Abstract
Objectives
Diagnosing chronic pancreatitis remains challenging. Endoscopic ultrasound (EUS) is utilized to evaluate pancreatic disease. Abnormal pancreas function test is considered the “non-histologic” gold standard for chronic pancreatitis. We derived a prediction model for abnormal endoscopic PFT (ePFT) by enriching EUS findings with patient demographic and pancreatitis behavioral risk characteristics.
Methods
Demographics, behavioral risk characteristics, EUS findings, and peak bicarbonate results were collected from patients evaluated for pancreatic disease. Abnormal ePFT was defined as peak bicarbonate <75 mEq/L. We fit a logistic regression model and converted it to a risk score system. The risk score was validated using 1,000 bootstrap simulations.
Results
A total of 176 patients were included; 61% were female with median age 48 (interquartile range 38, 57). Abnormal ePFT rate was 39.2% (69/176). Four variables formulated the risk score: alcohol or smoking status, number of parenchymal abnormalities, number of ductal abnormalities, and calcifications. Abnormal ePFT occurred in 10.7% with scores 4 or less versus 92.0% scoring 20 or greater. The model c-statistic was 0.78 (95% CI: 0.71, 0.85).
Conclusion
Number of EUS pancreatic duct and parenchymal abnormalities, presence of calcification, and smoking/alcohol status were predictive of abnormal ePFT. This simple model has good discrimination for ePFT results.
Keywords: chronic pancreatitis, EUS, pancreatic function test, endoscopic pancreatic function test, Rosemont classification
Introduction
Diagnosing chronic pancreatitis (CP) as a cause of chronic abdominal pain often presents a dilemma as histology is not readily available and diagnostic studies are imperfect. Current diagnostic studies include radiologic imaging with abdominal computed tomography (CT) scan and magnetic resonance imaging (MRI) with magnetic resonance cholangiopancreatography (MRCP), pancreatic function tests, and fecal elastase. Endoscopic procedures including endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasound (EUS) are often considered. EUS is safer and potentially more sensitive than ERCP for detecting CP and should be performed before ERCP in the diagnostic evaluation.1,2 Typically 9 parenchymal and ductal EUS criteria are evaluated in the diagnosis of CP with varying number of criteria chosen as the threshold for diagnosis of CP. With evidence that some EUS features may be physiologic and not pathologic especially in the presence of alcohol and cigarette use, the optimal threshold is unclear.2,3 More recently, the Rosemont classification weights various EUS criteria for CP based on expert opinion.4 The diagnostic accuracy of EUS features alone has come under considerable scrutiny.5
Secretin pancreatic function test (PFT) is the gold standard for detecting exocrine pancreatic insufficiency commonly observed in chronic pancreatitis.6–10 Traditional PFT using the Dreiling tube is a long, cumbersome procedure that can be difficult and uncomfortable for the unsedated patient. An endoscopic PFT (ePFT) has been developed with proven good correlation to the standard Dreiling PFT.8–10 A shortened ePFT test with timed aspirates from 15 to 45 minutes is highly accurate having 94% agreement with the full 1-hour test.8,11 This shortened ePFT can be combined with EUS to simultaneously assess gland morphology and secretory function.12
The aims of this retrospective study were to derive a CP prediction model using demographics, pancreatitis-specific behavioral risk factors, and EUS criteria, and to develop a diagnostic CP risk score using ePFT as the gold standard for CP.
Materials and Methods
Patient Population
Patients undergoing both EUS and ePFT either simultaneously or within 3 months of each other for suspected CP from April 2006 to October 2011 were eligible for study inclusion from 2 academic medical institutions (Cleveland Clinic Foundation, Brigham and Women’s Hospital). Patients were excluded from the study if they had a prior history of acute pancreatitis within 6 months of the procedures. The study protocol was approved by the Human Research Committee of the Partners Healthcare System.
The following demographic, behavior, EUS, and ePFT data were collected on all patients: age, gender, race; smoking and alcohol consumption; EUS parenchymal findings (hyperechoic foci, hyperechoic strands, lobularity, cysts), EUS ductal changes (hyperechoic duct wall, irregular duct margin, dilated main pancreatic duct [MPD], visible side branches, calcifications); peak bicarbonate concentration in secretin stimulated pancreas fluid. Smoking included any past or current smoking. Alcohol use was divided into >20 grams a day or less13.
EUS
Endosonography was performed using a curvilinear echoendoscope (Olympus GF-UC30P, GF-UCT 140-AL5; Olympus America, Center Valley, PA) for all cases. Sedation with intravenous midazolam, fentanyl, remifentanil, and/or propofol was used according to the judgement of the endoscopist and/or anesthesiologist. The pancreas was systematically examined with the pancreatic head visualized from the duodenal bulb to the pancreatic body and tail seen from the stomach. The standard 9-EUS criteria for diagnosis of CP were recorded based on changes in the pancreatic body and tail.14–16
Endoscopic Pancreatic Function Test
Performance of ePFT was standardized at both institutions. The modified ePFT was performed by administering 0.2mcg/kg secretin intravenously at time 0 minute. Gastric contents were completely aspirated and the suction channel of the endoscope was cleared by aspirating and discarding at least 2cc of duodenal fluid. Pancreas fluid (usually 5–10cc) was then aspirated from the 2nd to 3rd portion of the duodenum at 15, 30, and 45 minutes following secretin injection into a specimen container (Specimen Trap, Centurion, Howell, MI). All endoscopic biopsies and/or polypectomies were performed after the final pancreatic fluid collection to avoid contamination of the fluid specimen.
Pancreatic Fluid Analysis
Pancreatic fluid samples were frozen at −20 C and stored until analysis; all measurements were conducted within two weeks of sample collection. A separate in house laboratory validation study demonstrated no significant difference in pancreatic fluid electrolyte concentrations when stored for two weeks at −20 C (data not shown). Samples were thawed at room temperature, and an aliquot was passed through a serum filter (ML0550, MarketLab, Caledonia, MI) to remove particulates and fibrin microthrombi prior to analysis.
Laboratory analysis of fluid collected during ePFT was standardized at both institutions. Electrolyte (sodium, potassium, chloride, bicarbonate) measurements were conducted in CLIA-certified Clinical Chemistry Laboratories under standard operating procedures for plasma on an AU2700 (Olympus America, Center Valley, PA) automated chemistry analyzer. Sodium, potassium, and chloride were measured by indirect ion-selective electrodes, and total bicarbonate was measured by the two-step phosphoenolpyruvate carboxylase - malate dehydrogenase enzymatic-photometric method.17 Samples with results greater than the upper assay limit were diluted into the linear range with water. The highest bicarbonate concentration from the three samples was considered the peak bicarbonate value and a cutoff of 75 mEq/L was used to define abnormal (<75 mEq/L) or normal (≥75 mEq/L).16
Statistical Analysis
Model development
In order to identify the strongest predictors among potentially correlated EUS findings, data explorations were conducted using a logistic regression approach. Based on reiterative statistical testings and clinical plausibility evaluations, composite variables were created to optimally fit a predictive model. These variables were tested in continuous, multiple categorical, or dichotomous formats.
Risk score validation
The final logistic regression model coefficients were converted into an easy to use risk score system by dividing each variable coefficient (numerator) by the smallest variable coefficient (denominator) in the model and rounding it into integer point.18 Each patient’s risk score was calculated by summing up the total number of pertinent points. Validation of this risk score was conducted by 1,000 bootstrap simulations.19 The bootstrap simulations draw random samples with replacement from the study population, fit a regression model, and generate a c-statistic with each of the random samples. Overall, the 1,000 bootstrap simulations generated 1,000 random samples and c-statistics. The c-statistic values were ranked from the lowest to the highest, and the value at the 2.5th and 97.5th percentiles were used as the 95% confidence interval (CI) estimates for the model discrimination.
All analyses were conducted using Statistical Analysis Software (SAS; version 9.01, SAS Institute Inc., Cary, NC) and p-value <0.05 was considered significant.
Results
Patient Characteristics and Univariate Analysis
As shown in Table 1, a total of 176 patients were included in the study with 61% female; median age was 48 years (interquartile range [IQR] 38, 57). Four patients were excluded due to acute pancreatitis within 6 months of EUS and ePFT. Overall 39.2% (69/176) of patients had abnormal ePFT. Age, gender, and race were not associated with abnormal ePFT.
Table 1.
Demographic, behavior, and EUS data
| Variable | All Patients, n (%) |
Peak Bicarbonate < 75, n (%) |
Peak Bicarbonate ≥ 75, n (%) |
p-value | |
|---|---|---|---|---|---|
| Total | 176 | 69 (39.2) | 107 (60.8) | ||
| Demographics | |||||
| Age, median (Interquartile range) | 48 (38, 57) | 47 (39, 55) | 49 (37, 57) | 0.90 | |
| Male | 69 (39.2) | 27 (39.1) | 42 (60.9) | 0.99 | |
| Female | 107 (60.8) | 42 (39.3) | 65 (60.7) | ||
| White | 147 (83.5) | 62 (42.2) | 85 (57.8) | 0.07 | |
| Non-White | 29 (16.5) | 7 (24.1) | 22 (75.9) | ||
| Behavior | |||||
| Non-smoker | 88 (50.0) | 21 (23.9) | 67 (76.1) | <.0001 | |
| Current or previous smoker | 88 (50.0) | 48 (54.6) | 40 (45.5) | ||
| Alcohol use ≤20 grams/ day | 129 (73.3) | 44 (34.1) | 85 (65.9) | 0.02 | |
| Alcohol use >20 grams/ day | 47 (26.7) | 25 (53.2) | 22 (46.8) | ||
| EUS parenchymal findings | |||||
| Cysts - not present | 161 (91.5) | 61 (37.9) | 100 (62.1) | 0.24 | |
| present | 15 (8.5) | 8 (53.3) | 7 (46.7) | ||
| Hyperechoic strands - not present | 51 (29.0) | 15 (29.4) | 36 (70.6) | 0.09 | |
| present | 125 (71.0) | 54 (43.2) | 71 (56.8) | ||
| Hyperechoic foci - not present | 89 (50.6) | 28 (31.5) | 61 (68.5) | 0.03 | |
| present | 87 (49.4) | 41 (47.1) | 46 (52.9) | ||
| Lobularity - not present | 125 (71.0) | 39 (31.2) | 86 (68.8) | 0.0007 | |
| present | 51 (29.0) | 30 (58.8) | 21 (41.2) | ||
| EUS ductal findings | |||||
| Hyperechoic duct wall- not present | 74 (42.1) | 30 (40.5) | 44 (59.5) | 0.76 | |
| present | 102 (58.0) | 39 (38.2) | 63 (61.8) | ||
| Irregular MPD - not present | 143 (81.3) | 42 (29.4) | 101 (70.6) | <.0001 | |
| present | 33 (18.8) | 27 (81.8) | 6 (18.2) | ||
| Dilated MPD - not present | 140 (79.6) | 42 (30.0) | 98 (70.0) | <.0001 | |
| present | 36 (20.5) | 27 (75.0) | 9 (25.0) | ||
| Dilated side branches - not present | 153 (86.9) | 52 (34.0) | 101 (66.0) | 0.0003 | |
| present | 23 (13.1) | 17 (73.9) | 6 (26.1) | ||
| Calcifications - not present | 154 (87.5) | 49 (31.8) | 105 (68.2) | <.0001 | |
| present | 22 (12.5) | 20 (90.9) | 2 (9.1) | ||
The behavior variables, smoking and alcohol consumption, were associated with abnormal ePFT (p<0.05]. EUS parenchymal findings of hyperechoic foci and lobularity also predicted abnormal ePFT in 47% and 59% of patients, respectively (p<0.04). For EUS ductal criteria, irregular MPD, dilated MPD, dilated side branches, and calcification were all highly associated with abnormal secretory function (p<0.001) with abnormal ePFT prevalence rates ranging from 74% to 91%. The academic centers were compared as shown in Table 2; there were 114 patients from one academic center and 62 from the second center. The characteristics of patients from the two hospitals were similar in demographics, ePFT, and EUS findings.
Table 2.
Comparison of patient characteristics from two centers
| Variable | Center 1 (n=114) |
Center 2 (n=62) |
p- value |
|---|---|---|---|
| Age, median (IQR) | 49.5 (39,57) | 47 (35,56) | 0.42 |
| Female, n (%) | 68 (59.6) | 39 (62.9) | 0.67 |
| Peak bicarbonate, median (IQR) | 78 (62, 88) | 84 (64, 90) | 0.20 |
| Peak bicarbonate < 75, n (%) | 48 (42.1) | 21 (33.9) | 0.38 |
| EUS score, mean (standard deviation) | 2.9 (1.5) | 2.6 (2.4) | 0.24 |
IQR: interquartile range
EUS-ePFT Prediction Model and Risk Score
The logistic regression model identified four variables that predict ePFT: alcohol or smoking status, number of parenchymal abnormalities, number of ductal abnormalities, and calcifications (Table 3). Hyperechoic duct wall, as well as age, gender, race, were excluded in the multivariate analysis due to the insignificant associations with the outcome of the interest at the univariate level.
Table 3.
Prediction model for abnormal ePFT and weighted risk score
| Parameter | All Patients (n=176), n (%)* |
Abnormal ePFT (n=69), n (%)† |
Model Coefficient |
p-value | Risk Score‡ |
|---|---|---|---|---|---|
| Behavior (current or previous smoking/alcohol use) | |||||
| None | 76 (43) | 18 (24) | reference | N/A | 0 |
| Smoking OR alcohol status | 65 (37) | 29 (45) | 0.39 | 0.35 | 2 |
| Smoking AND alcohol status | 35 (20) | 22 (63) | 0.76 | 0.15 | 4 |
|
Number of parenchymal abnormalities present (cysts, strands, hyperechoic foci, and lobularity) | |||||
| 0 | 29 (16) | 4 (14) | reference | N/A | 0 |
| 1 or 2 | 112 (64) | 43 (38) | 1.05 | 0.10 | 6 |
| 3 or 4 | 35 (20) | 22 (63) | 1.46 | 0.05 | 8 |
|
Number of ductal abnormalities present (irregular MPD, dilated MPD, and dilated side branches) | |||||
| 0 | 124 (70) | 34 (27) | reference | 0 | |
| 1 | 23 (13) | 9 (39) | 0.19 | 0.71 | 1 |
| 2 or 3 | 29 (16) | 26 (90) | 2.14 | 0.002 | 11 |
| Calcifications | |||||
| No | 154 (88) | 49 (35) | reference | N/A | 0 |
| Yes | 22 (13) | 20 (91) | 1.79 | 0.03 | 9 |
Column % does not necessarily add to 100 due to rounding.
Row %
Risk score was calculated by dividing each variable coefficient by the smallest coefficient in the model and rounding the ratio to the integer.
The highest weighted predictor of abnormal ePFT in the multivariate model was the presence of 2 or 3 ductal abnormalities (irregular MPD, dilated MPD, and/ or dilated side branches) with a risk score of 11. Presence of calcifications had an independent score of 9 with the presence of 3 or 4 parenchymal abnormalities (cysts, strands, hyperechoic foci, and/ or lobularity) yielding a score of 8. Patients with both alcohol and smoking behavior had an independent score of 4 while those with either alcohol or smoking had a score of 2. The model c-statistic was 0.78. The 1,000 bootstrap simulations validated the model with 95% CI 0.71, 0.85.
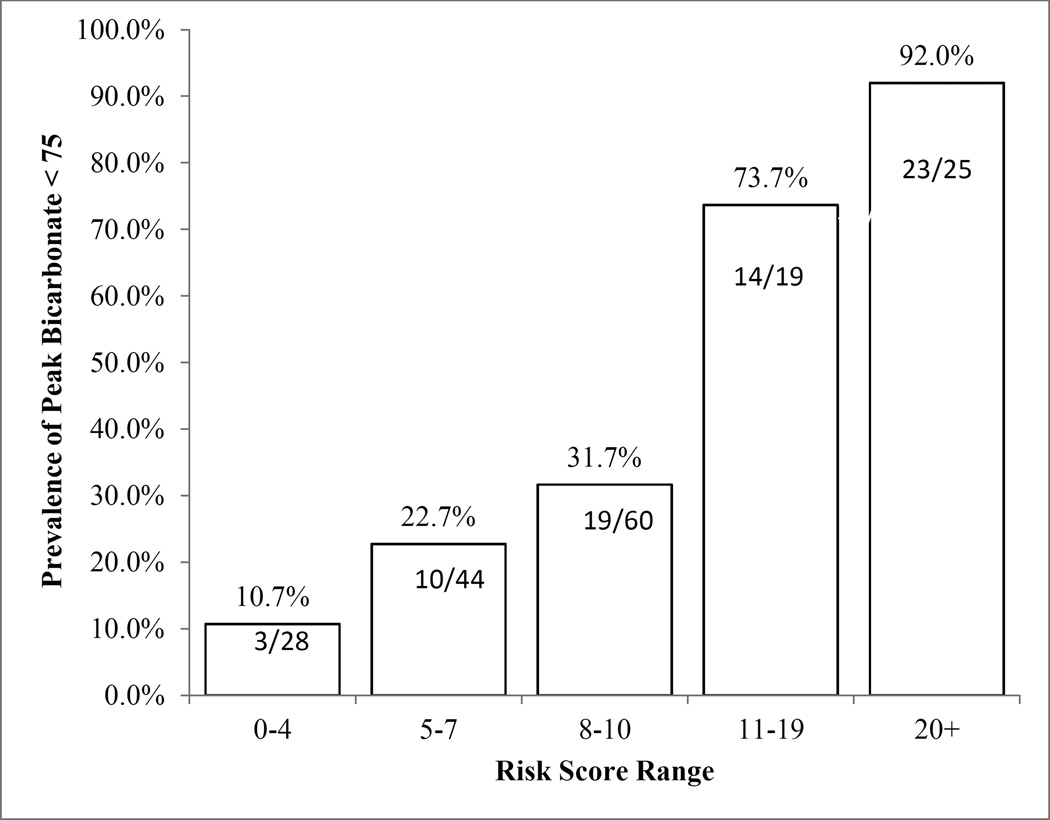
Risk scores ranged from 0 to 32 with median 8 (IQR 6, 11). Approximately 16% (28/176) of patients had a score of 4 or less, and the abnormal ePFT rate within this group was 10.7% (3/28) (Figure 1). Approximately 14% (25/176) of the patients had a score of 20 or higher, and among these patients abnormal ePFT rates were 92% (23/25) (Figure 1). The score distributions were similar among patients from the two study centers. The mean (standard deviation) of the risk score was 10.1 (6.6) versus 9.8 (10.1), p=0.80.
Figure 1.
Risk score strata and associated abnormal ePFT rates
Discussion
We have developed a more refined model for predicting abnormal pancreatic secretory function incorporating and weighting patient-specific pancreatitis behavioral risk factors and EUS findings. Our simple risk score allows stratification of patients into various probabilities of having abnormal pancreatic secretory function with very good discrimination. This scoring system can be used as an initial triage for further invasive testing.
Diagnosing CP can be difficult in early to mild forms of the disease despite availability of both structural and functional tests including MRI pancreas with MRCP, EUS, and PFT. No single test provides a definitive answer and a STEP-wise approach has been suggested.20 PFTs are time-consuming and have not gained widespread use. Although EUS is arguably the single most useful test for CP due to its ease of use, excellent patient tolerance, minimal patient risk and comprehensive imaging of the entire pancreas parenchyma and ductal system, limitations exist.21 Uncertainty remains over the appropriate EUS threshold for diagnosing CP. With recognition that the EUS criteria have different predictive potential for diagnosing CP, the Rosemont classification was developed which weights various EUS criteria. However, this is based on expert consensus opinion and has not been validated prospectively.4 Furthermore, it is uncertain whether the EUS findings are pathologic, age-related changes, asymptomatic fibrosis, or normal variants.5 At best EUS detects nonspecific fibrosis in the pancreas which can be observed in chronic pancreatitis, but cannot be interpreted in a vacuum and needs to be evaluated in the setting of a particular patient profile and pancreatitis risk behaviors.
Therefore, our goal was to enhance diagnosis of CP by incorporating EUS as well as patient demographic and pancreatitis behavioral risk factors into a more comprehensive model for CP. Both alcohol and smoking have been clearly identified as risk factors for CP, which make it important to include these features in a diagnostic model for CP.22–24 We found that presence of both alcohol and smoking is worse than the presence of either alone. No specific demographic factors including age, gender, and race were significantly associated with abnormal pancreatic function in our study. Consistent with previous studies, calcifications was a strong predictor of abnormal ePFT in addition to EUS ductal features of CP.12,25 Because secretin PFT relies on ductal cell secretion, higher correlation of ductal features with abnormal ePFT is expected. The uniqueness of our study results from not only the quantification of EUS criteria associated with CP, but also the addition of individual patient smoking and alcohol use to generate a more refined model for CP. Previous EUS studies have not objectively weighted the various EUS criteria nor included other variables to enhance EUS diagnosis of CP.
Our risk score is also practical in guiding the evaluation of suspected CP. If a patient has a risk score ≤4, PFT does not need to be performed because the risk of CP is very low. Similarly, if the patient’s risk score is ≥20, PFT can be avoided because the patient has a 92% likelihood of having an abnormal result. However, for those patients with scores between 5 and 19, pancreatic function testing may be performed to further assess for exocrine insufficiency.
The primary limitation of our study is the use of endoscopic PFT as the gold standard for diagnosing CP. However, in clinical reality histologic diagnosis of CP is rarely available nor is EUS-guided biopsy of the pancreas to diagnose CP recommended due to its relatively low sensitivity and safety concerns.21 Furthermore, secretin PFT is regarded as the non-histologic gold standard for diagnosis of CP.26 While external validation of our model using an independent cohort is desirable, our use of bootstrap methods is a widely accepted method of validation.19 However, our risk score does need validation in a prospective study, which will allow collection of more complete data including imaging findings that can be incorporated into our model.
In conclusion, we have developed a simple risk score for the diagnosis of CP which incorporates smoking and alcohol use in addition to quantitatively weighting EUS criteria for CP. This novel scoring system may enable more accurate classification of patients with suspected CP.
Acknowledgments
Source of Funding: Supported by an American College of Gastroenterology Clinical Research Award (DLC), an unrestricted research grant from ChiRhoClin, Inc. (DLC) and by the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK) and National Cancer Institute (NCI) under Award Number U01DK108327 (DLC).
Footnotes
Conflicts of Interest: No conflicts of interest for the authors.
References
- 1.Varadarajulu S, Eltoum I, Tamhane A, et al. Histopathologic correlates of noncalcific chronic pancreatitis by EUS: a prospective tissue characterization study. Gastrointest Endosc. 2007;66:501–509. doi: 10.1016/j.gie.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 2.Chong AK, Hawes RH, Hoffman BJ, et al. Diagnostic performance of EUS for chronic pancreatitis: a comparison with histopathology. Gastrointest Endosc. 2007;65:808–814. doi: 10.1016/j.gie.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 3.Petrone MC, Arcidiacono PG, Perri F, et al. Chronic pancreatitis-like changes detected by endoscopic ultrasound in subjects without signs of pancreatic disease: do these indicate age-related changes, effects of xenobiotics, or early chronic pancreatitis? Pancreatology. 2010;10:597–602. doi: 10.1159/000314599. [DOI] [PubMed] [Google Scholar]
- 4.Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc. 2009;69:1251–1261. doi: 10.1016/j.gie.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 5.Conwell DL, Lee LS, Yadav D, et al. American Pancreatic Association Practice Guidelines in Chronic Pancreatitis: evidence-based report on diagnostic guidelines. Pancreas. 2014;43:1143–1162. doi: 10.1097/MPA.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steer ML, Waxman I, Freedman S. Chronic pancreatitis. N Engl J Med. 1995;332:1482–1490. doi: 10.1056/NEJM199506013322206. [DOI] [PubMed] [Google Scholar]
- 7.Kitagawa M, Naruse S, Ishiguro H, et al. Evaluating exocrine function tests for diagnosing chronic pancreatitis. Pancreas. 1997;15:402–408. doi: 10.1097/00006676-199711000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Conwell DL, Zuccaro G, Jr, Vargo JJ, et al. An endoscopic pancreatic function test with synthetic porcine secretin for the evaluation of chronic abdominal pain and suspected chronic pancreatitis. Gastrointest Endosc. 2003;57:37–40. doi: 10.1067/mge.2003.14. [DOI] [PubMed] [Google Scholar]
- 9.Stevens T, Conwell DL, Zuccaro G, Jr, et al. A randomized crossover study of secretin-stimulated endoscopic and dreiling tube pancreatic function test methods in healthy subjects. Am J Gastroenterol. 2006;101:351–355. doi: 10.1111/j.1572-0241.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 10.Stevens T, Conwell DL, Zuccaro G, Jr, et al. A prospective crossover study comparing secretin-stimulated endoscopic and Dreiling tube pancreatic function testing in patients evaluated for chronic pancreatitis. Gastrointest Endosc. 2008;67:458–466. doi: 10.1016/j.gie.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 11.Stevens T, Conwell DL, Zuccaro G, Jr, et al. The efficiency of endoscopic pancreatic function testing is optimized using duodenal aspirates at 30 and 45 minutes after intravenous secretin. Am J Gastroenterol. 2007;102:297–301. doi: 10.1111/j.1572-0241.2006.00949.x. [DOI] [PubMed] [Google Scholar]
- 12.Stevens T, Dumot JA, Parsi MA, et al. Combined endoscopic ultrasound and secretin endoscopic pancreatic function test in patients evaluated for chronic pancreatitis. Dig Dis Sci. 2010;55:2681–2687. doi: 10.1007/s10620-009-1084-x. [DOI] [PubMed] [Google Scholar]
- 13.Schneider A, Lohr JM, Singer MV. The M-ANNHEIM classification of chronic pancreatitis: introduction of a unifying classification system based on a review of previous classifications of the disease. J Gastroenterol. 2007;42:101–119. doi: 10.1007/s00535-006-1945-4. [DOI] [PubMed] [Google Scholar]
- 14.Savides TJ, Gress FG, Zaidi SA, et al. Detection of embryologic ventral pancreatic parenchyma with endoscopic ultrasound. Gastrointest Endosc. 1996;43:14–19. doi: 10.1016/s0016-5107(96)70253-5. [DOI] [PubMed] [Google Scholar]
- 15.Sahai AV, Zimmerman M, Aabakken L, et al. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc. 1998;48:18–25. doi: 10.1016/s0016-5107(98)70123-3. [DOI] [PubMed] [Google Scholar]
- 16.Wiersema MJ, Hawes RH, Lehman GA, et al. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy. 1993;25:555–564. doi: 10.1055/s-2007-1010405. [DOI] [PubMed] [Google Scholar]
- 17.Forrester RL, Wataji LJ, Silverman DA, et al. Enzymatic method for determination of CO2 in serum. Clin Chem. 1976;22:243–245. [PubMed] [Google Scholar]
- 18.Sullivan LM, Massaro JM, D'Agostino RB., Sr Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23:1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 19.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. London, UK: Chapman and Hall; 1993. [Google Scholar]
- 20.Conwell DL, Wu BU. Chronic pancreatitis: making the diagnosis. Clin Gastroenterol Hepatol. 2012;10:1088–1095. doi: 10.1016/j.cgh.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Gardner TB, Levy MJ. EUS diagnosis of chronic pancreatitis. Gastrointest Endosc. 2010;71:1280–1289. doi: 10.1016/j.gie.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 22.Cote GA, Yadav D, Slivka A, et al. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2011;9:266–273. doi: 10.1016/j.cgh.2010.10.015. quiz e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yadav D, Hawes RH, Brand RE, et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med. 2009;169:1035–1045. doi: 10.1001/archinternmed.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed Ali U, Issa Y, Hagenaars JC, et al. Risk of Recurrent Pancreatitis and Progression to Chronic Pancreatitis After a First Episode of Acute Pancreatitis. Clin Gastroenterol Hepatol. 2016;14:738–746. doi: 10.1016/j.cgh.2015.12.040. [DOI] [PubMed] [Google Scholar]
- 25.Catalano MF, Lahoti S, Geenen JE, et al. Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc. 1998;48:11–17. doi: 10.1016/s0016-5107(98)70122-1. [DOI] [PubMed] [Google Scholar]
- 26.Chowdhury RS, Forsmark CE. Review article: Pancreatic function testing. Aliment Pharmacol Ther. 2003;17:733–750. doi: 10.1046/j.1365-2036.2003.01495.x. [DOI] [PubMed] [Google Scholar]