Abstract
Background
Reductions in N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) concentrations after treatment have been associated with improved survival in people with congestive heart failure (CHF), but have not been reported in cats with CHF.
Objectives
To evaluate changes in NT‐proBNP concentrations in cats with CHF after treatment and determine whether serial NT‐proBNP measurements provide prognostic information.
Animals
Thirty‐one client‐owned cats.
Methods
Prospective, observational study in cats with new onset CHF secondary to cardiomyopathy. Concentrations of NT‐proBNP were measured within 4 hours of admission to the hospital, on the day of discharge, and at re‐evaluation 7–10 days later.
Results
Median NT‐proBNP concentrations decreased significantly from admission (1,713 pmol/L [range, 160–3,784 pmol/L]) to discharge (902 pmol/L [range, 147–3,223 pmol/L]); P = .005) and from admission to re‐evaluation (1,124 pmol/L [range, 111–2,727 pmol/L]; P = .024). Median survival time was 109 days (range, 1–709 days), with 5 cats still alive at the time of analysis. Cats with a larger percent decrease in NT‐proBNP from admission to discharge had a longer survival time (P = .048). Cats with evidence of active CHF at the time of re‐evaluation (P = .010) and cats whose owners had difficulty administering medications (P = .045) had shorter survival times.
Conclusions and clinical importance
Cats with a larger percent decrease in NT‐proBNP during hospitalization and no evidence of CHF at the time of re‐evaluation had longer survival times. Additional studies are needed to determine whether NT‐proBNP can help guide treatment in cats with CHF.
Keywords: Biomarker, Cardiomyopathy, Feline, Prognosis
Abbreviations
- ATE
arterial thromboembolism
- BNP
B‐type natriuretic peptide
- CATCH
Cats’ Assessment Tool for Cardiac Health
- CHF
congestive heart failure
- DCM
dilated cardiomyopathy
- HCM
hypertrophic cardiomyopathy
- NT‐proBNP
N‐terminal pro‐B‐type natriuretic peptide
N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) is a stable, circulating peptide, which is cleaved from prohormone, proBNP that is synthesized and released from cardiomyocytes in the atria and ventricles in response to stretch.1 The degree of release is directly correlated with the severity of cardiac disease, making NT‐proBNP an important part of the assessment of human and veterinary patients with chronic heart disease.2, 3, 4, 5 In people with congestive heart failure (CHF), a decrease in NT‐proBNP or lower absolute NT‐proBNP concentrations have been correlated with a more favorable outcome.6, 7 Studies in humans have evaluated NT‐proBNP‐guided management of heart failure and found that care supported by BNP/NT‐proBNP‐guided therapy as an adjunct to standard treatment results in better outcomes (i.e, lower rate of adverse events).6, 7, 8
Single‐time measurement of NT‐proBNP has a wide array of clinical applications in veterinary medicine, including the prediction of first onset CHF in dogs with degenerative mitral valve disease,9 prognostication in dogs with acquired cardiac disease,10 differentiation between cardiac and noncardiac causes of respiratory signs in cats11 and dogs,12, 13, 14 detection of occult cardiomyopathy in cats15, 16 and screening for occult dilated cardiomyopathy (DCM) in Doberman pinschers.17, 18 In addition, changes in NT‐proBNP concentrations have been investigated in 1 study in dogs with degenerative mitral valve disease and CHF, although changes were not associated with survival, dogs with NT‐proBNP concentrations <965 pmol/L at the time of re‐evaluation had a longer survival time.19
To the authors’ knowledge, investigation of a relationship between changes in NT‐proBNP concentrations and outcome has not been reported in cats with CHF. An association between changes in NT‐proBNP and survival could provide useful prognostic information in cats with CHF. Therefore, the objective of this study was to evaluate changes in NT‐proBNP concentrations after treatment in cats with CHF and to determine whether serial NT‐proBNP measurements are associated with survival time.
Materials and Methods
Client‐owned cats >1 year of age presented to the Cummings Veterinary Medical Center at Tufts University with new onset CHF secondary to cardiomyopathy between March 2014 and February 2016 were eligible for the study. The Cummings Clinical Studies Review Committee approved the study, and all owners signed an informed consent form before enrollment.
The diagnosis of CHF was based on the presence of pleural effusion or pulmonary edema confirmed via thoracic radiography or thoracic ultrasound, in combination with a diagnosis of cardiomyopathy based on echocardiography. Cats were excluded if systemic hypertension (systolic blood pressure >180 mmHg) or hyperthyroidism was present, or if the blood urea nitrogen was greater than 50 mg/dL or creatinine was greater than 2.8 mg/dL. Cats also were excluded if blood for NT‐proBNP concentrations was not obtained within 4 hours of admission to the hospital or if they died or were euthanized before discharge from the hospital.
Within 4 hours of admission to the hospital for a first‐time episode of CHF, 4 mL of blood was collected by venipuncture under minimal restraint. Blood for a biochemistry profile and T4 (for cats >6 years of age) was submitted for analysis immediately.1,2 Blood for NT‐proBNP concentrations was centrifuged, and the serum was stored at −80°C until analysis. All NT‐proBNP samples were analyzed as a single batch at a commercial laboratory using a validated second generation feline enzyme‐linked immunosorbent assay.20 , 3 Cats were not treated according to a standardized protocol, but according to the clinical judgment of the clinician managing the case. Although not part of the study protocol, all cats had an electrocardiogram or telemetry performed during hospitalization. Body condition score was recorded on a 9‐point scale, and muscle condition score was recorded as normal, mild muscle loss, moderate muscle loss, or severe muscle loss. All cats had an echocardiogram.4 A board‐certified cardiologist or resident under the supervision of a board‐certified cardiologist obtained standard right and left parasternal echocardiographic views with standard echocardiographic measurements.21 A diagnosis of HCM was made if the interventricular septum or left ventricular free wall in diastole was >0.6 cm and diagnosis of DCM was made if fractional shortening was <26% and the left ventricular internal dimension in systole was >1.1 cm.22
In addition to the initial NT‐proBNP measurement, 2 additional NT‐proBNP measurements were obtained for all cats: at the time of discharge from the hospital and at re‐evaluation 7–10 days later. Additional information collected for each cat included body weight, heart rate and rhythm, respiratory rate, temperature, dose of furosemide received (in mg/kg/d) in first 24 hours after admission, medications at discharge, whether thoracocentesis was performed, presence of thrombus or spontaneous echogenic contrast, and Cats’ Assessment Tool for Cardiac Health (CATCH) quality of life questionnaire.23 Owners of all cats were contacted via telephone at 3 months post‐initial CHF presentation and every 3 months thereafter to obtain an update until death or euthanasia. Owners were also asked at the 7‐ to 10‐day re‐evaluation, at subsequent evaluations, and during each update call whether they were having any difficulties administering medications to their cats. The cause of death or reason for euthanasia was recorded as sudden death, worsening CHF, arterial thromboembolism (ATE), or noncardiac in origin. Date of death or euthanasia was also recorded.
Data were examined graphically and using Kolmogorov‐Smirnov tests to test for a normal distribution. Data that were normally distributed are reported as mean ± SD, whereas skewed data are reported as median (range). Concentrations of NT‐proBNP were compared between categories (e.g, whether or not a cat had active CHF at the time of re‐evaluation, medications administered) using Mann‐Whitney U‐tests, whereas NT‐proBNP concentrations were compared between continuous variables using Spearman correlation tests. For survival analyses, concentrations of NT‐proBNP for all cats at admission, discharge, and re‐evaluation were divided into categories by the median NT‐proBNP value into high (≥median) or low (<median) categories. The absolute and percent changes in NT‐proBNP concentrations from admission to discharge and from discharge to re‐evaluation were similarly divided into 2 categories using the median values (e.g, whether the change in NT‐proBNP from admission to discharge was larger than the median value [high] or smaller than the median value [low]). Finally, based on data from studies in humans with CHF in which a decrease of 30% in NT‐proBNP had prognostic value, cats were categorized into 2 groups: 1 group for cats whose NT‐proBNP concentrations achieved a decrease of at least 30% from admission to discharge, and the other group for cats whose NT‐proBNP concentrations did not achieve such a decrease.24 Categories of NT‐proBNP (absolute, percentage, and presence of a >30% decrease) were compared with survival times using Kaplan‐Meier curves and log‐rank tests. Cats still alive at the time of analysis were right‐censored. In addition, after transforming the data logarithmically, NT‐proBNP concentrations were compared over the course of the 3 study time points (i.e, presentation, discharge, re‐evaluation) using ANOVA with repeated measures test. Survival times were also analyzed for cardiac medications; echocardiographic measurements; CATCH Questionnaire scores; laboratory variables; age; body condition score; muscle condition score; the presence of arrhythmia, systolic anterior motion of the mitral valve, cardiac gallop, or low body temperature at admission or CHF at the time of re‐evaluation; and whether or not owners had difficulty administering medications to their cats. All continuous variables were transformed to categorical variables using the median value, except for laboratory values, which used the laboratory's reference ranges (e.g, hematocrit was categorized by cats above or below the lower end of reference range), for body condition score (categorized as ideal if 4‐5/9, thin if <4, and overweight if ≥6), and for muscle condition score (categorized as normal or any muscle loss). Commercial statistical software5 was used for all statistical analyses, and P values <.05 were considered statistically significant.
Results
Thirty‐four cats were screened for the study. One cat was excluded after the pleural effusion was diagnosed to be secondary to neoplasia, and another cat was excluded for hyperthyroidism. Thirty‐two cats were enrolled in the study, but 1 cat was euthanized on day 1 of hospitalization due to owner perceived poor prognosis. Therefore, 31 cats completed the study and all subsequent results refer to these 31 enrolled cats that survived to discharge. All cats were presented to the hospital for evaluation of respiratory distress. Mean age of the cats was 9.0 ± 4.3 years, with 24 males and 7 females (all neutered). The most common breed was domestic short‐ and longhaired cats (n = 27) and one each of Cornish rex, Maine Coon cat, Oriental shorthair, and Siamese. Median body weight was 5.5 kg (range, 3.5–9.4 kg), with a median body condition score of 5 (range, 3–9). Sixteen cats had normal muscle condition, but 14 cats had mild muscle loss, and 1 cat had moderate muscle loss. Twenty‐seven cats had hypertrophic cardiomyopathy (HCM) and 4 cats had DCM. Cats had pulmonary edema (n = 25), pleural effusion (n = 21), or pericardial effusion (n = 15) at the time of admission, with 23 cats having concurrent pulmonary edema, pleural effusion, or pericardial effusion. Mean heart rate at the time of admission was 190 ± 27 beats/min, mean temperature was 98.9 ± 2.0°F, and mean respiratory rate was 63 ± 20/min. At the time of cardiac consultation, physical examination findings included a cardiac gallop (n = 23), cardiac murmur (n = 20), and arrhythmia (n = 10, ventricular premature contractions [n = 7], atrial fibrillation [n = 2], paroxysmal supraventricular tachycardia [n = 1]).
Blood was collected for NT‐proBNP analysis from 3 to 240 minutes (median, 100 minutes) from the time of admission to the emergency room. Median hospitalization time was 2 days (range, 1–4 days). Median NT‐proBNP concentrations at admission and hospital discharge were 1,713 pmol/L (range, 160 to 3,784 pmol/L) and 902 pmol/L (range, 147–3,223 pmol/L), respectively. Median NT‐proBNP concentration at re‐evaluation 7–10 days after discharge was 1,124 pmol/L (range, 111–2,727 pmol/L). Median NT‐proBNP concentrations decreased significantly over the course of the 3 time points (P < .012), with significant changes from admission to discharge (P = .005) and from admission to re‐evaluation (P = .024), but not from discharge to re‐evaluation (P = .581; Fig 1). Nineteen cats had a decrease of >30% in NT‐proBNP concentrations during hospitalization (median = −56%; range, −94 to −33%). The only factor associated with the change in NT‐proBNP concentrations during hospitalization (i.e, from admission to discharge) was pimobendan administration. When comparing the change in NT‐proBNP concentrations from admission to discharge, cats that received pimobendan (n = 16) had a significantly greater decrease in NT‐proBNP concentrations (−821 pmol/L [range, −2,163 to −267 pmol/L]) compared to cats that did not receive pimobendan (−561 pmol/L [range, −2,137 to +889 pmol/L]; P = .045).
Figure 1.
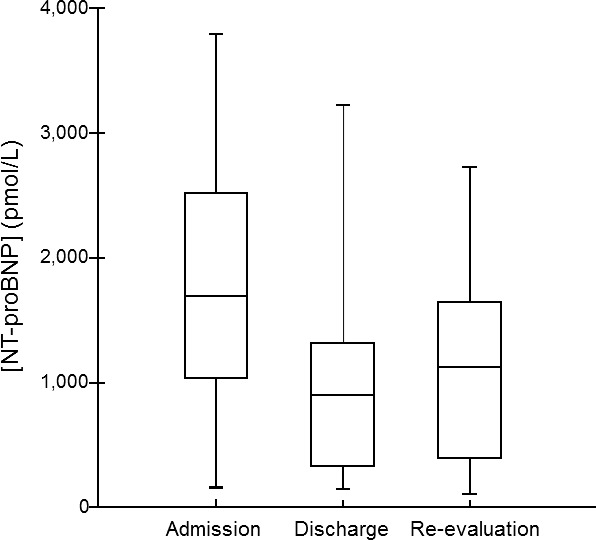
Box plot of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) concentrations in 31 cats with a first‐time episode of congestive heart failure secondary to cardiomyopathy. Concentrations of NT‐proBNP were measured at 3 time points in all cats: Within 4 hours of admission to the hospital (admission), at the time of discharge from the initial hospitalization (discharge), and at the re‐evaluation 7–10 days later (re‐evaluation). For each plot, the box represents the central 50% of the values. The horizontal line within the box represents the median NT‐proBNP concentration, and the bars indicate the range. Median NT‐proBNP concentrations decreased significantly over the course of the 3 time points (P < .012), with significant changes from admission to discharge (P = .005) and from admission to re‐evaluation (P = .024), but not from discharge to re‐evaluation (P = .58).
The 31 cats were discharged with instructions for owners to administer a variety of medications, including furosemide (n = 31), clopidogrel (n = 29), pimobendan (n = 16), angiotensin converting enzyme inhibitor (n = 12), low molecular weight heparin (n = 3), taurine (n = 3), carvedilol (n = 1), and spironolactone (n = 1). Twenty‐eight of the 31 cats (90%) survived to the re‐evaluation examination 7‐10 days after hospital discharge. The cause of death for the 3 cats that did not survive to the re‐evaluation included arterial thromboembolism (ATE; n = 1), sudden death (n = 1), and euthanasia for worsening CHF (n = 1). The median survival time for the 31 cats from the initial presentation was 109 days (range, 1–709 days), with 5 cats (15.6%) still alive at the time of analysis. Causes of death for the 26 cats no longer alive at the end of the study period included euthanasia for worsening CHF (n = 15; median survival time = 60 days, range 2–379 days), euthanasia due to ATE (n = 4; median survival time = 82 days, range, 6–192 days), sudden death (n = 4; median survival time = 120 days, range 4–419 days), and noncardiac causes (n = 3; median survival time = 60 days, range 41–377 days). Five cats developed ATE subsequent to the time of initial presentation for CHF, with the ATE occurring 6, 29, 82, 181, and 192 days after initial presentation for CHF.
Cats with a larger percent decrease in NT‐proBNP concentration (i.e, larger than the median) from admission to discharge had a significantly longer survival time (median survival time = 182 days [range, 2–379 days]) compared to cats with a smaller percent change during hospitalization (median survival time = 60 days [range, 6–709 days]; P = .048; Fig 2). Absolute NT‐proBNP concentrations at any of the 3 time points and achieving at least a 30% decrease in NT‐proBNP were not associated with survival time.
Figure 2.
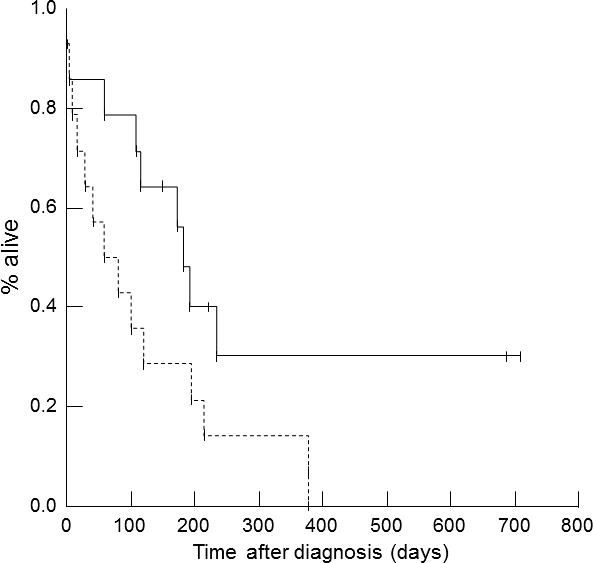
Kaplan‐Meier survival curve for 31 cats with a first‐time episode of congestive heart failure (CHF). Cats with CHF that had a decrease in the percent N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) concentration during hospitalization that was larger than or equal to the median NT‐proBNP concentration (solid line) had a significantly longer survival time (median survival time = 182 days [range, 2–379 days]) than cats with a decrease in percent NT‐proBNP concentrations that was less than the median (dashed line; median survival time = 60 days [range, 6–709 days]; P = .048).
Cats that did not have signs of active CHF (n = 18) at the time of the 7–10 day re‐evaluation had a longer survival time (median = 196 days [range, 11–709 days]) compared to cats with signs of active CHF at the re‐evaluation (n = 10, median = 82 days [range, 6–216 days]; P = .010). Cats whose owners had difficulty administering medications (n = 11) had a shorter survival time (median = 60 days [range, 2–234 days]) compared to owners who did not report difficulty (n = 20, median = 173 days [range, 1–709 days]; P = .045; Fig 3). None of the cardiac medications or dosages, echocardiographic variables, CATCH Questionnaire scores, or other variables were significantly associated with survival time. There were no significant associations in NT‐proBNP concentrations (or changes over time) between cats whose owners had difficulty administering medications and those who did not report difficulty (all P > .05), nor was there a significant association between cats with CHF at the time of re‐evaluation and owners’ difficulty in medicating their cats (P = .856).
Figure 3.
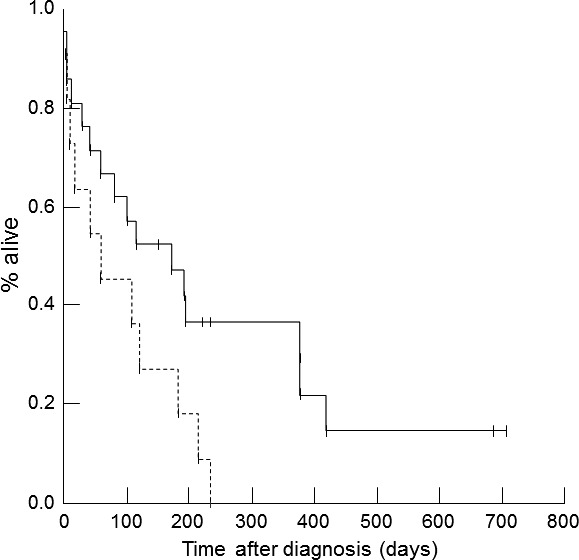
Kaplan‐Meier survival curve for 31 cats with a first‐time episode of congestive heart failure (CHF). Cats whose owners did not have trouble administering medications (solid line) had a longer survival time compared to cats whose owners had trouble administering medications (dashed line; P = .045).
Discussion
One finding in the current study in cats is that a larger percent decrease in NT‐proBNP concentrations during hospitalization for a first‐time episode of CHF was associated with a longer survival time. Although additional research is needed to confirm these results, a larger percent decrease in NT‐proBNP concentrations in cats could suggest better control and stabilization of CHF during hospitalization. The ability of NT‐proBNP concentrations to reflect stabilization of CHF is supported by the results of a study in dogs with CHF, in which NT‐proBNP concentrations <965 pmol/L at the time of re‐evaluation (7–30 days) were associated with a longer overall survival time.19 However, in that study, serial changes in NT‐proBNP concentrations were not reported. The current study was not designed to identify an absolute target number for NT‐proBNP concentrations that predict longer survival time but this would be valuable information to assess in future studies.
In people with CHF, the change in NT‐proBNP concentrations provides more valuable information than the absolute value in helping to guide therapy and predicting survival. A number of studies in people have found that care supported by BNP/NT‐proBNP‐guided therapy as an adjunct to standard treatment results in better outcomes.6, 8, 24, 25, 26 In people, a lowered or sustained NT‐proBNP ≤ 1000 pg/mL6 has been associated with a lower rate of adverse events and more favorable outcome. Another important predictor of outcome in people with CHF was baseline BNP and NT‐proBNP concentrations and their changes or trends over a 3‐month period,27 but longer term changes in NT‐proBNP were not assessed in the current study. Some studies in people have indicated a 20–25% reduction in mortality with biomarker‐guided therapy.25, 26 Having a biomarker‐guided therapy may be even more important as a complement to other testing in cats compared to people to improve clinicians’ confidence in diagnosing heart disease or making treatment decisions for cats with CHF. NT‐proBNP has already been shown to increase the accuracy and confidence of general practitioners in diagnosing CHF in dyspneic cats; accuracy without NT‐proBNP was 67%, and the accuracy improved to 87% with the addition of NT‐proBNP test results.28 In studies of people with CHF, a decrease in BNP >30% during hospitalization predicted a 45% decrease in mortality risk or >40% survival advantage.24 In addition, another study in people with CHF showed that a >30% decrease in NT‐proBNP concentrations was associated with fewer adverse events, such as readmission for CHF or death within 6 months.29 In the current study, whereas BNP was not measured, achieving >30% decrease in NT‐proBNP concentrations was not associated with survival time in cats, although this may be related to the small sample size. Additional research with larger populations of cats will be necessary to identify specific cutoff values or percent decreases in NT‐proBNP concentrations that can be used to help guide treatment.
In the current study, the absence of CHF at the 7‐ to 10‐day re‐evaluation was associated with a longer survival time compared to cats that had signs of CHF present at the re‐evaluation. Additional research is needed, but this finding suggests that more aggressive treatment for CHF at first admission may be beneficial. This finding also could be related to the fact that owners may be more willing to continue with treatment if the cat is doing well with a clear clinical improvement early on in the course of therapy (compared to cats whose CHF is harder to control). Another possible explanation for this finding is that cats whose owners had better medication adherence after discharge were less likely to be in CHF at the time of re‐evaluation. Although we did not specifically assess adherence, there was no association between difficulty in administering medications and the presence of CHF at the re‐evaluation or NT‐proBNP concentrations.
Given the option of euthanasia in veterinary medicine, the association between difficulty in administering medications and a shorter survival is not surprising. Administering medications can be distressing to cat owners. In a study by Reynolds et al., owners’ level of concern about medicating their cats increased as the number of medications and dosing frequencies increased.30 Such concern can negatively impact quality of life and potentially lead or contribute to a decision for euthanasia. The findings in the current study and other studies23, 30, 31 reinforce the importance of open communication with owners regarding issues that are perceived as negatively affecting their pet's quality of life. If difficulties with administering medications are identified, strategies to improve adherence can be recommended. In the current study, the CATCH score was not associated with survival, but use of the CATCH questionnaire may be useful. Although the CATCH questionnaire was not developed for using single items, the authors have found review of the individual items to be helpful to identify difficulties with medication administration or other concerns in owners of cats with cardiac disease, and to provide strategies to address them. Poor medication adherence is a common problem in people with heart failure and has been associated with a higher risk for hospital admission, poor outcome, and death.32, 33 Medication adherence interventions have been shown to significantly reduce mortality risk and improve hospital readmission rates for human patients with heart failure.32, 33, 34
Median survival time for cats with CHF in the current study is similar to that reported in previous studies, which ranges from 41 to 626 days.35, 36, 37, 38, 39, 40, 41, 42, 43 There were no associations between medications (including pimobendan) and survival time in the current study, which is likely due to the relatively small sample size and the fact that cats were receiving multiple medications. However, cats that received pimobendan had a significantly greater decrease in NT‐proBNP concentrations during hospitalization compared to cats that did not receive pimobendan. This may be related to improved cardiac function and reduction of myocardial stress secondary to positive inotropic or vasodilation effects, but further research is needed as results of the current study did not prove a cause and effect relationship. One retrospective study of cats with CHF secondary to HCM reported that the median survival time for cats receiving pimobendan was significantly longer than for cats that did not receive pimobendan (626 days versus 103 days, respectively).39 Additional research is warranted to assess the relationship between pimobendan, NT‐proBNP, and survival in cats with CHF.
Previously reported variables that have been shown to have an association with survival time in cats with CHF or HCM include body weight, age, body temperature at the time of hospital admission, left atrial size and function, extreme left ventricular hypertrophy, left ventricular systolic function, presence of systolic anterior motion, and cardiac troponin I.35, 36, 37, 44, 45, 46 Although troponin I was not measured, none of the other variables were found to be associated with survival time in the current study, which may be due to the small sample size or to a true lack of association in this population of cats.
There are a number of other limitations to the current study, including possible day‐to‐day and week‐to‐week variation in NT‐proBNP concentrations, which is not well understood in cats with cardiac disease and CHF. Median daily and weekly coefficient of variation for NT‐proBNP in adult healthy cats with no underlying cardiac disease was 13.1 and 21.2%, respectively.47 Therefore, if variability is similar or greater in cats with CHF than it is in healthy cats, it could have further impacted the results. In people, the week‐to‐week variation for BNP and NT‐proBNP concentrations can vary up to 60%, and reported day‐to‐day variation for NT‐proBNP concentrations is 26% in healthy people and 27% in patients with stable heart failure.48 Despite the intrinsic biologic variability, these biomarkers have still been shown to be valuable in providing prognostic information and therapeutic guidance in people with CHF. Other factors which could contribute to the variability in NT‐proBNP concentrations during hospitalization are medications administered, including dose and frequency, which were not standardized and were up to the discretion of the primary clinician in this study. In addition, using NT‐proBNP concentrations greater than a specific cutoff (e.g, 270) is not 100% sensitive or specific for CHF, which could be the reason for 1 cat in the current study that had a value of 160 pmol/L despite clear radiographic and echocardiographic evidence of CHF. Another limitation is that this study excluded cats with concurrent diseases so results might not be completely representative of the typical population of cats with CHF that frequently have comorbidities. In the current study, the investigators did not have results of the NT‐proBNP analysis until long after the cats were treated, so these values could not be used to guide treatment. Therefore, evaluating any potential benefits of NT‐proBNP‐guided treatment requires further study. Finally, nonmedical factors, such as owner finances or concerns for hospitalization, also can impact the management and clinical course of cats with CHF and likely changes in NT‐proBNP. Despite these limitations, the significant association between a larger decrease in NT‐proBNP concentrations and survival indicates that further investigation is warranted to evaluate the potential utility of NT‐proBNP as an adjunct in the management of cats with CHF.
Acknowledgments
The authors thank dedicated veterinary technicians, Kristen Antoon and Diane Welsh, for their important role in completing this study.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
The study was performed at Cummings School of Veterinary Medicine at Tufts University, 200 Westboro Road, North Grafton, MA 01536.
This study was supported by IDEXX, Cummings Companion Animal Health Fund, and the Barkley Fund.
Presented in abstract form at the 2016 ACVIM Forum, Denver, CO.
Footnotes
DRI T4 homogenous immunoassay, IDEXX Laboratories, North Grafton, MA
Nova Biomedical, Waltham, MA
CardioPet NT‐proBNP assay, IDEXX Laboratories, Westbrook, ME
GE Vivid 7 Dimension, General Electric Healthcare, Milwaukee, WI
Systat 13.0, Systat Software, Inc., San Jose, CA
References
- 1. Goetze JP. Biochemistry of pro‐B‐type natriuretic peptide‐derived peptides: The endocrine heart revisited. Clin Chem 2004;50:1503–1510. [DOI] [PubMed] [Google Scholar]
- 2. Baerts L, Gomez N, Vanderheyden M, et al. Possible mechanisms for brain natriuretic peptide resistance in heart failure with a focus on interspecies differences and canine BNP biology. Vet J 2012;194:34–39. [DOI] [PubMed] [Google Scholar]
- 3. Oyama MA, Boswood A, Connolly DJ, et al. Clinical usefulness of an assay for measurement of circulating N‐terminal pro‐B‐type natriuretic peptide concentration in dogs and cats with heart disease. J Am Vet Med Assoc 2013;243:71–82. [DOI] [PubMed] [Google Scholar]
- 4. Moe GW. B‐type natriuretic peptide in heart failure. Curr Opin Cardiol 2006;21:208–214. [DOI] [PubMed] [Google Scholar]
- 5. Oyama MA. Using cardiac biomarkers in veterinary practice. Clin Lab Med 2015;35:555–566. [DOI] [PubMed] [Google Scholar]
- 6. Januzzi JL, Troughton R. Serial natriuretic peptide measurements are useful in heart failure management. Circulation 2013;127:500–508. [DOI] [PubMed] [Google Scholar]
- 7. Richards AM. Tailored therapy for heart failure: Neurohormones. Can J Physiol Pharmacol 2011;89:603–607. [DOI] [PubMed] [Google Scholar]
- 8. Richards AM, Troughton RW. Use of natriuretic peptides to guide and monitor heart failure therapy. Clin Chem 2012;58:62–71. [DOI] [PubMed] [Google Scholar]
- 9. Reynolds CA, Brown DC, Rush JE, et al. Prediction of first onset of congestive heart failure in dogs with degenerative mitral valve disease: The PREDICT cohort study. J Vet Cardiol 2012;14:193–202. [DOI] [PubMed] [Google Scholar]
- 10. Chetboul V, Serres F, Tissier R, et al. Association of plasma N‐terminal pro‐B‐type natriuretic peptide concentration with mitral regurgitation severity and outcome in dogs with asymptomatic degenerative mitral valve disease. J Vet Intern Med 2009;23:984–994. [DOI] [PubMed] [Google Scholar]
- 11. Fox PR, Oyama MA, Reynolds C, et al. Utility of plasma N‐terminal pro‐brain natriuretic pepetide (NT‐proBNP) to distinguish between congestive heart failure and non‐cardiac causes of acute dyspnea in cats. J Vet Cardiol 2009;11:S51–S61. [DOI] [PubMed] [Google Scholar]
- 12. Fox PR, Oyama MA, Hezzell MJ, et al. Relationship of plasma N‐terminal pro‐brain natriuretic peptide concentrations to heart failure classification and cause of respiratory distress in dogs using a 2nd generation ELISA assay. J Vet Intern Med 2015;29:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oyama MA, Rush JE, Rozanski EA, et al. Assessment of serum N‐terminal pro‐B‐type natriuretic peptide concentration for differentiation of congestive heart failure from primary respiratory tract disease as the cause of respiratory signs in dogs. J Am Vet Med Assoc 2009;235:1319–1325. [DOI] [PubMed] [Google Scholar]
- 14. Fine DM, DeClue AE, Reinero CR. Evaluation of circulating amino terminal‐pro‐B‐type natriuretic peptide concentration in dogs with respiratory distress attributable to congestive heart failure or primary pulmonary disease. J Am Vet Med Assoc 2008;232:1674–1679. [DOI] [PubMed] [Google Scholar]
- 15. Machen MC, Oyama MA, Gordon SG, et al. Multi‐centered investigation of a point‐of‐care NT‐proBNP ELISA assay to detect moderate to severe occult (pre‐clinical) feline heart disease in cats referred for cardiac evaluation. J Vet Cardiol 2014;16:245–255. [DOI] [PubMed] [Google Scholar]
- 16. Fox PR, Rush JE, Reynolds CA, et al. Multicenter evaluation of plasma N‐terminal probrain natriuretic peptide (NT‐proBNP) as a biochemical screening test for asymptomatic (occult) cardiomyopathy in cats. J Vet Intern 2011;25:1010–1016. [DOI] [PubMed] [Google Scholar]
- 17. Wess G, Butz V, Mahling M, Hartmann K. Evaluation of N‐terminal pro‐B‐type natriuretic peptide as a diagnostic marker of various stages of cardiomyopathy in Doberman Pinschers. Am J Vet Res 2011;72:642–649. [DOI] [PubMed] [Google Scholar]
- 18. Singletary GE, Morris NA, O'Sullivan ML, et al. Prospective evaluation of NT‐proBNP assay to detect occult dilated cardiomyopathy and predict survival in Doberman Pinschers. J Vet Intern Med 2012;26:1330–1336. [DOI] [PubMed] [Google Scholar]
- 19. Wolf J, Gerlach N, Weber K, et al. Lowered N‐terminal pro‐B‐type natriuretic peptide levels in response to treatment predict survival in dogs with symptomatic mitral valve disease. J Vet Cardiol 2012;14:399–408. [DOI] [PubMed] [Google Scholar]
- 20. Mainville CA, Clark GH, Esty KJ, et al. Analytical validation of an immunoassay for the quantification of N‐terminal pro‐B‐type natriuretic peptide in feline blood. J Vet Diagn Invest 2015;27:414–421. [DOI] [PubMed] [Google Scholar]
- 21. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med 1993;7:247–252. [DOI] [PubMed] [Google Scholar]
- 22. MacDonald K. Myocardial disease: Feline In: Ettinger S, Feldman E. eds. Textbook of Veterinary Internal Medicine, 7th ed St Louis: Saunders Elsevier; 2010: 1328–1341. [Google Scholar]
- 23. Freeman LM, Rush JE, Oyama MA, et al. Development and evaluation of a questionnaire for assessment of health‐related quality of life in cats with cardiac disease. J Am Vet Med Assoc 2012;240:1188–1193. [DOI] [PubMed] [Google Scholar]
- 24. Lourenco P, Ribeiro A, Pintalhao M, et al. Predictors of six‐month mortality in BNP‐matched acute heart failure patients. Am J Cardiol 2015;116:744–748. [DOI] [PubMed] [Google Scholar]
- 25. Troughton RW, Frampton CM, Brunner‐La Rocca HP, et al. Effect of B‐type natriuretic peptide guided treatment of chronic heart failure on total mortality and hospitalization: An individual patient meta‐analysis. Eur Heart J 2014;35:1559–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Felker GM, Ahmad T, Anstrom KJ, et al. Rationale and design of the GUIDE‐IT study: Guiding evidence based therapy using biomarker intensified treatment in heart failure. JACC Heart Failure 2014;2:457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Masson S, Latini R, Anand IS, et al. Prognostic value of changes in N‐terminal pro‐brain natriuretic peptide in VaL‐HeFT (Valsartan heart failure trial). J Am Coll Cardiol 2008;52:997–1003. [DOI] [PubMed] [Google Scholar]
- 28. Singletary GE, Rush JE, Fox PR, et al. Effect of NT‐pro‐BNP assay on accuracy and confidence of general practitioners in diagnosing heart failure or respiratory disease in cats with respiratory signs. J Vet Intern Med 2012;26:542–546. [DOI] [PubMed] [Google Scholar]
- 29. Bettencourt P, Azevedo A, Pimenta J, et al. N‐terminal‐pro‐brain natriuretic peptide predicts outcome after hospitalize discharge in heart failure patients. Circulation 2004;110:2168–2174. [DOI] [PubMed] [Google Scholar]
- 30. Reynolds CA, Oyama MA, Rush JE, et al. Perceptions of quality of life and priorities of owners of cats with heart disease. J Vet Intern Med 2010;24:1421–1426. [DOI] [PubMed] [Google Scholar]
- 31. Rush JE, Roderick KV, Freeman LM, et al. Assessment of the responsiveness of the Cats’ Assessment Tool for Cardiac Health (CATCH) Questionnaire. J Vet Cardiol 2015;17(Suppl 1):S341–S348. [DOI] [PubMed] [Google Scholar]
- 32. Kripalani S, Goggins K, Nwosu S, et al. Vanderbilt Inpatient Cohort Study. Medication nonadherence before hospitalization for acute cardiac events. J Health Commun 2015;20(Suppl 2):S34–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ruppar TM, Cooper PS, Mehr DR, et al. Medication adherence interventions improve heart failure mortality and readmission rates: Systematic review and meta‐analysis of controlled trials. J Am Heart Assoc 2016;5:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davis EM, Packard KA, Jackevicius CA. The pharmacist role in predicting and improving medication adherence n heart failure patients. J Manag Care Spec Pharm 2014;20:741–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goutal CM, Keir I, Kenney S, et al. Evaluation of acute congestive heart failure in dogs and cats: 145 cases (2007–2008). J Vet Emerg Crit Care 2010;20:330–337. [DOI] [PubMed] [Google Scholar]
- 36. Rush JE, Freeman LM, Fenollosa NK, Brown DJ. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990–1999). J Am Vet Med Assoc 2002;220:202–207. [DOI] [PubMed] [Google Scholar]
- 37. Finn E, Freeman LM, Rush JE, Lee Y. The relationship between body weight, body condition and survival in cats with heart failure. J Vet Intern Med 2010;24:1369–1374. [DOI] [PubMed] [Google Scholar]
- 38. Hall DJ, Sofer F, Meier CK, Sleeper MM. Pericardial effusion in cats: A retrospective study of clinical findings and outcome in 146 cats. J Vet Intern Med 2007;21:1002–1007. [DOI] [PubMed] [Google Scholar]
- 39. Reina‐Doreste Y, Stern JA, Keene BW, et al. Case‐control study of the effects of pimobendan on survival time in cats with hypertrophic cardiomyopathy and congestive heart failure. J Am Vet Med Assoc 2014;245:535–539. [DOI] [PubMed] [Google Scholar]
- 40. Atkins CE, Gallo AM, Kurzman ID, Cowen P. Risk factors, clinical signs, and survival in cats with a clinical diagnosis of idiopathic hypertrophic cardiomyopathy: 74 cases (1985–1989). J Am Vet Med Assoc 1992;201:613–618. [PubMed] [Google Scholar]
- 41. Payne J, Luis Fuentes V, Boswood A, et al. Population characteristics and survival in 127 referred cats with hypertrophic cardiomyopathy (1997 to 2005). J Small Anim Pract 2010;51:540–547. [DOI] [PubMed] [Google Scholar]
- 42. MacGregor JM, Rush JE, Laste NJ, et al. Use of pimobendan in 170 cats (2006–2010). J Vet Cardiol 2011;13:251–260. [DOI] [PubMed] [Google Scholar]
- 43. Hambrook LE, Bennett PF. Effect of pimobendan on the clinical outcome and survival of cats with non‐taurine responsive dilated cardiomyopathy. J Fel Med Surg 2012;14:233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Payne JR, Borgeat K, Connolly DJ, et al. Prognostic indicators in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2013;27:1427–1436. [DOI] [PubMed] [Google Scholar]
- 45. Langhorn R, Tarnow I, Willesen JL, et al. Cardiac troponin I and T as prognostic markers in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2014;28:1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Borgeat K, Sherwood K, Payne JR, et al. Plasma cardiac troponin I concentration and cardiac death in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2014;28:1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Harris AN, Estrada AH, Gallagher AE, et al. Biologic variability of N‐terminal pro‐brain natriuretic peptide in adult healthy cats. J Feline Med Surg 2017;19:216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu AH. Serial testing of B‐type natriuretic peptide and NT pro‐BNP for monitoring therapy of heart failure: The role of biologic variation in the interpretation results. Am Heart J 2006;152:828–834. [DOI] [PubMed] [Google Scholar]


