Abstract
Objective
To investigate the impact of national implementation of age restriction on fluoroquinolone prescription in children and adolescents.
Methods
Data collected from the database of Health Insurance Review and Assessment Service in South Korea, a national health insurance system to analyze fluoroquinolone prescribing practice in children and adolescents younger than 18 years, between 2007 and 2015. The age restriction was implemented in December 2009. The annual prescription rate of FQ per 100,000 person-years was calculated and an autoregressive model was used to predict the prescription pattern if an intervention had not occurred.
Results
A total of 505,859 children received systemic fluoroquinolone during the study period—297,054 ciprofloxacin, and 208,805 levofloxacin. After implementation of the drug utilization review program, the annual prescription rate for ciprofloxacin declined by 97.5% (from 840 to 21 per 100,000 person-years, P < 0.001), and for levofloxacin by 96.4% (from 598 to 11 per 100,000 person-years, P < 0.001). The decline was more dramatic in the outpatient setting than in the inpatient setting for both drugs.
Conclusion
The dramatic and sustained decline in prescription number and change in prescription pattern after the regulatory action suggests that the implementation under drug utilization review program was successful in controlling excessive and inappropriate use of fluoroquinolones in children, possibly guiding towards more judicious and selective prescription behavior.
Introduction
Pediatric use of FQs has been restricted due to early findings of FQ-associated weight-bearing joint damage in juvenile animals [1–4]. However, the off-label pediatric use of FQ outside the regulatory boundaries is thought to be common and increasing worldwide [5–9]. Development of resistance to other antibiotics is thought to be the main driving factor behind the increasing use of FQs in children [10–15]. However, FQ itself is also associated with the rapid development of resistance, and thus, these agents should be used with caution and only when necessary [2, 16, 17]. A worldwide emergence of quinolone resistance has been observed, especially with Escherichia coli isolates, and this phenomenon is thought to be most prominent in the Asia–Pacific region [18–24]. Therefore, inappropriate and excessive use of FQs in children, especially in the ambulatory setting, is of concern in this era of increased antimicrobial resistance. Interventions have been often implemented on the individual hospital level to control the inappropriate use of antibiotics and restrict the use of certain broad-spectrum antibiotics. In South Korea, an intervention was implemented on a national level to control fluoroquinolone use of in children.
South Korea has a unique Drug Utilization Review (DUR) system that is concurrent and provides real-time information to prescribers and pharmacists [25]. It uses a predefined DUR criteria that include a list of medications that are contraindicated in pregnant women, drugs with drug–drug interactions, and drugs with age restrictions. After its initial establishment in 2004, 58 drugs were added to the list of contraindicated drugs in pediatrics in December 2009, which included the fluoroquinolone (FQ) class of antibiotics [26]. When a contraindicated drug is prescribed, an alert appears; at this point, the prescriber may change the prescription or provide a reason to justify the use and continue with the prescription. When a contraindicated drug is dispensed, a similar warning appears, and the pharmacist should contact the prescriber and confirm the prescription.
Before December 2009, there were no regulatory restrictions on FQ use in children and the Korean regulatory agencies reimbursed the use of FQs for patients of all ages. Such measure was in contrast to other countries where the regulatory approval status of FQs in children was expanded to allow the use in a wider range of indications, which was supported by the increasing availability of safety information showing that musculoskeletal events are rare and reversible [10–13]. Currently, the United States Food and Drug Administration (US FDA)-approved pediatric indications for FQs include inhalational anthrax, complicated urinary tract infections, and pyelonephritis for ciprofloxacin (CF), and inhalational anthrax for levofloxacin (LF) [27, 28]. Some European countries also allow the use of CF in children under certain indications [29].
To our knowledge, no quantitative studies have been conducted to quantify national prescription volume of fluoroquinolone in children. Although one study has assessed FQ use in Korean pediatric patients, it used patient sample data and short study period of two years, and thus limited in evaluating the overall, sustained impact of the nationwide intervention [30]. Thus, the aims of our study were 1) to quantify the national pediatric use of fluoroquinolones and 2) to evaluate the sustained as well as immediate effect of nationwide implementation of age restriction on fluoroquinolone use by analyzing the changing rates and patterns of prescription over 9 years. Based on the understanding of the national reimbursement policy, where the prescription of a contraindicated drug included in the list may be restrictively reimbursed, we hypothesized that the classification of FQ as drug contraindicated for pediatric use would result in a dramatic reduction in the number of FQ prescriptions.
Materials and methods
Data source
The Health Insurance Review and Assessment Service (HIRA), a Korean regulatory agency, is responsible for the review and assessment of reimbursement and plays a leading role in managing the DUR. The entire Korean population, with the exception of the 3% covered under medical aid, is enrolled in the national health insurance system, which achieved universal coverage of all citizens in 1989 [31]. The HIRA database contains nationwide information from medical facilities and pharmacies on patient demographics, diagnoses, prescriptions, and healthcare service providers. For reimbursement, the patient’s diagnosis must be confirmed by physicians, and the Korean National Health Insurance agency reviews it against the eligibility criteria, before submitting claims to the HIRA claim database. Thus, these data are considered reliable [32]. The ethics approval was obtained from the Institutional Review Boards of Chungnam National University and Chung-Ang University.
Study period and population
Data were collected from 2007 to 2015. Due to the implementation of the DUR age restriction on FQ use in December 2009, the time periods were separated as follows for purposes of comparison: “pre-DUR:” 2007 to 2009; “post-DUR:” 2010 to 2015. Both male and female patients who met the following criteria at the time of prescription were included: (i) younger than 18 years, (ii) visited a medical facility from 2007 to 2015, and (iii) received a prescription for systemic CF or LF. Data on topical FQ use were excluded.
Data collection and assessment
Drug information, such as the generic name of the drug, prescription date, duration, and route of administration were collected. Gender and age were determined at the time of hospital admission or on the first outpatient prescription. To evaluate the rationale behind prescription as well as its patterns, we analyzed the International Classification of Diseases, tenth revision (ICD-10) diagnostic codes and the specialties of prescribers. To determine which medical facility the DUR implementation had a greater impact on, the number of FQ prescriptions prescribed in each medical facility was analyzed. The medical facility at which FQ was prescribed was classified according to the domestic hospital classification system: clinic, hospital, general hospital, or tertiary referral hospital. As all personal data were de-identified, patient information and healthcare service information could not be matched.
To assess other factors that may have impacted pediatric FQ prescriptions, such as drug shortages and overall changes in pediatric antibiotic prescription patterns, data on the prescription volumes of five commonly prescribed pediatric antibiotics (amoxicillin, amoxicillin and clavulanate in combination, cefaclor, cefixime, and clarithromycin; selection based on literature review [33–35]) as well as FQs prescribed in adults were collected using the HIRA National Patients Sample (HIRA-NPS) database [36].
Statistical analysis
The annual prescription rate of FQ per 100,000 person-years was calculated as the number of patients prescribed FQ divided by the number of all Korean residents in the same age group, which was obtained from the national statistics data of 2010 [37]. Categorical variables were expressed as frequencies and proportions. A chi-square (χ2) test was performed to assess changes in prescription patterns after DUR implementation with regard to diagnostic codes, types of medical facility, and age groups. An autoregressive model was used to predict the prescription pattern if intervention had not occurred. The model coefficient and covariance were estimated based on the maximum-likelihood method. P < 0.05 were considered statistically significant, and all statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC).
Results
Characteristics of the study population and number of FQ prescriptions
Between 2007 and 2015, a total of 505,859 pediatric patients younger than 18 years received systemic FQs: 297,054 received CF, and 208,805 received LF (Table 1). No significant gender differences were observed between the pre-DUR and post-DUR groups (CF: P = 0.253; LF: P = 0.126); however, a significant increase in the mean age of patients was observed after the implementation of DUR (mean age ± SD; CF: 13.31 ± 4.14 vs. 14.36 ± 3.63 years, P < 0.001; LF: 14.15 ± 3.10 vs. 14.91± 3.31 years, P < 0.001).
Table 1. Demographic characteristics of pediatric patients who received systemic fluoroquinolone treatment.
| Characteristics (No, %) |
Ciprofloxacin | Levofloxacin | ||||||
|---|---|---|---|---|---|---|---|---|
| Inpatient | Outpatient | Inpatient | Outpatient | |||||
| Pre-DUR | Post-DUR | Pre-DUR | Post-DUR | Pre-DUR | Post-DUR | Pre-DUR | Post-DUR | |
| (n = 19284) | (n = 7553) | (n = 263513) | (n = 6704) | (n = 7435) | (n = 2474) | (n = 193955) | (n = 4941) | |
| Age | ||||||||
| <4 wks | 31 (0.2) | 21 (0.3) | 76 (0.0) | 0 (0) | 0 (0) | 10 (0.4) | 35 (0) | 1 (0) |
| 4 wks–11 mos | 143 (0.7) | 168 (2.2) | 3890 (1.5) | 18 (0.3) | 11 (0.1) | 35 (1.4) | 348 (0.2) | 7 (0.1) |
| 1–2 yrs | 482 (2.5) | 399 (5.3) | 7794 (3.0) | 36 (0.5) | 39 (0.5) | 35 (1.4) | 1809 (0.9) | 35 (0.7) |
| 3–5 yrs | 980 (5.1) | 616 (8.2) | 11816 (4.5) | 90 (2.4) | 60 (0.8) | 51 (2.1) | 5121 (2.6) | 99 (2.0) |
| 6–11 yrs | 2233 (11.6) | 771 (10.2) | 54743 (20.8) | 484 (7.2) | 711 (9.6) | 174 (7.0) | 35015 (18.1) | 312 (6.3) |
| 12–17 yrs | 15415 (79.9) | 5578 (73.9) | 185194 (70.3) | 6076 (90.6) | 6614 (89.0) | 2169 (87.7) | 151627 (78.2) | 4487 (90.8) |
| Gender | ||||||||
| Male | 10663 (55.3) | 4278 (56.6) | 137684 (52.2) | 3131 (46.7) | 4531 (60.9) | 1400 (56.6) | 100554 (51.8) | 2398 (48.5) |
Abbreviations: DUR, drug utilization review; wks, weeks; mos, months; yrs, years.
Annual FQ prescription rate
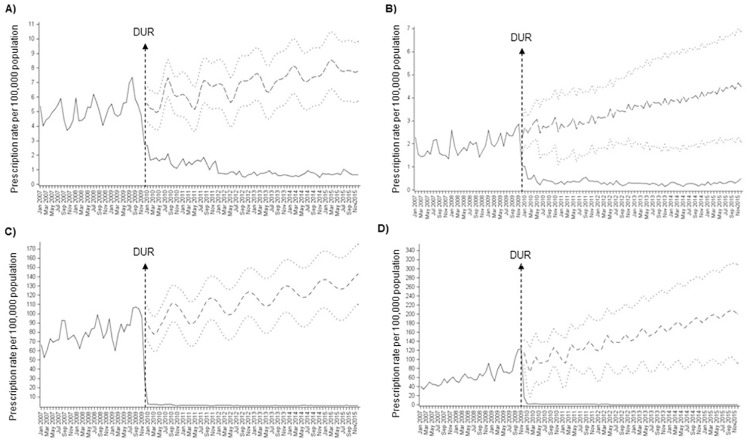
The monthly FQ prescription rate decreased significantly after the DUR implementation (Fig 1). The mean annual pre-DUR prescription rate of CF decreased from 840 to 21 per 100,000 person-years (relative reduction: 97.5%) and LF from 598 to 11 per 100,000 person-years (relative reduction: 98.2%). A greater reduction in the FQ prescription rate was observed in the outpatient (CF: from 782 to 10 per 100,000 person-years, 98.7%; LF: from 576 to 7 per 100,000 person-years, 98.8%) than in the inpatient group (CF: from 57 to 11 per 100,000 person-years, 80.7%; LF: from 22 to 4 per 100,000 person-years, 81.8%). Prescription rate of other commonly prescribed antibiotics in children and prescription of FQs in adults remained stable during the study period (Fig 2).
Fig 1. Impact of drug utilization review (DUR) intervention on the monthly fluoroquinolone prescription rates per 100,000 children younger than 18 years old.
(A) monthly prescription rate of CF in inpatient setting; (B) monthly prescription rate of LF in inpatient setting; (C) monthly prescription rate of CF in outpatient setting; (D) monthly prescription rate of LF in outpatient setting. Solid lines represent observed values, dashed lines represent predicted values and dotted lines represent 95% confidence intervals of predicted values. Abbreviations: CF, ciprofloxacin; LF, levofloxacin; DUR, drug utilization review.
Fig 2. Prescription of fluoroquinolones and other antibiotics to children and prescription of fluoroquinolone to adults (log scale).
Claimed diagnostic codes and specialties of prescribers
The top five most common diagnostic codes under which FQ was prescribed in children are shown in S1 Table. The most commonly used diagnostic code in the inpatient group remained unchanged after DUR (CF: certain infectious and parasitic diseases [A00–B99]; LF: diseases of the respiratory system [J00–J99]), and only minor changes occurred in the other top five diagnostic codes. In the outpatient setting, respiratory system diseases (J00-J99) were the most prevalent diagnostic codes associated with prescriptions of both CF and LF in the pre-DUR group. However, after DUR, significant changes in the diagnostic codes were observed with CF most commonly prescribed for certain infectious and parasitic diseases (A00–B99), and LF for diseases of the genitourinary system (N00–N99).
Furthermore, we analyzed the specialties of the physicians who prescribed FQ. The top five specialties of physicians who prescribed FQ in children are shown in Table 2. The most common specialties that prescribed FQs in the inpatient setting remained the same (both CF and LF: ‘Internal medicine’), and only minor changes between the pre-DUR and post-DUR periods were observed in the FQ prescription rates by the other top-ranked specialties. Notably, ‘Pediatrics’ ranked second in post-DUR LF inpatient prescriptions despite not being included in top five in pre-DUR. In the outpatient setting, significant changes in the distribution of FQ prescriptions across specialties were observed after DUR. For CF, ‘Obstetrics & Gynecology’ and ‘Surgery’ became newly ranked in top five after DUR replacing ‘Otolaryngology’ and ‘Ophthalmology’. Similarly, for LF, ‘Urology’ and ‘Obstetrics & Gynecology’ became newly ranked in top five after DUR replacing ‘Ophthalmology’ and ‘Pediatrics’.
Table 2. Five most common medical specialties of physicians prescribing pediatric fluoroquinolone prescriptions.
| Prescriber specialty, N (%) | ||||
|---|---|---|---|---|
| CF inpatient | Rank | Pre-DUR (n = 19284) | Rank | Post-DUR (n = 7553) |
| 1 | Internal Medicine: 9446 (49.0) | 1 | Internal Medicine: 2687 (35.6) | |
| 2 | Surgery: 2080 (10.8) | 2 | Otolaryngology: 1252 (16.6) | |
| 3 | Otolaryngology: 2047 (10.6) | 3 | Pediatrics: 1180 (15.6) | |
| 4 | Orthopedics: 1958 (10.2) | 4 | Orthopedics: 885 (11.7) | |
| 5 | Pediatrics: 1191 (6.2) | 5 | Surgery: 624 (8.3) | |
| CF outpatient | Rank | Pre-DUR (n = 263513) | Rank | Post-DUR (n = 6704) |
| 1 | Internal Medicine: 90469 (34.3) | 1 | Internal Medicine: 3005 (44.8) | |
| 2 | Otolaryngology: 64643 (24.5) | 2 | Urology: 678 (10.1) | |
| 3 | Pediatrics: 30836 (11.7) | 3 | Obstetrics & Gynecology: 627 (9.4) | |
| 4 | Ophthalmology: 24350 (9.2) | 4 | Pediatrics: 524 (7.8) | |
| 5 | Urology: 12358 (4.7) | 5 | Surgery: 322 (4.8) | |
| LF inpatient | Rank | Pre-DUR (n = 7435) | Rank | Post-DUR (n = 2474) |
| 1 | Internal Medicine: 2629 (35.4) | 1 | Internal Medicine: 1024 (41.4) | |
| 2 | Surgery: 1039 (14.0) | 2 | Pediatrics: 402 (16.2) | |
| 3 | Orthopedics: 913 (12.3) | 3 | Orthopedics: 252 (10.2) | |
| 4 | Otolaryngology: 849 (11.4) | 4 | Otolaryngology: 223 (9.0) | |
| 5 | Urology: 446 (6.0) | 5 | Surgery: 207 (8.4) | |
| LF outpatient | Rank | Pre-DUR (n = 193955) | Rank | Post-DUR (n = 4941) |
| 1 | Internal Medicine: 57390 (29.6) | 1 | Internal Medicine: 1575 (31.9) | |
| 2 | Otolaryngology: 30020 (15.5) | 2 | Urology: 822 (16.6) | |
| 3 | Ophthalmology: 28743 (14.8) | 3 | Obstetrics & Gynecology: 564 (11.4) | |
| 4 | Pediatrics: 22870 (11.8) | 4 | Otolaryngology: 356 (7.2) | |
| 5 | Dermatology: 16611 (8.6) | 5 | Dermatology: 324 (6.6) | |
Abbreviations: CF, ciprofloxacin; LF, levofloxacin; DUR, drug utilization review
Medical facility distribution of FQ prescriptions
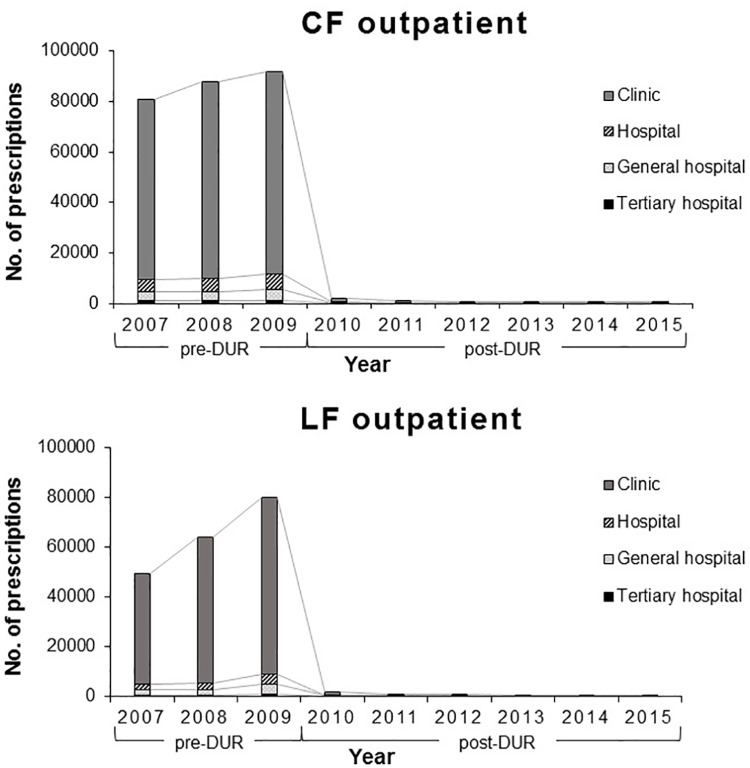
Significant changes in the distribution of FQ prescriptions by type of medical facility were observed after DUR implementation, (Fig 3; CF: inpatient P < .001, outpatient P < .001; LF: inpatient P < .001, outpatient P < .001). Such changes were more dramatic in the outpatient setting than in the inpatient setting and the changes in the yearly distribution of CF and LF prescriptions in the outpatient settings are shown in Fig 4. The proportion of FQ prescriptions issued by private clinics declined significantly after DUR, whereas those issued by other medical facilities, hospital, general hospital an tertiary hospital, increased.
Fig 3. Distribution of pediatric fluoroquinolone prescriptions in medical facilities before and after implementation of drug utilization review.
(A) distribution of CF prescriptions in medical facilities in inpatient setting, pre- and post-DUR (log scale); (B) distribution of LF prescriptions in medical facilities in inpatient setting, pre- and post-DUR (log scale); (C) distribution of CF prescriptions in medical facilities in outpatient setting, pre- and post-DUR (log scale); (D) distribution of LF prescriptions in medical facilities in outpatient setting, pre- and post-DUR (log scale).
Fig 4. Yearly distribution of pediatric fluoroquinolone prescriptions in medical facilities in outpatient setting.
Abbreviations: CF, ciprofloxacin; LF, levofloxacin; DUR, drug utilization review.
Discussion
Our study is first to assess the national off-label prescription of FQ in children, as no approved indications exist for FQ in South Korea. Prescription volume of FQ in all healthcare settings was observed over 9 years using a large-scale national database. Fluoroquinolone use declined substantially and immediately after the implementation of age restriction under DUR. The effect has been sustained for six years since implementation.
The nationwide incidence of FQ off-label prescription in children declined from 0.840% to 0.021% for CF and from 0.598% to 0.011% for LF after the regulatory action. A study conducted in British Columbia, Canada showed an incidence ranging between 0.06% and 0.09%, which is closer to the incidence after DUR implementation in South Korea [9]. Due to the lack of nationwide studies in other countries, direct comparisons are not possible. However, the pediatric FQ use in South Korea can be thought to be high as there are no approved indications in South Korea. The incidence of FQ prescription in the ambulatory setting was found to be higher than in the inpatient setting before the regulatory action, and a greater reduction occurred in the ambulatory setting than in the inpatient setting.
The pattern of the diagnostic codes associated with FQ prescription changed markedly after DUR implementation, especially in the outpatient setting. Before the implementation of DUR, ‘Diseases of the respiratory system (J00–J99)’ were the most commonly used diagnostic codes for both CF and LF outpatient prescriptions. However, after DUR implementation, ‘Certain infectious and parasitic diseases (A00–B99)’ and ‘Diseases of the genitourinary system (N00–N99)’ were the most commonly used diagnostic codes for CF and LF, respectively. In regards to medical specialties, ‘Internal medicine’ remained to be the most common medical specialty of physicians who prescribed FQs to children in both inpatient and outpatient settings for both CF and LF after DUR implementation. Prescribers with internal medicine as specialty are considered primary care physicians in Korea and internal medicine is a common specialty visited by patients of all ages [38]. The distribution of medical facilities at which FQs were prescribed also shifted significantly. After DUR implementation, the proportion of FQ prescriptions issued in clinics and primary care settings declined significantly; however, the proportion of prescriptions issued by referral hospitals increased, and such change was more dramatic in the ambulatory outpatient settings. The high volume of FQ prescriptions in primary care setting before DUR implementation is especially alarming, as unnecessary prescription of antibiotics frequently occur in an ambulatory setting [39, 40]. Additionally, current recommendations for pediatric FQ use are restricted to situations were no safe and effective alternative is available and the infection is caused by multidrug-resistant pathogens likely requiring inpatient treatment. Therefore, the shift in FQ prescription from primary care settings to referral hospitals, especially in the outpatient setting, is promising. The volume of prescriptions of other antibiotics for pediatric patients, as well as that of FQ to adults, was stable during the study period from 2009 to 2015, confirming that no other factors such as drug shortages or overall changes in pediatric antibiotic prescription are attributable to such significant reduction in FQ prescription volume in children.
Educational strategies, community-wide campaigns, and outpatient antimicrobial stewardship interventions often fail to exert a statistically significant and sustained effect on the issuance of antibiotic prescriptions [41–45]. Antimicrobial stewardship interventions by hospitals are commonly implemented to control for inappropriate use of antibiotics. However, in ambulatory settings, especially those involving primary care, these interventions or policies are rarely applied, which may be a reason behind the excessive antibiotic use in these settings. FQs have been suggested as the drugs of choice in treating complicated infections caused by drug-resistant pathogens in children [14, 15]. However, on the other hand, the inappropriate prescription of antibiotics is the main factor driving the emergence and spread of antibiotic resistance, and increasing FQ resistance is also of concern in pediatric populations [16, 46–50]. Therefore, it is important to promote judicious prescribing of FQ in children, only in situations where its use is required. The DUR system implemented in South Korea can contribute to such movement by encouraging reassessment of selecting FQ for use in pediatric patients.
The outcomes of the intervention under DUR program are not only positive in terms of reducing resistance but also limiting potential toxicity in pediatrics. Fluoroquinolones were initially restricted for use in children due to potential for cartilage toxicity. Recently, US FDA issued a FDA Drug Safety Communication recommending restriction of fluoroquinolones in adults due to side effects involving the tendons, muscles and joints [51]. Thus, it may be necessary to limit fluoroquinolones to absolutely needed conditions in both adults and children. Regulatory interventions, like DUR, may be required in other countries to effectively and sustainably restrict use of fluoroquinolone antibiotics. Additionally, it may be valuable to assess the impact of other public health interventions, such as the recent issue of the US FDA Drug Safety Communication [51], although, to the best of our knowledge, the interventions implemented in other systems are likely to be much less invasive the intervention under Korean DUR system.
Our results should be interpreted with caution. Claims data on individual-level do not contain information such as exact diagnosis, isolated pathogens, or antimicrobial susceptibility. Although diagnostic codes were collected for all prescribing events, the question of whether the changes in pediatric FQ prescription patterns align with the recommendations in the literature or with its approval status in other countries cannot be answered. Thus, we were unable to assess the appropriateness of any particular FQ prescription; therefore, it was not possible to determine whether most of the eliminated prescriptions were inappropriate. Also, domestic and international antimicrobial stewardship initiatives conducted during our study period may have influenced the results of the study and confounding factor from unmeasurable variables could not be excluded. Although we attempted to exclude the possible effect of drug shortages and overall changes in pediatric antibiotic prescription patterns by analyzing the prescription volume of five commonly prescribed antibiotics, it was not possible to determine which antibiotics had been used in place of FQ. Despite such limitations, it is evident that FQ use in children decreased in South Korea after DUR implementation and further studies on how the regulatory action influenced antibiotic prescribing and clinical outcomes are required.
This study is first to analyze the nationwide pediatric prescription of FQ. Age restriction under the DUR system resulted in an immediate, dramatic decline in FQ prescription and which effect was sustained afterwards. Regulatory action via the national DUR system appears to be especially effective in the ambulatory setting, promoting more judicious use of FQ as part of antimicrobial stewardship on a national level. Therefore, the potential use of similar regulatory intervention to control inappropriate and excessive use of certain medications in other countries, states or institutions appear promising.
Supporting information
(PDF)
Data Availability
Data are available from the HIRA database (http://opendata.hira.or.kr/op/opc/selectPatDataAplInfoView.do). For additional questions about data availability, please contact the person in charge (minji3701@hira.or.kr).
Funding Statement
This research was supported by a grant from the Korea Food and Drug Administration (1172KFDA294) and National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (NRF-2015R1A5A1008958). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Committee on Infectious Diseases. The Use of Systemic Fluoroquinolones. Pediatrics. 2006;118(3):1287 10.1542/peds.2006-1722 [DOI] [PubMed] [Google Scholar]
- 2.Bradley JS, Jackson MA, Brady MT, Bernstein HH, Byington CL, Edwards KM, et al. The use of systemic and topical fluoroquinolones. Pediatrics. 2011;128(4):E1034–E45. 10.1542/peds.2011-1496 [DOI] [PubMed] [Google Scholar]
- 3.Choi S-H, Kim EY, Kim Y-J. Systemic use of fluoroquinolone in children. Korean J Pediatr. 2013;56(5):196–201. 10.3345/kjp.2013.56.5.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burkhardt JE, Walterspiel JN. Quinolone Arthropathy in Animals versus Children. Clin Infect Dis. 1997;25(5):1196–204. 10.1086/516119 [DOI] [PubMed] [Google Scholar]
- 5.Yang Z, Zahar J, Méchaï F, Postaire M, Blanot S, Balfagon-Viel S, et al. Current ciprofloxacin usage in children hospitalized in a referral hospital in Paris. BMC Infect Dis. 2013;13(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimland E, Odlind V. Off-label drug use in pediatric patients. Clin Pharmacol Ther. 2012;91(5):796–801. 10.1038/clpt.2012.26 [DOI] [PubMed] [Google Scholar]
- 7.Porta A, Esposito S, Menson E, Spyridis N, Tsolia M, Sharland M, et al. Off-label antibiotic use in children in three European countries. Eur J Clin Pharmacol. 2010;66(9):919–27. 10.1007/s00228-010-0842-1 [DOI] [PubMed] [Google Scholar]
- 8.Genuini M, Prot-Labarthe S, Bourdon O, Doit C, Aujard Y, Naudin J, et al. Fluoroquinolones in pediatrics: review of hospital prescription use over 2 years. Int J Clin Pharmacol Ther. 2014;52(11):940–7. 10.5414/CP202103 [DOI] [PubMed] [Google Scholar]
- 9.Marra F, Patrick DM, Chong M, Bowie WR. Antibiotic use among children in British Columbia, Canada. J Antimicrob Chemother. 2006;58(4):830–9. 10.1093/jac/dkl275 [DOI] [PubMed] [Google Scholar]
- 10.Bradley JS, Kauffman RE, Balis DA, Duffy CM, Gerbino PG, Maldonado SD, et al. Assessment of musculoskeletal toxicity 5 years after therapy with levofloxacin. Pediatrics. 2014;134(1):e146 10.1542/peds.2013-3636 [DOI] [PubMed] [Google Scholar]
- 11.Grady R. Safety profile of quinolone antibiotics in the pediatric population. Pediatr Infect Dis J. 2003;22(12):1128–32. 10.1097/01.inf.0000101994.25947.12 [DOI] [PubMed] [Google Scholar]
- 12.Murray TS, Baltimore RS. Pediatric Uses of Fluoroquinolone Antibiotics. Pediatr Ann. 2007;36(6):336–42. [DOI] [PubMed] [Google Scholar]
- 13.Chalumeau M, Tonnelier S, d'Athis P, Treluyer J-M, Gendrel D, Breart G, et al. Fluoroquinolone Safety in Pediatric Patients: A Prospective, Multicenter, Comparative Cohort Study in France. Pediatrics. 2003;111(6):e714–E9. 10.1542/peds.111.6.e714 [DOI] [PubMed] [Google Scholar]
- 14.Pichichero ME, Casey JR. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA. 2007;298(15):1772–8. 10.1001/jama.298.15.1772 [DOI] [PubMed] [Google Scholar]
- 15.Principi N, Esposito S. Appropriate use of fluoroquinolones in children. Int J Antimicrob Agents. 2015;45(4):341–6. 10.1016/j.ijantimicag.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 16.Adam HJ, Hoban DJ, Gin AS, Zhanel GG. Association between fluoroquinolone usage and a dramatic rise in ciprofloxacin-resistant Streptococcus pneumoniae in Canada, 1997–2006. Int J Antimicrob Agents. 2009;34(1):82–5. 10.1016/j.ijantimicag.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 17.Sabharwal V, Marchant CD. Fluoroquinolone Use in Children. Pediatr Infect Dis J. 2006;25(3):257–8. 10.1097/01.inf.0000205799.35780.f3 [DOI] [PubMed] [Google Scholar]
- 18.Robicsek A, Jacoby GA, Hooper DC. The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect Dis. 2006;6(10):629 10.1016/S1473-3099(06)70599-0 [DOI] [PubMed] [Google Scholar]
- 19.Lau SM, Peng MY, Chang FY. Resistance rates to commonly used antimicrobials among pathogens of both bacteremic and non-bacteremic community-acquired urinary tract infection. J Microbiol Immunol Infect. 2004;37(3):185–91. [PubMed] [Google Scholar]
- 20.Neuhauser MM, Weinstein RA, Rydman R, Danziger LH, Karam G, Quinn JP. Antibiotic resistance among gram-negative bacilli in US intensive care units: implications for fluoroquinolone use. JAMA. 2003;289(7):885–8. [DOI] [PubMed] [Google Scholar]
- 21.Hsueh PR, Hoban DJ, Carmeli Y, Chen SY, Desikan S, Alejandria M, et al. Consensus review of the epidemiology and appropriate antimicrobial therapy of complicated urinary tract infections in Asia-Pacific region. J Infect. 2011;63(2):114–23. 10.1016/j.jinf.2011.05.015 [DOI] [PubMed] [Google Scholar]
- 22.Sarma JB, Marshall B, Cleeve V, Tate D, Oswald T, Woolfrey S. Effects of fluoroquinolone restriction (from 2007 to 2012) on resistance in Enterobacteriaceae: interrupted time-series analysis. J Hosp Infect. 2015;91(1):68–73. 10.1016/j.jhin.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 23.de Lastours V, Chau F, Roy C, Larroque B, Fantin B. Emergence of quinolone resistance in the microbiota of hospitalized patients treated or not with a fluoroquinolone. J Antimicrob Chemother. 2014;69(12):3393–400. 10.1093/jac/dku283 [DOI] [PubMed] [Google Scholar]
- 24.Low DE. Fluoroquinolone-resistant pneumococci: maybe resistance isn't futile? Clin Infect Dis. 2005;40(2):236–8. 10.1086/426822 [DOI] [PubMed] [Google Scholar]
- 25.Heo JH, Suh DC, Kim S, Lee EK. Evaluation of the pilot program on the real-time drug utilization review system in South Korea. Int J Med Inform. 2013;82(10):987–95. 10.1016/j.ijmedinf.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 26.Korea Ministry of Food and Drug Safety. Public Announcement on Additional List of Age-Contraindicated Drugs. 2009. http://www.mfds.go.kr/index.do?mid=916&seq=7145.
- 27.US Food and Drug Administration. Levaquin (levofloxacin) Label 2008. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021721s020_020635s57_020634s52_lbl.pdf.
- 28.US Food and Drug Administration. Cipro (ciprofloxacin hydrochloride) 2004. http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/019537s057,020780s019lbl.pdf.
- 29.European Medicines Agency. Ciprofloxacin Bayer—Article 30 referral—Annex I, II, III London 2008. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Ciprofloxacin_Bayer_30/WC500008075.pdf.
- 30.Shin JY, Kim MH, Shin SM, Lee SH, Park BJ. Dramatic decrease in fluoroquinolones in the pediatric population in Korea. Pharmacoepidemiol Drug Saf. 2014;23(12):1320–4. 10.1002/pds.3696 [DOI] [PubMed] [Google Scholar]
- 31.Song SO, Jung CH, Song YD, Park C-Y, Kwon H-S, Cha BS, et al. Background and Data Configuration Process of a Nationwide Population-Based Study Using the Korean National Health Insurance System. Diabetes & Metabolism Journal. 2014;38(5):395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Na K-H, Kim HJ, Kim KH, Han S, Kim P, Hann HJ, et al. Prevalence, Age at Diagnosis, Mortality, and Cause of Death in Retinitis Pigmentosa in Korea—A Nationwide Population-based Study. American Journal of Ophthalmology. 2017;176:157–65. 10.1016/j.ajo.2017.01.014 [DOI] [PubMed] [Google Scholar]
- 33.Yoon YK, Park GC, An H, Chun BC, Sohn JW, Kim MJ. Trends of Antibiotic Consumption in Korea According to National Reimbursement Data (2008–2012): A Population-Based Epidemiologic Study. Medicine. 2015;94(46):e2100 10.1097/MD.0000000000002100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Youngster I, Avorn J, Belleudi V, Cantarutti A, Díez-Domingo J, Kirchmayer U, et al. Antibiotic Use in Children—A Cross-National Analysis of 6 Countries. The Journal of Pediatrics. 2017;182:239–44.e1. 10.1016/j.jpeds.2016.11.027 [DOI] [PubMed] [Google Scholar]
- 35.Lee YS, Kwon JW, Oh OH, Sohn HS. Temporal decrease in overall antibiotic consumption accompanying antibiotic prescribing rate disclosure policy: evidence from analysis of national health insurance claims data in South Korea. Arch Pharm Res. 2014;37(10):1295–300. 10.1007/s12272-014-0333-5 [DOI] [PubMed] [Google Scholar]
- 36.Kim L, Sakong J, Kim Y, Kim S, Kim S, Tchoe B, et al. Developing the Inpatient Sample for the National Health Insurance Claims Data. Health Policy and Management. 2013;23(2):152–61. [Google Scholar]
- 37.Statistics Korea. Korean Satatistical Information Service 2014. http://kosis.kr/eng/statisticsList/statisticsList_01List.jsp?vwcd=MT_ETITLE&parentId=A#SubCont.
- 38.Lee JY, Eun SJ, Kim HJ, Jo M-W. Finding the Primary Care Providers in the Specialist-Dominant Primary Care Setting of Korea: A Cluster Analysis. PLoS One. 2016;11(8):e0161937 10.1371/journal.pone.0161937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finkelstein JA. Putting antibiotic prescribing for children into context. JAMA. 2013;309(22):2388–9. 10.1001/jama.2013.6288 [DOI] [PubMed] [Google Scholar]
- 40.Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128(6):1053–61. 10.1542/peds.2011-1337 [DOI] [PubMed] [Google Scholar]
- 41.Rubin MA, Bateman K, Alder S, Donnelly S, Stoddard GJ, Samore MH. A multifaceted intervention to improve antimicrobial prescribing for upper respiratory tract infections in a small rural community. Clin Infect Dis. 2005;40(4):546–53. 10.1086/427500 [DOI] [PubMed] [Google Scholar]
- 42.Weiss K, Blais R, Fortin A, Lantin S, Gaudet M. Impact of a Multipronged Education Strategy on Antibiotic Prescribing in Quebec, Canada. Clin Infect Dis. 2011;53(5):433–9. 10.1093/cid/cir409 [DOI] [PubMed] [Google Scholar]
- 43.Gerber JS, Prasad PA, Fiks AG, Localio AR, Grundmeier RW, Bell LM, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309(22):2345–52. 10.1001/jama.2013.6287 [DOI] [PubMed] [Google Scholar]
- 44.Perz JF, Craig AS, Coffey CS, Jorgensen DM, Mitchel E, Hall S, et al. Changes in antibiotic prescribing for children after a community-wide campaign. JAMA. 2002;287(23):3103–9. [DOI] [PubMed] [Google Scholar]
- 45.Gerber JS, Prasad PA, Fiks AG, Localio AR, Bell LM, Keren R, et al. Durability of benefits of an outpatient antimicrobial stewardship intervention after discontinuation of audit and feedback. JAMA. 2014;312(23):2569–70. 10.1001/jama.2014.14042 [DOI] [PubMed] [Google Scholar]
- 46.Goossens H, Ferech M, Vander Stichele R, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365(9459):579–87. 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 47.Mandell LA, Bartlett JG, Dowell SF, File TM Jr., Musher DM, Whitney C. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin Infect Dis. 2003;37(11):1405–33. 10.1086/380488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacDougall C, Powell JP, Johnson CK, Edmond MB, Polk RE. Hospital and Community Fluoroquinolone Use and Resistance in. Clin Infect Dis. 2005;41(4):435–40. 10.1086/432056 [DOI] [PubMed] [Google Scholar]
- 49.Steinke D, Davey P. Association between antibiotic resistance and community prescribing: a critical review of bias and confounding in published studies. Clin Infect [DOI] [PubMed] [Google Scholar]
- 50.Bryce A, Hay AD, Lane IF, Thornton HV, Wootton M, Costelloe C. Global prevalence of antibiotic resistance in pediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: systematic review and meta-analysis. BMJ. 2016;352:i939 10.1136/bmj.i939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.US Food and Drug Administration. FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together 2016. http://www.fda.gov/Drugs/DrugSafety/ucm500143.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
Data are available from the HIRA database (http://opendata.hira.or.kr/op/opc/selectPatDataAplInfoView.do). For additional questions about data availability, please contact the person in charge (minji3701@hira.or.kr).