Abstract
Saccharomyces cerevisiae responds to changes in extracellular inorganic phosphate (Pi) availability by regulating the activity of the phosphate-responsive (PHO) signaling pathway, enabling cells to maintain intracellular levels of the essential nutrient Pi. Pi-limitation induces upregulation of inositol heptakisphosphate (IP7) synthesized by the inositol hexakisphosphate kinase Vip1, triggering inhibition of the Pho80/Pho85 cyclin-cyclin dependent kinase (CDK) complex by the CDK inhibitor Pho81, which upregulates the PHO regulon through the CDK target and transcription factor Pho4. To identify genes that are involved in signaling upstream of the Pho80/Pho85/Pho81 complex and how they interact with each other to regulate the PHO pathway, we performed genome-wide screens with the synthetic genetic array method. We identified more than 300 mutants with defects in signaling upstream of the Pho80/Pho85/Pho81 complex, including AAH1, which encodes an adenine deaminase that negatively regulates the PHO pathway in a Vip1-dependent manner. Furthermore, we showed that even in the absence of VIP1, the PHO pathway can be activated under prolonged periods of Pi starvation, suggesting complexity in the mechanisms by which the PHO pathway is regulated.
Introduction
In the face of dynamic and unpredictable fluctuations in nutrient availability, microorganisms achieve cellular nutrient homeostasis through the action of nutrient responsive signaling pathways [1]. Pi is an essential nutrient required for synthesis of ATP and cellular constituents such as phospholipids. Saccharomyces cerevisiae (budding yeast) responds to changes in extracellular Pi availability by regulating the activity of the PHO pathway. Cells repress the activity of the PHO pathway under high Pi conditions, whereas the PHO pathway is activated and induces expression of the PHO regulon under low Pi conditions, presumably to rectify a transient decrease in Pi concentration in vivo [2, 3]. For example, cells increase the rate of Pi uptake from the environment under low Pi conditions by upregulating expression of the acid phosphatase Pho5 [2, 4] and the high-affinity Pi transporter Pho84 [5].
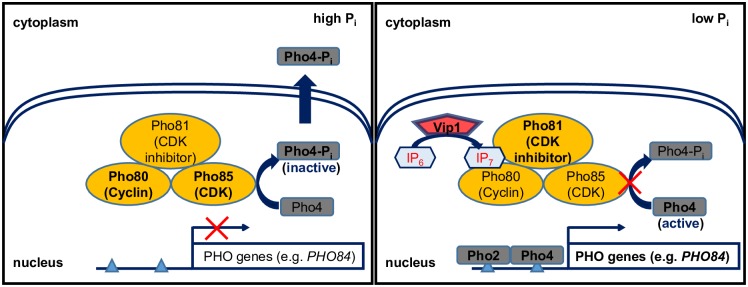
The core regulatory complex of the PHO pathway consists of the cyclin Pho80, cyclin-dependent kinase (CDK) Pho85 and CDK inhibitor Pho81 [6–8] (Fig 1). Under high Pi conditions, the Pho81 inhibitor is not active and the Pho80/Pho85 complex phosphorylates the transcription factor Pho4, causing its export from the nucleus [9–12]. Under low Pi conditions, (1/3)–diphosphoinositol pentakisphosphate ((1/3)-PP-IP5; referred to as IP7) is produced by Vip1 and binds to Pho81, leading to inhibition of Pho80/Pho85 complex kinase activity [13, 14], dephosphorylation and nuclear localization of Pho4, and transcriptional activation of the PHO regulon, including PHO5 and PHO84 [15].
Fig 1. Transcriptional regulation of the PHO regulon in high and low Pi conditions.
Compared to the well-elucidated pathway downstream of the Pho80/Pho85/Pho81 complex, little is known about upstream signaling processes. We do not understand how Pi availability is sensed and how information about Pi availability is transmitted to enzymes that regulate IP7 levels. Only three genes have been implicated in upstream signaling: genes encoding the adenosine kinase Ado1, the adenylate kinase Adk1, and the PP-IP5 kinase Vip1 [14, 16]. However, we do not understand how these enzymes are regulated under different Pi conditions or how they interact with each other to regulate the PHO pathway. Furthermore, other players beyond these three enzymes remain unknown.
To identify genes involved in signaling process upstream of the Pho80/Pho85/Pho81 complex, a previous study performed a high-throughput and quantitative screen of the yeast deletion library, consisting of 4848 haploid strains deleted for non-essential genes, searching for novel mutants defective in PHO5 expression [16]. Of the 90 most statistically significant candidates identified in the screen, 19 mutants were defective in PHO5 expression in a PHO-pathway specific manner, with ado1Δ and adk1Δ being the only mutants defective in signaling processes upstream of the Pho80/Pho85/Pho81 complex. VIP1, another upstream signaling factor [14], was not identified, suggesting that the screen was not comprehensive and more genes are left to be identified.
To perform genome-wide genetic screening of the PHO pathway, we adopted the synthetic genetic array (SGA) method. Originally, this methodology was developed for systematic construction of double mutants to create a global genetic interaction map [17–20]. The SGA method allows us to query large numbers of mutants. Additionally, by introducing a fluorescent reporter into each mutant in the library, we can improve the sensitivity and quantitative nature of the PHO pathway readout. Finally, we can investigate functional relationships between two genes from double mutant analysis, which compares the phenotype of a double mutant with that of its single mutants and estimates the extent to which deletion of one gene affects the phenotype of another deletion [17, 20].
Employing the SGA method, we identified more than 300 mutants defective in upstream signaling of the PHO pathway. In particular, we found that deletion of AAH1, whose product is involved in adenine nucleotide metabolism, de-represses the PHO pathway under high Pi conditions. We also characterized functional relationships between mutants such as aah1Δ and others defective in upstream signaling of the PHO pathway and showed that aah1Δ requires Vip1 for constitutive activation of the PHO pathway.
Materials and methods
Strains
All strains for screening used in this study are in the BY4741 background. A yeast library was obtained from the Weissman lab at UCSF consisting of 4974 knockout alleles of non-essential genes and 878 hypomorphic alleles of essential genes [17, 21]. All strains in the library are MATa haploids.
The PHO84 reporter strain was generated from yMJ003 (MATα his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 LYS+ can1Δ::STE2pr-spHIS5 lyp1Δ::STE3pr-LEU2 cyh2 ura3Δ::UPRE-GFP-TEF2pr-RFP-MET15-URA3) [17]. UPRE-GFP sequence in yMJ003 was replaced with PHO84 promoter sequence taken from -1000 to -1 base pairs from the ATG of the PHO84 open reading frame followed by Venus fluorescence protein sequences from pKT0090 [22]. To reduce PHO80 mRNA stability, the DAmP (Decreased Abundance by mRNA Perturbation) pho80 strain, pho80DΔ [23], was generated by inserting a nourseothricin marker (NatR) obtained from pFA6a-natMX4 [24] right after the stop codon of PHO80.
Insertion of the PHO84 reporter into each strain in the yeast library and generation of double mutants
The SGA method [17, 19] was applied to introduce the PHO84 reporter into each strain in the yeast library; the protocol for this process was the same as described in [17]. The PHO84 reporter strain was crossed to each of 5,852 strains in the library harboring G418 marker (KanR) in parallel with replicate-pinning tools (V&P Scientific, INC). After crossing, diploids carrying both the PHO84 reporter and the mutations (knockouts or hypomorphic alleles) were selected on SD—ura + G418 plates and put on sporulation plates. After sporulation, final MATα haploids carrying both the PHO84 reporter and KanR marker for the mutations were selected on SD—ura + G418 –leu—arg—lys + canavanine (Sigma-aldrich) + S-(2-Aminoethyl)-L-cysteine (Sigma-aldrich) + mono sodium glutamate (Sigma-aldrich) plates.
Double mutants were generated by crossing chosen MATα haploids carrying both the PHO84 reporter and KanR marker to other MATa haploids of interest whose knockout marker was NatR [24]. The selection process was identical to the case where the PHO84 reporter was inserted into the yeast library except that both nourseothricin (ClonNAT, Werner BioAgents) and G418 were used to simultaneously select for the NatR and KanR markers.
PHO84 expression level measurements from mutants
Cells were grown in synthetic complete (SC) medium at 30°C in a 384 well-plate on a plate shaker. SC medium with different Pi concentrations was prepared as described in [25]. Strains were inoculated from final haploid selection plates into 80 μl of 10 mM Pi SC medium and were grown overnight. To reduce Pi spillover from the overnight cultures, 5 μl of overnight cell culture was first inoculated into 75 μl of no Pi medium using a BioMek FX liquid handling robot (Beckman Coulter, Inc.,Fullerton, CA, USA). Subsequently, 5 μl of the inoculated cultures in no Pi medium were inoculated again into 75 μl of 50 μM and 1 mM Pi medium and were grown for 8 hours to measure the PHO84 expression levels of each strain. 50 μM Pi is near the maximum Pi concentration that leads to activation of the PHO pathway and 1 mM Pi is near the minimum Pi concentration to repress the PHO pathway. Note that the bimodality in PHO84 expression in the wild type strain arises in the conditions ~ 150 to 250 μM Pi (intermediate Pi); bimodality results in an off-population that expresses little PHO84 and an on-population that highly expresses PHO84 [26]. The intermediate Pi concentration regime was avoided because the relative ratio of the off- to on-population was too sensitive to small changes in Pi concentration to get reliable readouts from the measurements.
The PHO84 expression level of each strain was measured three times with a flow cytometer. The cell cultures were transferred to a Becton Dickinson High Throughput Sampler (BD, Franklin Lakes, NJ USA), which directly injected cells from the wells of the plate into the LSR Fortessa flow cytometer (BD). It took about 110 minutes to measure one 384-well plate and 2,000–6,000 cells/well were measured. Samples with low cell counts (<250/well) were disregarded. Venus was excited at 488nm and its fluorescence was collected through a 505 nm long-pass filter and a HQ 515/20 band-pass filter (YFP channel). mCherry was excited at 532 nm and fluorescence was collected through a 600nm long-pass filter and a 610/20 band-pass filter (RFP channel).
Extraction of the mean PHO84 reporter level of each strain from flow cytometry data
A customized MATLAB code was written to calculate the mean PHO84 reporter level of each strain from flow cytometry data. To import raw “.fcs” files obtained from the flow cytometer to our customized code, a function to read FCS 3.0 format written by Laszlo Balkay was used (available at the community File Exchange section at http://www.mathworks.com/matlabcentral). To adjust for non-Pi-specific perturbation of single-cell PHO84 expression levels (e.g. due to the different cell-cycle stages of cells), the signal in the YFP channel (PHO84 signal) was normalized by the signal in the RFP channel (TEF2 signal) for every cell. The distribution of log2(YFP/RFP) was obtained over all cells in the population and 5% of cells at either end of the distribution were removed to eliminate outliers. Then, the log2(YFP/RFP) of the remaining 90% of the cells was averaged to represent the PHO84 reporter level of each strain. Note that the mean value, not the median, of the cell population was calculated for the PHO84 reporter level of the sample. The distributions of the PHO84 signals in the YFP channel of some mutants were bimodal such that the median of those mutants was very sensitive to the relative ratio of the on- and off-populations and could differ greatly even if the ratio changed slightly due to measurement noise. All of the extracted mean PHO84 reporter levels measured in this study were reported in S1 Table.
Normalization of the mean PHO84 reporter levels of each strain
The mean PHO84 reporter level of each strain extracted from flow cytometry was normalized as described in [17]. In the genome-wide single mutant screen in 50 μM and 1mM Pi conditions, the mean PHO84 reporter levels of the single mutants were normalized by the median of the mean PHO84 reporter levels of all samples on the same plate. This normalization process is based on the premise that the number of genes expected to be involved in regulation of the PHO pathway is much smaller than the number of the strains in the library. In the epistasis analysis with pho80DΔ and pho81Δ, the mean PHO84 reporter levels of each double mutant were normalized by the value of pho80DΔ and pho81Δ, respectively. For the double mutant analysis with ado1Δ and aah1Δ, the wild type was included in 6 wells in the plate. The mean PHO84 reporter levels of the 6 wild type samples were averaged and the resulting value was used to normalize the mean PHO84 reporter levels of the double mutants.
Calculation of p-values for measurement errors
P-values for measurement errors in the genome-wide screen were calculated as described in [17]. The distribution of measurement errors was defined as (1-c1)*norm(0,σ1) + c1*norm(0,σ2) where c1 is the coefficient and norm(0,σ) is a Gaussian distribution with standard deviation σ and mean zero. C1, σ1, and σ2 were obtained using an iterative nonlinear fit to actual distribution of the difference between three replicate measurements of reporter levels in the library. The calculated distribution of measurement errors was used to generate the expected distribution of measured values for a strain with wild type PHO84 reporter levels [17]. Using this distribution, a p-value was calculated as a function of a measured reporter level (L) and number of measurements (N), an estimate of the probability of observing a reporter level equal to or more extreme than L upon averaging of N independent measurements of the wild type strain [17]. Strains with p-values <10−3 were designated as those showing PHO84 expression levels different from the wild type in 50 μM or 1mM Pi conditions.
Extraction of ATP, ADP, and AMP
The concentrations of ATP, ADP, and AMP were measured as described in [27]. Overnight cultures with O.D. 600 < 0.3 were diluted into 100 ml of fresh 10 mM Pi liquid medium and grown for at least 12 hours before harvest. The final O.D. 600 was ~ 0.2. 20 ml of this cell culture was used to extract metabolites from the wild type, adk1Δ, aah1Δ, and ado1Δ mutants grown in 10 mM Pi. For the time course measurements of the wild type in no Pi, the rest of the cell culture was transferred onto a vacuum filtration apparatus (Millipore nitrocellulose membrane with 0.8 μm pore size, Cat No. AAWG025000), washed with 30 ml of no Pi medium and resuspended into fresh no Pi medium. The resuspended cell culture was inoculated into fresh 20 mL of no Pi medium such that the final O.D. 600 at each time point (5, 15, 30, 60, and 90 minutes) was 0.2. For harvesting, cell culture was filtered onto a 50 mm nylon membrane filter, which was immediately transferred into -20°C extraction solvent (40:40:20 acetonitrile/methanol/water).
Cell extracts were analyzed by reversed phase ion-pairing liquid chromatography (LC) coupled with electrospray ionization (ESI) (negative mode) in a high-resolution, high-accuracy mass spectrometer (MS) (Exactive; Thermo Fisher Scientific) [28]. It was operated in full scan mode at 1 s scan time, 105 resolution, with compound identities verified by mass and retention time match to authenticated standard [28].
Calculation of the relative concentrations of ATP, ADP, and AMP
To convert raw LC-MS/MS ion counts to relative cellular concentrations, ion counts were first normalized by the cell density [29]. The normalized ion counts were converted to relative concentration by dividing the value for the samples by the corresponding value of the reference data (S2 Table) [29]. For the time course measurements in no Pi, data for each time point were divided by the corresponding values at time 0. For the measurements of adk1Δ, aah1Δ, and ado1Δ mutants, the values of the mutants were divided by the values of the wild type strain grown in 10 mM Pi.
Determination of array strains for double mutant analysis with ado1Δ and aah1Δ
320 array strains (293 less induced hits and 27 less repressed hits) exhibiting at least a 2-fold change in PHO84 expression levels compared to the wild type were selected for double mutant analysis. In addition, other mutants functionally related to one of the 320 array strains were included in the analysis, even though their PHO84 expression levels did not satisfy the conditions to be selected for the genome-wide single mutant screen. For example, ipk1Δ was included as an array strain as Ipk1 produces IP6 –a precursor for IP7 [30]–even though the PHO84 expression level of ipk1Δ is similar to that of wild type in 50 μM Pi (S3 Table). All strains measured as double mutants with ado1Δ and aah1Δ were listed in S4 Table.
Results
Design of screen to identify genes acting upstream of the Pho80/Pho85/Pho81 complex
We carried out two steps of systematic screening to identify genes involved in upstream PHO pathway signaling. First, we identified mutants defective in regulation of PHO84 expression in a yeast library containing deletion mutants of non-essential genes and hypomorphic alleles of essential genes. We chose PHO84 expression as a reporter instead of PHO5 because it is a more sensitive readout of pathway activity; since PHO5 expression requires more severe Pi-limited conditions than does PHO84 expression [25], mutants defective in PHO5 expression are a subset of those defective in PHO84 expression. We looked for mutants expressing less PHO84 than the wild type in low Pi (less induced hit) and mutants expressing more PHO84 than the wild type in high Pi conditions (less repressed hit). Second, to identify the subset of mutants defective in signaling upstream of the Pho80/Pho85/Pho81 complex, we performed epistasis analysis, which can determine the order of action between genes.
Genome-wide single mutant screening for mutants with altered PHO84 expression
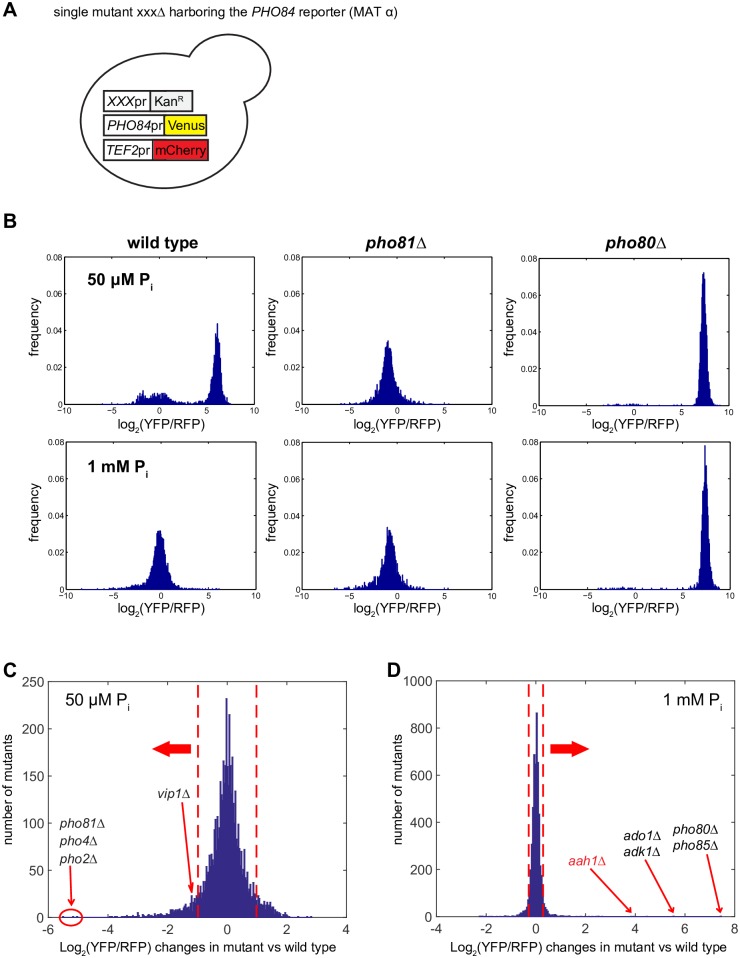
To measure the PHO84 expression level of each mutant in the library, we first constructed a reporter in which the PHO84 promoter drives expression of the yellow fluorescence protein (YFP) Venus (Fig 2A). To correct for Pi-independent expression changes, we co-expressed the red fluorescence protein (RFP) mCherry driven by the TEF2 constitutive promoter (Materials and methods), and used the log2 intensity ratio of YFP/RFP as a proxy for the activity of the PHO pathway (hereafter referred to as the PHO84 reporter level). We inserted the reporters into each mutant in the library using the SGA method (Fig 2A and Materials and methods) [17], obtained haploid strains harboring the reporters and each mutation, and then measured fluorescence from those haploid strains using flow cytometry (Materials and methods). To identify mutants with subtle defects such as different Pi activation/repression thresholds, as well as mutants with severe defects such as complete repression or constitutive derepression of the PHO pathway, we screened cells grown in 50 μM and 1 mM Pi, which are near the Pi threshold concentrations to turn on and off the PHO pathway, respectively. We validated our screening design by verifying that we could reproduce the phenotypes of mutants known to impair the activity of the PHO pathway such as pho80Δ and pho81Δ (Fig 2B–2D). Of the 19 mutants identified previously [16], 12 were detected with a reliable number of cells at least twice in this study and the PHO phenotypes of all 12 mutants were recapitulated.
Fig 2. Identification of mutants with altered PHO84 expression in low and high Pi conditions.
(A) Generation of single mutants harboring the PHO84 reporter with the SGA method. The PHO84 reporter consists of PHO84 promoter-driven Venus and TEF2 promoter-driven mCherry. Each single mutant in the library denoted by xxxΔ is kanamycin (G418)-resistant. (B) The distributions of the PHO84 reporter levels in single cells in the pho81Δ and pho80Δ strains. Log2 intensity ratio of Venus to mCherry (log2(YFP/RFP)) was used to quantify the PHO84 expression level. (C, D) The PHO84 reporter levels of single mutants in the library measured in 50 μM Pi and 1 mM Pi conditions. The PHO84 reporter level of each mutant was normalized to that of the wild type value in each Pi concentration (Materials and methods). Red dashed lines in (C) and (D) indicate the PHO84 reporter levels with p-values less than 0.001 estimating the maximum range of the PHO84 reporter levels that the wild type exhibits in each Pi concentration. The mutants in black are previously identified mutants and the one in red is identified in this study.
Calculating p-values of measurement errors, we estimated the maximum range of the PHO84 reporter levels (-0.98 and 0.29; red dashed lines in Fig 2C and 2D) that the wild type exhibits in low Pi (50 μM) and high Pi (1 mM) (Materials and methods) and used these as thresholds to identify mutants (“hits”) expressing PHO84 at levels different from those of the wild type. We identified 380 less induced hits from low Pi which exhibited the PHO84 reporter levels less than -0.98, and 243 less repressed hits from high Pi which exhibited the PHO84 reporter levels more than 0.29 (S3 Table).
Epistasis analysis to identify genes acting upstream of PHO80 or PHO81
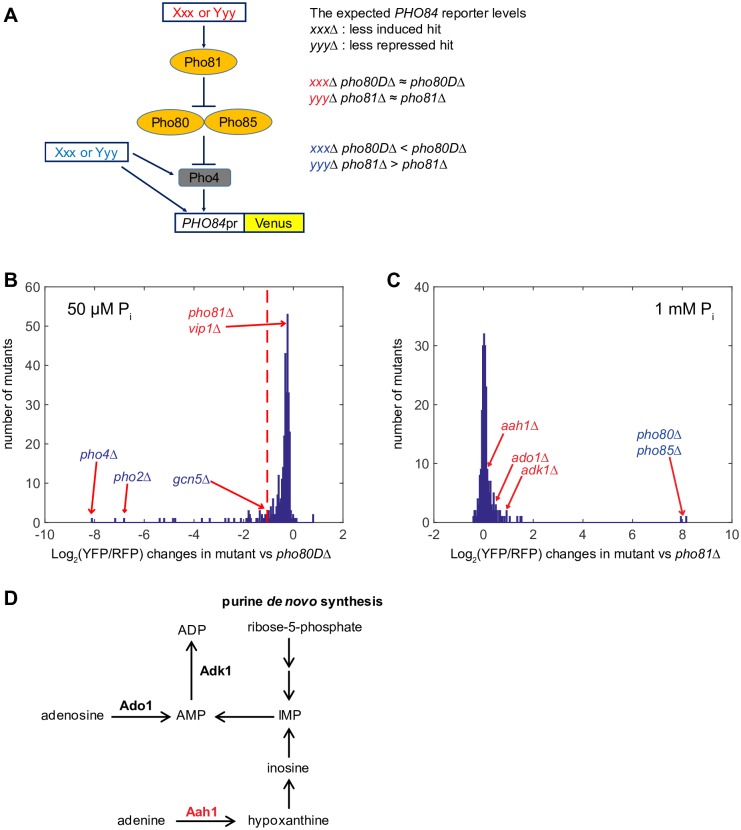
To identify genes acting upstream of the Pho80/Pho85/Pho81 kinase complex from the 623 hits (380 less induced and 243 less repressed), we performed epistasis analysis. Epistasis refers to a genetic interaction in which mutation of one gene influences the phenotypic effects of another [20, 31]; this approach can be used to infer the order of gene action in a signaling pathway [17]. For example, we can learn that gene A acts upstream of gene B from the observation that deletion of gene B masks the phenotypic effect of deletion of gene A.
For the less induced hits, we performed epistasis analysis in low Pi by generating double mutants with pho80. If the less induced gene hit XXX acts upstream of PHO80, mutation of PHO80 masks the effect of reduced PHO84 expression such that the xxxΔ pho80 double mutant expresses PHO84 at a level similar to the pho80 single mutant (Fig 3A). For this analysis we used pho80DΔ, a strain with attenuated PHO80 mRNA expression (Materials and methods) instead of complete deletion (pho80Δ) since PHO84 expression in the pho80Δ strain is extremely strong (Fig 2B and 2D). We hypothesized that pho80DΔ would sensitize PHO84 expression to the PHO pathway mutants so that epistasis analysis with pho80DΔ would allow us to identify genes acting upstream of PHO80.
Fig 3. Identification of genes acting upstream of the Pho80/Pho85/Pho81 kinase complex.
(A) A schematic diagram depicting the expected outcome of epistasis analysis depending on whether or not a mutant is defective in the signaling process upstream of Pho80/Pho85. Genes in red act upstream of Pho80/Pho85 and those in blue do not act upstream of Pho80/Pho85. (B) The PHO84 reporter levels of double mutants carrying the less induced hits and pho80DΔ in 50 μM Pi conditions. All 380 less induced hits were used to generate the double mutants. The PHO84 reporter levels of double mutants in (B) were normalized to that of pho80DΔ. A red dashed line in (B) indicates the maximum PHO84 reporter level of double mutants generated by one of the known downstream genes (pho80DΔ gcn5Δ). (C) The PHO84 reporter levels of double mutants carrying the less repressed hits and pho81Δ in 1 mM Pi conditions. All 243 less repressed hits were used to generate the double mutants. The PHO84 reporter levels of double mutants in (C) were normalized to that of pho81Δ. In (B) and (C), mutants in blue and red are defective in signaling process downstream and upstream of Pho80/Pho85, respectively. (D) A schematic diagram depicting adenine nucleotide metabolism. A gene in red is identified in this study and those in bold black are previously identified.
We validated our strategy for epistasis analysis with pho80DΔ by comparing the PHO84 reporter level of pho80DΔ with those of double mutants carrying mutations in genes known to act downstream or upstream (Fig 3B). Consistent with our hypothesis, pho80DΔ pho81Δ and pho80DΔ vip1Δ, which carry deletion mutations of upstream genes, expressed PHO84 at a level similar to pho80DΔ (-0.19 and -0.19, respectively; S5 Table). By contrast, double mutants of downstream genes expressed less PHO84 than did pho80DΔ (Fig 3B). For example, pho80DΔ pho4Δ expressed the lowest level of PHO84 (-8.13, S5 Table) of all the double mutants and pho80DΔ gcn5Δ (a catalytic subunit of ADA and SAGA histone acetyltransferase complexes [32, 33]) expressed PHO84 at levels lower than pho80DΔ (-1.07, S5 Table). To identify upstream genes, we needed to determine the lowest PHO84 reporter level, as a threshold, that double mutants of pho80DΔ and true upstream genes could exhibit. This threshold should lie between -1.07 and -0.19 since -1.07 is the maximum PHO84 reporter level of double mutants of pho80DΔ and known downstream genes (pho80DΔ gcn5Δ) and -0.19 is the PHO84 reporter level of double mutants of pho80DΔ and known upstream genes such as pho80DΔ vip1Δ. To try and ensure that no hits were lost due to a stringent threshold, we used -1.07 as the threshold in spite of the fact that this approach undoubtedly will allow false-positives. We classified 300 of the measured 334 less induced hits expressing PHO84 above the threshold as defective in signaling upstream of PHO80 (S5 Table).
For the less repressed hits, we performed epistasis analysis in high Pi with pho81Δ (Fig 3C). If gene YYY acts upstream of PHO81, deletion of PHO81 masks increased PHO84 expression such that pho81Δ yyyΔ expresses PHO84 at a level similar to pho81Δ (Fig 3A). As shown in Fig 3C, pho81Δ adk1Δ and pho81Δ ado1Δ, which carry deletions of genes known to act upstream of PHO81, expressed PHO84 at a level similar to pho81Δ (0.92 and 0.48, respectively; S6 Table). By contrast, pho81Δ pho80Δ and pho81Δ pho85Δ, which carry deletion mutation of genes known to act downstream of PHO81, expressed PHO84 at higher levels than pho81Δ (7.95 and 8.18, respectively; S6 Table). Because pho81Δ pho80Δ and pho81Δ pho85Δ were the only double mutants expressing PHO84 at significantly higher levels than pho81Δ, we classified the remaining 222 of the measured 224 less repressed hits as defective in signaling upstream of PHO81 (S6 Table).
Deletion of AAH1 encoding an adenine deaminase involved in adenine nucleotide metabolism derepresses the PHO pathway in high Pi
One of the genes we identified with the strongest phenotype was AAH1, a gene encoding an adenine deaminase that converts adenine into hypoxanthine. AAH1 acts upstream of PHO80 and loss of AAH1 leads to >15-fold induction of PHO84 expression in high Pi (Figs 2D and 3C, S1A Fig, S6 Table). Together with ADO1 and ADK1, AAH1 is involved in adenine nucleotide metabolism (Fig 3D). Based on the observation that three mutants defective in adenine nucleotide metabolism share the same PHO phenotype, it is plausible that intermediates or products of adenine nucleotide metabolism act as signaling factors for the PHO pathway.
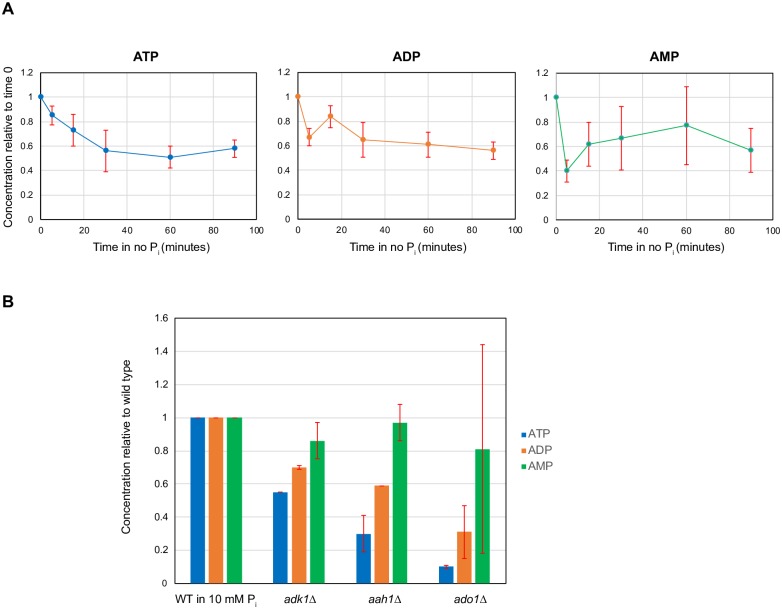
Based on a previous metabolome study in yeast [29], we speculated that a low ATP level might be involved in activation of the PHO pathway. Measuring the metabolic profiles in steady-state, chemostat grown cultures of S. cerevisiae with 3 different limiting nutrients (carbon, nitrogen and Pi), Boer et al. showed that metabolite concentrations were highly sensitive to the identity of the limiting nutrient, with Pi limitation leading to low nucleotide levels [29]. Furthermore, particularly strong responses occurred in metabolites closely linked to the limiting nutrient, for example, ATP in Pi limitation [29]. To test if we observe metabolic changes in response to changes in Pi availability in non-steady state experiments, we measured [ATP], [ADP], and [AMP] over time after transferring wild type cells from high Pi to no Pi medium (Materials and methods). As controls where the PHO pathway is activated, we also measured the adenine nucleotide levels in adk1Δ, aah1Δ, and ado1Δ grown in high Pi and compared them with those of the wild type grown in no Pi (Materials and methods). Consistent with the chemostat measurements [29], the adenine nucleotide levels of cells grown in no Pi were lower than those of cells grown in high Pi and ATP levels monotonically decreased over time in cells grown in no Pi (Fig 4A, S2 Table). Furthermore, ATP levels in adk1Δ, aah1Δ, and ado1Δ grown in high Pi were comparable to those in the wild type grown in no Pi (Fig 4B, S2 Table). The timescale of the reduction in the ATP level parallels the timescale for activation and nuclear accumulation of Pho4: after 15 minutes in no Pi ATP levels were reduced by ~25% relative to levels in 10 mM Pi and 50% of cells exhibited Pho4 nuclear localization, whereas after 60 minutes ATP levels were reduced by ~50% and essentially all cells had Pho4 localized to the nucleus [34]. These measurements support the speculation that a low ATP level might be involved in activation of the PHO pathway under low Pi conditions.
Fig 4. Adenine nucleotide levels in the wild type in no Pi and in adk1Δ, aah1Δ and ado1Δ in high Pi.
(A) [ATP], [ADP], and [AMP] in wild type (WT) cells over time grown in no Pi medium. All the adenine nucleotide concentrations at each time point were normalized to those in 10 mM Pi. Note that the PHO pathway in no Pi is activated within 15 minutes. Adenine nucleotide levels at each time point were measured three times and the error bars in (A) are standard errors. (B) [ATP], [ADP], and [AMP] in WT, adk1Δ, aah1Δ, and ado1Δ in 10 mM Pi. Adenine nucleotide levels in the three mutants were measured two times and the error bars in (B) are standard errors.
The PHO pathway in vip1Δ can be activated in a long period of time under Pi-limited conditions
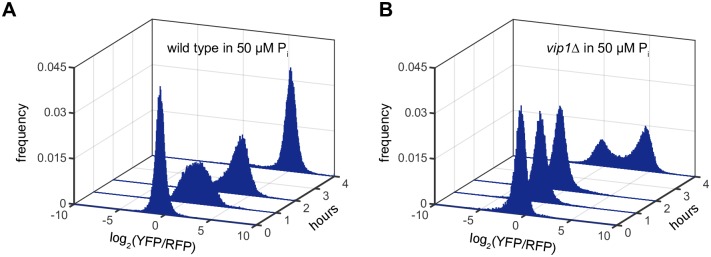
In this study, we observed that vip1Δ was able to induce PHO84 expression after 8 hours in low Pi, although to a lesser extent (~ 55%) than the wild type (Fig 2C, S1B Fig). This observation seems inconsistent with a previous study showing that deletion of VIP1 prevents activation of the PHO pathway under low Pi conditions [14]. However, the earlier work assayed pathway activity at 2 hours, rather than 8 hours, suggesting that PHO84 induction kinetics in vip1Δ are slower than in the wild type. To test if this is true, we measured PHO84 expression levels in vip1Δ in low Pi medium over time and compared them with those in the wild type strain. As shown in Fig 5, the wild type strain expressed PHO84 after 1 hour in low Pi medium but vip1Δ started to induce PHO84 expression only after 4 hours. Therefore, we conclude that vip1Δ is inducible, but activation of the PHO pathway in vip1Δ is slower than in the wild type.
Fig 5. The PHO pathway in vip1Δ mutant is inducible in 50 uM Pi, but its induction kinetics are slower than the wild type.
(A) The PHO84 reporter levels of the wild type over time in 50 uM Pi. (B) The PHO84 reporter levels of vip1Δ over time in 50 uM Pi. Time 0 data in (A) and (B) were obtained in 10 mM Pi before cells were inoculated into 50 μM Pi.
VIP1 is required for constitutive activation of the PHO pathway in both ado1Δ and aah1Δ mutants
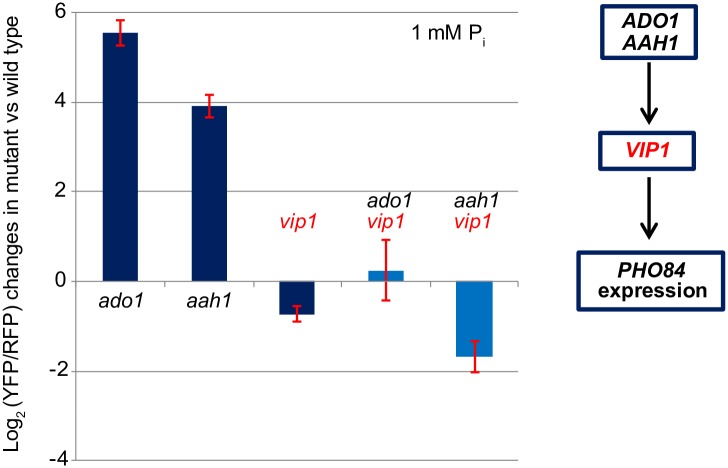
To understand the molecular mechanisms underlying the PHO phenotypes of ado1Δ, aah1Δ, and adk1Δ mutants defective in adenine nucleotide metabolism (Fig 3D), we sought to identify genes required for the constitutive activation phenotype of the three mutants. First, we tried to generate double mutants of ado1Δ, aah1Δ, and adk1Δ by crossing each of these mutants to the array strains that were chosen from those defective in signaling upstream of PHO80 or PHO81 (Materials and methods). The adk1Δ strain was defective in sporulation so we were unable to generate double mutants with this strain. We generated double mutants carrying aah1Δ and ado1Δ and measured PHO84 expression levels in high Pi conditions to identify those expressing <2-fold PHO84 more than the wild type. From these measurements, we identified 63 and 22 double mutants in the aah1Δ and ado1Δ backgrounds with elevated PHO84 expression, respectively, with 12 of them appearing in both mutant backgrounds (S4 Table). Although there was no enriched gene ontology term in the 12 gene set, we observed that deletion of VIP1 fully masked activation of the PHO pathway in ado1Δ and aah1Δ in high Pi conditions (Fig 6). Given that Vip1-synthesized IP7 is a signaling factor for the PHO pathway [14], this observation suggests that there may be interplay between adenine nucleotide metabolism and inositol polyphosphate synthesis.
Fig 6. VIP1 is required for constitutive activation of the PHO pathway in ado1Δ and aah1Δ.
The PHO84 reporter levels in all the strains in Fig 6 were averaged over 3 measurements and normalized to the PHO84 reporter level in the wild type in 1 mM Pi conditions. Error bars represent the standard deviation of the normalized PHO84 reporter levels in the mutants.
Discussion
In an effort to better understand signaling processes upstream of the Pho80/Pho85/Pho81 kinase complex in the PHO pathway, we used the SGA method to conduct systematic genome-wide screening. We found more than 300 mutants that are significantly defective in signaling upstream of PHO80 or PHO81 and investigated a subset in more detail. We found that genes acting in different metabolic pathways influence regulation of the PHO pathway. Deletion of AAH1, involved in adenine nucleotide metabolism, leads to derepression of the PHO pathway under high Pi conditions. Furthermore, Aah1 and Ado1 (another adenine metabolism gene) negatively regulate the activity of the PHO pathway in a Vip1-dependent manner, suggesting that interplay between adenine nucleotide metabolism and inositol polyphosphate metabolism may be important for regulation of the PHO pathway.
Our study provides new insight into how a decrease in ATP levels can be linked to elevation of Vip1-synthesized IP7 to activate the PHO pathway. This claim is supported by two observations: (1) ATP levels decrease when the PHO pathway is activated—in the wild type in low Pi and in ado1Δ, aah1Δ, and aak1Δ in high Pi; (2) Vip1 is required for constitutive activation of the PHO pathway in the ado1Δ and aah1Δ strains. We propose that changes in ATP levels resulting from changes in Pi availability affect the balance between the levels of different IP7 isomers. Two classes of kinases, Kcs1 (IP6 kinase) and Vip1 (PP-IP5 kinase), synthesize different IP7 isomers from IP6 [30]. Furthermore, they appear to have different Km values for ATP based on the values of their mammalian homologues (Km for ATP (mM): 0.13 (Vip1) vs.1 ~ 1.4 (Kcs1)) [35]. When ATP levels are high, like the wild type in high Pi conditions, Kcs1-synthesized IP7 appears to be the dominant IP7 isomer as deletion of KCS1 leads to near-complete removal of IP7; in kcs1Δ, IP6 levels are reduced by 20% and total IP7 levels are reduced by 83% compared to the wild type [36]. When ATP levels are low, as in the wild type in low Pi conditions (~50% decrease, Fig 4A) or in the three mutants (50 ~80% decrease, Fig 4B), we expect that Kcs1 kinase activity will be reduced but Vip1 kinase will still be active since physiological ATP concentrations in high Pi (1.1 ~ 1.4 mM) are near the Km for ATP of Kcs1 [37]. These changes in Kcs1 and Vip1 kinase activities in low ATP conditions may give rise to accumulation of Vip1-synthesized IP7 leading to activation of the PHO pathway. A prediction of this model is that in the absence of Kcs1, Vip1-synthesized IP7 levels may increase due to lack of competition over IP6. This prediction is consistent with our observation that the PHO84 reporter level in kcs1Δ is higher than that of the wild type in low Pi (1.33; S3 Table). To test this model, it will be necessary to carry out the following experiments: (1) to determine if IP7 levels in ado1Δ, aah1Δ, and adk1Δ mutants are comparable to those in the wild type in low Pi; (2) to measure Vip1 and Kcs1 kinase activities as a function of ATP concentrations, detecting different IP7 isomer levels to determine if Vip1 is more active than Kcs1 in the low ATP regime [38].
In addition, the reduction in ATP and ADP levels in the three mutants suggests that increases in AICAR (5-aminoimidazole-4-carboxamide ribonucleotide), an intermediate of purine de novo synthesis, may contribute to strong PHO84 reporter levels in the mutants. Decreases in ATP and ADP levels lead to loss of feedback inhibition of purine de novo synthesis [39], so it is expected that AICAR levels in these three mutants will increase. Pinson et al. showed that an increase in AICAR levels promotes interactions between Pho4 and Pho2, another co-transcription factor required for regulating the PHO pathway, leading to expression of the PHO regulon in a PHO pathway-independent manner [40]. Although AICAR is not likely to be acting upstream of the Pho80/Pho81/Pho85 complex [40], an increase in AICAR levels resulting from decreases in ATP and ADP levels may account for some of the increased PHO84 reporter levels in those mutants.
When we analyzed gene ontology (GO) enrichment for the less repressed hits with DAVID [41], we found that nine genes with the GO term of “transcription elongation from RNA polymerase II promoter” are significantly enriched (p-value: 3.6*10−4, S6 Table). This finding suggests a possible source of cell-to-cell variability in repression of PHO84 transcription, which may contribute to the bimodal distribution of PHO84 expression in intermediate Pi conditions. The interplay of feedback loops generated by Pi transporter regulation creates mutually exclusive states in which cells either express PHO84 or repress PHO84 in intermediate Pi conditions [26]. Wykoff et al. have speculated that cell-to-cell variability leads to the bimodality in PHO84 expression [26]. Since low copies of antisense PHO84 RNA are expressed sporadically and are sufficient to repress PHO84 mRNA expression within individual cells [42, 43], cells that harbor antisense PHO84 RNA are expected to repress PHO84 expression and the remaining cells are expected to express PHO84, leading to the bimodality in PHO84 expression. As antisense PHO84 RNA can act in trans [42], our PHO84 reporter is expected to be subject to this antisense regulation. Thus, these nine genes may be involved in transcription of antisense PHO84 RNA. This hypothesis could be evaluated by measuring antisense PHO84 RNA in these nine mutants with single molecule RNA FISH (fluorescence in situ hybridization).
The observation that the PHO pathway can be activated in vip1Δ after a long period of time in Pi-limited conditions demonstrates the complexity of molecular mechanisms underlying regulation of the PHO pathway. This phenotype of vip1Δ can be explained by two possible mechanisms: (i) alternative IP7 synthesis pathways where other kinases can take over the synthesis of IP7 in the absence of Vip1, or (ii) a novel IP7-independent regulation of Pho80/Pho85 kinase activity. In a future study, it may be possible to test this hypothesis by measuring IP7 levels in the vip1Δ strain over time under Pi-limited conditions.
In conclusion, our screening results provide a resource for further studies on the molecular mechanisms by which the PHO pathway is regulated. Metabolic profiling with the mutants that we identified in this study, as a complement to genetic screening, could be useful for monitoring how different metabolic pathways—in particular, adenine nucleotide metabolism and inositol polyphosphate metabolism—respond to changes in Pi availability and elucidating how these pathways influence the activity of the PHO pathway. Given the complexity of the metabolic networks involved in regulation of the PHO pathway, an extensive analysis with the array strains will yield important information on the signaling network that allows budding yeast cells to respond properly to changes in environmental nutrient availability.
Supporting information
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We thank Martin Jonikas and Jonathan Weissman for sharing with us the yeast library and Matlab analysis codes; Joseph Markson for insightful discussions; members of the O’Shea laboratory for discussions; and Joseph Markson, Dennis Wykoff and Harold Kim for critical reading the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funded by Howard Hughes Medical Institute (http://www.hhmi.org): JC AR EKO. Samsung Scholarship (http://www.ssscholarship.com): JC. Mary Fieser fellowship (http://chemistry.harvard.edu): AR. DOE Grant DE-SC0012461 (https://science.energy.gov/) : YX JDR.
References
- 1.Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature. 2015;517(7534):302–10. 10.1038/nature14190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oshima Y. The phosphatase system in Saccharomyces cerevisiae. Genes Genet Syst. 1997;72(6):323–34. [DOI] [PubMed] [Google Scholar]
- 3.Lenburg ME, O'Shea EK. Signaling phosphate starvation. Trends in Biochemical Sciences. 1996;21(10):383–7. [PubMed] [Google Scholar]
- 4.Toh-e A, Ueda Y, Kakimoto S-I, Oshima Y. Isolation and Characterization of Acid Phosphatase Mutants in Saccharomyces cerevisiae. J Bacteriol. 1973;113(2):727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bun-Ya M, Nishimura M, Harashima S, Oshima Y. The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol Cell Biol. 1991;11(6):3229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider KR, Smith RL, O'Shea EK. Phosphate-regulated inactivation of the kinase PHO80-PHO85 by the CDK inhibitor PHO81. Science. 1994;266(5182):122–6. [DOI] [PubMed] [Google Scholar]
- 7.O'Neill EM, Kaffman A, Jolly ER, O'Shea EK. Regulation of PHO4 nuclear localization by the PHO80-PHO85 cyclin-CDK complex. Science. 1996;271(5246):209–12. [DOI] [PubMed] [Google Scholar]
- 8.Kaffman A, Herskowitz I, Tjian R, O'Shea EK. Phosphorylation of the transcription factor PHO4 by a cyclin-CDK complex, PHO80-PHO85. Science. 1994;263(5150):1153–6. [DOI] [PubMed] [Google Scholar]
- 9.Byrne M, Miller N, Springer M, O'Shea EK. A distal, high-affinity binding site on the cyclin-CDK substrate Pho4 is important for its phosphorylation and regulation. J Mol Biol. 2004;335(1):57–70. [DOI] [PubMed] [Google Scholar]
- 10.Jeffery DA, Springer M, King DS, O'Shea EK. Multi-site phosphorylation of Pho4 by the cyclin-CDK Pho80-Pho85 is semi-processive with site preference. J Mol Biol. 2001;306(5):997–1010. 10.1006/jmbi.2000.4417 [DOI] [PubMed] [Google Scholar]
- 11.Springer M, Wykoff DD, Miller N, O'Shea EK. Partially phosphorylated Pho4 activates transcription of a subset of phosphate-responsive genes. PLoS Biol. 2003;1(2):E28 10.1371/journal.pbio.0000028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaffman A, Rank NM, O'Neill EM, Huang LS, O'Shea EK. The receptor Msn5 exports the phosphorylated transcription factor Pho4 out of the nucleus. Nature. 1998;396(6710):482–6. 10.1038/24898 [DOI] [PubMed] [Google Scholar]
- 13.Lee Y-S, Huang K, Quiocho FA, O'Shea EK. Molecular basis of cyclin-CDK-CKI regulation by reversible binding of an inositol pyrophosphate. Nat Chem Biol. 2008;4(1):25–32. 10.1038/nchembio.2007.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y-S, Mulugu S, York JD, O'Shea EK. Regulation of a cyclin-CDK-CDK inhibitor complex by inositol pyrophosphates. Science. 2007;316(5821):109–12. 10.1126/science.1139080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaffman A, Rank NM, O'Shea EK. Phosphorylation regulates association of the transcription factor Pho4 with its import receptor Pse1/Kap121. Genes Dev. 1998;12(17):2673–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang S, O'Shea EK. A systematic high-throughput screen of a yeast deletion collection for mutants defective in PHO5 regulation. Genetics. 2005;169(4):1859–71. 10.1534/genetics.104.038695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonikas MC, Collins SR, Denic V, Oh E, Quan EM, Schmid V, et al. Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science. 2009;323(5922):1693–7. 10.1126/science.1167983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins SR, Miller KM, Maas NL, Roguev A, Fillingham J, Chu CS, et al. Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature. 2007;446(7137):806–10. 10.1038/nature05649 [DOI] [PubMed] [Google Scholar]
- 19.Tong AHY, Boone C. Synthetic Genetic Array Analysis in Saccharomyces cerevisiae In: Xiao W, editor. Yeast Protocol. Methods in Molecular Biology: Humana Press; p. 171–91. [DOI] [PubMed] [Google Scholar]
- 20.Boone C, Bussey H, Andrews BJ. Exploring genetic interactions and networks with yeast. Nat Rev Genet. 2007;8(6):437–49. 10.1038/nrg2085 [DOI] [PubMed] [Google Scholar]
- 21.Schuldiner M, Collins SR, Thompson NJ, Denic V, Bhamidipati A, Punna T, et al. Exploration of the function and organization of the yeast early secretory pathway through an epistatic miniarray profile. Cell. 2005;123(3):507–19. 10.1016/j.cell.2005.08.031 [DOI] [PubMed] [Google Scholar]
- 22.Sheff MA, Thorn KS. Optimized cassettes for fluorescent protein tagging in Saccharomyces cerevisiae. Yeast. 2004;21(8):661–70. 10.1002/yea.1130 [DOI] [PubMed] [Google Scholar]
- 23.Breslow DK, Cameron DM, Collins SR, Schuldiner M, Stewart-Ornstein J, Newman HW, et al. A comprehensive strategy enabling high-resolution functional analysis of the yeast genome. Nat Meth. 2008;5(8):711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldstein AL, McCusker JH. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast. 1999;15(14):1541–53. [DOI] [PubMed] [Google Scholar]
- 25.Lam FH, Steger DJ, O'Shea EK. Chromatin decouples promoter threshold from dynamic range. Nature. 2008;453(7192):246–50. 10.1038/nature06867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wykoff DD, Rizvi AH, Raser JM, Margolin B, O'Shea EK. Positive feedback regulates switching of phosphate transporters in S. cerevisiae. Mol Cell. 2007;27(6):1005–13. 10.1016/j.molcel.2007.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu YF, Zhao X, Glass DS, Absalan F, Perlman DH, Broach JR, et al. Regulation of yeast pyruvate kinase by ultrasensitive allostery independent of phosphorylation. Molecular cell. 2012;48(1):52–62. Epub 2012/08/21. 10.1016/j.molcel.2012.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabinowitz JD, Lu WY, Clasquin MF, Melamud E, Amador-Noguez D, Caudy AA. Metabolomic Analysis via Reversed-Phase Ion-Pairing Liquid Chromatography Coupled to a Stand Alone Orbitrap Mass Spectrometer. Analytical Chemistry. 2010;82(8):3212–21. 10.1021/ac902837x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boer VM, Crutchfield CA, Bradley PH, Botstein D, Rabinowitz JD. Growth-limiting intracellular metabolites in yeast growing under diverse nutrient limitations. Mol Biol Cell. 2010;21(1):198–211. 10.1091/mbc.E09-07-0597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson MSC, Livermore TM, Saiardi A. Inositol pyrophosphates: between signalling and metabolism. Biochem J. 2013;452(3):369–79. 10.1042/BJ20130118 [DOI] [PubMed] [Google Scholar]
- 31.Avery L, Wasserman S. Ordering gene function: the interpretation of epistasis in regulatory hierarchies. Trends Genet. 1992;8(9):312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W, Bone JR, Edmondson DG, Turner BM, Roth SY. Essential and redundant functions of histone acetylation revealed by mutation of target lysines and loss of the Gcn5p acetyltransferase. The EMBO Journal. 1998;17(11):3155 10.1093/emboj/17.11.3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grant PA, Duggan L, Côté J, Roberts SM, Brownell JE, Candau R, et al. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes & Development. 1997;11(13):1640–50. [DOI] [PubMed] [Google Scholar]
- 34.Thomas MR, O'Shea EK. An intracellular phosphate buffer filters transient fluctuations in extracellular phosphate levels. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9565–70. 10.1073/pnas.0501122102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wundenberg T, Mayr Georg W. Synthesis and biological actions of diphosphoinositol phosphates (inositol pyrophosphates), regulators of cell homeostasis. 2012. p. 979. [DOI] [PubMed]
- 36.Saiardi A, Caffrey JJ, Snyder SH, Shears SB. The Inositol Hexakisphosphate Kinase Family: CATALYTIC FLEXIBILITY AND FUNCTION IN YEAST VACUOLE BIOGENESIS. Journal of Biological Chemistry. 2000;275(32):24686–92. 10.1074/jbc.M002750200 [DOI] [PubMed] [Google Scholar]
- 37.Gancedo JM, Gancedo C. Concentrations of intermediary metabolites in yeast. Biochimie. 1973;55(2):205–11. [DOI] [PubMed] [Google Scholar]
- 38.Lin H, Lindner K, Mayr GW. Synthesis and Nonradioactive Micro-analysis of Diphosphoinositol Phosphates by HPLC with Postcolumn Complexometry In: Barker CJ, editor. Inositol Phosphates and Lipids: Methods and Protocols. Totowa, NJ: Humana Press; 2010. p. 103–22. [DOI] [PubMed] [Google Scholar]
- 39.Rebora K, Desmoucelles C, Borne F, Pinson B, Daignan-Fornier B. Yeast AMP pathway genes respond to adenine through regulated synthesis of a metabolic intermediate. Mol Cell Biol. 2001;21(23):7901–12. 10.1128/MCB.21.23.7901-7912.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinson B, Vaur S, Sagot I, Coulpier F, Lemoine S, Daignan-Fornier B. Metabolic intermediates selectively stimulate transcription factor interaction and modulate phosphate and purine pathways. Genes Dev. 2009;23(12):1399–407. 10.1101/gad.521809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protocols. 2008;4(1):44–57. [DOI] [PubMed] [Google Scholar]
- 42.Camblong J, Beyrouthy N, Guffanti E, Schlaepfer G, Steinmetz LM, Stutz F. Trans-acting antisense RNAs mediate transcriptional gene cosuppression in S. cerevisiae. Genes Dev. 2009;23(13):1534–45. 10.1101/gad.522509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castelnuovo M, Rahman S, Guffanti E, Infantino V, Stutz F, Zenklusen D. Bimodal expression of PHO84 is modulated by early termination of antisense transcription. Nat Struct Mol Biol. 2013;20(7):851–8. 10.1038/nsmb.2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.