Abstract
Background and Objectives
The prevalence of ADHD is greater in substance use disorders than the general population, and ADHD and substance use disorders share neurobiological features such as dysregulation of reward circuitry. We tested the hypothesis that stimulants would decrease marijuana use in a randomized controlled trial of extended release mixed amphetamine salts (MAS-XR) for treatment of co-occurring ADHD and cocaine use disorders.
Methods
Marijuana users were defined as participants reporting use in the 30 days before study initiation, collected with timeline follow-back. The original 14-week trial utilized a 3-arm randomized design, comparing placebo, MAS-XR 60 mg, and MAS-XR 80 mg. For this analysis, both MAS-XR groups were combined, leaving n = 20 in the placebo group and n = 37 in the MAS-XR group. The primary outcome was proportion of subjects reporting any marijuana use per study week. Comparisons between groups were made using a logistic mixed effects model incorporating multiple predictors and modeling time-by-treatment interactions.
Results
There were no significant baseline differences in marijuana use frequency and quantity. There was a significant decrease in the proportion of participants using marijuana over time in the MAS-XR group, but no difference in the proportion of marijuana-use days over time.
Discussion and Conclusions
Treatment of ADHD and comorbid cocaine use disorders with MAS-XR is associated with increased weekly abstinence from marijuana but not with a decrease in the proportion of marijuana using days per week.
Scientific Significance
Stimulant treatment of ADHD and cocaine use disorders may diminish co-occurring cannabis use.
INTRODUCTION
The prevalence of Attention Deficit/Hyperactivity Disorder (ADHD) in all adults has been estimated between 2.5% and 5.29%.1–3 In those with substance use disorders (SUD), ADHD prevalence has been estimated at 10.8%, and patients with adult ADHD are three times as likely to have any alcohol or drug use disorder compared to the general population.1 ADHD is associated with earlier onset of substance use, increased substance use in adulthood4 and poorer response to treatment.5 Furthermore, increased substance use in adolescents with ADHD has been linked with lower attention scores on neuropsychiatric assessments.6,7 ADHD and SUD may share common pathophysiology, such as dysfunction of the dopaminergic reward system of the midbrain and basal ganglia, and of frontal cortical regions involved in executive functioning and response inhibition.8,9
Given these findings, increased attention has been paid to the consequences of co-morbid ADHD and substance use for clinical treatment including medication development. Though there are no FDA-approved medications for cocaine dependence, agents that reverse dopamine transporter activity and boost dopamine transmission like amphetamines have shown promise, with dextroamphetamine and methamphetamine shown to reduce cocaine use in patients with cocaine dependence alone,10,11 and mixed amphetamine salts shown to reduce cocaine use in co-occurring cocaine dependence and ADHD.12 Dextroamphetamine and mixed amphetamine salts are FDA-approved treatments for ADHD, and are believed to work by boosting dopamine levels in the forebrain. Different drugs of abuse including cocaine and marijuana have been posited to alleviate aspects of ADHD symptomatology, as theorized in the self-medication hypothesis of addictive disorders including cocaine13 and echoed by patient surveys and anecdotal accounts.14,15
Marijuana use and marijuana dependence commonly co-occur with ADHD and SUDs like cocaine dependence.16–19 The psychoactive component of marijuana, delta-9-tetrahydrocannabinol (THC) acts as a partial agonist at CB1 receptors, broadly distributed in the CNS with a variety of generally inhibitory effects on cognitive, affective and attentional systems, and indirectly increases dopamine levels in the nucleus accumbens.20 Childhood hyperactivity symptoms have been associated with earlier initiation of cannabis use, while childhood and current inattentive symptoms are associated more severe cannabis use, craving, and worse cannabis outcomes in young adults.21 Beyond ADHD, cannabis is frequently used with other drugs22 often to either enhance intoxication or manage the side effects of other drugs, which could help to reinforce behaviors with long-term medical and social consequences, though moderate cannabis use has been associated with treatment retention in a prior study of the comorbid ADHD and cocaine dependent population.23
Despite this apparent interrelationship, atomoxetine, an approved non-stimulant pharmacotherapy for ADHD, has shown no impact on marijuana use in a comorbid ADHD and marijuana dependent population.24 In a study of methamphetamine users, however, the stimulant methylphenidate-SR was associated with fewer marijuana-positive urine drug screens in the late phase of the trial compared to placebo,25 arguing that stimulants such as methylphenidate or amphetamines reduce marijuana use in that population. Together, this suggests that in a comorbid ADHD and marijuana-using population stimulants like methylphenidate or amphetamines (but not atomoxetine) could potentially reduce marijuana use as well as ADHD symptoms.
In this study, to begin to examine this question we addressed whether treatment of patients with co-occurring ADHD and cocaine use disorders with extended-release mixed amphetamine salts (MAS-XR) also led to reduction in use of marijuana. We conducted a secondary analysis with data collected from a double-blinded placebo-controlled clinical trial of patients with co-occurring ADHD and cocaine dependence where participants were randomized to receive MAS-XR 60 mg daily, MAS-XR 80 mg daily or placebo with 45.2% of study participants reporting marijuana use, and with the findings of the original trial notable for reduction of ADHD symptoms in both MAS-XR groups compared to placebo, and increased likelihood of weekly and sustained abstinence from cocaine compared to placebo at both doses of MAS-XR. We hypothesized that treatment with MAS-XR would increase abstinence from marijuana over the course of the study as well as decrease the quantity and frequency of marijuana use.
METHODS
Participants and Criteria
The methods utilized for the antecedent clinical trial and primary analysis have been described elsewhere.12 Briefly, patients seeking treatment for cocaine use disorders were enrolled at the Columbia University/New York State Psychiatric Institute (NYSPI) Substance Treatment and Research Service (STARS) or at the Ambulatory Research Center (ARC) at the University Of Minnesota Department Of Psychiatry. Inclusion criteria for the study required participants to be aged 18–60, to meet criteria for adult ADHD and current cocaine dependence as diagnosed by the Conners Adult ADHD Diagnostic Interview for DSM-IV26 and the SCID for DSM-IV,27 respectively, and to be medically and psychiatrically stable. Exclusion criteria included a history of mania, schizophrenia, or any psychosis beyond transient symptoms related to drug use, evidence of abnormal cardiac function or any unstable medical or psychiatric conditions. For this analysis, only those participants who reported marijuana use in the 30 days prior to study enrollment were included.
Procedures
Study Design
The original clinical trial was approved by the Institutional Review Board at both the University of Minnesota as well as at NYSPI. A Data Safety and Monitoring Board met yearly to review significant adverse events, enrollment, and medication tolerability. All participants provided written informed consent. Participants were enrolled in the trial from December 2007 through March 2013, with study completion in June 2013. The study was a three-arm, randomized, double-blinded placebo-controlled parallel group 14-week trial comparing placebo, MAS-XR 60 mg daily, and MAS-XR 80 mg daily which included a placebo lead-in during week 1 for all participants, followed by randomization as shown in Fig. 1.
FIGURE 1.
CONSORT flow diagram of participants through the trial.
Randomization was in fixed blocks of four stratified by baseline cocaine use (measured via quantitative urine testing) during the lead-in week, supervised by statisticians independent from the study group at both sites. Participants unable to tolerate the assigned MAS-XR dose underwent dose reduction based on clinical evaluation and a predetermined schedule. All participants were tapered off the study medication in the final week of the trial. All participants received a standardized weekly Cognitive-Behavioral Therapy/Relapse Prevention Treatment that focused on addressing cocaine use, conducted by experienced therapists.
Study Measures
Self-reported drug use data were obtained via the timeline follow-back method, which has been validated for cannabis and cocaine use.28 This information included reports of all substance use by day for 28 days prior to evaluation and then continuing weekly throughout the study. Quantity of marijuana used was reported by either estimated weight or the number of consumable units used (joints, blunts, bowls etc.) by participant preference. Participants were scheduled for three study visits per week; urine samples to test for cocaine use were collected at each visit, however, marijuana use was assessed solely by TLFB. A once weekly assessment of ADHD symptoms, side effects and adverse events, global clinical status, and medication adherence was also performed. Vital signs, blood work, electrocardiograms, and pregnancy status were monitored regularly throughout the study with cutoff parameters for continued participation.
Outcome Measures
We focus on two major outcomes, both assessed for each subject separately: Weekly abstinence during the trial (marijuana abstinence outcome) and weekly proportion of marijuana use days during the trial (change in marijuana use). Because no differences between the 60 mg and 80 mg doses were expected with respect to the two outcomes in this paper, to simplify our analyses subjects who received MAS-XR medication (60 mg or 80 mg daily) were combined into one MAS-XR group and compared to the placebo arm as seen in Fig. 1. All participant-reported marijuana data were standardized to joints via conversions based on prior measurements of marijuana use practices at STARS, with 1 blunt = 1.52 joints and 1 bowl/pipe = .60 joints.29 Each week after randomization was scored as marijuana positive, negative or missing. A marijuana-abstinent week was defined when all self-reported marijuana use for the week was negative. A marijuana-positive week was defined as at least 1 positive self-report. Weeks with insufficient data to determine use was designated as missing.
Statistical Analysis
Both outcomes were analyzed using longitudinal mixed effects models that allow the existence of missing data; the inference from these models is valid and unbiased as long as any missing outcome data are missing at random.
To compare the weekly proportion of subjects abstinent between groups, a longitudinal logistic mixed effects model was used with predictors including study arm (placebo vs. combined MAS-XR), study week (continuous), study arm by week interaction, baseline marijuana days used, baseline marijuana joints per using day, and treatment center (NYSPI vs. University of Minnesota). The within-subject correlation between weeks was modeled using an autoregressive AR(1) structure, the differences between subjects were modeled using random effects. Significant time by treatment interaction indicates differential slopes over time between treatment groups. To better describe the significant time by treatment interaction, contrasts were tested at each time point between treatment groups.
To examine the proportion of marijuana use days per week between the groups,a longitudinal mixed effects model wasused with predictors including study arm, week, study arm by week interaction, baseline marijuana days used, baseline joints per using day, and center. The within-subject weekly correlations were again modeled using autoregressive AR(1) structure, and subjects were modeled using random effects. A non-significant time-by-treatment interaction was removed from the model and the main effect of treatment and time were tested.
Additionally, baseline marijuana joints per using day and the number of marijuana using days in the past 30 were compared between the groups using T-tests. All analyses were performed using PROC GLIMMIX in SAS 9.3. All statistical tests were 2-tailed and with a significance level of 5%.
RESULTS
Including only participants with marijuana use in the 30 days prior to enrollment resulted in two groups, 20 receiving placebo and 37 receiving MAS-XR. At baseline, there were no significant differences between the groups on the number of marijuana joints per using day (t55 = .95, p = .3450) or in the number of days of marijuana use in the month prior to enrollment (t55 = .93, p = .3578) as shown in Table 1. There were no differences between the groups in age, sex distribution, education level, race or ethnicity. The dropout rate in the placebo group was 30% compared to a dropout rate of 24.3% in the active arm.
TABLE 1.
Demographics (n = 57)
| Placebo
|
MAS-XR
|
|||
|---|---|---|---|---|
| (N = 20) | (N = 37) | |||
| Demographic characteristics | Mean | SD | Mean | SD |
| Age (years) | 36.6 | 5.8 | 39.3 | 8.8 |
| Education (years) | 14.2 | 2.0 | 14.1 | 2.3 |
| n | %* | n | %* | |
| Male | 17 | 85.0 | 28 | 75.7 |
| Race/ethnicity | ||||
| Hispanic | 3 | 15.0 | 3 | 8.1 |
| Black | 3 | 15.0 | 7 | 18.9 |
| White | 13 | 65.0 | 24 | 64.9 |
| Asian | 0 | 0 | 1 | 2.7 |
| NA/AN | 1 | 5.0 | 2 | 5.4 |
| Clinical characteristics | ||||
| Marijuana joints per using day | 2.3 | 1.9 | 1.9 | 1.5 |
| Marijuana days used (past month) | 14.5 | 11.3 | 11.6 | 11.3 |
Percentages may not add up to 100 due to rounding.
Subject-Wise Weekly Abstinence During Trial
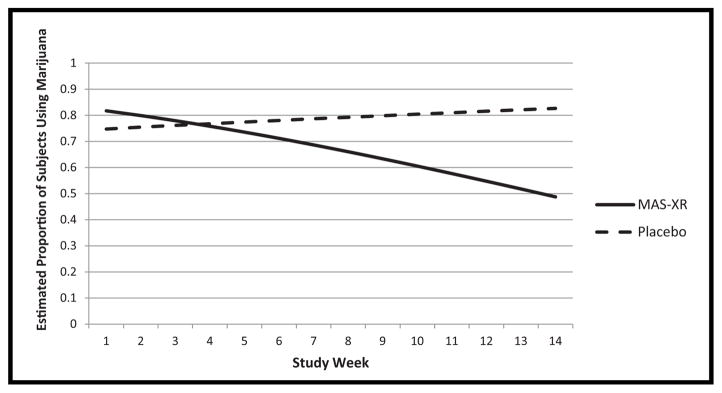
Analysis of the proportion of subjects using marijuana per week revealed significant interaction between study arm and week (F1,658 = 5.39, p = .0206), indicating significant differential slopes between treatment groups as seen in Fig. 2. The different trends of proportion of subjects using marijuana per week for each treatment group resulted in a significant contrast difference at study week 14 (OR = 4.99, t658 = 2.00, p = .0454): the odds of smoking marijuana in week 14 for subjects in the placebo group were almost five times the odds of those in the MAS-XR group (OR = 4.99). The proportion of subjects using marijuana per week decreased significantly over the duration of the study for the MAS-XR group (t658 = −3.72, p = .0002) and no such significant decreasing trend was found for the placebo group. The proportion of subjects using marijuana per week was not significantly different between sites (F1,658 = .65, p = .4210). Baseline days of marijuana use were significantly related to weekly marijuana use by subjects (F1,658 = 16.10, p <.0001) indicating that subjects with higher number of days of marijuana use at baseline are more likely to use marijuana during later study weeks. Number of joints per using day at baseline was not significantly related to weekly marijuana use by subjects (F1,658 = 2.46, p = .1173).
FIGURE 2.
Estimated proportion of subjects using marijuana by study week.
Weekly Proportion of Marijuana Use Days During Trial
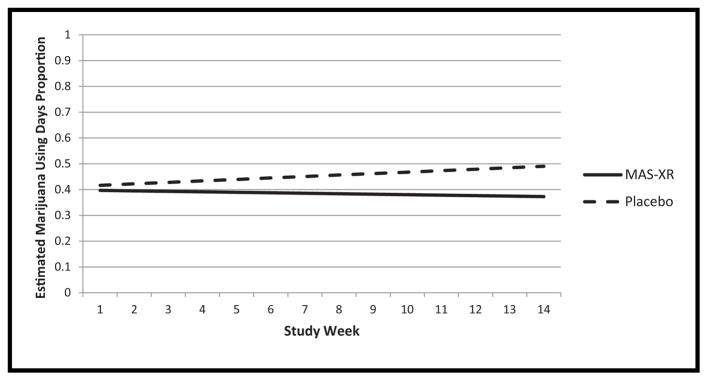
Analysis of proportion of marijuana use days per week suggested that there were no significant differences between slopes for each group over time (see Fig. 3), thus the interaction between study arm and week was not significant (F1,658 = .62, p = .4326) and was omitted from the final model. Additionally, there was no significant difference between the MAS-XR group and the placebo group overall (F1,659 = 1.07, p = .3004), over time (F1,659 = .02, p = .8809) nor across sites (F1,659 = .17, p = .6793). Weekly proportion of marijuana use days were significantly related to baseline days of marijuana use (F1,659 = 42.02, p <.0001) and number of joints per using day (F1,659 = 8.02, p = .0048) with a higher number of days of marijuana use and a higher number of joints per using day at baseline significantly related to a higher weekly proportion of marijuana use days during the trial.
FIGURE 3.
Estimated proportion of days using marijuana by study week.
DISCUSSION
Our results confirmed our hypothesis that treatment with extended-release mixed amphetamine salts (MAS-XR) is associated with increased abstinence from marijuana in a predominantly male population with comorbid ADHD and cocaine use disorders, in contrast to prior studies of this population with non-stimulant pharmacotherapies for ADHD.24 There was no difference between the groups, however, in the weekly proportion of marijuana use days throughout the study. The finding of increased abstinence from marijuana by study week over time in the MAS-XR group suggests that in this dual use population stimulants may promote clinically significant durations of marijuana abstinence, increasing over time with exposure to the medication. Use of stimulants like MAS-XR in this comorbid population could help to extinguish marijuana use, which could be of significant clinical importance, as the importance of comorbid substance use in perpetuating other drug use has been documented in the literature, mostly focusing on tobacco and cannabis30 and tobacco and other drug use disorders.31,32
Whether this effect is mediated by treatment of ADHD symptoms by the study medication, treatment of cocaine dependence, dopamine-enhancing effects of stimulants reducing reinforcing effects of marijuana, or some other factor is unclear, however, and ultimately beyond the scope of this analysis due to power analysis limitations. Baseline differences in the amount (joints/day) of marijuana had no significant impact on this association; however, the number of days of marijuana use at baseline did, implying that frequent users were more likely to continue use by week throughout the trial. Though marijuana abstinence by week was increased during the study, weekly proportion of marijuana use days in the MAS-XR group did not decrease and did not significantly differ from placebo group. Frequent heavier marijuana users may be more likely to experience withdrawal symptoms with reduction in use or abstinence, which is associated with relapse risk33 an effect consistent with results for other substances of abuse such as alcohol.34
There are other possible explanations for this effect, however; first, to the extent that concurrent marijuana use might enhance cocaine use or vice versa, reduction of cocaine use via a medication intervention could also reduce use of marijuana. Second, decreasing cocaine use, the result of the primary study, could be associated with decreased marijuana use due to less need to reduce acute cocaine intoxication or withdrawal effects like agitation, irritability or insomnia with marijuana rather than any impact of the stimulant on cognitive or executive control deficits. That those unpleasant symptoms may be mediated by decreased dopamine transmission after the initiation of cocaine abstinence may help explain why participants not seeking treatment for marijuana use and using less cocaine on aggregate, but now on a dopamine-boosting medication like MAS-XR, would also reduce their use of another dopamine-enhancing substance given the vulnerability to drug craving after onset of abstinence.35
Lending credence to this idea is the finding that bupropion, another dopamine enhancer, has been associated with reduced use of both cocaine and heroin in a methadone-dependent cocaine-using population in conjunction with contingency management,36 suggesting a broad impact on substance use from altering dopamine transmission beyond “like for like” or agonist substitution. Regardless of the precise mechanism of action of our intervention, our findings suggest the importance of thorough assessment of ADHD and co-morbid substance use disorders, as stimulant treatment may help patients with comorbid cocaine dependence and ADHD moderate or extinguish marijuana use that could contribute to an overall lower level of functioning.
There are several limitations to our findings. As a secondary analysis, our study is not primarily powered to assess marijuana outcomes in this relatively small sample. As the primary study was focused on cocaine dependence and ADHD, there was no minimum baseline of marijuana use or severity among participants, insufficient statistical power to stratify by the quantity of marijuana used by participants and a lack of data on which marijuana-using participants categorically met DSM-IV criteria for cannabis dependence. In addition, because the original analysis focused on cocaine outcomes, urine cannabinoids were not available for analysis and though self-reported marijuana use has been validated as an outcome measure in past studies28 this remains a limitation. Due to the complexity of the study’s longitudinal design and the sample size, mediation analyses to clarify the impact of changes in ADHD symptoms or cocaine use with stimulant treatment were not performed. The male predominance in this sample population also reduces the generalizability of our results. Further research is warranted to duplicate this finding in participants with more homogenous marijuana use patterns and a more balanced gender representation and to explore whether any changes in marijuana use are mediated by changes in ADHD symptomatology, cocaine use or measures of dopamine signaling in the CNS.
CONCLUSIONS
Treatment of ADHD and comorbid cocaine use disorders with extended release mixed amphetamine salts is associated with increased abstinence from marijuana in those reporting baseline marijuana use.
Acknowledgments
Work on this presentation was supported by the National Institute on Drug Abuse (NIDA; Rockville, MD) grants DA023652 (Dr. Frances Levin) and DA023651 (Dr. John Grabowski). Dr. Levin is also supported by NIDA K24 DA029647, and Dr. Notzon is supported by NIDA T32 DA007294-22.
We would like to acknowledge C. Jean Choi of the Columbia University Mailman School of Public Health for her contributions, as well as the staff of the Substance Treatment and Research Service (STARS) at Columbia University and the New York State Psychiatric Institute.
Footnotes
Declaration of Interest
Dr. Notzon, Dr. Mariani, Dr. Pavlicova, Dr. Grabowski, Andrew Glass, Amy Mahony, and Daniel Brooks have no disclosures. Dr. Levin currently receives medication from US WorldMed for an ongoing study that is sponsored by the National Institute on Drug Abuse and served as a consultant to GW Pharmaceuticals, Eli Lily and served on an advisory board to Shire in 2005–2007.
References
- 1.Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: Results from the national comorbidity survey replication. Am J Psychiatry. 2006;163:716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 3.Simon V, Czobor P, Balintn S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: Meta-analysis. Br J Psychiatry. 2009;194:204–211. doi: 10.1192/bjp.bp.107.048827. [DOI] [PubMed] [Google Scholar]
- 4.Wilens TE, Biederman J, Mick E, et al. Attention deficit hyperactivity disorder (ADHD) is associated with early onset substance use disorders. J Nerv Ment Dis. 1997;185:475–482. doi: 10.1097/00005053-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Levin FR, Evans SM, Vosburg SK, et al. Impact of attention-deficit hyperactivity disorder and other psychopathology on treatment retention among cocaine abusers in a therapeutic community. Addict Behav. 2004;29:1875–1882. doi: 10.1016/j.addbeh.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 6.Tercyak KP, Lerman C, Audrain J. Association of attention-deficit/hyperactivity disorder symptoms with levels of cigarette smoking in a community sample of adolescents. J Am Acad Child Adolesc Psychiatry. 2002;41:799–805. doi: 10.1097/00004583-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Tapert SF, Baratta MV, Abrantes AM, et al. Attention dysfunction predicts substance involvement in community youths. J Am Acad Child Adolesc Psychiatry. 2002;41:680–686. doi: 10.1097/00004583-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tripp G, Wickens JR. Neurobiology of ADHD. Neuropharmacology. 2009;57:579–589. doi: 10.1016/j.neuropharm.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 10.Grabowski J, Rhoades H, Schmitz J, et al. Dextroamphetamine for cocaine-dependence treatment: A double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Mooney ME, Herin DV, Schmitz JM, et al. Effects of oral methamphetamine on cocaine use: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2009;101:34–41. doi: 10.1016/j.drugalcdep.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levin FR, Mariani JJ, Specker S, et al. Extended-Release mixed amphetamine salts vs placebo for comorbid adult attention-Deficit/Hyperactivity disorder and cocaine use disorder: A randomized clinical trial. JAMA Psychiatry. 2015;72:593–602. doi: 10.1001/jamapsychiatry.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khantzian EJ. Addiction as a self-regulation disorder and the role of self-medication. Addiction. 2013;108:668–669. doi: 10.1111/add.12004. [DOI] [PubMed] [Google Scholar]
- 14.Wilens TE, Adamson J, Sgambati S, et al. Do individuals with ADHD self-medicate with cigarettes and substances of abuse? Results from a controlled family study of ADHD. Am J Addict. 2007;16:14–21. doi: 10.1080/10550490601082742. quiz 22-13. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell JT, Sweitzer MM, Tunno AM, McClernon FJ, et al. “I Use Weed for My ADHD“: A qualitative analysis of online forum discussions on cannabis use and ADHD. PLoS ONE. 2016;11:e0156614. doi: 10.1371/journal.pone.0156614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilens TE. Attention deficit hyperactivity disorder and substance use disorders. Am J Psychiatry. 2006;163:2059–2063. doi: 10.1176/ajp.2006.163.12.2059. [DOI] [PubMed] [Google Scholar]
- 17.Murphy KR, Barkley RA, Bush T. Young adults with attention deficit hyperactivity disorder: Subtype differences in comorbidity, educational, and clinical history. J Nerv Ment Dis. 2002;190:147–157. doi: 10.1097/00005053-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Ameringer KJ, Leventhal AM. Associations between attention deficit hyperactivity disorder symptom domains and DSM-IV lifetime substance dependence. Am J Addict. 2013;22:23–32. doi: 10.1111/j.1521-0391.2013.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daigre C, Roncero C, Grau-Lopez L, et al. Attention deficit hyperactivity disorder in cocaine-dependent adults: A psychiatric comorbidity analysis. Am J Addict. 2013;22:466–473. doi: 10.1111/j.1521-0391.2013.12047.x. [DOI] [PubMed] [Google Scholar]
- 20.Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu rev psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 21.Bidwell LC, Henry EA, Willcutt EG, et al. Childhood and current ADHD symptom dimensions are associated with more severe cannabis outcomes in college students. Drug Alcohol Depend. 2014;135:88–94. doi: 10.1016/j.drugalcdep.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quek LH, Chan GC, White A, et al. Concurrent and simultaneous polydrug use: Latent class analysis of an Australian nationally representative sample of young adults. Front Public Health. 2013;1:61. doi: 10.3389/fpubh.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aharonovich E, Garawi F, Bisaga A, et al. Concurrent cannabis use during treatment for comorbid ADHD and cocaine dependence: Effects on outcome. Am J Drug Alcohol Abuse. 2006;32:629–635. doi: 10.1080/00952990600919005. [DOI] [PubMed] [Google Scholar]
- 24.McRae-Clark AL, Carter RE, Killeen TK, et al. A placebo-controlled trial of atomoxetine in marijuana-dependent individuals with attention deficit hyperactivity disorder. Am J Addict. 2010;19:481–489. doi: 10.1111/j.1521-0391.2010.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ling W, Chang L, Hillhouse M, et al. Sustained-release methylphenidate in a randomized trial of treatment of methamphetamine use disorder. Addiction. 2014;109:1489–1500. doi: 10.1111/add.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epstein JN, Johnson DE, Conners CK. Conners’ Adult ADHD Diagnostic Interview for DSM-IV (CAADID) North Tonawanda, NY: Multi-Health Systems; 2001. [Google Scholar]
- 27.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-I/P), Version 2.0. New York: Biometrics Research, New York State Psychiatric Institute; 1995. [Google Scholar]
- 28.Robinson SM, Sobell LC, Sobell MB, et al. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav. 2014;28:154–162. doi: 10.1037/a0030992. [DOI] [PubMed] [Google Scholar]
- 29.Mariani JJ, Brooks D, Haney M, et al. Quantification and comparison of marijuana smoking practices: Blunts, joints, and pipes. Drug Alcohol Depend. 2011;113:249–251. doi: 10.1016/j.drugalcdep.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agrawal A, Budney AJ, Lynskey MT. The co-occurring use and misuse of cannabis and tobacco: A review. Addiction. 2012;107:1221–1233. doi: 10.1111/j.1360-0443.2012.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein MD, Caviness CM, Kurth ME, et al. Varenicline for smoking cessation among methadone-maintained smokers: A randomized clinical trial. Drug Alcohol Depend. 2013;133:486–493. doi: 10.1016/j.drugalcdep.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winhusen TM, Kropp F, Theobald J, et al. Achieving smoking abstinence is associated with decreased cocaine use in cocaine-dependent patients receiving smoking-cessation treatment. Drug Alcohol Depend. 2014;134:391–395. doi: 10.1016/j.drugalcdep.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haney M, Cooper ZD, Bedi G, et al. Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacology. 2013;38:1557–1565. doi: 10.1038/npp.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moos RH, Moos BS. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction. 2006;101:212–222. doi: 10.1111/j.1360-0443.2006.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pickens CL, Airavaara M, Theberge F, et al. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poling J, Oliveto A, Petry N, et al. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch Gen Psychiatry. 2006;63:219–228. doi: 10.1001/archpsyc.63.2.219. [DOI] [PubMed] [Google Scholar]