Side branch dilation |
| Stones | Calcified stones |
Footnotes
Disclosure: The authors have no conflicts of interest or funding to disclose.
References
- 1.Steer ML, Waxman I, Freedman S. Chronic pancreatitis. N Engl J Med. 1995;332:1482–90. doi: 10.1056/NEJM199506013322206. [DOI] [PubMed] [Google Scholar]
- 2.Witt H, Apte MV, Keim V, et al. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology. 2007;132:1557–73. doi: 10.1053/j.gastro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Whitcomb DC. Hereditary pancreatitis: a model for understanding the genetic basis of acute and chronic pancreatitis. Pancreatology. 2001;1:565–70. doi: 10.1159/000055864. [DOI] [PubMed] [Google Scholar]
- 4.Yadav D, Slivka A, Sherman S, et al. Smoking is underrecognized as a risk factor for chronic pancreatitis. Pancreatology. 2010;10:713–9. doi: 10.1159/000320708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoun E, Chang CC, Greer JB, et al. Pathways to injury in chronic pancreatitis: decoding the role of the high-risk SPINK1 N34S haplotype using meta-analysis. PLoS One. 2008;3:e2003. doi: 10.1371/journal.pone.0002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitcomb DC. Mechanisms of disease: Advances in understanding the mechanisms leading to chronic pancreatitis. Nat Clin Pract Gastroenterol Hepatol. 2004;1:46–52. doi: 10.1038/ncpgasthep0025. [DOI] [PubMed] [Google Scholar]
- 7.Forsmark CE. The early diagnosis of chronic pancreatitis. Clin Gastroenterol Hepatol. 2008;6:1291–3. doi: 10.1016/j.cgh.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Forsmark CE. The diagnosis of chronic pancreatitis. Gastrointest Endosc. 2000;52:293–8. doi: 10.1067/mge.2000.106889. [DOI] [PubMed] [Google Scholar]
- 9.Conwell DL, Wu BU. Chronic Pancreatitis I: Making the Diagnosis. Clin Gastroenterol Hepatol. 2012 doi: 10.1016/j.cgh.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682–707. doi: 10.1053/gast.2001.22586. [DOI] [PubMed] [Google Scholar]
- 11.Whitcomb DC, Yadav D, Adam S, et al. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American Pancreatitis Study 2 (NAPS2) Pancreatology. 2008;8:520–31. doi: 10.1159/000152001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yadav D, Hawes RH, Brand RE, et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med. 2009;169:1035–45. doi: 10.1001/archinternmed.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mullady DK, Yadav D, Amann ST, et al. Type of pain, pain-associated complications, quality of life, disability and resource utilisation in chronic pancreatitis: a prospective cohort study. Gut. 2011;60:77–84. doi: 10.1136/gut.2010.213835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cote GA, Yadav D, Slivka A, et al. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2011;9:266–73. doi: 10.1016/j.cgh.2010.10.015. quiz e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cahen DL, Gouma DJ, Nio Y, et al. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med. 2007;356:676–84. doi: 10.1056/NEJMoa060610. [DOI] [PubMed] [Google Scholar]
- 16.Bouwense SA, Olesen SS, Drewes AM, et al. Effects of pregabalin on central sensitization in patients with chronic pancreatitis in a randomized, controlled trial. PLoS One. 2012;7:e42096. doi: 10.1371/journal.pone.0042096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dite P, Ruzicka M, Zboril V, et al. A prospective, randomized trial comparing endoscopic and surgical therapy for chronic pancreatitis. Endoscopy. 2003;35:553–8. doi: 10.1055/s-2003-40237. [DOI] [PubMed] [Google Scholar]
- 18.Deviere J, Bell RH, Jr, Beger HG, et al. Treatment of chronic pancreatitis with endotherapy or surgery: critical review of randomized control trials. J Gastrointest Surg. 2008;12:640–4. doi: 10.1007/s11605-007-0448-9. [DOI] [PubMed] [Google Scholar]
- 19.Lieb JG, 2nd, Forsmark CE. Review article: pain and chronic pancreatitis. Aliment Pharmacol Ther. 2009;29:706–19. doi: 10.1111/j.1365-2036.2009.03931.x. [DOI] [PubMed] [Google Scholar]
- 20.Pezzilli R. Pain in chronic pancreatitis: from the bench to the bedside. JOP. 2012;13:245–6. [PubMed] [Google Scholar]
- 21.Fasanella KE, Davis B, Lyons J, et al. Pain in chronic pancreatitis and pancreatic cancer. Gastroenterol Clin North Am. 2007;36:335–64. ix. doi: 10.1016/j.gtc.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Raimondi S, Lowenfels AB, Morselli-Labate AM, et al. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010;24:349–58. doi: 10.1016/j.bpg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Stevens T, Conwell DL, Zuccaro G. Pathogenesis of chronic pancreatitis: an evidence-based review of past theories and recent developments. Am J Gastroenterol. 2004;99:2256–70. doi: 10.1111/j.1572-0241.2004.40694.x. [DOI] [PubMed] [Google Scholar]
- 24.Vardanyan M, Rilo HL. Pathogenesis of chronic pancreatitis-induced pain. Discov Med. 2010;9:304–10. [PubMed] [Google Scholar]
- 25.Apte M, Pirola R, Wilson J. The fibrosis of chronic pancreatitis: new insights into the role of pancreatic stellate cells. Antioxid Redox Signal. 2011;15:2711–22. doi: 10.1089/ars.2011.4079. [DOI] [PubMed] [Google Scholar]
- 26.Lankisch PG, Assmus C, Maisonneuve P, et al. Epidemiology of pancreatic diseases in Luneburg County. A study in a defined german population. Pancreatology. 2002;2:469–77. doi: 10.1159/000064713. [DOI] [PubMed] [Google Scholar]
- 27.Dite P, Stary K, Novotny I, et al. Incidence of chronic pancreatitis in the Czech Republic. Eur J Gastroenterol Hepatol. 2001;13:749–50. doi: 10.1097/00042737-200106000-00024. [DOI] [PubMed] [Google Scholar]
- 28.Yadav D, Timmons L, Benson JT, et al. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. The American Journal of Gastroenterology. 2011;106:2192–9. doi: 10.1038/ajg.2011.328. [DOI] [PubMed] [Google Scholar]
- 29.Lin Y, Tamakoshi A, Matsuno S, et al. Nationwide epidemiological survey of chronic pancreatitis in Japan. J Gastroenterol. 2000;35:136–41. doi: 10.1007/s005350050026. [DOI] [PubMed] [Google Scholar]
- 30.Tinto A, Lloyd DA, Kang JY, et al. Acute and chronic pancreatitis--diseases on the rise: a study of hospital admissions in England 1989/90–1999/2000. Aliment Pharmacol Ther. 2002;16:2097–105. doi: 10.1046/j.1365-2036.2002.01367.x. [DOI] [PubMed] [Google Scholar]
- 31.Yang AL, Vadhavkar S, Singh G, et al. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med. 2008;168:649–56. doi: 10.1001/archinte.168.6.649. [DOI] [PubMed] [Google Scholar]
- 32.Spanier BW, Dijkgraaf MG, Bruno MJ. Trends and forecasts of hospital admissions for acute and chronic pancreatitis in the Netherlands. Eur J Gastroenterol Hepatol. 2008;20:653–8. doi: 10.1097/MEG.0b013e3282f52f83. [DOI] [PubMed] [Google Scholar]
- 33.Yadav D, Muddana V, O’Connell M. Hospitalizations for chronic pancreatitis in allegheny county, pennsylvania, USA. Pancreatology: official journal of the International Association of Pancreatology. 2011;11:546–52. doi: 10.1159/000331498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider A, Lohr JM, Singer MV. The M-ANNHEIM classification of chronic pancreatitis: introduction of a unifying classification system based on a review of previous classifications of the disease. J Gastroenterol. 2007;42:101–19. doi: 10.1007/s00535-006-1945-4. [DOI] [PubMed] [Google Scholar]
- 35.Ammann RW, Heitz PU, Kloppel G. Course of alcoholic chronic pancreatitis: a prospective clinicomorphological long-term study. Gastroenterology. 1996;111:224–31. doi: 10.1053/gast.1996.v111.pm8698203. [DOI] [PubMed] [Google Scholar]
- 36.Ammann RW, Muellhaupt B, Meyenberger C, et al. Alcoholic nonprogressive chronic pancreatitis: prospective long-term study of a large cohort with alcoholic acute pancreatitis (1976–1992) Pancreas. 1994;9:365–73. [PubMed] [Google Scholar]
- 37.Cavallini G, Frulloni L, Pederzoli P, et al. Long-term follow-up of patients with chronic pancreatitis in Italy. Scandinavian journal of gastroenterology. 1998;33:880–9. doi: 10.1080/00365529850171567. [DOI] [PubMed] [Google Scholar]
- 38.Dani R, Penna FJ, Nogueira CE. Etiology of chronic calcifying pancreatitis in Brazil: a report of 329 consecutive cases. Int J Pancreatol. 1986;1:399–406. doi: 10.1007/BF02801872. [DOI] [PubMed] [Google Scholar]
- 39.Frulloni L, Gabbrielli A, Pezzilli R, et al. Chronic pancreatitis: report from a multicenter Italian survey (PanCroInfAISP) on 893 patients. Dig Liver Dis. 2009;41:311–7. doi: 10.1016/j.dld.2008.07.316. [DOI] [PubMed] [Google Scholar]
- 40.Lankisch PG, Lohr-Happe A, Otto J, et al. Natural course in chronic pancreatitis. Pain, exocrine and endocrine pancreatic insufficiency and prognosis of the disease. Digestion. 1993;54:148–55. doi: 10.1159/000201029. [DOI] [PubMed] [Google Scholar]
- 41.Layer P, Yamamoto H, Kalthoff L, et al. The different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology. 1994;107:1481–7. doi: 10.1016/0016-5085(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 42.Marks IN, Bank S, Louw JH. Chronic pancreatitis in the Western Cape. Digestion. 1973;9:447–53. doi: 10.1159/000197473. [DOI] [PubMed] [Google Scholar]
- 43.Robles-Diaz G, Vargas F, Uscanga L, et al. Chronic pancreatitis in Mexico City. Pancreas. 1990;5:479–83. doi: 10.1097/00006676-199007000-00017. [DOI] [PubMed] [Google Scholar]
- 44.Whitcomb DC, Yadav D, Adam S, et al. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American Pancreatitis Study 2 (NAPS2) Pancreatology: official journal of the International Association of Pancreatology. 2008;8:520–31. doi: 10.1159/000152001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nojgaard C, Becker U, Matzen P, et al. Progression from acute to chronic pancreatitis: prognostic factors, mortality, and natural course. Pancreas. 2011;40:1195–200. doi: 10.1097/MPA.0b013e318221f569. [DOI] [PubMed] [Google Scholar]
- 46.Yadav D, Eigenbrodt ML, Briggs MJ, et al. Pancreatitis: prevalence and risk factors among male veterans in a detoxification program. Pancreas. 2007;34:390–8. doi: 10.1097/mpa.0b013e318040b332. [DOI] [PubMed] [Google Scholar]
- 47.Kristiansen L, Gronbaek M, Becker U, et al. Risk of pancreatitis according to alcohol drinking habits: a population-based cohort study. Am J Epidemiol. 2008;168:932–7. doi: 10.1093/aje/kwn222. [DOI] [PubMed] [Google Scholar]
- 48.Katz M, Carangelo R, Miller LJ, et al. Effect of ethanol on cholecystokinin-stimulated zymogen conversion in pancreatic acinar cells. Am J Physiol. 1996;270:G171–5. doi: 10.1152/ajpgi.1996.270.1.G171. [DOI] [PubMed] [Google Scholar]
- 49.Lu Z, Karne S, Kolodecik T, et al. Alcohols enhance caerulein-induced zymogen activation in pancreatic acinar cells. American journal of physiology Gastrointestinal and liver physiology. 2002;282:G501–7. doi: 10.1152/ajpgi.00388.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pandol SJ, Periskic S, Gukovsky I, et al. Ethanol diet increases the sensitivity of rats to pancreatitis induced by cholecystokinin octapeptide. Gastroenterology. 1999;117:706–16. doi: 10.1016/s0016-5085(99)70465-8. [DOI] [PubMed] [Google Scholar]
- 51.Deng X, Wang L, Elm MS, et al. Chronic alcohol consumption accelerates fibrosis in response to cerulein-induced pancreatitis in rats. American Journal of Pathology. 2005;166:93–106. doi: 10.1016/S0002-9440(10)62235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bourliere M, Barthet M, Berthezene P, et al. Is tobacco a risk factor for chronic pancreatitis and alcoholic cirrhosis? Gut. 1991;32:1392–5. doi: 10.1136/gut.32.11.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin Y, Tamakoshi A, Hayakawa T, et al. Cigarette smoking as a risk factor for chronic pancreatitis: a case-control study in Japan. Research Committee on Intractable Pancreatic Diseases. Pancreas. 2000;21:109–14. doi: 10.1097/00006676-200008000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Lowenfels AB, Zwemer FL, Jhangiani S, et al. Pancreatitis in a native American Indian population. Pancreas. 1987;2:694–7. doi: 10.1097/00006676-198711000-00012. [DOI] [PubMed] [Google Scholar]
- 55.Morton C, Klatsky AL, Udaltsova N. Smoking, coffee, and pancreatitis. The American Journal of Gastroenterology. 2004;99:731–8. doi: 10.1111/j.1572-0241.2004.04143.x. [DOI] [PubMed] [Google Scholar]
- 56.Talamini G, Bassi C, Falconi M, et al. Cigarette smoking: an independent risk factor in alcoholic pancreatitis. Pancreas. 1996;12:131–7. [PubMed] [Google Scholar]
- 57.Tolstrup JS, Kristiansen L, Becker U, et al. Smoking and risk of acute and chronic pancreatitis among women and men: a population-based cohort study. Arch Intern Med. 2009;169:603–9. doi: 10.1001/archinternmed.2008.601. [DOI] [PubMed] [Google Scholar]
- 58.Yen S, Hsieh CC, MacMahon B. Consumption of alcohol and tobacco and other risk factors for pancreatitis. American Journal of Epidemiology. 1982;116:407–14. doi: 10.1093/oxfordjournals.aje.a113425. [DOI] [PubMed] [Google Scholar]
- 59.Andriulli A, Botteri E, Almasio PL, et al. Smoking as a cofactor for causation of chronic pancreatitis: a meta-analysis. Pancreas. 2010;39:1205–10. doi: 10.1097/MPA.0b013e3181df27c0. [DOI] [PubMed] [Google Scholar]
- 60.Talamini G, Bassi C, Falconi M, et al. Smoking cessation at the clinical onset of chronic pancreatitis and risk of pancreatic calcifications. Pancreas. 2007;35:320–6. doi: 10.1097/mpa.0b013e31812e965e. [DOI] [PubMed] [Google Scholar]
- 61.Lankisch PG, Breuer N, Bruns A, et al. Natural history of acute pancreatitis: a long-term population-based study. Am J Gastroenterol. 2009;104:2797–805. doi: 10.1038/ajg.2009.405. quiz 806. [DOI] [PubMed] [Google Scholar]
- 62.Cohn JA, Friedman KJ, Noone PG, et al. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N Engl J Med. 1998;339:653–8. doi: 10.1056/NEJM199809033391002. [DOI] [PubMed] [Google Scholar]
- 63.Felderbauer P, Hoffmann P, Einwachter H, et al. A novel mutation of the calcium sensing receptor gene is associated with chronic pancreatitis in a family with heterozygous SPINK1 mutations. BMC Gastroenterol. 2003;3:34. doi: 10.1186/1471-230X-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Masson E, Chen JM, Scotet V, et al. Association of rare chymotrypsinogen C (CTRC) gene variations in patients with idiopathic chronic pancreatitis. Hum Genet. 2008;123:83–91. doi: 10.1007/s00439-007-0459-3. [DOI] [PubMed] [Google Scholar]
- 65.Muddana V, Lamb J, Greer JB, et al. Association between calcium sensing receptor gene polymorphisms and chronic pancreatitis in a US population: role of serine protease inhibitor Kazal 1type and alcohol. World J Gastroenterol. 2008;14:4486–91. doi: 10.3748/wjg.14.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pfutzer RH, Barmada MM, Brunskill AP, et al. SPINK1/PSTI polymorphisms act as disease modifiers in familial and idiopathic chronic pancreatitis. Gastroenterology. 2000;119:615–23. doi: 10.1053/gast.2000.18017. [DOI] [PubMed] [Google Scholar]
- 67.Santhosh S, Witt H, te Morsche RH, et al. A loss of function polymorphism (G191R) of anionic trypsinogen (PRSS2) confers protection against chronic pancreatitis. Pancreas. 2008;36:317–20. doi: 10.1097/MPA.0b013e31815db4b3. [DOI] [PubMed] [Google Scholar]
- 68.Sharer N, Schwarz M, Malone G, et al. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. N Engl J Med. 1998;339:645–52. doi: 10.1056/NEJM199809033391001. [DOI] [PubMed] [Google Scholar]
- 69.Whitcomb DC, Gorry MC, Preston RA, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nature genetics. 1996;14:141–5. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 70.Witt H, Luck W, Hennies HC, et al. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000;25:213–6. doi: 10.1038/76088. [DOI] [PubMed] [Google Scholar]
- 71.Witt H, Sahin-Toth M, Landt O, et al. A degradation-sensitive anionic trypsinogen (PRSS2) variant protects against chronic pancreatitis. Nat Genet. 2006;38:668–73. doi: 10.1038/ng1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ooi CY, Dorfman R, Cipolli M, et al. Type of CFTR mutation determines risk of pancreatitis in patients with cystic fibrosis. Gastroenterology. 2011;140:153–61. doi: 10.1053/j.gastro.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 73.Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nature reviews Gastroenterology & hepatology. 2010;7:131–45. doi: 10.1038/nrgastro.2010.6. [DOI] [PubMed] [Google Scholar]
- 74.Schneider A, Singer MV. Alcoholic pancreatitis. Dig Dis. 2005;23:222–31. doi: 10.1159/000090169. [DOI] [PubMed] [Google Scholar]
- 75.Zhang L, Chari S, Smyrk TC, et al. Autoimmune pancreatitis (AIP) type 1 and type 2: an international consensus study on histopathologic diagnostic criteria. Pancreas. 2011;40:1172–9. doi: 10.1097/MPA.0b013e318233bec5. [DOI] [PubMed] [Google Scholar]
- 76.Kloppel G. Chronic pancreatitis, pseudotumors and other tumor-like lesions. Mod Pathol. 2007;20:S113–31. doi: 10.1038/modpathol.3800690. [DOI] [PubMed] [Google Scholar]
- 77.Alpern MB, Sandler MA, Kellman GM, et al. Chronic pancreatitis: ultrasonic features. Radiology. 1985;155:215–9. doi: 10.1148/radiology.155.1.3883420. [DOI] [PubMed] [Google Scholar]
- 78.Tsao TF, Kang RJ, Tyan YS, et al. Color Doppler twinkling artifact related to chronic pancreatitis with parenchymal calcification. Acta Radiol. 2006;47:547–8. doi: 10.1080/02841850600690371. [DOI] [PubMed] [Google Scholar]
- 79.Lankisch PG, Otto J, Erkelenz I, et al. Pancreatic calcifications: no indicator of severe exocrine pancreatic insufficiency. Gastroenterology. 1986;90:617–21. doi: 10.1016/0016-5085(86)91115-7. [DOI] [PubMed] [Google Scholar]
- 80.Ammann RW, Muench R, Otto R, et al. Evolution and regression of pancreatic calcification in chronic pancreatitis. A prospective long-term study of 107 patients. Gastroenterology. 1988;95:1018–28. doi: 10.1016/0016-5085(88)90178-3. [DOI] [PubMed] [Google Scholar]
- 81.Shawker TH, Linzer M, Hubbard VS. Chronic pancreatitis: the diagnostic significance of pancreatic size and echo amplitude. J Ultrasound Med. 1984;3:267–72. doi: 10.7863/jum.1984.3.6.267. [DOI] [PubMed] [Google Scholar]
- 82.Remer EM, Baker ME. Imaging of chronic pancreatitis. Radiol Clin North Am. 2002;40:1229–42. v. doi: 10.1016/s0033-8389(02)00044-1. [DOI] [PubMed] [Google Scholar]
- 83.Siddiqi AJ, Miller F. Chronic pancreatitis: ultrasound, computed tomography, and magnetic resonance imaging features. Semin Ultrasound CT MR. 2007;28:384–94. doi: 10.1053/j.sult.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 84.Kim DH, Pickhardt PJ. Radiologic assessment of acute and chronic pancreatitis. Surg Clin North Am. 2007;87:1341–58. viii. doi: 10.1016/j.suc.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 85.Miller FH, Keppke A, Balthazar E. Imaging Pancreatitis. In: Gore RMLM, editor. Textbook of Gastrointestinal Radiology. 3. WB. Saunders; 2008. pp. 1885–914. [Google Scholar]
- 86.Luetmer PH, Stephens DH, Ward EM. Chronic pancreatitis: reassessment with current CT. Radiology. 1989;171:353–7. doi: 10.1148/radiology.171.2.2704799. [DOI] [PubMed] [Google Scholar]
- 87.Scuro LA, Cavallini G, Benini L, et al. Pancreatic calcifications in patients with chronic pancreatitis. A sign of long-lasting or severe disease? Int J Pancreatol. 1990;6:139–50. [PubMed] [Google Scholar]
- 88.Ichikawa T, Sou H, Araki T, et al. Duct-penetrating sign at MRCP: usefulness for differentiating inflammatory pancreatic mass from pancreatic carcinomas. Radiology. 2001;221:107–16. doi: 10.1148/radiol.2211001157. [DOI] [PubMed] [Google Scholar]
- 89.Johnson PT, Outwater EK. Pancreatic carcinoma versus chronic pancreatitis: dynamic MR imaging. Radiology. 1999;212:213–8. doi: 10.1148/radiology.212.1.r99jl16213. [DOI] [PubMed] [Google Scholar]
- 90.Yamada Y, Mori H, Matsumoto S, et al. Pancreatic adenocarcinoma versus chronic pancreatitis: differentiation with triple-phase helical CT. Abdom Imaging. 2010;35:163–71. doi: 10.1007/s00261-009-9579-7. [DOI] [PubMed] [Google Scholar]
- 91.Wiersema MJ, Wiersema LM. Endosonography of the pancreas: normal variation versus changes of early chronic pancreatitis. Gastrointestinal Endoscopy Clinics of North America. 1995;5:487–96. [PubMed] [Google Scholar]
- 92.Wallace MB, Hawes RH, Durkalski V, et al. The reliability of EUS for the diagnosis of chronic pancreatitis: interobserver agreement among experienced endosonographers. Gastrointestinal Endoscopy. 2001;53:294–9. doi: 10.1016/s0016-5107(01)70401-4. [DOI] [PubMed] [Google Scholar]
- 93.Catalano MF, Lahoti S, Alcocer E, et al. Dynamic imaging of the pancreas using real-time endoscopic ultrasonography with secretin stimulation. Gastrointestinal Endoscopy. 1998;48:580–7. doi: 10.1016/s0016-5107(98)70039-2. [DOI] [PubMed] [Google Scholar]
- 94.Stevens T, Lopez R, Adler DG, et al. Multicenter comparison of the interobserver agreement of standard EUS scoring and Rosemont classification scoring for diagnosis of chronic pancreatitis. Gastrointestinal Endoscopy. 2010;71:519–26. doi: 10.1016/j.gie.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 95.Rajan E, Clain JE, Levy MJ, et al. Age-related changes in the pancreas identified by EUS: a prospective evaluation. Gastrointestinal Endoscopy. 2005;61:401–6. doi: 10.1016/s0016-5107(04)02758-0. [DOI] [PubMed] [Google Scholar]
- 96.Nattermann C, Goldschmidt AJ, Dancygier H. Endosonography in chronic pancreatitis--a comparison between endoscopic retrograde pancreatography and endoscopic ultrasonography. Endoscopy. 1993;25:565–70. doi: 10.1055/s-2007-1010406. [DOI] [PubMed] [Google Scholar]
- 97.Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointestinal Endoscopy. 2009;69:1251–61. doi: 10.1016/j.gie.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 98.Topazian M, Enders F, Kimmey M, et al. Interobserver agreement for EUS findings in familial pancreatic-cancer kindreds. Gastrointestinal Endoscopy. 2007;66:62–7. doi: 10.1016/j.gie.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 99.NIH state-of-the-science statement on endoscopic retrograde cholangiopancreatography (ERCP) for diagnosis and therapy. NIH Consens State Sci Statements. 2002;19:1–26. [PubMed] [Google Scholar]
- 100.Adler DG, Lichtenstein D, Baron TH, et al. The role of endoscopy in patients with chronic pancreatitis. Gastrointest Endosc. 2006;63:933–7. doi: 10.1016/j.gie.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 101.Axon AT, Classen M, Cotton PB, et al. Pancreatography in chronic pancreatitis: international definitions. Gut. 1984;25:1107–12. doi: 10.1136/gut.25.10.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ladas SD, Tassios PS, Giorgiotis K, et al. Pancreatic duct width: its significance as a diagnostic criterion for pancreatic disease. Hepatogastroenterology. 1993;40:52–5. [PubMed] [Google Scholar]
- 103.Kang JK, Chung JB, Moon YM, et al. The normal endoscopic pancreatogram in Koreans. Korean J Intern Med. 1989;4:74–9. doi: 10.3904/kjim.1989.4.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reuben A, Johnson AL, Cotton PB. Is pancreatogram interpretation reliable?--a study of observer variation and error. Br J Radiol. 1978;51:956–62. doi: 10.1259/0007-1285-51-612-956. [DOI] [PubMed] [Google Scholar]
- 105.Anand BS, Vij JC, Mac HS, et al. Effect of aging on the pancreatic ducts: a study based on endoscopic retrograde pancreatography. Gastrointest Endosc. 1989;35:210–3. doi: 10.1016/s0016-5107(89)72760-7. [DOI] [PubMed] [Google Scholar]
- 106.Jones SN, McNeil NI, Lees WR. The interpretation of retrograde pancreatography in the elderly. Clin Radiol. 1989;40:393–6. doi: 10.1016/s0009-9260(89)80132-1. [DOI] [PubMed] [Google Scholar]
- 107.Schmitz-Moormann P, Himmelmann GW, Brandes JW, et al. Comparative radiological and morphological study of human pancreas. Pancreatitis like changes in postmortem ductograms and their morphological pattern. Possible implication for ERCP. Gut. 1985;26:406–14. doi: 10.1136/gut.26.4.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Angelini G, Cavallini G, Pederzoli P, et al. Long-term outcome of acute pancreatitis: a prospective study with 118 patients. Digestion. 1993;54:143–7. doi: 10.1159/000201028. [DOI] [PubMed] [Google Scholar]
- 109.Forsmark CE, Toskes PP. What does an abnormal pancreatogram mean? Gastrointest Endosc Clin N Am. 1995;5:105–23. [PubMed] [Google Scholar]
- 110.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–81. doi: 10.1016/j.cgh.2006.02.005. quiz 665. [DOI] [PubMed] [Google Scholar]
- 111.Brentnall TA, Bronner MP, Byrd DR, et al. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med. 1999;131:247–55. doi: 10.7326/0003-4819-131-4-199908170-00003. [DOI] [PubMed] [Google Scholar]
- 112.Vitale GC, Davis BR, Zavaleta C, et al. Endoscopic retrograde cholangiopancreatography and histopathology correlation for chronic pancreatitis. Am Surg. 2009;75:649–53. doi: 10.1177/000313480907500803. discussion 53. [DOI] [PubMed] [Google Scholar]
- 113.Sica GT, Braver J, Cooney MJ, et al. Comparison of endoscopic retrograde cholangiopancreatography with MR cholangiopancreatography in patients with pancreatitis. Radiology. 1999;210:605–10. doi: 10.1148/radiology.210.3.r99fe55605. [DOI] [PubMed] [Google Scholar]
- 114.Calvo MM, Bujanda L, Calderon A, et al. Comparison between magnetic resonance cholangiopancreatography and ERCP for evaluation of the pancreatic duct. Am J Gastroenterol. 2002;97:347–53. doi: 10.1111/j.1572-0241.2002.05468.x. [DOI] [PubMed] [Google Scholar]
- 115.Parsi MA, Conwell DL, Zuccaro G, et al. Findings on endoscopic retrograde cholangiopancreatography and pancreatic function test in suspected chronic pancreatitis and negative cross-sectional imaging. Clin Gastroenterol Hepatol. 2008;6:1432–6. doi: 10.1016/j.cgh.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 116.Malfertheiner P, Buchler M, Stanescu A, et al. Exocrine pancreatic function in correlation to ductal and parenchymal morphology in chronic pancreatitis. Hepatogastroenterology. 1986;33:110–4. [PubMed] [Google Scholar]
- 117.Chowdhury RS, Forsmark CE. Review article: Pancreatic function testing. Aliment Pharmacol Ther. 2003;17:733–50. doi: 10.1046/j.1365-2036.2003.01495.x. [DOI] [PubMed] [Google Scholar]
- 118.Pandol SJ. Pancreatic Secretion. In: Feldman MLFaLB., editor. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 8. Philadelphia, PA: Saunders-Elsevier; 2006. [Google Scholar]
- 119.Lieb JG, 2nd, Draganov PV. Pancreatic function testing: here to stay for the 21st century. World J Gastroenterol. 2008;14:3149–58. doi: 10.3748/wjg.14.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hahn JU, Kerner W, Maisonneuve P, et al. Low fecal elastase 1 levels do not indicate exocrine pancreatic insufficiency in type-1 diabetes mellitus. Pancreas. 2008;36:274–8. doi: 10.1097/MPA.0b013e3181656f8. [DOI] [PubMed] [Google Scholar]
- 121.Amann ST, Bishop M, Curington C, et al. Fecal pancreatic elastase 1 is inaccurate in the diagnosis of chronic pancreatitis. Pancreas. 1996;13:226–30. doi: 10.1097/00006676-199610000-00002. [DOI] [PubMed] [Google Scholar]
- 122.Gullo L, Ventrucci M, Tomassetti P, et al. Fecal elastase 1 determination in chronic pancreatitis. Dig Dis Sci. 1999;44:210–3. doi: 10.1023/a:1026691209094. [DOI] [PubMed] [Google Scholar]
- 123.Schneider A, Funk B, Caspary W, et al. Monoclonal versus polyclonal ELISA for assessment of fecal elastase concentration: pitfalls of a new assay. Clin Chem. 2005;51:1052–4. doi: 10.1373/clinchem.2004.046888. [DOI] [PubMed] [Google Scholar]
- 124.Johnson SG, Levitt MD. Relation between serum pancreatic isoamylase concentration and pancreatic exocrine function. Am J Dig Dis. 1978;23:914–8. doi: 10.1007/BF01072466. [DOI] [PubMed] [Google Scholar]
- 125.Yamamura J, Grosse R, Jarisch A, et al. Pancreatic exocrine function and cardiac iron in patients with iron overload and with thalassemia. Pediatr Blood Cancer. 2011;57:674–6. doi: 10.1002/pbc.22990. [DOI] [PubMed] [Google Scholar]
- 126.Stormon MO, Ip WF, Ellis L, et al. Evidence of a generalized defect of acinar cell function in Shwachman-Diamond syndrome. J Pediatr Gastroenterol Nutr. 2010;51:8–13. doi: 10.1097/MPG.0b013e3181d67e78. [DOI] [PubMed] [Google Scholar]
- 127.Cleghorn G, Benjamin L, Corey M, et al. Serum immunoreactive pancreatic lipase and cationic trypsinogen for the assessment of exocrine pancreatic function in older patients with cystic fibrosis. Pediatrics. 1986;77:301–6. [PubMed] [Google Scholar]
- 128.Jacobson DG, Curington C, Connery K, et al. Trypsin-like immunoreactivity as a test for pancreatic insufficiency. N Engl J Med. 1984;310:1307–9. doi: 10.1056/NEJM198405173102007. [DOI] [PubMed] [Google Scholar]
- 129.Nousia-Arvanitakis S. Fecal elastase-1 concentration: an indirect test of exocrine pancreatic function and a marker of an enteropathy regardless of cause. J Pediatr Gastroenterol Nutr. 2003;36:314–5. doi: 10.1097/00005176-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 130.Herzig KH, Purhonen AK, Rasanen KM, et al. Fecal pancreatic elastase-1 levels in older individuals without known gastrointestinal diseases or diabetes mellitus. BMC Geriatr. 2011;11:4. doi: 10.1186/1471-2318-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Le Moine O, Devaster JM, Deviere J, et al. Trypsin activity. A new marker of acute alcoholic pancreatitis. Dig Dis Sci. 1994;39:2634–8. doi: 10.1007/BF02087701. [DOI] [PubMed] [Google Scholar]
- 132.Hahn JU, Bochnig S, Kerner W, et al. A new fecal elastase 1 test using polyclonal antibodies for the detection of exocrine pancreatic insufficiency. Pancreas. 2005;30:189–91. doi: 10.1097/01.mpa.0000153617.40513.34. [DOI] [PubMed] [Google Scholar]
- 133.Dominguez-Munoz JE, Iglesias-Garcia J, Vilarino-Insua M, et al. 13C-mixed triglyceride breath test to assess oral enzyme substitution therapy in patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2007;5:484–8. doi: 10.1016/j.cgh.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 134.Keller J, Bruckel S, Jahr C, et al. A modified (1)(3)C-mixed triglyceride breath test detects moderate pancreatic exocrine insufficiency. Pancreas. 2011;40:1201–5. doi: 10.1097/MPA.0b013e318220ad98. [DOI] [PubMed] [Google Scholar]
- 135.Weintraub A, Blau H, Mussaffi H, et al. Exocrine pancreatic function testing in patients with cystic fibrosis and pancreatic sufficiency: a correlation study. J Pediatr Gastroenterol Nutr. 2009;48:306–10. doi: 10.1097/mpg.0b013e318180af4f. [DOI] [PubMed] [Google Scholar]
- 136.Amann ST, Josephson SA, Toskes PP. Acid steatocrit: a simple, rapid gravimetric method to determine steatorrhea. Am J Gastroenterol. 1997;92:2280–4. [PubMed] [Google Scholar]
- 137.Patane R, Bottaro G, Ricca O, et al. “Qualitative test of fecal fat” and “steatocrit”, simple complementary methods for the evaluation of steatorrhea in childhood. Pediatr Med Chir. 1988;10:403–8. [PubMed] [Google Scholar]
- 138.Goldberg DM. Proteases in the evaluation of pancreatic function and pancreatic disease. Clin Chim Acta. 2000;291:201–21. doi: 10.1016/s0009-8981(99)00229-6. [DOI] [PubMed] [Google Scholar]
- 139.Pieramico O, Nelson DK, Glasbrenner B, et al. Impaired interdigestive pancreatic polypeptide release. Early hormonal disorder in chronic pancreatitis? Dig Dis Sci. 1994;39:69–74. doi: 10.1007/BF02090063. [DOI] [PubMed] [Google Scholar]
- 140.Owyang C, Scarpello JH, Vinik AI. Correlation between pancreatic enzyme secretion and plasma concentration of human pancreatic polypeptide in health and in chronic pancreatitis. Gastroenterology. 1982;83:55–62. [PubMed] [Google Scholar]
- 141.Kitagawa M, Naruse S, Ishiguro H, et al. Evaluating exocrine function tests for diagnosing chronic pancreatitis. Pancreas. 1997;15:402–8. doi: 10.1097/00006676-199711000-00011. [DOI] [PubMed] [Google Scholar]
- 142.Catalano MF, Lahoti S, Geenen JE, et al. Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc. 1998;48:11–7. doi: 10.1016/s0016-5107(98)70122-1. [DOI] [PubMed] [Google Scholar]
- 143.Chowdhury R, Bhutani MS, Mishra G, et al. Comparative analysis of direct pancreatic function testing versus morphological assessment by endoscopic ultrasonography for the evaluation of chronic unexplained abdominal pain of presumed pancreatic origin. Pancreas. 2005;31:63–8. doi: 10.1097/01.mpa.0000164451.69265.80. [DOI] [PubMed] [Google Scholar]
- 144.Stevens T, Dumot JA, Parsi MA, et al. Combined endoscopic ultrasound and secretin endoscopic pancreatic function test in patients evaluated for chronic pancreatitis. Dig Dis Sci. 2010;55:2681–7. doi: 10.1007/s10620-009-1084-x. [DOI] [PubMed] [Google Scholar]
- 145.Heij HA, Obertop H, van Blankenstein M, et al. Relationship between functional and histological changes in chronic pancreatitis. Dig Dis Sci. 1986;31:1009–13. doi: 10.1007/BF01300251. [DOI] [PubMed] [Google Scholar]
- 146.Hayakawa T, Kondo T, Shibata T, et al. Relationship between pancreatic exocrine function and histological changes in chronic pancreatitis. Am J Gastroenterol. 1992;87:1170–4. [PubMed] [Google Scholar]
- 147.Varadarajulu S, Eltoum I, Tamhane A, et al. Histopathologic correlates of noncalcific chronic pancreatitis by EUS: a prospective tissue characterization study. Gastrointest Endosc. 2007;66:501–9. doi: 10.1016/j.gie.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 148.Chong AK, Hawes RH, Hoffman BJ, et al. Diagnostic performance of EUS for chronic pancreatitis: a comparison with histopathology. Gastrointest Endosc. 2007;65:808–14. doi: 10.1016/j.gie.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 149.Albashir S, Bronner MP, Parsi MA, et al. Endoscopic ultrasound, secretin endoscopic pancreatic function test, and histology: correlation in chronic pancreatitis. Am J Gastroenterol. 2010;105:2498–503. doi: 10.1038/ajg.2010.274. [DOI] [PubMed] [Google Scholar]
- 150.Ammann RW, Heitz PU, Kloppel G. Course of alcoholic chronic pancreatitis: a prospective clinicomorphological long-term study. Gastroenterology. 1996;111:224–31. doi: 10.1053/gast.1996.v111.pm8698203. [DOI] [PubMed] [Google Scholar]
- 151.Vega-Peralta J, Manivel C, Attam R, et al. Accuracy of EUS for Diagnosis of Minimal Change Chronic Pancreatitis (MCCP): Correlation with Histopathology in 50 Patients Undergoing Total Pancreatectomy (TP) with Islet Autotransplantion (IAT) (Abstract). 42nd Annual Meeting of the American Pancreatic Association; Chicago, IL: Lippincott Williams & Wilkins; 2011. [Google Scholar]