Abstract
Background
There is conflicting data regarding the efficacy of tulathromycin for the treatment of foals with bronchopneumonia.
Hypotheses
Tulathromycin is effective for the treatment of bronchopneumonia in foals and noninferior to the combination of azithromycin and rifampin.
Animals
A total of 240 foals on a farm endemic for infections caused by Rhodococcus equi.
Methods
In a controlled, randomized, and double‐blinded clinical trial, foals with ultrasonographic pulmonary lesions (abscess score 10–15 cm) were allocated to 3 groups: 1—tulathromycin IM q 7 days (n = 80); 2—azithromycin‐rifampin, orally q24h (n = 80); or 3—untreated controls (n = 80). Physical examination and thoracic ultrasonography were performed by individuals unaware of treatment group assignment. Foals that worsened were considered treatment failures and removed from the study.
Results
The proportion of foals that recovered was significantly higher for foals treated with tulathromycin (70 of 79) or azithromycin‐rifampin (76 of 80) compared to that of control foals (22 of 80). The difference in the percentage of efficacy of azithromycin‐rifampin versus tulathromycin was 6.4% (90% CI = −0.72–13.5%). Given that the confidence interval crossed the predetermined noninferiority limit of 10%, the null hypothesis that the response rate in the azithromycin‐rifampin group is superior to that of the tulathromycin group could not be rejected. Resolution of ultrasonographic lesions occurred faster in foals treated with azithromycin‐rifampin than in foals treated with tulathromycin.
Conclusion and clinical importance
Tulathromycin was effective for the treatment of bronchopneumonia in foals at this farm but not as effective as the combination of azithromycin‐rifampin.
Keywords: Macrolide, Pneumonia, Rhodococcus equi
Abbreviations
- AZM
azithromycin
- MIC
minimum inhibitory concentration
- RIF
rifampin
- TUL
tulathromycin
Pneumonia is a leading cause of morbidity and mortality in foals.1 Streptococcus equi subspecies zooepidemicus (S. zooepidemicus) and Rhodococcus equi are the 2 most common causes of pneumonia in foals between 1 and 6 months of age.2, 3 In the absence of an effective vaccine, control of infections caused by R. equi at endemic farms relies on early detection of disease using thoracic ultrasonography with antimicrobial therapy for affected foals.4, 5, 6 Based on a consensus statement from the American College of Veterinary Internal Medicine, the combination of a macrolide (erythromycin or clarithromycin) or azalide (azithromycin) with rifampin is the recommended treatment for infection caused by R. equi.7 However, these antimicrobial agents occasionally result in adverse effects such as hyperthermia and diarrhea.8, 9 Furthermore, their use requires multiple daily oral administrations, which is labor intensive, particularly when large numbers of foals must be treated.
Tulathromycin (TUL) is a semisynthetic long‐acting macrolide approved for the treatment of respiratory disease in swine and cattle. Intramuscular administration of TUL to healthy foals at a dosage of 2.5 mg/kg was well tolerated and resulted in drug concentrations in pulmonary epithelial lining fluid and bronchoalveolar cells considerably above concurrent plasma concentrations.10, 11 In a prospective study, 36 of 37 foals with small ultrasonographic pulmonary lesions (abscess score of 1.5 to 4.4 cm) treated with IM TUL once a week responded to therapy suggesting that the drug might be effective for the treatment of bronchopneumonia in foals.6 However, more recent studies have demonstrated that the majority of foals with abscess scores <6 cm recover spontaneously and that antimicrobial therapy of affected foals does not provide any benefit relative to administration of a placebo.12, 13 In addition, it is now known that TUL is poorly active against R. equi in vitro with a MIC inhibiting at least 90% of the isolates (MIC90) ≥64 μg/mL,14, 15 which is well above concentrations achievable in plasma, pulmonary epithelial lining fluid and bronchoalveolar cells in healthy foals. As a result, it is unknown if TUL is truly effective for the treatment of bronchopneumonia in foals.
The objectives of this study were to determine the relative efficacy and safety of TUL and AZM in combination with RIF for the treatment of foals with pulmonary lesions on a farm with endemic infections caused by R. equi.
Materials and Methods
Study Population
The study protocol was reviewed and approved in accordance with ethics guidelines of Hanover University and the German Animal Welfare Act. The study was a controlled, randomized, and double‐blinded clinical trial performed during the 2016 breeding season at a farm breeding Warmblood horses in Germany. The farm has a history of recurrent foal pneumonia attributable to R. equi. Multiple studies performed at the farm demonstrated that R. equi could be isolated from tracheobronchial aspirates of 39% (17 of 44) to 54% (118 of 217) of foals with ultrasonographic evidence of pneumonia.16, 17, 18, 19, 20 In addition, postmortem examination of 24 foals from the same farm confirmed the presence of R. equi in lung tissue of all foals with ultrasonographic lesions.21
Monitoring of the Foals
From birth to 5 months of age, each foal was subjected weekly to a complete physical examination and blood collection by jugular venipuncture for determination of WBC counts. Foals with any clinical sign(s) of respiratory disease (cough, nasal discharge, abnormal lung sounds, dyspnea, or temperature >39.5°C) or with a WBC count >13,000/μL22 were subjected to thoracic ultrasonography. Thoracic ultrasonography was performed twice weekly using a portable unit1 with a 7.5 MHz linear transducer. The chest was drenched with alcohol, and the entire surface of both lungs was imaged. For the purpose of the study, abscesses were defined as focal hypoechoic areas of consolidation with a diameter ≥1.0 cm. The number of abscesses noted during a given exam was recorded. In addition, the diameters of each abscess were added to generate a total abscess score in centimeters. For asymmetrical lesions, the average of the largest and smallest diameter was used for data analysis.
Criteria for Inclusion of the Treatment Study and Study Design
Foals 21 days of age or older with abscess scores between 10.0 and 15.0 cm were enrolled for participation in the study. Foals with abscess scores >15.0 cm and foals with dyspnea (regardless of their abscess scores) were excluded from participation in the study. Prior to the beginning of the study, numbers from 1 to 240 were randomly assigned to 3 equal treatment groups using a computer software, with the number 1 representing the first foal meeting the inclusion criteria. Treatment groups were as follows: 1—TUL at a dose of 2.5 mg/kg body weight administered IM in the semimembranosus/semitendinosus muscles once a week (n = 80); 2—AZM at a dose of 10 mg/kg PO once daily in combination with RIF at a dose of 10 mg/kg PO once daily (n = 80); and 3—no antimicrobial therapy (controls; n = 80). All the foals also received acetylcysteine at a dose of 10 mg/kg PO once daily to ensure that foals in each group were subjected to similar daily handling.
Foals were monitored daily for adverse reactions, such as diarrhea, lameness, colic, or swelling at the injection site. Each foal enrolled in the study was subjected to physical examination and thoracic ultrasonography twice weekly until resolution of the lesions and discontinuation of therapy. Physical examination parameters were used to generate a clinical score based on respiratory rate (< or >80 bpm), presence and type of nasal discharge (none, serous, purulent), submandibular lymph nodes (normal or enlarged), dyspnea (absent or present), and auscultation of the lungs and trachea (normal versus abnormal) as previously described.6, 23 In addition, blood was obtained weekly by jugular venipuncture for determination of WBC counts. Criteria for discontinuation of therapy were resolution of clinical signs and no evidence of consolidation upon thoracic ultrasonography for 2 consecutive weeks after a minimum of 42 days of treatment. The individuals responsible for thoracic ultrasonography, physical examination, and for determining the need for therapy were unaware of specific treatment group assignment for a given foal. All treatments were given by different individuals who were not involved in monitoring and who had no control over treatment assignment.
Criteria for Removal from the Study
The study protocol included a rescue mechanism to reduce the risk of mortality. Foals that developed dyspnea or an abscess score ≥18 cm were removed from the study protocol. Foals removed from the study were switched to the combination of gamithromycin (6 mg/kg IV once weekly) with rifampin (10 mg/kg PO once daily).
Additional Data Collection
For each foal enrolled in the study, data collected included sex, age, and body weight at onset of clinical signs, clinical score, number of abscesses, abscess score, and adverse effects. For each treatment group, the proportion of foals removed from the study (rescue mechanism) was recorded. For foals that were removed from the study, time from initiation of therapy to removal from the study was recorded.
Data Analysis
Sample size analysis based on the ability to detect an estimated response rate of 94% in the TUL treatment group as being significantly superior from an estimated response rate of 75% in the control group with alpha set at 0.05 and power set at 80% indicated that 66 foals per group would be required. Sample size calculations for a noninferiority trial testing the null hypothesis that the response rate in the AZM‐RIF treatment group is superior to that of the TUL treatment group was based on estimated efficacy of either treatment of 94%, statistical power of 80%, alpha of 0.05, and a noninferiority limit of 10%. The estimated number of foals required was 70 per group. We elected to enroll 80 foals per group to allow for potential losses to follow‐up and variation in observed values from hypothetical values used for sample size calculations.
Comparisons of the proportion of foals that responded to therapy or developed adverse effects between treatment groups were performed using the Chi‐squared test. Cox proportional hazard regression was used to compare the rate of treatment failure between treatment groups after adjusting for age, sex, and abscess score. The proportional hazard assumption was tested graphically using log‐log plots and statistically on the basis of Schoenfeld residuals. Kaplan‐Meier curves were generated to display the distribution of events over time for the treatments groups. Abscess score over time data for foals treated with AZM‐RIF or TUL were analyzed using linear mixed‐effects modeling with foal modeled as a random effect, and treatment group, time and group by time interactions modeled as fixed nominal effect. Baseline abscess score was used as a covariate to account for potential imbalances in abscess scores at the time of initiation of therapy. Model fit was assessed using Akaike information criterion values. Normality of the data was assessed based on examination of histograms and normal Q‐Q plots of the residuals. For all analyzes, values of P were adjusted for multiple comparisons using the method of Holm‐Sidak. P < .05 was considered significant.
Results
Baseline variables at the time of initiating therapy with TUL, AZM‐RIF, or in untreated controls for the 240 foals included in this study were similar between treatment groups (Table 1). One foal randomly allocated to therapy with TUL was removed from the study due to an illness unrelated to respiratory disease. All the other foals completed the study. The proportion of foals that recovered without the need for a change in therapy was significantly (P < .001) higher for foals treated with TUL (70 of 79; 88.6%) or AZM‐RIF (76 of 80; 95.0%) compared to that of control foals (22 of 80; 27.5%). The proportion of foals that recovered without the need for a change in therapy in foals treated with TUL was not significantly (P = .142) different from that of foals treated with AZM‐RIF. The difference in the percentage of efficacy of AZM‐RIF versus TUL was 6.4% (90% CI = −0.72 to 13.5%). Given that the confidence interval crossed the predetermined noninferiority limit of 10%, the null hypothesis that the response rate in the AZM‐RIF treatment group is superior to that of the TUL treatment group could not be rejected.
Table 1.
Baseline variables at the time of initiation of therapy with TUL or AZM‐RIF. The control group did not receive antimicrobial therapy
| Variables | TUL (n = 80) | AZM‐RIF (n = 80) | CONTROL (n = 80) |
|---|---|---|---|
| Males (%) | 42 (52.5) | 49 (61.3) | 40 (50.0) |
| Females (%) | 38 (47.5) | 31 (38.8) | 40 (50.0) |
| Age at diagnosis (days) | 99 (50–126)a | 102 (56–133) | 103 (52–123) |
| Body weight at diagnosis (kg) | 165 (120–195) | 170 (132–200) | 168 (118–190) |
| Clinical score | 2 (2–4) | 2 (2 – 4) | 2 (1–4) |
| Abscess score (cm) | 11.8 (10.0–14.0) | 12.0 (10.0–14.5) | 12.5 (10.0–15.0) |
| Number of lesions ≥ 1 cm | 8 (5–10) | 8 (5–11) | 8 (5–11) |
| Number of lesions < 1 cm | 1 (0–4) | 1 (0–4) | 1 (0–4) |
| WBC (×103/μL) | 14.4 (9.4–20.1) | 14.7 (10.3–18.2) | 14.3 (10.0–20.6) |
Median (10th and 90th percentiles).
Even after adjusting for potential imbalances in baseline characteristics and differences in lesion severity using Cox proportional hazard regression, the risk of treatment failure was reduced by approximately 95.4% (hazard ratio = 0.046; 95% CI = 0.017 to 0.128; P < .001) in foals treated with AZM‐RIF and by approximately 89.3% (hazard ratio = 0.107; 95% CI = 0.052 to 0.216; P < .001) in foals treated with TUL relative to controls (Fig 1). The risk of treatment failure was reduced by approximately 56.4% (hazard ratio = 0.436; 95% CI = 0.130 to 1.420; P = .168) in foals treated with AZM‐RIF relative to foals treated with TUL, but this difference was not statistically significant (Fig 1). There was a significant effect of treatment group (P = .003), time (P < .001), and a significant interaction between treatment group and time (P = .005) on the abscess score. The abscess score was significantly smaller in foals treated with AZM‐RIF compared to that of foals treated with TUL after 1, 1.5, and 2.5 weeks of therapy (Fig 2). The proportion of foals that developed diarrhea was not significantly different (P = .123) between treatment groups (3 of 80 for controls, 6 of 80 for TUL, and 8 of 80 for AZM‐RIF). One foal treated with TUL developed transient swelling at the injection site. No other adverse effects were noted.
Figure 1.
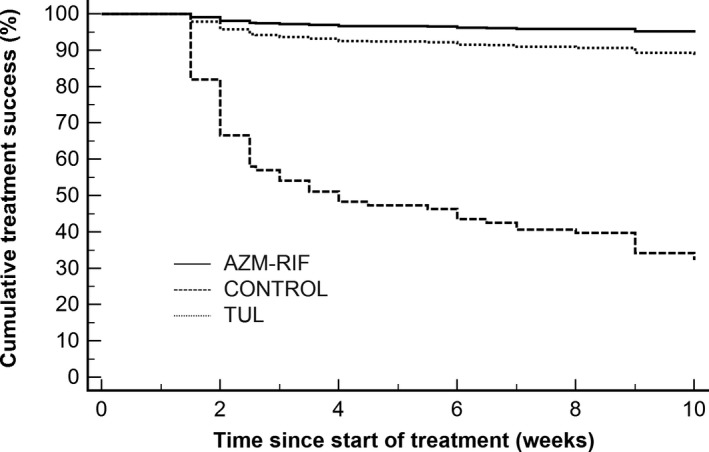
Kaplan‐Meier curves of the probability of treatment success in foals with bronchopneumonia treated with AZM‐RIF (n = 80) or TUL (n = 79). The control group (n = 80) did not receive antimicrobial therapy.
Figure 2.
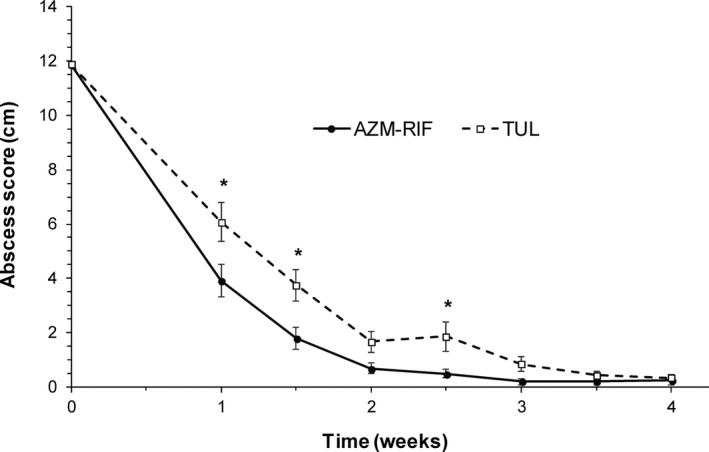
Least square mean abscess score (±SE) in foals with bronchopneumonia for the first 4 weeks after initiation of therapy with AZM‐RIF (n = 80) or TUL (n = 79). *Indicates a significant difference between foals treated with AZM‐RIF and foals treated with TUL at a given time point (P < .05).
Discussion
The present study demonstrated that TUL is effective for the treatment of foals with clinical signs of lower respiratory tract disease and ultrasonographic lesion scores between 10 and 15 cm. This finding is surprising given the fact that TUL concentrations in plasma, pulmonary epithelial lining fluid and bronchoalveolar cells achievable in vivo in healthy foals are more than 30‐fold lower than the MIC90 of TUL against R. equi (≥64 μg/mL). The in vitro activity of TUL against S. zooepidemicus has not been investigated. However, TUL is poorly active against S. suis with a MIC90 >64 μg/mL.24 It is possible that measurement of TUL concentrations in whole BAL cells underestimate the concentration of the drug in R. equi‐containing vacuoles within macrophages. In addition, concentrations of TUL in the lungs of pneumonic foals might be higher than those observed in healthy foals, as documented in swine experimentally infected with Actinobacillus pleuropneumoniae.25 Alternatively, the efficacy of TUL for the treatment of bronchopneumonia in foals might be unrelated to its direct antimicrobial activity.
TUL is highly effective in the treatment of bovine respiratory disease26, 27 despite concentrations of the drug in interstitial lung fluid and pulmonary epithelial lining fluid being below the MIC90 of Mannheimia haemolytica and Pasteurella multocida.28 Similarly, there is no association between in vitro MIC and clinical response in cattle treated with tilmicosin, another long‐acting macrolide approved for the treatment of bovine respiratory disease.29 These findings suggest that the direct antimicrobial activity of macrolides does not solely explain their superior clinical efficacy in the treatment of bovine respiratory disease. Macrolides exert a wide variety of anti‐inflammatory or immunomodulatory effects that might enhance their clinical efficacy including decreased mucus production by epithelial cells and reduced secretion of pro‐inflammatory cytokines by mononuclear and epithelial cells.30 Recent studies indicate that TUL induces apoptosis in bovine neutrophils and macrophages, and inhibits production of pro‐inflammatory mediators by these cells.31, 32, 33 Additional studies will be required to determine the exact mechanism responsible for the efficacy of TUL in the treatment of bronchopneumonia in foals.
Recent double‐blinded randomized controlled studies have demonstrated that many foals with small pulmonary lesions (median abscess score ≤6 cm) recover without antimicrobial therapy and that antimicrobial treatment of these foals does not significantly hasten lesion resolution, compared to administration of a placebo.12, 13 In contrast, therapy of foals with larger ultrasonographic lesion scores with AZM‐RIF or with gamithromycin provides a significant benefit compared to administration of a placebo.34, 35 These findings underscore the importance of a blinded controlled design in studies assessing the relative efficacy of various therapies. Despite being significantly more effective than a placebo and leading to complete resolution in 88.6% of treated foals in this study, TUL was not quite as effective as standard therapy on the basis of delayed lesion resolution relative to that of foals treated with AZM‐RIF. These findings are similar to those of a prior study in which the abscess score after 1 week of therapy was significantly higher in foals treated with TUL compared to that of foals treated with AZM‐RIF.6 The faster lesion resolution in foals treated with AZM‐RIF suggests that a shorter treatment course might be sufficient. This hypothesis could not be tested in the present study because of the minimum treatment duration of 42 days as per established farm protocols.
It is unknown whether the slightly lower efficacy of TUL documented in this study is due to its lower antimicrobial activity against R. equi and possibly other bacterial pathogens of horses relative to AZM or if it is due to the fact that TUL was used in monotherapy without concurrent administration of RIF. Although TUL monotherapy was significantly more effective than a placebo, these results must be interpreted with caution because the foals enrolled in the present study had either mild clinical signs of pneumonia or subclinical pulmonary disease. Therefore, TUL monotherapy might not be effective in foals with advanced pulmonary lesions and severe signs of respiratory disease. The combination of a macrolide and rifampin is synergistic both in vitro and in vivo.36, 37, 38 In addition, the use of the 2 classes of drugs in combination reduces the likelihood of development of resistance to either drug in vitro.36, 39 Although TUL was used in monotherapy in the present study to assess the efficacy of the drug, the treatment of choice for pneumonia caused by R. equi remains the combination of a macrolide with rifampin, as recommended in the ACVIM consensus statement.7
The definitive diagnosis of bronchopneumonia caused by R. equi should be based on bacteriologic culture or amplification of the vapA gene by polymerase chain reaction from a tracheobronchial aspirate obtained from a foal with 1—clinical signs of lower respiratory tract disease, 2—cytological evidence of septic airway inflammation, or 3—radiographic or ultrasonographic evidence of bronchopneumonia.7 Although prior studies documented that R. equi could be cultured from most pneumonic foals at the farm, tracheobronchial aspirates were not performed in the present study. Although the etiology of the pulmonary lesions could not be determined, the presents study is an accurate reflection of what is occurring in clinical practice because tracheobronchial aspirates are not typically performed as part of ultrasonographic screening programs.5
The mucolytic acetylcysteine was used in all foals enrolled in the study to ensure similar daily handling. Acetylcysteine does not impair the bioavailability of beta‐lactam antimicrobial agents such as cefpodoxime in people,40 but its effect on the bioavailability of AZM or RIF is unknown. It is unlikely that acetylcysteine decreased the clinical efficacy of AZM‐RIF because the treatment success of this combination in the present study (95%) was identical to that observed in a prior study evaluating the efficacy AZM‐RIF in the absence of co‐administration of acetylcysteine in foals with similar lesion scores.13 Similarly, it is unlikely that administration of acetylcysteine to the control group improved outcome because the proportion of foals that recovered without the need for antimicrobial therapy in this study (22%) was lower than that observed in a prior study conducted at the same farm in which the control group received saline as a placebo.13
In conclusion, the present study demonstrates that TUL is effective for the treatment of bronchopneumonia in foals but not as effective as the combination of AZM‐RIF. The extent to which the results of the present study may be extrapolated to other farms is unknown because the proportion of foals that recover without therapy may vary by farm, geographical region, and age at which ultrasonographic lesions are detected.
Acknowledgments
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Off‐label use of azithromycin, rifampin, and tulathromycin.
Footnote
Esaote Tringa Linear, Milano, Italy
References
- 1. Cohen ND. Causes of and farm management factors associated with disease and death in foals. J Am Vet Med Assoc 1994;204:1644–1651. [PubMed] [Google Scholar]
- 2. Hoffman AM, Viel L, Prescott JF, et al. Association of microbiologic flora with clinical, endoscopic, and pulmonary cytologic findings in foals with distal respiratory tract infection. Am J Vet Res 1993;54:1615–1622. [PubMed] [Google Scholar]
- 3. Giguère S, Gaskin JM, Miller C, et al. Evaluation of a commercially available hyperimmune plasma product for prevention of naturally acquired pneumonia caused by Rhodococcus equi in foals. J Am Vet Med Assoc 2002;220:59–63. [DOI] [PubMed] [Google Scholar]
- 4. Slovis NM, McCracken JL, Mundy G. How to use thoracic ultrasound to screen foals for Rhodococcus equi at affected farms. Proc Am Assoc Equine Pract 2005;51:274–278. [Google Scholar]
- 5. McCracken JL, Slovis NM. Use of thoracic ultrasound for the prevention of Rhodococcus equi pneumonia on endemic farms. Proc Am Assoc Equine Pract 2009;55:38–44. [Google Scholar]
- 6. Venner M, Kerth R, Klug E. Evaluation of tulathromycin in the treatment of pulmonary abscesses in foals. Vet J 2007;174:418–421. [DOI] [PubMed] [Google Scholar]
- 7. Giguère S, Cohen ND, Keith CM, et al. Diagnosis, treatment, control, and prevention of infections caused by Rhodococcus equi in Foals. J Vet Intern Med 2011;25:1209–1220. [DOI] [PubMed] [Google Scholar]
- 8. Giguère S, Jacks S, Roberts GD, et al. Retrospective comparison of azithromycin, clarithromycin, and erythromycin for the treatment of foals with Rhodococcus equi pneumonia. J Vet Intern Med 2004;18:568–573. [DOI] [PubMed] [Google Scholar]
- 9. Stratton‐Phelps M, Wilson WD, Gardner IA. Risk of adverse effects in pneumonic foals treated with erythromycin versus other antibiotics: 143 cases (1986–1996). J Am Vet Med Assoc 2000;217:68–73. [DOI] [PubMed] [Google Scholar]
- 10. Scheuch E, Spieker J, Venner M, et al. Quantitative determination of the macrolide antibiotic tulathromycin in plasma and broncho‐alveolar cells of foals using tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2007;850:464–470. [DOI] [PubMed] [Google Scholar]
- 11. Venner M, Peters J, Hohensteiger N, et al. Concentration of the macrolide antibiotic tulathromycin in broncho‐alveolar cells is influenced by comedication of rifampicin in foals. Naunyn Schmiedebergs Arch Pharmacol 2010;381:161–169. [DOI] [PubMed] [Google Scholar]
- 12. Venner M, Rodiger A, Laemmer M, et al. Failure of antimicrobial therapy to accelerate spontaneous healing of subclinical pulmonary abscesses on a farm with endemic infections caused by Rhodococcus equi . Vet J 2012;192:293–298. [DOI] [PubMed] [Google Scholar]
- 13. Venner M, Astheimer K, Lammer M, et al. Efficacy of mass antimicrobial treatment of foals with subclinical pulmonary abscesses associated with Rhodococcus equi . J Vet Intern Med 2013;27:171–176. [DOI] [PubMed] [Google Scholar]
- 14. Carlson K, Kuskie K, Chaffin K, et al. Antimicrobial activity of tulathromycin and 14 other antimicrobials against virulent Rhodococcus equi in vitro. Vet Ther 2010;11:E1–E9. [PubMed] [Google Scholar]
- 15. Riesenberg A, Fessler AT, Erol E, et al. MICs of 32 antimicrobial agents for Rhodococcus equi isolates of animal origin. J Antimicrob Chemother 2014;69:1045–1049. [DOI] [PubMed] [Google Scholar]
- 16. Kilian K. Comparative studies of the detection of Rhodococcus equi in the breath, in tracheobronchial fluids and in faeces of foals. Thesis, Veterinary School of Hanover, 2008. 128 pages.
- 17. Lammer M. Detection of Rhodococcus equi in faeces and tracheobronchial secretions of foals on a farm with endemic rhodococcus: comparison of healthy foals and foals with pulmonary abscesses. Thesis, Veterinary School of Hanover, 2010. 116 pages.
- 18. Venner M, Heyers P, Strutzberg‐Minder K, et al. Detection of Rhodococcus equi by microbiological culture and by polymerase chain reaction in samples of tracheobronchial secretions of foals. Berl Munch Tierarztl Wochenschr 2007;120:126–133. [PubMed] [Google Scholar]
- 19. Venner M, Meyer‐Hamme B, Verspohl J, et al. Genotypic characterization of VapA positive Rhodococcus equi in foals with pulmonary affection and their soil environment on a warmblood horse breeding farm in Germany. Res Vet Sci 2007;83:311–317. [DOI] [PubMed] [Google Scholar]
- 20. Hagist C. Genotypisierung von Rhodococcus equi stämmen aus Deutschland, isoliert bei fohlen und anderen tierarten. Thesis, Veterinary School of Hanover, 2016. 134 pages.
- 21. Weimar BM. Lung abscesses in foals: Evaluation of clinical, ultrasonic, endoscopical, pathomorphological and microbiological findings. Thesis, Veterinary School of Hanover, 2006. 157 pages.
- 22. Giguère S, Hernandez J, Gaskin JM, et al. Evaluation of WBC concentration, plasma fibrinogen concentration, and an agar gel immunodiffusion test for early identification of foals with Rhodococcus equi pneumonia. J Am Vet Med Assoc 2003;222:775–781. [DOI] [PubMed] [Google Scholar]
- 23. Venner M, Reinhold B, Beyerbach M, et al. Efficacy of azithromycin in preventing pulmonary abscesses in foals. Vet J 2009;179:301–303. [DOI] [PubMed] [Google Scholar]
- 24. El GF, de JA, Simjee S, et al. Monitoring of antimicrobial susceptibility of respiratory tract pathogens isolated from diseased cattle and pigs across Europe, 2009–2012: VetPath results. Vet Microbiol 2016;194:11–22. [DOI] [PubMed] [Google Scholar]
- 25. Gajda A, Bladek T, Jablonski A, et al. The influence of Actinobacillus pleuropneumoniae infection on tulathromycin pharmacokinetics and lung tissue disposition in pigs. J Vet Pharmacol Ther 2016;39:176–182. [DOI] [PubMed] [Google Scholar]
- 26. Schunicht OC, Booker CW, Guichon PT, et al. An evaluation of the relative efficacy of tulathromycin for the treatment of undifferentiated fever in feedlot calves in Nebraska. Can Vet J 2007;48:600–606. [PMC free article] [PubMed] [Google Scholar]
- 27. Wellman NG, O'Connor AM. Meta‐analysis of treatment of cattle with bovine respiratory disease with tulathromycin. J Vet Pharmacol Ther 2007;30:234–241. [DOI] [PubMed] [Google Scholar]
- 28. Foster DM, Martin LG, Papich MG. Comparison of active drug concentrations in the pulmonary epithelial lining fluid and interstitial fluid of calves injected with enrofloxacin, florfenicol, ceftiofur, or tulathromycin. PLoS ONE 2016;11:e0149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McClary DG, Loneragan GH, Shryock TR, et al. Relationship of in vitro minimum inhibitory concentrations of tilmicosin against Mannheimia haemolytica and Pasteurella multocida and in vivo tilmicosin treatment outcome among calves with signs of bovine respiratory disease. J Am Vet Med Assoc 2011;239:129–135. [DOI] [PubMed] [Google Scholar]
- 30. Giamarellos‐Bourboulis EJ. Macrolides beyond the conventional antimicrobials: A class of potent immunomodulators. Int J Antimicrob Agents 2008;31:12–20. [DOI] [PubMed] [Google Scholar]
- 31. Fischer CD, Beatty JK, Zvaigzne CG, et al. Anti‐Inflammatory benefits of antibiotic‐induced neutrophil apoptosis: Tulathromycin induces caspase‐3‐dependent neutrophil programmed cell death and inhibits NF‐kappaB signaling and CXCL8 transcription. Antimicrob Agents Chemother 2011;55:338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fischer CD, Duquette SC, Renaux BS, et al. Tulathromycin exerts proresolving effects in bovine neutrophils by inhibiting phospholipases and altering leukotriene B4, prostaglandin E2, and lipoxin A4 production. Antimicrob Agents Chemother 2014;58:4298–4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fischer CD, Beatty JK, Duquette SC, et al. Direct and indirect anti‐inflammatory effects of tulathromycin in bovine macrophages: Inhibition of CXCL‐8 secretion, induction of apoptosis, and promotion of efferocytosis. Antimicrob Agents Chemother 2013;57:1385–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Venner M, Credner N, Lammer M, et al. Comparison of tulathromycin, azithromycin and azithromycin‐rifampin for the treatment of mild pneumonia associated with Rhodococcus equi . Vet Rec 2013;173:397. [DOI] [PubMed] [Google Scholar]
- 35. Hildebrand F, Venner M, Giguere S. Efficacy of gamithromycin for the treatment of foals with mild to moderate bronchopneumonia. J Vet Intern Med 2015;29:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nordmann P, Ronco E. In‐vitro antimicrobial susceptibility of Rhodococcus equi . J Antimicrob Chemother 1992;29:383–393. [DOI] [PubMed] [Google Scholar]
- 37. Prescott JF, Nicholson VM. The effects of combinations of selected antibiotics on the growth of Corynebacterium equi . J Vet Pharmacol Ther 1984;7:61–64. [DOI] [PubMed] [Google Scholar]
- 38. Giguère S, Lee EA, Guldbech KM, et al. In vitro synergy, pharmacodynamics, and postantibiotic effect of 11 antimicrobial agents against Rhodococcus equi . Vet Microbiol 2012;160:207–213. [DOI] [PubMed] [Google Scholar]
- 39. Berghaus LJ, Giguère S, Guldbech K. Mutant prevention concentration and mutant selection window for 10 antimicrobial agents against Rhodococcus equi . Vet Microbiol 2013;166:670–675. [DOI] [PubMed] [Google Scholar]
- 40. Kees F, Wellenhofer M, Brohl K, et al. Bioavailability of cefpodoxime proxetil with co‐administered acetylcysteine. Arzneimittelforschung 1996;46:435–438. [PubMed] [Google Scholar]


