Abstract
Background
Hyperkalemia is a frequently observed electrolyte imbalance in dehydrated neonatal diarrheic calves that can result in skeletal muscle weakness and life‐threatening cardiac conduction abnormalities and arrhythmias.
Hypothesis
Intravenous administration of a small‐volume hypertonic NaHCO3 solution is clinically more effective in decreasing the plasma potassium concentration (cK) in hyperkalemic diarrheic calves than hypertonic NaCl or glucose solutions.
Animals
Twenty‐two neonatal diarrheic calves with cK >5.8 mmol/L.
Methods
Prospective randomized clinical trial. Calves randomly received either 8.4% NaHCO3 (6.4 mL/kg BW; n = 7), 7.5% NaCl (5 mL/kg BW; n = 8), or 46.2% glucose (5 mL/kg BW; n = 7) IV over 5 minutes and were subsequently allowed to suckle 2 L of an electrolyte solution. Infusions with NaHCO3 and NaCl provided an identical sodium load of 6.4 mmol/kg BW.
Results
Hypertonic NaHCO3 infusions produced an immediate and sustained decrease in plasma cK. Hypertonic glucose infusions resulted in marked hyperglycemia and hyperinsulinemia, but cK remained unchanged for 20 minutes. Between 30 and 120 minutes after initiation of treatment, the most marked decrements in cK from baseline occurred in group NaHCO3, which were significantly (P < .05) larger during this period of time than in calves in group NaCl, but not group glucose. After 120 minutes, the mean decrease in cK from baseline was −26 ± 10%, −9 ± 8%, and −22 ± 6% in groups NaHCO3, NaCl, and glucose, respectively.
Conclusions/Clinical Importance
Small‐volume hypertonic NaHCO3 infusions appear to have clinical advantages for the rapid resuscitation of hyperkalemic diarrheic calves, compared to hypertonic NaCl or glucose solutions.
Keywords: Dehydration, Electrolyte imbalances, Insulin, Sodium, Strong ion (metabolic) acidosis
Diarrhea in neonatal calves can result in metabolic derangements including azotemia, hemoconcentration, D‐lactatemia, and development of a strong ion (metabolic) acidosis.1, 2, 3 Electrolyte imbalances are also common in diarrheic calves and are closely linked to derangements of acid‐base status.2, 4, 5, 6 Although neonatal diarrheic calves have a negative potassium balance due to intestinal losses and low milk intake,7 they usually have normo‐ or hyperkalemic plasma concentrations in the presence of acidemia.4, 8 Hyperkalemia is a clinically relevant electrolyte imbalance in diarrheic calves and has historically been attributed to impaired intracellular translocation of potassium ions due to acidemia and decreased intracellular pH.9, 10 However, recent studies indicate that hyperkalemia in diarrheic calves is dependent on the nature of the existing acidosis but not on acidemia per se, with D‐lactic acidosis being rarely associated with increased plasma potassium concentrations (cK). More importantly, the cK in diarrheic calves is most closely associated with clinical and laboratory indices of dehydration, indicating that a decrease in renal glomerular filtration rate plays a central role in the development of a hyperkalemic state.4, 8
Clinical effects of hyperkalemia are related to impaired neuromuscular excitability, which is further exacerbated by the presence of hyponatremia and metabolic acidosis,11 conditions that are usually present in affected calves. Due to the potential cardiotoxicity, acute hyperkalemia represents a potentially life‐threatening state and has historically been considered to be an important cause of death in neonatal calves with diarrhea.12 Electrocardiographic manifestations of hyperkalemia typically include flattened or missing P‐waves, increased QRS duration, large and spiked T‐waves, and R‐R irregularities.9, 13, 14, 15 Additionally, the clinical picture of hyperkalemic diarrheic calves is characterized by severe clinical dehydration, cyanosis, and impaired ability to stand8 and affected calves are frequently presented with signs of shock. Acute hyperkalemia should therefore be considered as an emergency, and treatment objectives should focus on rapid correction of hyperkalemia. Although it is well known that the correction of a hyperkalemic state can be achieved by intravenous administration of large volumes of fluids containing sodium bicarbonate, sodium chloride, or glucose solutions,16, 17, 18, 19 the underlying mechanisms and the efficacy of different infusion solutions in the initial treatment have not been fully explored. Specifically, it needs to be determined how much of the return of potassium homeostasis is due to volume expansion (dilutional effect), rehydration with concomitant restoration of renal function (excretional effect), or intracellular translocation of potassium ions in response to alkalinization, a sodium‐induced strong ion effect, or the action of endogenous insulin.
Intravenous administration of hypertonic (8.4%) sodium bicarbonate solution induces an immediate and sustained decrease in cK, which is most closely associated with a rapid increase in venous blood pH and a rapid improvement in hydration status.17 In general, hypertonic sodium‐containing infusion solutions such as 8.4% sodium bicarbonate or 7.2% sodium chloride (saline) have a sound physiologic basis in the initial treatment of affected calves as these solutions not only induce rapid plasma volume expansion and correct hyperkalemia, but they also enhance the redistribution of potassium ions into cells. Consequently, hypertonic sodium solutions can rapidly reverse the electrocardiographic manifestations of hyperkalemia,20 which has been demonstrated for hypertonic saline in hyperkalemic humans, dogs, and a calf.14, 21, 22 However, hypertonic saline might be inferior to hypertonic sodium bicarbonate as hypertonic saline does not correct the concomitant acidemia, which is usually present in affected calves. In human medicine, hyperkalemia is most commonly treated by the intravenous administration of insulin and dextrose,11, 23 as insulin stimulates cellular potassium uptake through activation of the Na+/K+‐ATPase mediated by an inward flux of sodium ions.24 Intravenous administration of a hypertonic glucose solution might therefore represent an alternative option in the initial treatment of hyperkalemic diarrheic calves, as hypertonic glucose solutions induce an endogenous insulin release that has been associated with a decrease in cK in healthy eukalemic cows.25 However, the insulin‐mediated potassium‐lowering effect could be hampered in diarrheic calves20 as even mild acidemia with measured blood pH values of 7.27 ± 0.01 and 7.37 ± 0.02 was reported to result in insulin resistance in humans.26, 27
Consequently, the aim of this study was to compare the potassium‐lowering effects of hypertonic sodium chloride‐, sodium bicarbonate‐, and glucose‐containing infusion solutions in the initial treatment of hyperkalemic diarrheic calves. As hypertonic saline and glucose solutions do not have alkalinizing capacity, we hypothesized that administration of a hypertonic sodium bicarbonate solution would be associated with a more rapid, marked, and sustained decrease in plasma cK than administration of hypertonic sodium chloride or glucose solutions.
Materials and Methods
Methods of this study were approved by the Animal Welfare and Ethics Committee of the government of Upper Bavaria (permit no. 55.2‐1‐54‐2532‐211‐13).
Calves
Between February 2015 and May 2016, a prospective study was conducted involving 26 calves that were admitted to the Clinic for Ruminants with Ambulatory and Herd Health Services, LMU Munich. Criteria for inclusion into the study were a clinical diagnosis of neonatal diarrhea, age ≤21 days, and a measured plasma potassium concentration >5.8 mmol/L. General exclusion criteria included the presence of hypernatremia (plasma sodium concentration >160 mmol/L), venous blood pH ≤6.80, and severe concurrent health problems (e.g, advanced bronchopneumonia). A total of 4 calves were subsequently excluded from the analysis due to a postmortem diagnosis of generalized peritonitis and infarction of the caudal part of the spinal cord (n = 1), dosage error in the volume of infused solution (n = 1), and pretreatment with hypertonic sodium bicarbonate infusions in 2 calves shortly before admission to the hospital (n = 2). Therefore, a total of 22 calves remained in the study. Written informed consent was obtained from the owners of the calves before inclusion into the study.
Due to regional preferences, 20 of 22 calves belonged to the Simmental breed (German Fleckvieh), the most common dairy breed in Bavaria. The mean age and body mass of the calves were 8 ± 3 days and 42.3 ± 6.3 kg, respectively.
Experimental Protocol
After weighing and an initial clinical examination, a catheter1 was placed in a jugular vein and secured in place with suture material. For this purpose, the area over the respective jugular vein was clipped, antiseptically prepared, and 2 mL of a 2% procaine solution injected into and under the skin before catheterization. Calves were randomly allocated to 1 of 3 treatment groups, which was conducted by drawing a lot out of a pool of 3 possible lots representing each treatment group:
Sodium bicarbonate group (NaBic; n = 7): Calves received a commercially available 8.4% sodium bicarbonate solution2 (theoretical osmolarity 2000 mOsm/L) in a dosage of 6.4 mL/kg BW over a period of 5 minutes. This dosage was chosen to provide the same sodium load as in calves of group NaCl.
Saline group (NaCl, n = 8): Calves received a commercially available 7.5% sodium chloride solution3 (theoretical osmolarity, 2566 mOsm/L) in a dosage of 5 mL/kg body weight (BW) over 5 minutes. This provided a sodium load of 6.4 mmol per kg BW.
Glucose group (Gluc, n = 7): Calves received a 46.2% glucose solution in a dosage of 5 mL/kg body mass over a period of 5 minutes. For this purpose, a commercially available 50% glucose solution4 was diluted with sterile water5 to provide an infusion solution that had a comparable osmolarity (2564 mOsm/L) to the hypertonic saline solution.
All infusion solutions were injected at room temperature through the jugular vein catheter with 60‐ml polypropylene syringes. Blood samples were taken from the same catheter at −15, 0, 7, 10, 15, 20, 30, 40, 50, 60, 75, 90, and 120 minutes relative to the onset of the injection. After the injection of the infusion solution and blood sampling, the catheter was flushed with heparinized 0.9% NaCl (40 U of heparin/mL).
At 20 min after the start of IV infusion, calves were allowed to suckle 2 L of a commercially available oral electrolyte solution.6 This oral electrolyte solution contained 2.34 g NaCl (40 mmol), 1.12 g KCl (15 mmol), 6.72 g sodium bicarbonate (80 mmol), 3.84 g citric acid (20 mmol), 32.44 g lactose monohydrate (90 mmol), and 2.25 g glycine (30 mmol) per liter. The theoretical calculated osmolarity and effective strong ion difference of this solution were 410 mOsm/L and 80 mEq/L, respectively. If the respective volume of the solution was not suckled entirely within 10 minutes, the remainder of the solution was tube‐fed after blood sampling at 30 minutes.
After the end of the study period at 120 minutes, calves were treated according to clinic principles and received further infusions based on the current acid‐base and clinical dehydration status.
Clinical Examination
Physical examination followed a standardized protocol3 and included the clinical assessment of posture/ability to stand, behavior, suckling and palpebral reflex, and extent of enophthalmos (in mm) before administration of infusion solutions and at the end of the study period at 120 minutes. Posture was scored as: 1 = standing up by itself; 2 = standing up after encouragement; 3 = standing securely after lifting; 4 = insecurely, able to correct position; 5 = insecurely, unable to correct position; 6 = sternal recumbency; and 7 = lateral recumbency. Behavior was scored as: 1 = adequate reaction; very bright and alert; 2 = adequate reaction; 3 = delayed reaction; 4 = calf reacts only to painful stimuli; 5 = no reaction to painful stimuli. Suckling reflex was categorized as: 1 = strong, 2 = weak, 3 = absent. The palpebral reflex was scored as: 1 = eyelids are closed immediately and fully; 2 = eyelids are closed immediately but not fully; 3 = eyelids are closed with delay and not fully; and 4 = eyelids are not closed at all. The severity of enophthalmos was quantified by measuring the distance (in mm) between the medial canthus and the eyeball.28, 29 Subjective measurements could not be masked due to differences of administered volumes of infusion solution and repeated blood gas measurement, which were performed by the same investigator.
Laboratory Analyses
Lithium‐heparinized blood samples were anaerobically collected with a 2‐mL polypropylene syringe and blood pH, partial pressure of carbon dioxide (pCO2), sodium, chloride, potassium, and ionized calcium concentrations were determined with direct potentiometry at all sampling times with a blood pH, gas, and electrolyte analyzer with ion‐selective electrodes.7 Blood samples were kept at room temperature until blood gas analysis, which was performed within 15 minutes. Blood pH and pCO2 were corrected for rectal temperature by standard algorithms.30 After blood gas analysis, syringes were immediately refrigerated and centrifuged within 60 minutes after collection at 1,500 × g for 10 minutes.
Harvested plasma samples were assayed for concentrations of insulin, glucose (hexokinase), total protein (biuret), and inorganic phosphorus (molybdenum) at all sampling times. Plasma urea (urease), creatinine (picric acid), D‐lactate (D‐lactate dehydrogenase), L‐lactate (L‐lactate dehydrogenase), and total magnesium (xylidyl blue) concentrations were determined at 0, 15, 30, 60, 90, and 120 minutes. An automatic analyzing system8 was used for biochemical analysis except for insulin determination, which was performed with a commercially available ELISA kit9 on plasma samples that had been stored at −25°C until analyzed. This assay is a species‐optimized test, which has a reported inter‐ and intra‐assay coefficient of variation of ≤7% and ≤5.3%, respectively.10 Based on recommendations from the test provider, insulin concentrations below a concentration of 0.05 μg/L were not calculated from the calibration curve and therefore entered into the analysis as 0.05 μg/L.
Hematologic variables were determined at 0, 15, 30, 60, 90, and 120 minutes from an additional EDTA blood sample with a hematologic analyzer.11
Calculations
Plasma insulin concentrations were converted from μg/L to μIU/mL by multiplying values with a factor of 20.56.31 If laboratory variables were measured at −15 and 0 minutes, those values were used to calculate baseline values as the mean of those 2 measurements. Otherwise, baseline values were based on a single measurement at 0 minutes.
Actual bicarbonate concentration (cHCO3 −) was automatically calculated by the blood gas unit by the Henderson‐Hasselbalch equation with measured blood pH and pCO2 at 37°C:
| (1) |
Values for the negative logarithm of the dissociation constant of carbonic acid (pK1′) and solubility of carbon dioxide (S) for plasma were 6.105 and 0.0307 mmol/L per mmHg, respectively. After measuring the hemoglobin concentration (Hb in g/dL) photometrically, blood base excess (in vitro base excess) was automatically calculated in units of mmol/L by the van Slyke equation32 with measured blood pH at 37°C and the determined actual bicarbonate concentration:
| (2) |
An estimate of the unmeasured anion concentration was obtained by calculating the anion gap (AG) in mEq/L, whereby:
| (3) |
In addition to the traditional Henderson‐Hasselbalch acid‐base model, the simplified quantitative physicochemical strong ion approach33 was used to allow a more comprehensive assessment of acid‐base status of calves of this study population. The strong ion difference in mEq/L calculated from the plasma concentrations of sodium, potassium, and chloride (SID3) was obtained as follows2:
| (4) |
The measured strong ion difference obtained from 7 strong ions34 (SID7, mEq/L) was calculated by the measured value for [Ca2+] determined by ion‐selective potentiometry and assigning a charge of +1.38 to magnesium assuming 69% dissociation35 and −1 to D‐lactate and L‐lactate assuming 100% dissociation such that:
| (5) |
The concentration of nonvolatile weak acids (A tot) in mmol/L was calculated from plasma concentrations of total protein:2
| (6) |
The strong ion gap (SIG) was calculated to obtain an estimate of the unmeasured strong anion concentration by the experimentally determined value for A tot, the experimentally determined value for the negative logarithm of dissociation constant of plasma nonvolatile weak acids (pKa = 7.08), and the following equation:2
| (7) |
The first expression on the right‐hand side of the SIG equation represents the net negative charge in mEq/L of nonvolatile weak acids (A−) in plasma. The percent changes in plasma volume at each time point x relative to a previous time point y were extrapolated from the changes in plasma total protein concentrations36 such that:
| (8) |
The changes (differences) in cK (Diff K) between baseline and a time point y were determined by the following equation:37
| (9) |
Also the area under the time curve of cK and Diff K (AUCK, AUCDiff K) for the study period of 120 minutes after the start of administration of respective infusion solutions was calculated by the trapezoidal method.
Statistical Analysis
Commercially available software programs12 , 13 , 14 were used for the statistical analysis of the results, and P‐values < .05 were considered to be statistically significant. A normal distribution of data was assessed by the Shapiro‐Wilk W test and visual inspection of QQ plots. Continuous data are reported as mean ± standard deviation (SD) or median and interquartile ranges. If necessary, data were log‐transformed to achieve a normal distribution of respective variables. A two‐way repeated‐measures ANOVA was used to detect differences of continuous variables over time and between treatment groups. If only a single comparison between treatment groups had to be performed, a one‐way ANOVA was used. Bonferroni‐adjusted P‐values were used whenever the F‐test was significant to assess differences within and between treatment groups.
Scores of clinical variables were expressed as median and corresponding minimum and maximum values and compared by a paired Wilcoxon test (within‐group comparisons) or a Kruskal‐Wallis test (between‐group comparisons).
The primary outcome variable of interest was the decrease in plasma potassium concentration from baseline, with a 15% difference in reduction at the end of the study period being the effect size of interest. Based on anticipated mean and SD values for the plasma potassium concentration of 7.45 ± 1.28 mmol/L for the studied population based on data from 234 hyperkalemic calves of a previously published study,4 an alpha of 0.05 and a group size of 7, we calculated the power of the study to be 0.80.
Results
Clinical Conditions
Clinical scores of calves before and at the end of the study period are given in Table 1. All calves were moderately to severely dehydrated based on the magnitude of eye recession into the orbit, with the degree of enophthalmos ranging from 3 to 8 mm. A total of 5 calves were presented in sternal (n = 2) or lateral recumbency (n = 3), whereas 9 calves presented an impairment of ability to stand (score 4 or 5). The suckling reflex was weak or absent in each of ten calves, but the strength of the palpebral reflex was not altered (Score 1) in 20 out of 22 calves.
Table 1.
Scores of clinical examination findings and degree of enophthalmos in 22 neonatal hyperkalemic diarrheic calves before and 120 minutes after start of infusions of either hypertonic 8.4% sodium bicarbonate (n = 7), 7.5% sodium chloride (n = 8), or 46.2% glucose (n = 7) and subsequent suckling of an oral electrolyte solution
| Variable | Before treatment | 120 minutes After Start of Treatment | P‐value |
|---|---|---|---|
| Posture (Score) | |||
| NaBic | 4 (2–7) | 2 (2–4) | .041 |
| NaCl | 4 (2–7) | 3.5 (2–6) | .059 |
| Gluc | 3 (2–6) | 2 (2–6) | .10 |
| Behavior (Score) | |||
| NaBic | 4 (2–4) | 2 (2–4) | .034 |
| NaCl | 3.5 (1–4) | 3 (1–4) | .025 |
| Gluc | 3 (3–5) | 2 (2–4) | .025 |
| Suckling reflex (Score) | |||
| NaBic | 2 (1–3) | 2 (2–3) | 1.0 |
| NaCl | 2.5 (1–3) | 2 (1–3) | .32 |
| Gluc | 2 (2–3) | 3 (1–3) | .56 |
| Palpebral reflex (Score) | |||
| NaBic | 1 (1–3) | 1 (1–3) | 1.0 |
| NaCl | 1 (1–1) | 1 (1–1) | 1.0 |
| Gluc | 1 (1–2) | 1 (1–2) | 1.0 |
| Enophthalmos (mm) | |||
| NaBic | 5 (3–7) | 3 (1–5) | .026 |
| NaCl | 5 (3–8) | 3.5 (2–8) | .024 |
| Gluc | 5 (3–8) | 3 (2–8) | .038 |
P‐values are based on a within‐group comparison. Values are reported as medians and corresponding ranges (minimum–maximum).
After injections of infusion solutions, a total of ten calves (4 calves of group NaBic, and each of 3 calves of groups NaCl and Gluc) suckled the entire volume of the offered electrolyte solution. The mean volumes of voluntarily suckled ORS in groups NaBic, NaCl, and Gluc were 1.5, 1.0, and 1.2 L, respectively (P = .61).
Clinical scores for posture, behavior, and degree of enophthalmos improved in all treatment groups, with no difference between treatment groups at the end of the study period (Table 1). However, a total of 18 calves still showed signs of moderate to severe dehydration as indicated by eye recession into the orbit. Two calves of group NaCl and 1 calf of group Gluc remained unable to stand at the end of the investigation period.
Changes in Plasma Potassium Concentrations
Changes in plasma cK over the study period in the 3 treatment groups are shown in Figure 1A. There was no significant effect of group (P = .40), but the effect of time and the interaction of time × group was statistically significant (P < .001). A similar decline in cK was observed in groups NaCl and NaBic until 10 minutes, but a subsequent increase in cK was observed in group NaCl, whereas in calves of group NaBic a further decline in cK was observed until 120 minutes. Plasma cK values in calves of group NaCl were similar to baseline between 50 and 120 minutes. In calves of group Gluc, cK remained unchanged between 7 minutes and 20 minutes, but continuously decreased thereafter until 120 minutes. The area under the cK time curve was 692 ± 160 mmol/L*min in group NaBic, 812 ± 141 mmol/L*min in group NaCl, and 700 ± 115 mmol/L*min in group Gluc (P = .20).
Figure 1.
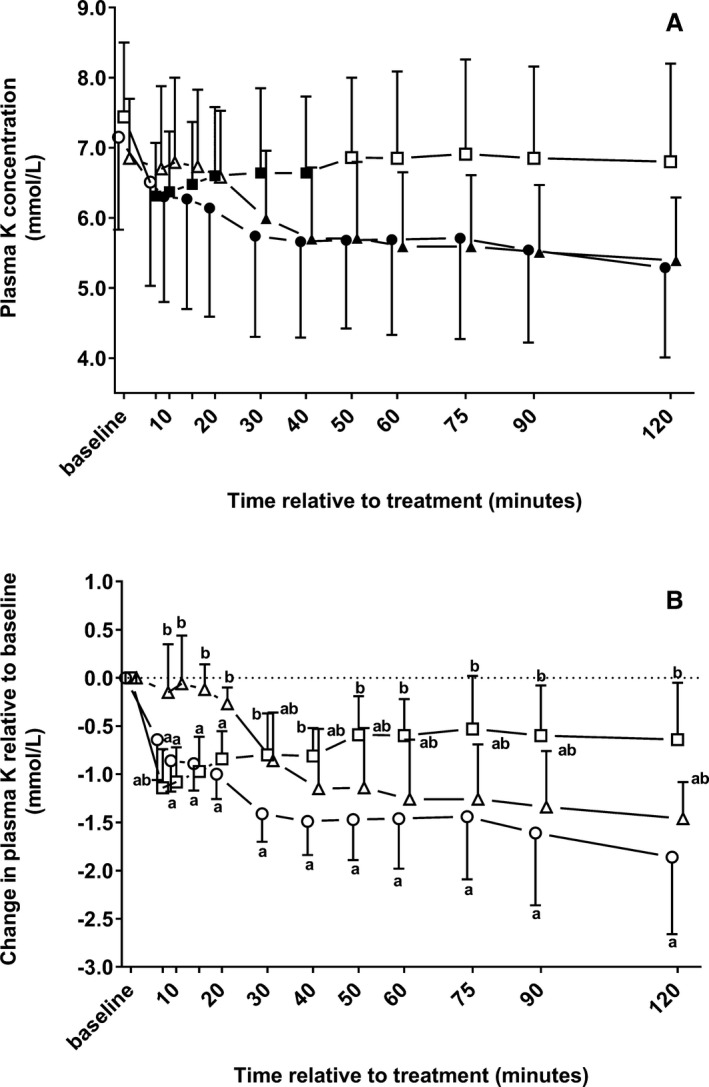
Mean ± SD of plasma potassium concentrations (A) and changes in plasma potassium concentrations relative to baseline (B) in 22 neonatal diarrheic calves after injections of an 8.4% sodium bicarbonate solution in a dosage of 6.4 mL/kg body mass (ο; n = 7), 7.5% sodium chloride solution in a dosage of 5 mL/kg body mass (□; n = 8), or a 46.2% glucose solution in a dosage of 5 mL/kg body mass (Δ; n = 7) over a period of five min and subsequent administration of an oral electrolyte solution. Values with different letters differed significantly between groups (P < .05). Values with a filled symbol in graph A differ significantly (P < .05) from baseline (within‐group comparisons for the change in plasma potassium concentrations relative to baseline were not possible due to the statistical methods applied). Values for groups NaBic and Gluc were slightly offset at each time point to improve readability.
Changes in plasma potassium concentrations relative to baseline (Diff K) in the 3 treatment groups during the study period are shown in Figure 1B. There was a statistically significant effect of group (P = .007), time (P < .001), and the interaction of time and group (P < .001) as indicated by different slopes and amplitudes of respective Diff K time curves. Similar decrements of cK were observed in groups NaCl and NaBic between 10 and 20 minutes, which were significantly higher than in calves of group Gluc during the same period of time. Thereafter, an increase in the Diff K time curve was observed in calves of group NaCl, whereas a decrease in the Diff K time curve was observed in calves of group Gluc. Between 30 minutes and 120 minutes, the most marked decrement of cK was observed in calves group NaBic, which was reflected by Diff K values that differed significantly from calves of group NaCl, but not from calves of group Gluc. At 120 minutes, the observed decrements were equivalent to −26.0 ± 10.4%, −9.1 ± 8.3%, and −21.6 ± 6.4% of baseline cK in groups NaBic, NaCl, and Gluc, respectively. Those values differed significantly between groups NaCl and NaBic (P = .003), NaCl and Gluc (P = .032), but not between NaBic and Gluc (P = 1.00).
The calculated area under the Diff K time curve was −166 ± 50 mmol/L × min for group NaBic, −82 ± 43 mmol/L × min for group NaCl, and −122 ± 49 mmol/L × min for group Gluc. The AUCDiff K values also differed significantly between groups NaBic and NaCl (P = .008), but not between NaBic and Gluc (P = .28), and NaCl and Gluc (P = .35).
Acid‐Base Variables
Changes in acid‐base variables during the study period and results of the repeated‐measures ANOVA are presented in Table 2. Infusion of sodium bicarbonate caused an immediate and marked increase in venous blood pH and bicarbonate concentration (Fig 2). Venous blood pH and bicarbonate concentration did not change significantly from baseline in groups NaCl and Gluc throughout the study period, except at 10 minutes where a statistically significant decrease in bicarbonate concentration was detectable in group NaCl. Also, IV administration of sodium bicarbonate resulted in an increase in measured plasma strong ion difference as indicated by values for SID3 and SID7 that were significantly higher than at baseline throughout the study period. In contrast, a decrease in plasma SID3 and SID7 was observed in groups NaCl and Gluc, which was statistically significant for SID7 at 15 and 30 minutes, and for SID3 from 7 minutes until 20 minutes. No group effect, but statistically significant time and time x group effects were observed for AG and SIG.
Table 2.
Laboratory findings in 22 neonatal diarrheic calves before and after administration of infusions with a hypertonic 8.4% sodium bicarbonate (n = 7), 7.5% sodium chloride (n = 8), or 46.2% glucose solution (n = 7) over a period of 5 minutes and subsequent suckling of an oral electrolyte solution
| Variable | Baseline | Time After Start of Treatment | P‐value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 15 minutes | 30 minutes | 60 minutes | 90 minutes | 120 minutes | Group | Time | Time × group | ||
| Henderson‐Hasselbalch acid‐base model | |||||||||
| pCO2 (mmHg) | |||||||||
| NaBic | 41 ± 7 | 47 ± 8 | 49 ± 8 | 46 ± 8 | 46 ± 9 | 44 ± 8 | .44 | .3 | .014 |
| NaCl | 49 ± 12 | 44 ± 10 | 46 ± 8 | 44 ± 10 | 44 ± 10 | 45 ± 9 | |||
| Gluc | 52 ± 7 | 49 ± 5 | 50 ± 5 | 51 ± 8 | 54 ± 5 | 53 ± 6 | |||
| BE (mmol/L) | |||||||||
| NaBic | −12.2 ± 9.4a | 5.3 ± 7.6*a | 0.6 ± 8.5*a | −0.9 ± 8.5*a | 0.0 ± 9.4*a | −0.3 ± 9.4*a | .001 | <.001 | <.001 |
| NaCl | −15.4 ± 7.5a | −16.7 ± 6.4b | −16.1 ± 6.5b | −15.3 ± 6.3b | −14.0 ± 6.7b | −12.8 ± 7.1b | |||
| Gluc | −9.9 ± 6.8a | −10.5 ± 6.3b | −10.8 ± 6.0b | −9.2 ± 7.2ab | −8.0 ± 7.2ab | −7.5 ± 7.9ab | |||
| AG (mEq/L) | |||||||||
| NaBic | 22.8 ± 4.1 | 20.5 ± 4.7 | 22.8 ± 4.8 | 22.4 ± 4.3 | 21.9 ± 5.3 | 21.7 ± 5.7 | .49 | <.001 | .01 |
| NaCl | 27.3 ± 5.9 | 24.2 ± 5.7* | 24.7 ± 5.1 | 25.1 ± 5.7 | 23.4 ± 5.6* | 22.4 ± 5.4* | |||
| Gluc | 24.8 ± 5.3 | 20.8 ± 4.8* | 22.5 ± 4.8 | 22.8 ± 4.2 | 22.7 ± 4.3 | 21.6 ± 3.8 | |||
| Strong ion difference acid‐base model | |||||||||
| SID3 (mEq/L) | |||||||||
| NaBic | 38.5 ± 5.1*a | 50.7 ± 6.1*a | 48.9 ± 5.8*a | 47.2 ± 6.6*a | 47.5 ± 6.9*a | 46.7 ± 6.8*a | .012 | .008 | <.001 |
| NaCl | 41.6 ± 5.1a | 36.9 ± 5.0*b | 38.1 ± 4.7b | 38.8 ± 5.0b | 38.0 ± 5.1b | 38.0 ± 4.8b | |||
| Gluc | 43.5 ± 7.9a | 38.4 ± 7.4*b | 39.9 ± 7.3b | 41.7 ± 6.9ab | 43.0 ± 6.3ab | 42.1 ± 6.2ab | |||
| SID7 (mEq/L) | |||||||||
| NaBic | 36.0 ± 6.0a | 45.9 ± 7.1*a | 43.9 ± 6.5*a | 42.6 ± 7.7*a | 43.1 ± 7.7*a | 42.9 ± 8.0*a | .14 | .011 | <.001 |
| NaCl | 39.2 ± 4.0a | 34.8 ± 4.3*b | 36.2 ± 3.9*b | 37.1 ± 4.1a | 36.5 ± 5.0a | 36.7 ± 4.7a | |||
| Gluc | 41.1 ± 5.3a | 35.3 ± 4.7*b | 36.4 ± 4.8*b | 38.7 ± 4.4a | 40.5 ± 4.4a | 39.9 ± 4.4a | |||
| A− (mEq/L) | |||||||||
| NaBic | 13.0 ± 2.7a | 12.9 ± 1.4a | 13.1 ± 1.7a | 13.3 ± 1.9a | 13.2 ± 2.0a | 13.4 ± 2.2a | .068 | <.001 | <.001 |
| NaCl | 13.0 ± 2.3a | 10.1 ± 1.9*b | 10.8 ± 1.9*a | 11.4 ± 1.8*a | 11.7 ± 1.7a | 11.9 ± 1.9a | |||
| Gluc | 13.7 ± 1.6a | 9.7 ± 1.4*b | 10.8 ± 1.6*a | 12.0 ± 1.7*a | 12.5 ± 1.8a | 12.9 ± 1.8a | |||
| SIG (mEq/L) | |||||||||
| NaBic | −9.3 ± 5.8a | −7.6 ± 5.1*a | −9.7 ± 5.6a | −9.1 ± 4.8a | −8.6 ± 5.9a | −8.2 ± 6.1a | .25 | <.001 | <.001 |
| NaCl | −14.3 ± 7.4a | −14.0 ± 6.9b | −13.9 ± 6.2a | −13.7 ± 6.5a | −11.7 ± 6.0a | −10.5 ± 6.1a | |||
| Gluc | −11.2 ± 5.0a | −11.1 ± 5.1ab | −11.7 ± 4.8a | −10.8 ± 4.5a | −10.2 ± 4.6a | −8.7 ± 4.3a | |||
| Clinical biochemistry analysis | |||||||||
| l‐lactate (mmol/L) | |||||||||
| NaBic | 3.0 ± 1.9 | 4.6 ± 3.0* | 5.0 ± 3.1* | 4.7 ± 3.0* | 4.3 ± 2.8 | 4.0 ± 2.7 | .86 | .001 | .002 |
| NaCl | 4.6 ± 3.6 | 4.3 ± 3.6 | 4.1 ± 3.4 | 3.8 ± 3.5 | 3.6 ± 3.2 | 3.4 ± 3.1 | |||
| Gluc | 4.6 ± 2.8 | 4.9 ± 2.6 | 5.4 ± 2.4 | 5.0 ± 2.6 | 4.6 ± 2.4 | 4.2 ± 2.4 | |||
| d‐lactate (mmol/L) | |||||||||
| NaBic | 2.25 (0.46–2.91) | 2.52 (0.54–3.47) | 2.47 (0.59–3.40) | 2.48 (0.60–3.28) | 2.47 (0.58–3.17) | 2.33 (0.58–3.21) | .79 | .27 | .079 |
| NaCl | 1.47 (0.78–3.82) | 1.40 (0.61–3.21) | 1.41 (0.71–3.40) | 1.48 (0.83–3.57) | 1.46 (0.72–3.69) | 1.47 (0.87–3.51) | |||
| Gluc | 1.37 (0.25–4.94) | 1.41 (0.23–4.86) | 1.37 (0.30–4.85) | 1.36 (0.34–5.03) | 1.38 (0.31–4.99) | 1.38 (0.24–5.23) | |||
| Urea (mmol/L) | |||||||||
| NaBic | 18.6 ± 7.7a | 18.3 ± 7.6a | 18.3 ± 7.8a | 18.3 ± 7.8a | 18.0 ± 7.7a | 17.9 ± 7.8a | .015 | .019 | .68 |
| NaCl | 31.7 ± 12.2b | 31.2 ± 12.0*b | 31.2 ± 12.0b | 31.2 ± 12.3b | 31.1 ± 12.5b | 30.8 ± 12.5b | |||
| Gluc | 18.2 ± 5.1a | 18.0 ± 5.0a | 18.0 ± 5.1a | 17.9 ± 5.2a | 17.9 ± 5.3a | 17.9 ± 5.4a | |||
| Creatinine (μmol/L) | |||||||||
| NaBic | 317 (150–401) | 300 (142–377)* | 307 (138–370)* | 297 (136–357)* | 290 (129–342)* | 287 (125–331)* | .4 | <.001 | .32 |
| NaCl | 406 (206–708) | 380 (190–666)* | 385 (186–683)* | 384 (180–694)* | 379 (175–699)* | 371 (170–702) | |||
| Gluc | 327 (245–406) | 317 (246–392) | 319 (238–404) | 314 (230–413) | 311 (228–425) | 306 (219–426) | |||
| Total protein (g/L) | |||||||||
| NaBic | 68.9 ± 7.0a | 54.9 ± 4.8*ab | 59.2 ± 4.9*a | 60.1 ± 6.1*a | 59.5 ± 6.4*a | 59.9 ± 7.1*a | .22 | <.001 | <.001 |
| NaCl | 76.0 ± 7.0a | 59.9 ± 5.8*a | 63.6 ± 5.1*a | 65.0 ± 4.7*a | 65.1 ± 5.2*a | 64.6 ± 5.0*a | |||
| Gluc | 72.9 ± 8.8a | 51.4 ± 5.9*b | 57.8 ± 7.3*a | 63.0 ± 8.2*a | 65.4 ± 8.1*a | 66.7 ± 8.6*a | |||
| P (mmol/L) | |||||||||
| NaBic | 3.7 ± 1.1 | 3.4 ± 1.0* | 3.4 ± 0.9* | 3.3 ± 0.9 | 3.2 ± 0.9* | 3.1 ± 0.9* | .78 | <.001 | .052 |
| NaCl | 4.1 ± 1.3 | 3.6 ± 1.1* | 3.6 ± 1.1* | 3.6 ± 1.1* | 3.5 ± 1.0* | 3.4 ± 1.0* | |||
| Gluc | 3.9 ± 0.9 | 3.4 ± 0.9* | 3.3 ± 0.9* | 3.1 ± 0.9* | 3.1 ± 0.9* | 3.0 ± 0.9* | |||
| Electrolytes | |||||||||
| Cl (mmol/L) | |||||||||
| NaBic | 97 ± 12a | 92 ± 12*a | 93 ± 12*a | 94 ± 12*ab | 94 ± 12*a | 94 ± 12*a | .012 | <.001 | <.001 |
| NaCl | 94 ± 7a | 110 ± 7*b | 108 ± 7*b | 106 ± 7*a | 106 ± 7*a | 106 ± 7*a | |||
| Gluc | 98 ± 10a | 89 ± 9*a | 91 ± 10*a | 93 ± 10*b | 94 ± 10*a | 96 ± 11*a | |||
| Ca (mmol/L) | |||||||||
| NaBic | 1.18 (1.16–1.21)a | 0.97 (0.94–1.01)*a | 1.02 (0.98–1.08)*a | 0.99 (0.92–1.02)*a | 0.99 (0.90–1.09)*a | 1.02 (0.94–1.08)*a | .018 | <.001 | <.001 |
| NaCl | 1.20 (1.10–1.28)a | 1.17 (1.09–1.23)b | 1.18 (1.13–1.26)a | 1.20 (1.16–1.23)b | 1.18 (1.15–1.23)b | 1.17 (1.13–1.22)b | |||
| Gluc | 1.18 (1.14–1.33)a | 1.08 (1.05–1.23)*b | 1.12 (1.11–1.26)*a | 1.18 (1.12–1.27)b | 1.16 (1.12–1.27)b | 1.18 (1.13–1.23)b | |||
| Mg (mmol/L) | |||||||||
| NaBic | 1.06 ± 0.23a | 0.88 ± 0.18*a | 0.92 ± 0.18*a | 0.91 ± 0.17*a | 0.91 ± 0.16*a | 0.91 ± 0.13*a | .03 | <.001 | .013 |
| NaCl | 1.36 ± 0.30a | 1.22 ± 0.24*b | 1.24 ± 0.24*b | 1.27 ± 0.23b | 1.25 ± 0.25b | 1.23 ± 0.24*b | |||
| Gluc | 1.30 ± 0.32a | 1.09 ± 0.24*ab | 1.14 ± 0.24*ab | 1.19 ± 0.25ab | 1.22 ± 0.24b | 1.23 ± 0.24b | |||
| Hematology | |||||||||
| PCV (%) | |||||||||
| NaBic | 46 ± 13 | 37 ± 10* | 39 ± 11* | 39 ± 11* | 39 ± 11* | 39 ± 11* | .78 | <.001 | <.001 |
| NaCl | 46 ± 7 | 38 ± 7* | 40 ± 7* | 40 ± 7* | 40 ± 7* | 41 ± 8* | |||
| Gluc | 51 ± 12 | 37 ± 10* | 40 ± 10* | 43 ± 11* | 45 ± 11* | 45 ± 11* | |||
| Hb (mmol/L) | |||||||||
| NaBic | 9.0 ± 2.4 | 7.4 ± 1.9* | 7.8 ± 1.9* | 7.8 ± 2.0* | 7.7 ± 2.0* | 7.7 ± 2.0* | .89 | <.001 | <.001 |
| NaCl | 9.0 ± 1.5 | 7.6 ± 1.3* | 7.9 ± 1.4* | 8.0 ± 1.4* | 8.0 ± 1.4* | 8.0 ± 1.5* | |||
| Gluc | 9.6 ± 2.2 | 7.2 ± 1.8* | 7.7 ± 1.7* | 8.3 ± 1.9* | 8.6 ± 2.0* | 8.7 ± 2.0* | |||
pCO2, partial pressure of carbon dioxide; BE, base excess; AG, anion gap; SID3, strong ion difference calculated from 3 strong cations and anions; SID7, strong ion difference calculated from 7 strong cations and anions; A−, total net anion charge of nonvolatile weak acids; SIG, strong ion gap; PCV, packed cell volume; Hb, hemoglobin; Δ Plasma vol., Change in plasma volume extrapolated from the change in total protein concentration; P, inorganic phosphorus. Laboratory findings at t = 7, 10, 20, 40, 50, and 75 minutes after start of treatment are not shown but were included in the repeated‐measures ANOVA if available.
Values are reported as mean ± SD or median and interquartile ranges. Different letters indicate a statistical significant difference between groups at the respective time point (P < .05). Asterisks indicate values that are significantly different from baseline (P < .05).
Figure 2.
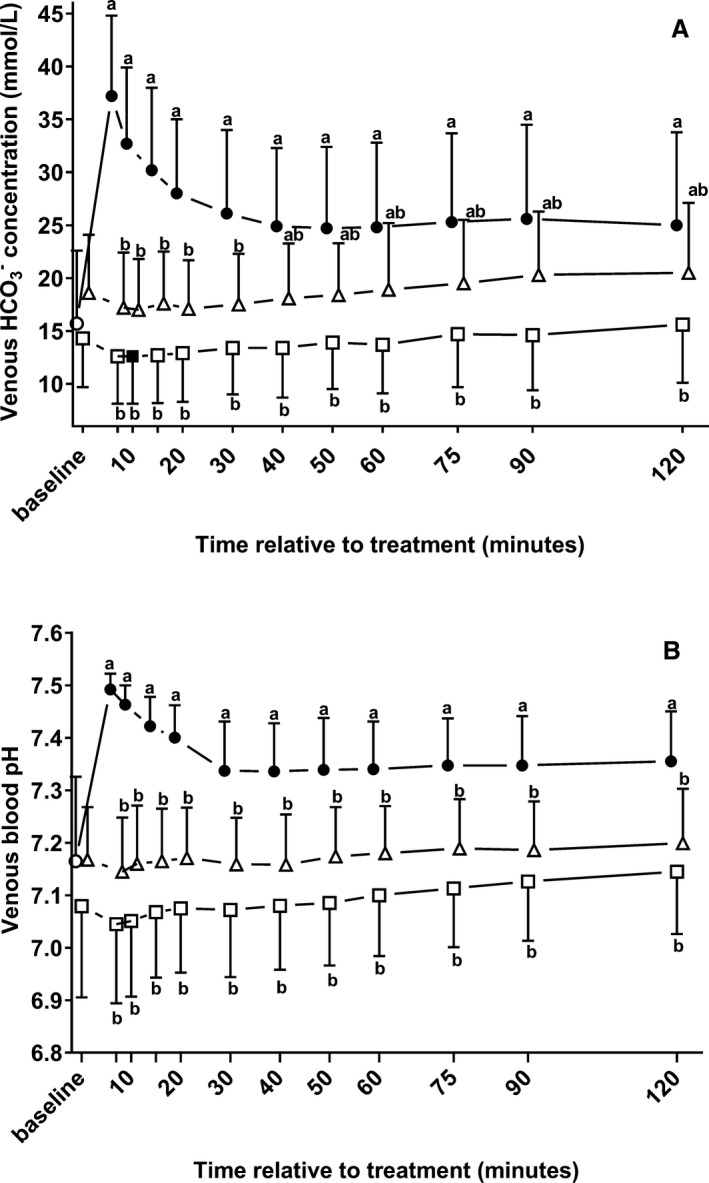
Changes (mean ± SD) in venous bicarbonate concentrations (A) and venous blood pH values (B) in 22 neonatal diarrheic calves after injections of an 8.4% sodium bicarbonate solution in a dosage of 6.4 mL/kg body mass (ο; n = 7), 7.5% sodium chloride solution in a dosage of 5 mL/kg body mass (□; n = 8), or a 46.2% glucose solution in a dosage of 5 mL/kg body mass (Δ; n = 7) over a period of five min and subsequent administration of an oral electrolyte solution. Values with different letters differed significantly between groups (P < .05). Values with a filled symbol differ significantly from baseline (P < .05). Values for groups NaBic and Gluc were slightly offset at each time point to improve readability.
Plasma Sodium Concentrations, Sodium‐to‐Potassium Ratio, and Changes in Plasma Volume
A similar increase in plasma cNa was observed after treatment in groups NaBic and NaCl, with values that differed significantly from baseline throughout the study period (Fig 3). IV administration of a sodium‐free glucose solution resulted in a significant decrease in cNa with values that differed significantly from calves of groups NaBic and NaCl between 7 minutes and 20 minutes. Despite these findings, no group (P = .41), but a statistically significant time (P < .001) and time x group (P < .001) effect was observed for the calculated Na/K ratio. Administration of sodium bicarbonate was the only treatment that resulted in an increase in the Na/K‐ratio, which was significantly different from baseline during the whole study period. In contrast, a statistically significant increase in the Na/K ratio was only detectable until 40 minutes in group NaCl and at 90 minutes and 120 minutes in calves of group Gluc.
Figure 3.
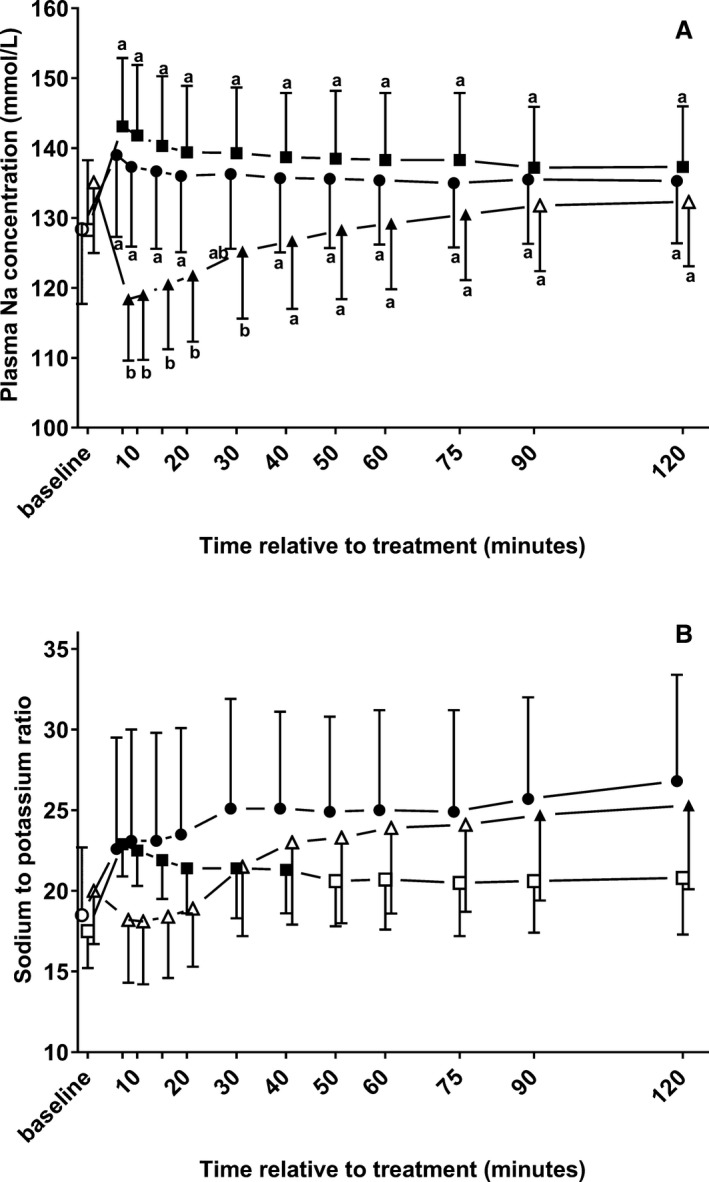
Changes (mean ± SD) in plasma sodium concentrations (A) and the sodium‐to‐potassium ratio (B) in 22 neonatal diarrheic calves after injections of an 8.4% sodium bicarbonate solution in a dosage of 6.4 mL/kg body mass (ο; n = 7), 7.5% sodium chloride solution in a dosage of 5 mL/kg body mass (□; n = 8), or a 46.2% glucose solution in a dosage of 5 mL/kg body mass (Δ; n = 7) over a period of five min and subsequent administration of an oral electrolyte solution. Values with different letters differed significantly between groups (P < .05). Values with a filled symbol differ significantly from baseline (P < .05). Values for groups NaBic and Gluc were slightly offset at each time point to improve readability.
Changes in plasma volume during the study period are illustrated by Figure 4. There was a statistically significant effect (P < .001) of time and time × group, but the effect of group was not significant (P = .13). The changes in plasma volume relative to baseline were significantly higher in calves of group Gluc between 7 and 20 minutes than for calves of groups NaCl and NaBic.
Figure 4.
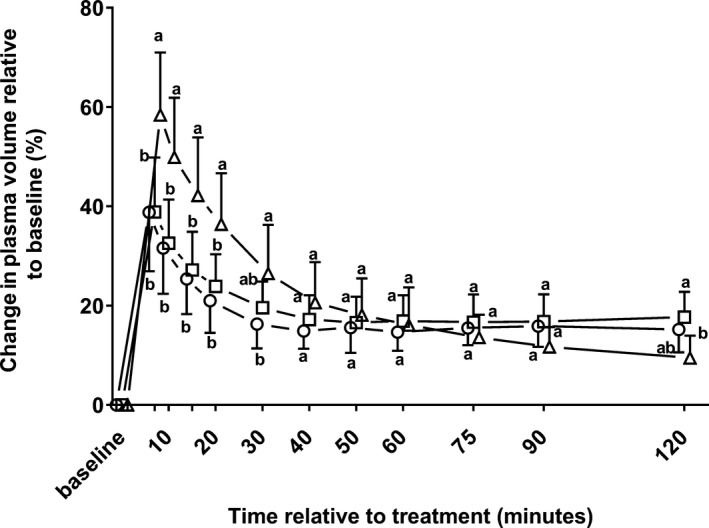
Mean plasma volume changes ± SD in 22 neonatal diarrheic calves after injections of an 8.4% sodium bicarbonate solution in a dosage of 6.4 mL/kg body mass (ο; n = 7), 7.5% sodium chloride solution in a dosage of 5 mL/kg body mass (□; n = 8), or a 46.2% glucose solution in a dosage of 5 mL/kg body mass (Δ; n = 7) over a period of five min and subsequent administration of an oral electrolyte solution (please see text for details). Values with different letters differed significantly between groups (P < .05). Values for groups NaBic and Gluc were slightly offset at each time point to improve readability.
Clinical Biochemistry, Hematologic Analysis, and Changes in Ionized Calcium Concentrations
Hematologic and plasma biochemical variables stratified by treatment groups and sampling times and respective results of the repeated‐measures ANOVA are also presented in Table 2. Infusions of the 46.2% glucose solution resulted in a large increase in plasma glucose and insulin concentration that differed significantly from baseline and from calves of groups NaCl and NaBic throughout the study period (Fig 5).
Figure 5.
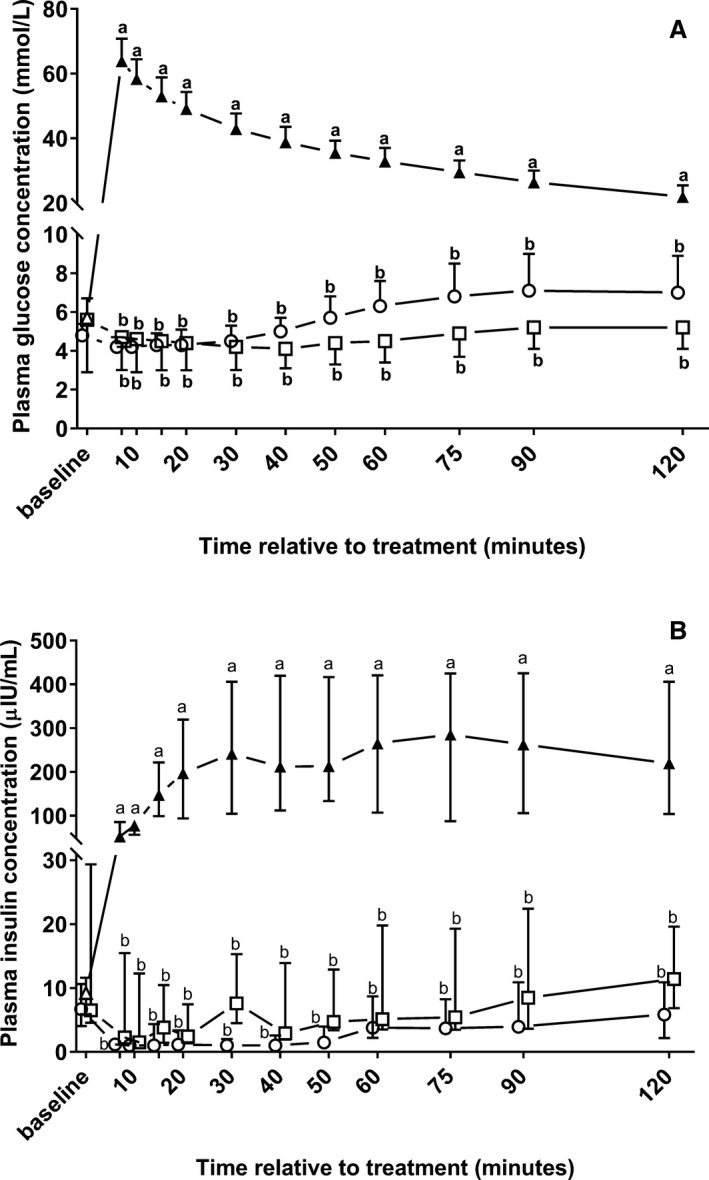
Changes in plasma glucose (A; mean ± SD) and insulin (B; median and interquartile ranges) concentrations in 22 neonatal diarrheic calves after injections of an 8.4% sodium bicarbonate solution in a dosage of 6.4 mL/kg body mass (ο; n = 7), 7.5% sodium chloride solution in a dosage of 5 mL/kg body mass (□; n = 8), or a 46.2% glucose solution in a dosage of 5 mL/kg body mass (Δ; n = 7) over a period of five min and subsequent administration of an oral electrolyte solution. Values with different letters differed significantly between groups (P < .05). Values with a filled symbol differ significantly from baseline (P < .05). Values for groups NaBic and NaCl were slightly offset at each time point to improve readability.
No statistically significant group, but time effects were observed for plasma concentrations of creatinine, phosphorus, and total protein as well as for PCV and blood hemoglobin concentrations. A significant group and time, but no time × group effect was detectable for plasma urea concentrations. D‐lactate concentrations did not change significantly from baseline values in all treatment groups. However after start of treatment, a statistically significant rise of plasma L‐lactate concentrations was detectable in calves of group NaBic. Statistically significant group, time, and time × group effects were also observed for ionized calcium concentrations (Table 2).
Outcome of Treatment
After a mean duration of 12 ± 5 days of hospitalization 21 out of the 22 calves were discharged in a healthy state. One calf of group NaCl had to be euthanized for reasons of an advanced pneumonia, which had progressed during hospitalization.
Discussion
The aim of the present study was to document the plasma potassium‐lowering effect of hypertonic saline‐, glucose‐, and sodium bicarbonate‐containing infusion solutions in the initial treatment of hyperkalemic diarrheic calves. Central findings of this study suggest a treatment advantage of sodium bicarbonate over the use of hypertonic saline‐ or glucose‐containing infusion solutions as indicated by Diff K values in calves of group NaBic being 1.2 mmol/L lower at the end of the 120 minutes study period than in calves of groups NaCl, and also by a more rapid initial potassium‐lowering response in calves of group NaBic when compared to calves of group Gluc.
Rapid correction of hyperkalemia is considered decisive in the treatment of affected calves and associated clinical alterations, which are characterized by cardiac conduction abnormalities and arrhythmias, marked dehydration, and skeletal muscle weakness in spite of normal or only slightly elevated D‐lactate concentrations,8, 13 as also observed in calves of the present study. An immediate and sustained potassium‐lowering effect of hypertonic (8.4%) sodium bicarbonate infusion solution has already been demonstrated in previous studies,17, 18, 38 with the observed initial decrements of cK being most closely associated with increases in venous blood pH.17 Despite those findings, it still remained to be elucidated if the potassium‐lowering effect of sodium bicarbonate‐containing infusion solutions is related to the administered sodium load, plasma volume expansion, or alkalinization. In the present study, the administered infusion solutions in calves of groups NaBic and NaCl differed only in the alkalinizing capacity as the sodium load of those solutions was identical and differences in osmolarity were counterbalanced by a higher infusion volume in calves of group NaBic, which resulted in a similar increase in plasma volume in those groups. The findings that administration of hypertonic saline resulted only in a short‐term decrease in cK and that administration of sodium bicarbonate was associated with a more pronounced and sustained potassium‐lowering effect therefore indicates that alkalinization represents an effective potassium‐lowering mechanism in neonatal hyperkalemic diarrheic calves. This finding is also consistent with the current understanding of cellular transport processes that are involved in the extrarenal potassium homeostasis in as much that increases in extracellular induce a compartmental shift of potassium ions by enhancing cellular Na+ uptake via a Na+/ cotransport and Na+/H+ exchange, which results in stimulation of Na+/K+‐ATPase activity and consequently in a net cellular uptake of potassium ions.39
The use of sodium bicarbonate in the treatment of acute hyperkalemia has been a controversial issue,11 which is particularly based on studies in dogs with experimentally induced hyperkalemia (after KCl infusions) and human patients with end‐stage renal disease where intravenous administration of isotonic or hypertonic sodium bicarbonate solution was documented to be ineffective in lowering cK.40, 41, 42, 43 However, there is a fundamental difference between these studies in that the occurrence of hyperkalemia in patients with end‐stage renal disease is mainly related to a disturbance of external potassium balance (i.e, dysbalance between potassium intake and excretion), which is different to the situation in neonatal diarrheic calves. Therefore, results of studies that are based on treatment observations in patients with end‐stage renal disease or an experimental setting where dogs are infused with KCl are unlikely suitable for extrapolation of treatment strategies to neonatal diarrheic calves. Interestingly, there are also studies that documented a potassium‐lowering effect of sodium bicarbonate in acidotic humans.44, 45 As an explanation for the existent discrepancies a recent review article39 discussed that the potassium‐lowering effect of sodium bicarbonate depends on the presence of metabolic acidosis and more importantly on the degree of intracellular acidosis as intracellular Na+ entry by Na+–H+ exchange and Na+‐bicarbonate cotransport is greater when intracellular pH and are reduced. This would explain why sodium bicarbonate has a marked potassium‐lowering effect in acidemic neonatal diarrheic calves.
In the light of those issues, it also needs to be considered that treatment of hyperkalemia in diarrheic calves is usually not addressed as an isolated problem because treatment objectives should also focus on correction of concomitant dehydration and metabolic acidosis; especially correction of metabolic acidosis is considered decisive in the treatment of critically ill diarrheic calves46 and sodium bicarbonate has been shown to be the alkalinizing agent of choice in this case.47
Despite the fact that administration of sodium bicarbonate was associated with a significantly higher decrement of cK than in group NaCl at the end of the 120 minutes study period, clinical findings such as posture, behavior, and strength of the suckling reflex were not significantly differing between treatment groups. This might be related to the finding that irrespective of treatment groups, many calves were still clinically dehydrated and therefore still in a critical condition. However, in the light of those findings it needs to be clearly emphasized that it was the aim of the present study to compare the potassium‐lowering efficiency of different hypertonic infusion solutions in the initial treatment of hyperkalemic calves and not to test the resuscitative effect of a single injection of hypertonic infusion solution and subsequent suckling of an oral electrolyte solution. Nevertheless, given the facts that hyperkalemia in neonatal diarrheic calves is associated with marked dehydration and that an incomplete restoration of potassium homeostasis was even observed in calves of groups NaBic in spite of alkalinization and correction of acidosis, our findings suggest that rehydration should be another goal in the treatment of hyperkalemic calves as also indicated by the results of a recent observational study.17 Although not assessed in the present study, it is likely that administration of an additional volume of crystalloid infusion solutions would have resulted in a more rapid decline in cK through a more sustained plasma volume expansion and renal potassium excretion.
In recent years, hypertonic rehydration strategies consisting of administration of small volumes of hypertonic (7.2 or 7.5%) sodium chloride with or without dextran in combination with oral electrolyte solutions have been evaluated as an alternative to traditional isotonic IV fluid administration or oral rehydration in dehydrated neonatal diarrheic calves.18, 48, 49, 50, 51, 52 Some of those studies49, 50, 52 reported a similar or even better treatment success of hypertonic rehydration when compared to IV administration of different amounts of isotonic fluids. Also a sustained plasma potassium‐lowering effect of hypertonic saline solution was reported in some studies,48, 49, 51 which is different than the results of the present investigation. However, it needs to be considered that those investigations were predominantly performed in calves with experimentally induced diarrhea and dehydration. Although some research groups48, 49, 51 were also able to induce an increase in plasma potassium concentrations, they usually observed only slight derangements of acid‐base status and failed to reproduce the complex metabolic alterations that are usually seen in markedly acidemic calves with naturally acquired diarrhea, which likely explains the differences to results of the study reported here. Also in a 2008 study18 evaluating the resuscitative effect of oral rehydration in combination with a 5.85% saline solution (5 mL/kg BW) or 8.4% sodium bicarbonate (10 mL/kg BW) in profoundly acidemic calves with naturally acquired diarrhea, serum potassium concentrations remained unchanged (7.7 ± 2.0 mmol/L) after a period of 60 minutes after administration of hypertonic saline and subsequent suckling or tube feeding of 3 L of an electrolytes solution, as it was also the case in the present study. An important finding of that study18 was also that hypertonic rehydration with saline had a lower success rate (63%) than that with sodium bicarbonate (92%), which was related to an incomplete correction of acidosis and associated clinical alterations in calves that were initially presented with severe acidemia.
Induction of a paradoxical intracellular and cerobrospinal fluid (CSF) acidosis has been listed as a potential adverse effect of rapid administration of (hypertonic) sodium bicarbonate solutions, as the resulting buffer reaction might not only result in a rapid and large increase in CO2 in the blood, but also in the CSF and intracellular space, as CO2 is able to rapidly diffuse across cell membranes and the blood‐brain barrier.46, 53, 54 However, experimental studies55, 56 failed to induce a paradoxical intracellular and CSF acidosis after rapid administration of hypertonic55 or isotonic56 sodium bicarbonate solutions to acidotic calves, strongly indicating that spontaneously ventilating animals are sufficiently able to handle the resulting increase in pCO2. Those experimental studies55, 56 were performed in normovolemic calves, but also in the present study, only a slight and not statistically significant increase in blood pCO2 from 41.3 ± 7.3 mmHg at baseline to 50 ± 8.6 and 46.7 ± 7.2 mmHg was even detectable in dehydrated calves of group NaBic at 7 minutes and 10 minutes, respectively. Demyelinating brain lesions such as pontine myelinolysis might theoretically represent another complication of rapid IV administration of hypertonic sodium solutions as those conditions have been described as an adverse effect of rapid correction of chronic and severe hyponatremia in rats (cNa 95 ± 0.7 mmol/L)57 and humans (cNa 97.3 ± 6.7 mmol/L).58 Therefore, rapid increase in plasma sodium concentration might potentially result in neurologic sequelae if hypertonic sodium solutions are administered to chronically hyponatremic calves. However, central pontine myelinolysis after IV administration of hypertonic sodium solutions has to the best of our knowledge so far not been described in neonatal diarrheic calves and it needs to be considered that hyponatremia in those animals is usually only moderate and less pronounced4, 18, 19, 34, 38 than it was the case in the aforementioned studies.57, 58
Remarkably, infusions with hypertonic sodium bicarbonate resulted in a statistically significant increase in plasma L‐Lactate concentration that was not observed in all other treatment groups. Similar observations were also made in dogs with experimentally induced lactic acidosis and hemorrhagic shock59, 60 as well as in endotoxemic ponies.61 Also oral administration of sodium bicarbonate before high‐intensity exercise testing resulted in a significant more pronounced rise of blood L‐lactate concentrations during strenuous exercise in humans and horses when compared to control interventions.62, 63 A shift of lactate from the intracellular to the extracellular space in response to alkalinization represents a plausible explanation for those observations, as a rise of the pH gradient between compartments favored lactate release from muscle in in vitro studies.64, 65 A similar increase after administration of sodium bicarbonate was not observed for D‐lactate in the present study. However, it needs to be emphasized that D‐lactate concentrations of most calves were within an established reference range for bucket‐fed calves of ≤3.96 mmol/L,66 which is in agreement with our previous findings that hyperkalemia in diarrheic calves is rarely associated with D‐lactic acidosis.4, 8 However given the fact that D‐lactate is only produced in minimal amounts in the methylglyoxal pathway in mammals,67 a therapeutic effect on D‐lactate concentrations could still be expected. Previous studies on the dynamics of plasma D‐lactate concentration during the course of treatment have shown that complete normalization of plasma D‐lactate concentration can require a period of 24 hours or more.19, 68, 69 The underlying mechanisms have still not been completely clarified, but are most likely due to alkalinization and increased renal elimination triggered by enhancement of renal perfusion after rehydration.70 Unchanged plasma D‐lactate concentration in calves of the present study can therefore be explained by the short study period, incomplete rehydration of calves, and persistent acidemia in calves of groups NaCl and Gluc.
Infusions with sodium bicarbonate also resulted in a statistically significant decrease in the ionized calcium concentration with values that were significantly lower at most time points than in calves of groups Gluc or NaCl (Table 2). This was likely a pH‐dependent effect due to increased binding at negatively charged sites at albumin that became available with increased blood pH.71 A similar decrease in the ionized calcium concentration as in NaBic‐treated calves of the present study has also been previously observed during intravenous fluid therapy with sodium bicarbonate‐containing infusions in diarrheic calves.72 The authors discussed that this decrease could be of clinical relevance, but obvious side reactions such as tetanic convulsions or muscle cramps were not observed in the calves of the present study. The ionized calcium concentration in bovine plasma can be corrected for change in pH from 7.40 by use of the following equation:73 cCa2+ corrected = cCa2+ × 10(−0.24×[7.40−pH]). By use of that equation, the median values (and interquartile ranges) for cCa2+ in calves of group NaBic at baseline, and at 30, 60, and 120 minutes after start of treatment would be 1.06 (0.99–1.15), 1.02 (0.94–1.09), 0.99 (0.89–1.01), and 1.03 (0.89–1.09), respectively.
Findings of the present study also indicate that acidemic diarrheic calves can release considerable amounts of insulin in response to a hyperglycemic glucose challenge. The presence of an acidemia‐induced insulin resistance was recently suggested as one potential mechanism that might impact the insulin‐mediated potassium‐lowering effect of glucose‐containing infusion solutions in neonatal diarrheic calves.20 Unfortunately, the design of the present study does not allow any conclusion on the significance of this potential mechanism, but we observed a potassium‐lowering response in glucose‐treated calves despite ongoing acidemia with similar decrements of cK at 120 minutes and values for AUCDiff K than in calves of group NaBic. However, in contrast to calves of groups NaBic and NaCl, cK values remained unchanged until 30 min in glucose‐treated calves, which requires explanation as a marked increase in plasma volume was observed during the same period of time, which should have had a dilutional effect on cK. As an explanation, increased cK have also been described in response to hyperglycemia in the absence of sufficient insulin or insulin responsiveness in diabetic human patients.74, 75 In the absence of sufficient insulin, glucose acts as an effective osmole as it is unable to rapidly pass cell membranes, leading to extracellular hypertonicity, cellular shrinkage, and a subsequent increase in intracellular potassium concentration, which favors an efflux of potassium ions.76 Loss of intracellular potassium therefore likely explains why no net change in cK was observed in spite of a marked increase in plasma volume during the first 20 minutes after hypertonic glucose infusion. Sodium chloride as well as sodium bicarbonate likely acted to a lesser extent as effective osmoles than glucose, as sodium and chloride ions can pass the cellular membrane and bicarbonate ions dissipate in water and carbon dioxide, which can be subsequently eliminated through the lungs. Therefore, a higher extent of cellular shrinkage might have occurred in glucose‐treated calves until sufficient insulin was released, which would also explain the significantly higher increase in plasma volume during the first 20 minutes after treatment when compared to calves of groups NaCl and NaBic.
Another observed effect of hypertonic glucose infusions was a significant decrease in plasma sodium concentration, which was evident until 90 minutes and which resulted (together with the observed changes in cK) in an unchanged sodium‐to‐potassium ratio during the same period of time. This effect together with a delayed potassium‐lowering response represents a potential disadvantage of that solution over the use of hypertonic sodium bicarbonate or sodium chloride in calves with acute hyperkalemia and associated cardiac conduction abnormalities as an immediate increase in the sodium‐to‐potassium ratio is required to reverse the cardiotoxic effects of hyperkalemia.20, 21, 77 Also, administration of a hypertonic glucose solution (which has an effective SID of 0 mEq/L) caused a significant decrease in measured plasma SID resulting in a slight acidifying effect, which was also observed in NaCl‐treated calves. Although no negative clinical side reactions were observed in glucose‐treated calves and all of those calves survived the study, the acidifying and hyponatremic effect of an isolated IV administration of glucose might be even detrimental in calves with a more extreme hyperkalemia than in the 7 calves of the present study (cK 6.8 ± 0.9 mmol/L) because the cardiotoxic effects of hyperkalemia can be exacerbated by the presence of acidemia and hyponatremia.11, 21 However, a significantly more pronounced decrease in cK after a combined treatment of sodium bicarbonate, glucose, and insulin when compared to administration of sodium bicarbonate alone has been reported in hyperkalemic humans78 and in a recent study on hyperkalemic diarrheic calves.16
Although our study provided valuable information in respect to the plasma potassium‐lowering efficacy of different hypertonic infusions in the initial treatment of hyperkalemic diarrheic calves, our analyses have also some limitations. One limitation is the small number of calves, which was related to definition of strict criteria for inclusion into the study. Another limitation is the observed variation of basal clinical and laboratory conditions between calves and resulting differences between treatment groups that could not be prevented in spite of randomization and that might have had an effect on the results of our analyses.
Conclusions
Hypertonic (8.4%) sodium bicarbonate solution has a sound physiologic basis in the initial treatment of neonatal hyperkalemic diarrheic calves, as those solutions induce rapid plasma volume expansion, correct concomitant acidemia and have a marked and sustained potassium‐lowering effect. Results of the present study suggest a treatment advantage of sodium bicarbonate over the use of a hypertonic sodium chloride infusion with an identical sodium load, indicating that alkalinization is an effective potassium‐lowering mechanism. Acidemic neonatal diarrheic calves can release considerable amounts of insulin in response to a hyperglycemic glucose challenge, which resulted in a similar decline in cK than in calves after administration of sodium bicarbonate. However, a delayed potassium‐lowering effect and a resultant decrease in plasma sodium concentration negatively influencing the plasma sodium‐to‐potassium ratio, as well as nonalkalinizing capacity, are potential disadvantages over the use of sodium bicarbonate solutions. However, the resultant endogenous insulin release of glucose solutions makes them potentially useful in the treatment of hyperkalemic calves as an additive to sodium bicarbonate‐containing infusion solutions.
Acknowledgments
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This work was financed by a research grant of the German Research Foundation (Deutsche Forschungsgemeinschaft; TR 1321/1‐1).
Footnotes
Splittocan Infusionskatheter, 16‐gauge, 150 mm, Walter Veterinär Instrumente, Baruth, Germany
Natriumhydrogencarbonat, 8.4% B. Braun Infusionslösung, B. Braun Melsungen AG, Melsungen, Germany
Hypertone Natriumchlorid‐Lösung, 7.5 g/100 ml, B. Braun Melsungen AG, Melsungen, Germany
Glucose 500 mg/mL B. Braun Infusionslösung, B. Braun Melsungen AG, Melsungen, Germany
Ampuwa®, Fresenius Kabi Deutschland GmbH, Bad Homburg, Germany
Effydral®, Zoetis Deutschland GmbH, Berlin, Germany
Rapidpoint 405, Siemens Healthcare Diagnostics Inc., Tarrytown
Cobas c 311, Roche Diagnostics, Mannheim, Germany
Insulin Bovine ELISA, kit EIA‐4748, DRG Instruments GmbH, Germany; provided by Mercodia, Uppsala, Sweden
Johansson A, Olander S, Ludvigsen E. A novel sandwich ELISA for the measurement of insulin in bovine serum and plasma. Available at: https://www.mercodia.com/mercodia-bovine-insulin-elisa. Accessed February 28, 2017
poCH‐100iV Diff, Sysmex Corporation, Kobe, Japan
SPSS, version 18.0, IBM, New York
GraphPad Prism, version 7.01, GraphPad Software Inc., La Jolla
R, version 3.3.1, R Foundation for statistical computing, Vienna, Austria
References
- 1. Lorenz I. Influence of D‐lactate on metabolic acidosis and on prognosis in neonatal calves with diarrhoea. J Vet Med A Physiol Pathol Clin Med 2004;51:425–428. [DOI] [PubMed] [Google Scholar]
- 2. Constable PD, Stämpfli HR, Navetat H, et al. Use of a quantitative strong ion approach to determine the mechanism for acid‐base abnormalities in sick calves with or without diarrhea. J Vet Intern Med 2005;19:581–589. [DOI] [PubMed] [Google Scholar]
- 3. Trefz FM, Lorch A, Feist M, et al. Metabolic acidosis in neonatal calf diarrhea‐clinical findings and theoretical assessment of a simple treatment protocol. J Vet Intern Med 2012;26:162–170. [DOI] [PubMed] [Google Scholar]
- 4. Trefz FM, Constable PD, Sauter‐Louis C, et al. Hyperkalemia in neonatal diarrheic calves depends on the degree of dehydration and the cause of the metabolic acidosis but does not require the presence of acidemia. J Dairy Sci 2013;96:7234–7244. [DOI] [PubMed] [Google Scholar]
- 5. Trefz FM, Constable PD, Lorenz I. Quantitative physicochemical analysis of acid‐base balance and clinical utility of anion gap and strong ion gap in 806 neonatal calves with diarrhea. J Vet Intern Med 2015;29:678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trefz FM, Lorch A, Zitzl J, et al. Risk factors for the development of hypokalemia in neonatal diarrheic calves. J Vet Intern Med 2015;29:688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewis LD, Phillips RW. Water and electrolyte losses in neonatal calves with acute diarrhea. A complete balance study. Cornell Vet 1972;62:596–607. [PubMed] [Google Scholar]
- 8. Trefz FM, Lorch A, Feist M, et al. The prevalence and clinical relevance of hyperkalaemia in calves with neonatal diarrhoea. Vet J 2013;195:350–356. [DOI] [PubMed] [Google Scholar]
- 9. Lewis L, Phillips R. Diarrheic induced changes in intracellular and extracellular ion concentrations in neonatal calves. Ann Rech Vétér 1973;4:99–111. [Google Scholar]
- 10. Sweeney RW. Treatment of potassium balance disorders. Vet Clin North Am Food Anim Pract 1999;15:609–617. [DOI] [PubMed] [Google Scholar]
- 11. Evans KJ, Greenberg A. Hyperkalemia: A review. J Intensive Care Med 2005;20:272–290. [DOI] [PubMed] [Google Scholar]
- 12. Fisher EW, McEwan AD. Death in neonatal calf diarrhoea. Pt. II: The role of oxygen and potassium. Br Vet J 1967;123:4–7. [Google Scholar]
- 13. Weldon AD, Moise NS, Rebhun WC. Hyperkalemic atrial standstill in neonatal calf diarrhea. J Vet Intern Med 1992;6:294–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Constable PD. Hypertonic saline. Vet Clin North Am Food Anim Pract 1999;15:559–585. [DOI] [PubMed] [Google Scholar]
- 15. Özkan C, Altuğ N, Yüksek N, et al. Assessment of electrocardiographic findings, serum nitric oxide, cardiac troponins and some enzymes in calves with hyperkaliemia related to neonatal diarrhoea. Revue Méd Vét 2011;162:171–176. [Google Scholar]
- 16. Altuğ N, Yüksek N, Özkan C, et al. Serum potassium‐lowering effects of insulin plus dextrose and adrenalin treatment that enhance intracellular potassium transitions in hyperkalemic diarrheic calves. Pak Vet J 2016;36:140–144. [Google Scholar]
- 17. Trefz FM, Lorch A, Zitzl J, et al. Effects of alkalinization and rehydration on plasma potassium concentrations in neonatal calves with diarrhea. J Vet Intern Med 2015;29:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koch A, Kaske M. Clinical efficacy of intravenous hypertonic saline solution or hypertonic bicarbonate solution in the treatment of inappetent calves with neonatal diarrhea. J Vet Intern Med 2008;22:202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Trefz FM, Lorch A, Feist M, et al. Construction and validation of a decision tree for treating metabolic acidosis in calves with neonatal diarrhea. BMC Vet Res 2012;8:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Constable PD, Grünberg W. Hyperkalemia in diarrheic calves: Implications for diagnosis and treatment. Vet J 2013;195:271–272. [DOI] [PubMed] [Google Scholar]
- 21. Garcia‐Palmieri MR. Reversal of hyperkalemic cardiotoxicity with hypertonic saline. Am Heart J 1962;64:483–488. [DOI] [PubMed] [Google Scholar]
- 22. Kaplan JL, Eynon CA, Dalsey WC, et al. Hypertonic saline treatment of severe hyperkalemia in nonnephrectomized dogs. Acad Emerg Med 2000;7:965–973. [DOI] [PubMed] [Google Scholar]
- 23. Weisberg LS. Management of severe hyperkalemia. Crit Care Med 2008;36:3246–3251. [DOI] [PubMed] [Google Scholar]
- 24. Rosic NK, Standaert ML, Pollet RJ. The mechanism of insulin stimulation of (Na+, K+)‐ATPase transport activity in muscle. J Biol Chem 1985;260:6206–6212. [PubMed] [Google Scholar]
- 25. Grünberg W, Morin DE, Drackley JK, et al. Effect of continuous intravenous administration of a 50% dextrose solution on phosphorus homeostasis in dairy cows. J Am Vet Med Assoc 2006;229:413–420. [DOI] [PubMed] [Google Scholar]
- 26. DeFronzo RA, Beckles AD. Glucose intolerance following chronic metabolic acidosis in man. Am J Physiol 1979;236:E328–E334. [DOI] [PubMed] [Google Scholar]
- 27. Mak RH. Effect of metabolic acidosis on insulin action and secretion in uremia. Kidney Int 1998;54:603–607. [DOI] [PubMed] [Google Scholar]
- 28. Niethammer FM. Untersuchungen zur Dehydratation bei Kälbern mit akuter Diarrhoe unter Berücksichtigung ausgewählter klinischer und labordiagnostischer Parameter. Doctoral thesis. Munich: Ludwig‐Maximilians‐University; 2007. [Google Scholar]
- 29. Constable PD, Walker PG, Morin DE, Foreman JH. Clinical and laboratory assessment of hydration status of neonatal calves with diarrhea. J Am Vet Med Assoc 1998;212:991–996. [PubMed] [Google Scholar]
- 30. Thomas LJ. Algorithms for selected blood acid‐base and blood gas calculations. J Appl Physiol 1972;33:154–158. [DOI] [PubMed] [Google Scholar]
- 31. Abuelo A, De Koster J, Hernandez J, et al. Quantifying bovine insulin: Conversion of units. Vet Clin Pathol 2012;41:308–310. [DOI] [PubMed] [Google Scholar]
- 32. Siggaard‐Andersen O. The van Slyke equation. Scand J Clin Lab Invest Suppl 1977;37(Suppl 146):15–20. [DOI] [PubMed] [Google Scholar]
- 33. Constable PD. A simplified strong ion model for acid‐base equilibria: Application to horse plasma. J Appl Physiol 1997;83:297–311. [DOI] [PubMed] [Google Scholar]
- 34. Müller KR, Gentile A, Klee W, Constable PD. Importance of the effective strong ion difference of an intravenous solution in the treatment of diarrheic calves with naturally acquired acidemia and strong ion (metabolic) acidosis. J Vet Intern Med 2012;26:674–683. [DOI] [PubMed] [Google Scholar]
- 35. Riond JL, Kocabagli N, Spichiger UE, Wanner M. The concentration of ionized magnesium in serum during the periparturient period of non‐paretic dairy cows. Vet Res Commun 1995;19:195–203. [DOI] [PubMed] [Google Scholar]
- 36. Van Beaumont W, Greenleaf JE, Juhos L. Disproportional changes in hematocrit, plasma volume, and proteins during exercise and bed rest. J Appl Physiol 1972;33:55–61. [DOI] [PubMed] [Google Scholar]
- 37. Grünberg W, Hartmann H, Burfeind O, et al. Plasma potassium‐lowering effect of oral glucose, sodium bicarbonate, and the combination thereof in healthy neonatal dairy calves. J Dairy Sci 2011;94:5646–5655. [DOI] [PubMed] [Google Scholar]
- 38. Coskun A, Sen I, Guzelbektes H, et al. Comparison of the effects of intravenous administration of isotonic and hypertonic sodium bicarbonate solutions on venous acid‐base status in dehydrated calves with strong ion acidosis. J Am Vet Med Assoc 2010;236:1098–1103. [DOI] [PubMed] [Google Scholar]
- 39. Aronson PS, Giebisch G. Effects of pH on potassium: New explanations for old observations. J Am Soc Nephrol 2011;22:1981–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blumberg A, Weidmann P, Shaw S, Gnädinger M. Effect of various therapeutic approaches on plasma potassium and major regulating factors in terminal renal failure. Am J Med 1988;85:507–512. [DOI] [PubMed] [Google Scholar]
- 41. Gutierrez R, Schlessinger F, Oster JR, et al. Effect of hypertonic versus isotonic sodium bicarbonate on plasma potassium concentration in patients with end‐stage renal disease. Miner Electrolyte Metab 1991;17:297–302. [PubMed] [Google Scholar]
- 42. Blumberg A, Weidmann P, Ferrari P. Effect of prolonged bicarbonate administration on plasma potassium in terminal renal failure. Kidney Int 1992;41:369–374. [DOI] [PubMed] [Google Scholar]
- 43. Kaplan JL, Braitman LE, Dalsey WC, et al. Alkalinization is ineffective for severe hyperkalemia in nonnephrectomized dogs. Acad Emerg Med 1997;4:93–99. [DOI] [PubMed] [Google Scholar]
- 44. Fraley DS, Adler S. Correction of hyperkalemia by bicarbonate despite constant blood pH. Kidney Int 1977;12:354–360. [DOI] [PubMed] [Google Scholar]
- 45. Schwarz KC, Cohen BD, Lubash GD, Rubin AL. Severe acidosis and hyperpotassemia treated with sodium bicarbonate infusion. Circulation 1959;19:215–220. [DOI] [PubMed] [Google Scholar]
- 46. Berchtold J. Treatment of calf diarrhea: Intravenous fluid therapy. Vet Clin North Am Food Anim Pract 2009;25:73–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kasari TR, Naylor JM. Clinical evaluation of sodium bicarbonate, sodium L‐lactate, and sodium acetate for the treatment of acidosis in diarrheic calves. J Am Vet Med Assoc 1985;187:392–397. [PubMed] [Google Scholar]
- 48. Constable PD, Gohar HM, Morin DE, Thurmon JC. Use of hypertonic saline‐dextran solution to resuscitate hypovolemic calves with diarrhea. Am J Vet Res 1996;57:97–104. [PubMed] [Google Scholar]
- 49. Walker PG, Constable PD, Morin DE, et al. Comparison of hypertonic saline‐dextran solution and lactated Ringer's solution for resuscitating severely dehydrated calves with diarrhea. J Am Vet Med Assoc 1998;213:113–121. [PubMed] [Google Scholar]
- 50. Sentürk S. Effects of a hypertonic saline solution and dextran 70 combination in the treatment of diarrhoeic dehydrated calves. J Vet Med A Physiol Pathol Clin Med 2003;50:57–61. [DOI] [PubMed] [Google Scholar]
- 51. Leal ML, Fialho SS, Cyrillo FC, et al. Intravenous hypertonic saline solution (7.5%) and oral electrolytes to treat of calves with noninfectious diarrhea and metabolic acidosis. J Vet Intern Med 2012;26:1042–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zafar MA, Muhammad G, Iqbal Z, et al. Evaluation of relative resuscitative effects of hypertonic and isotonic saline solutions as an adjunct to ceftiofur HCl in bovine neonatal diarrhea associated with Escherichia coli . Int J Agric and Biol 2015;17:953–960. [Google Scholar]
- 53. Eigenmann U, Rüdiger B, Schoon H, Grunert E. Natriumbikarbonat‐ und Glukosebehandlung bei der Asphyxie des Kalbes. Dtsch tierärztl Wschr 1982;89:228–234. [PubMed] [Google Scholar]
- 54. Iwabuchi S, Suzuki K, Abe I, Asano R. Comparison of the effects of isotonic and hypertonic sodium bicarbonate solutions on acidemic calves experimentally induced by ammonium chloride administration. J Vet Med Sci 2003;65:1369–1371. [DOI] [PubMed] [Google Scholar]
- 55. Berchtold JF, Constable PD, Smith GW, et al. Effects of intravenous hyperosmotic sodium bicarbonate on arterial and cerebrospinal fluid acid‐base status and cardiovascular function in calves with experimentally induced respiratory and strong ion acidosis. J Vet Intern Med 2005;19:240–251. [DOI] [PubMed] [Google Scholar]
- 56. Abeysekara S, Zello GA, Lohmann KL, et al. Infusion of sodium bicarbonate in experimentally induced metabolic acidosis does not provoke cerebrospinal fluid (CSF) acidosis in calves. Can J Vet Res 2012;76:16–22. [PMC free article] [PubMed] [Google Scholar]
- 57. Sterns RH, Thomas DJ, Herndon RM. Brain dehydration and neurologic deterioration after rapid correction of hyponatremia. Kidney Int 1989;35:69–75. [DOI] [PubMed] [Google Scholar]
- 58. Brunner JE, Redmond JM, Haggar AM, et al. Central pontine myelinolysis and pontine lesions after rapid correction of hyponatremia: A prospective magnetic resonance imaging study. Ann Neurol 1990;27:61–66. [DOI] [PubMed] [Google Scholar]
- 59. Iberti TJ, Kelly KM, Gentili DR, et al. Effects of sodium bicarbonate in canine hemorrhagic shock. Crit Care Med 1988;16:779–782. [DOI] [PubMed] [Google Scholar]
- 60. Benjamin E, Oropello JM, Abalos AM, et al. Effects of acid‐base correction on hemodynamics, oxygen dynamics, and resuscitability in severe canine hemorrhagic shock. Crit Care Med 1994;22:1616–1623. [PubMed] [Google Scholar]
- 61. Gossett KA, French DD, Cleghorn B, Church GE. Blood biochemical response to sodium bicarbonate infusion during sublethal endotoxemia in ponies. Am J Vet Res 1990;51:1370–1374. [PubMed] [Google Scholar]
- 62. Ferrante PL, Taylor LE, Kronfeld DS, Meacham TN. Blood lactate concentration during exercise in horses fed a high‐fat diet and administered sodium bicarbonate. J Nutr 1994;124:2738S–2739S. [DOI] [PubMed] [Google Scholar]
- 63. Egger F, Meyer T, Such U, Hecksteden A. Effects of sodium bicarbonate on high‐intensity endurance performance in cyclists: A double‐blind, randomized cross‐over trial. PLoS ONE 2014;9:e114729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Seo Y. Effects of extracellular pH on lactate efflux from frog sartorius muscle. Am J Physiol 1984;247:C175–C181. [DOI] [PubMed] [Google Scholar]
- 65. Spriet LL, Lindinger MI, Heigenhauser GJ, Jones NL. Effects of alkalosis on skeletal muscle metabolism and performance during exercise. Am J Physiol 1986;251:R833–R839. [DOI] [PubMed] [Google Scholar]
- 66. Lorenz I, Hartmann I, Gentile A. Determination of D‐lactate in calf serum samples – an automated enzymatic assay. Comp Clin Path 2003;12:169–171. [Google Scholar]
- 67. Ewaschuk JB, Naylor JM, Zello GA. D‐lactate in human and ruminant metabolism. J Nutr 2005;135:1619–1625. [DOI] [PubMed] [Google Scholar]
- 68. Reischer N. Dynamik der Serumkonzentration und Ausscheidung von D‐Laktat bei jungen Kälbern mit Durchfall. Doctoral thesis. Munich: Ludwig‐Maximilians‐University; 2012. [Google Scholar]
- 69. Lorenz I, Vogt S. Investigations on the association of D‐lactate blood concentrations with the outcome of therapy of acidosis, and with posture and demeanour in young calves with diarrhoea. J Vet Med A Physiol Pathol Clin Med 2006;53:490–494. [DOI] [PubMed] [Google Scholar]
- 70. Lorenz I, Gentile A. D‐lactic acidosis in neonatal ruminants. Vet Clin North Am Food Anim Pract 2014;30:317–331. [DOI] [PubMed] [Google Scholar]
- 71. Pedersen KO. Binding of calcium to serum albumin. II. Effect of pH via competitive hydrogen and calcium ion binding to the imidazole groups of albumin. Scand J Clin Lab Invest 1972;29:75–83. [DOI] [PubMed] [Google Scholar]
- 72. Grove‐White DH, Michell AR. Iatrogenic hypocalcaemia during parenteral fluid therapy of diarrhoeic calves. Vet Rec 2001;149:203–207. [DOI] [PubMed] [Google Scholar]
- 73. Constable PD, Hinchcliff KW, Done SH, Grünberg W. Disturbances of free water, electrolytes, acid‐base balance, and oncotic pressure In: Constable PD, Hinchcliff KW, Done SH, Grünberg W, eds. Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats, 11th ed St. Louis: Elsevier; 2017:113–152. [Google Scholar]
- 74. Nicolis GL, Kahn T, Sanchez A, Gabrilove JL. Glucose‐induced hyperkalemia in diabetic subjects. Arch Intern Med 1981;141:49–53. [PubMed] [Google Scholar]
- 75. Viberti GC. Glucose‐induced hyperkalaemia: A hazard for diabetics? Lancet 1978;1:690–691. [DOI] [PubMed] [Google Scholar]
- 76. Weiner ID, Wingo CS. Hyperkalemia: A potential silent killer. J Am Soc Nephrol 1998;9:1535–1543. [DOI] [PubMed] [Google Scholar]
- 77. Ballantyne F, Davis LD, Reynolds EW. Cellular basis for reversal of hyperkalemic electrocardiographic changes by sodium. Am J Physiol 1975;229:935–940. [DOI] [PubMed] [Google Scholar]
- 78. Kim HJ. Combined effect of bicarbonate and insulin with glucose in acute therapy of hyperkalemia in end‐stage renal disease patients. Nephron 1996;72:476–482. [DOI] [PubMed] [Google Scholar]


