Abstract
Background
Worsening renal function (WRF) is a common endpoint in decompensated heart failure clinical trials because of associations between WRF and adverse outcomes. However, WRF has not universally been identified as a poor prognostic sign, challenging the validity of WRF as a surrogate endpoint. Our aim was to describe the associations between changes in creatinine and adverse outcomes in a clinical trial of decongestive therapies.
Methods and Results
We investigated the association between changes in creatinine and the composite endpoint of death, rehospitalization or emergency room visit within 60 days in 301 patients in the Diuretic Optimization Strategies Evaluation (DOSE) trial. WRF was defined as an increase in creatinine >0.3 mg/dL and improvement in renal function (IRF) as a decrease >0.3 mg/dL. When examining linear changes in creatinine from baseline to 72 hours (the coprimary endpoint of DOSE), increasing creatinine was associated with lower risk for the composite outcome (HR = 0.81 per 0.3 mg/dL increase, 95% CI 0.67–0.98, P = .026). Compared with patients with stable renal function (n = 219), WRF (n = 54) was not associated with the composite endpoint (HR = 1.17, 95% CI = 0.77–1.78, P = .47). However, compared with stable renal function, there was a strong relationship between IRF (n = 28) and the composite endpoint (HR = 2.52, 95% CI = 1.57–4.03, P <.001).
Conclusion
The coprimary endpoint of the DOSE trial, a linear increase in creatinine, was paradoxically associated with improved outcomes. This was driven by absence of risk attributable to WRF and a strong risk associated with IRF. These results argue against using changes in serum creatinine as a surrogate endpoint in trials of decongestive strategies.
Keywords: Worsening in renal function, improvement in renal function, cardio-renal syndrome, renal dysfunction, acute decompensated heart failure
Worsening renal function (WRF) is a common complication of acute decompensated heart failure (ADHF) treatment and has been associated with adverse outcomes including longer length of stay, hospital readmission, and increased mortality.1 This in part has led to the use of changes in serum creatinine as endpoints of ADHF clinical trials.2–6 However, the associations between WRF and poor outcomes in observational studies is complicated by the fact that patients who experience WRF often have a greater disease severity and are less responsive to ADHF therapies including diuretics; as a result, they are intrinsically at greater risk of adverse events independent of the renal dysfunction.1 Notably, it has recently been observed that WRF in the setting of interventions that are otherwise beneficial, such as aggressive diuresis or initiation of renin-angiotensin-aldosterone system antagonists, may have limited prognostic importance.7–9 Furthermore, some observational studies have actually found that improvement in renal function (IRF) can be associated with a similarly poor prognosis to WRF.10–12 As such, the assumption that a change in serum creatinine is in fact a meaningful clinical trial endpoint in ADHF deserves further investigation.
The Diuretic Optimization Strategies Evaluation (DOSE) trial was a prospective multicenter trial investigating strategies of loop diuretic administration, such as high vs low-dose intensification, with a coprimary endpoint of a change in serum creatinine from baseline to 72 hours.2 Per protocol, loop diuretic dosing was aggressive in all patients, with a median daily dose over the intervention period of ~120 mg/day of IV furosemide in the low-intensity group and ~260 mg/day in the high-intensity group. Importantly, unlike many other recent ADHF trials, the high-dose diuretic intervention in the DOSE trial was associated with significantly greater fluid and weight loss. Given the aggressive dosing of diuretics and the protocol-driven ascertainment of changes in renal function (RF), the DOSE trial represents a unique opportunity to further investigate the implications of changes in creatinine during trials of decongestive therapies. We hypothesized that if change in serum creatinine during a decongestion intervention was a meaningful surrogate endpoint, WRF should be associated with poor outcomes and IRF should be associated with improved outcomes.
Methods
The analysis was conducted using a limited dataset from the Biologic Specimen and Data Repository from the National Heart, Lung, and Blood Institute Heart Failure Network’s DOSE trial, for which the study design and results have been published previously.2 Briefly, DOSE was a multicenter, randomized, double-blind, placebo-controlled trial of diuretic strategies in ADHF patients. Using a 2 × 2 factorial design, patients were randomized to a strategy of high vs low intensification of furosemide and continuous infusion vs every 12-hour bolus furosemide administration. High dose consisted of 2.5 times the baseline oral diuretic dose; low dose consisted of 1.0 times the baseline oral diuretic dose both administered as IV furosemide. Eligibility criteria included an oral loop diuretic dose of 80–240 mg of furosemide equivalents for at least 1 month, systolic blood pressure ≥90 mmHg, and creatinine ≤3.0 mg/dL. The randomized treatment was continued for 72 hours with an option for the treating physician to adjust the dose at 48 hours while maintaining study treatment concealment.
The primary goals of the current analysis were to examine the relationship between changes in RF and outcomes in an ADHF clinical trial, focusing on the coprimary endpoint of change in serum creatinine from baseline to 72 hours and the secondary endpoint of a >0.3 mg/dL change in creatinine at any time from baseline to 72 hours. Given the previously observed nonlinear relationship between change in creatinine and outcomes (ie, where both IRF and WRF identify higher risk), the shape of the relationship between change in creatinine across the spectrum of values was interrogated.11–13 For a more relevant clinical interpretation of these relationships, patients were divided into RF groups: WRF, IRF, and Stable RF. Outcomes in those with WRF and IRF were first compared with the remainder of the cohort (ie, WRF to patients with no WRF with the comparator including patients with IRF) and then only to those with Stable RF.
Change in serum creatinine from baseline to 72 hours was defined as the 72-hour creatinine minus the baseline creatinine in mg/dL with the 48-hour creatinine substituted for 72 hours in patients discharged before 72 hours (n = 30). To recapitulate the DOSE trial definition, WRF and IRF were defined as >0.3 mg/dL increase or decrease in serum creatinine at any point from baseline to 72 hours respectively. As a sensitivity analysis, IRF was defined as a ≥20% increase in estimated glomerular filtration rate (eGFR) from baseline to 72 hours and WRF as a ≥20% decrease in eGFR from baseline to 72 hours to account for the nonlinear relationship between creatinine and outcomes and consistent with previously published studies of IRF and WRF.8,10,11,13–15 Patients who had a change in RF less than the thresholds used to define WRF or IRF were considered to have Stable RF. The chronic kidney disease epidemiology collaboration equation was used to calculate eGFR.16 Additional detail on rationale for dealing with missing creatinine values in the DOSE trial is provided in the Supplementary Methods.
Loop diuretic doses were converted to furosemide equivalents with 1 mg bumetanide = 20 mg torsemide = 40 mg furosemide and expressed as IV furosemide equivalents.17,18 For oral diuretics, a bioavailability of 50% was assumed for furosemide, whereas torsemide and bumetanide were converted 1:1.19 The investigation conforms with the Declaration of Helsinki and qualified as exempt by the institutional review boards at Medical University of South Carolina and Yale University.
Statistical Analysis
Values reported are mean ± standard deviation, median (25th–75th percentile), and percentage. Analysis of variance or the Kruskal-Wallis test was used to compare continuous variables between change in RF groups: WRF, IRF and Stable RF. Student t test or the Mann Whitney U test was used to compare continuous variables in subjects with IRF vs WRF. The chi-square test was used to evaluate associations between categorical variables except in those cases when the expected cell counts were less than 5 and Fisher’s exact test was used (stroke, in-house thiazide use, in-house inotrope use, and congestion free at 72 hours). Cox proportional hazards modeling was used to evaluate time-to-event associations with the composite endpoint of death, rehospitalization, or emergency room visit at 60 days. To capture the nonlinearities in the relationship between change in RF and outcomes, both change in creatinine and percent change in eGFR from baseline to 72 hours were each modeled with cubic splines using three degrees of freedom and presented graphically. For more relevant clinical interpretation of these relationships, multivariate Cox regression models were constructed to evaluate the effects of IRF vs no-IRF and WRF vs no-WRF (using multiple definitions) as well as the effects of IRF vs Stable RF and WRF vs Stable RF (using multiple definitions) on the composite endpoint. These models were subsequently adjusted for baseline eGFR and all baseline characteristics with <10% missing values and a univariate association with the composite endpoint of P ≤ .2, in addition to any parameter which differed significantly across RF groups (Stable RF, WRF, IRF; Table 1). Additional multivariable models were further adjusted for in-hospital characteristics with a theoretical basis for potential confounding regardless of the relationship with mortality. Finally, models were also adjusted for the study interventions. Baseline characteristics entered into the multivariable models included age, diabetes, hypertension, gout, ejection fraction, dose and intensification strategies, outpatient furosemide dose, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use, beta-blocker use, systolic blood pressure, heart rate, jugular venous pressure, baseline eGFR, amino terminal pro B-type natriuretic peptide, serum sodium, hemoglobin, and blood urea nitrogen. In-hospital characteristics entered into the final multivariable model consisted of total diuretic received during the study period and inpatient inotrope use. Models were built using backward elimination (likelihood ratio) where covariates with a P <.2 were retained.20 Survival curves were plotted comparing the three change in RF groups. The x-axis was terminated at 60 days and statistical significance was determined using the log-rank test. Statistical analyses were performed using IBM SPSS Statistics version 22 (IBM Corp., Armonk, NY) and Stata 13.1 (Statacorp, College Station, TX). Statistical significance was defined as a 2-tailed P <.05 for all analyses except for tests of interaction, where P <.1 was considered significant.
Table 1.
Baseline Characteristics by Improvement, Worsening, or Stable Renal Function Over the 72-H Intervention Period
| Characteristic | Stable RF (n = 219) | WRF (n = 54) | IRF (n = 28) | P Value Overall | P Value IRF vs WRF |
|---|---|---|---|---|---|
| Demographics/medical history | |||||
| Age | 66.4 ± 13.4 | 64.7 ± 14.7 | 65.9 ± 13.5 | .719 | .706 |
| Male sex | 72.6% | 70.4% | 85.7% | .286 | .125 |
| Black race | 23.7% | 27.8% | 25.0% | .826 | .788 |
| HF hospitalization in past year | 74.9% | 75.9% | 67.9% | .697 | .434 |
| Nonischemic etiology | 49.8% | 53.7% | 53.6% | .835 | .991 |
| Diabetes | 49.3% | 72.2% | 39.3% | .004* | .004* |
| Hypertension | 78.1% | 92.6% | 75.0% | .042* | .027* |
| Stroke | 13.2% | 7.4% | 14.3% | .472 | .435 |
| Gout | 20.5% | 33.3% | 21.4% | .132 | .261 |
| Ejection fraction (%) | 33.2 ± 17.2 | 37.2 ± 18.6 | 39.7 ± 16.7 | .089 | .545 |
| Physical examination | |||||
| Systolic blood pressure (mmHg) | 119 ± 19.6 | 122 ± 20.9 | 111 ± 16.8 | .073 | .025* |
| Heart rate (beats/min) | 78 ± 16 | 79 ± 16 | 78 ± 14 | .953 | .880 |
| Jugular venous pressure ≥8 cm | 61.3% | 44.7% | 59.3% | .111 | .227 |
| Edema >2+ | 29.2% | 25.9% | 39.3% | .444 | .213 |
| Rales | 56.9% | 66.0% | 53.6% | .419 | .272 |
| Medications | |||||
| ACE or ARB | 66.7% | 61.1% | 46.4% | .100 | .204 |
| Beta blocker | 83.1% | 81.5% | 89.3% | .651 | .359 |
| Loop diuretic dose (mg furosemide equivalents) | 120 (80, 160) | 160 (80, 240) | 160 (120, 230) | .072 | .150 |
| Digoxin | 30.6% | 29.6% | 25.0% | .830 | .658 |
| Aldosterone antagonist | 27.4% | 29.6% | 25.0% | .900 | .658 |
| Laboratory values | |||||
| Sodium (mEq/L) | 138 ± 3.7 | 139 ± 3.3 | 136 ± 4.1 | .008* | .003* |
| Blood urea nitrogen (mg/dL) | 34 ± 20 | 38 ± 25 | 61 ± 25 | <.001* | <.001* |
| Creatinine (mg/dL) | 1.5 ± 0.5 | 1.5 ± 0.5 | 2.1 ± 0.4 | <.001* | <.001* |
| eGFR (mL/min/1.73 m2) | 50.6 ± 23.7 | 63.8 ± 25.3 | 38.3 ± 12.3 | <.001* | .001* |
| NT-proBNP (pg/mL) | 4195 (2560, 10,429) | 4238 (1991, 8066) | 7174 (3676, 18,754) | .071 | .041* |
| Hemoglobin (g/dL) | 11.7 ± 2.0 | 11.6 ± 2.1 | 11.4 ± 2.0 | .658 | .721 |
IRF and (WRF) defined by a >0.3 change in serum creatinine.
ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; eGFR: estimated glomerular filtration rate; HF, heart failure, IRF, improvement in renal function; NT-proBNP: amino terminal pro B-type natriuretic peptide; RF, renal function; WRF, worsening renal function.
Significant P value.
Results
Overall, 301 of 308 patients had at least 1 serum creatinine other than baseline; 292 patients had a 72-hour or day of discharge creatinine available. In the overall population, the mean change in serum creatinine at 72 hours was 0.04 ± 0.31 mg/dL corresponding to a mean loss in eGFR of 1.94 ± 12.1 mL/min/1.73 m2. Eighteen percent of the population experienced WRF with a mean increase in creatinine of 0.54 ± 0.27 mg/dL and mean decrease in eGFR in these patients of 27.3 ± 17.0%. IRF at 72 hours was less common and occurred in 9.1% of patients with a mean decrease in creatinine of 0.43 ± 0.14 mg/dL and a mean increase in eGFR of 35.8 ± 17.8%. When WRF and IRF were defined by ≥20% decrease or increase in eGFR respectively, WRF (15.4%) and IRF (11.0%) had similar incidences.
A detailed description of baseline characteristics stratified by WRF, IRF, and Stable RF over 72 hours is provided in Table 1 and Table S1. Aside from higher eGFR, greater prevalence of diabetes and hypertension, higher serum sodium and blood urea nitrogen, and lower baseline NT-proBNP levels at admission with WRF compared with IRF, baseline characteristics were otherwise similar between patients with IRF and WRF (Table 1). In-hospital and treatment-related parameters of patients with Stable RF, WRF, and IRF are presented in Table 2. Randomization to the high-dose strategy tended to be more common in the WRF group compared with IRF, but this did not reach statistical significance (Table 2). However, WRF patients received study drug for a significantly shorter duration than patients with Stable RF or IRF (Table 2). Patients with WRF demonstrated a lower prevalence of jugular venous distension at 72 hours with statistically insignificant trends toward more complete decongestion during treatment with respect to peripheral edema, “congestion-free” status at 72 hours, and change in NT-proBNP (Table 2). When WRF and IRF were defined by ≥20% change in eGFR, similar trends were noted (Table S2).
Table 2.
In-hospital Characteristics by Improvement, Worsening, or Stable Renal Function at 72 H
| Characteristic | Stable RF (n = 219) | WRF (n = 54) | IRF (n = 28) | P Value Overall | P Value IRF vs WRF |
|---|---|---|---|---|---|
| Diuretic treatment and strategy | |||||
| Randomized to continuous IV infusion treatment | 47.0% | 51.9% | 53.6% | .698 | .882 |
| Randomized to high-intensity treatment | 47.5% | 64.8% | 53.6% | .071 | .322 |
| Time on study drug between randomization and 72 h | 68 (48, 72) | 49 (48, 68) | 71 (48, 72) | .041* | .055 |
| 72-h cumulative IV furosemide dose equivalents (mg) | 524 (314, 847) | 582 (375, 774) | 596 (439, 992) | .350 | .338 |
| Changed to oral diuretics at 48 h | 24.2% | 25.9% | 21.4% | .903 | .653 |
| Days from randomization to discharge | 5 (3, 8) | 6 (4, 11) | 6 (4, 11) | .153 | .953 |
| Medication use | |||||
| In-hospital thiazide use | 15.1% | 14.8% | 17.9% | .897 | .756 |
| In-hospital inotropes | 10.0% | 11.1% | 14.3% | .671 | .729 |
| Physical examination and laboratory findings | |||||
| Weight change at 72 h (lb) | 6.8 (3.1, 11.9) | 5.5 (0.2, 11.4) | 5.5 (1.4, 10.8) | .429 | .926 |
| Net fluid loss at 72 h (mL) | 3835 (2190, 5888) | 2721 (1367, 5051) | 4412 (2151, 6527) | .139 | .118 |
| Change in baseline to 72-h NT-proBNP | −779 (−2769, 58.5) | −1027 (−2980, −56) | −1078 (−3078, 601) | .686 | .597 |
| Dyspnea VAS at 72 h | 4820 (3851, 5821) | 4437 (3395, 5542) | 4734 (3334, 5405) | .275 | .607 |
| Global VAS at 72 h | 4537 (3601, 5440) | 4418 (3347, 5023) | 3803 (3337, 5016) | .157 | .703 |
| Congestion free at 72 h | 13.3% | 18.8% | 7.7% | .411 | .309 |
| JVP ≥8 cm at 72 h | 27.7% | 20.0% | 44.0% | .109 | .038* |
| Peripheral Edema at 72 h | 12.6% | 10.0% | 11.5% | .882 | .836 |
IRF and WRF defined by a >0.3 change in serum creatinine.
JVP, jugular venous pressure; VAS, visual analog scale. Other abbreviations as in Table 1.
Significant P value.
Changes in RF and Outcomes
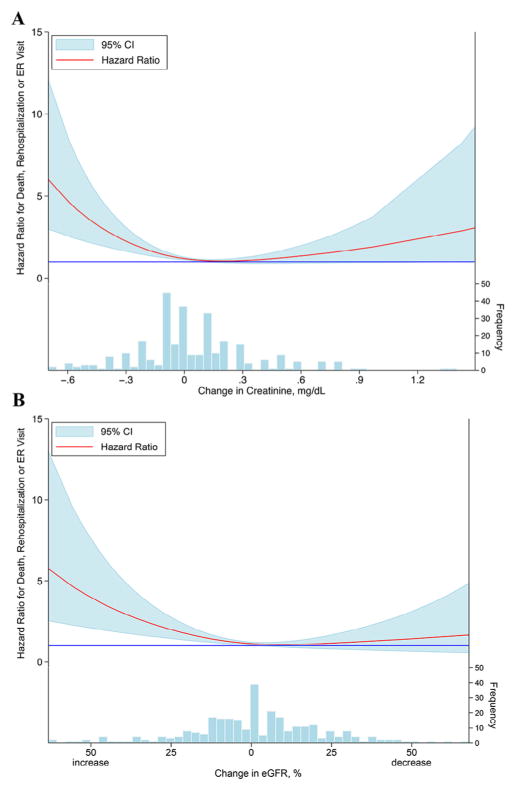
Over a follow-up period of 60 days, 139 patients (45%) in this subset experienced the composite outcome of death, rehospitalization, or an emergency room visit. When evaluating the coprimary endpoint for the DOSE trial of a linear change in serum creatinine from baseline to 72 hours, increasing creatinine over the intervention period was associated with significantly improved outcomes (hazard ratio [HR] 0.81 per 0.3 mg/dL increase, 95% confidence interval [CI] 0.67–0.98, P = .026). The relative change in eGFR from baseline to 72 hours demonstrated a similar relationship; for every 10% worsening in eGFR, the risk of adverse outcomes decreased by more than 10% (HR 0.88 per 10% decrease, 95% CI 0.81–0.96, P = .003). Importantly, the relationships between change in RF and the composite endpoint were nonlinear with a marked increase in adverse outcomes with improvement in creatinine, which drove the findings described above (Fig. 1). As a result, the linear change in creatinine, which was the primary safety endpoint of the trial, violates the linearity assumption required for Cox models and subsequently poorly captures the true source of the associations with worsened outcomes. Thus, further exploration of the associations between RF and outcomes are described below using WRF, IRF, and Stable RF groups.
Fig. 1.
Relationship between changes in renal function and clinical outcomes. Relationship between the absolute change in serum creatinine (A) and the percent change in eGFR (B) from baseline to 72 hours and the composite outcome death, rehospitalization, or emergency room visit within 60 days. The solid blue line represents a hazard ratio of 1. Histograms denote number of patients in each change in creatinine or eGFR group. CI, confidence interval; eGFR, estimated glomerular filtration rate; ER, emergency room.
When evaluating WRF as defined for the secondary safety endpoint of DOSE (>0.3 mg/dL increase in creatinine at any time from baseline to 72 hours) vs those without WRF, there was no evidence of increased risk for the composite endpoint (HR 1.08, 95% CI 0.71–1.63, P = .72; comparison group being any patient without WRF). Similar results were obtained when defining WRF by a ≥20% decrease in eGFR (HR 1.14, 95% CI 0.73–1.77, P = .57). However, compared with the rest of the cohort, IRF demonstrated a statistically significant and strong relationship with death, rehospitalization, or emergency room visit (HR 2.46, 95% CI 1.54–3.93, P <.001; ≥20% increase in eGFR HR 2.35, 95% CI 1.50–3.69, P <.001). Although limited by sample size, a sensitivity analysis of the individual components of the composite outcome revealed similarly increased risks associated with IRF (Table S3).
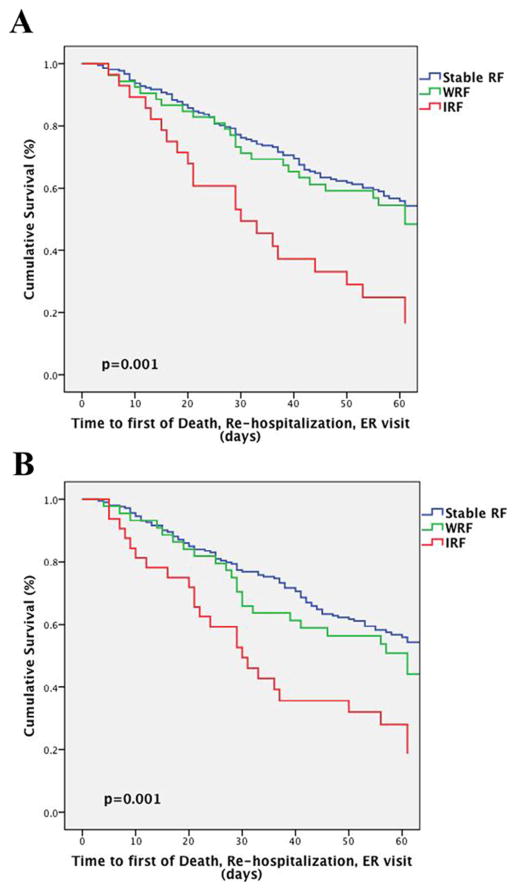
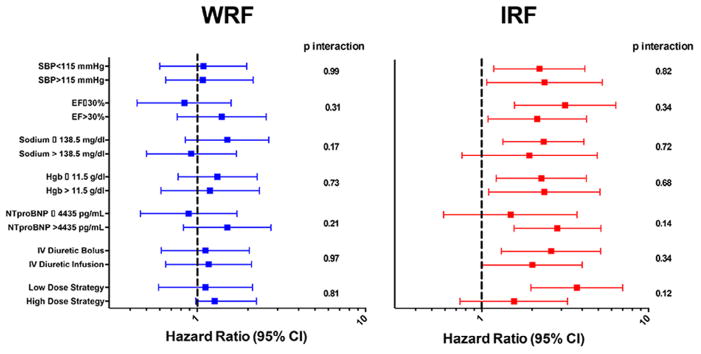
In models comparing WRF and IRF only to patients with Stable RF, WRF continued to be free from significantly increased risk of the composite endpoint, whereas IRF remained strongly associated with increased risk of adverse outcomes (Table 3, Fig. 2). These associations persisted with adjustment for baseline characteristics (including eGFR), and inhospital treatment-related factors (Table 3). Notably, the associations between both IRF (compared with Stable RF) and WRF (compared with Stable RF) and outcomes persisted across numerous subgroups and were not modified by randomized dose-intensification strategy or route of diuretic administration (Fig. 3). These findings were similar between patients with preserved and reduced EF (p-interaction = 0.23).
Table 3.
Risk of Death, Rehospitalization, or Emergency Room Visit with IRF and WRF at 72 H Compared With Patients With Stable RF
| Unadjusted |
Adjusted for Baseline eGFR
|
Adjusted for Baseline
Characteristics |
Adjusted for Baseline and
In-Hospital Characteristics |
|||||
|---|---|---|---|---|---|---|---|---|
| HR 95% CI | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| IRF and WRF defined using a >0.3 absolute change in creatinine | ||||||||
| IRF | 2.52 (1.57–4.03) | <.001* | 2.21 (1.35–3.61) | .002* | 2.21 (1.34–3.68) | .002* | 2.19 (1.32–3.64) | .003* |
| WRF | 1.17 (0.77–1.78) | .47 | 1.18 (0.78–1.79) | .44 | 1.36 (0.85–2.18) | .20 | 1.34 (0.83–2.15) | .23 |
| IRF and WRF defined using a ≥20% change in eGFR | ||||||||
| IRF | 2.47 (1.57–3.92) | < .001* | 2.25 (1.41–3.60) | .001* | 2.01 (1.22–3.33) | .007* | 2.08 (1.27–3.41) | .003* |
| WRF | 1.30 (0.83–2.03) | .26 | 1.45 (0.91–2.31) | .11 | 1.47 (0.88–2.45) | .14 | 1.28 (0.77–2.13) | .34 |
Patients were divided into 3 renal function groups: WRF, IRF, and Stable RF. Hazard ratios are presented for WRF compared with Stable RF and IRF compared with Stable RF.
CI, confidence interval; HR, hazard ratio. Other abbreviations as in Table 1.
Significant P value.
Fig. 2.
Survival plots of the risk of death, rehospitalization, or emergency department visit by stable, worsening, or improvement in renal function. WRF and IRF were defined by >0.3 mg/dL change in serum creatinine (A) and a ≥20% change in eGFR (B). Patients who did not meet criteria for WRF or IRF were classified as having Stable RF. IRF, improvement in renal function; RF, renal function; WRF: worsening renal function.
Fig. 3.
Subgroup analysis of the relationship between WRF and IRF and the risk of death, rehospitalization, or emergency department visit. Subgroups of continuous parameters were dichotomized about the median value. Patients with WRF and IRF were each compared with those patients with Stable RF within each subgroup. WRF and IRF were defined as a >0.3 mg/dL increase or decrease in serum creatinine respectively. P values represent formal tests of interaction for each subgroup. CI, confidence interval; EF, ejection fraction; Hgb, hemoglobin; IRF, improvement in renal function; NTproBNP, N-terminal probrain natriuretic peptide; SBP, systolic blood pressure; WRF, worsening in renal function.
Discussion
The primary finding of the current analysis is that, in the setting of a multicenter trial of decongestive strategies in ADHF, worsening in serum creatinine during the randomized intervention was not associated with an increase in adverse outcomes. This observation was consistent across several different approaches to analyzing change in creatinine, including the trial’s coprimary and secondary renal endpoints. On the contrary, decreases in serum creatinine, when incorrectly assumed to be linearly related to outcomes, were paradoxically associated with a substantially increased risk for adverse events. These observations, from one of the only randomized ADHF trials with protocolized aggressive diuretic dosing and where the intervention actually improved diuresis, suggest the practice of using mild to moderate-sized changes in serum creatinine as an endpoint in ADHF clinical trials may be inappropriate.
The kidney plays a critical role in maintenance of fluid and sodium homeostasis, processes integral to ADHF pathogenesis. As a result, it is intuitive that WRF would both identify high-risk patients and potentially contribute directly to adverse events such as increased length of stay, readmission, and even mortality.21,22 It therefore is not surprising that WRF has been associated with a nearly 2-fold mortality increase in multiple studies.1 These data appropriately motivated many contemporary ADHF trials to include changes in RF as endpoints. In fact, the rationale described in the DOSE trial protocol for selection of change in creatinine as a coprimary endpoint was the “known association of WRF with other adverse outcomes.”2 However, subsequent to the design and initiation of many of these trials, evidence has accumulated that WRF may not universally be associated with adverse outcomes, and WRF that occurs in the setting of otherwise beneficial escalation of HF therapy (aggressive decongestion or initiation of renin-angiotensin-aldosterone system blockade) may have limited prognostic importance.8,9,14 Furthermore, the observation that IRF may signify a prognosis similar to or worse than WRF has been reported in several studies.10–13,15 In light of the recent data regarding changes in RF described previously and the aggressive diuretic dosing in the DOSE trial, the current findings are not necessarily surprising.
In part because of the early reported associations between WRF and decreased survival, preserving RF has now become an important focus of ADHF clinical trials with changes in RF as a primary or secondary endpoint.2–6,23,24 The current findings, in the setting of an ADHF randomized trial, the primary safety endpoint of worsening in creatinine over the intervention period was paradoxically associated with improved outcomes, further questions the adequacy of changes in serum creatinine as a meaningful surrogate endpoint. A requisite for a good surrogate endpoint is that it will reliably and strongly associate with the true outcome of interest.25 For a parameter to be considered a valid surrogate, (1) it must correlate with the outcome (preferably secondary to that parameter being on the causal pathway) and more importantly, (2) the effect of the intervention in a clinical trial on the proposed surrogate should mirror the intervention’s effect on the outcome.26 The fact that HF therapies (neurohormonal antagonists) can increase creatinine yet favorably affect survival argues against changes in creatinine as the primary mediator of a treatment’s effect on outcomes. Furthermore, the assumption that a therapy that decreases creatinine will improve outcomes may not hold true. In the recent BEACON trial (Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease), bardoxolone methyl significantly improved RF in diabetic patients, but the trial was stopped early because of a higher rate of cardiovascular events with bardoxolone.27 This growing literature in conjunction with the current findings from the DOSE trial suggests that we cannot always infer an effect on meaningful outcomes from the change in serum creatinine induced by any given treatment. As such, we may be in danger of dismissing potentially beneficial ADHF therapies by continuing to design therapeutic trials to avoid WRF or induce IRF.
Although conclusions regarding change in creatinine as a surrogate outcome in clinical trials may be relatively straightforward, the implications with respect to routine clinical care are less so. While the surrogate clearly “didn’t work” because decreasing creatinine was paradoxically associated with adverse outcomes, the reason why the outcomes were paradoxical is critically important to managing patients. The outcomes observed in this trial represent the complex interaction between the biology leading to the change in creatinine (ie, aggressive decongestion, worsening/improving in HF status) and how the physician responded to those changes in creatinine. Notably, in the current analysis, WRF was in fact associated with significantly shorter duration of study drug treatment. This earlier discontinuation of therapy could have prevented more severe forms of kidney injury and thus be responsible for the lack of detrimental impact of WRF on outcomes in the DOSE trial. However, given that only 18.8% of patients in the WRF group were considered “congestion free” by their physicians at 72 hours, it is possible that potentially beneficial therapy was prematurely discontinued in part because of small changes in creatinine. Furthermore, it is highly implausible that the IRF itself is directly harmful. Rather, patient and treatment-related factors are likely responsible for the association between IRF and increased adverse events. Patients with IRF presented with more symptoms and signs of congestion and were more likely to receive longer duration of study drug treatment, which in turn may have led to subsequently decreased creatinine with decongestion.10 However, despite a longer duration of therapy than those with WRF, only 7.7% of patients with IRF were “congestion free” at 72 hours, potentially indicative of a greater severity of illness and suboptimal decongestion despite the IRF. It is also possible that physicians, encouraged by the IRF, may have discharged patients prematurely; there was no difference in days from randomization to discharge between groups despite evidence of persistent congestion in those with IRF. Similar findings have been reported in prior studies of IRF.10,11,13,15 Furthermore, it has previously been described that in-hospital IRF appears to be the resolution of outpatient WRF as the majority of these patients have a worsening in creatinine prior to admission. Finally, the IRF experienced in these patients is transient in the majority of cases.10 Unfortunately, it will likely require a randomized trial to truly understand how small to moderate sized changes in creatinine should influence our treatment decisions, if at all.
Limitations
Given the post hoc nature of this study, causality is impossible to demonstrate and residual confounding cannot be excluded. Although WRF was a prespecified secondary endpoint, the DOSE trial was not designed to examine the effects of changes in RF on outcomes. In fact, up or down titration of diuretics at 48 hours and use of additional diuretics were both permitted and common in the DOSE trial. The relatively small number of patients with IRF increases the likelihood that our results may be due to chance; however, given that prior studies of ADHF have demonstrated similar relationships between IRF and adverse outcomes, coupled with the strength of the associations in this analysis despite the small numbers and covariate adjustment, argues against this possibility. Still, subtle yet important differences between RF groups may not have reached statistical significance as a result of the small sample size. Although patients included in the DOSE trial were enrolled within 24 hours of presentation, information on symptom duration prior to presentation was not collected and therefore the potential effect of time from symptom onset to treatment on the observed outcomes across RF groups cannot be determined. Finally, physicians were not blinded to changes in serum creatinine and likely modified treatment in response to these data. As a result, we are unable to determine if WRF was not associated with adverse outcomes because small to moderate increases in creatinine are truly irrelevant during ADHF treatment, or if early physician response to these changes caused the lack of negative association.
Conclusion
The coprimary safety endpoint of the DOSE trial, a linear increase in serum creatinine, was paradoxically associated with improved outcomes. This artifact was driven by an absence of risk attributable to WRF and a strong risk associated with IRF. These results add to the growing literature that changes in serum creatinine may not be a reliable prognostic measure because these changes can be directionally inconsistent with subsequent clinical outcomes. As a result, we should reconsider whether the use of small to moderate sized changes in serum creatinine as clinical trial endpoints is valid. Additionally, these data should further motivate clinicians to critically evaluate changes in RF during the treatment of ADHF in the context of the overall clinical status of the patient until a randomized trial clarifies whether changes in creatinine should influence our diuretic strategies or treatment decisions.
Supplementary Material
Acknowledgments
This manuscript was prepared using Diuretic Optimization Strategies Evaluation (DOSE) research materials obtained from the National Heart, Lung, and Blood Institute (NHLBI) Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the DOSE study investigators or the NHLBI.
Funding sources: This work was supported by the National Institutes of Health, K23HL128933 (MAB), K23HL114868 and L30HL115790 (JT), and K23DK097201 (FPW).
Footnotes
Disclosures
None.
Supplementary data related to this article can be found at doi:10.1016/j.cardfail.2016.06.423.
References
- 1.Damman K, Valente MAE, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35:455–69. doi: 10.1093/eurheartj/eht386. [DOI] [PubMed] [Google Scholar]
- 2.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296–304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. 2013;310:2533–43. doi: 10.1001/jama.2013.282190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massie BM, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, et al. Committees PIa. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med. 2010;363:1419–28. doi: 10.1056/NEJMoa0912613. [DOI] [PubMed] [Google Scholar]
- 6.van Deursen VM, Hernandez AF, Stebbins A, Hasselblad V, Ezekowitz JA, Califf RM, et al. Nesiritide, renal function, and associated outcomes during hospitalization for acute decompensated heart failure: results from ASCEND-HF. Circulation. 2014;130:958–65. doi: 10.1161/CIRCULATIONAHA.113.003046. [DOI] [PubMed] [Google Scholar]
- 7.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–72. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail. 2011;4:685–91. doi: 10.1161/CIRCHEARTFAILURE.111.963256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lesogor A, Cohn JN, Latini R, Tognoni G, Krum H, Massie B, et al. Interaction between baseline and early worsening of renal function and efficacy of renin-angiotensin-aldosterone system blockade in patients with heart failure: insights from the Val-HeFT study. Eur J Heart Fail. 2013;15:1236–44. doi: 10.1093/eurjhf/hft089. [DOI] [PubMed] [Google Scholar]
- 10.Testani JM, McCauley BD, Chen J, Coca SG, Cappola TP, Kimmel SE. Clinical characteristics and outcomes of patients with improvement in renal function during the treatment of decompensated heart failure. J Card Fail. 2011;17:993–1000. doi: 10.1016/j.cardfail.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brisco MA, Coca SG, Chen J, Owens AT, McCauley BD, Kimmel SE, et al. Blood urea nitrogen/creatinine ratio identifies a high-risk but potentially reversible form of renal dysfunction in patients with decompensated heart failure. Circ Heart Fail. 2013;6:233–9. doi: 10.1161/CIRCHEARTFAILURE.112.968230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brisco MA, Kimmel SE, Coca SG, Putt ME, Jessup M, Tang WW, et al. Prevalence and prognostic importance of changes in renal function after mechanical circulatory support. Circ Heart Fail. 2014;7:68–75. doi: 10.1161/CIRCHEARTFAILURE.113.000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Testani J, McCauley B, Kimmel S, Shannon R. Characteristics of patients with improvement or worsening in renal function during treatment of acute decompensated heart failure. Am J Cardiol. 2010;106:1763–9. doi: 10.1016/j.amjcard.2010.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Testani JM, Coca SG, Shannon RP, Kimmel SE, Cappola TP. Influence of renal dysfunction phenotype on mortality in the setting of cardiac dysfunction: analysis of three randomized controlled trials. Eur J Heart Fail. 2011;13:1224–30. doi: 10.1093/eurjhf/hfr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupont M, Mullens W, Finucan M, Taylor DO, Starling RC, Tang WH. Determinants of dynamic changes in serum creatinine in acute decompensated heart failure: the importance of blood pressure reduction during treatment. Eur J Heart Fail. 2013;15:433–40. doi: 10.1093/eurjhf/hfs209. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brater DC, Day B, Burdette A, Anderson S. Bumetanide and furosemide in heart failure. Kidney Int. 1984;26:183–9. doi: 10.1038/ki.1984.153. [DOI] [PubMed] [Google Scholar]
- 18.Vargo DL, Kramer WG, Black PK, Smith WB, Serpas T, Brater DC. Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide and furosemide in patients with congestive heart failure. Clin Pharmacol Ther. 1995;57:601–9. doi: 10.1016/0009-9236(95)90222-8. [DOI] [PubMed] [Google Scholar]
- 19.Brenner B, Rector F. Brenner & Rector’s The Kidney. Philadelphia, PA: Elsevier; 2008. [Google Scholar]
- 20.Mickey R, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–37. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 21.Cowie M, Komajda M, Murray-Thomas T, Underwood J, Ticho B POSH Investigators. Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: results of the prospective outcomes study in heart failure (POSH) Eur Heart J. 2006;27:1216–22. doi: 10.1093/eurheartj/ehi859. [DOI] [PubMed] [Google Scholar]
- 22.Forman D, Butler J, Wang Y, Abraham W, O’Connor C, Gottlieb S, et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–7. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 23.O’Connor C, Starling R, Hernandez A, Armstrong P, Dickstein K, Hasselblad V, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 24.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 25.Temple RJ. A regulatory authority’s opinion about surrogate endpoints. In: Nimmo WS, Tucker GT, editors. Clinical measurement in drug evaluation. New York: John Wiley & Sons Ltd; 1995. [Google Scholar]
- 26.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8:431–40. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 27.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492–503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.