Abstract
Background
Symmetric dimethylarginine (SDMA) is considered a biomarker for early detection of renal dysfunction in human patients with acute kidney injury (AKI). At present, no studies exist analyzing the relevance of SDMA in dogs with AKI.
Hypothesis/objectives
SDMA would correctly identify dogs with renal disease but would not be able to differentiate between AKI and CKD.
Animals
Eighteen healthy control dogs, 48 dogs with AKI, and 29 dogs with CKD.
Methods
Prospective study. Dogs with kidney disease were categorized as having AKI or CKD according to the history, clinical signs, laboratory findings, and results of diagnostic imaging. Plasma SDMA concentration was measured by IDEXX Laboratories. SDMA/creatinine ratio was calculated in dogs with AKI or CKD.
Results
Median SDMA concentrations were 8.5 μg/dL (6–12 μg/dL), 39.5 μg/dL (8–>100 μg/dL), and 35 μg/dL (12–>100 μg/dL), in healthy, AKI, and CKD, respectively. SDMA concentrations were significantly higher in dogs with AKI (P < .0001) or CKD (P < .0001) in comparison with healthy dogs. Median SDMA/creatinine ratio in dogs with AKI and CKD was 6.5 (1.7–20.9) and 10 (2.4–33.9) (P = .0004), respectively. Although there was overlap of the SDMA/creatinine ratio in dogs with AKI or CKD, it was significantly higher in dogs with CKD compared to dogs with AKI (P = .0004).
Conclusions and Clinical Importance
In this population, SDMA was suitable for identifying dogs affected by AKI or CKD, but could not differentiate between them.
Keywords: Azotemia, Methylated arginines, Renal biomarker, Renal function
Abbreviations
- AKI
acute kidney injury
- CKD
chronic kidney disease
- GFR
glomerular filtration rate
- JLU
Justus‐Liebig University Giessen
- MAT
microagglutination test
- NGAL
neutrophil gelatinase‐associated lipocalin
- SDMA
symmetric dimethylarginine
- UPC
urine protein‐to‐creatinine ratio
- USG
urine specific gravity
- VFUB
Vetsuisse Faculty University of Bern
Despite the availability of intensive care treatments, AKI has a high fatality rate.1, 2, 3, 4 Early recognition and intervention is a necessity to effectively treat dogs with AKI, as in contrast to CKD, AKI is reversible.5 Therefore markers for early detection of kidney injury are needed.
At present, renal disease is mainly diagnosed based on an elevation of serum creatinine concentration. Creatinine concentration can be affected by age, sex, muscle mass, and hydration status. A loss of about 75% of renal function is considered necessary for serum creatinine concentration to rise above the upper reference interval.6 Creatinine is usually considered an insensitive marker to reliably diagnose AKI, although sensitivity might be improved by applying the most recent AKI grading criteria from the International Renal Interest Society (IRIS).1
The use of special biomarkers to recognize ongoing kidney injury facilitates the differentiation of AKI from CKD in human medicine and allows an early detection of kidney damage.7 Because of their simple determination, these markers even seem to be superior to the gold standard of clearance methods for determining glomerular filtration rate (GFR).8, 9, 10
Symmetric dimethylarginine (SDMA) is a relatively newly discovered renal biomarker. SDMA is primarily eliminated by renal excretion. Therefore, it is an endogenous marker of GFR. It is not influenced by muscle mass, which is an advantage in comparison with creatinine.11 So far, SDMA has been used successfully to diagnose CKD in dogs and cats.12, 13, 14, 15, 16
SDMA is an important biomarker for early detection of renal dysfunction in human nephrology studies about patients with AKI.17, 18, 19 However, we are not aware of data on SDMA for dogs with AKI.
The aims of this study were to report SDMA concentration in dogs with AKI and to investigate whether SDMA might be a suitable biomarker to differentiate AKI from CKD. Secondly, we aimed to assess whether SDMA might have prognostic value in dogs with AKI.
Material and Methods
Study Design
Eighteen healthy dogs were included as a control group. The dogs were owned by staff members of the small animal clinic of the Justus‐Liebig University Giessen (JLU). They were considered healthy based on physical examination, complete blood cell count, plasma biochemistry profile, and urinalysis with urine culture. GFR was measured by plasma sinistrin clearance as described previously.20 The study was approved by the state ethics and welfare committee Hessia (number 35/2011).
Blood samples collected for a previous study to investigate renal biomarkers in dogs with AKI or CKD were analyzed for SDMA. Dogs with AKI and CKD were prospectively included and classified as previously published.10 Briefly, dogs presented between November 2010 and November 2011 with current or historical renal azotemia were eligible for the study. Dogs also were included if they developed renal azotemia during hospitalization. Two university clinics were involved: the internal medicine service of the JLU and the Small Animal Clinic of the Vetsuisse Faculty of the University of Bern (VFUB). Inclusion criteria were similar to the previous study.10 Briefly, renal azotemia was defined as a plasma creatinine concentration ≥1.4 mg/dL (upper laboratory reference value) and plasma urea concentration ≥54 mg/dL (upper laboratory reference value) persisting at least 24 hours after correction of prerenal factors21 together with a urine specific gravity (USG) <1.025. If no initial urine was available, response to fluid therapy was used to determine whether a dog had prerenal azotemia. If azotemia resolved within 24 hours of fluid therapy, the dog was considered to have prerenal azotemia and was subsequently excluded. Dogs with known or suspected acute‐on‐chronic kidney disease were excluded. Dogs with known CKD in IRIS stage 1 were included. As no urinary biomarkers were assessed, dogs with suspected or proven urinary tract infection were included.
In each dog, one heparin plasma sample was obtained in the first 24 hours after presentation or after development of azotemia. The plasma samples were frozen within 6 hours, stored at −80°C for a maximum of 5 years, and batched for analysis.
Based on history, clinical course, laboratory, and ultrasonographic findings, dogs were assigned either to have AKI or CKD. Criteria used to assign a dog to the AKI group were acute onset of clinical signs and azotemia in a previously healthy dog, signs of acute tubular injury on urinalysis (e.g, glucosuria, urinary casts), imaging findings compatible with AKI such as perirenal free fluid or enlarged kidneys, as well as the absence of chronic changes or resolution or marked improvement of azotemia within 30 days of discharge. IRIS AKI grading criteria were used for further classification into AKI grade I–V on the day of inclusion.1 On the basis of the cause for AKI, dogs in this group were further subdivided into dogs having leptospirosis or other disease. All dogs with leptospirosis were presented to the VFUB. Dogs were diagnosed with leptospirosis based on a positive PCR on urine, a 4‐fold titer increase in paired microagglutination tests (MAT), a single MAT titer ≥1:800 for nonvaccinal serovars or a single MAT titer ≥1:3200 for vaccinal serovars.10, 21, 22 Dogs that were suspected to have leptospirosis based on laboratory results and clinical course but died before diagnosis could be proven were considered not to have leptospirosis (n = 4).10 Criteria used to assign a dog to the CKD group were previous history of CKD in the absence of acute deterioration, laboratory findings compatible with CKD (e.g, nonregenerative anemia), imaging findings compatible with CKD, such as cystic lesions or irregular kidneys, or stable azotemia within 30 days after discharge.1, 23
Dogs with acute‐on‐chronic kidney disease, prerenal, and postrenal azotemia were excluded.
SDMA Assay
Plasma SDMA concentration was measured by liquid chromatography‐mass spectrometry.16 The sample analysis for SDMA was performed by API4000 coupled with Shimadzu Nexera as previously described.13,2 In dogs with SDMA concentration above the upper detection limit of 100 μg/dL, a value of 101 μg/dL was assigned for statistical analysis.
SDMA/Creatinine Ratio
In dogs with kidney disease, a SDMA/creatinine ratio (μg/mg) was calculated by dividing the SDMA concentration (μg/dL) by the creatinine concentration (mg/dL).3 In dogs with SDMA concentrations above the upper detection limit of the assay (100 μg/dL), the ratio was not calculated. A SDMA/creatinine ratio >10 has a poor prognosis in dogs and cats with CKD.3 This cutoff was therefore used to analyze survival data in dogs with CKD or AKI.
Statistics
Statistical analysis was performed by statistical software Prism 6.4 Results of statistical analysis are shown as median and range or median and 25th‐75th percentile. Distribution was tested by D'Agostino and Pearson omnibus normality test. As some data were not normally distributed, nonparametric tests were used for statistical analysis. Spearman's correlation was calculated between SDMA and creatinine concentration in all groups (healthy, AKI, CKD). Mann‐Whitney test was used to compare creatinine concentration in dogs with AKI or CKD as well as SDMA and SDMA/creatinine ratio in dogs with kidney disease alive or deceased 30 days after discharge. Fisher's Exact test was used to compare survival data (yes/no) in dogs with AKI or CKD with SDMA/creatinine ratio >10 or ≤10. SDMA concentration and SDMA/creatinine ratio between the different groups were compared by the Kruskal‐Wallis test. Post hoc assessment to calculate significant differences among the groups was performed by the Dunn's Test with correction of alpha for multiple comparisons. Statistical significance was set at P < .05.
Results
Plasma SDMA concentration was measured in a total of 95 dogs. Included were 18 healthy dogs, 48 dogs with AKI and 29 dogs with CKD.
There were 49 male (34 neutered) and 46 female dogs (22 spayed). The median age was 5.3 years (range 1–16 years). A variety of breeds were included.
Healthy dogs
The clinicopathologic data at inclusion are listed in Table 1. SDMA concentration was significantly lower in the healthy dogs (8.5 μg/dL; range 6.0–12.0 μg/dL) in comparison with the dogs with AKI (39.5 μg/dL; 8.0–>100 μg/dL; P < .0001) or CKD (35.0 μg/dL; 12.0–>100 μg/dL; P = .0077) (Fig 1). Glomerular filtration rate was 3.6 mL/kg/min (2.2–5.5 mL/kg/min) and was significantly correlated with both the creatinine (r = −0.61, P = .0072) and the SDMA concentration (r = −0.49, P = .041). Creatinine and SDMA concentration also were significantly correlated (r = 0.55, P = .017; Fig 2).
Table 1.
Clinical and clinicopathologic data in healthy dogs and dogs with acute kidney injury (AKI) and dogs with chronic kidney disease (CKD)
| Variable [reference range] | Healthy Median (range) | AKI Median (range) | CKD Median (range) |
|---|---|---|---|
| Age (years) | 3.5 (1.0–11.0) n = 18 |
7.0 (1.0–12.0)a
n = 48 |
6.0 (1.0–15.0)a
n = 29 |
| Body Weight (kg) |
27.5 (8.1–58) n = 18 |
23.5 (1.9–64.0) n = 47 |
18.6 (2.4–43.0) n = 29 |
|
Urea (mg/dL) [19.8–58.9] |
40.7 (25.0–75.6) n = 18 |
143.0 (31.4–306.9)a, b
n = 48 |
244.5 (54.1–701.9)a
n = 29 |
|
Creatinine (mg/dL) [0.6–1.4] |
0.9 (0.6–1.2) n = 18 |
6.6 (1.8–21.0)a, b
n = 48 |
3.6 (1.0–13.2)a
n = 29 |
|
Potassium (mEq/L) [3.4–4.4] |
4.0 (3.7–4.7) n = 18 |
4.6 (2.9–7.9)a
n = 48 |
4.3 (3.2–7.0) n = 29 |
|
Phosphorus (mg/dL) [2.5–6.5] |
4.2 (2.6–5.4) n = 18 |
11.9 (1.8–30.3)a, b
n = 48 |
5.2 (1.9–27.4)a
n = 29 |
| USG |
1.038 (1.021–1.050) n = 18 |
1.016 (1.008–1.035)a
n = 26 |
1.014 (1.006–1.024)a
n = 26 |
USG, urine specific gravity.
P < .05 versus healthy.
P < .05 versus CKD.
Figure 1.
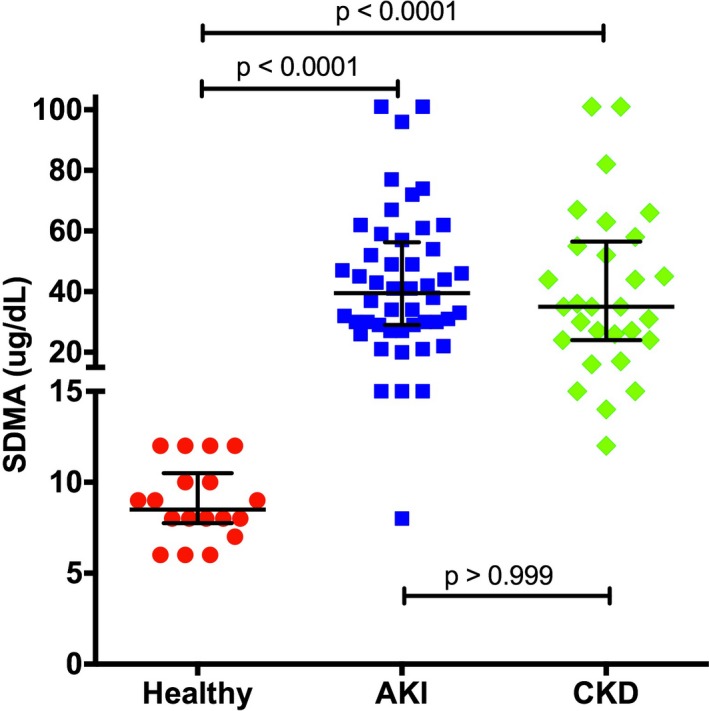
Symmetric dimethylarginine (SDMA) concentration in healthy dogs, dogs with acute kidney injury (AKI), and dogs with chronic kidney disease (CKD). Lines represent median and 25th–75th percentile.
Figure 2.
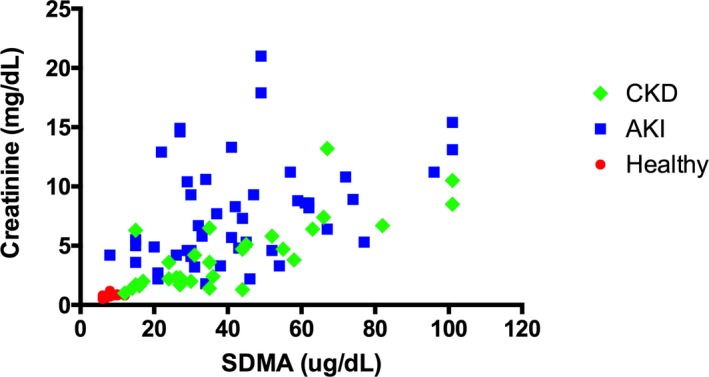
Creatinine and symmetric dimethylarginine (SDMA) in healthy dogs and dogs with acute kidney injury (AKI) or chronic kidney disease (CKD).
Dogs with Kidney Disease
Clinicopathologic data at inclusion of dogs with AKI or CKD are shown in Table 1. SDMA concentration in dogs with AKI or CKD was not significantly different between the two groups (Fig 1). Creatinine concentration was higher in dogs with AKI (6.6 mg/dL; 1.8–21.0 mg/dL) compared to dogs with CKD (3.6 mg/dL; 1.0–13.2 mg/dL) (P = .0002). As in healthy dogs, creatinine and SDMA concentration showed a significant correlation (AKI: r = 0.40, P = .0051, CKD: r = 0.74, P < .0001; Fig 2). Correlation over all groups was moderate, but significant (r = 0.74, P = <.0001).
Using the AKI grading criteria, AKI grade II was detected in 3 dogs (6%), AKI grade III in 15 dogs (31%), AKI grade IV in 17 dogs (35%), and AKI grade V in 13 dogs (28%). Median SDMA concentration in dogs with AKI grade II, III, IV, and V was 34 μg/dL (21–46 μg/dL), 30 μg/dL (8–54 μg/dL), 45 μg/dL (15–77 μg/dL), and 49 μg/dL (22–>100 μg/dL), respectively. As only three dogs were classified as AKI grade II, AKI grade II and III were combined for statistical analysis (AKI grade II + III). AKI grade II + III had significantly lower SDMA concentration than AKI grade IV (P = .0218), but not significantly lower than AKI grade V (P = .1176). SDMA concentrations in AKI grade IV and V were similar (P = >.99).
Among dogs with AKI, leptospirosis was identified in 23 of 48 dogs (48%). SDMA concentration was similar (P > .99) in dogs with AKI caused by leptospirosis (34 μg/dL; 8.0–>100 μg/dL) compared to dogs with AKI from other causes (41 μg/dL; 15.0–>100 μg/dL).
The SDMA/creatinine ratio could be calculated in 46/48 dogs with AKI and 27/29 dogs with CKD. It was significantly (P = .0004) lower in dogs with AKI (6.4 μg/mg; 1.7–20.9 μg/mg) compared to dogs with CKD (10 μg/mg; 2.4–33.9 μg/mg), but showed significant overlap between the groups (Fig 3). SDMA/creatinine ratio was similar (P = .0576) in dogs with mild CKD (n = 9, IRIS CKD stage 1 & 2: 12 μg/mg; 8.5–33.9 μg/mg) compared to dogs with more progressed azotemia (n = 18, IRIS CKD stage 3 & 4: 9.6 μg/mg; 2.4–15.3 μg/mg).
Figure 3.
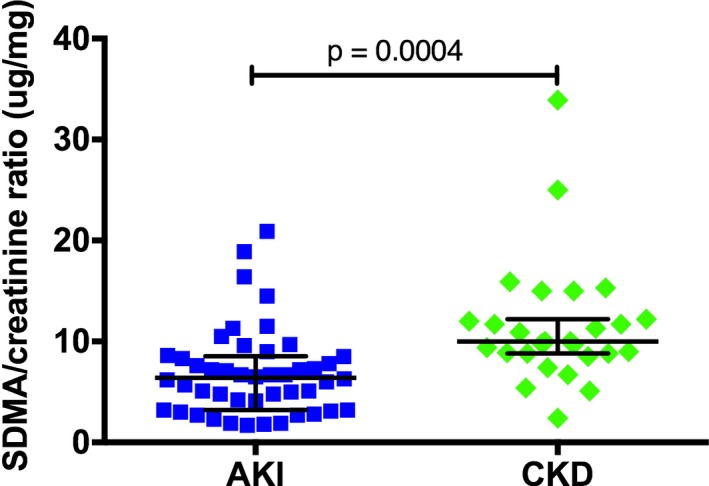
Symmetric dimethylarginine (SDMA) to creatinine ratio in dogs with acute kidney injury (AKI) and chronic kidney disease (CKD). Lines represent median and 25th–75th percentile.
Survival data (alive or dead 30 days post discharge) was available for all dogs. SDMA and SDMA/creatinine ratio were similar between survivors and nonsurvivors in all dogs with kidney disease (P = .11; P = .40) and in dogs with AKI (P = .24, and P = .29, respectively) and CKD (P = .33, and P = .44, respectively). Dogs with AKI or CKD with a SDMA/creatinine ratio >10 were not more likely to die within 30 days of discharge than dogs with a SDMA/creatinine ratio ≤10 (P = .46; AKI: P = .16; CKD P = 1).
Discussion
Creatinine and SDMA concentrations were significantly higher in dogs with renal azotemia compared to healthy dogs. Both creatinine and SDMA are markers of GFR, and creatinine, as well as SDMA, correlates inversely with GFR.5 SDMA concentration in dogs with CKD significantly correlates with creatinine concentration.5 This was confirmed in the current study. Based on previous study results, SDMA allows earlier detection of chronic kidney disease in dogs than creatinine.24,6 Our study design did not allow evaluation of SDMA as a marker of early kidney disease in AKI.
No significant difference was seen in the plasma concentration of SDMA between dogs with AKI and dogs with CKD. This was expected, as SDMA is mainly a marker of filtration.13, 16, 5 Reduction of GFR is seen in both AKI and CKD. A direct comparison of SDMA with GFR in these groups would have been valuable, but GFR was measured only in the group of healthy dogs in our study.
Creatinine concentration was significantly higher in dogs with AKI compared to CKD (Table 1), although SDMA concentration was similar between groups. Despite the significant correlation of SDMA and creatinine in the examined population of dogs, this correlation was much lower in the dogs with AKI compared to the dogs with CKD. This was unexpected because of the potential lower muscle mass in dogs with advanced CKD and the influence of muscle mass on creatinine concentration.6 A lower correlation of SDMA and creatinine in the dogs with CKD would be expected. The reason of this discrepant correlation result in our study was unclear. Also, SDMA/creatinine ratio was significantly higher in dogs with CKD in comparison with dogs with AKI (Fig 3). Other than by GFR, creatinine concentration is mainly influenced by muscle mass, which is reduced in many dogs with CKD.6, 25 Dogs with AKI are expected to have a normal body condition at presentation.1 As CKD might lead to more profound muscle wasting compared to AKI, a higher SDMA/creatinine ratio in dogs with progressed CKD might be a result of decreased muscle mass. SDMA/creatinine ratio in dogs with mild and advanced stages of CKD was similar in the present study. However, a larger number of dogs in the different IRIS stages including assessment of a muscle score might be useful in assessing the influence of muscle mass on SDMA/creatinine ratio in future prospective studies. Although SDMA/creatinine ratio was significantly different in dogs with AKI or CKD, a large overlap was present between the groups (Fig 3) and these results should not be over‐interpreted. Further studies, ideally including GFR assessment, are necessary to elaborate the disproportional elevation of SDMA as compared with creatinine in dogs with AKI.
Most dogs included in the AKI group had advanced kidney disease. Only three of the included dogs with AKI could be classified with AKI grade II.1 A conclusion about SDMA in dogs with earlier grades of AKI is therefore not possible. Further studies are needed to analyze SDMA concentration in dogs with early AKI, such as hospital acquired kidney disease. SDMA might be of additional benefit if early measurement is possible.
A SDMA/creatinine ratio >10 has been reported to carry a poor prognosis in dogs and cats with CKD.3 The prognostic value of SDMA/creatinine ratio is thought to be a result of a disproportional increase of SDMA compared to creatinine in animals with CKD. The typical SDMA/creatinine ratio is <10 in the majority of animals with CKD. The higher the ratio (>10), the higher the risk of death in dogs and cats with CKD.3 However, SDMA/creatinine ratio was independent of short‐term survival for both dogs with AKI and dogs with CKD in this study.
One dog with leptospirosis had a SDMA concentration well within the reference range (8 μg/dL) despite having marked azotemia (creatinine concentration 4.2 mg/dL). A laboratory error was initially suspected, but repeated measurements confirmed the values. Although this dog had hyperbilirubinemia in addition to the severe azotemia, interference of biological substances with SDMA assay is highly unlikely, because of lack of interference with hemoglobin, lipids, and bilirubin at the tested concentrations.16, 26 Normalization of GFR before normalization of creatinine would also be possible, as creatinine usually lags behind. However, this dog was anuric and blood work performed approximately 18 h later showed an increase of creatinine concentration from 4.2 mg/dL to 7.2 mg/dL, indicating that the dog's GFR was not recovering or normalizing. Therefore the discrepancy in this dog remains unclear. Storage time did not have an influence on the SDMA concentration in a previous study.16 Whereas samples in our study were stored for several years at −80°C, in this individual dog influence of storage time is unlikely because the storage time was comparable to that of the other samples of the azotemic or healthy control dogs. Two dogs with CKD also had a SDMA concentration within the reference range. Both were classified as CKD stage 1 with high normal creatinine concentration, potentially explaining the normal SDMA concentration.
This study has several limitations. The first limitation is the lack of GFR measurements in the azotemic dogs. Previous studies and also the healthy population in our study show a good correlation between GFR, creatinine, and SDMA concentration, but no information about the relation of SDMA and GFR in dogs with AKI is available.13, 15, 16,5 A further limitation is that SDMA/creatinine ratio could not be calculated in dogs with SDMA values above the upper detection limit. As this was only the case in four dogs, it is likely that this has not confounded the analysis. All dogs with acute‐on‐chronic kidney disease were excluded from further analysis. However, because of the lack of clear identification criteria, some dogs might have been misclassified, possibly affecting the results. Follow‐up to 30 days after discharge was included in all reported dogs to ascertain the disease status of the dogs, making misclassification less likely. Lastly the study lacks the determination of a muscle score. This would have been helpful to evaluate extrarenal influences on creatinine concentration.
In conclusion, plasma SDMA concentration is a suitable marker for identifying acute and chronic kidney disease in dogs. Dogs with AKI have similar plasma SDMA concentration as dogs with CKD and markedly elevated SDMA concentration compared to healthy dogs. In this population of dogs, the SDMA/creatinine ratio was significantly lower in the AKI group compared with the CKD group for yet unknown reasons, and further, prospective studies including a variety of cases spanning across all IRIS AKI grades might help to better understand the performance of this biomarker.
Acknowledgment
We acknowledge the help of Giosi Farace, IDEXX Laboratories, Inc.
Conflict of Interest Declaration: T. Francey has acted as a paid independent consultant to IDEXX Laboratories, Inc. in 2014 and as a paid speaker in IDEXX‐organized lectures. A. Schweighauser has acted as a paid speaker in IDEXX‐organized lectures. S. Steinbach has acted as a paid independent consultant to IDEXX Laboratories, Inc. in 2014. M. Yerramilli and E. Obare are currently employed by IDEXX Laboratories, Inc. SDMA was measured free of charge by IDEXX Laboratories Inc.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
The work was done at the Justus‐Liebig University Giessen and the Vetsuisse Faculty University of Bern. Measurement of SDMA concentration was performed by IDEXX Laboratories, Inc. The results of this research were presented in part as a poster abstract at the Annual Congress of the European College of Veterinary Internal Medicine in Gothenburg, Sweden, September 2016.
Footnotes
IRIS grading of AKI. International Renal Interest Society website. Iris‐kidney.com/guidelines/grading. html. 2013, © 2016 International Renal Interest Society (IRIS)
IDEXX Laboratories, Inc., Westbrook, Maine 04092
Yerramilli M, Yerramilli M, Obare E, et al. Prognostic value of symmetric dimethylarginine (SDMA) to creatinine ratio in dogs and cats with chronic kidney disease (CKD). J Vet Intern Med 2015;29:1122‐1256. (abstract)
Prism 6 for Mac OS X, GraphPad Software Inc, La Jolla, CA
Nabity MB, Lees GE, Boggess M, et al. Correlation of symmetric dimethylarginine with glomerular filtration rate in dogs with chronic progressive renal disease. J Vet Intern Med 2013;27(3):733. (abstract)
Yerramilli M, Yeramilli M, Obare E, et al. Symmetric dimethylarginine (SDMA) increases earlier than serum creatinine in dogs with chronic kidney disease (CKD). J Vet Intern Med 2014;28(3):1084‐1085. (abstract)
References
- 1. Langston C. Acute uremia In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine: Diseases of the Dog and the Cat, 7th ed St. Louis, MO: Saunders Elsevier; 2010:1969–1984. [Google Scholar]
- 2. Harison E, Langston C, Palma D, Lamb K. Acute azotemia as a predictor of mortality in dogs and cats. J Vet Intern Med 2012;26:1093–1098. [DOI] [PubMed] [Google Scholar]
- 3. Vaden SL, Levine J, Breitschwerdt EB. A retrospective case‐control of acute renal failure in 99 dogs. J Vet Intern Med 1997;11:58–64. [DOI] [PubMed] [Google Scholar]
- 4. Thoen ME, Kerl ME. Characterization of acute kidney injury in hospitalized dogs and evaluation of a veterinary acute kidney injury staging system. J Vet Emerg Crit Care 2011;21:648–657. [DOI] [PubMed] [Google Scholar]
- 5. Gerber B, Glaus TM, Unterer S, Reusch CE. Beurteilung von Parametern zur Unterscheidung von akuter und chronischer Niereninsuffizienz beim Hund. Schweiz Arch Tierheilk 2004;146:365–373. [DOI] [PubMed] [Google Scholar]
- 6. Braun JP, Lefebvre HP, Watson ADJ. Creatinine in the dog: A review. Vet Clin Path 2003;32:162–179. [DOI] [PubMed] [Google Scholar]
- 7. Endre ZH, Kellum JA, DiSomma S, et al. Differential diagnosis of AKI in clinical practice by functional and damage biomarkers: Workgroup statements from the tenth acute dialysis quality initiative consensus conference In: McCullough PA, Kellum JA, Mehta RL, Murray PT, Ronco C, eds. ADQI Consensus on AKI Biomarkers and Cardiorenal Syndromes. Basel: Contrib. Nephrol; 2013;182:30–44. [DOI] [PubMed] [Google Scholar]
- 8. Lee YJ, Hu YY, Lin YS, et al. Urine neutrophil gelatinase‐associated lipocalin (NGAL) as a biomarker for acute canine kidney injury. Vet Res 2012;8:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cobrin AR, Blois SL, Kruth SA, et al. Biomarkers in the assessment of acute and chronic kidney diseases in the dog and cat. J Small Anim Pract 2013;54:647–655. [DOI] [PubMed] [Google Scholar]
- 10. Steinbach S, Weis J, Schweighauser A, et al. Plasma and urine neutrophil gelatinase‐associated lipocalin (NGAL) in dogs with acute kidney injury and chronic kidney disease. J Vet Intern Med 2014;28:264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hall JA, Yerramilli M, Obare M, et al. Relationship between lean body mass and serum renal biomarkers in healthy dogs. J Vet Intern Med 2015;29:803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jepson RE, Syme HM, Vallance C, Elliott J. Plasma asymmetric dimethylarginine, symmetric dimethylarginine, L‐Arginine, and nitrite/nitrate concentrations in cats with chronic kidney disease and hypertension. J Vet Intern Med 2008;22:317–324. [DOI] [PubMed] [Google Scholar]
- 13. Braff J, Obare E, Yerramilli M, et al. Relationship between serum symmetric dimethylarginine concentration and glomerular filtration rate in cats. J Vet Intern Med 2014;28:1699–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hall JA, Yerramilli M, Obare E, et al. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in healthy geriatric cats fed reduced protein foods enriched with fish oil, L‐carnitine, and medium‐chain triglycerides. Vet J 2014;202:588–596. [DOI] [PubMed] [Google Scholar]
- 15. Hall JA, Yerramilli M, Obare E, et al. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J Vet Intern Med 2014;28:1676–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nabity MB, Lees GE, Bogges MM, et al. Symmetric dimethylarginine assay validation, stability, and evaluation as a marker for the early detection of chronic kidney disease in dogs. J Vet Intern Med 2015;29:1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kielstein JT, Salpeter SR, Bode‐Boeger SM, et al. Symmetric dimethylarginine (SDMA) as endogenous marker of renal function ‐ a meta‐analysis. Nephrol Dial Transplant 2006;21:2446–2451. [DOI] [PubMed] [Google Scholar]
- 18. Kielstein JT, Veldink H, Martens‐Lobenhoffer J, et al. SDMA is an early marker of change in GFR after living‐related kidney donation. Nephrol Dial Transplant 2011;26:324–328. [DOI] [PubMed] [Google Scholar]
- 19. Lüneburg N, Von Holten RA, Töpper RF, et al. Symmetric dimethylarginine is a marker of detrimental outcome in the acute phase after ischaemic stroke: Role of renal function. Clin Sci 2012;122:105–111. [DOI] [PubMed] [Google Scholar]
- 20. Haller M, Müller W, Estelberger W, Arnold P. Single‐injection inulin clearance – a simple method for measuring glomerular filtration rate in dogs. Res Vet Sci 1998;64:151–156. [DOI] [PubMed] [Google Scholar]
- 21. Geigy CA, Schweighauser A, Doherr M, Francey T. Occurrence of systemic hypertension in dogs with acute kidney injury and treatment with amlodipine besylate. J Small Anim Pract 2011;52:340–346. [DOI] [PubMed] [Google Scholar]
- 22. Sykes JE, Hartmann K, Lunn KF, et al. 2010 ACVIM small animal consensus statement on leptospirosis: Diagnosis, epidemiology, treatment, and prevention. J Vet Intern Med 2011;25:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Polzin DJ. Chronic kidney disease In: Ettinger SJ, Feldmann EC, eds. Textbook of Veterinary Internal Medicine: Diseases of the Dog and the Cat, 7th ed St. Louis, MO: Saunders Elsevier; 2010:1990–2021. [Google Scholar]
- 24. Hall JA, Yerramilli M, Obare E, et al. Serum concentrations of symmetric dimethylarginine and creatinine in dogs with naturally occurring chronic kidney disease. J Vet Intern Med 2016;30:794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Relford R, Robertson J, Clements C. Symmetric dimethylarginine improving the diagnosis and staging of chronic kidney disease in small animals. Vet Clin Small Anim 2016;46:941–960. [DOI] [PubMed] [Google Scholar]
- 26. Martens‐Lobenhoffer J, Bode‐Böger SM. Amino acid N‐acetylation: Metabolic elimination of symmetric dimethylarginine as symmetric Nɑ‐acetyldimethylarginine, determined in human plasma and urine by LC‐MS/MS. J Chromatogr B 2015;975:59–64. [DOI] [PubMed] [Google Scholar]


