Abstract
Background
The poor permeability of the plasma and nuclear membranes to DNA plasmids are two major barriers for the development of these therapeutic molecules. Therefore, success in gene therapy approaches depends on the development of efficient and safe non-viral delivery systems.
Objectives
The aim of this study was to investigate the in vitro delivery of plasmid DNA encoding HPV16 E7 gene using cell penetrating peptide delivery system to achieve the best conditions for cell transfection and protein expression. For this purpose, we have used a cationic peptide delivery system, MPG which forms stable non-covalent complexes with nucleic acids for delivery of pEGFP-E7 as a model antigen in vitro.
Materials and Methods
DNA construct encoding HPV16 E7 (pEGFP-E7) was prepared in large scale with high purity. MPG peptide/ DNA complexes were prepared at different N/P (nitrogen/phosphate) ratios and physicochemical characterization and stability of nanoparticles were investigated. In vitro peptide-mediated E7-GFP DNA transfection, and its expression was evaluated in three cell types. To quantify the transfection efficiency of this delivery system, transfected cells were harvested and assessed for GFP-positive cells by flow cytometry. Furthermore, E7-GFP expression was confirmed by western blot analysis.
Results
The cellular uptake of MPG based nanoparticles was shown to be comparable with standard reagent PEI. The COS-7 cells transfected by MPG-based nanoparticles at an N/P ratio of 15:1 showed the highest transfection efficiency and gene expression.
Conclusions
The results indicated that the efficient gene expression depends on both cell type and N/P ratio applied, in vitro. The efficient protein expression detected by western blotting and flow cytometry supports the potential of MPGbased nanoparticles as a potent gene delivery system.
Keywords: Cell penetrating peptide, E7, Gene delivery, Human papillomavirus, MPG-based nanoparticles
1. Background
Application of gene therapy and nucleic acids in medicine has been identified as a promising treatment strategy for various disorders such as cancer during the past decades (1). However, the lack of an efficient delivery system has led to fail the potential application of gene therapies. Unfortunately, these therapeutic molecules are usually unable to cross cellular barriers efficiently by passive diffusion, due to their strong negative charge, high molecular weight (MW) and hydrophilicity which make cellular membrane impermeable to them. Therefore, the major challenge for nucleic acids delivery is the use of appropriate vectors that can protect cargo, shipping thoroughly to the target sites within cell (1-3). Generally, there are two different gene delivery systems: viral and non-viral vectors. Viral vectors have been shown to be very efficient, but their practical use is limited by safety concerns (3). In recent years, non-viral gene delivery systems have attracted special interest (4). However, they suffer from disadvantages such as low gene transfection efficiency and significant toxicity (2-3). Therefore, it is critical to develop new carriers with precisely defined structures and properties for efficient delivery into the cells with minimal toxicity. Recently, among the different available non-viral delivery systems such as cationic polymers, cationic liposomes and inorganic nano-particles, cell-penetrating peptides (CPPs) represent an interesting alternative to bypass the problem of poor membrane permeability to nucleic acids (5-6). These peptides consist of less than 30 amino acids; mostly, possess cationic and hydrophobic residues that help them to establish interactions with the cell-surface negative charges (7).
MPG with 27 residues, GALFLGFLGAAGSTMGAWSQPKKKRKV, is a primary amphipathic peptide, composed of three domains: an N-terminus hydrophobic domain derived from the fusion sequence of the HIV gp41 and is required for efficient targeting to the cell membrane and cellular uptake; a hydrophilic lysine-rich domain, which is derived from the nuclear localization sequence (NLS) of SV40 large T-antigen (KKKRKV), and is necessary for the main interactions with nucleic acids, intracellular transportation of the cargo and solubility of the peptide vector; and a spacer domain (WSQP), that improves the flexibility and integrity of the hydrophobic and hydrophilic domains (6, 8-9).
Human papillomavirus (HPV) infection is a necessary cause of cervical cancer (10). HPV oncogenes, especially E7, are known to contribute to the progression towards malignancy (11). E7 is constantly expressed by the HPV-positive tumor cells. Therefore, it represents an ideal target for development of the immunotherapy in HPV-positive cervical cancers (12).
2. Objectives
To improve non-viral gene delivery in vitro, we hypothesized that a peptide-based gene delivery system, MPG, which forms stable non-covalent complexes with nucleic acids would enhance transfection efficiency of plasmid DNA encoding HPV16 E7 gene. For this purpose, MPG/ pEGFP-E7 complexes were prepared and the plasmid DNA stability during formulation and protection of its structure in serum was evaluated. After MPG cytotoxicity assay (MTT), transfection efficiency of the nanoparticles in three cell types (COS-7, HEK293T and TC-1) was determined by fluorescent microscopy and flow cytometry. Furthermore, E7-GFP expression was confirmed by western blot analysis.
3. Materials and Methods
3.1. Materials
The MPG peptide was purchased from Biomatik Corporation, Canada. MTT (3-(4, 5-dimethyl-thiazol-2-yl)-2, 5-diphenyl tetrazolium bromide) and high molecular weight linear polyethylenimine (PEI) 25 kDa were obtained from Sigma-Aldrich and Polysciences, respectively. Fetal calf serum (FCS) was purchased from Invitrogen, USA. RPMI 1640 and HEPES were provided by Sigma, USA. L-glutamine and gentamicin were purchased from Sigma, Germany.
TC-1 (ATCC number: CRL-2785) tumor cell line, Human Embryonic Kidney (HEK-293T), and African Green Monkey Kidney (COS-7, ATCC number: CRL-1651) cell lines were obtained from National Cell Bank of Iran (NCBI, Pasteur Institute of Iran, Tehran, Iran).
3.2. Preparation of Endotoxin-Free Plasmid DNA Expressing E7 Protein
Purification of pEGFP-E7 obtained from (13), was accomplished by ion exchange chromatography with an endo-free plasmid Giga kit (QIAGEN, Valencia, CA) according to the manufacturer’s instructions. Plasmid DNA was analyzed by agarose gel electrophoresis, quantified by spectrophotometry and stored in endotoxin-free PBS1X at -20ºC.
3.3. Preparation of Peptide/DNA Complexes
The stock solution of the synthetic MPG peptide was prepared in sterile water at a final concentration of 2 mg.mL-1. Peptide solution was added dropwise to 1 μg of plasmid DNA at different molar ratios of basic amino acid residues in the MPG peptide to DNA phosphates in PBS (pH 7.4), and incubated for 60 min at 22°C to allow complete electrostatic interaction between peptide and DNA, ensuring the formation of complex.
3.4. DNA Binding and Neutralization
The gel retardation or electrophoretic mobility shift assay is a sensitive method to confirm the condensation between peptide and DNA and neutralization of the negative charges on DNA. For this purpose, 10 μL of each peptide/DNA complex was mixed with 2 μL of a 6 loading buffer and loaded onto a 1% agarose gel containing ethidium bromide.
3.5. Scanning Electron Microscopy
The MPG/DNA nanoparticles were prepared at an N/P ratio of 15:1 and a thin gold layer was sputtered on the surface. The size and morphology of nanoparticles were analyzed using a scanning electron microscope (SEM; KYKY-EM3200 model, China).
3.6. Stability and Protection Assay of Peptide Delivery System
To assess the stability of MPG/DNA complexes against DNA nucleases, DNase I was added to the nanoparticles (at different N/P ratios of 2:1 to 25:1) with a final concentration of 1.37 U enzyme per 1 μg DNA and the mixtures were incubated at 37ºC for 1 h followed by addition of stop solution (200 mM sodium chloride, 20 mM EDTA and 1% SDS) (14). To evaluate the serum stability, the nanoparticles at ratio of N/P: 10 and 15 were exposed to 10% serum and incubated for 5 h at 37ºC. Plasmid DNAs were released from peptide by adding 10% SDS solution for 2 h. Samples were analyzed by electrophoresis on 1% agarose gel containing ethidium bromide and the integrity of the DNA was visualized and compared with the naked DNA as control.
3.7. Cell Viability Assay
For cell viability assay, COS-7 cells (104 cells/ well) were seeded into 96-well microtiter plates in RPMI-1640 supplemented with 5% FCS and cultured for 16 h at 37°C in humidified incubator with 5% CO2 atmosphere. After replacement of media with fresh RPMI-1640, different concentration of MPG peptide was added to the cells and left for 24 and 48 h without exchanging the media. In a parallel assay, different N/P ratios were prepared and added to the cells. After 24 or 48 h of incubation, the media was removed and 20 μL of sterile filtered MTT stock solution (5 mg.mL-1) in fresh RPMI-1640 media was added to each well. After 3 h, the media was removed and 100 μL dimethyl sulfoxide (DMSO) was added to each well to dissolve the formazan products. The color intensity, which is proportional to the number of viable cells, assessed by an ELISA reader (Model 550, Bio-Rad) at 570 nm. Non-treated cells were used as a control (100% cell viability). The data were reported as the mean of each condition ± SD.
3.8. Transfection Assay
COS-7, HEK-293T and TC-1 cell lines were placed at a density of 0.5 × 105 cells/well in a 4-well plate (Greiner, Germany) in complete RPMI-1640 supplemented with 5% heat-inactivated FCS. Cells were approximately 80% confluent at the time of transfection. Peptide/DNA nanoparticles at N/P ratio of 10:1 and 15:1 as transfection reagent were prepared in a total volume of 100 μL and incubated for 1 h at 22ºC. MPG/DNA nanoparticles were added to the cells in the presence of 10% serum. The medium was replaced after 8 h incubation at 37°C with complete RPMI 10% FCS. Polyethyleneimine (LINPEI 25 kDa, Polyscience, N/P: 7) was used as a positive control. The un-transfected cells were used as a negative control. The level of GFP expression (transfection efficacy) was monitored by fluorescence microscopy (Envert Fluorescent Ceti, Korea) at 24 and 48 h after transfection and also quantified by a FACS Calibur flow cytometer (BD Biosciences) at 48 h post-transfection. For flow cytometry analysis, the transfected cells were harvested and scored for GFP-positive cells with appropriate gating using the green channel FL-1H. A total of 5×104 events were counted for each sample. The data were reported as means ± SD. E7-GFP expression was detected by western blot analysis.
3.9. Western Blot Analysis
The cells (i.e., un-transfected and transfected cells with MPG/DNA or PEI/DNA complexes) were scraped from their dishes and washed in PBS1X at 48 h after transfection. The extracted protein samples were separated by SDS-PAGE in a 12.5% (w/v) polyacrylamide gel. The proteins were resolved on gel and transferred onto protran nitrocellulose transfer membrane (Schleicher and Schuell Bioscience, Dassel, Germany). The membrane was pre-equilibrated with Tris-buffered saline Tween-20 (TBST) solution containing 2.5 percent bovine serum albumin (BSA) for overnight and reacted with anti-HPV16 E7 monoclonal antibody (1:10000 v/v, USBiological) or anti-GFP polyclonal antibody (1:5000 v/v; Acris antibodies GmbH) under standard procedures for 2 h at 22°C. After three washes with TBS, the membrane was incubated with anti-mouse IgG-HRP (1:2000, Sigma, USA) for 1.5 h at room temperature. The immunoreactive protein bands were visualized using peroxidase substrate 3, 3'-diaminobenzidine (DAB, Sigma, St. Louis, MO).
4. Results
4.1. Formation of MPG/DNA Complexes
pEGFP-E7 (1 μg) was mixed with increasing amounts of MPG at different N/P ratios for 1 h at 22°C. Herein, the N/P ratio is a molar ratio of 4 lysine residue and one arginine residue (N) in the peptide to phosphate group (P) in the DNA molecule. N/P ratio was calculated as previously described (15-16). Gel shift assay was used to examine peptide/DNA interaction. As shown in Figure 1A, the polynucleotide molecule did not migrate into the agarose gel at N/P ratio of 5: 1, indicating the formation of MPG/DNA complex. SEM analysis of nanoparticles at N/P ratio of 15:1 showed the spherical shape with a narrow size distribution (Figure 1B).
Figure 1 .
A: Gel retardation assay of MPG peptide complexed with pEGFP-E7 at different N/P ratios; Lane 1: naked plasmid DNA (pEGFP-E7), Lane 2: N/P = 2:1, Lane 3: N/P = 5:1, Lane 4: N/P = 10:1, Lane 5: N/P = 15:1, Lane 6: N/P = 20:1, Lane 7: N/P = 25:1 and lane 8: N/P = 30:1. B: The SEM micrograph of the spherical nanoparticles formed at N/P = 15:1 at 20,000× magnification
4.2. Stability of MPG-Based Nanoparticles in Presence of Serum
For serum protection assay, the N/P ratios of 10:1 and 15:1 of nanoparticles were selected. Agarose gel electrophoresis showed that unprotected plasmid DNA was degraded in the presence of serum after 5 h incubation with FCS as shown in Figure 2. In contrast, recovered DNA from nanoparticles remained intact. DNase degradation of DNA was observed at lower N/P ratios, but the bands corresponding to plasmid (intact plasmid) showed similar intensity at the N/P ratios higher than 10:1.
Figure 2 .
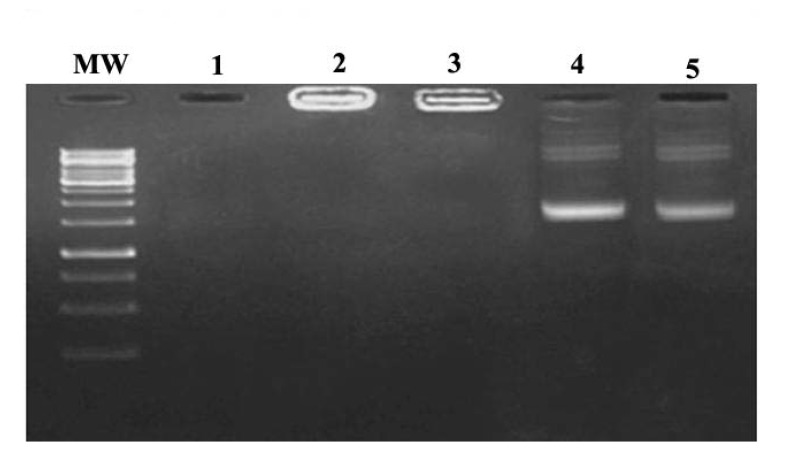
Serum stability assay. Lane 1: naked plasmid DNAin the presence of 10% FCS, Lane 2: N/P = 15:1 in the presence of 10% FCS, Lane 3: N/P= 15:1 without FCS, Lane 4 and 5: released plasmid DNA from nanoparticle (with N/P ratio of 10:1 and 15:1 respectively) after incubation with 10 % SDS
4.3. In vitro Cytotoxicity of Nanoparticles
To evaluate the potential of the MPG peptide as delivery system, the cytotoxicity of MPG complexed with pEGFP-E7 at various N/P ratios and different amounts of MPG peptide alone were determined (Figure 3). For this purpose, we investigated MTT assay in COS-7 cell line at 24 and 48 h after incubation. As shown in Figure 3A, MPG did not induce any cytotoxic effects up to concentration of 32 μM over a period of 48 h. The cell viability started to decrease at concentrations of peptide more than 40 μM as compared to untreated cells (~ 10%, p < 0.05). MPG was not cytotoxic at the concentrations used for gene delivery, and the complex of MPG with DNA could reduce cytotoxicity of MPG at higher concentrations (Figure 3B).
Figure 3 .
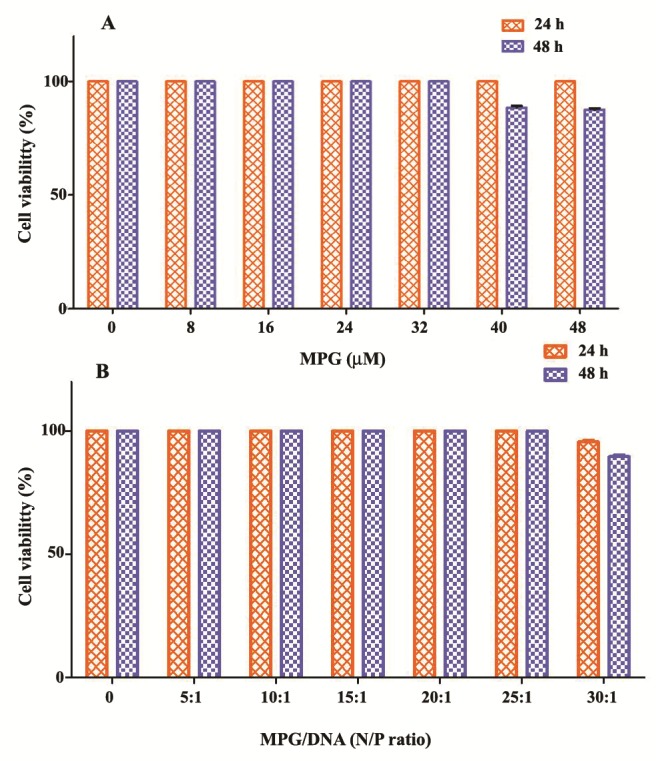
In vitro cytotoxicity of MPG/pEGFP-E7 in COS- 7 cells. A: Cell viability was examined using MTT assay in the presence of various amounts of peptide and B: different N/P ratios of peptide/plasmid after 24 and 48 h. Data are the means ± SD
4.4. In vitro Cell Transfection
The GFP expression efficiency induced by MPG/DNA nanoparticle was investigated at N/P ratios of 10:1 and 15:1, against COS-7, HEK-293T and TC-1 cell lines for 8 h with plasmid DNA encoding E7-GFP. Our results indicated that MPG/DNA nanoparticles at both ratios of 10:1 and 15:1 were capable to transfect HEK-293T and COS-7 cells efficiently, but not TC-1 cells at both times. For confirmation of the E7 DNA delivery in vitro, transfection efficiency was compared with PEI 25 kDa as a transfection reagent and it was 22.53% and 71.5% for HEK-293T and COS-7 cells, respectively (data not shown). As shown in Figure 4, strong E7-GFP expression was detected in approximately 17.3% of HEK- 293T and 67.6% of COS-7 cells treated with the N/P ratio of 15:1 of MPG-based nanoparticles. In the N/P ratio of 10:1, percentage of cells expressing E7-GFP was 13.22% and 66% for HEK293 and COS-7 cells, respectively. In the N/P ratio of 15:1, the efficiency of transfection mediated by MPG-based nanoparticles is comparable with the standard transfection agent (PEI) at its optimal N/P ratio of 7. However, MPG/DNA nanoparticles have some benefits such as no cytotoxicity to mammalian cells and no sensitivity to serum in comparison with PEI/DNA complexes.
Figure 4 .

A: Transfection efficiency of pEGFP-E7 using MPG in HEK, B: COS-7 and C: TC-1 (C) cells after 48 h: The expression of E7-GFP using GFP reporter was monitored by Epi-fluorescent microscopy and flow cytometry. E7-GFP expression in HEK, COS-7 and TC-1 cells was 13.22%, 66% and 2.04% at ratio of 10: 1 and 17.3 %, 67.6 % and 4.61 % at ratio of 15: 1 as compared to negative control
Our results in Figures 4 and 5 indicated that TC-1 transfection with MPG/DNA nanoparticles did not show any significant fluorescence at the N/P ratios of 10:1 and 15:1 (2.09% and 4.61% for N/P ratio of 10:1 and 15:1, respectively) suggesting that TC-1 cells internalize polyplexes in a different mechanism from that of COS-7 and HEK 293T cells.
Figure 5 .
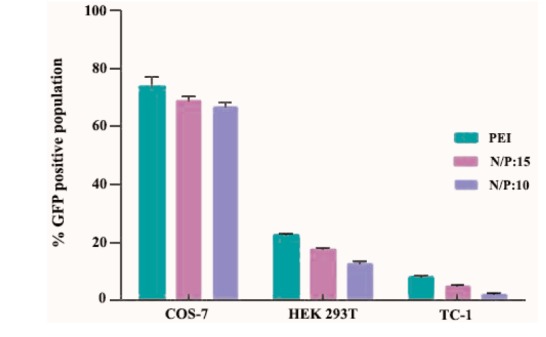
The percentage of EGFP-expressing cells transfected with PEI/DNA and MPG/DNA nanoparticles at N/P ratios of 10:1 and 15:1. Data are the means ±SD
4.5. Western Blotting
E7-GFP expression was confirmed by western blot analysis. E7 expression was detectable in HEK and COS-7 cells transfected with either PEI/DNA or MPG/DNA nanoparticles at N/P ratios of 10:1 and 15:1 by western blotting at 48 h after transfection (data not shown). The dominant bands of 38 (i.e. 11 kDa E7 + 27 kDa GFP) and 50 kDa (i.e. 23 kDa E7 + 27 kDa GFP) were detected in transfected cells expressing E7-GFP using the anti-GFP antibody. The corresponding bands were not detected in the untransfected cells, showing that expression of the E7 protein was specifically induced in the transfected cells. In addition, a 50 kDa E7-GFP protein was detected in transfected cells using the anti-HPV16 E7 monoclonal antibody. The theoretical molecular mass of HPV16E7 protein is approximately 11 kDa. However, our results in this study showed that E7 protein migrate as a 23 kDa protein (an abnormal protein) in SDS-PAGE. This abnormal migration pattern of E7 protein was reported previously (17). These results indicate that the substantial net negative charge of the wild type E7 protein (high content of acidic amino acid residues) is responsible for its anomalous electrophoretic behavior.
5. Discussion
The DNA condensation capability into small campact particles is a critical prerequisite for efficient gene delivery into cells. This property was demonstrated in MPG when it was used to complex with pEGFPE7 to form particles of a spherical nature with a size range of 150 nm to 1 μm in diameter at different N/P ratios. In fact, negatively charged pEGFP-E7 interacted with positively charged cationic peptide. Other studies have also shown that self-assembly between MPG and oligonucleotides mainly involves electrostatic interactions, which take place between the negative charges of the nucleic acids (phosphate groups) and the positively charged moiety of MPG (6, 18). The electrostatic interactions of the cationic peptides with the negatively charged phosphate backbone of DNA leads to nanometer-sized particles with a net positive charge that is able to interact with cell membranes and internalize into the cell to promote gene expression (19).
Current study indicates that the MPG/DNA nanoparticles were stable in transfection media containing serum and overcame the intracellular barriers led to significant E7 expression in transfected cells. One of the unique and essential features of MPG in comparison with other non-viral delivery systems is the presence of a nuclear localization signal (NLS), which plays a crucial role in both electrostatic interactions with DNA and nuclear uptake. The NLS sequence (KKKRKV) has been shown to actively facilitate the transport of nucleic acids through the nuclear pore by interaction with importin a (20). To the best of our knowledge, this study is the first report of HPV16E7 DNA delivery mediated by MPG-based nanoparticles in vitro.
Cell-penetrating peptides represent a promising non-viral transmembrane delivery systems in preference to the polymer and lipid-based DNA delivery systems, because they are relatively stable, easy to synthesize and functionalize, and less toxic or immunogenic than other vectors (5, 21). Vector cytotoxicity is an important parameter during its design. For example, PEI as the most known polymeric vector although has a good transfection efficiency but its use in vivo is limited because of high toxicity (22). PEI has shown significant cytotoxicity at concentration of 10 μg and an IC50 of < 0.01 mg.mL-1 was calculated (23-24).
In our experimental analysis, MPG did not show any cytotoxic effects up to 32 μM. It has been shown that MPG is not cytotoxic at the concentrations used for gene delivery. The transfection efficiency of E7-GFP gene using MPG peptide showed that MPG/DNA nanoparticles facilitate uptake of DNA by cells that leads to the protein expression. Some studies have shown that transport efficiency of CPPs depends on the properties of both CPP and cargo as well as on the transfection conditions and the cell lines (18-19). We found that three cell lines (COS-7, HEK-293T and TC-1) represent different efficiency to DNA transfection. Keller et al. (2013) showed that cancerous cell lines such as HeLa and NIH-3T3 show strong protease activity. In these cells, membrane-bound and secreted proteases degraded cell penetrating peptides (CPPs) within 60 min. In comparison with these cancerous cell lines, COS-7 and NB-4 cells were less proteolytic (25). Thus, our results provide evidence for the differences between HPV16E7 expressing tumor cell (TC-1) and normal cells in extracellular matrix and secreted proteases. These differences likely result from different membrane compositions and extracellular matrix, cell characteristics and a selective mechanism of the nanoparticle uptake. For different delivery systems, endocytosis has been suggested to be the main mechanism of internalization. Recently, it has been claimed that the mechanism of gene delivery by MPG does not follow the endosomal pathway. Morris et al. (1997) showed that efficient delivery of the MPG/DNA complex into the nucleus was observed immediately after 30 min incubation at 37ºC, suggesting that internalization is faster than endocytosis events and another mechanism should be the cause (9). Supplementary experiments of transfection at low temperature (4ºC) to block the endosomal pathway confirmed that the cellular uptake of oligonucleotides is independent of endosomal pathway (9, 20). Here, an experiment suggesting non-endosomal pathway of MPG was carried out. Briefly, the cells were transfected with MPG/DNA in different N/P ratios (5:1, 10:1, 15:1 and 30:1) in the absence and presence of 100 μM chloroquine (CQ), as an endosome disrupting agent, according to the protocol reported before (22). The results showed that CQ has not any positive effect on improving transfection efficiency (data not shown).
As shown by Keller et al. (2013), enhancing the molar ratios of CPP peptide to DNA can significantly increase the uptake of cargoes even for CPPs, which form only weak complexes or exhibit only low uptake efficiencies (26). Current results showed that transfection efficiency was improved with the increase of N/P ratios from 5:1 to 15:1. This suggests that higher MPG doses increase the permeability of cell membranes for plasmid DNA. Therefore, according to our results it seems that in higher ratios of MPG peptide to plasmid DNA, the attachment of nanoparticles to the cell surface has been improved by ionic interactions, resulting in the efficient translocation of nanoparticles.
6. Conclusions
Internalization of DNA into live cells using cell penetrating peptides is a practical and efficient approach for gene therapy by formation of non-covalent complexes. In the present study, we have implemented a peptide-based gene delivery system, MPG which forms stable non-covalent nanoparticles with pDNA for delivery of HPV16E7 as a tumor antigen, in vitro. The efficiency of transfection mediated by MPG was comparable with PEI 25 kDa as a standard reagent with some preferences for MPG-based nanoparticles including no cytotoxicity to mammalian cells and no sensitivity to serum. Based on our results, the transfection efficiency of MPG/DNA nanoparticles depends on both cell type and applied N/P ratio. Interestingly, E7 protein expression increased by higher MPG/DNA charge ratios (N/P: 10 and 15) in COS-7 cells as compared to other cells (HEK-293 and TC-1). This report highlights the potential of MPG-based nanoparticles as a pEGFP-E7 plasmid carrier in vitro, the differences in cell membranes’ properties for gene delivery and the need to investigate each step of intracellular mechanism separately.
Acknowledgments
Financial support of this work was provided by Research Council of Tarbiat Modares University and Pasteur Institute of Iran.
References
- 1.Verma IM, Somia N. Gene therapy-promises, problems and prospects. Nature. 1997;389(6648):239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Satterlee A, Huang L. In Vivo Gene Delivery by Nonviral Vectors: Overcoming Hurdles & quest. Mol Ther. 2012;20(7):1298–1304. doi: 10.1038/mt.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang T, Upponi JR, Torchilin VP. Design of multifunctional non-viral gene vectors to overcome physiological barriers: Dilemmas and strategies. Int J Pharm. 2012;427(1):3–20. doi: 10.1016/j.ijpharm.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4(5):346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 5.Bolhassani A. Potential efficacy of cell-penetrating peptides for nucleic acid and drug delivery in cancer. Biochim Biophys Acta Rev Cancer. 2011;1816:232–246. doi: 10.1016/j.bbcan.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Morris MC, Deshayes S, Heitz F, Divita G. Cell-penetrating peptides: from molecular mechanisms to therapeutics. Biol Cell. 2008;100(4):201–217. doi: 10.1042/BC20070116. [DOI] [PubMed] [Google Scholar]
- 7.Lee SH, Castagner B, Leroux J-C. Is there a future for cell-penetrating peptides in oligonucleotide delivery? Eur J Pharm Biopharm. 2013;85(1):5–11. doi: 10.1016/j.ejpb.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Deshayes S, Morris M, Heitz F, Divita G. Delivery of proteins and nucleic acids using a non-covalent peptide-based strategy. Adv Drug Deliv Rev. 2008;60(4-5):537–547. doi: 10.1016/j.addr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Morris M, Vidal P, Chaloin L, Heitz F, Divita G. A new peptide vector for efficient delivery of oligonucleotides into mammalian cells. Nucleic Acids Res. 1997;25(14):2730–2736. doi: 10.1093/nar/25.14.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. J Adolesc Health. 2010;46(4):S20–S26. doi: 10.1016/j.jadohealth.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 12.Bolhassani A, Mohit E, Rafati S. Different spectra of therapeutic vaccine development against HPV infections. Hum Vaccine. 2009;5(10):671–689. doi: 10.4161/hv.5.10.9370. [DOI] [PubMed] [Google Scholar]
- 13.Bolhassani A, Zahedifard F, Taghikhani M, Rafati S. Enhanced immunogenicity of HPV16E7 accompanied by Gp96 as an adjuvant in two vaccination strategies. Vaccine. 2008;26(26):3362–3370. doi: 10.1016/j.vaccine.2008.03.082. [DOI] [PubMed] [Google Scholar]
- 14.Lee M, Nah J-W, Kwon Y, Koh JJ, Ko KS, Kim SW. Water-soluble and low molecular weight chitosan-based plasmid DNA delivery. Pharm Res. 2001;18(4):427–431. doi: 10.1023/a:1011037807261. [DOI] [PubMed] [Google Scholar]
- 15.Tang Q, Cao B, Wu H, Cheng G. Cholesterol-Peptide hybrids to form liposome-like vesicles for gene delivery. PloS one. 2013;8(1):e54460. doi: 10.1371/journal.pone.0054460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SH, Kim SH, Park TG. Intracellular siRNA delivery system using polyelectrolyte complex micelles prepared from VEGF siRNA-PEG conjugate and cationic fusogenic peptide. Biochem Biophys Res Commun. 2007;357(2):511–516. doi: 10.1016/j.bbrc.2007.03.185. [DOI] [PubMed] [Google Scholar]
- 17.Bolhassani A, Zahedifard F, Taslimi Y, Taghikhani M, Nahavandian B, Rafati S. Antibody detection against HPV16 E7 & GP96 fragments as biomarkers in cervical cancer patients. Indian J Med Res. 2009;130(5) [PubMed] [Google Scholar]
- 18.Laufer S, Restle T. Peptide-mediated cellular delivery of oligonucleotide-based therapeutics in vitro: quantitative evaluation of overall efficacy employing easy to handle reporter systems. Curr Pharm Des. 2008;14(34):3637. doi: 10.2174/138161208786898806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang XX, Eden HS, Chen X. Peptides in cancer nanomedicine: Drug carriers, targeting ligands and protease substrates. J Control Release. 2012;159:2–13. doi: 10.1016/j.jconrel.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simeoni F, Morris MC, Heitz F, Divita G. Insight into the mechanism of the peptide-based gene delivery system MPG: implications for delivery of siRNA into mammalian cells. Nucleic Acids Res. 2003;31(11):2717–2724. doi: 10.1093/nar/gkg385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Margus H, Padari K, Pooga M. Cell-penetrating peptides as versatile vehicles for oligonucleotide delivery. Mol Ther. 2012;20(3):525–533. doi: 10.1038/mt.2011.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114(1):100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Florea BI, Meaney C, Junginger HE, Borchard G. Transfection efficiency and toxicity of polyethylenimine in differentiated Calu-3 and nondifferentiated COS-1 cell cultures. Aaps Pharmsci. 2002;4(3):1–11. doi: 10.1208/ps040312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunath K, von Harpe A, Fischer D, Petersen H, Bickel U, Voigt K, Kissel T. Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: comparison of physicochemical properties, transfection efficiency and in vivo distribution with high-molecular-weight polyethylenimine. J Control Release. 2003;89(1):113–125. doi: 10.1016/S0168-3659(03)00076-2. [DOI] [PubMed] [Google Scholar]
- 25.Keller A-A, Mussbach F, Breitling R, Hemmerich P, Schaefer B, Lorkowski S, Reissmann S. Relationships between cargo, cell penetrating peptides and cell type for uptake of non-covalent complexes into live cells. Pharmaceuticals. 2013;6(2):184–203. doi: 10.3390/ph6020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiraishi T, Nielsen PE. Enhanced delivery of cell-penetrating peptide–peptide nucleic acid conjugates by endosomal disruption. Nat Protocol. 2006;1(2):633–636. doi: 10.1038/nprot.2006.92. [DOI] [PubMed] [Google Scholar]



