Abstract
Background
Bezafibrate (BZF) is effective in the treatment of hypertriglyceridemia in human patients, but there are no data on its use in dogs.
Objective
To assess the safety of BZF in hyperlipidemic dogs and its efficacy in decreasing serum triglyceride (TG) and cholesterol (CHO) concentrations.
Animals
Forty‐six dogs, 26 females and 20 males, mean (±SD) age of 9 (±3) years, with TG ≥150 mg/dL (33 dogs also were hypercholesterolemic [>300 mg/dL]).
Methods
Prospective, uncontrolled clinical trial. Dogs were treated with bezafibrate once daily, using 200 mg tablets at a dosage of 4–10 mg/kg (depending on body weight). Serum TG and CHO concentrations and alanine aminotransferase (ALT) and creatine kinase (CK) activity before and after 30 days of treatment were compared.
Results
Sixteen dogs (34.8%) had primary hyperlipidemia, and 30 dogs (65.2%) had secondary hyperlipidemia (including spontaneous hyperadrenocorticism [41.3%, n = 19/46], chronic treatment with glucocorticoids [10.8%, n = 5/46], and hypothyroidism [15.2%, n = 7/46]). After 30 days, serum TG concentration normalized (<150 mg/dL) in 42 dogs (91.3%) and CHO concentration normalized (<270 mg/dL) in 22 of 33 dogs (66.7%). There was no difference in baseline TG concentration between the primary and secondary hyperlipidemia subgroups, but the decrease in TG concentration after treatment was greater in the primary hyperlipidemia subgroup. No adverse effects were observed, but ALT activity decreased significantly after 30 days of treatment.
Conclusions and Clinical Importance
Over 30 days, BZF was safe and effective in treatment of primary and secondary hyperlipidemia in dogs.
Keywords: Cholesterol, Hyperadrenocorticism, Peroxisome proliferator‐activated receptor, Schnauzer, Triglyceride
Abbreviations
- ALT
alanine aminotransferase
- BZF
bezafibrate
- CHO
cholesterol
- CK
creatine kinase
- PPAR
peroxisome proliferator‐activated receptor
- TG
triglyceride
- VLDL
very low density lipoproteins
Hyperlipidemia (or dyslipidemia) is defined as an increase in serum triglyceride (TG) concentration (hypertriglyceridemia) or serum cholesterol (CHO) concentration (hypercholesterolemia) or both.1, 2 Hyperlipidemia is common in dogs, and it can be present as a primary or secondary condition. Secondary hyperlipidemia is more common and can be caused by endocrine disorders (e.g, hypothyroidism, hyperadrenocorticism, diabetes mellitus) and less commonly by obesity, pancreatitis, protein‐losing nephropathy, hepatic cholestasis, high fat feeding, and therapy with corticosteroids.3, 4, 5, 6 Primary hyperlipidemia usually is a familial or hereditary disorder of lipoprotein metabolism.1, 2, 7 It has been reported in Miniature Schnauzers, Shetland sheepdogs, Beagles, and other breeds.8, 9, 10 The etiology of primary hyperlipidemia is not well understood. Based on the various phenotypes in different breeds, there is likely >1 etiology. Primary hyperlipidemia might be caused by genetic defects in lipoprotein lipase or absence of apoprotein IIC, but mutations in these genes have not yet been identified in dogs.2, 11, 12
Hyperlipidemia is a challenging disease because most hyperlipidemic dogs are asymptomatic. Two studies investigated primary hyperlipidemia in seemingly healthy Schnauzers and found that the frequency of hyperlipidemia was 32.8% and 56.4%.8 , 1 Therefore, predisposed breeds and dogs with endocrinopathies should be regularly screened for early recognition of this metabolic disorder.
Hyperlipidemia in dogs has been associated with several clinical complications such as pancreatitis, insulin resistance, increased liver enzyme activities, gall bladder mucocele, seizures, behavioral changes, peripheral neuropathies, and ocular abnormalities.13, 14, 15, 16, 17, 18, 19 Hyperlipidemia can be the cause of atherosclerotic disease and endothelial lesions that can lead to cardiovascular dysfunction and affect other organs such as the brain and kidney.20, 21 Because of these potential complications, there is a need for effective therapy to decrease or normalize of the serum TG and CHO concentrations.
The initial approach to treatment of hyperlipidemia consists of a low‐fat diet and, in cases of secondary hyperlipidemia, treatment of the underlying disease. These treatment strategies, however, may be only partially effective, and then, pharmacological intervention should be considered.11 , 2 The choice of a lipid‐lowering drug should be based on the underlying disturbance causing the hyperlipidemia (e.g, excessive fat in the diet, increased free fatty acid production in the liver, increased lipid mobilization, and ineffective clearance of lipids from the blood).11, 22
Bezafibrate is a derivative of fibric acid that is used in people as lipid‐lowering drug. Fibrates are specifically effective in treating hypertriglyceridemia. Mediated by peroxisome proliferator‐activated receptor (PPAR) α, the effects of fibrates include induction of hepatic fatty acid uptake and a decrease in hepatic triglyceride and very low density lipoproteins (VLDL) synthesis, increased activity of lipoprotein lipase, increased gall bladder excretion of hepatic CHO, and increased production of HDL. The combination of decreased production with increased excretion results in substantial lowering of circulating TG (in the form of VLDL) concentration and a slight decrease in CHO concentration.23, 24 Common adverse effects of fibrate therapy in people include muscle pain, emesis, diarrhea, increased creatine kinase (CK) and alanine aminotransferase (ALT) activity, and predisposition to the formation of gallstones.24, 25
Fibrates are the first drug of choice for treatment of moderate‐to‐severe hyperlipidemia in people.23, 25 In dyslipidemic dogs, the therapeutic effects of fibrates were reported only in isolated cases.3 Therefore, the objectives of our study were to investigate the therapeutic efficacy of BZF in decreasing the serum concentrations of TG and CHO and to assess possible adverse effects within a 30‐day treatment period.
Materials and Methods
Ours was a prospective study that included dogs that were admitted to the Veterinary Service of University of Guarulhos, Brazil. The study was approved by the University of Guarulhos Bioethics committee. All dogs presented with hyperlipidemia between January 2011 and December 2011 were considered for inclusion. Dogs were included only if their primary disease status was stable and if treatment of the primary disease could be postponed for 30 days (e.g, levothyroxine for hypothyroidism, trilostane for hyperadrenocorticism). Similarly, dogs that were treated with long‐term glucocorticoid drugs for atopy were included if their treatment regimen was stable and not expected to change during the study. Dogs with increased CK or ALT activity (1× and 3×, respectively, above the reference interval) were excluded because of the risk of hepatotoxicity and rhabdomyolysis in people.24
All blood tests were performed after at least 12 hours of fasting. Dogs were included if they had a diagnosis of hyperlipidemia based on increased TG or CHO concentrations above the laboratory's reference interval (TG ≥150 mg/dL, CHO ≥300 mg/dL). Primary hyperlipidemia was diagnosed based on exclusion of diabetes mellitus, hyperadrenocorticism, hypothyroidism, nephrotic syndrome, and therapy with glucocorticoid drugs or phenobarbital. Tests for diagnosis and exclusion of these diseases were performed at the discretion of the attending clinician based on clinical presentation (including CBC, biochemistry panels, dexamethasone suppression test [DST], serum total thyroxine concentrations [TT4], thyroid‐stimulating hormone [TSH] concentrations, and abdominal ultrasonography).26
All dogs were treated with BZF once daily, immediately after the main meal for 30 days. Because there are no standardized dosages for BZF in the veterinary literature, and because BZF was available only as 200 mg tablets, the dosage was extrapolated from people and based on body weight category as described in Table 1.
Table 1.
Dosing regimen of bezafibrate based on body weight
| Body Weight | Bezafibrate 200 mg tablet, once daily | |
|---|---|---|
| Dose in mg | Tablet scoring | |
| ≤12 kg | 50 | ¼ |
| 12.1–25 kg | 100 | ½ |
| >25 kg | 200 | 1 |
Serum concentrations of TG and CHO as well as ALT and CK activity were measured before treatment and at the end of 30 days of treatment.24 All biochemical tests were performed on an automated biochemistry analyzer3 in the clinical pathology laboratory at the School of Veterinary Medicine, Guarulhos University, São Paulo, Brazil. All hormone tests were performed in a reference laboratory.4
Statistical analysis was performed using commercially available computer software.5 All variables were calculated for the group of 46 hyperlipidemic dogs and then separately for the 2 subgroups of primary hyperlipidemia dogs and secondary hyperlipidemia dogs. The change in TG, CHO, ALT, and CK from time zero (T0: before initiation of treatment) to time 30 (T30: 30 days after initiation of treatment) was defined as ∆TG, ∆CHO, ∆ALT, and ∆CK, respectively. Data are reported as mean ± SD, and median (range). Data were assessed for normal distribution using the Shapiro–Wilk test. All data were not normally distributed, and therefore, all comparisons were performed with nonparametric tests. The Wilcoxon matched‐pairs signed rank test was used to compare the change from before and after treatment. The Mann‐Whitney U‐test (nonpaired) was used to compare groups of dogs with primary vs. secondary hyperlipidemia. Significance level was set at P < .05.
Results
Forty‐six dogs were included (26 female and 20 males) with a mean (±SD) age of 9 ± 3 years. Most dogs were purebred including Miniature Schnauzer (n = 11), Poodle (n = 8), Cocker Spaniel (n = 4), Dachshund (n = 4), Yorkshire Terrier (n = 3), Shetland Sheepdog (n = 2), Beagle (n = 2), Maltese (n = 2), 1 Bichon Frise (n = 1), Lhasa Apso (n = 1), Rottweiler (n = 1), and Labrador Retriever (n = 1). Sixteen dogs (34.8%) were diagnosed with primary hyperlipidemia (all Miniature Schnauzers and Shetland Sheepdogs and 3 Cocker Spaniels) and 30 (65.2%) with secondary hyperlipidemia (hyperadrenocorticism, n = 19/46 [41.3%]; hypothyroidism, n = 7/46 [15.2%], long‐term treatment with glucocorticoid drugs, n = 5/46 [10.8%]). No diabetic dogs were included. All dogs with hypothyroidism or hyperadrenocorticism were newly diagnosed at the time of inclusion and were not treated for their underlying disease during the 30 days of the study. All 46 dogs (100%) were hypertriglyceridemic at the time of inclusion, and 30 (65.2%) of them also were hypercholesterolemic.
All 46 dogs completed the trial. No adverse effects were reported by the owners, and there was no change in body weight or body condition score during the study.
The BZF dosage was 6.0 ± 1.5 mg/kg (range, 4–10 mg/kg). At the end of the trial, 42 dogs had normal serum TG concentration (42/46 = 91.3%). Of the 30 dogs that were initially hypercholesterolemic, 20 had normal serum CHO concentrations at the end of the trial (66.7%). Serum TG concentrations in the 46 dogs decreased significantly from median (range) of 487 (350–4356) mg/dL at T0 to 92 (41–443) mg/dL at T30 (P = .0001; Table 2; Fig 1). Compared to T0, TG was decreased at T30 by a median (range) of 84% (68–95%). There was a strong negative correlation between TG at T0 and ∆TG (r = −0.995, P < .0001; Fig 2). There was also a significant decrease in serum CHO from 352 (125–973) mg/dL to 238 (131–491) mg/dL after treatment (median decrease 32% [ranging from an increase of 24% to a maximum decrease of 73%, P = .0001; Fig 3). Baseline CHO concentration was negatively correlated to ∆CHO (r = −0.95, P < .0001; Fig 4). In dogs that were hypercholesterolemic at T0 (n = 30), CHO decreased at T30 by 45% (13–73%, P = .001), and in 66% of them, CHO concentrations were <300 mg/dL. Of the 16 dogs that had normal CHO at T0, CHO decreased significantly (P = .01) from baseline but still remained in the normal range at T30, with a median decrease of 12% (ranging from a 24% increase to a 41% decrease). Five of these dogs had an increase in CHO at T30 (range at T30, 155–260mg/dL).
Table 2.
Serum concentrations of triglycerides (mg/dL), cholesterol (mg/dL), ALT activity (U/L), and CK activity (U/L) before (T0) and after 30 days (T30) use of bezafibrate
| T0 | T30 | P value | |
|---|---|---|---|
| TG | |||
| Mean ± SD | 752 ± 663 | 110 ± 82 | .0001 |
| Median (Range) | 487 (350–4,356) | 91.5 (41–443) | |
| CHO | |||
| Mean ± SD | 428 ± 217 | 244 ± 72 | .0001 |
| Median (Range) | 352 (125–973) | 238 (131–491) | |
| ALT | |||
| Mean ± SD | 128 ± 96 | 100 ± 56 | .01 |
| Median (Range) | 87 (14–422) | 93 (14–312) | |
| CK | |||
| Mean ± SD | 105 ± 32 | 111 ± 42 | .34 |
| Median (Range) | 95 (57–178) | 107 (57–274) | |
CK, creatine kinase; CHO, cholesterol; ALT, alanine aminotransferase transaminase; TG, triglycerides; SD, standard deviation.
Figure 1.
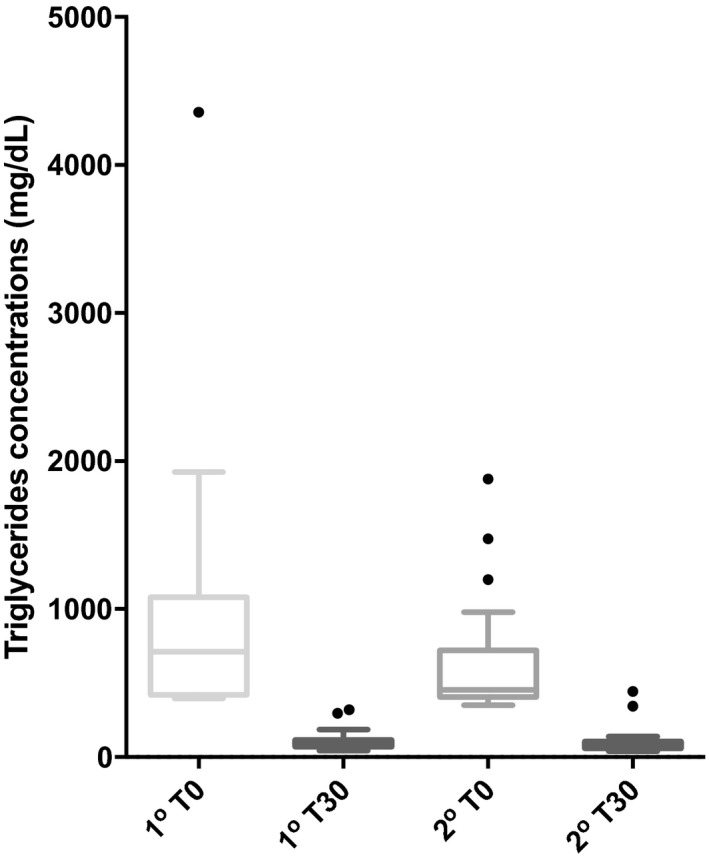
Box‐and‐whiskers graph of serum concentrations of triglycerides (TG), before (T0) and 30 days after (T30) therapy with bezafibrate in 15 dogs with primary hyperlipidemia (1o) and 31 dogs with secondary hyperlipidemia (2o). Boxes represent 25th and 75th percentiles with median in between. The whiskers represent values at 1.5× IQR (interquartile range) and the individual points represent outliers.
Figure 2.
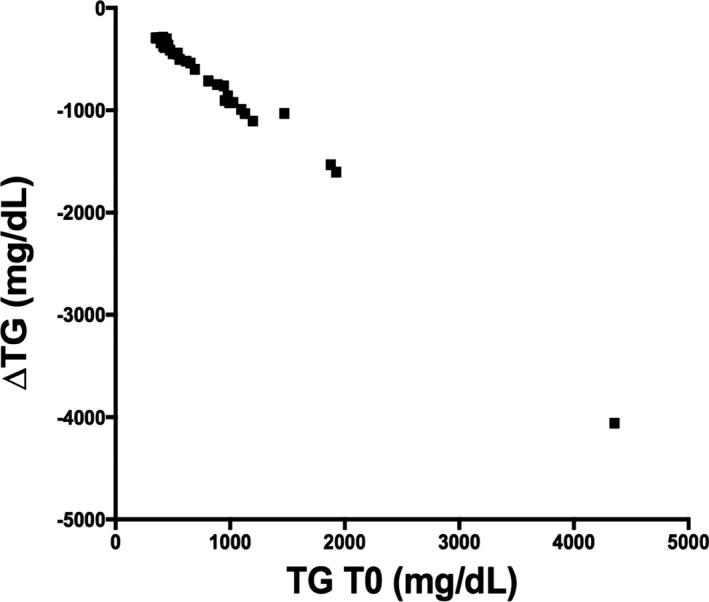
Correlation between TG at T0 and the change in TG from T0 to T30.
Figure 3.
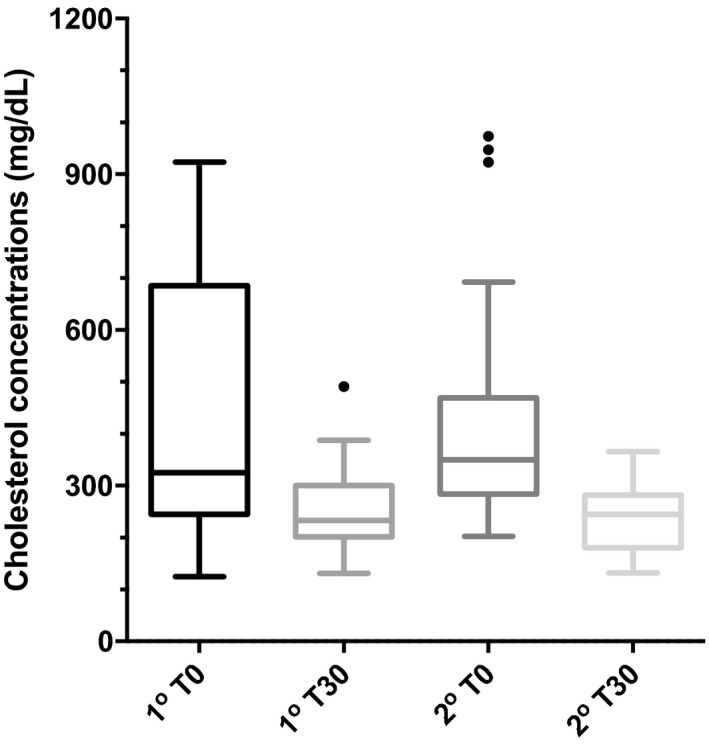
Box‐and‐whiskers graph of serum concentrations of cholesterol (CHO), before (T0) and 30 days after (T30) therapy with bezafibrate in 15 dogs with primary hyperlipidemia (1st) and 31 dogs with secondary hyperlipidemia (2nd). Boxes represent 25th and 75th percentiles with median in between. The whiskers represent values at 1.5× IQR (interquartile range) and the individual points represent outliers.
Figure 4.
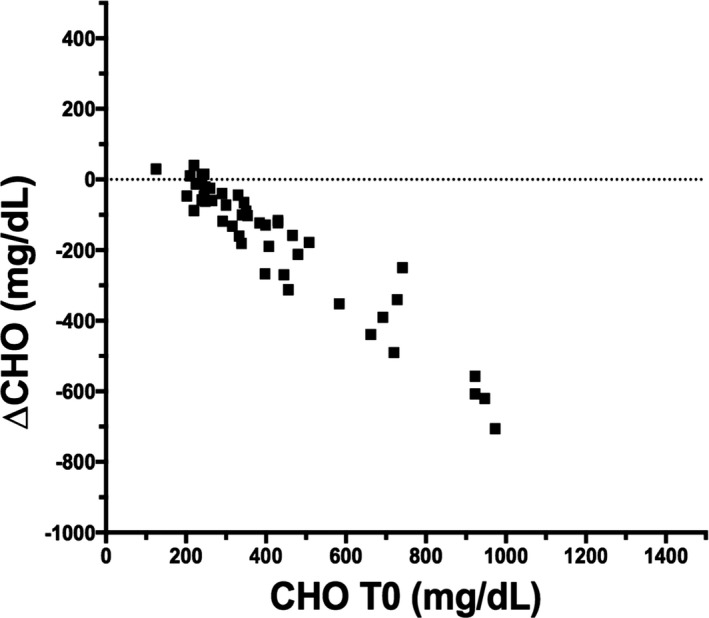
Correlation between CHO at T0 and the change in CHO from T0 to T30.
Comparing primary and secondary hyperlipidemia groups, there was no difference in baseline CHO (P = .7) or baseline TG (P = .1). However, ∆TG was higher in the primary group (median [range] = −616 [−307 to −4059] mg/dL) vs. the secondary group (−379 [−288 to −1533] mg/dL, P = .046). In contrast, there was no difference in ∆CHO between primary and secondary groups (P = .4).
There was a significant decrease in ALT activity (P = .01) in the group as a whole (Fig 5). In the secondary hyperlipidemia subgroup, ALT activity decreased significantly (P = .001), but in the primary hyperlipidemia subgroup, the decrease in ALT was not significant (P = .07). There was no significant difference in ∆ALT between the subgroups (P = .8). There was no change in CK activity (P = .3) in the group as a whole or in the 2 subgroups (data not shown).
Figure 5.
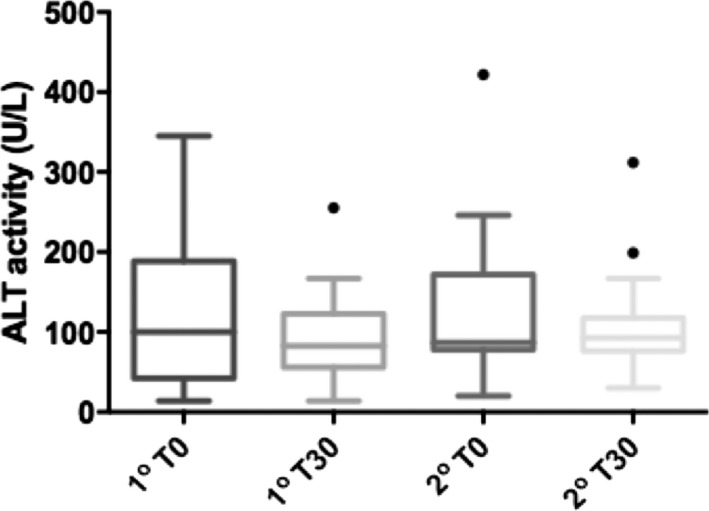
Box‐and‐whiskers graph of serum ALT activity before (T0) and 30 days after (T30) therapy with Bezafibrate in 15 dogs with primary hyperlipidemia (1o) and 31 dogs with secondary hyperlipidemia (2o). Boxes represent 25th and 75th percentiles with median in between. The whiskers represent values at 1.5× IQR (interquartile range) and the individual points represent outliers.
Discussion
Treatment options for hyperlipidemia in dog are limited. We evaluated the effect of BZF in a large group of hyperlipidemic dogs. Normalization of serum TG concentrations (≤150 mg/dL) was observed in 91.3% of dogs, with a median decrease of 84% within 1 month. This a larger effect than observed in people (based on meta‐analysis) in which serum TG decreased by 14–38%.27 The study population in people, however, was mostly composed of patients with cardiovascular disease, with a minority of type 2 diabetics. The goal of fibrates in these cardiovascular patients was not to decrease their TG concentration, but to decrease their total CHO concentration and increase their HDL concentration. Indeed, in these studies, BZF led to a 26–52% increase in HDL.27
Hypercholesterolemia was not the main target of treatment in our study, but it also resolved in the majority of dogs (66%, n = 20/30). Cholesterol concentrations decreased even in dogs that started the study with normal CHO. Our study cannot determine whether this observation is a result of improvement in TG or an independent effect. With these results, however, and considering the mechanism of action of fibrates, it seems reasonable to also study the effect of BZF in dogs that are hypercholesterolemic but not hypertriglyceridemic. Fibrates alter free fatty acid (FFA) metabolism by activating PPAR, especially PPARα.24 As a result, fibrates augment the oxidation of FFA in the liver and divert them from TG synthesis, which leads to decrease in synthesis of VLDL. Peripherally, fibrates increase the expression of the LPL gene, with consequent increase in HDL production and stimulation of reverse CHO transport.23, 24
The use of lipid‐lowering drugs in general, and fibrates in particular, has seldom been reported in dogs. Gemfibrozil6 was one of the first fibrates to be used in dogs with hypertriglyceridemia (10 mg/kg q12h PO), but there are no studies evaluating the efficacy and safety of gemfibrozil in dogs. Anecdotally, gemfibrozil in dogs has been associated with various adverse effects (e.g, vomiting, diarrhea, decreased appetite). Moreover, clinical experience suggests that the lipid‐lowering effect of gemfibrozil is insufficient in some dogs with primary hyperlipidemia.1, 11 In people, gemfibrozil is less efficacious than BZF and 15.8% of patients discontinue using it because of gastrointestinal adverse effects.28 To date, there are no reported studies in dogs comparing the use of different lipid‐lowering drugs, and only a few isolated reports on fenofibrate and BZF.11, 29 , 3 In experimentally induced hyperlipidemia, fenofibrate (10 mg/kg PO immediately after feeding) was associated with a significant decrease in TG and CHO in 6 obese beagles. Dogs with higher baseline lipid concentrations had the largest responses,30 as observed in our study with the use of BZF.
In people, BZF is administered every 8 or 12 hours, with food.24 The use of BZF for treatment of hyperlipidemia in dogs was first reported in 2003 in 2 Miniature Schnauzers that were not responsive to dietary therapy.7 In these 2 cases, BZF administered at a dosage of 2.5 mg/kg q12h was associated with marked decrease in TG (from 891 mg/dL and 4,398 mg/dL pretreatment to 306 mg/dL and 419 mg/dL after treatment, respectively). We used a higher dosage and once daily administration because BZF was only available commercially as a 200 mg tablet8 and we wanted to avoid scoring of tablets inaccurately or compounding the drug. Apparently, this more convenient regimen still is effective and nontoxic.
Adverse effects most frequently associated with the use of fibrates in humans are muscle pain, emesis, diarrhea, increased CK and ALT activity, and increased risk of biliary calculi. Fibrates therefore are contraindicated in patients with renal dysfunction and in those with liver and gallbladder diseases.24, 25 None of these adverse effects, however, were observed in our study. Moreover, ALT activity decreased in dogs that first presented with increased ALT activity. This improvement in ALT is likely related to a decrease in circulating TG concentrations and improvement of hepatic lipidosis.23, 24 Contrary to what is observed in people, myopathy secondary to fibrate therapy seems infrequent in dogs.29 We found no clinical signs associated with myopathy and no increase in CK activity in our study. Importantly, the lack of adverse effects (and indeed, even the improvement in ALT activity) should be interpreted with caution, considering the relatively short duration of this study. Regardless of the underlying disease that is causing hyperlipidemia, most dogs that were in need of BZF therapy most likely would need lifelong therapy for their hyperlipidemia. Therefore, the safety and efficacy of BZF should be further evaluated in long‐term clinical trials before final conclusions are drawn regarding its use.
One limitation of our study was lack of uniform screening for causes of secondary hyperlipidemia. Screening tests were done at the discretion of the attending clinician. As such, cases of secondary hyperlipidemia might have been categorized incorrectly as primary. Doing so could have obscured differences in response to treatment, but even so, the magnitude of decrease in hypertriglyceridemia was higher in cases of primary vs. secondary hyperlipidemia. This finding could be explained by the fact that in secondary dyslipidemias, hormonal influence contributes a more negative impact on the genesis of hyperlipidemia. In hypercortisolism (naturally occurring and iatrogenic) in dogs, for example, about 75% of patients are hypercholesterolemic, hypertriglyceridemic, or both because of the stimulation of lipolysis of visceral fat by glucocorticoids, increased activity of hormone‐sensitive lipase and impaired removal of plasma TG due to inhibition of lipoprotein lipase.2, 3 Similarly, about 80% of dogs with hypothyroidism are hyperlipidemic.30 This hyperlipidemia is caused by a decrease in the lipid degradation rate and increased lipid synthesis, decreased biliary excretion of CHO, and a decrease in the activity of lipoprotein lipase, leading to the accumulation of HDL, LDL, and VLDL.11, 30 The difference in magnitude of decrease between groups also could be related to progression of the underlying disease in the secondary hyperlipidemia group. The underlying disease was not treated during the 30 days of the study, which could have offset the benefit of BZF treatment.
In primary hyperlipidemia cases, or in cases of secondary hyperlipidemia that fail to completely respond to treatment of the underlying disease, dietary fat restriction usually is the next line of treatment in hyperlipidemic dogs. In those cases in which dietary fat restriction is ineffective, lipid‐lowering drugs are indicated, especially with the advent of safe and effective drugs.11, 22 Based on the results of our study, we conclude that once daily BZF at a dosage of 4–10 mg/kg is effective in controlling hypertriglyceridemia and hypercholesterolemia in dogs with primary and secondary dyslipidemia. No clinical adverse effects were observed in our study as well as no increases in CK and ALT activity during the treatment period. Long‐term evaluation of BZF therapy in dogs with hyperlipidemia is necessary to determine its safety and efficacy not only in decreasing serum lipid concentrations in the blood but also in preventing the complications of hyperlipidemia.
Acknowledgments
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Where the work was done: Veterinary Hospital of University of Guarulhos.
Study support: none.
Meeting presentations: 2013 ACVIM Forum, Seattle, WA.
Footnotes
De Marco V, Netto MFG, Mello GFR, et al. Investigation of basal and postprandial hypertriglyceridemia and insulin resistance in healthy Miniature Schnauzers (abstract). J Vet Intern Med 2013;27:694
Xenoulis PG, Suchodolski, J. S., Steiner, J. M., et al. Effect of a low‐fat diet on serum triglyceride, cholesterol, and pancreatic lipase immunoreactivity concentrations in Miniature Schnauzers with hypertriglyceridemia (abstract). J Vet Intern Med 2011;25:687
SB‐190, CELM
Provet laboratory – Veterinary Medicine Diagnostics, Moema, São Paulo, Brazil
GraphPadPrism; GraphPad Software Inc, CA, USA, and SPSS 14.0 for Mac; SPSS Inc 2005, Chicago, IL
Lopid®, Pfizer Inc
Jericó MM, Maschietto LA. Emprego do Bezafibrato no tratamento da hipertrigliceridemia primária em Schnauzer. Relato de 2 casos (abstract). Brazil J Vet Res Anim Sci 2003;40:193
Cedur®, Roche Ltd
References
- 1. Bauer JE. Evaluation and dietary considerations in idiopathic hyperlipidemia in dogs. J Am Vet Med Assoc 1995;206:1684–1688. [PubMed] [Google Scholar]
- 2. Xenoulis PG, Steiner JM. Lipid metabolism and hyperlipidemia in dogs. Vet J 2010;183:12–21. [DOI] [PubMed] [Google Scholar]
- 3. Feldman EC, Nelson RW. Canine and Feline Endocrinology and Reproduction, 3rd ed Philadelphia, PA: WB Saunders; 2004, p. 86–538. [Google Scholar]
- 4. Jeusette IC, Lhoest ET, Istasse LP, Diez MO. Influence of obesity on plasma lipid and lipoprotein concentrations in dogs. Am J Vet Res 2005;66:81–86. [DOI] [PubMed] [Google Scholar]
- 5. Jericó MM, Chiquito FDC, Kajihara K, et al. Chromatographic analysis of the lipid fractions in healthy dogs and dogs with obesity or hyperadrenocorticism. J Vet Diagn Invest 2009;21:203–207. [DOI] [PubMed] [Google Scholar]
- 6. Ford RB, Ludlow CL. Disorders of lipid metabolism In: Hand MS, Thatcher CD, Remillard RL, Roudebush P, Novotny BJ, eds. Small Animal Clinical Nutrition. Topeka, KS: Mark Morris Institute; 2010:545–557. [Google Scholar]
- 7. Xenoulis PG, Suchodolski JS, Levinski MD, Steiner JM. Investigation of hypertriglyceridemia in healthy miniature schnauzers. J Vet Intern Med 2007;21:1224–1230. [DOI] [PubMed] [Google Scholar]
- 8. Rogers WA, Donovan EF, Kociba GJ. Idiopathic hyperlipoproteinemia in dogs. J Am Vet Med Assoc 1975;166:1087–1091. [PubMed] [Google Scholar]
- 9. Sato K, Agoh H, Kaneshige T, et al. Hypercholesterolemia in Shetland sheepdogs. J Vet Med Sci 2000;62:1297–1301. [DOI] [PubMed] [Google Scholar]
- 10. Wada M, Minamisono T, Ehrhart LA, et al. Familial hyperlipoproteinemia in Beagles. Life Sci 1977;20:999–1008. [DOI] [PubMed] [Google Scholar]
- 11. Xenoulis PG, Steiner JM. Canine hyperlipidaemia. J Small Anim Pract 2015;56:595–605. [DOI] [PubMed] [Google Scholar]
- 12. Schickel R. Identification of the nucleotide sequence of the lipoprotein lipase gene as well as its role in the development of hyperlipidemia and pancreatitis in the Miniature Schnauzer. (Dissertation). Munich, Germany: Ludwig‐Maximilians University; 2005. [Google Scholar]
- 13. Aguirre AL, Center SA, Randolph JF, et al. Gallbladder disease in Shetland sheepdogs: 38 cases (1995–2005). J Am Vet Med Assoc 2007;231:79–88. [DOI] [PubMed] [Google Scholar]
- 14. Xenoulis PG, Suchodolski JS, Levinski MD, Steiner JM. Serum liver enzyme activities in healthy miniature schnauzers with and without hypertriglyceridemia. J Am Vet Med Assoc 2008;232:63–67. [DOI] [PubMed] [Google Scholar]
- 15. Xenoulis PG, Levinski MD, Suchodolski JS, Steiner JM. Association of hypertriglyceridemia with insulin resistance in healthy miniature schnauzers. J Am Vet Med Assoc 2011;238:1011–1016. [DOI] [PubMed] [Google Scholar]
- 16. Xenoulis PG, Levinski MD, Suchodolski JS, Steiner JM. Serum triglyceride concentrations in miniature schnauzers with and without a history of probable pancreatitis. J Vet Intern Med 2011;25:20–25. [DOI] [PubMed] [Google Scholar]
- 17. Kutsunai M, Kanemoto H, Fukushima K, et al. The association between gall bladder mucoceles and hyperlipidaemia in dogs: A retrospective case control study. Vet J 2014;199:76–79. [DOI] [PubMed] [Google Scholar]
- 18. Vitale CL, Olby NJ. Neurologic dysfunction in hypothyroid, hyperlipidemic Labrador retrievers. J Vet Intern Med 2007;21:1316–1322. [DOI] [PubMed] [Google Scholar]
- 19. Crispin SM. Ocular manifestations of hyperlipoproteinemia. J Small Anim Pract 1993;34:500–506. [Google Scholar]
- 20. Hess RS, Kass PH, Van Winkle TJ. Association between diabetes mellitus, hypothyroidism or hyperadrenocorticism, and atherosclerosis in dogs. J Vet Intern Med 2003;17:489–494. [DOI] [PubMed] [Google Scholar]
- 21. Furrow E, Jaeger JQ, Parker VJ, et al. Proteinuria and lipoprotein lipase activity in Miniature Schnauzer dogs with and without hypertriglyceridemia. Vet J 2016;212:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elliot DA. Dietary and medical considerations in hyperlipidemia In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. St. Louis, MO: Saunders Elsevier; 2005:592–595. [Google Scholar]
- 23. Staels B, Dallongeville J, Auwerx J, et al. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 1998;98:2088–2093. [DOI] [PubMed] [Google Scholar]
- 24. Sando KR, Knight M. Nonstatin therapies for management of dyslipidemia: A review. Clin Ther 2015;37:2153–2179. [DOI] [PubMed] [Google Scholar]
- 25. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012;97:2969–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Behrend EN. Canine hyperadrenocorticism In: Feldman EC, Nelson RW, Reush CE, et al., eds. Canine and Feline Endocrinology, 4th ed St Louis, MO: WB Saunders; 2015:377–451. [Google Scholar]
- 27. Abourbih S, Filion KB, Joseph L, et al. Effect of fibrates on lipid profiles and cardiovascular outcomes: A systematic review. Am J Med 2009;122:962.e1–962.e8. [DOI] [PubMed] [Google Scholar]
- 28. Beggs PW, Clark DW, Williams SM, Coulter DM. A comparison of the use, effectiveness and safety of bezafibrate, gemfibrozil and simvastatin in normal clinical practice using the New Zealand Intensive Medicines Monitoring Programme (IMMP). Br J Clin Pharmacol 1999;47:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Serisier S, Briand F, Ouguerram K, et al. Fenofibrate lowers lipid parameters in obese dogs. J Nutr 2006;136(7 Suppl):2037S–2040S. [DOI] [PubMed] [Google Scholar]
- 30. Mooney CT, Shiel RE. Canine hypothyroidism In: Mooney CT, Peterson ME, eds. BSAVA Manual of Canine and Feline Endocrinology, 4th ed Gloucester, UK: British Small Animal Veterinary Association; 2012:63–85. [Google Scholar]


