Abstract
Background
In the absence of ocular target organ damage (ocular‐TOD), diagnosis of hypertension is challenging in cats. Biomarkers would provide additional support for the diagnosis of hypertension.
Hypothesis
Vascular endothelial growth factor (VEGF), N‐terminal probrain natriuretic peptide (NT‐proBNP), cardiac troponin I (cTnI), and urine protein‐to‐creatinine ratio (UPC) are predictors of systemic hypertension, will be increased in cats with hypertension with or without ocular‐TOD, and will decrease with antihypertensive treatment.
Methods
Plasma VEGF, NT‐proBNP, and cTnI concentrations and UPC were determined in healthy geriatric cats, normotensive cats with chronic kidney disease (CKD), hypertensive cats with evidence of hypertensive retinopathy (HT‐ocular‐TOD), and hypertensive cats without hypertensive ocular‐TOD (HT‐noTOD). Comparisons among groups were performed. Multivariable binary logistic regression models were built to identify independent biomarkers of hypertension and ocular‐TOD. Receiver operator characteristic (ROC) curves were drawn to assess clinical use.
Results
Cats with HT‐ocular‐TOD had significantly higher VEGF than all other groups (P < .05) and significantly higher NT‐proBNP than healthy cats (P < .001). Healthy cats had significantly lower cTnI than all other groups (P < .05). No differences were found among groups for UPC (P = .08). Cardiac troponin I and VEGF were independent predictors of hypertension (P < .05), but none of the biomarkers were independent predictors of ocular‐TOD. N‐terminal probrain natriuretic peptide concentrations decreased with antihypertensive treatment (P < .001). The ROC curves indicated that none of the biomarkers met the criteria to function as diagnostic tests for the diagnosis of hypertension or associated ocular‐TOD.
Conclusions and Clinical Significance
Despite statistical significance and changes with ocular‐TOD, antihypertensive treatment, or both, VEGF, NT‐proBNP, and cTnI did not function as useful diagnostic tests for hypertension. Persistently increased systolic blood pressure (SBP) measurements in combination with fundoscopy remains the preferred method for diagnosis of feline hypertension.
Keywords: Chronic kidney disease, Hypertension
Abbreviations
- CKD
chronic kidney disease
- cTnI
cardiac troponin I
- cTSH
canine thyroid‐stimulating hormone
- CV
coefficient of variation
- GFR
glomerular filtration rate
- HCM
hypertrophic cardiomyopathy
- IRIS
International Renal Interest Society
- NT‐proBNP
N‐terminal probrain natriuretic peptide
- ROC
receiver operator characteristic
- SBP
systolic blood pressure
- TOD
target organ damage
- UPC
urine protein‐to‐creatinine ratio
- USG
urine specific gravity
- VEGF
vascular endothelial growth factor
White coat hypertension is reported to occur frequently in cats, with systolic blood pressure (SBP) increasing on average 22.3 mmHg during clinical examination, but with a highly unpredictable interindividual effect and SBP increasing by as much as 75 mmHg in some cats.1 Without evidence of hypertensive retinopathy (ocular target organ damage [ocular‐TOD]), it therefore can be challenging to diagnose hypertension, and clinicians must rely on documentation of consistently high SBP measurements in affected cats.2 Hypertension has many immediate effects on the body and results in several physiologic responses in an attempt to decrease blood pressure. In hypertensive human patients, a number of these physiologic responses have been shown to involve substances that could function as biomarkers of hypertension, hypertensive TOD, efficacy of treatment, or some combination of these. The heart and blood vessels are considered target organs of hypertensive damage in both humans and cats.2 Vascular endothelial growth factor (VEGF) secreted by endothelial cells and N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) and cardiac troponin I (cTnI) secreted by cardiomyocytes are examples of biomarkers that have been investigated for use in hypertensive human patients.3, 4 Urine protein‐to creatinine ratio (UPC) is a potential marker of TOD and renal injury.5, 6 Increased blood pressure leads to increased glomerular pressure and therefore leakage of protein into the urine. The UPC has been shown to be increased in hypertensive cats when compared to normotensive cats.7
Vascular endothelial growth factor is secreted in response to shear stress of endothelial cells8 and mechanical stretch of vascular smooth muscle cells.9 Plasma VEGF concentrations are increased in human patients with hypertension3, 10 and in patients with hypertensive retinopathy.11 In addition, plasma VEGF decreases with antihypertensive treatment in humans.12 Concentrations of NT‐proBNP are increased in hypertensive human patients with peripheral arterial disease.4 Human subjects with poor blood pressure control have higher plasma NT‐proBNP concentrations, indicating its potential use as a marker for effectiveness of treatment.13 Cats with hypertension and evidence of ocular‐TOD have significantly higher NT‐proBNP concentrations than do healthy cats and those with normotensive chronic kidney disease (CKD).14 Hypertensive cats have been reported to have lower concentrations of NT‐proBNP if hypertension is not accompanied by ocular‐TOD, although these cats also had significantly lower blood pressure than did their counterparts with evidence of ocular changes.15 Cardiac troponin I is produced only by cardiomyocytes and is a sensitive and specific marker of myocardial cell necrosis in humans16 and dogs.17 Cats with hypertrophic cardiomyopathy (HCM) have increased cTnI concentrations.18 During hypoxic stress, cardiomyocytes release cTnI,16 and it is possible that cTnI also is released when hypertension causes hypertrophy, stretch, strain, or damage. Human patients admitted to hospital for severe hypertension often have increased cTnI concentrations.19
The aims of our study were to assess UPC, VEGF, NT‐proBNP, and cTnI as potential biomarkers of hypertension in cats with and without ocular‐TOD, and to assess whether VEGF, NT‐proBNP, and cTnI are informative in terms of assessment of blood pressure control.
Materials and Methods
Clinics and Case Selection
Cats diagnosed with hypertension at 2 first opinion practices in central London (People's Dispensary for Sick Animals in Bow and Beaumont Sainsbury Animal Hospital in Camden) between May 2007 and July 2015 were retrospectively identified. A standardized visit protocol was followed for each cat, which included full history and physical examination. A noninvasive Doppler technique1 was used to measure SBP after a period of acclimatization. Average SBP was calculated from 5 consecutive readings after discarding the first reading. If average SBP was ≥160 mmHg, indirect fundoscopy was performed by the attending clinician with an independent light source and a lens, after applying 1 drop of tropicamide 1% to both eyes.
At the first visit to the clinic, informed owner consent was obtained to collect blood samples by jugular venipuncture and urine samples by cystocentesis. The standard clinic protocols, information sheets, and consent forms for use of residual samples for research were approved by the Royal Veterinary College's Ethics and Welfare Committee. Blood samples were collected into lithium heparin tubes and EDTA tubes and held on ice (4°C) for a maximum of 6 hours before centrifugation2 and separation. Plasma biochemistry was performed at an external laboratory.3 Not all cats included in this study had serum total thyroxine (TT4) concentration measured, but cases were excluded if they had a TT4 > 40 nmol/L (reference range, 10–55 nmol/L), had clinical signs consistent with hyperthyroidism (eg, polyphagia, weight loss, palpable goiter, tachycardia), or were currently undergoing treatment for hyperthyroidism. Cats also were excluded if they had evidence of other concurrent disease (eg, palpation of an abdominal mass) and if they were on medications that were likely to alter blood pressure (eg, benazepril). Urine protein‐to‐creatinine ratios were determined at the same laboratory that was used for plasma biochemistry and TT4 measurements, and urine samples from cats with evidence of a urinary tract infection were excluded from analysis.
Four groups were included: cats diagnosed with azotemic CKD (CKD), healthy geriatric cats (Healthy), hypertensive cats with evidence of ocular hypertensive TOD (HT‐ocular‐TOD), and hypertensive cats without evidence of hypertensive retinopathy (HT‐noTOD). The hypertensive visit for the HT‐ocular‐TOD and HT‐noTOD groups and 1 visit for the CKD and healthy groups were used for the cross‐sectional analyses. Azotemic CKD was diagnosed based on a plasma creatinine concentration ≥2.0 mg/dL either on 2 consecutive visits or on a single occasion if in conjunction with urine specific gravity (USG) <1.035. Healthy cats had unremarkable history and physical examination, SBP <160 mmHg, and plasma biochemistry lacked abnormalities deemed to be clinically relevant by the attending clinician. Cats that had urine samples available and USG <1.035 were only included as healthy if they had follow‐up visits at which the cat was assessed as healthy by the attending clinician and no azotemia developed within 6 months. All cats included in the HT‐ocular‐TOD group had SBP ≥160 mmHg and evidence of hypertensive retinopathy or choroidopathy on indirect ophthalmologic examination, whereas all cats included in the HT‐noTOD group were diagnosed based on a SBP ≥170 mmHg on 2 consecutive visits, but did not have evidence of ocular hypertensive TOD. A small number of the cats had undergone echocardiographic assessment, but to prevent bias in the selection, the authors were blinded to the results of the cardiac evaluation.
Only hypertensive cats were included in the longitudinal study. All hypertensive cats were treated with amlodipine besylate.4 A blood sample was taken at the time of diagnosis of hypertension (included in the cross‐sectional study), at the first visit antihypertensive control was achieved (defined as SBP <160 mmHg and receiving 0.625–1.25 mg amlodipine/cat/day) and approximately 4 months later (approximately 5 months after diagnosis of hypertension). All cats must have had residual stored heparinized plasma and EDTA plasma samples available for all visits included in both the cross‐sectional and longitudinal studies to be eligible for inclusion.
Measurement of UPC, VEGF, NT‐proBNP, and cTnI
The VEGF assay validated in this study was a commercially available canine VEGF ELISA.5 Precision and reproducibility of the assay for heparinized feline plasma samples were determined by calculating intra‐ and interassay coefficients of variation (CV). Dilutional parallelism was assessed, as was the effect of 4 freeze‐thaw cycles.
Plasma EDTA samples previously stored at −80°C were used for batch analysis of NT‐proBNP and cTnI at a commercial laboratory.6 Batch analysis of UPC on urine samples previously stored at −80°C was performed at the same laboratory. The UPC was only determined for samples included in the cross‐sectional study, and not post‐treatment with amlodipine in the hypertensive groups.
Statistical Analyses
Statistical analyses were performed by 2 different statistical software packages.5 Results are presented as median [25th, 75th percentiles], and statistical significance was determined as P < .05. Variables were assessed for normality by visual inspection of histograms. The number of cats that was needed for the cross‐sectional study was estimated by a parametric power calculation on unpublished VEGF data on healthy cats and hypertensive cats without ocular‐TOD, and estimated data for cats with ocular‐TOD, with power set at 80% and significance at 5%. This power calculation was repeated after all measurements were performed, and an actual mean concentration was known for ocular‐TOD cats. The parametric power calculation was adjusted for the fact that the data were skewed by multiplying it by 1.15 (15% additional cats).
Cross‐sectional comparisons among HT‐ocular‐TOD, HT‐noTOD, CKD, and healthy cats were made using Kruskal–Wallis tests with posthoc comparisons by Dunn's test or a 1‐way ANOVA with posthoc Tukey's test. The proportion of cats with concurrent CKD was compared between HT‐ocular‐TOD and HT‐noTOD using Fisher's exact test. Comparisons among related visits within the hypertensive groups were made using the nonparametric Wilcoxon signed‐rank test and Friedman test with posthoc comparisons using the Bonferroni method.
To identify predictors of hypertension, HT‐ocular‐TOD and HT‐noTOD were grouped as hypertensive, and the healthy cats and cats with CKD functioned as the control group. To identify predictors of ocular‐TOD, the HT‐noTOD cats functioned as the control group and were compared to the cats with HT‐ocular‐TOD. Plasma VEGF, NT‐proBNP, and cTnI concentrations and UPC all were included as explanatory variables in univariable binary logistic regression models with hypertension or ocular‐TOD as dependent variable. All variables were log‐transformed to meet normality criteria. Variables significant at the 20% level were included in a multivariable binary logistic regression to identify the biomarkers that were independent predictors of hypertension and ocular‐TOD.
Receiver operating characteristic curves (ROC) were used to determine whether VEGF, NT‐proBNP, cTnI, and UPC could be used to predict hypertension and ocular‐TOD in a clinical setting. Sensitivity and specificity were used to describe potential cutoffs for the variables that had an area under the curve (AUC) significantly >0.5. The combined use of VEGF, NT‐proBNP, and cTnI to diagnose hypertension or ocular‐TOD was explored. The cutoff value for each variable was determined based on the greatest combined sensitivity and specificity. Sensitivity and specificity were determined using each possible combination of the significant values to diagnose hypertension and ocular‐TOD.
Results
Validation of the Canine VEGF ELISA for Feline Plasma
The intra‐assay CVs for samples measuring 99.5, 100.9, and 101.5 pg/mL were 9.9, 5.0, and 4.5%, respectively. The interassay CVs for samples measuring 63.2, 100.3, and 109.5 pg/mL were 13.9, 7.2 and 4.3%, respectively. Dilutional parallelism was demonstrated: 94.9 (±13.6) % recovery. Measurement after 4 freeze‐thaw cycles gave a recovery of 119.4 (±4.3) % (n = 3).
Subjects
The power calculation indicated 25 cats were needed per group. A total of 25 hypertensive cats with ocular‐TOD, 24 hypertensive cats without ocular‐TOD, 25 healthy cats, and 25 CKD cats were included (Table 1). As expected, both hypertensive groups had significantly higher SBP than did the healthy and CKD cats. The healthy cats were significantly younger and had lower plasma urea and creatinine concentrations, and higher USG than all other groups. Three healthy cats had USG <1.035 at the included visit. All 3 had follow‐up visits at which they remained nonazotemic and were deemed healthy by the attending veterinarian. Two of these cats had urine samples available at follow‐up visits, and both had concentrated urine at those visits. Twenty‐nine cats had NT‐proBNP >270 pmol/L, which makes cardiac disease more likely (http://www.idexx.co.uk/pdf/en_gb/smallanimal/reference-laboratories/clinical-guidance-cardiac-disease.pdf, downloaded on 21/09/2015). Twelve cats with NT‐proBNP>270 pmol/L had an echocardiographic result available, of which 4 were classified as normal (1 in the HT‐ocular‐TOD group; 3 in the CKD group), 5 had evidence of severe hypertrophy (3 in the HT‐noTOD group, all were already on amlodipine treatment at the time of echocardiography; 2 had CKD), 1 had pseudohypertrophy likely because of dehydration (HT‐ocular‐TOD group, echocardiography was performed on the day hypertension was diagnosed), 1 had possible left ventricular hypertrophy (CKD group), and 1 cat had dynamic left ventricular outflow tract obstruction (HT‐noTOD group, echocardiography performed on the day hypertension was diagnosed). The majority of cats (n = 88) did not have an echocardiographic assessment available.
Table 1.
Clinicopathologic variables for healthy, CKD, HT‐ocular‐TOD, and HT‐noTOD cats
| Healthy (n = 25) | CKD (n = 25) | HT‐noTOD (n = 24) | HT‐Ocular‐TOD (n = 25) | |
|---|---|---|---|---|
| VEGF (pg/mL) | 49.4 [39.0, 94.4]a | 70.4 [51.2, 78.5]a | 53.3 [39.9, 106.6]a | 105.6 [77.3, 142.5]b |
| NT‐proBNP (pmol/L) | 67.0 [28.5, 118.0]a | 126.0 [75.0, 290.5]ab | 120.0 [75.0, 289.0]ab | 236.0 [114.5, 519.0]b |
| cTnI (ng/mL) | 0.02 [0.01, 0.03]a | 0.04 [0.02, 0.05]b | 0.04 [0.02, 0.06]b | 0.07 [0.05, 0.14]b |
| SBP (mmHg) | 131.2 [113.0, 139.4]a | 136.0 [133.2, 141.4]a | 179.4 [175.3, 188.2]b | 184.8 [168.4, 203.6]b |
| UPC | 0.18 [0.13, 0.22] | 0.24 [0.15, 0.58] | 0.25 [0.20, 0.50] | 0.17 [0.14, 0.30] |
| Heart rate (bpm) | 180 [165, 199] | 192 [180, 210] | 180.0 [180.0, 204.0] | 182 [164, 205] |
| Age (years) | 12.1 [11.1, 13.2]a | 15.3 [12.4, 16.7]ab | 15.3 [12.8, 17.0]b | 15.8 [13.5, 18.0]b |
| Weight (kg) | 4.5 [3.8, 5.4]a | 3.8 [3.3, 4.6]bc | 4.6 [3.5, 5.7]ab | 3.6 [3.0, 4.4]c |
| PCV (%) | 36 [34, 40] | 35 [29, 39] | 37 [34, 40] | 35 [30, 39] |
| Albumin (g/dL) | 3.2 [3.0, 3.3] | 3.1 [3.0, 3.2] | 3.3 [3.1, 3.4] | 3.1 [2.9, 3.3] |
| Creatinine (mg/dL) | 1.4 [1.3, 1.6]a | 2.4 [2.1, 2.9]b | 2.1 [1.7, 2.9]b | 2.3 [1.6, 2.8]b |
| Urea (mmol/L) | 9.6 [8.1, 11.4]a | 15.0 [12.5, 19.2]b | 15.8 [11.8, 21.6]b | 16.2 [11.4, 22.3]b |
| Phosphate (mg/dL) | 3.56 [3.25, 4.15] | 3.62 [3.28, 4.30] | 4.09 [3.34, 4.74] | 3.84 [3.34, 4.92] |
| Total calcium (mg/dL) | 9.8 [9.5, 10.2] | 10.4 [9.8, 11.1] | 10.0 [9.8, 10.4] | 10.0 [9.5, 10.6] |
| Sodium (mEq/L) | 153.7 [152.5, 154.7] | 155.8 [153.5, 157.6] | 154.1 [151.3, 156.5] | 154.2 [152.3, 156.7] |
| Potassium (mEq/L) | 4.1 [3.7, 4.3] | 4.1 [3.8, 4.3] | 4.1 [3.7, 4.5] | 4.0 [3.7, 4.3] |
| Chloride (mEq/L) | 119.6 [118.2, 122.5]a | 119.7 [118.1, 120.9]ab | 117.5 [115.3, 119.7]b | 118.8 [116.3, 120.3]ab |
| Cholesterol (mmol/L) | 181.5 [142.9, 212.4] | 223.9 [181.5, 278.0] | 202.3 [177.6, 239.4] | 200.8 [179.5, 219.3] |
| USG | 1.047 [1.036, 1.057]a | 1.018 [1.017, 1.024]b | 1.018 [1.015,1.028]b | 1.019 [1.016, 1.025]b |
CKD, chronic kidney disease; HT‐ocular‐TOD, hypertensive cats with evidence of ocular target organ damage; HT‐noTOD, hypertensive cats without evidence of ocular target organ damage; VEGF, vascular endothelial growth factor; NT‐proBNP, N‐terminal probrain natriuretic peptide; cTnI, cardiac troponin I; UPC, urine protein‐to‐creatinine ratio; SBP, systolic blood pressure; PCV, packed cell volume; USG, urine specific gravity.
Comparisons between groups were performed using a Kruskal–Wallis with posthoc Dunn's test or 1‐Way ANOVA with posthoc Tukey's test. Medians bearing the same superscript letter are not significantly different from one another. All values are presented as median [25th, 75th percentiles].
Vascular Endothelial Growth Factor, NT‐proBNP, cTnI, and UPC as Predictors of Hypertension and Ocular‐TOD
Univariable Analyses
Comparison among groups determined that HT‐ocular‐TOD had significantly higher plasma VEGF concentrations than did HT‐noTOD, CKD, and healthy cats (P < .05; Fig 1). The power calculation with the actual values indicated that 35 cats were needed per group to show a potential difference between HT‐ocular‐TOD and HT‐noTOD. A sample size of 58 per group would be needed to detect a decrease of 20% in response to treatment when grouping HT‐ocular‐TOD and HT‐noTOD, assuming a standard deviation (SD) of 46 on the change in VEGF with treatment (80% power with significance set at 5%). Univariable binary logistic regression indicated that log‐transformed plasma VEGF concentration was a positive predictor of hypertension (P < .05; Table 2). The ROC curve indicated that plasma VEGF could be used to predict hypertension (AUC, 0.64; P = .016) with an optimal value of >76.1 pg/mL (specificity, 72%; sensitivity, 59%). Concentrations of VEGF were found to be a positive and significant predictor of ocular‐TOD in the univariable analysis (P = .01; Table 3). The cutoff to diagnose ocular‐TOD was defined at >71.6 pg/mL (AUC, 0.73; sensitivity, 80%; specificity, 62.5%; P < .01).
Figure 1.

Plasma vascular endothelial growth factor (VEGF) concentration in healthy cats (n = 25), cats with chronic kidney disease (n = 25), hypertensive cats without evidence of hypertensive ocular target organ damage (HT‐noTOD) (n = 24), and hypertensive cats with evidence of hypertensive retinopathy (HT‐ocular‐TOD) (n = 25). HT‐ocular‐TOD had significantly higher plasma VEGF concentration than all other groups (P < .05 for all comparisons).
Table 2.
Univariable analysis of VEGF, NT‐proBNP, and cTnI as predictors of hypertension in cats
| Variable | n | β | SE | P‐Value | 95% CI for β |
|---|---|---|---|---|---|
| log(cTnI) | 99 | 0.814 | 0.237 | .0006 | 0.375 to 1.311 |
| log(NT‐proBNP) | 99 | 0.565 | 0.200 | .0046 | 0.190 to 0.978 |
| log(VEGF) | 99 | 0.843 | 0.351 | .0164 | 0.185 to 1.574 |
| log(UPC) | 50 | 0.310 | 0.429 | .4700 | −0.519 to 1.199 |
VEGF, vascular endothelial growth factor; NT‐proBNP, N‐terminal probrain natriuretic peptide; cTnI, cardiac troponin I; UPC, urine protein‐to‐creatinine ratio; n, number of cats; 95% CI, 95% confidence interval.
Log(cTnI), log(NT‐proBNP), and log(VEGF) were significant at the 20% level and included in the multivariable analysis.
Table 3.
Univariable analysis of VEGF, NT‐proBNP, and cTnI as predictors of TOD in cats
| Variable | n | β | SE | P‐Value | 95% CI for β |
|---|---|---|---|---|---|
| log(VEGF) | 49 | 1.405 | 0.546 | .01 | 0.426 to 2.594 |
| log(cTnI) | 49 | 0.770 | 0.350 | .028 | 0.136 to 1.528 |
| log(UPC) | 27 | 1.034 | 0.660 | .118 | −0.153 to 2.518 |
| log(NT‐proBNP) | 49 | 0.3853 | 0.279 | .167 | −0.145 to 0.966 |
VEGF, vascular endothelial growth factor; NT‐proBNP, N‐terminal probrain natriuretic peptide; cTnI, cardiac troponin I, UPC; urine protein‐to‐creatinine ratio; n, number of cats; 95% CI, 95% confidence interval.
All 4 variables were significant at the 20% level and included in the multivariable analysis.
Plasma NT‐proBNP concentration was found to be significantly higher in HT‐ocular‐TOD when compared with healthy cats (P < .001; Fig 2). Log‐transformed plasma NT‐proBNP concentration was a positive predictor of hypertension in the univariable analysis (P < .01; Table 2). The optimum cut‐point for NT‐proBNP to predict hypertension was >134.5 pmol/L (AUC, 0.68; sensitivity, 59%; specificity, 72%; P = .002). Log‐transformed NT‐proBNP was not a significant predictor of ocular‐TOD in the univariable analysis (P = .167; Table 3). The ROC had an AUC that was not significantly different from the line of zero discrimination (AUC = 0.64, P = .08). A cutoff point for using NT‐proBNP to predict ocular‐TOD therefore was not determined.
Figure 2.

Plasma N‐terminal probrain natriuretic peptide (NT‐proBNP) concentrations in healthy cats, (n = 25), cats with chronic kidney disease (n = 25), hypertensive cats without evidence of hypertensive ocular target organ damage (HT‐noTOD) (n = 24), and hypertensive cats with evidence of hypertensive retinopathy (HT‐ocular‐TOD) (n = 25). HT‐TOD had significantly higher plasma NT‐proBNP concentration than healthy cats (P < .001).
Plasma cTnI concentration was significantly lower in healthy cats when compared with HT‐ocular‐TOD, HT‐noTOD, and CKD (Fig 3). Log‐transformed cTnI was a positive significant predictor of hypertension in the univariable analysis (P < .001; Table 2). The ROC curve indicated that the cutoff value for cTnI to determine hypertension to be >0.045 ng/mL (AUC, 0.73; sensitivity, 63%; specificity, 78%; P < .0001). Plasma cTnI concentration was significantly associated with ocular‐TOD in the univariable analysis (P = .028; Table 3). The drawn ROC curve indicated that cTnI could be used to predict ocular‐TOD, with a cutoff value of >0.045 pg/mL (AUC = 0.71; sensitivity, 84%; specificity, 58%; P = .01).
Figure 3.

Plasma cardiac troponin I (cTnI) concentrations in healthy cats, (n = 25), cats with chronic kidney disease (n = 25), hypertensive cats without evidence of hypertensive ocular target organ damage (HT‐noTOD) (n = 24), and hypertensive cats with evidence of hypertensive retinopathy (HT‐ocular‐TOD) (n = 25). Healthy cats had significantly lower plasma cTnI concentrations than all other groups (P < .05).
The UPC was not found to be different among groups (Fig 4). Log‐transformed UPC was not a significant predictor of hypertension in the univariable analysis (P = .47; Table 2). The ROC for determination of hypertension therefore was not drawn. The UPC was associated with ocular‐TOD at the 20% level in the univariable analysis (P = .118; Table 3). However, the ROC curve indicated that UPC could not be used to predict ocular‐TOD (AUC = 0.67, P = .14).
Figure 4.
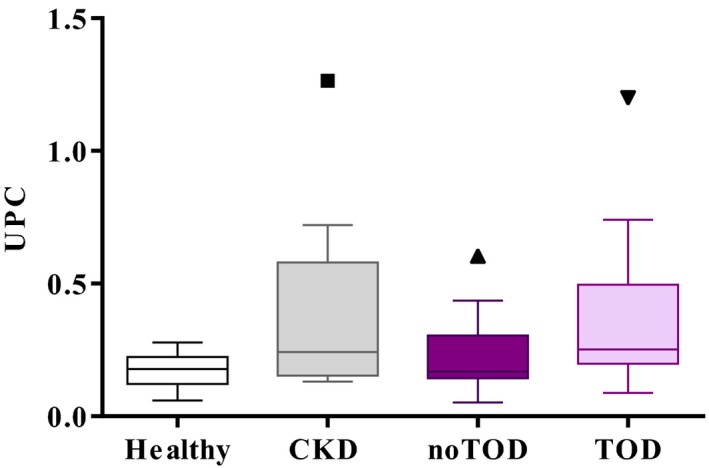
Urine protein‐to‐creatinine ratio (UPC) in healthy cats, (n = 13), cats with chronic kidney disease (n = 10), hypertensive cats without evidence of hypertensive ocular target organ damage (HT‐noTOD) (n = 14), and hypertensive cats with evidence of hypertensive retinopathy (HT‐ocular‐TOD) (n = 13). No significant differences were found between groups.
Multivariable Analyses
Plasma VEGF, NT‐proBNP, and cTnI concentrations all were predictive of hypertension at the 20% level in the univariable logistic regression and were included in the multivariable model. This indicated that log‐transformed plasma VEGF and cTnI concentrations remained significant and positive predictors of hypertension (Table 4). A 10‐fold increase in plasma VEGF concentration was associated with an odds ratio (OR) of 2.16 (95% confidence interval [CI], 1.06–4.65; P < .05), and a 10‐fold increase in plasma cTnI concentration had an OR of 2.38 (95% CI, 1.17–5.28; P < .05).
Table 4.
Multivariable analysis including VEGF, NT‐proBNP, and cTnI to identify independent predictors of hypertension in cats
| Variable | β | SE | P‐Value | 95% CI for β |
|---|---|---|---|---|
| log(cTnI) | 0.866 | 0.380 | .023 | 0.157 to 1.663 |
| log(VEGF) | 0.768 | 0.373 | .04 | 0.059 to 1.536 |
| log(NT‐proBNP) | −0.102 | 0.334 | .761 | −0.777 to 0.546 |
| (Intercept) | 0.006 | 2.970 | .998 | −5.815 to 5.958 |
cTnI, cardiac troponin I; NT‐proBNP, N‐terminal probrain natriuretic peptide; VEGF, vascular endothelial growth factor; n, number of cats; 95% CI, 95% confidence interval.
Both cTnI and VEGF remained independent positive predictors of hypertension in the multivariable analysis.
All 4 variables were significant at the 20% level to predict ocular‐TOD and therefore were included in the multivariable analysis. None of the variables remained significantly associated with ocular‐TOD (Table 5).
Table 5.
Multivariable analysis including VEGF, NT‐proBNP, and cTnI to identify independent predictors of TOD in cats
| Variable | β | SE | P‐Value | 95% CI for β |
|---|---|---|---|---|
| log(VEGF) | 1.520 | 0.794 | .056 | 0.155 to 3.389 |
| log(cTnI) | 1.526 | 0.970 | .115 | −0.150 to 3.768 |
| log(NT‐proBNP) | −0.424 | 0.736 | .564 | −1.956 to 1.038 |
| log(UPC) | 0.428 | 0.802 | .594 | −1.076 to 2.224 |
| (Intercept) | 0.113 | 6.688 | .987 | −13.96 to 13.25 |
VEGF, vascular endothelial growth factor; cTnI, cardiac troponin I; NT‐proBNP, N‐terminal probrain natriuretic peptide, UPC, urine protein‐to‐creatinine ratio; 95% CI, 95% confidence interval; TOD, target organ damage.
No independent positive or negative predictors of ocular‐TOD were found.
The ROC curves indicated that cTnI had the highest specificity for diagnosing hypertension (78%). The UPC could not reliably diagnose hypertension, and only combinations of cTnI, VEGF, and NT‐proBNP were explored. Using a combination of VEGF and either NT‐proBNP or cTnI, specificity of 94% could be achieved, with sensitivity of 37% and 45%, respectively. The specificity could be increased to 96% by incorporating all 3 variables, which was associated with a decrease in sensitivity to 31%.
Assessment of the ROC curves indicated that NT‐proBNP concentration and UPC could not reliably be used to diagnose ocular‐TOD, and therefore, only the combination of VEGF and cTnI was explored for this purpose. Plasma cTnI concentration >0.045 pg/mL in combination with plasma VEGF concentration >71.6 pg/mL had a specificity of 88% to diagnose ocular‐TOD; sensitivity was 76%.
Changes in VEGF, NT‐proBNP, and cTnI With Treatment of Hypertension
Cats with HT‐ocular‐TOD had received amlodipine for 20 ± 8 days, and HT‐noTOD for 25 ± 15 days at the first controlled visit. The third visit was 141 ± 43 days after starting treatment for the HT‐ocular‐TOD group and 157 ± 42 days for the HT‐noTOD group. At the diagnosis of hypertension, 20 cats in the HT‐ocular‐TOD group and 16 cats in the HT‐noTOD group had concurrent CKD (P = .35). Plasma creatinine concentration did not significantly change over the course of 5 months (P = .58), and no cats showed evidence of CKD progression (defined as a >25% increase in plasma creatinine concentration).
At the hypertensive visit, median plasma VEGF concentration in the HT‐ocular‐TOD group was 105.6 [77.30, 142.5] pg/mL. At the first controlled visit, the median concentration was 91.0 [64.2, 162.7] pg/mL, and at visit 3, the concentration was 86.2 [64.0, 124.7] pg/mL. No significant change over time was found (P = .07). The VEGF concentration for the HT‐noTOD group was 53.3 [39.9, 106.6] pg/mL at baseline, and 62.4 [45.8, 105.3] pg/mL at the first controlled visit, followed by a concentration of 54.3 [38.6, 105.3] at visit 3 (not significant, P = .19).
Because of financial considerations, NT‐proBNP concentrations from both the hypertensive visit and the first controlled visit were determined initially, before making a decision with regard to measuring the sample concentration of a visit after approximately 5 months of amlodipine treatment. In HT‐ocular‐TOD, NT‐proBNP concentration decreased significantly with approximately 2 weeks of amlodipine treatment, from 236.0 [114.5, 519.0] pmol/L to 94.0 [56.0, 245.5] pmol/L (P < .001). The NT‐proBNP concentration also significantly decreased in the HT‐noTOD‐group, from 120.0 [75.0, 289.0] pmol/L to 78.0 [55.0, 192.8] pmol/L (P < .001; Fig 5). Because NT‐proBNP showed a significant decrease at the first controlled visit, this marker was not measured after 5 months of antihypertensive treatment.
Figure 5.
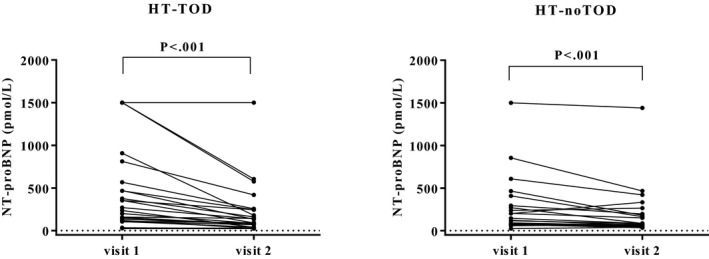
Plasma N‐terminal probrain natriuretic peptide (NT‐proBNP) in hypertensive cats at visit hypertension was diagnosed (visit 1) and first controlled visit on amlodipine treatment (defined as systolic blood pressure below 160 mmHg) (visit 2; for HT‐ocular‐TOD after 20 ± 8 days and HT‐noTOD 25 ± 15 days). The median NT‐proBNP concentration decreased by 60% in cats with evidence of hypertensive ocular target organ damage (HT‐ocular‐TOD) and by 35% in cats without evidence of hypertensive retinopathy (HT‐noTOD).
Plasma cTnI concentration did not significantly change with antihypertensive treatment in either group (HT‐ocular‐TOD: hypertensive visit, 0.07 [0.05, 0.14] ng/mL; first controlled visit, 0.06 [0.04, 0.12] ng/mL; after approximately 5 months of treatment, 0.07 [0.05, 0.13] ng/mL; P = .25); HT‐noTOD: hypertensive visit, 0.04 [0.02, 0.06] ng/mL; first controlled visit, 0.04 [0.03, 0.11] ng/mL; after approximately 5 months of treatment, 0.05 [0.03, 0.08] ng/mL; P = .48).
Discussion
Plasma VEGF and cTnI concentrations were found to be independent predictors of hypertension. Plasma NT‐proBNP decreased significantly with antihypertensive treatment. These results seem promising for the use of these markers for the diagnosis and management of hypertension, but the ROC curves indicated that all markers evaluated had fairly low sensitivity. Additionally, all groups had substantial overlap in VEGF, NT‐proBNP, and cTnI concentrations, making the markers less able to differentiate among groups. The power calculation that was performed using the VEGF concentrations indicated that a larger sample size could have led to significant results. The cats included in the current study were diagnosed with hypertension based on either high SBP with evidence of ocular‐TOD or had persistently high SBPs >170 mmHg. The groups were chosen in such a way to be able to identify markers of definitive hypertension and ocular‐TOD.2 The repeatedly high SBP in some cats without ocular‐TOD may or may not have been because of white coat hypertension, which is a limitation that is challenging to manage. Use of biomarkers would therefore be an attractive alternative to the current way of diagnosing hypertension by repeated blood pressure measurements.
The UPC previously has been shown to be increased in hypertensive cats.7 Additionally, UPC can be increased in CKD and is a predictor of CKD progression.6 The fact that most of the hypertensive cats included in the current study had concurrent CKD therefore may be an explanation for why this marker was not found to be a significant predictor of hypertension or ocular‐TOD. Only half of the included cats had a palpable bladder and therefore a urine sample available for UPC measurement. The smaller sample size therefore also could explain the lack of significance. However, the UPC distribution was fairly atypical, with 25% of normotensive CKD cases having UPC of 0.58, and 25% of healthy cats having UPC ≥0.22. Why this distribution occurred in the current group is unclear, but possibly because of the low number of cats. None of the included cases had urinary tract infection.
One consideration is the speed with which the concentrations of these markers change in response to increases in blood pressure in cats, which currently is unknown.20 The rate of elimination of the biomarkers also must be considered, especially because many cats included in this study had concurrent CKD, which may decrease the clearance of VEGF,21 NT‐proBNP,14, 22, 23 and cTnI.24, 25 For this reason, both healthy and CKD cats were included as control groups. Increased plasma VEGF concentrations have been reported in human patients with CKD when compared to healthy controls, and concentrations were higher with more advanced CKD.26, 27 In our study, large overlap was found among plasma VEGF concentrations of hypertensive cats, healthy cats, and cats with CKD, but cats with CKD did not have significantly higher plasma VEGF concentrations compared to healthy cats. The concentration of NT‐proBNP also has been reported to increase with decreasing glomerular filtration rate (GFR) in some species.28 Cats with CKD included in our study did not have significantly higher NT‐proBNP concentrations than did healthy cats, which conflicts with previously published results.14 However, in the previous study, the cats with CKD were in more advanced International Renal Interest Society (IRIS) stages. The majority of cats in our study were diagnosed in IRIS stage 2, which could explain the absence of significant differences among the groups. The cTnI concentrations were higher in CKD, HT‐ocular‐TOD, and HT‐noTOD cats when compared to the healthy group. This difference is likely to be because of underlying CKD in the hypertensive cats. Previous studies that evaluated cTnI in cats with CKD have reported concentrations above the reference interval.29 Human patients with renal failure often have increased cTnI concentrations.30 However, it is possible that cats with CKD, like human patients, develop concurrent heart disease or suffer from uremic damage to the heart, and whether the increase found in our study is caused by increased production or decreased excretion warrants further research.
It seems unlikely that any of the biomarkers studied would change measurably because of the transient increases in blood pressure that occur during a visit to the clinic. Increases in VEGF occur rapidly in response to stretch, deformation, and shear stress in cell culture,31 but take up to 3 days to increase in animals with hypertension.32, 33 Brain natriuretic peptide (BNP) gene expression and mRNA levels increase rapidly (within 2 hours) with stretch and pressure overload, and BNP gene expression increases after 2 hours of stretch in mouse cardiac tissue specimens.34 The N‐terminal fragment of BNP (NT‐proBNP) is spliced off after BNP secretion, and, because elimination of NT‐proBNP is much slower, it is considered a more suitable assessment method for change in BNP in clinical patients. Because of the slow elimination, the concentration in the blood will reflect prevailing concentrations over a much longer period than the active hormone.35 When inducing necrosis in cell culture studies, cTnI release does not occur until the cardiomyocytes are irreversibly damaged (after >18 hours), whereas in the reversible phase, the concentrations do not increase.36 A few studies have described cTnI release from viable cardiomyocytes in response to stretch, indicating that myocardial strain rather than cell injury could lead to cTnI increases.37 Overall, however, the evidence suggests that increases in cTnI do not occur rapidly with transient increases in SBP.
In our study, VEGF appeared to hold the greatest promise as a marker of ocular‐TOD caused by hypertension, but the marker did not prove to be significant in the multivariable analysis. Plasma VEGF concentration was not increased in cats that were diagnosed with hypertension in the absence of ocular‐TOD. Vascular endothelial growth factor has been proposed as a marker for hypertension in humans, but it is not yet clear whether it is a marker of, or a player in, the development of hypertension. Hypertensive human patients have increased plasma VEGF concentrations compared to normotensive controls.10, 12, 38, 39 Plasma VEGF concentrations may increase with increasing pressure, and the development of ocular‐TOD could simply be caused by higher pressure in the arteries and arterioles of target organs. However, the SBP of cats without ocular‐TOD was not significantly lower than the SBP of cats with ocular‐TOD. It therefore seems more likely that those cats with ocular‐TOD have a different pathophysiologic response to increased pressure than cats without ocular‐TOD or that the underlying pathophysiology for the development of hypertension is different. Considering the edematous ocular lesions often seen in hypertensive cats,2 a permeability factor like VEGF may play a role in the development of retinopathy and choroidopathy. However, if a systemic response was damaging to the retina, the damage is likely also to occur in other target organs, such as the kidney, and cats with hypertensive ocular‐TOD did not have significantly higher plasma creatinine concentrations or UPC than those without. It therefore also is possible that certain cats in the HT‐noTOD group were incorrectly classified and that both groups suffered similar hypertensive damage to their kidneys.
If deranged angiogenesis underlies the hypertension observed in cats with ocular‐TOD, it could be accompanied by increased plasma VEGF concentrations.40 In human patients, VEGF has been reported to decrease with a comparable duration of antihypertensive treatment as that studied here.38 Plasma VEGF concentration did not decrease with effective antihypertensive treatment in the cats in our study, something that would have been expected to happen if VEGF was increased as a physiologic response. This observation could support a role for VEGF in the causation of hypertensive ocular‐TOD in cats. Additional studies including a larger number of cats, both before and after they develop hypertension with and without ocular‐TOD, are required to investigate this hypothesis.
Our study is the second to assess NT‐proBNP concentrations in hypertensive cats.14 Other studies published to date have evaluated NT‐proBNP as a biomarker of myocardial disease,41 and for differentiation of cardiac and noncardiac causes of respiratory distress.41 The concentration of NT‐proBNP was not found to be an independent predictor of hypertension or ocular‐TOD in the cats in our study, although many cats had increased concentrations, including in the control groups. This finding could be a result of subclinical heart disease, which is very prevalent in the feline population.42, 43 Up to 16% of cats have subclinical cardiomyopathy,44 and therefore, exclusion of cats with evidence of heart disease may have introduced bias.45 Furthermore, echocardiography cannot differentiate between primary heart disease and secondary hypertrophy because of hypertension. Additionally, not having the opportunity to perform echocardiography on all cats is representative of general practice. It was therefore decided to keep all cats, including those with evidence of severe hypertrophy on echocardiographic assessment, with or without an increased NT‐proBNP concentration, in the study. Additionally, thyroid status was assessed in all cats based on history and clinical findings, but was not always based on TT4 measurement. An important consideration in clinical practice is that NT‐proBNP concentrations are significantly increased in hyperthyroidism.46, 47
Limitations of our study that must be recognized are that cats with primary cardiac disease with or without occult hyperthyroidism could have been included. Target organ damage was determined by fundic examination. However, the eye is not the only organ susceptible to hypertensive damage. The kidneys, brain, and heart also are possible targets, most cats included in the HT‐noTOD group had CKD, and 3 cats had evidence of severe hypertrophy on echocardiography. It is not possible to differentiate hypertrophy as a consequence of primary cardiac disease versus that secondary to the effects of systemic hypertension, and therefore, the cardiac changes identified on echocardiography may have reflected TOD. For this reason, 2 separate analyses were performed—1 with the hypertensive cats grouped together and 1 with the ocular‐TOD cats separately.
A few studies describe NT‐proBNP as a marker of TOD in human medicine.4, 48 Hypertensive cats with ocular‐TOD had significantly higher plasma concentrations NT‐proBNP than did healthy cats, but HT‐ocular‐TOD cats did not have significantly different NT‐proBNP from HT‐noTOD. Studies of humans have reported similar results when comparing BNP and NT‐proBNP in patients with hypertension and TOD to controls.49 The finding that HT‐noTOD and HT‐ocular‐TOD did not differ significantly is in contrast with results previously presented,15 and might be explained by the absence of a difference in SBP between the 2 groups included in our study. The concentration of NT‐proBNP significantly decreased in both hypertensive groups with antihypertensive treatment, suggesting that it may be useful to assess the efficacy of treatment. Studies following cats over a longer period of time and comparing animals in different SBP categories over time would be a useful next step.
Cardiac troponins (cTns) historically have been accepted as markers for cardiac injury and myocardial cell necrosis.36, 50 In cats included in our study, cTnI was an independent predictor of hypertension, but not of ocular‐TOD. With treatment of hypertension, cTnI did not decrease, which indicates that it is less useful for assessing the effect of treatment. The human medical literature currently is still confusing, with most studies reporting increases in cTnI in hypertensive patients,19, 51, 52 but a recent study reported decreased cTnI concentrations in hypertensive HCM patients.53 Increased SBP can lead to cardiac remodeling, with initial hypertrophy, followed by apoptotic loss of cardiomyocytes and fibrosis.54 Hypertension may lead to some cardiomyocyte damage and necrosis, but the majority of cells may not have a severely damaged sarcolemma, and therefore, release of cTnI may occur in an alternative manner. Indeed, cell culture studies have confirmed that stimulation of integrins (mechanotransducing molecules in cardiomyocytes) leads to the release of intact cTnI,37 and excised rat hearts put under increased pressure secrete cTnI products independent of ischemia.55 Stretch of cardiomyocytes therefore may induce cTnI release, which may explain why, in our study, cTnI was identified as an independent predictor of hypertension. Despite cTnI being an independent predictor of hypertension, the hypertensive cats did not have significantly higher cTnI concentrations than did the CKD cats. Most hypertensive cats (36 of 49) had underlying CKD, and damage to the heart caused by CKD is a potential explanation for the higher concentrations found in all groups when compared to healthy cats. Additionally, effective antihypertensive treatment did not decrease plasma cTnI concentration. Cardiac troponin I has a long plasma half‐life,16, 56 but hyperthyroid cats undergoing radioactive iodine treatment have decreased cTnI concentrations 3 months after treatment, indicating that the lack of a decrease at the 5‐month time point in our study cannot be explained by the long half‐life of cTnI.46, 57 This observation strengthens the hypothesis that cTnI is higher in all groups when compared to healthy cats because of underlying CKD. Because CKD is a risk factor for the development of hypertension,58 the association between CKD and cTnI may have resulted in the predictive relationship between cTnI and hypertension.
The ultimate aim of identifying biomarkers would be to develop a diagnostic test that can help veterinarians in the challenging diagnosis of hypertension in the absence of ocular‐TOD. When assessing the ROC curves (sensitivity and specificity), none of the biomarkers alone met the criteria to diagnose hypertension.59, 60 When combining all 3 potential markers with an AUC that was significantly different from the line of zero discrimination, using a cutoff for VEGF of 76.1 pg/mL, NT‐proBNP of 134.5 pmol/L, and cTnI of 0.045 ng/mL, the specificity can be increased to 96%. This is however associated with a sensitivity of 31%. A combination of cTnI and VEGF has a specificity 88% and sensitivity of 76% to diagnose hypertensive ocular‐TOD. One cannot argue in favor of replacing a noninvasive method (retinal examination) with an invasive blood test with a lower sensitivity and specificity. This indicates that, although associated with blood pressure, hypertension, and ocular‐TOD, these biomarkers are unlikely to be of clinical utility in the differentiation of white coat from true hypertension when no ocular‐TOD is present.
In conclusion, plasma VEGF and cTnI concentrations are independent predictors of hypertension. Vascular endothelial growth factor may play a role in the development of hypertension or ocular‐TOD associated with hypertension, and further research is warranted. Both plasma cTnI and VEGF concentrations may be indicative of less reversible tissue injury, and neither factor decreased with antihypertensive treatment. Plasma NT‐proBNP concentration possibly could be used to assess efficacy of antihypertensive treatment. However, none of the biomarkers performed sufficiently well in predicting ocular‐TOD to serve as a clinical test for hypertension or ocular‐TOD.
Acknowledgments
Grant support: This study was supported by Zoetis Ltd.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This study was performed at the Royal Veterinary College, London, UK.
Results of this study were presented at the ECVIM Congress 2015, Lisbon, Portugal.
Footnotes
Parks Electronic Doppler Model 811B, Perimed UK, Bury St Edmunds, UK
Mistral 3000, Sanyo‐Gallenkamp, Leicestershire, UK
IDEXX Laboratories, Wetherby, Yorkshire, UK
Istin, Pfizer, Kent, UK
Canine VEGF Quantikine ELISA, R&D systems, Minneapolis, USA
GraphPad Prism version 6 for Windows, GraphPad Software, La Jolla, California, USA and R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
References
- 1. Belew AM, Barlett T, Brown SA. Evaluation of the white‐coat effect in cats. J Vet Intern Med 1999;13:134–142. [DOI] [PubMed] [Google Scholar]
- 2. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007;21:542–558. [DOI] [PubMed] [Google Scholar]
- 3. Belgore FM, Blann AD, Li‐Saw‐Hee FL, et al. Plasma levels of vascular endothelial growth factor and its soluble receptor (SFlt‐1) in essential hypertension. Am J Cardiol 2001;87:805–807, A809. [DOI] [PubMed] [Google Scholar]
- 4. Hoshide S, Kario K, Yano Y, et al. Association of morning and evening blood pressure at home with asymptomatic organ damage in the J‐HOP Study. Am J Hypertens 2014;27:939–947. [DOI] [PubMed] [Google Scholar]
- 5. Jepson RE, Brodbelt D, Vallance C, et al. Evaluation of predictors of the development of azotemia in cats. J Vet Intern Med 2009;23:806–813. [DOI] [PubMed] [Google Scholar]
- 6. Chakrabarti S, Syme HM, Elliott J. Clinicopathological variables predicting progression of azotemia in cats with chronic kidney disease. J Vet Intern Med 2012;26:275–281. [DOI] [PubMed] [Google Scholar]
- 7. Jepson RE, Syme HM, Elliott J. Plasma renin activity and aldosterone concentrations in hypertensive cats with and without azotemia and in response to treatment with amlodipine besylate. J Vet Intern Med 2014;28:144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. dela Paz NG, Walshe TE, Leach LL, et al. Role of shear‐stress‐induced VEGF expression in endothelial cell survival. J Cell Sci 2012;125:831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feng Y, Yang JH, Huang H, et al. Transcriptional profile of mechanically induced genes in human vascular smooth muscle cells. Circ Res 1999;85:1118–1123. [DOI] [PubMed] [Google Scholar]
- 10. Nadar SK, Blann A, Beevers DG, et al. Abnormal angiopoietins 1&2, angiopoietin receptor Tie‐2 and vascular endothelial growth factor levels in hypertension: Relationship to target organ damage [a sub‐study of the Anglo‐Scandinavian Cardiac Outcomes Trial (ASCOT)]. J Intern Med 2005;258:336–343. [DOI] [PubMed] [Google Scholar]
- 11. Tsai WC, Li YH, Huang YY, et al. Plasma vascular endothelial growth factor as a marker for early vascular damage in hypertension. Clin Sci 2005;109:39–43. [DOI] [PubMed] [Google Scholar]
- 12. Nadar SK, Blann AD, Lip GY. Plasma and platelet‐derived vascular endothelial growth factor and angiopoietin‐1 in hypertension: Effects of antihypertensive therapy. J Intern Med 2004;256:331–337. [DOI] [PubMed] [Google Scholar]
- 13. Andreadis EA, Georgiopoulos DX, Tzavara CK, et al. Plasma brain natriuretic peptide: A biochemical marker of effective blood pressure management? J Hypertens 2009;27:425–432. [DOI] [PubMed] [Google Scholar]
- 14. Lalor SM, Connolly DJ, Elliott J, et al. Plasma concentrations of natriuretic peptides in normal cats and normotensive and hypertensive cats with chronic kidney disease. J Vet Cardiol 2009;11(Suppl. 1):S71–S79. [DOI] [PubMed] [Google Scholar]
- 15. Lalor SM, Elliott J, Syme HM. Do cats with hypertension but no ocular lesions have increases in plasma NT‐proBNP concentration? (Abstract). J Vet Intern Med 2010;24:679. [Google Scholar]
- 16. Adams JE 3rd, Bodor GS, Davila‐Roman VG, et al. Cardiac troponin I. A marker with high specificity for cardiac injury. Circulation 1993;88:101–106. [DOI] [PubMed] [Google Scholar]
- 17. Sleeper MM, Clifford CA, Laster LL. Cardiac troponin I in the normal dog and cat. J Vet Intern Med 2001;15:501–503. [DOI] [PubMed] [Google Scholar]
- 18. Borgeat K, Sherwood K, Payne JR, et al. Plasma cardiac troponin I concentration and cardiac death in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2014;28:1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Afonso L, Bandaru H, Rathod A, et al. Prevalence, determinants, and clinical significance of cardiac troponin‐I elevation in individuals admitted for a hypertensive emergency. J Clin Hypertens 2011;13:551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mata‐Greenwood E, Grobe A, Kumar S, et al. Cyclic stretch increases VEGF expression in pulmonary arterial smooth muscle cells via TGF‐beta1 and reactive oxygen species: A requirement for NAD(P)H oxidase. Am J Physiol Lung Cell Mol Physiol 2005;289:L288–L289. [DOI] [PubMed] [Google Scholar]
- 21. Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int 2004;65:2003–2017. [DOI] [PubMed] [Google Scholar]
- 22. Vickery S, Price CP, John RI, et al. B‐type natriuretic peptide (BNP) and amino‐terminal proBNP in patients with CKD: Relationship to renal function and left ventricular hypertrophy. Am J Kidney Dis 2005;46:610–620. [DOI] [PubMed] [Google Scholar]
- 23. Srisawasdi P, Vanavanan S, Charoenpanichkit C, et al. The effect of renal dysfunction on BNP, NT‐proBNP, and their ratio. Am J Clin Pathol 2010;133:14–23. [DOI] [PubMed] [Google Scholar]
- 24. deFilippi C, Seliger SL, Kelley W, et al. Interpreting cardiac troponin results from high‐sensitivity assays in chronic kidney disease without acute coronary syndrome. Clin Chem 2012;58:1342–1351. [DOI] [PubMed] [Google Scholar]
- 25. Kraus MS, Kaufer BB, Damiani A, et al. Elimination half‐life of intravenously administered equine cardiac troponin I in healthy ponies. Equine Vet J 2013;45:56–59. [DOI] [PubMed] [Google Scholar]
- 26. Harper SJ, Downs L, Tomson CR, et al. Elevated plasma vascular endothelial growth factor levels in non‐diabetic predialysis uraemia. Nephron 2002;90:341–343. [DOI] [PubMed] [Google Scholar]
- 27. Chen YT, Cheng BC, Ko SF, et al. Value and level of circulating endothelial progenitor cells, angiogenesis factors and mononuclear cell apoptosis in patients with chronic kidney disease. Clin Exp Nephrol 2013;17:83–91. [DOI] [PubMed] [Google Scholar]
- 28. Tagore R, Ling LH, Yang H, et al. Natriuretic peptides in chronic kidney disease. Clin J Am Soc Nephrol 2008;3:1644–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Porciello F, Rishniw M, Herndon WE, et al. Cardiac troponin I is elevated in dogs and cats with azotaemia renal failure and in dogs with non‐cardiac systemic disease. Aust Vet J 2008;86:390–394. [DOI] [PubMed] [Google Scholar]
- 30. Bjurman C, Petzold M, Venge P, et al. High‐sensitive cardiac troponin, NT‐proBNP, hFABP and copeptin levels in relation to glomerular filtration rates and a medical record of cardiovascular disease. Clin Biochem 2015;48:302–307. [DOI] [PubMed] [Google Scholar]
- 31. Haga JH, Li YS, Chien S. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomech 2007;40:947–960. [DOI] [PubMed] [Google Scholar]
- 32. Kuwahara F, Kai H, Tokuda K, et al. Hypoxia‐inducible factor‐1alpha/vascular endothelial growth factor pathway for adventitial vasa vasorum formation in hypertensive rat aorta. Hypertension 2002;39:46–50. [DOI] [PubMed] [Google Scholar]
- 33. Suzuma I, Hata Y, Clermont A, et al. Cyclic stretch and hypertension induce retinal expression of vascular endothelial growth factor and vascular endothelial growth factor receptor‐2: Potential mechanisms for exacerbation of diabetic retinopathy by hypertension. Diabetes 2001;50:444–454. [DOI] [PubMed] [Google Scholar]
- 34. Raskin AM, Hoshijima M, Swanson E, et al. Hypertrophic gene expression induced by chronic stretch of excised mouse heart muscle. Mol Cell Biomech 2009;6:145–159. [PMC free article] [PubMed] [Google Scholar]
- 35. Sisson DD. Neuroendocrine evaluation of cardiac disease. Vet Clin North Am Small Anim Pract 2004;34:1105–1126. [DOI] [PubMed] [Google Scholar]
- 36. Hessel MH, Michielsen EC, Atsma DE, et al. Release kinetics of intact and degraded troponin I and T after irreversible cell damage. Exp Mol Pathol 2008;85:90–95. [DOI] [PubMed] [Google Scholar]
- 37. Hessel MH, Atsma DE, van der Valk EJ, et al. Release of cardiac troponin I from viable cardiomyocytes is mediated by integrin stimulation. Pflugers Arch 2008;455:979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Felmeden DC, Spencer CG, Belgore FM, et al. Endothelial damage and angiogenesis in hypertensive patients: Relationship to cardiovascular risk factors and risk factor management. Am J Hypertens 2003;16:11–20. [DOI] [PubMed] [Google Scholar]
- 39. Stumpf C, Jukic J, Yilmaz A, et al. Elevated VEGF‐plasma levels in young patients with mild essential hypertension. Eur J Clin Invest 2009;39:31–36. [DOI] [PubMed] [Google Scholar]
- 40. Sane DC, Anton L, Brosnihan KB. Angiogenic growth factors and hypertension. Angiogenesis 2004;7:193–201. [DOI] [PubMed] [Google Scholar]
- 41. Connolly DJ. Natriuretic peptides: The feline experience. Vet Clin North Am Small Anim Pract 2010;40:559–570. [DOI] [PubMed] [Google Scholar]
- 42. Wagner T, Fuentes VL, Payne JR, et al. Comparison of auscultatory and echocardiographic findings in healthy adult cats. J Vet Cardiol 2010;12:171–182. [DOI] [PubMed] [Google Scholar]
- 43. Nakamura RK, Rishniw M, King MK, et al. Prevalence of echocardiographic evidence of cardiac disease in apparently healthy cats with murmurs. J Feline Med Surg 2011;13:266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paige CF, Abbott JA, Elvinger F, et al. Prevalence of cardiomyopathy in apparently healthy cats. J Am Vet Med Assoc 2009;234:1398–1403. [DOI] [PubMed] [Google Scholar]
- 45. Berger ED, Bader BD, Ebert C, et al. Reduction of proteinuria; combined effects of receptor blockade and low dose angiotensin‐converting enzyme inhibition. J Hypertens 2002;20:739–743. [DOI] [PubMed] [Google Scholar]
- 46. Sangster JK, Panciera DL, Abbott JA, et al. Cardiac biomarkers in hyperthyroid cats. J Vet Intern Med 2014;28:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Menaut P, Connolly DJ, Volk A, et al. Circulating natriuretic peptide concentrations in hyperthyroid cats. J Small Anim Pract 2012;53:673–678. [DOI] [PubMed] [Google Scholar]
- 48. Svensson P, de Faire U, Niklasson U, et al. Plasma NT‐proBNP concentration is related to ambulatory pulse pressure in peripheral arterial disease. Blood Press 2005;14:99–106. [DOI] [PubMed] [Google Scholar]
- 49. Bettencourt P, Ferreira A, Sousa T, et al. Brain natriuretic peptide as a marker of cardiac involvement in hypertension. Int J Cardiol 1999;69:169–177. [DOI] [PubMed] [Google Scholar]
- 50. Wells SM, Sleeper MM. Cardiac troponins. J Vet Emerg Crit Care 2008;18:235–245. [Google Scholar]
- 51. Shafi T, Zager PG, Sozio SM, et al. Troponin I and NT‐proBNP and the association of systolic blood pressure with outcomes in incident hemodialysis patients: The Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. Am J Kidney Dis 2014;64:443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sato Y, Yamamoto E, Sawa T, et al. High‐sensitivity cardiac troponin T in essential hypertension. J Cardiol 2011;58:226–231. [DOI] [PubMed] [Google Scholar]
- 53. Zhang C, Liu R, Yuan J, et al. Significance and determinants of cardiac troponin I in patients with obstructive hypertrophic cardiomyopathy. Am J Cardiol 2015;116:1744–1751. [DOI] [PubMed] [Google Scholar]
- 54. Fortuno MA, Ravassa S, Fortuno A, et al. Cardiomyocyte apoptotic cell death in arterial hypertension: Mechanisms and potential management. Hypertension 2001;38:1406–1412. [DOI] [PubMed] [Google Scholar]
- 55. Feng J, Schaus BJ, Fallavollita JA, et al. Preload induces troponin I degradation independently of myocardial ischemia. Circulation 2001;103:2035–2037. [DOI] [PubMed] [Google Scholar]
- 56. Langhorn R, Persson F, Ablad B, et al. Myocardial injury in dogs with snake envenomation and its relation to systemic inflammation. J Vet Emerg Crit Care (San Antonio) 2014;24:174–181. [DOI] [PubMed] [Google Scholar]
- 57. Connolly DJ, Guitian J, Boswood A, et al. Serum troponin I levels in hyperthyroid cats before and after treatment with radioactive iodine. J Feline Med Surg 2005;7:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bijsmans ES, Jepson RE, Chang YM, et al. Changes in systolic blood pressure over time in healthy cats and cats with chronic kidney disease. J Vet Intern Med 2015;29:855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Altman DG, Bland JM. Diagnostic tests 3: Receiver operating characteristic plots. BMJ 1994;309:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Soreide K. Receiver‐operating characteristic curve analysis in diagnostic, prognostic and predictive biomarker research. J Clin Pathol 2009;62:1–5. [DOI] [PubMed] [Google Scholar]


