Abstract
Background
Endothelin (ET)‐1 is a 21‐amino‐acid peptide with potent vasoactive properties, which increases intrahepatic resistance in patients with chronic hepatitis (CH) or cirrhosis. ET‐1 concentrations have not been investigated in dogs with CH.
Hypothesis/Objectives
This study compared hepatic and plasma ET‐1 levels in healthy dogs and in dogs with CH, and examined the relationship between the plasma ET‐1 level and portal vein pressure in dogs with CH.
Animals
Fourteen healthy dogs and twenty dogs with CH were used in this study.
Methods
Prospective case‐control study. Hepatic ET‐1 mRNA expression was determined by real‐time reverse transcription polymerase chain reaction, and hepatic and plasma ET‐1 levels were assessed using ELISA. Splenic pulp pressure (SPP), as an indicator of portal vein pressure, was measured laparoscopically.
Results
Hepatic ET‐1 mRNA levels were 3.7 times higher in dogs with CH than in healthy dogs (P = .008). The median hepatic and plasma ET‐1 protein levels were significantly higher in dogs with CH than in healthy dogs (13.20 pg/mg wet liver vs. 3.42 pg/mg wet liver, P = .004, and 0.99 pg/mL vs. 0.71 pg/mL, P = .013, respectively). Moreover, there was a weak but significant correlation between plasma ET‐1 level and SPP in dogs with CH (P = .036; r s = 0.53).
Conclusions and clinical importance
The results indicate that ET‐1 might play an important role in the pathogenesis of portal hypertension caused by CH.
Keywords: Canine, Chronic liver disease, Portal hypertension, Vasoactive peptide
Abbreviations
- APSCs
acquired portosystemic collaterals
- CH
chronic hepatitis
- CI
confidence interval
- ET
endothelin
- HPRT
hypoxanthine phosphoribosyl transferase
- PDGF
platelet‐derived growth factor
- PH
portal hypertension
- PHPV
primary hypoplasia of the portal vein
- ROC
receiver operating characteristic
- RT‐PCR
reverse transcription polymerase chain reaction
- SPP
splenic pulp pressure
- TGF‐β
transforming growth factor‐β
Chronic hepatitis (CH) is a common liver disease in dogs and is histologically defined by the presence of hepatocellular apoptosis or necrosis, a variable mononuclear or mixed inflammatory cell infiltrate, regenerative nodes of hepatocytes, and fibrosis.1 Cirrhosis is the final stage of CH, and the progression of CH can lead to portal hypertension (PH) as a result of fibrosis and increased resistance, increased blood flow, or both the conditions in the portal circulation.2 The clinical consequences of PH include acquired portosystemic collaterals (APSCs), ascites, hepatic encephalopathy, or their combination.3, 4, 5
Endothelin (ET)‐1 is a 21‐amino‐acid peptide with potent vasoactive properties6, 7 and is markedly overexpressed in the human patients with cirrhotic liver, particularly in sinusoidal endothelial cells and hepatic stellate cells.7 The physiological functions of ET‐1 are mediated by two ET receptors, ETA and ETB. ET‐1 is produced and released from sinusoidal endothelial cells and exerts paracrine effects on ETA receptors in vascular smooth muscle and hepatic stellate cells, thereby inducing their contraction. ET‐1 also exerts autocrine, intracrine, or both effects on sinusoidal endothelial cells via induction of ETB receptor‐mediated release of vascular‐relaxing factors, such as nitric oxide and prostacyclin. The major function of these receptors appears to be regulation of vascular tone.7, 8, 9, 10 ET‐1 levels in plasma and hepatic tissue are elevated in human patients with CH or cirrhosis, and this increase is proportional to the severity of liver disease.11, 12, 13 Current evidence indicates that elevated plasma and hepatic ET‐1 levels might also contribute to PH caused by CH in humans.11, 12, 13
Plasma ET‐1 levels are elevated in dogs with heart failure or respiratory failure and are used as diagnostic biomarkers to assess the severity of heart failure or respiratory failure.14, 15, 16, 17, 18 However, the role of ET‐1 in the pathogenesis of liver failure has not been evaluated.
This study aimed to determine whether hepatic ET‐1 mRNA expression and hepatic and plasma ET‐1 protein levels in dogs with CH are different from those observed in healthy beagle dogs. Additionally, it is investigated whether the plasma ET‐1 level correlated with portal vein pressure in dogs with CH.
Materials and Methods
Dogs
In this study, 20 dogs were laparoscopically examined for the histopathological evaluation of CH. Liver biopsy specimens were examined histopathologically by an American College of Veterinary Pathologists board‐certified pathologist, and CH was diagnosed in the dogs according to the criteria developed by the World Small Animal Veterinary Association Liver Standardization Group.1 It was confirmed that the dogs had no concurrent diseases, such as congestive heart failure or pulmonary disease, based on clinical signs, auscultation, thoracic radiography, and echocardiography. The presence of APSCs was determined using laparoscopic splenoportography. The study controls comprised 14 beagle dogs. The College of Bioresource Sciences, Nihon University, granted ethical approval for use of the control dogs, and the study proceeded in accordance with the Guide for Animal Experimentation published by the College of Bioresource Sciences, Nihon University. The beagle dogs were confirmed as healthy according to a clinical examination, a complete blood cell count, serum biochemistry, and abdominal radiography and ultrasonography. The median age and body weight of the control group (9 male dogs and 5 female dogs) were 2.6 years (range, 0.9–5.9 years) and 10.8 kg (range, 9.2–11.7 kg), respectively.
Sampling and Treatment of Biopsy Specimens
After giving general anesthesia, laparoscopic liver biopsy was performed in 20 CH dogs and 3 healthy beagle dogs. Additionally, laparotomic liver biopsy was performed in three other healthy beagle dogs. Biopsy of liver tissue was performed in five to six samples from each dog. The samples were extracted using biopsy forceps and subsequently used for mRNA, hepatic ET‐1 protein, and histopathological analyses in all dogs. Liver specimens for total RNA extraction were immediately preserved in RNAlater1 and stored at −20°C until use. Liver specimens for hepatic ET‐1 concentration analysis were immediately immersed in liquid nitrogen and stored at −80°C until use. Blood samples collected in ethylenediaminetetraacetic acid tubes were centrifuged at 1,600 × g for 15 minutes at 4°C, and the plasma was stored at −80°C until use. Samples for histopathology were immediately placed in 10% neutral buffered formalin, and hematoxylin and eosin‐stained sections were prepared.
Hepatic ET‐1 mRNA Expression
ET‐1 mRNA levels were measured in the liver tissue samples of the 20 dogs with CH and the 6 healthy beagle dogs using real‐time reverse transcription polymerase chain reaction (RT‐PCR). Stabilized liver tissue (20 mg) was ground thoroughly with a mortar and pestle, and frozen at −80°C prior to addition of 600 μL of lysis buffer containing β‐mercaptoethanol. The lysate was transferred onto a QIAshredder spin columna in a 2 mL collection tube and centrifuged at 7,000 × g for 2 minutes in a microcentrifuge. The supernatant was used for total RNA extraction with the RNeasy Protect Mini Kit.1 RNA was quantified spectrophotometrically using a Nanodrop ND‐2000b system and diluted to a concentration of 0.1 μg/μL. Single‐stranded cDNA was synthesized from 1 μg of total RNA using a PrimeScript RT‐PCR kit2 according to the manufacturer's instructions. Thermal conditions for reverse transcription were an initial incubation at 42°C for 15 minutes, followed by incubation at 95°C for 5 minutes and at 4°C for 5 minutes. Real‐time RT‐PCR was performed using Fast SYBR Green Master Mix3 in an Applied Biosystems 7500 Fast RT‐PCR system.3 The thermal cycles were carried out as follows: 95°C for 30 seconds; 40 cycles of 95°C for 5 seconds and 60°C for 20 seconds; and dissociation at 95°C for 1 second, 65°C for 15 seconds, and 95°C for 1 second. mRNA levels of hypoxanthine phosphoribosyl transferase (HPRT) were determined for each sample as a control and to normalize the data. All reactions were performed in triplicate and included a no‐template control. The amplification efficiency for ET‐1 (89%) and HPRT (87%) in the samples was determined in triplicate using serial 10‐fold sample dilutions. The relative quantity of each transcript was calculated using the 2−ΔΔCT method19 as described by the manufacturer (Applied Biosystems). The primers used are summarized in Table 1.20
Table 1.
Primer sequences used in real‐time RT‐PCR
| Gene | Primer | Sequence (5′–3′) | Product size (bp) | Source |
|---|---|---|---|---|
| ET‐1 | Forward | GCC CCG CCG ATC CA | 61 | AC: AB115087 |
| Reverse | GGT GGC AGAAGT AGACACACTCTTT | 61 | AC: AB115087 | |
| HPRT | Forward | AGC TTG CTG GTG AAA AGG AC | 114 | Brinkhof et al.20 |
| Reverse | TTA TAG TCA AGG GCA TAT CC | 114 | Brinkhof et al.20 |
AC, GenBank accession number; HPRT, hypoxanthine phosphoribosyltransferase.
Measurement of ET‐1 Concentration
ET‐1 concentrations were determined in blood samples collected from 20 dogs with CH and 14 healthy beagle dogs, and in liver tissue samples collected from the 20 dogs with CH and 6 of the healthy beagle dogs. Briefly, 100 mg of each minced liver tissue was boiled in a 1mL mixture of 1 M acetate and 20 mM hydrochloride for 10 minutes at 100°C, followed by centrifugation at 7,000 × g for 10 minutes at 4°C. The supernatant was filtered, lyophilized, and dissolved in 300 μL of buffer solution according to the manufacturer's instructions (Endothelin‐1 Assay Kite). The extracted peptide solution was used for ELISA using a human sandwich ELISA kit that had been validated previously for use with canine plasma.18, 21 The kit exhibited a sensitivity of 0.23 pg/mL and was highly specific for ET‐1. Prior to measurement, pre‐extraction with a Sep‐Pac C‐18 column4 was required to obtain a 2‐fold concentration of the sample.18 The ELISA used was specific for ET‐1 and did not cross‐react with ET‐2, ET‐3, big ET‐1, big ET‐2, or big ET‐3 (cross‐reactivity, <0.1%).
Splenic Pulp Pressure Measurement
Splenic pulp pressure (SPP) was measured in cases of severe liver change or grossly abnormal blood vessels. SPP was not measured in dogs with grossly normal livers. Splenoportography was performed simultaneously. SPP was measured laparoscopically in the 16 dogs with CH by placing the dogs in the right lateral recumbent position and applying CO2 gas insufflation (Endoflatorg) of the abdominal cavity. Intra‐abdominal pressure was automatically maintained between 8 and 10 mmHg. Two cannulae were placed in the abdomen, and the abdominal cavity was visualized through a laparoscope (Hopkins II, 5 mm, 0°g). An 18‐gauge over‐the‐needle intravenous catheter (SRFF 1832; length, 6.4 cmh) was percutaneously inserted into the spleen parallel to the long axis. After the pneumoperitoneum was stopped and gas was removed completely from the abdominal cavity, SPP was measured by a laparoscopist (M.S.) via the catheter as previously described.22 The normal level for SPP in healthy dogs is 6.2 ± 0.8 mmHg, as previously reported.22
Statistical Analysis
Data were expressed as the median (range). Differences in hepatic ET‐1 mRNA expression and plasma and hepatic ET‐1 concentrations between the dogs with CH and healthy dogs were statistically analyzed using the Mann Whitney test. Area under the receiver operating characteristic (ROC) curve and sensitivity and specificity with 95% confidence intervals (CIs) were calculated. The diagnostic validity of CH in dogs with regard to hepatic and plasma ET‐1 concentrations was investigated based on the ROC curve. Correlations between the plasma ET‐1 concentration and SPP were evaluated using Spearman's correlation coefficient. All data were analyzed using GraphPad PRISM for Mac OS X version 5.0b.5 Differences were considered significant at P < .05.
Results
Dogs
The 20 dogs with CH (n = 20; 3 intact males, 7 castrated males, 3 intact females, and 7 spayed females) included 12 breeds, including American cocker spaniel, Labrador retriever, miniature dachshund (n = 3 each), English cocker spaniel, Welsh corgi (n = 2 each), Irish setter, Jack Russell terrier, Cairn terrier, Maltese, mixed breeds, Shiba, and West Highland white terrier (n = 1 each). The median age and body weight of dogs with CH were 7.1 years (range, 2.2–11.9 years) and 9.4 kg (range, 4.2–38.3 kg), respectively. The median SPP was 10.0 mmHg (range, 7.0–19.0 mmHg) in the 16 CH dogs. The presence of APSCs was confirmed in 12 dogs with CH. In these 12 dogs, the median SPP was 10.5 mmHg (range, 8.0–19.0 mmHg). In contrast, in the 4 dogs without APSCs, the median SPP was 7.0 mmHg (range, 7.0–9.0 mmHg).
Gene Expression of ET‐1 and Hepatic and Plasma ET‐1 Concentrations
Hepatic ET‐1 mRNA expression was significantly higher in dogs with CH than in healthy dogs (P = .008; Fig 1). Hepatic ET‐1 protein concentrations were significantly higher in dogs with CH (median, 13.20 pg/mg wet liver; range, 3.56–62.60 pg/mg wet liver) than in the healthy dogs (median, 3.42 pg/mg wet liver; range, 1.70–8.68 pg/mg wet liver; P = .004; Fig 2). Similarly, plasma ET‐1 concentrations in dogs with CH were significantly higher (median, 0.99 pg/mL; range, 0.37–2.98 pg/mL) than those in healthy beagle dogs (median, 0.71 pg/mL; range, 0.25–1.28 pg/mL; P = .013; Fig 3). The median plasma ET‐1 concentrations in CH dogs with APSCs (1.33 pg/mL; range, 0.64–2.98 pg/mL) were slightly but not significantly higher than those measured in CH dogs without APSCs (0.86 pg/mL; range, 0.37–1.53 pg/mL; P = .114). The sensitivity and specificity of hepatic ET‐1 concentration (cut‐off, 6.66 pg/mg wet liver) were 80.0% (95% CI, 56.3–94.3%) and 83.3% (95% CI, 35.9–99.6%), respectively. The sensitivity and specificity of plasma ET‐1 concentrations (cut‐off, 0.82 pg/mL) were 70% (95% CI, 45.7–88.1%) and 64.3% (95% CI, 35.1–87.2%), respectively. The areas under the ROC curves were 0.90 (95% CI, 0.76–1) and 0.75 (95% CI, 0.59–0.92) for hepatic and plasma ET‐1 concentrations, respectively.
Figure 1.
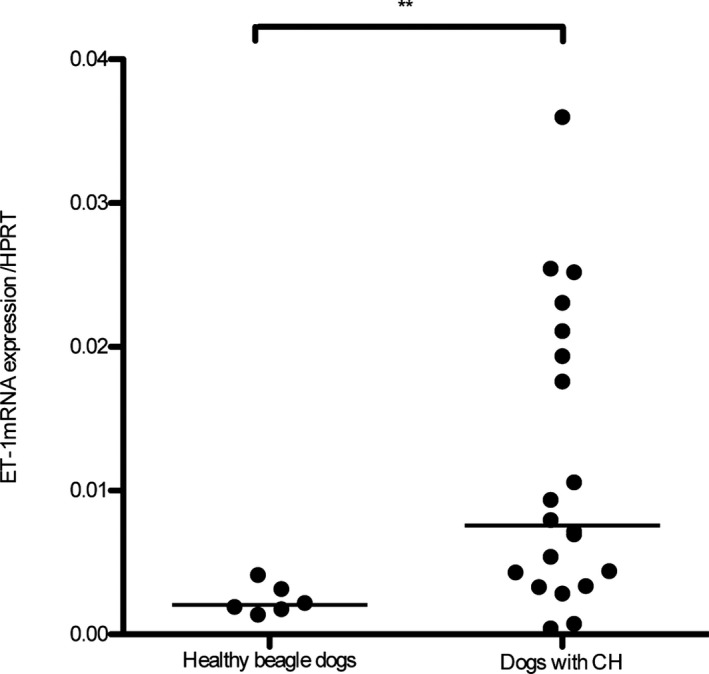
Expression of hepatic ET‐1 mRNA in dogs with chronic hepatitis (CH; n = 20) and healthy beagle dogs (n = 6). The expression of hepatic ET‐1 mRNA was significantly higher in dogs with CH than in healthy beagle dogs (**P = .008). The line represents the median.
Figure 2.
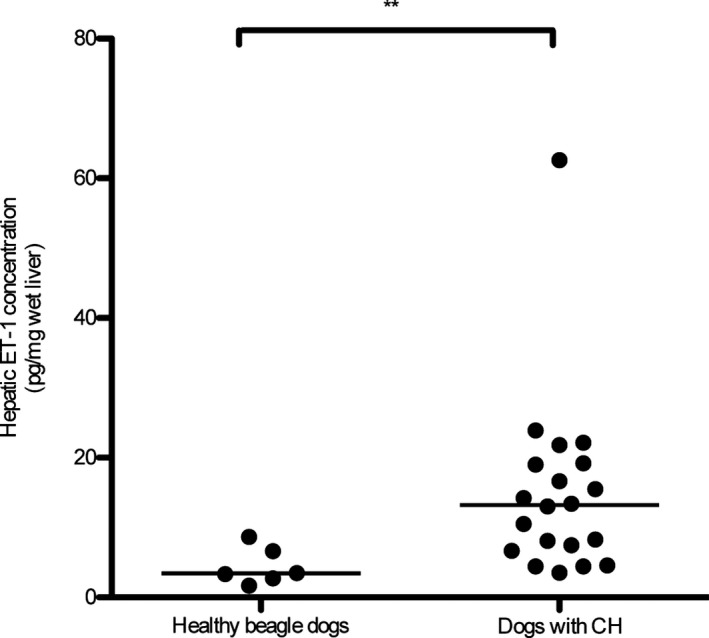
Hepatic ET‐1 concentration in dogs with chronic hepatitis (CH; n = 20) and healthy beagle dogs (n = 6). The hepatic ET‐1 concentration was significantly higher in dogs with CH than in healthy beagle dogs (**P = .004). The line represents the median.
Figure 3.
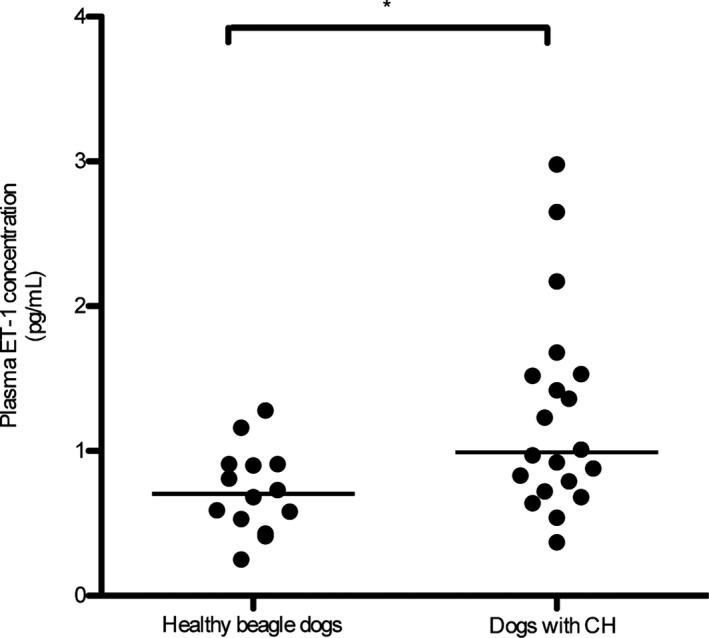
Plasma ET‐1 concentration in dogs with chronic hepatitis (CH; n = 20) and healthy beagle dogs (n = 14). The plasma ET‐1 concentration was significantly higher in dogs with CH than in healthy beagle dogs (*P = .013). The line represents the median.
Relationship between Plasma ET‐1 Concentration and SPP
There was a weak but significant correlation between plasma ET‐1 concentration and SPP in dogs with CH (r s = 0.53; P = .036, n = 16; Fig 4).
Figure 4.
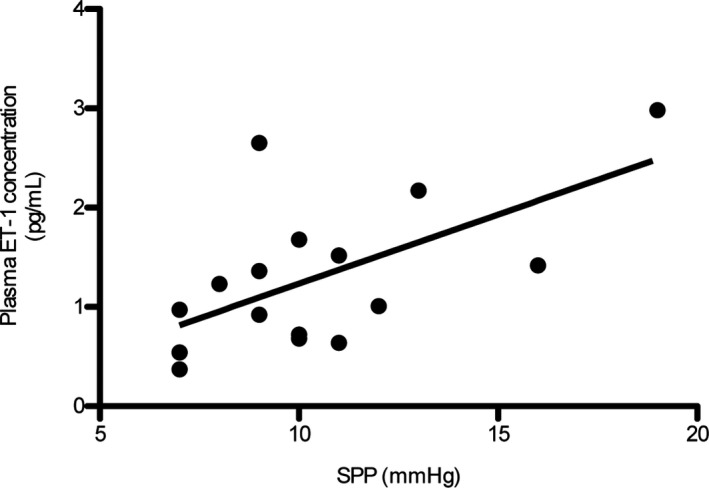
Relationship between plasma ET‐1 concentration and splenic pulp pressure (SPP) in dogs with chronic hepatitis (CH). There was a weak but significant correlation between plasma ET‐1 levels and SPP in dogs with CH (r s = 0.53; P = .036, n = 16).
Discussion
Dogs with PH caused by CH and cirrhosis exhibited PH symptoms. Portal vein pressure is rarely measured, and the presence of APSCs indicates PH in dogs. The main treatment for PH is infusion and diuretics. The ET family consists of three members, ET‐1, ET‐2, and ET‐3, among which ET‐1 is a potent vasoconstrictor, that are widely expressed in a variety of tissues such as the blood vessels, heart, lungs, and liver.7 Elevated hepatic ET‐1 mRNA expression and circulating ET‐1 protein in human patients with PH caused by liver diseases, such as CH and cirrhosis, have been reported,11, 12, 13, 23 and hepatic and plasma ET‐1 levels are closely related to the severity of liver fibrosis and PH.11, 12, 13 Elevated circulating ET‐1 levels in dogs with pulmonary hypertension caused by idiopathic pulmonary fibrosis and heartworm disease have been reported.18, 24 Plasma ET‐1 levels are significantly higher in dogs with congestive heart failure than in dogs with heart disease without congestive heart failure.17 Therefore, elevated ET‐1 levels are an exacerbating factor in dogs with pulmonary hypertension and heart disease. However, hepatic ET‐1 mRNA and plasma and hepatic ET‐1 protein levels in dogs with PH caused by liver disease have not been reported.
This study demonstrated that hepatic ET‐1 mRNA expression was significantly higher in dogs with CH than in healthy beagle dogs. Similar observations were previously reported by Leivas et al.23 and Tièche et al.25 for expression of the ET‐1 gene in humans and rats. Additionally, ET‐1 mRNA is upregulated by cytokines, including transforming growth factor‐β (TGF‐β), and growth factors, such as platelet‐derived growth factor (PDGF), in a variety of cells.7 Although we did not investigate the levels of TGF‐β and PDGF, previous studies showed that increased expression of the PDGF and TGF‐β genes and elevated plasma TGF‐β contribute to fibrosis in dogs with CH.26, 27 Additionally, several studies reported a 2‐ to 5‐fold increase in plasma ET‐1 levels in human patients with cirrhosis.28, 29, 30
Plasma ET‐1 concentrations are significantly higher in patients exhibiting higher scores associated with liver disease, and hepatic ET‐1 concentrations are correlated with the extent and severity of hepatic fibrosis in human patients.12, 13 In accordance with the increased mRNA expression, hepatic and plasma ET‐1 protein concentrations in dogs with CH were significantly higher than those in healthy beagle dogs. Elevated hepatic ET‐1 concentrations indicated that ET‐1 might contribute to increased intrahepatic resistance by constriction of the sinusoid, and may exacerbate PH caused by CH and cirrhosis. The factors involved in the elevation of plasma ET‐1 concentrations include increased hepatic, splanchnic, and renal ET‐1 production as well as decreased hepatic clearance.28 Moreover, downregulation of the ETB receptor was previously reported.7, 31 Hepatic ET‐1 concentrations may be more suitable than plasma ET‐1 concentrations for diagnosis of CH. Plasma ET‐1 concentrations may change relative to hepatic ET‐1 levels through changes in the portal hemodynamics depending on the presence or absence of APSCs32 and the effect of clearance in the lungs and liver. However, the cut‐off value for the hepatic ET‐1 concentration in the present study was 6.66 pg/mg wet liver, whereas the concentrations measured in healthy beagle dogs and CH dogs were within a range of 3.56–8.68 pg/mg wet liver. Therefore, we believe that cases presenting values such as these require attention for potential diagnosis of CH.
Plasma ET‐1 levels in the hepatic veins have been found to be significantly correlated with the hepatic venous pressure gradient in human patients with cirrhosis.33, 34 Notably, we found a weak correlation between plasma ET‐1 levels and SPP in the 16 dogs with CH, in which SPP could be measured. Therefore, these results suggest that plasma ET‐1 might be involved in increased portal vein pressure in dogs with CH.
It has been reported that administration of ET‐1‐receptor antagonists reduces portal vein pressure in carbon tetrachloride‐treated mice.35 Additionally, nonselective ET‐1 antagonists exhibit antifibrotic effects.35 The ETB receptor mediates nitric oxide production, which is presumably important in maintaining sinusoidal relaxation in normal liver tissue, whereas in the injured liver, the ETB receptor mediates fibrogenic and contractile responses in hepatic stellate cells. In this state, blocking this effect leads to specific antifibrogenic and portal‐hypotensive responses.35 The efficacy of ET receptor antagonists has been evaluated in dogs with experimental congestive heart failure.36, 37 Therefore, ET‐1 receptor antagonists may be applied to treat dogs with PH caused by CH.
Liver diseases that cause PH in dogs include primary hypoplasia of the portal vein (PHPV), CH, and cirrhosis.1 In humans, idiopathic PH is a disease similar to PHPV in dogs. Although plasma ET‐1 concentrations are high in humans with idiopathic PH, hepatic ET‐1 concentrations and immunoreactivity are low in these patients.38 Nitric oxide levels and expression of vascular cell adhesion molecule‐1 are reportedly elevated in the spleen and blood of patients with idiopathic PH as compared to healthy subjects according to immunochemical staining results. Therefore, to clarify the role of ET‐1 and other vasoactive agents, such as nitric oxide and vascular cell adhesion molecule‐1, in disease pathogenesis, it may be useful to reveal the pathogenic mechanisms in dogs with PHPV to promote the discovery of therapeutic targets.
This study had the following six limitations: (1) a small number of dogs were included, and the controls were of a single breed; (2) all dogs included were diagnosed with CH, although various subtypes of this disease may exist in dogs; (3) we do not know the specific site of ET‐1 production in the liver; (4) although reports have indicated that ET‐1 levels do not increase with age in dogs, in contrast to humans,14, 18 the control beagle dogs used in this study were very young relative to the dogs with CH; (5) we did not apply a grading classification to the stained liver tissues, despite correlations between the severity of liver disease and hepatic and plasma ET‐1 concentrations being reported in humans;11, 13 and (6) despite the MIQE guidelines for real‐time RT‐PCR published in 2009,39 we used only one reference gene in this study.
In conclusion, we found significantly higher plasma ET‐1 levels, hepatic ET‐1 mRNA levels, and hepatic ET‐1 levels in dogs with CH than in control beagle dogs. Based on these results, ET‐1 may be involved in the pathogenesis of PH in dogs with CH. These findings suggest an avenue for the treatment of PH caused by CH in dogs.
Acknowledgments
Conflict of Interest Declaration: The authors declared that there is no conflict of interest.
Off‐label Antimicrobial Declaration: The authors declared that there is no off‐label use of antimicrobials.
This work was completed in the Laboratory of Veterinary Internal Medicine, Department of Veterinary Medicine, College of Bioresource Sciences, Nihon University, Kanagawa, Japan.
A part of this study was supported by the Institutional Program for Young Researcher Overseas Visits from the Japanese Society for the Promotion of Science (JSPS) and by a Grant‐in‐Aid for Young Scientists B (20780228) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan.
Parts of this article were presented at the 2012 ACVIM Forum, New Orleans, LA.
Footnotes
Qiagen, Valencia, CA
Thermo Scientific, Yokohama, Japan
TaKaRa, Shiga, Japan
Applied Biosystems, Branchburg, NJ
IBL, Gunma, Japan
Waters Corporation, Milford, MA
Karl Storz GmbH & Co. KG, Tuttlingen, Germany
SR‐FF1832; length, 6.4 cm; Terumo, Tokyo, Japan
GraphPad Prism; GraphPad Software Inc., San Diego, CA
References
- 1. van den Ingh TSGAM, van Winke T, Cullen JM, et al. Morphological classification of parenchymal disorders of the canine and feline liver. 2. Hepatocellular death, hepatitis and cirrhosis In: WSAVA standardization Group , ed. WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases. Philadelphia, PA: Elsevier, 2006:85–101. [Google Scholar]
- 2. Buob S, Johnston AN, Webster CRL. Portal hypertension: Pathophysiology, diagnosis, and treatment. J Vet Intern Med 2011;25:169–186. [DOI] [PubMed] [Google Scholar]
- 3. Raffan E, McCallum A, Scase TJ, et al. Ascites is a negative prognostic indicator in chronic hepatitis in dogs. J Vet Intern Med 2009;23:63–66. [DOI] [PubMed] [Google Scholar]
- 4. Sevelius E. Diagnosis and prognosis of chronic hepatitis and cirrhosis in dogs. J Small Anim Pract 1995;36:521–528. [DOI] [PubMed] [Google Scholar]
- 5. Poldervaart JH, Favier RP, Penning LC, et al. Primary hepatitis in dogs: A retrospective review (2002–2006). J Vet Intern Med 2009;23:72–80. [DOI] [PubMed] [Google Scholar]
- 6. Masaki T, Yanagisawa M, Goto K. Physiology and pharmacology of endothelins. Med Res Rev 1992;12:391–421. [DOI] [PubMed] [Google Scholar]
- 7. Mallat A, Lotersztajn S. Multiple hepatic functions of endothelin‐1: Physiopathological relevance. J Hepatol 1996;25:405–413. [DOI] [PubMed] [Google Scholar]
- 8. Bosch J, Abraldes JG, Fernández M, et al. Hepatic endothelial dysfunction and abnormal angiogenesis: New targets in the treatment of portal hypertension. J Hepatol 2010;53:558–567. [DOI] [PubMed] [Google Scholar]
- 9. Sanyal AJ, Bosch J, Blei A, et al. Portal hypertension and its complications. Gastroenterology 2008;134:1715–1728. [DOI] [PubMed] [Google Scholar]
- 10. Hennenberg M, Tebicka J, Sauerbruch T, et al. Mechanisms of extrahepatic vasodilation in portal hypertension. Gut 2008;57:1300–1314. [DOI] [PubMed] [Google Scholar]
- 11. Curgunlu A, Vural P, Canbaz M, et al. Plasma nitrate/nitrite and endothelin‐1 in patients with liver cirrhosis. J Clin Lab Anal 2005;19:177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ikura Y, Ohsawa M, Naruko T, et al. Expression of the hepatic endothelin system in human cirrhotic livers. J Pathol 2004;204:304–310. [DOI] [PubMed] [Google Scholar]
- 13. Alam I, Bass NM, Bacchetti P, et al. Hepatic tissue endothelin‐1 levels in chronic liver disease correlate with disease severity and ascites. Am J Gastroenterol 2000;95:199–203. [DOI] [PubMed] [Google Scholar]
- 14. Tessier‐Vetzel D, Tissier R, Chetboul V, et al. Diagnostic and prognostic value of endothelin‐1 plasma concentrations in dogs with heart and respiratory disorders. Vet Rec 2006;158:783–788. [DOI] [PubMed] [Google Scholar]
- 15. Piantedosi D, Cortese L, Loria AD, et al. Plasma atrial natriuretic peptide (pro ANP 31–67), B‐type natriuretic peptide (Nt–pro BNP) and endothelin‐1 (ET‐1) concentrations in dogs with chronic degenerative valvular disease (CDVD). Vet Res Commun 2009;33:S197–S200. [DOI] [PubMed] [Google Scholar]
- 16. O'Sullivan ML, O'Grady MR, Minors SL. Plasma big endothelin‐1, atrial natriuretic peptide, aldosterone, and norepinephrine concentration in normal doberman pinschers and doberman pinschers with dilated cardiomyopathy. J Vet Intern Med 2007;21:92–99. [DOI] [PubMed] [Google Scholar]
- 17. Prošek R, Sisson DD, Oyama MA, et al. Plasma endothelin‐1 immunoreactivity in normal dogs and dogs with acquired heart disease. J Vet Intern Med 2004;18:840–844. [DOI] [PubMed] [Google Scholar]
- 18. Krafft E, Heikkilä HP, Jespers P, et al. Serum and bronchoalveolar lavage fluid endothelin‐1 concentrations as diagnostic biomarkers of canine idiopathic pulmonary fibrosis. J Vet Intern Med 2011;1:1–7. [DOI] [PubMed] [Google Scholar]
- 19. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2−ΔΔCt method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 20. Brinkfoh B, Spee B, Rothuizen J, et al. Development and evaluation of canine reference genes for accurate quantification of gene expression. Anal Biochem 2006;356:36–43. [DOI] [PubMed] [Google Scholar]
- 21. Schellenberg S, Grenacher B, Kaufmann K, et al. Analytical validation of commercial immunoassays for the measurement of cardiovascular peptides in the dog. Vet J 2008;178:85–90. [DOI] [PubMed] [Google Scholar]
- 22. Sakamoto Y, Sakai M, Watari T. Three minimally invasive methods of measuring of portal vein pressure in healthy dogs. J Vet Med Sci 2012;74:1299–1302. [DOI] [PubMed] [Google Scholar]
- 23. Leivas A, Jiménez W, Bruix J, et al. Gene expression of endothelin‐1 and ETA and ETB receptors in human cirrhosis: Relationship with hepatic hemodynamics. J Vasc Res 1998;35:186–193. [DOI] [PubMed] [Google Scholar]
- 24. Uchide T, Saida K. Elevated endothelin‐1 expression in dogs with heartworm disease. J Vet Med Sci 2005;67:1155–1161. [DOI] [PubMed] [Google Scholar]
- 25. Tièche S, Gottardi AD, Kappeler A, et al. Overexpression of endothelin‐1 in bile duct ligated rats: Correlation with activation of hepatic stellate cells and portal pressure. J Hepatol 2001;34:38–45. [DOI] [PubMed] [Google Scholar]
- 26. Kanemoto H, Ohno K, Sakai M, et al. Expression of fibrosis‐related genes in canine chronic hepatitis. Vet Pathol 2011;48:839–845. [DOI] [PubMed] [Google Scholar]
- 27. Nemann S, Kaup FJ, Beardi B. Plasma concentration of transforming growth factor‐β1 and hepatic fibrosis in dogs. Can J Vet Res 2008;72:428–431. [PMC free article] [PubMed] [Google Scholar]
- 28. Gerbes AL, Moller S, Gülberg V, et al. Endothelin‐1 and ‐3 plasma concentration in patients with cirrhosis: Role of splanchnic and renal passage and liver function. Hepatology 1995;21:735–739. [PubMed] [Google Scholar]
- 29. Uchihara M, Izumio N, Sato C, et al. Clinical significance of elevated plasma endothelin concentration in patients with cirrhosis. Hepatology 1992;16:95–99. [DOI] [PubMed] [Google Scholar]
- 30. Moore K, Wendon J, Frazer M, et al. Plasma endothelin immunoreactivity in liver disease and the hepatorenal syndrome. N Engl J Med 1992;327:1774–1778. [DOI] [PubMed] [Google Scholar]
- 31. Fukuroda T, Fujikawa T, Ozaki S, et al. Clearance of circulating endothelin‐1 by ETB receptors in rats. Biochem Biophys Res Commun 1994;199:1461–1465. [DOI] [PubMed] [Google Scholar]
- 32. Beltolini G. Acquired portal collateral circulation in the dog and cat. Vet Radiol Ultrasound 2010;51:25–33. [DOI] [PubMed] [Google Scholar]
- 33. Møller S, Gülberg V, Henriksen JH, et al. Endothelin‐1 and endothelin‐3 in cirrhosis: Relations to systemic and splanchnic heamodynamics. J Hepatol 1995;23:135–144. [DOI] [PubMed] [Google Scholar]
- 34. Hartleb M, Kirstetter P, Moreau R, et al. Relationships between plasma endothelin concentrations and the severity of cirrhosis. Gastroenterol Clin Biol 1994;18:407–412. [PubMed] [Google Scholar]
- 35. Feng HQ, Weymouth ND, Rockey DC. Endothelin antagonism in portal hypertensive mice: Implications for endothelin receptor‐specific signaling in liver disease. Am J Physiol Gastrointest Liver Physiol 2009;297:G27–G33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Borgeson DD, Grantham JA, Williamson EE, et al. Chronic oral endothelin type A receptor antagonism in experimental heart failure. Hypertension 1998;31:766–770. [DOI] [PubMed] [Google Scholar]
- 37. Clavell AL, Mattingly MT, Stevens TL, et al. Angiotensin converting enzyme inhibition modulates endogenous endothelin in chronic canine thoracic inferior vena caval constriction. J Clin Invest 1996;97:1286–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Däbritz J, Worch J, Materna U, et al. Life‐threatening hypersplenism due to idiopathic portal hypertension in early childhood: Case report and review of the literature. BMC Gastroenterol 2010;10:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: Minimum information for publication of quantitative real‐time PCR experiments. Clin Chem 2009;55:611–622. [DOI] [PubMed] [Google Scholar]


