Abstract
With the current wealth of new inhalers available and insurance policy driven inhaler switching, the need for insights in optimal education on inhaler use is more evident than ever. We aimed to systematically review educational inhalation technique interventions, to assess their overall effectiveness, and identify main drivers of success. Medline, Embase and CINAHL databases were searched for randomised controlled trials on educational inhalation technique interventions. Inclusion eligibility, quality appraisal (Cochrane’s risk of bias tool) and data extraction were performed by two independent reviewers. Regression analyses were performed to identify characteristics contributing to inhaler technique improvement. Thirty-seven of the 39 interventions included (95%) indicated statistically significant improvement of inhaler technique. However, average follow-up time was relatively short (5 months), 28% lacked clinical relevant endpoints and all lacked cost-effectiveness estimates. Poor initial technique, number of inhalation procedure steps, setting (outpatient clinics performing best), and time elapsed since intervention (all, p < 0.05), were shown to have an impact on effectiveness of the intervention, explaining up to 91% of the effectiveness variation. Other factors, such as disease (asthma vs. chronic obstructive pulmonary disease), education group size (individual vs. group training) and inhaler type (dry powder inhalers vs. pressurised metered dose inhalers) did not play a significant role. Notably, there was a trend (p = 0.06) towards interventions in adults being more effective than those in children and the intervention effect seemed to wane over time. In conclusion, educational interventions to improve inhaler technique are effective on the short-term. Periodical intervention reinforcement and longer follow-up studies, including clinical relevant endpoints and cost-effectiveness, are recommended.
Introduction
Bronchodilators and corticosteroids play a key role in maintaining disease control in asthma and chronic obstructive pulmonary disease (COPD) patients. Delivery of these drugs is mainly achieved by inhalers, which can be categorised into three types: pressurised metered dose inhalers (pMDIs), dry powder inhalers (DPIs) and nebulisers. Previous studies, performed in controlled settings, showed that all inhalers are equally capable of delivering an appropriate medication dose.1, 2 In daily use however, a large majority of patients make inhalation errors.3 Suboptimal inhaler technique is associated with worsened health outcomes, such as increased risk of hospitalisation and poor disease control.4–6 Consequences can also be found in the financial context as studies estimate that a considerable amount of resources spent on inhalers are wasted.7 Important inter-patient differences have repeatedly been shown, with as few as 25% of the patients able to demonstrate a correct technique.8–11 As such, it is of utmost importance to properly train patients on inhaler technique.4, 12, 13 Various educational interventions to do so have been reported. However, so far there has been no systematic review of these interventions, leaving the key characteristics of successful interventions to remain obscure. With little improvement shown over time,14 the current wealth of new inhalers available and frequent health insurance policy driven inhaler switches, the need for optimal education on inhaler use is more evident than ever.
This review aims to provide a systematic overview of educational interventions focusing on inhaler technique in asthma and COPD patients, assess their overall effectiveness, and identify their main drivers of success.
Results
Inclusion
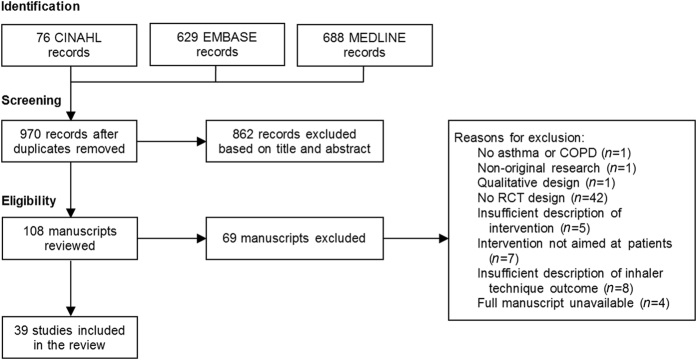
The literature search yielded a total of 1393 results. Of the 970 unique articles, 862 were excluded based on title and abstract, while a further 69 articles were excluded during full-text screening (Fig. 1). Initial agreement between reviewers on eligibility was 87% (Cohen’s κ = 0.72). After one consensus round, full agreement was reached (e-Appendix 2) and 39 articles were eventually included.15–53 Full manuscripts of four studies were unavailable, all dating from 2001 or before.54–57 Three authors were contacted, but did not reply. The remaining author could not be traced.
Fig. 1.
Flow diagram on article inclusion
Study quality
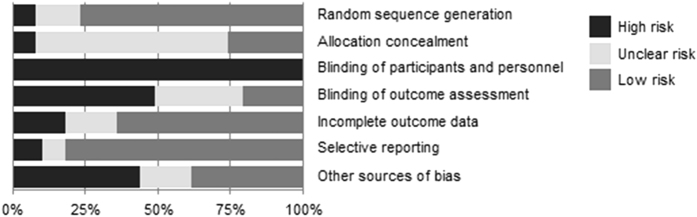
Inter-reviewer agreement on the study quality assessment was of moderate strength (Cohen’s weighted κ = 0.51), but consensus was reached after one consensus round. Twelve of the 39 studies scored a low risk of bias on four or more of the seven categories (see e-Appendix 3). Random sequence generation and selective reporting were found to be best addressed by the studies (Fig. 2). Allocation concealment was frequently not described, and it was therefore difficult to determine whether concealment was sufficient but not reported, or insufficient and a potential source of bias. As was already established beforehand, blinding of participants was not possible, which is reflected in the quality appraisal results. Blinding of outcome assessment was possible, but in almost half of the studies not implemented. Regression analysis showed that quality of the study was not associated with intervention outcome results (p > 0.05), irrespective of the type of outcome reported.
Fig. 2.
Quality assessment of included studies. Percentages represent the percentage of included articles having a high risk (black bar), unclear risk (light grey bar) or low risk (medium-grey bar) of bias for each category in the Cochrane Collaboration’s risk of bias assessment tool
Study characteristics
An overview of the 39 studies and their 56 intervention groups is provided in Table 1. Full details of the studies can be found in e-Appendix 4. The majority covered patients with asthma (n = 35), of which six also included COPD patients. Studies exclusively performed in COPD-patients were rare (n = 4). The interventions mainly took place in outpatient clinics (n = 17) or community pharmacy settings (n = 15).
Table 1.
Study and intervention characteristics
| FFirst author | Disease | Setting | Sessions | Session length (h:mm) | Delivery | Deliverer | N | Inhaler type | Maximum follow-up (months) | Outcome type | Inhaler technique Improvement | Clinical outcomes | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (p.p.) | ||||||||||||
| Al-Showair 200715 | Asthma | Outpatient clinic | 1 | – | Individual | – | 107 | MDI | 1.5 | IFR | – | Peak flow:↑, AQLQ: ↑ | |
| Armour 201316 | Asthma | Pharmacy | 4 | 00:26 | Individual | Pharmacist | 398 | MDI | 6 | Patients | 50 p.p. | ACQ:≈ | |
| Basheti 200817 | Asthma | Pharmacy | 4 | 00:03 | Individual | Pharmacist | 97 | DPI | 6 | Steps | 49% | Asthma severity: ↓ | |
| Basheti 200518 | Asthma | Pharmacy | 1 | 00:08 | Individual | Researcher | 17 | DPI | 0.5 | Steps | 75% | None | |
| Asthma | Pharmacy | 1 | 00:08 | Individual | Researcher | 17 | DPI | 0.5 | Steps | 80% | |||
| Bosnic-Anticevich 201019 | Both | Pharmacy | 3 | – | Individual | Researcher | 52 | MDI | 4 | Steps | 38% | None | |
| Bynum 200120 | Asthma* | Outpatient clinic | 1 | 00:15 | Individual | Pharmacist | 49 | MDI | 1 | Steps | 77% | None | |
| Carpenter 201521 | Asthma* | Outpatient clinic | 1 | 00:03 | Individual | Researcher | 91 | MDI | 1 | Steps | 16% | ACT: ≈ | |
| Chan 200722 | Asthma* | Outpatient clinic | 5 | – | Individual | Other | 60 | MDI | 12 | Steps | 8% | QOL: ≈, hospitalisations: ≈ | |
| Asthma* | Outpatient clinic | 5 | – | Individual | Other | 60 | DPI | 12 | Steps | 12% | |||
| Chan 200323 | Asthma* | Outpatient clinic | 4 | – | Individual | Other | 10 | Both | 6 | Steps | – | Peak flow: ↑, QOL: ≈ | |
| Cicutto 201324 | Asthma* | School | 6 | 00:53 | Group | – | 1316 | – | 12 | Steps | 48% | QOL:↑, Urgent care:↓ | |
| Cordina 200125 | Asthma | Pharmacy | 1 | – | Individual | – | 152 | – | 12 | Patients | 36 p.p. | QOL: ↑, peak flow: ↑, hospitalisations: ↓ | |
| Crane 201426 | Asthma | – | 1 | – | Individual | – | 123 | Both | 12 | Patients | 16 p.p. | None | |
| De Blaquiere 198927 | Both | Outpatient clinic | 1 | 00:17 | Individual | Researcher | 100 | MDI | 2 | Patients | 81 p.p. | Hospitalisations: ≈ | |
| De Oliveira 199928 | Asthma | Outpatient clinic | 8 | – | – | Researcher | 42 | MDI | 6 | Patients | 73 p.p. | ER visits:↓, symptoms:↓, QOL:↑ | |
| Garcia-Cardenas 201329 | Asthma | Pharmacy | 3 | – | Individual | Pharmacist | 336 | DPI | 6 | Patients | 56 p.p. | ACQ:↑ | |
| Goodyer 200630 | Asthma | – | 1 | – | Individual | Pharmacist | 35 | MDI | 0 | Steps | – | None | |
| Asthma | – | 1 | – | Individual | Pharmacist | 34 | MDI | 0 | Steps | – | |||
| Goris 201331 | COPD | Outpatient clinic | 1 | – | Individual | – | 24 | MDI | 3 | Steps | 100% | QOL:↑, attacks:↓, hospitalisations:≈ | |
| COPD | Outpatient clinic | 1 | – | Individual | – | 110 | DPI | 3 | Steps | 43% | |||
| Hesselink 200432 | Both | Other | 2 | 00:30 | Individual | Nurse | 276 | – | 24 | Patients | – | QOL: ≈ | |
| Horner 200833 | Asthma* | School | 16 | 00:15 | Group | Other | 183 | MDI | 1.5 | Steps | 36% | None | |
| Kiser 201234 | COPD | Hospital | 1 | 00:23 | Individual | Researcher | 99 | MDI | 1.25 | Steps | 29% | None | |
| COPD | Hospital | 1 | 00:23 | Individual | Researcher | 41 | DPI | 1.25 | Steps | 22% | |||
| COPD | Hospital | 1 | 00:23 | Individual | Researcher | 27 | DPI | 1.25 | Steps | 20% | |||
| Kools 200635 | Asthma | – | 1 | 00:01 | Individual | Researcher | 50 | MDI | 0 | Steps | – | None | |
| Kritikos 200736 | Asthma | Pharmacy | 1 | 02:30 | Group | Pharmacist | 22 | MDI | 3 | Patients | 73 p.p. | Poor control: ↓, AQOL: ↑ | |
| Asthma | Pharmacy | 1 | 02:30 | Group | Pharmacist | 25 | DPI | 3 | Patients | 79 p.p. | |||
| Asthma | Pharmacy | 1 | 02:30 | Group | Researcher | 20 | MDI | 3 | Patients | 86 p.p. | |||
| Asthma | Pharmacy | 1 | 02:30 | Group | Researcher | 26 | DPI | 3 | Patients | 84 p.p. | |||
| Kumar 200937 | Both | Hospital | 4 | – | Individual | Pharmacist | 98 | MDI | 2 | Steps | 115% | FEV1:↑ | |
| Both | Hospital | 4 | – | Individual | Pharmacist | 18 | DPI | 2 | Steps | 100% | |||
| Martin 201538 | Asthma* | Other | 4 | – | Individual | Nurse | 51 | – | 12 | Steps | 50% | Control: ≈ | |
| Asthma* | Other | 4 | – | Individual | Nurse | 50 | – | 12 | Steps | 29% | |||
| Mehuys 200839 | Asthma | Pharmacy | 3 | – | Individual | Pharmacist | 201 | Both | 6 | Steps | 25% | Nighttime symptoms:↓, ACT:↑ | |
| Mulloy 199640 | Asthma | Outpatient clinic | 1 | – | Individual | Nurse | 60 | – | 12 | Steps | 20% | Symptoms: ↓, peak flow: ≈ | |
| Patterson 200541 | Asthma* | School | 8 | – | Group | Nurse | 173 | – | 4 | Patients | 38 p.p. | QOL: ≈ | |
| Perneger 200242 | Asthma | Hospital | 3 | 01:15 | Group | Other | 131 | – | 6 | Patients | 28 p.p. | QOL: ≈, healthcare utilisation: ≈ | |
| Petkova 200843 | Asthma | Pharmacy | 5 | – | – | Researcher | 50 | – | 4 | Patients | 14 p.p. | Hospitalisation: ↓, QOL:↑ | |
| Press 201244 | Both | Hospital | 1 | 00:06 | Individual | Researcher | 50 | MDI | 0 | Patients | 52 p.p. | Health-related events:↓ | |
| Both | Hospital | 1 | 00:06 | Individual | Researcher | 18 | DPI | 0 | Patients | 50 p.p. | |||
| Rahmati 201445 | Asthma | – | 3 | – | Group | – | 60 | MDI | 1 | Steps | 60% | Peak flow: ↑ | |
| Asthma | – | 3 | – | Group | – | 60 | MDI | 1 | Steps | 90% | |||
| Rootmensen 200846 | Both | Outpatient clinic | 1 | 00:45 | Individual | Nurse | 191 | – | 6 | Steps | 3% | QOL: ≈, Exacerbations: ↓ | |
| Rydman 199947 | Asthma | Outpatient clinic | 1 | – | Individual | Researcher | 68 | MDI | 3 | Patients | 36 p.p. | None | |
| Santos 201048 | Asthma | Outpatient clinic | 2 | 01:00 | Individual | Pharmacist | 28 | MDI | 2 | Steps | 167% | None | |
| Asthma | Outpatient clinic | 2 | 01:00 | Individual | Pharmacist | 28 | DPI | 2 | Steps | 33% | |||
| Tommelein 201449 | COPD | Pharmacy | 2 | 00:20 | Individual | Pharmacist | 734 | Both | 3 | Steps | 38% | Hospitalisations↓ | |
| Toumas-Shehata 201450 | Asthma | Pharmacy | 1 | – | Individual | Pharmacist | 101 | DPI | 1 | Steps | 40% | ACQ:↑ | |
| Van der Palen 199751 | COPD | Outpatient clinic | 1 | 00:45 | Group | Nurse | 70 | Both | 9 | Steps | 26% | None | |
| COPD | Outpatient clinic | 1 | 00:45 | Individual | Other | 73 | Both | 9 | Steps | 18% | |||
| COPD | Other | 1 | 00:45 | Individual | Other | 71 | Both | 9 | Steps | 26% | |||
| Verver 199652 | Asthma | Other | 1 | – | Individual | Nurse | 48 | DPI | 0.5 | Steps | 9% | Dyspnoea: ↓ | |
| Wilson 199353 | Asthma | Hospital | 4 | 00:45 | Individual | Nurse | 227 | MDI | 12 | Steps | – | Asthma status: ↑, physical activity: ↑, Acute visits:↓ | |
| Asthma | Hospital | 4 | 01:30 | Group | Nurse | 229 | MDI | 12 | Steps | – | |||
*: children, ↑: increase/improvement, ↓: decrease/worsening, ≈: no difference, Outcome type: mean number or percentage of correct steps (in the table: “Steps”), the percentage of patients who showed a correct technique (in the table: “Patients”), or inhalation flow rate (IFR). Improvement over baseline is either reported as percentage (%) or as percentage points (p.p.)
Sample sizes ranged from 10 to 1316 participants with a median of 60 participants. One fifth of the studies targeted children. Of the studies that reported the included inhaler types, 82.8% included pMDIs, whereas DPIs were included in 58.6% of the studies. Ten studies did not specify which inhaler types were included. The average follow-up time was five months; six studies had ≥1 year follow-up.
Outcomes were most frequently recorded as correct-steps (64.1%), whereas correct-patients outcome reporting was less common (33.3%). One study reported outcomes as improvements in inhalation flow rate.15 Improvements over baseline displayed a large difference between studies, with correct-patients studies reporting improvements of 3% to 167% and correct-steps studies 14 to 86 percentage points. Eleven studies (28%) did not report any relevant clinical outcomes besides inhalation scores.
Educational interventions
Almost all interventions (89%) included a physical or video demonstration of inhaler use. Physical demonstrations were most common, whereas video demonstrations were used in six studies.21, 25, 30, 31, 40, 51 The form of the demonstration did not have a significant effect on improvement of inhaler technique over baseline (p > 0.05). Whether or not patients were requested to demonstrate own inhaler use after demonstration was frequently not reported.
Approximately half of the studies (n = 22) provided additional disease education or embedded the inhaler education in a more complex intervention. Disease education usually addressed topics such as disease pathophysiology49 and disease triggers.16 Complex interventions also included counselling on self-management skills38 and health beliefs.29
The mean number of sessions was 2.6. The mean duration of a session was 30 min, excluding an outlier.36 The interventions in outpatient clinics and pharmacy settings were similar in the sense that they were mostly individual educational interventions. Furthermore, the mean number of sessions (outpatient clinics:2.6; pharmacies:2.7) and total intervention time (both 1.5 h) did not statistically differ (p > 0.05). However, videos and internet-based education were more common in outpatient settings.20–23, 31, 40
Inhaler technique improvement
Over 90% of studies reported a significant improvement in inhaler technique after intervention. Two studies reported no effect over usual care.27, 46 These studies were both single-session interventions in outpatient clinics. Martin et al.38 reported a significant improvement only in a subgroup. Younger children did not have significant changes, whereas inhaler technique in older children significantly improved. Several studies reported a (partial) loss of effect of the intervention over time.17, 19, 24, 47 This waning effect did not seem to be related to the intervention’s characteristics, the setting in which it was performed, or any patient characteristics. The study with the longest follow-up time showed in a subgroup analysis that patients who attended multiple sessions had an increased inhaler technique over patients who only attended one session.32
Regression models (Table 2) showed that several intervention characteristics influenced the intervention’s effectiveness. For correct-step studies (n = 21), using a forward selection procedure, these were the total number of steps evaluated, setting (outpatient clinics performing best, community pharmacies and non-categorised settings performing worst), adults improved more than children, and baseline performance. The model had an excellent fit (adjusted R 2: 0.906). Using a backward selection procedure, the total number of steps evaluated and the baseline performance were the only study characteristics that showed a significant influence on the intervention’s effectiveness. For correct-patients studies (n = 12, with 16 intervention groups), the percentage of patients with baseline correct technique, and follow-up time were significant. Both selection procedures, forward selection and backward elimination, led to the same result and the model had a good fit (adjusted R 2: 0.862). In both models, publication year, general disease education, number of sessions, session length, total length of intervention, delivery form, sample size, disease, inhaler and gender did not significantly influence improvement inhaler technique improvement.
Table 2.
Linear regression models with improvement over baseline as dependent variable
| Correct-steps interventions (n = 32) | Correct-patients interventions (n = 16) | |||||||
|---|---|---|---|---|---|---|---|---|
| β | 95% CI | p-value | β | 95% CI | p-value | |||
| min | max | min | max | |||||
| Total number of steps evaluated | 0.065 | 0.027 | 0.104 | 0.002 | ||||
| Intervention setting | ||||||||
| Community pharmacy | [ref] | |||||||
| Hospital | 0.089 | −0.051 | 0.228 | 0.200 | ||||
| Outpatient clinic | 0.149 | 0.024 | 0.274 | 0.022 | ||||
| School | 0.025 | −0.224 | 0.275 | 0.835 | ||||
| Other | −0.004 | −0.172 | 0.164 | 0.958 | ||||
| Age group (adults vs. children) | 0.153 | −0.003 | 0.310 | 0.055 | ||||
| Baseline performance | −2.720 | −3.101 | −2.338 | <0.001 | −1.498 | −1.921 | −1.075 | <0.001 |
| Intervention provider | ||||||||
| Pharmacist | [ref] | |||||||
| Researcher | −0.052 | −0.190 | 0.087 | 0.414 | ||||
| Nurse | −0.224 | −0.450 | 0.001 | 0.051 | ||||
| Other | −0.226 | −0.458 | 0.007 | 0.056 | ||||
| Follow-up time | −0.034 | −0.068 | −0.001 | 0.046 | ||||
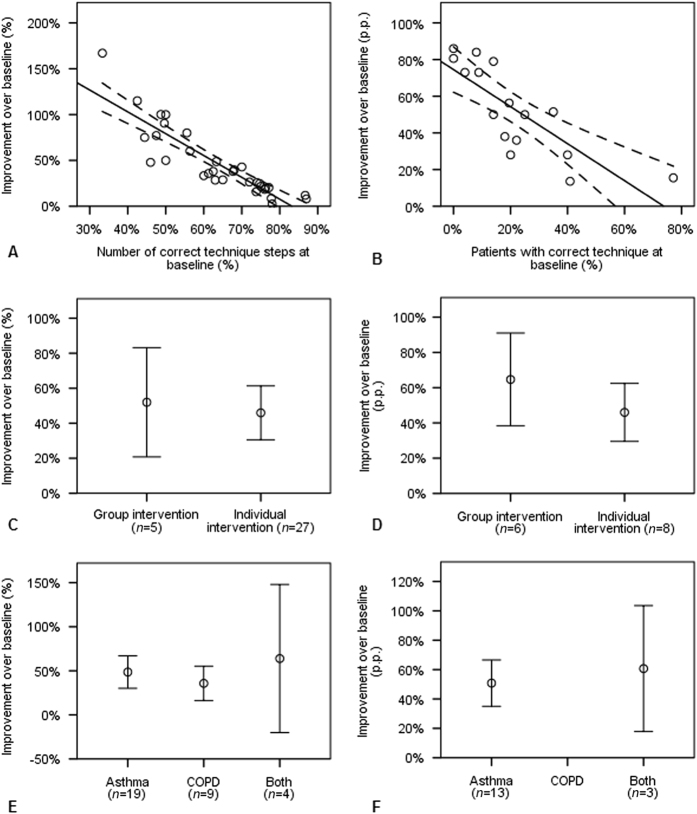
Baseline performance explained a large percentage of intervention’s effectiveness, independent of outcome measurement used (Fig. 3a, b). Patients with good baseline technique showed little improvement after intervention. Delivery form (group or individual) was not significantly correlated to inhaler technique improvement (Fig. 3c, d). However, only few interventions were delivered to a group (n = 11), whereas the majority was delivered to individuals (n = 35). Differences between diseases were difficult to determine, as there were no correct-patients studies in COPD-patients and confidence intervals were large (Fig. 3e, f).
Fig. 3.
Improvement in inhaler technique plotted against baseline performance (a, b), type of intervention (c, d), and patients’ disease background (e, f) with 95% confidence intervals. The left column (a, c, and e) displays results for correct-steps studies, the right column shows results for correct-patients studies
Clinical outcomes
Twenty-eight studies (72%) reported additional clinical outcomes (Table 1). Those outcomes included a measure of control or quality of life (44%), lung function (FEV1, peak flow) (15%), symptoms (e.g. night-time symptoms/dyspnoea) (10%), healthcare utilisation (e.g. ER visits, hospitalisations) (28%). Cost-effectiveness was never reported. The majority indicated favourable results for the intervention group, with highest discrepancy regarding effects on quality of life.
Discussion
Main findings
This review showed that educational interventions on inhaler technique are effective, at least on the short term. All studies showed improvements and 95% indicated statistical significance with a mean intervention time of 30 min and an average follow-up of five months. Regression analysis revealed several key characteristics that influenced intervention’s effectiveness. Major predictors for success were low baseline performance, outpatient setting and short follow-up time, with setting only being significant when outcomes were assessed in terms of correct number of inhalation steps. Other factors that predicted effectiveness were higher number of steps evaluated, and higher age group. Duration of the intervention, scale (group or individual), executor (pharmacist, nurse or other), inhaler (pMDI or DPI) and disease (asthma or COPD) were not associated with intervention effectiveness. Of note, a trend (p = 0.06) was observed in interventions being more effective in adults than in children, however relatively few studies targeted children specifically. The studies that included clinical relevant endpoints mostly indicated favourable clinical effects with highest discrepancy regarding effects on quality of life. Cost-effectiveness was never reported.
Interpretation of findings in relation to previously published work
There are several factors which were found to explain the variation in improvement of inhaler technique. Of these factors, baseline performance was found to be associated with positive outcomes in both correct-steps and correct-patients studies. Intervention setting and intervention provider showed a relative strong dependency (Fisher exact test: 0.00296). This might explain why inclusion of one of the two factors may have led to exclusion of the other. The higher number of technique steps evaluated being positively correlated to the effectiveness of the intervention might point to a methodological issue. A potential explanation for this correlation could be that interventions are improving parts of the patient’s technique which are not measured by all studies. Considering the large variability of outcome measures in use, ranging from five to eleven different steps in inhaler technique,24, 45, 48 this seems plausible. Equally important are the factors which were found to not be associated with improvements in inhaler technique. Duration and type of intervention, individual or group based, did not have a significant impact on outcomes. This bears important consequences for health-economic decisions in clinical practice, as less time-consuming and group interventions can be selected without sacrificing effectiveness. The addition of general disease education, or in more general terms, embedding the intervention in a multi-component intervention did not provide a benefit for improving inhaler technique either. Contrary to reports of multi-component interventions on adherence which showed mixed results,58 inhaler technique did not suffer negative effects. Intervention effectiveness was shown to be independent of disease, but note that COPD-specific studies were scarce and patient populations were heterogeneous, warranting further investigation of specific subgroups (such as children and patients with low literacy). Patients benefited from interventions, irrespective of the type of inhaler they used. This is in line with previous studies, which report improvements made with multiple types of inhalers.59 It also confirms a previous recommendation to educate patients on their inhaler instead of switching inhalers.60
Strength and limitations
To our knowledge, this is the first systematic review on educational inhaler technique interventions in asthma and COPD patients and it provides definitive evidence on their effectiveness and success factors. Especially given the current wealth of new inhalers available and insurer-driven inhaler switches, we feel this study is very relevant and timely. This systematic review was however limited to RCTs and did therefore exclude useful observational studies. A limiting factor was the wide variety of interventions and outcome measures, hampering the performance of a meta-analysis. From a clinical perspective it is unrealistic to combine the outcome effects of studies that focus on different patient populations (e.g. school children vs. adults) and implement interventions with vastly different characteristics (e.g. short video vs. interactive disease management sessions). Performing a meta-analysis would imply comparability of interventions and may lead to false interpretation of outcomes.
Assessment of relevant independent variables in the regression analysis was based on forward selection and backward elimination procedures. These procedures are sometimes referred to as data dredging methods and come with their own flaws, such as an overestimation of the variance explained by the model.61 Furthermore, the backward elimination models suffered from problems with multicollinearity resulting in the exclusion of several potential predicting variables. Nonetheless, considering the explorative nature of this study and the lack of clinical guidance on relevant independent predictors of improvement of inhaler technique over baseline, this methodology was assessed to be a relevant option to use.
The vast majority of studies showed a positive effect of their intervention on inhaler technique, a warning marker for potential publication (or reporting) bias. An alternative explanation for the positive results could be the relatively short follow-up time of most studies. Lastly, it should be noted that studies were conducted by well-trained healthcare professionals with plenty of time available. In clinical practice however, available consultation time, knowledge and skills regarding inhalers among healthcare professionals are often limited, highlighting that well-trained intervention deliverers with sufficient time available are essential.62, 63
Implications for future research, policy and practice
Focusing efforts and resources on educational interventions could result in improved inhaler technique and clinical outcomes in asthma and COPD patients.64 This is an important finding underlining the value of educational interventions. Switching of inhaler devices is associated with several disadvantages to the patient, such as an increase in the number of errors made and reduced compliance.65 In this light, clinicians may prefer to opt for an educational intervention to improve inhaler technique of the device currently in use by the patient.
The effectiveness of interventions holds true for patients with an insufficient initial technique, whereas interventions may be less valuable for patients with an already moderate to good technique. Therefore, the patient population targeted by an intervention could affect its cost-effectiveness. Unfortunately, only few cost-effectiveness studies have been conducted on improving inhaler technique in COPD 66 and asthma.67, 68 Considering constraints on budgets and time available, clinicians may wish to provide intervention on inhaler technique to patients who have been identified to suffer from a poor inhaler technique, instead of indiscriminately providing these interventions to a more general patient population. Regular reviewing of inhaler technique is a recommendation that has been voiced previously 65 and enables a more appropriate application of interventions.
Evidence on effectiveness of educational inhaler technique interventions in COPD patients is scarce and the rate of inhaler errors has not decreased over time.14 To ease comparability, we recommend that studies use a uniform method to assess inhaler technique. Unfortunately, no golden standard exists yet, but technological developments, including acoustic sound based technology and eHealth applications are promising.69, 70
Lastly, the positive effect of interventions seems to wane over time, stressing the need for continuous monitoring and periodically reinforcement of inhalation instructions. In conjunction with continuous monitoring and periodical retraining it may be important to match the inhaler device to the patient, ensuring a high baseline performance of inhaler technique.71 This could potentially reduce the need for retraining of patients.
Considering the important role of inhaler medication in asthma and COPD, future research should try to understand the type of educational interventions that could be effective in different patient groups, the optimal duration of the interventions, their maintenance and ways to improve their cost-effectiveness.
Conclusions
Educational interventions on inhaler technique in asthma and COPD patients are effective on the short-term. Key predictors for success are patient’s initial technique and time elapsed since intervention. Disease and inhaler do not play a significant role. Periodical intervention reinforcement and longer follow-up studies, including clinical relevant endpoints and cost-effectiveness, are recommended.
Methods
Study design
The study design was a systematic review, performed as per PRISMA-guideline.72
Inclusion and exclusion criteria
All articles reporting randomised controlled trials (RCTs) on interventions aimed at improving inhaler technique in asthma or COPD patients (no age restriction) vs. usual care, published before 31 March 2015, were eligible for inclusion. Exclusion criteria were non-English manuscripts, no asthma or COPD, non-original research, qualitative studies, non-RCT design and interventions not aimed at patients. In addition, articles that did not operationalise their outcome measures or interventions without individual components description were excluded.
Search strategy
Manuscripts were retrieved from the Medline, Embase and CINAHL-databases. It is advisable to use a combination of both Medline and Embase as they return only partly overlapping results.73, 74 CINAHL was added as it provides additional coverage on the nursing subfield.
Keywords in the search strategy (please refer to e-Appendix 1 for a full overview) related to both intervention and disease. Intervention keywords included a combination of variations on ‘inhaler’ and ‘technique’ or ‘instructions’, whereas disease keywords included variations on ‘asthma’ and ‘COPD’. Disease specific keywords were based on previous publications.58 A high sensitivity therapy filter based on the work of the Hedges Project was selected to limit search results to clinical trials, while reducing the probability of excluding relevant studies.75, 76 The filter was extensively validated for all three databases included within this review and was shown to have a sensitivity of 94.6% to 99.4%.77–79
Initial screening based on title and abstract was conducted by one reviewer (S.K.). Afterwards, each potentially eligible full-text manuscript was independently reviewed by at least two reviewers (S.K., J.B., and M.H.). Disagreements were resolved in consensus round(s).
Quality assessment
All included articles were independently assessed by two different reviewers (S.K., J.B., M.H., M.R.) using the Cochrane Collaboration’s tool for assessing risk of bias in randomised trials.80 Scoring was carried out as described in the tool’s guidelines,74 even though risk of performance bias was not fully applicable, due to lack of feasible blinding options of participants. This is characteristic of educational interventions. Inter-reviewer discrepancies in scoring were resolved in a consensus procedure.
Data extraction
Study characteristics, study population, and outcomes were systematically recorded for all included articles using a pre-structured spreadsheet.
Study characteristics
If multiple intervention groups were included within a single study, or outcomes were separately reported for pMDI and DPI users, each group was recorded separately. Data were extracted by a single researcher (S.K.) in order to maintain consistency throughout coding. Extracted data of 10% of the included studies was validated by a second reviewer (M.H. or J.B.), based on a random sample. Extracted study characteristics included country, intervention, comparator, setting, executor, delivery form, sample size and follow-up time. Setting was categorised as community pharmacies, hospitals, schools, outpatient clinics or other. Executors were labelled as researchers, pharmacists, nurses (including assistants and community healthcare workers) or other. Delivery form was either group education or individual education. Follow-up time was recorded as the time elapsed between baseline and last measurement.
Study population
Study population data covered disease, inhaler, mean age and sex. Disease was either asthma or COPD. In case both asthma and COPD patients were included and outcomes were not segregated by disease, disease type was recorded as asthma and COPD. Inhaler type was generalised into two main categories, i.e. pMDI and DPI. pMDI included inhalers with and without spacers. DPI included inhalers such as Turbuhaler and Diskus. Age was originally recorded as mean age and afterwards dichotomised into children (<18 years) or adults. Sex was recorded as the percentage of males.
Outcomes
Outcomes were categorised a priori into two main classes: studies reporting the number of correct technique steps, and studies reporting the percentage of patients with a correct technique. For ease of reading, the former group of studies will be referred to as ‘correct-steps’ studies, the latter as ‘correct-patients’ studies. Based on the clinical experience of two of the authors (M.R.R. and T.v.d.M.) as well as opinions from an external clinical expert showed that patient handling of inhalers was in clinical practice usually evaluated on different steps. Hence, if a study reported both outcomes, preference in the data extraction was given to the number of correct steps. Furthermore, this outcome measure provided richer data. If studies also reported relevant clinical endpoints, these data were extracted as well and assessed in a descriptive manner.
Analysis
Agreement between reviewers on inclusion eligibility was tested with Cohen’s Kappa and interpreted according to guidelines.81 Inter-reviewer agreement on the quality appraisal was tested with a weighted Cohen’s Kappa test which gives more weight to larger differences in ordinal values.82 Data were initially evaluated in a descriptive way. In a second step, linear regression models were created considering inhalation technique improvement as dependent variable. Potentially associated independent variables were tested for significant predictive value. These included year of publication, country, setting, executor, delivery form, sample size, follow-up time, disease, inhaler, age, sex and baseline performance. The number of steps evaluated, and performance required from a participant to be coded as showing correct technique were tested when applicable. Due to the difference in outcome measurement, one regression model was created for correct-steps studies, and another model was created for correct-patients studies. Both forward selection and backward elimination methods were used to assess the inclusion of independent variables, at a significance threshold of 0.05. All quantitative analyses were conducted in SPSS® Statistics version 22.
Electronic supplementary material
Author contributions
This study was designed by M.H. and J.V.B. Data extraction was performed by S.K. and J.V.B. Data review was done by all authors. First draft was written by S.K. and J.V.B. All authors commented on the first draft and agreed with the final version. J.V.B. is the guarantor of the study.
Competing interests
Mr. S.K. reports current employment (Pharmerit International), during the study he was a student at Maastricht University. Dr. M.R.R. reports personal fees from Astra Zeneca, personal fees from Boehringer Ingelheim, grants and personal fees from GSK, personal fees from Mundipharma, personal fees from Novartis, personal fees from TEVA, outside the submitted work. Dr. T.v.d.M. reports grants and personal fees from Glaxo, grants and personal fees from Astra Zeneca, personal fees and non-financial support from Teva, personal fees from Mundifarma, personal fees from Boehringer Ingelheim, outside the submitted work. Dr. J.F.M.v.B. reports grants (GSK, Boehringer Ingelheim), consultancy fees (AstraZeneca) and travel fees (European COPD Coalition, Respiratory Effectiveness Group) outside the submitted work. Other authors report no disclosures.
Footnotes
Electronic supplementary material
Supplementary Information accompanies the paper on the npj Primary Care Respiratory Medicine website (doi:10.1038/s41533-017-0022-1).
References
- 1.Dolovich MB, et al. Device selection and outcomes of aerosol therapy: evidence-based guidelines: American college of chest physicians/American college of asthma, allergy, and immunology. Chest. 2005;127:335–371. doi: 10.1378/chest.127.1.335. [DOI] [PubMed] [Google Scholar]
- 2.Brocklebank D, et al. Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature. Health Technol. Assess. 2001;5:1–149. doi: 10.3310/hta5260. [DOI] [PubMed] [Google Scholar]
- 3.Leiva-Fernandez F, et al. Efficacy of two educational interventions about inhalation techniques in patients with chronic obstructive pulmonary disease (COPD). TECEPOC: study protocol for a partially randomized controlled trial (preference trial) Trials. 2012;13:64. doi: 10.1186/1745-6215-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melani AS, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir. Med. 2011;105:930–938. doi: 10.1016/j.rmed.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Lindgren S, Bake B, Larsson S. Clinical consequences of inadequate inhalation technique in asthma therapy. Eur. J. Respir. Dis. 1987;70:93–98. [PubMed] [Google Scholar]
- 6.Virchow JC, et al. Importance of inhaler devices in the management of airway disease. Respir. Med. 2008;102:10–19. doi: 10.1016/j.rmed.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 7.Newman S. Improving inhaler technique, adherence to therapy and the precision of dosing: major challenges for pulmonary drug delivery. Expert Opin. Drug Deliv. 2014;11:365–378. doi: 10.1517/17425247.2014.873402. [DOI] [PubMed] [Google Scholar]
- 8.Hilton S. An audit of inhaler technique among asthma patients of 34 general practitioners. Br. J. Gen. Pract. 1990;40:505–506. [PMC free article] [PubMed] [Google Scholar]
- 9.Diggory P, Fernandez C, Humphrey A, Jones V, Murphy M. Comparison of elderly people’s technique in using two dry powder inhalers to deliver zanamivir: randomised controlled trial. BMJ. 2001;322:577–579. doi: 10.1136/bmj.322.7286.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molimard M, et al. Assessment of handling of inhaler devices in real life: an observational study in 3811 patients in primary care. J. Aerosol. Med. 2003;16:249–254. doi: 10.1089/089426803769017613. [DOI] [PubMed] [Google Scholar]
- 11.Khassawneh BY, et al. Handling of inhaler devices in actual pulmonary practice: metered-dose inhaler versus dry powder inhalers. Respir. Care. 2008;53:324–328. [PubMed] [Google Scholar]
- 12.Crompton GK, et al. The need to improve inhalation technique in Europe: a report from the Aerosol Drug Management Improvement Team. Respir. Med. 2006;100:1479–1494. doi: 10.1016/j.rmed.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Broeders ME, et al. The ADMIT series--issues in inhalation therapy. 2. Improving technique and clinical effectiveness. Prim. Care. Respir. J. 2009;18:76–82. doi: 10.4104/pcrj.2009.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchis, J., Gich, I. & Pedersen, S. Systematic review of errors in inhaler use: has patient technique improved over time? Chest150, doi:10.1016/j.chest.2016.03.041 (2016). [DOI] [PubMed]
- 15.Al-Showair RAM, Pearson SB, Chrystyn H. The potential of a 2Tone trainer to help patients use their metered-dose inhalers. Chest. 2007;131:1776–1782. doi: 10.1378/chest.06-2765. [DOI] [PubMed] [Google Scholar]
- 16.Armour CL, et al. Feasibility and effectiveness of an evidence-based asthma service in australian community pharmacies: a pragmatic cluster randomized trial. J. Asthma. 2013;50:302–309. doi: 10.3109/02770903.2012.754463. [DOI] [PubMed] [Google Scholar]
- 17.Basheti IA, Armour CL, Bosnic-Anticevich SZ, Reddel HK. Evaluation of a novel educational strategy, including inhaler-based reminder labels, to improve asthma inhaler technique. Patient Educ. Couns. 2008;72:26–33. doi: 10.1016/j.pec.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Basheti IA, Reddel HK, Armour CL, Bosnic-Anticevich SZ. Counseling about Turbuhaler technique: needs assessment and effective strategies for community pharmacists. Resp. Care. 2005;50:617–623. [PubMed] [Google Scholar]
- 19.Bosnic-Anticevich SZ, Sinha H, So S, Reddel HK. Metered-dose inhaler technique: the effect of two educational interventions delivered in community pharmacy over time. J. Asthma. 2010;47:251–256. doi: 10.3109/02770900903580843. [DOI] [PubMed] [Google Scholar]
- 20.Bynum A, Hopkins D, Thomas A, Copeland N, Irwin C. The effect of telepharmacy counseling on metered-dose inhaler technique among adolescents with asthma in rural Arkansas. Telemed. J. E. Health. 2001;7:207–217. doi: 10.1089/153056201316970902. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter DM, et al. Using videos to teach children inhaler technique: a pilot randomized controlled trial. J. Asthma. 2015;52:81–87. doi: 10.3109/02770903.2014.944983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan DS, et al. Internet-based home monitoring and education of children with asthma is comparable to ideal office-based care: results of a 1-year asthma in-home monitoring trial. Pediatrics. 2007;119:569–578. doi: 10.1542/peds.2006-1884. [DOI] [PubMed] [Google Scholar]
- 23.Chan DS, Callahan CW, Sheets SJ, Moreno CN, Malone FJ. An internet-based store-and-forward video home telehealth system for improving asthma outcomes in children. Am. J. Health-Syst. Ph. 2003;60:1976–1981. doi: 10.1093/ajhp/60.19.1976. [DOI] [PubMed] [Google Scholar]
- 24.Cicutto L, To T, Murphy S. A randomized controlled trial of a public health nurse-delivered asthma program to elementary schools. J. Sch. Health. 2013;83:876–884. doi: 10.1111/josh.12106. [DOI] [PubMed] [Google Scholar]
- 25.Cordina M, McElnay JC, Hughes CM. Assessment of a community pharmacy-based program for patients with asthma. Pharmacotherapy. 2001;21:1196–1203. doi: 10.1592/phco.21.15.1196.33894. [DOI] [PubMed] [Google Scholar]
- 26.Crane MA, Jenkins CR, Goeman DP, Douglass JA. Inhaler device technique can be improved in older adults through tailored education: findings from a randomised controlled trial. NPJ Prim. Care Respir. Med. 2014;24:14034. doi: 10.1038/npjpcrm.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Blaquiere P, Christensen DB, Carter WB, Martin TR. Use and misuse of metered-dose inhalers by patients with chronic lung disease. A controlled, randomized trial of two instruction methods. Am. Rev. Respir Dis. 1989;140:910–916. doi: 10.1164/ajrccm/140.4.910. [DOI] [PubMed] [Google Scholar]
- 28.De Oliveira MA, Faresin SM, Bruno VF, De Bittencourt AR, Fernandes ALG. Evaluation of an educational programme for socially deprived asthma patients. Eur. Respir. J. 1999;14:908–914. doi: 10.1034/j.1399-3003.1999.14d30.x. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Cardenas V, et al. Effect of a pharmacist intervention on asthma control. A cluster randomised trial. Respir. Med. 2013;107:1346–1355. doi: 10.1016/j.rmed.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Goodyer L, Savage I, Dikmen Z. Inhaler technique in Turkish people with poor English: a case of information discrimination? Pharm. World Sci. 2006;28:107–114. doi: 10.1007/s11096-006-9019-5. [DOI] [PubMed] [Google Scholar]
- 31.Goris S, Tasci S, Elmali F. The effects of training on inhaler technique and quality of life in patients with COPD. J. Aerosol. Med. Pulm. Drug. Deliv. 2013;26:336–344. doi: 10.1089/jamp.2012.1017. [DOI] [PubMed] [Google Scholar]
- 32.Hesselink AE, et al. Effectiveness of an education programme by a general practice assistant for asthma and COPD patients: results from a randomised controlled trial. Patient Educ. Couns. 2004;55:121–128. doi: 10.1016/j.pec.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Horner SD, Fouladi RT. Improvement of rural children’s asthma self-management by lay health educators. J. Sch. Health. 2008;78:506–513. doi: 10.1111/j.1746-1561.2008.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiser K, et al. A randomized controlled trial of a literacy-sensitive self-management intervention for chronic obstructive pulmonary disease patients. J. Gen. Intern. Med. 2012;27:190–195. doi: 10.1007/s11606-011-1867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kools M, van de Wiel MWJ, Ruiter RAC, Kok G. Pictures and text in instructions for medical devices: effects on recall and actual performance. Patient Educ. Couns. 2006;64:104–111. doi: 10.1016/j.pec.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Kritikos V, Armour CL, Bosnic-Anticevich SZ. Interactive small-group asthma education in the community pharmacy setting: a pilot study. J. Asthma. 2007;44:57–64. doi: 10.1080/02770900601125755. [DOI] [PubMed] [Google Scholar]
- 37.Kumar DSA, Adepu R, Parthasarathi G, Mahesh PA. Impact of community pharmacist provided patient education in asthma patients on treatment outcomes - a study. Indian J. Pharm. Educ. Res. 2009;43:125–133. [Google Scholar]
- 38.Martin MA, et al. Results from a community-based trial testing a community health worker asthma intervention in Puerto Rican youth in Chicago. J. Asthma. 2015;52:59–70. doi: 10.3109/02770903.2014.950426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehuys E, et al. Effectiveness of pharmacist intervention for asthma control improvement. Eur. Respir. J. 2008;31:790–799. doi: 10.1183/09031936.00112007. [DOI] [PubMed] [Google Scholar]
- 40.Mulloy E, Donaghy D, Quigley C, McNicholas WT. A one-year prospective audit of an asthma education programme in an out-patient setting. Ir. Med. J. 1996;89:226–228. [PubMed] [Google Scholar]
- 41.Patterson EE, et al. A cluster randomised intervention trial of asthma clubs to improve quality of life in primary school children: the school care and asthma management project (SCAMP) Arch. Dis. Child. 2005;90:786–791. doi: 10.1136/adc.2004.062612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perneger TV, et al. Effect of patient education on self-management skills and health status in patients with asthma: a randomized trial. Am. J. Med. 2002;113:7–14. doi: 10.1016/S0002-9343(02)01136-1. [DOI] [PubMed] [Google Scholar]
- 43.Petkova VB. Pharmaceutical care for asthma patients: a community pharmacy-based pilot project. Allergy Asthma Proc. 2008;29:55–61. doi: 10.2500/aap2008.29.3083. [DOI] [PubMed] [Google Scholar]
- 44.Press VG, et al. Teaching the use of respiratory inhalers to hospitalized patients with asthma or COPD: a randomized trial. J. Gen. Intern. Med. 2012;27:1317–1325. doi: 10.1007/s11606-012-2090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahmati H, Ansarfard F, Ghodsbin F, Ghayumi MA, Sayadi M. The effect of training inhalation technique with or without spacer on maximum expiratory flow rate and inhaler usage skills in asthmatic patients: a randomized controlled trial. Int. J. Community Based Nurs. Midwifery. 2014;2:211–219. [PMC free article] [PubMed] [Google Scholar]
- 46.Rootmensen GN, et al. The effects of additional care by a pulmonary nurse for asthma and COPD patients at a respiratory outpatient clinic: results from a double blind, randomized clinical trial. Patient Educ. Couns. 2008;70:179–186. doi: 10.1016/j.pec.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 47.Rydman RJ, Sonenthal K, Tadimeti L, Butki N, McDermott MF. Evaluating the outcome of two teaching methods of breath actuated inhaler in an inner city asthma clinic. J. Med. Syst. 1999;23:349–356. doi: 10.1023/A:1020525116505. [DOI] [PubMed] [Google Scholar]
- 48.Santos DO, et al. Pharmaceutical care for patients with persistent asthma: assessment of treatment compliance and use of inhaled medications. J. Bras. Pneumol. 2010;36:14–22. doi: 10.1590/S1806-37132010000100005. [DOI] [PubMed] [Google Scholar]
- 49.Tommelein E, et al. Effectiveness of pharmaceutical care for patients with chronic obstructive pulmonary disease (PHARMACOP): a randomized controlled trial. Br. J. Clin. Pharmacol. 2014;77:756–766. doi: 10.1111/bcp.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toumas-Shehata M, Price D, Basheti IA, Bosnic-Anticevich S. Exploring the role of quantitative feedback in inhaler technique education: a cluster-randomised, two-arm, parallel-group, repeated-measures study. NPJ Prim Care Respir Med. 2014;24:14071. doi: 10.1038/npjpcrm.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Der Palen J, Klein JJ, Kerkhoff AHM, Van Herwaarden CLA, Seydel ER. Evaluation of the long-term effectiveness of three instruction modes for inhaling medicines. Patient Educ. Couns. 1997;32:S87–S95. doi: 10.1016/S0738-3991(97)00100-6. [DOI] [PubMed] [Google Scholar]
- 52.Verver S, Poelman M, Bogels A, Chisholm SL, Dekker FW. Effects of instruction by practice assistants on inhaler technique and respiratory symptoms of patients. A controlled randomized videotaped intervention study. Fam. Pract. 1996;13:35–40. doi: 10.1093/fampra/13.1.35. [DOI] [PubMed] [Google Scholar]
- 53.Wilson SR, et al. A controlled trial of two forms of self-management education for adults with asthma. Am. J. Med. 1993;94:564–576. doi: 10.1016/0002-9343(93)90206-5. [DOI] [PubMed] [Google Scholar]
- 54.Neri M, et al. Short and long-term evaluation of two structured self management programmes on asthma. Monaldi Arch. Chest Dis. 2001;56:208–210. [PubMed] [Google Scholar]
- 55.Turgeon JP, et al. Teaching inhalation techniques to asthmatic children: a randomized clinical trial. Amb. Child Health. 1996;1:205–213. [Google Scholar]
- 56.Grainger-Rousseau TJ, Mc Elnay JC. A model for community pharmacist involvement with general practitioners in the management of asthma patients. J. Appl. Therap. 1996;1:145–161. [Google Scholar]
- 57.Nimmo CJR, et al. Assessment of patient acceptance and inhalation technique of a pressurized aerosol inhaler and two breath-actuated devices. Ann. Pharmacother. 1993;27:922–927. doi: 10.1177/106002809302700721. [DOI] [PubMed] [Google Scholar]
- 58.Bryant J, et al. Improving medication adherence in chronic obstructive pulmonary disease: a systematic review. Respir. Res. 2013;14:109. doi: 10.1186/1465-9921-14-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dantic DE. A critical review of the effectiveness of ‘teach-back’ technique in teaching COPD patients self-management using respiratory inhalers. Health Educ. J. 2014;73:41–50. doi: 10.1177/0017896912469575. [DOI] [Google Scholar]
- 60.Melani AS, et al. Inhalation technique and variables associated with misuse of conventional metered-dose inhalers and newer dry powder inhalers in experienced adults. Ann. Allergy Asthma Immunol. 2004;93:439–446. doi: 10.1016/S1081-1206(10)61410-X. [DOI] [PubMed] [Google Scholar]
- 61.Freedman LS, Pee D, Midthune DN. The problem of underestimating the residual error variance in forward stepwise regression. J. R. Stat. Soc. Series B Stat. Methodol. 1992;41:405–412. [Google Scholar]
- 62.Hanania NA, Wittman R, Kesten S, Chapman KR. Medical personnel’s knowledge of and ability to use inhaling devices. Metered-dose inhalers, spacing chambers, and breath-actuated dry powder inhalers. Chest. 1994;105:111–116. doi: 10.1378/chest.105.1.111. [DOI] [PubMed] [Google Scholar]
- 63.Self TH, Arnold LB, Czosnowski LM, Swanson JM, Swanson H. Inadequate skill of healthcare professionals in using asthma inhalation devices. J. Asthma. 2007;44:593–598. doi: 10.1080/02770900701554334. [DOI] [PubMed] [Google Scholar]
- 64.Garcia-Cardenas V, et al. Pharmacists’ interventions on clinical asthma outcomes: a systematic review. Eur. Respir. J. 2015 doi: 10.1183/13993003.01497-2015. [DOI] [PubMed] [Google Scholar]
- 65.Levy ML, et al. Inhaler technique: facts and fantasies. A view from the aerosol drug management improvement team (ADMIT) NPJ Prim Care Respir Med. 2016;26:16028. doi: 10.1038/npjpcrm.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Boven JF, et al. Improving inhaler adherence in patients with chronic obstructive pulmonary disease: a cost-effectiveness analysis. Respir. Res. 2014;15:66. doi: 10.1186/1465-9921-15-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Windsor RA, et al. Evaluation of the efficacy and cost effectiveness of health education methods to increase medication adherence among adults with asthma. Am. J. Public Health. 1990;80:1519–1521. doi: 10.2105/AJPH.80.12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zafari Z, Lynd LD, FitzGerald JM, Sadatsafavi M. Economic and health effect of full adherence to controller therapy in adults with uncontrolled asthma: a simulation study. J. Allergy Clin. Immunol. 2014;134:908–915 e903. doi: 10.1016/j.jaci.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 69.D’Arcy S, et al. A method to assess adherence in inhaler use through analysis of acoustic recordings of inhaler events. PLoS ONE. 2014;9:e98701. doi: 10.1371/journal.pone.0098701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Boven JF, Trappenburg JC, van der Molen T, Chavannes NH. Towards tailored and targeted adherence assessment to optimise asthma management. NPJ Prim Care Respir. Med. 2015;25:15046. doi: 10.1038/npjpcrm.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haughney J, et al. Choosing inhaler devices for people with asthma: current knowledge and outstanding research needs. Respir. Med. 2010;104:1237–1245. doi: 10.1016/j.rmed.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 72.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suarez-Almazor ME, Belseck E, Homik J, Dorgan M, Ramos-Remus C. Identifying clinical trials in the medical literature with electronic databases: MEDLINE alone is not enough. Control. Clin. Trials. 2000;21:476–487. doi: 10.1016/S0197-2456(00)00067-2. [DOI] [PubMed] [Google Scholar]
- 74.Higgins, J. P. T. & Green, S. (eds) Cochrane handbook for systematic reviews of Interventions, edn 5.1.0 (The Cochrane Collaboration, 2011).
- 75.Haynes RB, et al. Optimal search strategies for retrieving scientifically strong studies of treatment from Medline: analytical survey. BMJ. 2005;330:1179. doi: 10.1136/bmj.38446.498542.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Health Information Research Unit - McMaster University. Hedges, http://hiru.mcmaster.ca/hiru/HIRU_Hedges_home.aspx (2016).
- 77.Wilczynski NL, McKibbon KA, Walter SD, Garg AX, Haynes RB. MEDLINE clinical queries are robust when searching in recent publishing years. J. Am. Med. Inform. Assoc. 2013;20:363–368. doi: 10.1136/amiajnl-2012-001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilczynski NL, Haynes RB, Hedges T. EMBASE search strategies achieved high sensitivity and specificity for retrieving methodologically sound systematic reviews. J. Clin. Epidemiol. 2007;60:29–33. doi: 10.1016/j.jclinepi.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 79.Wong SS, Wilczynski NL, Haynes RB. Optimal CINAHL search strategies for identifying therapy studies and review articles. J. Nurs. Sch. 2006;38:194–199. doi: 10.1111/j.1547-5069.2006.00100.x. [DOI] [PubMed] [Google Scholar]
- 80.Higgins JP, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 82.Fleiss JL, Cohen J. The equivalence of weighted kappa and the interclass correlation coefficent as measures of reliability. Educ. Psychol. Meas. 1973;33:613–619. doi: 10.1177/001316447303300309. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.