Abstract
Background
The assessment of left atrial (LA) function by 2‐dimensional speckle tracking echocardiography (STE) holds important clinical implications in human medicine. Few similar data are available in dogs.
Objectives
To assess LA function by STE in dogs with and without myxomatous mitral valve disease (MMVD), analyzing LA areas, systolic function, and strain.
Animals
One hundred and fifty dogs were divided according to the American College of Veterinary Internal Medicine classification of heart failure: 23 dogs in class A, 52 in class B1, 36 in class B2, and 39 in class C + D.
Methods
Prospective observational study. Conventional morphologic and Doppler variables, LA areas, and STE‐based LA strain analysis were performed in all dogs and results were compared among groups. Correlation analysis was carried out between LA STE variables and other echocardiographic variables.
Results
Variability study showed good reproducibility for all the tested variables (coefficient of variation <16%). Left atrial areas, fractional area change, peak atrial longitudinal strain (PALS), peak atrial contraction strain, and contraction strain index (CSI) differed significantly between groups B2 and C + D and all the other groups (overall P < .001), whereas only PALS differed between groups B1 and A (P = .01). Left atrial areas increased with progression of the disease, whereas LA functional parameters decreased. Only CSI increased nonsignificantly from group A to group B1 and then progressively decreased. Thirty‐one significant correlations (P < .001, r > .3) were found between conventional left heart echocardiographic variables and LA areas and strain variables.
Conclusions and Clinical Importance
Left atrial STE analysis provides useful information on atrial function in the dog, highlighting a progressive decline in atrial function with worsening of MMVD.
Keywords: Canine, Cardiology, Left atrium, Strain
Abbreviations
- 2D
2‐dimensional
- Ao
aortic root
- BW
body weight
- CHF
congestive heart failure
- CSI
contraction strain index
- CV
coefficient of variation
- FAC
fractional area change
- LAAmax
left atrial maximal area
- LAAmin
left atrial minimal area
- LA
left atrium
- LV
left ventricle
- MMVD
myxomatous mitral valve disease
- MR
mitral regurgitation
- MV
mitral valve
- PACS
peak atrial contraction strain
- PALS
peak atrial longitudinal strain
- STE
speckle tracking echocardiography
- TDI
tissue Doppler imaging
- TTE
transthoracic echocardiography
The left atrium (LA) plays an essential role in cardiac performance by means of 3 main functions, namely reservoir, conduit, and booster pump function.1 Reservoir phase represents the collection of pulmonary venous flow during ventricular systole, conduit phase is the passage of blood from the pulmonary veins to the left ventricle (LV) in early diastole, whereas booster pump function represents the active contraction in late diastole. Assessment of LA function is essential for clinical evaluation and prognostic purposes in dogs affected by myxomatous mitral valve disease (MMVD).2 Several methods have been used to assess LA function in humans, including 2‐dimensional (2D) and 3‐dimensional echocardiography, computed tomography, and magnetic resonance.3, 4 In dogs, the majority of studies have been focused on conventional echocardiographic variables, such as calculation of LA phasic sizes (areas and volumes),5, 6, 7, 8 and pulsed‐wave Doppler evaluation of transmitral and pulmonary venous flow.9 Only 1 study evaluated the prognostic relevance of LA function in dogs with MMVD, showing a worse prognosis in those animals with a poorer LA systolic function.2 However, most of the studied echocardiographic parameters of LA function are highly preload dependent and they are influenced by changes in hemodynamic load associated with mitral regurgitation (MR), thus raising the need for more sophisticated echocardiographic techniques.10 Assessment of atrial deformation profiles obtained by tissue Doppler imaging (TDI) and its derived variables, strain and strain rate, has been recently proposed as an alternative method of exploring LA mechanics in both humans3, 4, 10 and dogs.11 Nevertheless, a number of potential drawbacks of this approach should be considered, including suboptimal reproducibility, angle dependence, and the confounding effect of noise artifacts.3, 4, 10, 11 Many of these limitations might be overcome by 2D speckle tracking echocardiography (STE), the most recent and promising ultrasound technology for the direct evaluation of LA function from standard grayscale echocardiographic images.3, 4, 10 Specifically, regional myocardial deformation can be calculated from continuous frame‐by‐frame tracking of the tiny echo‐dense speckles created by the interference between the ultrasound beam and myocardium. The movement of the speckle during the cardiac cycle follows myocardial movement, and a change between speckles is assumed to represent myocardial deformation. In this manner, STE allows for the assessment of myocardial function independent of cardiac translation and angle of insonation.3, 4, 10 In people, reference range values, feasibility, and reproducibility of STE for the study of LA mechanics have been extensively documented.12, 13, 14, 15 Furthermore, the clinical and prognostic importance of STE variables has been demonstrated in numerous pathophysiologic conditions typically associated with abnormal LA function, including MMVD, atrial fibrillation, systemic hypertension, and dilated, as well as hypertrophic, cardiomyopathy.3, 4, 10, 16, 17 The majority of STE studies in dogs to date focused on the evaluation of mechanical ventricular properties.18, 19, 20, 21, 22, 23, 24, 25, 26, 27 Few data are available regarding the application of this technique for LA assessment28, 29, 30, and no studies have focused on the evaluation of LA STE‐derived strain in dogs with cardiac disease.
Therefore, the aims of this study were (1) to document the feasibility and reproducibility of STE in the assessment of LA function in dogs; (2) to compare selected STE indices of LA function between healthy dogs and dogs with MMVD at different stages; and (3) to evaluate the association between these indices and traditional echocardiographic variables.
Materials and Methods
Animals
Dogs were presented to the cardiology unit of the Veterinary Teaching Hospital of the University of Bologna for cardiac evaluation on the basis of cardiac murmur assessment, clinical signs referable to heart disease (ie, dyspnea, cough, and syncope), for breed screening, or for pre‐anesthetic assessment. Dogs were prospectively recruited from January 2011 to December 2014 and included in the study if they had clinical and echocardiographic signs typical for MMVD and a body weight (BW) <21 kg. Dogs with congenital cardiac malformations, dilated cardiomyopathy, or other acquired cardiac diseases were excluded. Dogs were permitted to receive cardiac medications for congestive heart failure (CHF) if considered necessary by the clinician. Healthy dogs were recruited from students, university staff, and owners as the control group. These animals were considered clinically healthy based on cardiovascular examination, noninvasive systemic arterial blood pressure measurements, and complete transthoracic echocardiographic examination.
Procedures
All dogs underwent a complete physical examination, cardiac auscultation, and noninvasive systemic arterial blood pressure measurement. Complete transthoracic echocardiography (TTE) was performed according to standard techniques31 by 2 investigators (MBT and MC) using ultrasound units1 , 2 equipped with phased array transducers of various frequencies and continuous ECG tracing. The diagnosis of MMVD was made based on clinical examination and echocardiographic criteria including thickening, prolapse, or both thickening and prolapse of the mitral valve (MV) leaflets on 2D echocardiography and MR on color Doppler examination. Wall thickness and chamber diameter of the LV were obtained from 2D guided M‐mode images acquired from a right parasternal short‐axis view at the level of the papillary muscles. In particular, LV end‐diastolic and end‐systolic dimensions were normalized to the aortic root (Ao) diameter and compared with previously published reference intervals, in order to identify the presence of LV dilatation.32 Left atrial diameter, Ao diameter, and Ao area were measured on 2D images obtained from a right parasternal short‐axis view at the basilar level,5 and a LA/Ao ratio <1.5 was considered normal.33 Left ventricular diastolic inflow was recorded from the left apical 4‐chamber view placing the sample volume at the level of the MV on the ventricular side. Three consecutive measurements were averaged for each variable. Thoracic radiography in 2 orthogonal projections was performed to exclude or confirm the presence of CHF (pulmonary edema, pleural effusion, or both) in all dogs with left atrial dilatation or according to clinical signs of cough, dyspnea, or both. Radiographic evidence of interstitial opacity in the caudo‐dorsal fields in the presence of cardiomegaly was considered indicative of CHF. If CHF was present at the time of presentation, dogs were first stabilized and a complete TTE was then performed for the first time or repeated. Only dogs in sinus rhythm during echocardiography were included in the study.
According to the guidelines for the diagnosis and treatment of MMVD,34 dogs were divided into different groups as follows: control, healthy dogs with normal LV and LA dimensions (group A); dogs having MMVD with normal LV and LA dimensions (group B1); dogs having MMVD with LV, LA, or both dimensions above the reference limits32, 33 but without confirmed radiographic evidence of CHF (group B2); dogs with symptomatic MMVD and radiographic evidence of pulmonary edema/pleural effusion; or dogs with at least 1 confirmed episode of CHF in the past (group C + D).
Left Atrial Areas and Speckle Tracking Echocardiography
The same operator (MBT) that was blinded to the dogs’ classification performed this part of the analysis. For the analysis of LA areas and STE, a left apical 4‐chamber view was optimized in order to visualize the LA walls. Then, 3 consecutive cardiac beats cine loops were acquired and stored in DICOM format for subsequent offline analysis. Dedicated software3 was used to review the cine loops, the beat with the best image quality was chosen, and the endocardial border of the LA was automatically drawn by the software and LA areas provided during the entire cardiac cycle. If necessary, manual editing of the tracing was done in order to correct software errors in tracking the endocardial border. Left atrial maximal area (LAAmax) and minimal area (LAAmin) expressed in cm2 were automatically calculated by the software and were then indexed to Ao area. Left atrial fractional area change (FAC) was calculated with the formula: FAC = [(LAAmax − LAAmin)/LAAmax] × 100, and expressed as a percentage.2
The same cine loops and beats used to measure LA areas were successively employed for the STE analysis. The operator selected 3 points on the LA (2 on the MV annulus and 1 on the LA roof), and the software automatically drew a region of interest on the entire LA wall dividing it into 7 regions (from the basilar segments to the roof of the LA) (Fig 1). When necessary, the operator manually edited the tracking of each segment, and those segments that showed artifactual values (due to lung interposition or echo dropout at the level of the region of the fossa ovalis or the pulmonary veins inlet) were excluded from the analysis. The number of segments considered for each dog was recorded. Time/intensity curves were then generated for each segment displacing strain values (as percentages) on the y‐axis and time (in seconds) on the x‐axis over an entire cardiac cycle. The software also generated a mean curve of all the segments that was used by the operator to measure the peak strain value during LV contraction (peak atrial longitudinal strain [PALS]), and just before LA active contraction, on the peak of the P wave of the ECG trace (peak atrial contraction strain [PACS]). Finally, the contraction strain index (CSI) was calculated by the formula: CSI = [(PALS/PACS) × 100] (Fig 1). This last parameter represents the contribution of LA contraction on LV filling.17
Figure 1.
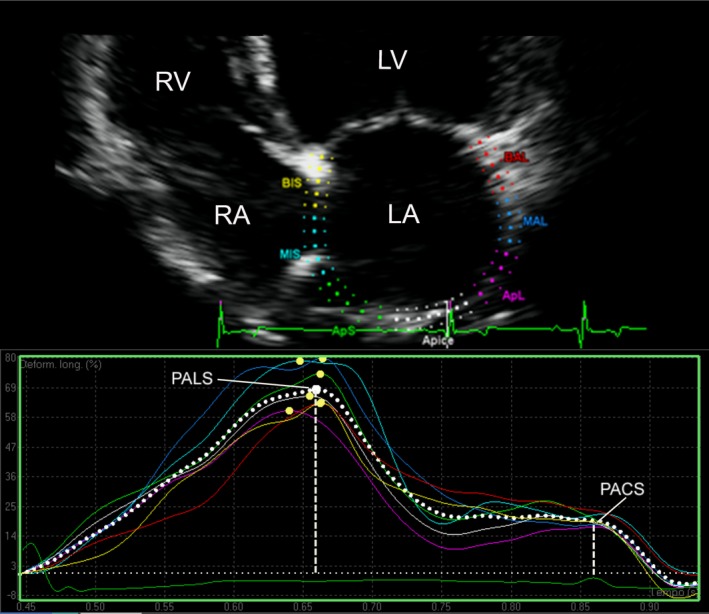
Representative image of the LA strain profile of a dog. On the top of the panel, the LA visualization is optimized in the left apical 4‐chamber view. A region of interest is manually drawn to include the LA wall, which is automatically divided into 7 different segments with different colors. On the bottom of the panel, a trace screen of the speckle tracking echocardiography‐derived strain (ε) is shown, which is expressed in percentage on the y‐axis and time (in seconds) on the x‐axis. Different colored lines are traced for each LA segment, and the mean ε value over time is presented as a dotted white line. The profile is characterized by 2 positive deflections, 1 during ventricular systole (PALS), and 1 just before LA contraction, on the P wave (PACS, peak contraction strain). The CSI is calculated from PALS and PACS as their difference expressed in percentage (see text for details). LA, left atrial; LV, left ventricle; RA, right atrium; RV, right ventricle; PACS, peak atrial contraction strain; PALS, peak atrial longitudinal strain; CSI, contraction strain index.
Variability Study
To assess measurement variability, 6 dogs enrolled into the study were randomly selected. For each dog 1 single echocardiographic study was then chosen, thus a total of 6 different echocardiographic studies were analyzed. For interobserver variability, 2 operators with different levels of experience in echocardiography (MBT, GR) analyzed and measured the LA areas (LAAmax and LAAmin) and FAC, and LA STE variables (PALS, PACS, CSI). To assess interday intra‐observer variability, the same operator (MBT) repeated the measurements for each of the 6 dogs on different days (at least 7 days apart). Attention was made to analyze the same cardiac cycle for each loop. Finally, to assess interday intra‐observer acquisition variability, 5 echocardiographic studies from the same number of dogs from those enrolled in the control group, were repeated 1 week apart. Then, the same operator (MBT) measured LA areas and LA STE variables. The variability was then quantified as the coefficient of variation (CV) by use of the equation CV = (mean difference between measurement/mean of measurements)/100 and expressed as a percentage. The degree of variability was arbitrarily defined as follows: CV <5%, very low variability; 5–15%, low variability; 16–25%, moderate variability; or >25%, high variability.35
Statistical Analysis
Descriptive statistics were used for age, sex, and BW. A 1‐way ANOVA and a chi‐square test were performed to evaluate the group effect (A, B1, B2, C + D) on BW and age, and sex, respectively. Normal distribution of the echocardiographic, Doppler, and STE‐derived variables was assessed by a Shapiro–Wilks test. Variables not normally distributed were log‐transformed. An ANCOVA model was used to test the fixed effects of the group of dogs, sex, as well as the continuous variables of heart rate, BW and age as covariates on echocardiographic, Doppler, and STE‐derived variables. The hypothesis of the linear model was assessed by visual inspection of the residuals plots.
When a significant main effect was detected, a post hoc pairwise contrast were conducted by a Bonferroni correction. Data were reported as Least Squares means (LS‐means) ± Standard error (SE). The association between STE‐derived variables and left heart measurements was tested with a Pearson correlation index (r) and considered significant for P < .001 and r > .3, then a linear regression model was applied.36
All statistical analyses were performed by 2 commercial dedicated software programs.4 , 5 An overall value of P < .01 was considered significant.
Results
General Characteristics of the Study Population and Conventional Echocardiographic Variables
One hundred and fifty dogs met the inclusion criteria. Of these dogs, 23 (15%) were clinically healthy (group A), 52 (35%) had MMVD without cardiac enlargement (group B1), 36 (24%) had MMVD with cardiac enlargement (group B2), and 39 (26%) had symptomatic MMVD (group C + D). Dogs in group A were younger compared with all the other groups, whereas dogs in group B1 were younger than those in group B2 and C + D (P < .001). Dogs of group B1 were significantly heavier than those in group C + D (P = .028). Male dogs were overrepresented in groups B1, B2, and C + D, whereas female dogs were overrepresented in group A; however, a statistically relevant difference was only observed between groups B2 and A (P = .042). Mixed‐breed dogs were overrepresented (n = 68; 45%), followed by Cavalier King Charles Spaniels (n = 10; 7%), Yorkshire Terriers (n = 9; 6%), Miniature Poodles (n = 7; 5%), Maltese (n = 6; 4%), Shih Tzu (n = 6; 4%), and Cocker Spaniels (n = 3; 2%). All descriptive characteristics of the study population and all drugs used with potential impact on hemodynamic variables are summarized in Table 1.
Table 1.
Demographic data from 150 dogs used in this study
| Variable | A | B1 | B2 | C + D | Overall P |
|---|---|---|---|---|---|
| No. of dogs | 23 | 52 | 36 | 39 | |
| Age (years) | 7.0 ± 0.6 | 9.3 ± 0.4 | 11.1 ± 0.5 a | 12.8 ± 0.5 a , b | <.001 |
| Body weight (kg) | 10.5 ± 0.9 | 11.1 ± 1.0 | 9.3 ± 1.1 | 8.1 ± 0.9 | .028 |
| Sex (male/female) | 8/15 | 30/22 | 26/10 | 21/18 | .042 |
| Breed (no. of dogs) | Mixed breed (9) | Mixed breed (23) | Mixed breed (11) | Mixed breed (25) | |
| Cocker Spaniel (3) | CKCS (8) | Yorkshire Terrier (5) | Miniature Poodle (5) | ||
| Yorkshire Terrier (4) | Beagle, Bolognese, CKCS, Dachshund, Lagotto Romagnolo, Shih Tzu (2) | Shih Tzu (4) | |||
| Maltese, Miniature Schnauzer (2) | English Setter, Jack Russell Terrier, Maltese, Miniature Poodle (2) | Maltese (2) | |||
| Medications received (no. of dogs) [mean dose received] |
Other breeds (7) None |
Other breeds (9) None |
Other breeds (8) None |
Other breeds (3) Benazepril (39) [0.38 mg/kg q12–24h] Pimobendan (35) [0.31 mg/kg q24h] Amlodipine (5) [0.3 mg/kg q24h] Spironolactone (3) [2 mg/kg q24h] Furosemide (39) [2 mg/kg q12h] |
A, healthy control group; B1, dogs with MMVD without cardiac enlargement; B2, dogs with MMVD and cardiac enlargement; C + D, dogs with MMVD and clinical signs due to congestive heart failure; CKCS, Cavalier King Charles Spaniel; MMVD, myxomatous mitral valve disease.
Data are expressed as Least Squares‐Means ± SE and numerical data for sex prevalence. In bold values with statistical differences
P < .001 compared with group A.
P < .001 compared with group B1.
All echocardiographic variables were normally distributed, except LAAmax/AoArea and LAAmin/AoArea. The heart rate was higher in dogs of group C + D compared with those of all other groups (P < 0.001). There was a progressive increase of MV E velocity, MV A velocity, and MV E:A ratio when comparing dogs of groups A and B1 to those of groups B2 and C + D (P < .001) (Table 2). None of the dogs had systemic arterial hypertension on noninvasive measurement.
Table 2.
Conventional echocardiographic and left atrial STE‐derived variables from a population of 23 healthy dogs (A) and 127 dogs with MMVD at different stages
| Variable | A (n = 23) | B1 (n = 52) | B2 (n = 36) | C + D (n = 39) | Overall P Value |
|---|---|---|---|---|---|
| Conventional | |||||
| Heart rate (bpm) | 137 ± 6 | 130 ± 4 | 135 ± 4.8 | 160 ± 4.6 a , c , d | <.001 |
| MV E vel (cm/s) | 71.3 ± 5.64 | 77.2 ± 3.71 | 112.7 ± 4.45 b , c | 139.1 ± 4.31 b , c , d | <.001 |
| MV A vel (cm/s) | 63.2 ± 5.24 | 71.7 ± 3.49 | 83.7 ± 4.19 a | 97.2 ± 4.03 b , c | <.001 |
| MV E/A | 1.2 ± 0.09 | 1.1 ± 0.06 | 1.4 ± 0.07 c | 1.6 ± 0.07 b , c | <.001 |
| STE | |||||
| LAAmax/AoArea | 2.2 (1.9–2.4) | 2.7 (2.4–3.0) | 3.6 (3.3–4.1) b , c | 5.2 (4.7–5.9) b , c , d | <.001 |
| LAAmin/AoArea | 0.9 (0.8–1.1) | 1.3 (1.2–1.5) | 2.0 (1.8–2.3) b , c | 3.5 (3.0–4.0) b , c , d | <.001 |
| FAC (%) | 55.1 ± 2.09 | 49.2 ± 1.39 | 44.7 ± 1.67 b | 36.2 ± 1.60 b , c , d | <.001 |
| PALS (%) | 60.4 ± 2.36 | 49.8 ± 1.57 b | 40.6 ± 1.89 b , c | 28.9 ± 1.81 b , c , d | <.001 |
| PACS (%) | 26.3 ± 1.77 | 24.3 ± 1.18 | 16.5 ± 1.42 b , c | 10.6 ± 1.32 b , c , d | <.001 |
| CSI (%) | 42.8 ± 3.24 | 49 ± 2.16 | 40.0 ± 2.59 b | 33.9 ± 2.49 c | <.001 |
C + D, dogs with MMVD and clinical signs due to congestive heart failure; MV E vel, mitral valve E wave peak velocity; MV A vel, mitral valve A wave peak velocity; E/A, mitral valve peak E; and A wave velocity ratio; LAAmax/AoArea, left atrial maximal area‐to‐aortic area ratio; LAAmin/AoArea, left atrial minimal area‐to‐aortic area ratio; FAC, left atrial fractional area change; PALS, peak atrial longitudinal strain; PACS, peak atrial contraction strain; CSI, contraction strain index; MMVD, myxomatous mitral valve disease; STE, speckle tracking echocardiography.
Data are expressed as LSmeans ± SE. LAAmax/AoArea and LAAmin/AoArea mean values are reported after anti‐log transformation and CI are reported into parentheses. In bold values with statistical differences.
P < .05 compared with group A (healthy dogs).
P < .01 compared with group A.
P ≤ .001 compared with group A.
P < .05 compared with group B1 (dogs with MMVD without heart enlargement).
P < .01 compared with group B1.
P < .001 compared with group B1.
P < .05 compared with group B2 (dogs with MMVD and heart enlargement).
P < .01 compared with group B2.
P ≤ .001 compared with group B2.
Left Atrial Areas, LA STE Variables, and Correlation Analysis
For each dog, it was possible to obtain a good tracking profile of the atria and meaningful values for LA areas and strain. Similar to the linear measurements, LA areas also increased with progression of MMVD (Table 2). Specifically, LAAmax/AoArea and LAAmin/AoArea were higher in dogs of group B2 compared with those of groups A and B1, whereas dogs of group C + D had higher values compared with those of all other groups (P < .001). Fractional area change was lower in dogs of group B2 compared with that of group A, whereas dogs of group C + D had lower values compared with those of all the other groups (P < .001).
Of 1,050 LA segments (7 for each dog), only 61 segments (5.8%) were censored due to artifacts whereas the remaining 989 segments (94.2%) were analyzed. Regarding the strain values, dogs of group C + D had lower values for PALS compared with those of all the other groups, and lower PACS values compared only to groups A and B1; dogs of group B2 had lower values compared with dogs of groups B1 and A, whereas dogs of group B1 had lower PALS values compared with those of group A (P < .001). The CSI had significantly lower values in dogs of group C + D compared with those of group B1 and in dogs of group B2 compared with those of group A (P < .001) (Table 2).
Results of the ANCOVA model did not show a significant effect of heart rate or age on each of the tested variables. Body weight had a significant negative effect on FAC, PALS and PACS, a positive effect on LAAmax/AoArea and LAAmin/AoArea, and no effect on CSI.
The correlation analysis demonstrated several positive and negative correlations between the LA STE‐derived variables and morphologic and Doppler variables of the LA and LV. In particular, 31 significant correlations (P < .001) with values of r > .3 were found between LA areas and functional variables (FAC, PALS, PACS, and CSI) and conventional left heart echocardiographic variables (Table 3; Fig 2).
Table 3.
Correlation analysis between speckle tracking echocardiography‐derived variables and some selected conventional echocardiographic parameters in the 150 dogs used in this study
| Variable | LAAmax/AoArea | LAAmin/AoArea | FAC | PALS | PACS | CSI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | r | P | |
| Heart rate (bpm) | .268 | <.001 | .300 | <.001 | −.129 | .116 | −.183 | .025 | −.113 | .170 | −.025 | .757 |
| LA/Ao | .750 | <.001 | .756 | <.001 | −.538 | <.001 | −.609 | <.001 | −.539 | <.001 | −.303 | .002 |
| LVIDd/Ao | .697 | <.001 | .693 | <.001 | −.473 | <.001 | −.540 | <.001 | −.521 | <.001 | −.332 | <.001 |
| LVIDs/Ao | .452 | <.001 | .458 | <.001 | −.356 | <.001 | −.315 | <.001 | −.309 | .001 | −.187 | .022 |
| MV E vel (cm/s) | .644 | <.001 | .669 | <.001 | −.515 | <.001 | −.593 | <.001 | −.586 | <.001 | −.385 | <.001 |
| MV A vel (cm/s) | .405 | <.001 | .384 | <.001 | −.200 | .014 | −.279 | .001 | −.206 | .012 | −.028 | .731 |
| E/A | .312 | <.001 | .362 | <.001 | −.400 | <.001 | −.398 | <.001 | −.460 | <.001 | −.385 | <.001 |
LAAmax/AoArea, left atrial maximal area‐to‐aortic area ratio; LAAmin/AoArea, left atrial minimal area‐to‐aortic area ratio; FAC, left atrial fractional area change; PALS, peak atrial longitudinal strain; PACS, peak atrial contraction strain; CSI, contraction strain index; LA/Ao, left atrium‐to‐aorta ratio; LVIDd/Ao, left ventricular internal diameter in diastole‐to‐aorta ratio; LVIDs/Ao, left ventricular internal diameter in systole‐to‐aorta ratio; MV E vel, mitral valve E wave peak velocity; MV A vel, mitral valve A wave peak velocity; E/A, mitral valve peak E and A wave velocity ratio.
In bold values with significant correlation (P < .001, r > .3).
Figure 2.
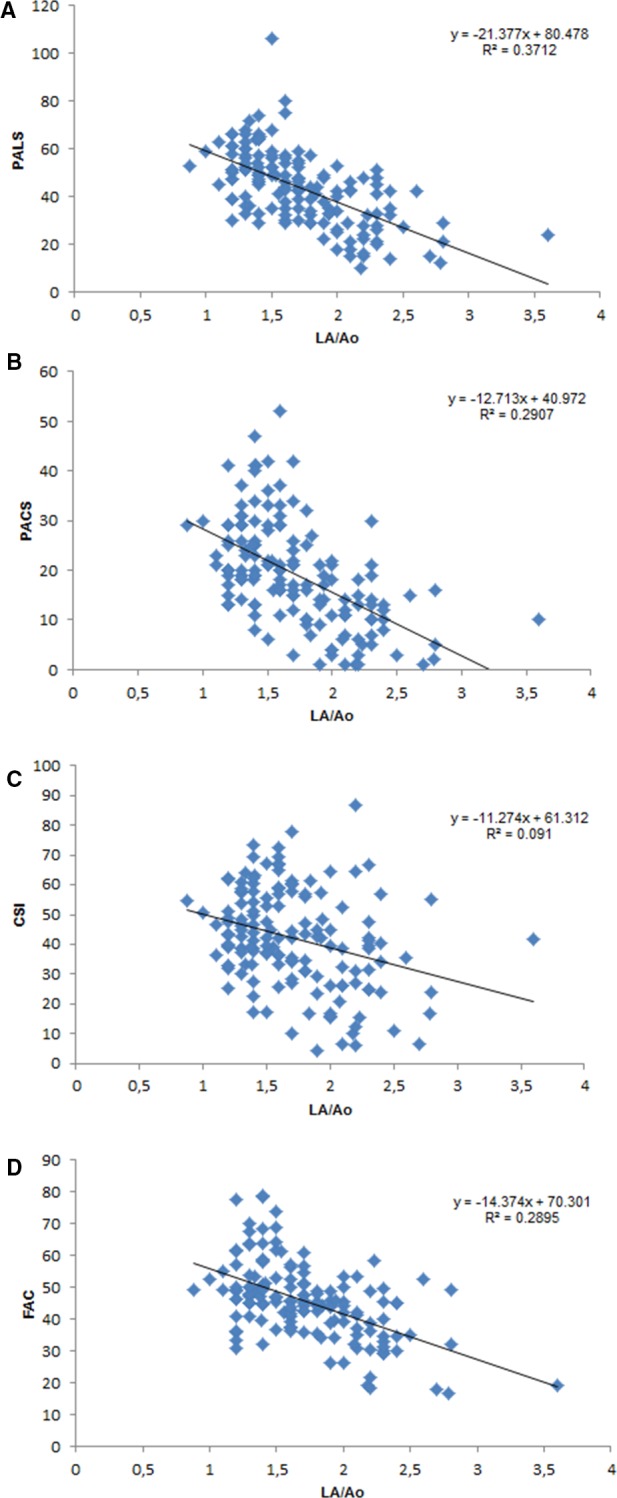
Linear regression analysis of 4 selected LA strain variables and FAC obtained from LA areas, compared with LA linear dimension normalized to aortic root diameter (LA/Ao). LA, left atrial diameter; AO, aortic diameter; PALS, peak atrial longitudinal strain; PACS, peak atrial contraction strain; CSI, contraction strain index; FAC, fraction area change.
Variability Study
The interobserver measurement variability was low for the 6 tested STE variables, with CV values always below 16%. Intra‐observer interday measurement variability was always very low or low, with CV values always below 13% for PALS, PACS and CSI and below 7% for LAAmax, LAAmin and FAC. Interday intra‐observer acquisition variability was low (between 5% and 15%) for all variables expect FAC, where the variability was 16%.
Discussion
In the present study we evaluated novel echocardiographic indices of LA function in healthy dogs and dogs with MMVD. The major findings were: (1) LA STE analysis is feasible and reproducible in the dog, (2) several statistical significant differences exist in LA STE variables between healthy dogs and dogs with MMVD at different stages of severity, indicating a progressive impairment of LA function with the disease progression, and (3) a strong association is present between LA STE‐derived indices and some conventional echocardiographic variables, supporting a reduction of LA function as the heart dilates.
Two‐dimensional STE was feasible and repeatable for evaluation of LA longitudinal strain in awake, unsedated dogs with and without MMVD, with a lower variability compared with TDI‐derived LA strain imaging in healthy dogs.11 Specifically, intra‐ and interobserver variability was considered clinically acceptable (CV <16%) for all STE measurements. In addition, the obtained images were of sufficient quality to allow STE analysis, both in dogs with a structurally normal LA and in dogs with an enlarged LA. Similar data have been reported in healthy people, where adequate tracking quality was achieved in >95% of segments analyzed and inter‐ and intra‐observer variability coefficients of measurements ranged approximately between 3% and 6%.13, 37 The CV relative to LAAmax, LAAmin and FAC were slightly lower than those obtained for the STE variables (PALS, PACS, and CSI). This finding could be explained by the fact that the more complex software analysis needed to calculate the STE variables and the need of analyzing high‐quality images, might increase their variability, whereas the simple area measurements, which require less computation and not necessarily high‐quality images, allow higher repeatability. Speckle tracking echocardiography analysis allows an excellent assessment of the LA deformation profile during an entire cardiac cycle, closely following LA physiology. In particular, during the period of LV systole, the LA acts as a reservoir allowing collection of blood from the pulmonary veins whereas the MV is closed. Consequently, longitudinal elongation of the LA walls occurs, resulting in a LA strain increase characterized by a peak at the end of LA filling, just before MV opening. During the successive conduit phase, the MV opens and the LA empties quickly; the shortening of LA walls results in a strain decrease, up to a plateau corresponding to the phase of diastole. Finally, after the P wave of the ECG, the LA acts as an active contractile chamber in order to complete the LV filling, reaching a second negative peak at the end of LA contraction.11, 13, 16 Starting from the above‐described strain curve, specific STE variables of LA function can be calculated, including PALS and PACS, which reflect the LA function during its reservoir and booster pump phase, respectively.17 In addition, CSI can be also obtained from these 2 variables, representing the contribution of LA active contraction to the LV filling phase.17 Our results showed a linear decrease of LA STE‐derived measurements including FAC, another index of LA function, from healthy dogs to dogs with progressively more severe MMVD. In addition, a negative correlation was found between the above‐mentioned STE‐derived variables and conventional echocardiographic measurements of left cardiac chamber size. These data are in agreement with previous reports focused on conventional echocardiographic assessment of LA function in dogs with MMVD,2, 7 as well as human studies in patients investigated by STE,38, 39, 40, 41, 42, 43, 44 and suggest progressive LA dysfunction with worsening of MMVD and LA dilatation. These findings might be explained by the progressive LA volume overload due to MR and remodeling and by an increase in LV filling pressures in the advanced phases of the disease. Specifically, LA remodeling includes adaptive and maladaptive processes, such as alterations in the composition of extracellular matrix with excessive fibroblast proliferation, and myocyte hypertrophy, necrosis, and apoptosis.45, 46 All of these ultrastructural changes significantly affect LA myocardial wall properties, such as relaxation and compliance, ultimately leading to the observed reduction of LA phasic functions assessed by STE.40, 47, 48 An interesting finding of the present study was the observed lower PALS in dogs with MMVD class B1 compared with that of healthy dogs, whereas LA areas and almost all STE‐derived variables were not different between these groups of dogs. This finding suggests a possible early effect of MR on LA structure and function that was only evident by strain analysis in this study. Although canine MMVD progresses in the absence of significant ventricular myocardial fibrosis,49 PALS has been proven to highly correlate with LA fibrosis in human patients with MR.38 At present, studies investigating the role of LA fibrosis in dogs in the early stage of MMVD are lacking. On the other hand, because dogs of group A were significantly younger than those of the other groups, it cannot be excluded that an age‐related effect might also have influenced this finding. Contraction strain index, a parameter indicative of LA active systolic function, showed a transient increase from dogs of group A to those of group B1 followed by a progressive reduction. This supernormal pattern has already been observed in humans with mild MR, and it is likely secondary to Starling's law usually applied to the ventricles.37 In fact, the atria also enhance their contractile function in response to an increased preload during the early phase of MR, but this function subsequently declines as the LV filling pressure increases.37
In the present study, we recorded relatively higher values of PALS, PACS, and CSI in our control group compared with those recently reported in healthy dogs.30 This difference might be explained by the different equipment and software used for the analysis in the 2 studies, and also by the different demographic populations. The dogs enrolled in that previous study had a relatively broad range of breeds, age, and BW, whereas dogs of the present study were comparably older and smaller in size. Although no association was found between STE variables and BW and age in the reported study,30 we cannot exclude a possible BW and age effect on STE variables.
Some limitations of the present study must be highlighted. First, technical aspects of STE‐derived variables should be considered, because the reliability of measurements might be questioned in dogs with extremely remodeled LA. In these cases, excessive dilatation of the pulmonary vein inlet and displacement of the interatrial septum might complicate the tracking procedure. These structures were excluded from tracking analysis, therefore reducing the risk of producing artifactual data among the global LA strain. Moreover, we documented a low intra‐ and interobserver variability in all groups of dogs, including those of group C + D, where the described anatomical modifications are common. Second, dogs affected by MMVD were older than those of the control group. However, given the acquired and progressive nature of MMVD, it is difficult to find a perfectly age‐ and BW‐matched control group of dogs without some degree of MR, and age did not have any significant effect on STE‐derived variables in the ANCOVA model. Third, a possible influence of cardiac medications on LA function could not be evaluated in the present study. However, only 39 of 127 (31%) dogs with MMVD received cardiac medications at the time of enrollment, and different drug combinations and dosages were employed. Finally, LA mechanical properties were not assessed for functional impairment by gold standard imaging modalities, such as cardiac computed tomography and magnetic resonance. However, given the need of general anesthesia and the related risks (especially in dogs with decompensated MMVD), these procedures were not carried out for ethical reasons.
In conclusion, we described the use of STE for the evaluation of LA strain and other functional indexes in healthy dogs and dogs with MMVD. These variables can be effectively obtained also in dogs in the advanced stages of MMVD and are useful to track the progressive decline in LA function as the disease progresses.
Acknowledgments
Grant support: No grant support.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Presented in part as an abstract at the 25th Congress of the European Society of Veterinary Internal Medicine—Companion Animals, Lisbon, Portugal, 10th–12th September 2015.
This work was done at the Department of Veterinary Medical Sciences, Alma Mater Studiorum‐University of Bologna.
Footnotes
iU22 ultrasound system, Philips Healthcare, Monza, Italy
iE33 ultrasound system, Philips Healthcare, Monza, Italy
QLAB quantification software version 9.1, Philips Healthcare, Monza, Italy
SAS version 9.3, SAS Institute Inc., Cary, NC
MedCalc version 12.6.1.0, MedCalc Software, Ostend, Belgium
References
- 1. Hoit BD. Left atrial function: Basic physiology In: Klein AL, Garcia MJ, ed. Diastology. Clinical Approach to Diastolic Heart Failure, 1st ed Philadelphia, PA: Saunders Elsevier; 2008:33–41. [Google Scholar]
- 2. Nakamura K, Osuga T, Morishita K, et al. Prognostic value of left atrial function in dogs with chronic mitral valvular heart disease. J Vet Intern Med 2014;28:1746–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. To ACY, Flamm SD, Marwick TH, et al. Clinical utility of multimodality LA imaging. Assessment of size, function, and structure. JACC Cardiovasc Imaging 2011;4:788–798. [DOI] [PubMed] [Google Scholar]
- 4. Vizzardi E, D'Aloia A, Rocco E, et al. How should we measure left atrial size and function? J Clin Ultrasound 2012;40:155–166. [DOI] [PubMed] [Google Scholar]
- 5. Rishniw M, Erb HN. Evaluation of four 2‐dimensional echocardiographic methods of assessing left atrial size in dogs. J Vet Intern Med 2000;14:429–435. [DOI] [PubMed] [Google Scholar]
- 6. Hollmer M, Willesen JL, Tolver A, et al. Left atrial volume and phasic function in clinically healthy dogs of 12 different breeds. Vet J 2013;197:639–645. [DOI] [PubMed] [Google Scholar]
- 7. Tidholm A, Höglund K, Häggström J, et al. Left atrial ejection fraction assessed by real‐time 3‐dimensional echocardiography in normal dogs and dogs with myxomatous mitral valve disease. J Vet Intern Med 2013;27:884–889. [DOI] [PubMed] [Google Scholar]
- 8. Hansson K, Häggström J, Kvart C, et al. Left atrial to aortic root indices using two‐dimensional and M‐mode echocardiography in cavalier king Charles spaniels with and without left atrial enlargement. Vet Radiol Ultrasound 2002;43:568–575. [DOI] [PubMed] [Google Scholar]
- 9. Schober KE, Hart TM, Stern JA, et al. Detection of congestive heart failure in dogs by Doppler echocardiography. J Vet Intern Med 2010;24:1358–1368. [DOI] [PubMed] [Google Scholar]
- 10. Roşca M, Lancellotti P, Popescu BA, et al. Left atrial function: Pathophysiology, echocardiographic assessment, and clinical applications. Heart 2011;97:1982–1989. [DOI] [PubMed] [Google Scholar]
- 11. Baron Toaldo M, Guglielmini C, Diana A, et al. Feasibility and reproducibility of echocardiographic assessment of regional left atrial deformation and synchrony by tissue Doppler ultrasonographic imaging in healthy dogs. Am J Vet Res 2014;75:59–66. [DOI] [PubMed] [Google Scholar]
- 12. Sirbu C, Herbots L, D'hooge J, et al. Feasibility of strain and strain rate imaging for the assessment of regional left atrial deformation: A study in normal subjects. Eur J Echocardiogr 2006;7:199–208. [DOI] [PubMed] [Google Scholar]
- 13. Cameli M, Caputo M, Mondillo S, et al. Feasibility and reference value of left atrial longitudinal strain imaging by two‐dimensional speckle tracking. Cardiovasc Ultrasound. 2009;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vianna‐Pinton R, Moreno CA, Baxter CM, et al. Two‐dimensional speckle tracking echocardiography of the left atrium: Feasibility and regional contraction and relaxation differences in normal subjects. J Am Soc Echocardiogr 2009;22:299–305. [DOI] [PubMed] [Google Scholar]
- 15. Kim DB, Lee KJ, Jeong SY, et al. Feasibility of two‐dimensional global longitudinal strain and strain rate imaging for the assessment of left atrial function: A study in subjects with a low probability of cardiovascular disease and a normal exercise capacity. Echocardiography 2009;26:1179–1187. [DOI] [PubMed] [Google Scholar]
- 16. Vieira MJ, Teixeira R, Gonçalves L, et al. Left atrial mechanics: Echocardiographic assessment and clinical implications. J Am Soc Echocardiogr 2014;27:463–478. [DOI] [PubMed] [Google Scholar]
- 17. Cameli M, Lisi M, Righini FM, et al. Novel echocardiographic techniques to assess left atrial size, anatomy and function. Cardiovasc Ultrasound 2012;10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zois NE, Tidholm A, Nägga KM, et al. Radial and longitudinal strain and strain rate assessed by speckle‐tracking echocardiography in dogs with myxomatous mitral valve disease. J Vet Intern Med 2012;26:1309–1319. [DOI] [PubMed] [Google Scholar]
- 19. Suzuki R, Matsumoto H, Teshima T, et al. Noninvasive clinical assessment of systolic torsional motion by two‐dimensional speckle‐tracking echocardiography in dogs with myxomatous mitral valve disease. J Vet Intern Med 2013;27:69–75. [DOI] [PubMed] [Google Scholar]
- 20. Suzuki R, Matsumoto H, Teshima T, et al. Clinical assessment of systolic myocardial deformations in dogs with chronic mitral valve insufficiency using two‐dimensional speckle‐tracking echocardiography. J Vet Cardiol 2013;15:41–49. [DOI] [PubMed] [Google Scholar]
- 21. Suzuki R, Matsumoto H, Teshima T, et al. Influence of heart rate on myocardial function using two‐dimensional speckle‐tracking echocardiography in healthy dogs. J Vet Cardiol 2013;15:139–146. [DOI] [PubMed] [Google Scholar]
- 22. Zois NE, Olsen NT, Moesgaard SG, et al. Left ventricular twist and circumferential strain in dogs with myxomatous mitral valve disease. J Vet Intern Med 2013;27:875–883. [DOI] [PubMed] [Google Scholar]
- 23. Westrup U, McEvoy FJ. Speckle tracking echocardiography in mature Irish wolfhound dogs: Technical feasibility, measurement error and reference intervals. Acta Vet Scand 2013;55:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Suzuki R, Matsumoto H, Teshima T, et al. Dobutamine stress echocardiography for assessment of systolic function in dogs with experimentally induced mitral regurgitation. J Vet Intern Med 2014;28:386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen HY, Lien YH, Huang HP. Assessment of left ventricular function by two‐dimensional speckle‐tracking echocardiography in small breed dogs with hyperadrenocorticism. Acta Vet Scand 2014;56:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Visser LC, Scansen BA, Schober KE, et al. Echocardiographic assessment of right ventricular systolic function in conscious healthy dogs: Repeatability and reference intervals. J Vet Cardiol 2015;17:83–96. [DOI] [PubMed] [Google Scholar]
- 27. Visser LC, Scansen BA, Brown NV, et al. Echocardiographic assessment of right ventricular systolic function in conscious healthy dogs following a single dose of pimobendan versus atenolol. J Vet Cardiol 2015;17:161–172. [DOI] [PubMed] [Google Scholar]
- 28. Osuga T, Nakamura K, Lim SY, et al. Repeatability and reproducibility of measurements obtained via two‐dimensional speckle tracking echocardiography of the left atrium and time‐left atrial area curve analysis in healthy dogs. Am J Vet Res 2013;74:864–869. [DOI] [PubMed] [Google Scholar]
- 29. Osuga T, Nakamura K, Morita T, et al. Effect of various cardiovascular drugs on indices obtained with two‐dimensional speckle tracking echocardiography of the left atrium and time‐left atrial area curve analysis in healthy dogs. Am J Vet Res 2015;76:702–709. [DOI] [PubMed] [Google Scholar]
- 30. Caivano D, Rishniw M, Patata V, et al. Left atrial deformation and phasic function determined by 2‐dimensional speckle tracking echocardiography in healthy dogs. J Vet Cardiol 2016;18:146–155. [DOI] [PubMed] [Google Scholar]
- 31. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med 1993;7:247–252. [DOI] [PubMed] [Google Scholar]
- 32. Brown DJ, Rush JE, MacGregor J, et al. M‐mode echocardiographic ratio indices in normal dogs, cats and horses: A novel quantitative method. J Vet Intern Med 2003;17:653–662. [DOI] [PubMed] [Google Scholar]
- 33. Wesselowski S, Borgarelli M, Bello NM, Abbott J. Discrepancies in identification of left atrial enlargement using left atrial volume versus left atrial‐to‐aortic root ration in dogs. J Vet Intern Med 2014;28:1527–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Atkins C, Bonagura J, Ettinger S, et al. Guidelines for diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med 2009;23:1142–1150. [DOI] [PubMed] [Google Scholar]
- 35. Bland M. Clinical measurement In: Bland M, ed. An Introduction to Medical Statistics, 3rd ed Oxford, UK: Oxford University Press; 2000:268–293. [Google Scholar]
- 36. Curtin F, Schulz P. Multiple correlation and Bonferroni's correction. Biol Psychiatry 1998;44:775–777. [DOI] [PubMed] [Google Scholar]
- 37. Sun JP, Yang Y, Guo R, et al. Left atrial regional phasic strain, strain rate and velocity by speckle‐tracking echocardiography: Normal values and effect of aging in a large group of normal subjects. Int J Cardiol 2013;168:3473–3479. [DOI] [PubMed] [Google Scholar]
- 38. Cameli M, Lisi M, Giacomin E, et al. Chronic mitral regurgitation: Left atrial deformation analysis by two‐dimensional speckle tracking echocardiography. Echocardiography 2011;28:327–334. [DOI] [PubMed] [Google Scholar]
- 39. Her AY, Choi EY, Shim CY, et al. Prediction of left atrial fibrosis with speckle tracking echocardiography in mitral valve disease: A comparative study with histopathology. Korean Circ J 2012;42:311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cameli M, Lisi M, Righini FM, et al. Left atrial speckle tracking analysis in patients with mitral insufficiency and history of paroxysmal atrial fibrillation. Int J Cardiovasc Imaging 2012;28:1663–1670. [DOI] [PubMed] [Google Scholar]
- 41. Cameli M, Lisi M, Righini FM, et al. Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol 2013;111:595–601. [DOI] [PubMed] [Google Scholar]
- 42. Debonnaire P, Leong DP, Witkowski TG, et al. Left atrial function by two‐dimensional speckle‐tracking echocardiography in patients with severe organic mitral regurgitation: Association with guidelines‐based surgical indication and postoperative (long‐term) survival. J Am Soc Echocardiogr 2013;26:1053–1062. [DOI] [PubMed] [Google Scholar]
- 43. Yang LT, Shih JY, Liu YW, et al. Effects of left atrial strain on functional capacity in chronic severe mitral regurgitation. Int J Cardiol 2013;168:e151–e153. [DOI] [PubMed] [Google Scholar]
- 44. Yang LT, Liu YW, Shih JY, et al. Predictive value of left atrial deformation on prognosis in severe primary mitral regurgitation. J Am Soc Echocardiogr 2015;28:1309–1317. [DOI] [PubMed] [Google Scholar]
- 45. Janus I, Noszczyk‐Nowak A, Nowak M, et al. A comparison of the histopathologic pattern of the left atrium in canine dilated cardiomyopathy and chronic mitral valve disease. BMC Vet Res 2016;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cha YM, Dzeja PP, Shen WK, et al. Failing atrial myocardium; energetic deficits accompany structural remodeling and electrical instability. Am J Physiol Heart Circ Physiol 2003;284:H313–H320. [DOI] [PubMed] [Google Scholar]
- 47. Le Tourneau T, Messika‐Zeitoun D, Russo A, et al. Impact of left atrial volume on clinical outcome in organic mitral regurgitation. J Am Coll Cardiol 2010;56:570–578. [DOI] [PubMed] [Google Scholar]
- 48. Moustafa SE, Alharthi M, Kansal M, et al. Global left atrial dysfunction and regional heterogeneity in primary chronic mitral insufficiency. Eur J Echocardiogr 2011;12:384–393. [DOI] [PubMed] [Google Scholar]
- 49. Falk T, Ljungvall I, Zois NE, et al. Cardiac troponin‐I concentration, myocardial arteriosclerosis, and fibrosis in dogs with congestive heart failure because of myxomatous mitral valve disease. J Vet Intern Med 2013;27:500–506. [DOI] [PubMed] [Google Scholar]


