Abstract
Background & aims
Availability of directly-acting antivirals (DAAs) has changed the treatment landscape of hepatitis C virus (HCV) infection. The high price of DAAs has restricted their use in several countries. However, in some countries such as India, generic DAAs are available at much cheaper price. This study examined whether generic DAAs could be cost-saving and how long it would take for the treatment to become cost-saving/effective.
Methods
A previously-validated, mathematical model was adapted to the HCV-infected population in India to compare the outcomes of no treatment versus treatment with DAAs. Model parameters were estimated from published studies. Cost-effectiveness of HCV treatment using available DAAs was calculated, using a payer’s perspective. We estimated quality-adjusted life years (QALYs), disability-adjusted life years (DALYs), total costs, and incremental cost-effectiveness ratio of DAAs versus no treatment. One-way and probabilistic sensitivity analyses were conducted.
Results
Compared with no treatment, the use of generic DAAs in Indian HCV patients would increase the life expectancy by 8.02 years, increase QALYs by 3.89, avert 19.07 DALYs, and reduce the lifetime healthcare costs by $1,309 per-person treated. Treatment became cost-effective within 2 years, and cost-saving within 10 years of its initiation overall and within 5 years in persons with cirrhosis. Treating 10,000 HCV-infected persons could prevent 3400–3850 decompensated cirrhosis, 1800–2500 HCC, and 4000–4550 liver-related deaths. The results were sensitive to the costs of DAAs, pre- and post-treatment diagnostic tests and management of cirrhosis, and quality of life after sustained virologic response.
Conclusions
Treatment with generic DAAs available in India will improve patient outcomes, provide a good value for money within 2 years, and be ultimately cost-saving. Therefore, in this and similar settings, HCV treatment should be a priority from a public health as well an economic perspective.
Introduction
Hepatitis C virus (HCV) infects more than 70 million people worldwide and between 6–11 million in India [1]. Chronic HCV infection is the leading cause for cirrhosis, hepatocellular carcinoma (HCC) and liver-related deaths worldwide. Untreated HCV infection also leads to substantial economic burden [2]. In India, HCV infection was estimated to be responsible for 59,000 deaths in the year 2015 [3].
New directly-acting antivirals (DAAs) for HCV treatment are highly effective, with DAA-based regimens providing rates of sustained virological response (SVR) exceeding 95%. These regimens are also very safe and convenient, needing administration of oral drugs once or twice daily for 12–24 weeks [4]. Thus, these drugs offer a hope of reducing the burden of HCV. However, these drugs are very costly in several countries, limiting treatment to those with advanced disease. The median nominal price of a 12-week regimen of sofosbuvir across 26 Organisation for Economic Co-operation and Development (OECD) member countries was US$42,017, ranging from US$37,729 in Japan to US$64,680 in the United States [5].
However, in India, three DAAs (sofosbuvir, ledipasvir and daclatasvir) are available from several generic manufacturers at a price as low as $300 [6]. Despite this, treatment rates remain very low in India [1]. This is partially because of limited budget allocated to HCV and lack of data on the cost-effectiveness of HCV treatment. Almost all published cost-effectiveness analyses were conducted in high- and middle-income countries [7–12], where DAAs are costly. While it is expected that the DAAs will be cost-effective at low prices prevalent in India, it is not known how long it would take them to become cost-effective, and if DAAs could also be cost-saving; i.e. improve life expectancy and reduce costs at the same time. If it were indeed so, this would encourage advocacy by various stakeholders including the patient groups, physicians and public health professionals towards public funding of HCV treatment, and may make it easier for health administrators and political leaders to take such a decision.
We therefore estimated the cost-effectiveness of treatment of HCV-infected persons in India using low-priced DAAs, and evaluated if/when the upfront cost of treatment for HCV infection could result in cost-savings. This question is also of interest for other countries where it may be possible to obtain DAAs at low prices.
Methods
Model overview
We have previously developed and validated an individual-level Markov state-transition model, called the Markov-based Analyses of Treatments for Chronic Hepatitis C (MATCH) [9], using C++, a general-purpose computer programming language. The natural history output from this model has been validated with the results of a multicenter follow-up study of patients with advanced fibrosis, and with previously published cost-effectiveness studies [13–16]. For the current study, we adapted this model to the HCV-infected population in India (MATCH-India), following the principles recommended by the World Health Organization (WHO) on economic analyses in the field of viral hepatitis [17].
Characteristics of base case population
Our base case population included HCV-infected person, aged 35 years, in India. We defined a total of 30 unique patient profiles based on HCV genotype (G1, G3, or G4), and the patient’s sex (male or female) and METAVIR fibrosis score (no fibrosis [F0], portal fibrosis without septa [F1], portal fibrosis with few septa [F2], numerous septa without fibrosis [F3], or cirrhosis [F4]) [18]. The relative frequencies of these profiles in the HCV-infected Indian cohort were based on the available data (Table A in S1 Appendix). Patients with HIV or hepatitis B virus co-infection, and those belonging to special groups at higher risk of HCV reinfection (e.g. hemodialysis, thalassemia, and haemophilia) were excluded. All patients were considered treatment-naïve because the percentage of treatment-experienced patients in India is negligible.
Treatment regimens and efficacy
The treatment regimens used were based on the DAAs available in India, depending on HCV genotype and fibrosis stage (Table B in S1 Appendix) [19]. Treatment efficacy, adverse events and premature treatment discontinuation rates were modeled on data from recent clinical trials of DAAs in treatment-naïve patients [20–23].
Natural history of HCV infection
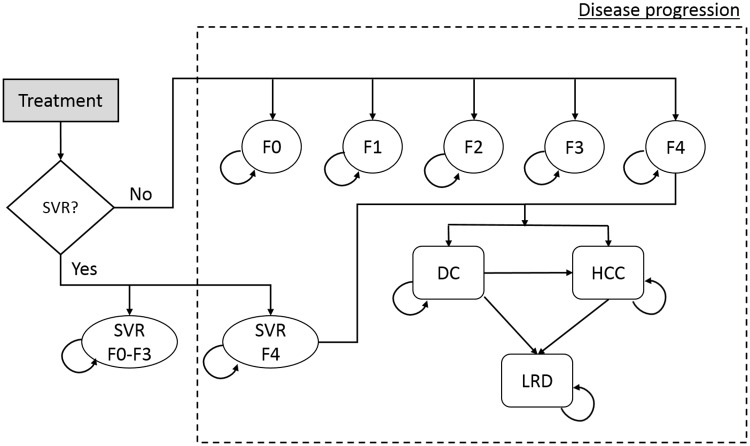
The natural history of HCV infection and progression were defined as transitions between Markov health states. Each patient started in one of five METAVIR liver fibrosis states (F0–F4) (Fig 1), and could, at the end of each cycle, remain in the same state, die from background mortality, or move into a higher fibrosis state, or to decompensated cirrhosis and/or HCC, or liver-related death. Patients in F0-F3 states who achieved SVR were assumed to be cured and to follow background mortality thereafter; however, those in F4 state who achieved SVR could progress to more advanced states, albeit at a slower rate [24]. Patients who failed to achieve SVR or who discontinued treatment continued to progress over time at the original rate. The cycle length used was one week.
Fig 1. State-transition model schematic of showing the natural history of hepatitis C virus infection.
Abbreviations: DC = decompensated cirrhosis; HCC = hepatocellular carcinoma; HCV = hepatitis C virus; LRD = liver-related death; SVR = sustained virologic response. (Adapted from Chhatwal et al.[9])
We used data from a previously-published meta-regression analysis to model fibrosis progression rates from F0 to F4 [25], and from published observational studies to model progression from cirrhosis to decompensated cirrhosis and HCC (Table 1) [26, 27]. Patients with decompensated cirrhosis or HCC had a higher liver-related mortality than those in early stages of HCV infection [28]. Because the number of liver transplants performed in India is negligible, our model disregarded the option of liver transplantation.
Table 1. Annual transition probabilities, healthcare costs and quality of life weights for different Markov states.
| Input | Base case | Values for sensitivity analysis | |||
|---|---|---|---|---|---|
| Range | Distribution | Parameter 1a | Parameter 2b | ||
| Transition probabilities (annual) | |||||
| F0 to F1 [25] | 0.117 | 0.104–0.130 | Beta | 274.98 | 2,075.30 |
| F1 to F2 [25] | 0.085 | 0.075–0.096 | Beta | 210.06 | 2,261.18 |
| F2 to F3 [25] | 0.120 | 0.109–0.133 | Beta | 288.05 | 2,112.38 |
| F3 to F4 [25] | 0.116 | 0.104–0.129 | Beta | 270.61 | 2,062.22 |
| F4 to DC [26] | 0.039 | 0.010–0.079 | Beta | 3.51 | 86.48 |
| F4 to HCC [26] | 0.014 | 0.010–0.079 | Beta | 0.18 | 12.38 |
| Post F4-SVR to DC [24] | 0.008 | 0.002–0.036 | Beta | 0.31 | 38.58 |
| Post F4-SVR to HCC [24] | 0.005 | 0.002–0.013 | Beta | 1.49 | 297.13 |
| DC to HCC [27] | 0.068 | 0.030–0.083 | Beta | 73.58 | 1008.49 |
| DC (first year) to death from liver disease [27] | 0.182 | 0.065–0.190 | Beta | 1626.40 | 7309.88 |
| DC (subsequent year) to death from liver disease [27] | 0.112 | 0.065–0.190 | Beta | 7.03 | 55.77 |
| HCC to death from liver disease (26) | 0.427 | 0.330–0.860 | Beta | 2.14 | 2.87 |
| Health state costs (annual in INR) | |||||
| F0-F3 [29, 30] | 2000 ($30)c | 0.5 to 2.0 fold | Gamma | 7.11 | 281.25 |
| Compensated cirrhosis [30] | 10,000 ($149) | 0.5 to 2.0 fold | Gamma | 7.11 | 281.25 |
| Decompensated Cirrhosis [30] | 40,000 ($596) | 0.5 to 2.0 fold | Gamma | 7.11 | 1406.25 |
| Hepatocellular Cancer [30] | 60,000 ($894) | 0.5 to 2.0 fold | Gamma | 7.11 | 5625.00 |
| Testing cost (INR) | |||||
| Pre-treatment (diagnosis) | 8,000 ($119) | 0.5 to 2.0 fold | Gamma | 7.11 | 1406.25 |
| Post-treatment | 6,000 ($89) | 0.5 to 2.0 fold | Gamma | 7.11 | 1125.00 |
| Health state quality-of-life weights | |||||
| Anemia multiplier d [31] | 0.83 | 0.75–0.97 | Beta | 22.95 | 4.70 |
| F0–F3 [32] | 0.93 | 0.84–0.99 | Beta | 47.47 | 3.57 |
| Compensated cirrhosis [32] | 0.90 | 0.81–0.99 | Beta | 31.12 | 3.46 |
| DC [32] | 0.80 | 0.57–0.99 | Beta | 12.29 | 3.07 |
| HCC [32] | 0.79 | 0.54–0.99 | Beta | 11.42 | 3.03 |
| Post-SVR | 1.00 | 0.92–1.00 | Beta | 3833.92 | 3.84 |
| Disutility weights | |||||
| F0-F4 (assumption) | 0 | 0–0.1 | Beta | 0.15 | 14.69 |
| DC [33] | 0.194 | 0.127–0.273 | Beta | 22.58 | 93.79 |
| HCC [33] | 0.508 | 0.348–0.670 | Beta | 19.08 | 18.48 |
Abbreviations: SVR, sustained virologic response; F0–F4, METAVIR fibrosis score; DC, decompensated cirrhosis; HCC, hepatocellular carcinoma; F4-SVR. Post-SVR state of treated cirrhotic patient. INR, Indian rupees (1 USD = 67.11 INR)
aParameter 1 corresponds to α parameter for beta distribution and k (shape) parameter for gamma distribution
bParameter 2 corresponds to β parameter for beta distribution and θ (scale) parameter for gamma distribution
cconversion rate:
dFor patients experienced anemia during treatment, quality of life was multiplied by this factor
Medical costs
The model represented a healthcare payer’s perspective. The cost of sofosbuvir-ledipasvir (with or without ribavirin), or sofosbuvir-daclatasvir combination treatment was taken as Indian Rupees (INR) 6711 (USD 100) for every 28 days’ supply [6]. The costs of pre-treatment testing (for disease staging and HCV genotyping) was taken as INR 8000 (USD 119), and those for tests while on and after treatment was taken as INR 6000 (USD 89); these costs were estimated using expert opinion, because such data in India are not available (Table 1). We used relatively low costs for treatment of sequelae because many Indian patients, given their limited financial resources, do not access highly-specialized care for these diseases, and because we wanted our model to represent a conservative scenario by underestimating the savings resulting from DAA use; however, this was supplemented by sensitivity analyses using a wide range of costs.
Quality of life and disability weights
For effectiveness outcomes, we estimated quality-adjusted life years (QALYs), a commonly used metric in cost-effectiveness analysis, and disability-adjusted life years (DALYs), as recommended by the WHO [33]. For QALYs, we assigned quality-of-life (QoL) weights for each health state from previous studies [16, 32, 34]. We assumed the QoL of patients who achieved SVR to be equivalent to that of the general population [32]. However, for those patients with SVR who progressed, the QoL of the respective advanced states was used. To calculate DALYs, we used expected years-of-life lost and disability weights defined by the Global Burden of Disease study (0 for METAVIR scores F0–F4, 0.194 [range 0.127–0.273] for decompensated cirrhosis, and 0.508 [0.348–0.67] for HCC) [33].
Cost-effectiveness analysis
The MATCH-India model was used to simulate the clinical course of HCV-infected persons in India with and without DAA-based treatment. We ran 10,000 iterations for each profile and projected the expected life-years, discounted QALYs, DALYs and discounted costs. From these, we estimated the incremental cost-effectiveness ratio (ICER; US$ per discounted QALY and US$ per DALY-averted) of DAA-based treatment in comparison with no treatment. We used a lifetime horizon, and discounted both future costs and QALYs at 3% per year, with additional sensitivity analyses with 0% and 5% rates. In addition, we projected the cumulative incidence of decompensated cirrhosis and HCC, and liver-related deaths.
Since HCV progresses slowly, the full benefits of successful HCV treatment may appear several years later. Therefore, we also estimated the ICERs over time. Specifically, we estimated how long would it take for the cost offsets resulting from prevention of advanced disease to exceed the upfront cost of DAA-based treatment.
Sensitivity analyses
We performed one-way sensitivity analyses to estimate the effects of changes in transition probabilities, QOL weights, disability weights, cost inputs and patient’s age on the cost-effectiveness of treatment. Since our estimates of outcomes of various disease states were derived from developed countries, we conducted a sensitivity analysis considering that mortality rates for various liver conditions in India could be 1.0 to 3.0-fold the baseline rates, and rates of progression of liver fibrosis in HCV infection could be 0.6 to 1.2-fold the baseline. We also performed probabilistic sensitivity analysis using 10,000 first-order and 5000 second-order samples by simultaneously varying all key model inputs using the recommended statistical distributions (Table 1).
Results
Baseline cost-effectiveness analysis
Compared with no treatment, the use of DAAs in Indian patients with HCV infection was estimated to increase overall life expectancy by 8.02 years and discounted QALYs by 3.89. The improvement in outcomes was more marked in patients with cirrhosis than those without, and was similar across viral genotypes (Table 2). The no-treatment arm had a lifetime cost of $1,988 per person, entirely for management of advanced sequelae of HCV infection. In contrast, the DAA arm had a lower lifetime cost of $679, including $324 on DAAs, $208 on pre/post-treatment tests, and $147 on management of advanced sequelae that still occurred in a few persons. In other words, antiviral treatment in India was found to be cost-saving, i.e., it increased QALYs by 3.89 years while simultaneously decreasing total healthcare costs by $1,309.
Table 2. Cost-effectiveness results comparing model outcomes of no treatment versus treatment with direct-acting antivirals in India.
| Patient group | Life Years | Quality-adjusted Life Years (Discounted*) | Total Life-time Cost (Discounted* $) | ICER ($/QALY)** | |||||
|---|---|---|---|---|---|---|---|---|---|
| No treatment | With DAA-based treatment | Increase in LYs | No treatment | With DAA-based treatment | Increase in QALY | No treatment | With DAA-based treatment | ||
| Non-cirrhosis (F0–F3) | |||||||||
| Genotype 1 | 30.25 | 37.92 | 7.677 | 15.05 | 18.75 | 3.71 | 1,803 | 536 | Cost-saving |
| Genotype 3 | 30.25 | 37.82 | 7.572 | 15.05 | 18.71 | 3.66 | 1,803 | 558 | Cost-saving |
| Genotype 4 | 30.25 | 37.70 | 7.452 | 15.05 | 18.65 | 3.60 | 1,803 | 590 | Cost-saving |
| All F0–F3 | 30.25 | 37.85 | 7.600 | 15.05 | 18.72 | 3.67 | 1,803 | 553 | Cost-saving |
| Cirrhosis (F4) | |||||||||
| Genotype 1 | 19.16 | 30.28 | 11.115 | 10.37 | 15.86 | 5.49 | 3,182 | 1,192 | Cost-saving |
| Genotype 3 | 19.16 | 29.65 | 10.487 | 10.37 | 15.51 | 5.15 | 3,182 | 1,672 | Cost-saving |
| Genotype 4 | 19.16 | 30.51 | 11.350 | 10.37 | 15.97 | 5.60 | 3,182 | 1,152 | Cost-saving |
| All F4 | 19.16 | 29.89 | 10.728 | 10.37 | 15.65 | 5.28 | 3,182 | 1,494 | Cost-saving |
| All patients | 28.76 | 36.78 | 8.020 | 14.42 | 18.31 | 3.89 | 1,988 | 679 | Cost-saving |
*QALYs were discounted at 3% per year rate
**Cost-saving implies the ICER was negative.
Abbreviations: DAA, direct-acting antivirals; ICER, incremental cost-effectiveness ratio; LYs, life years; QALY, quality-adjusted life year
Further, we found that HCV treatment with low-cost DAAs in India would avert 19.07 DALYs per-person treated (Table 3). This gain was similar across HCV genotypes and irrespective of presence/absence of cirrhosis. Cost per DALY averted was negative, i.e., HCV treatment with DAAs was cost-saving.
Table 3. Disability-adjusted life years (DALYs) averted with DAA-based treatment in HCV-infected patients in India, in relation to presence or absence of cirrhosis and viral genotype.
| Population group | DALY of no treatment | DALY with DAA-based treatment | DALY averted using DAA-based treatment | Cost per DALY averted ($) |
|---|---|---|---|---|
| Non-cirrhosis (F0-F3) | ||||
| Genotype 1 | 19.63 | 0.35 | 19.28 | -88 (cost-saving) |
| Genotype 3 | 19.63 | 0.58 | 19.05 | -88 (cost-saving) |
| Genotype 4 | 19.63 | 0.94 | 18.69 | -88 (cost-saving) |
| All genotypes | 19.63 | 0.53 | 19.11 | -88 (cost-saving) |
| Cirrhosis (F4) | ||||
| Genotype 1 | 36.36 | 16.73 | 19.62 | -107 (cost-saving) |
| Genotype 3 | 36.36 | 17.98 | 18.38 | -88 (cost-saving) |
| Genotype 4 | 36.36 | 16.37 | 19.99 | -107 (cost-saving) |
| All genotypes | 36.36 | 17.51 | 18.85 | -95 (cost-saving) |
| All patients | 21.87 | 2.80 | 19.07 | -89 (cost-saving) |
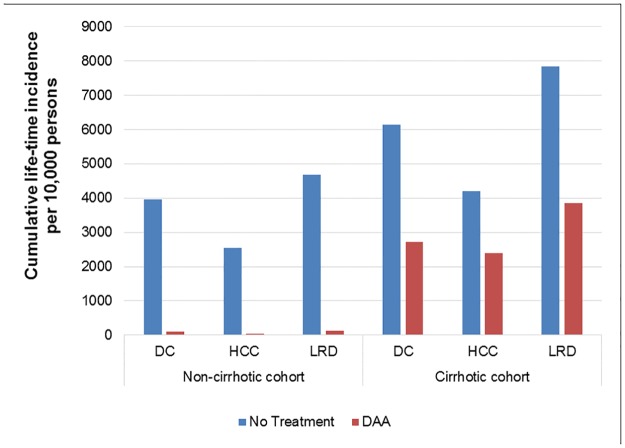
Compared with the no-treatment arm, treating 10,000 persons without cirrhosis using DAAs could prevent ~3850 decompensated cirrhosis, ~2500 HCC, and ~4550 liver-related deaths. In cirrhotic patients, treatment could prevent ~3400 decompensated cirrhosis, ~1800 HCC and ~4000 liver-related deaths (Fig 2).
Fig 2. Change in adverse clinical outcomes in patients with hepatitis C virus infection in India following treatment using regimens based on directly-acting antiviral drugs available there at low cost.
Abbreviations: DAA, direct-acting antivirals; DC, decompensated cirrhosis; HCC, hepatocellular carcinoma; LRD, liver-related deaths.
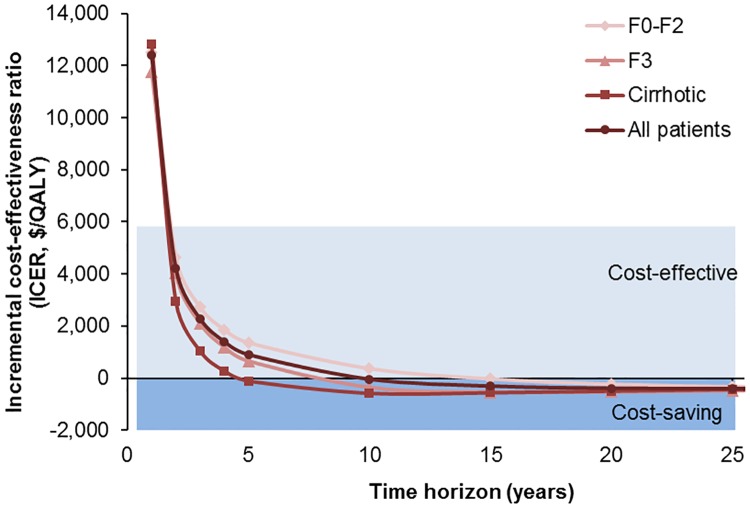
Cost-effectiveness over time
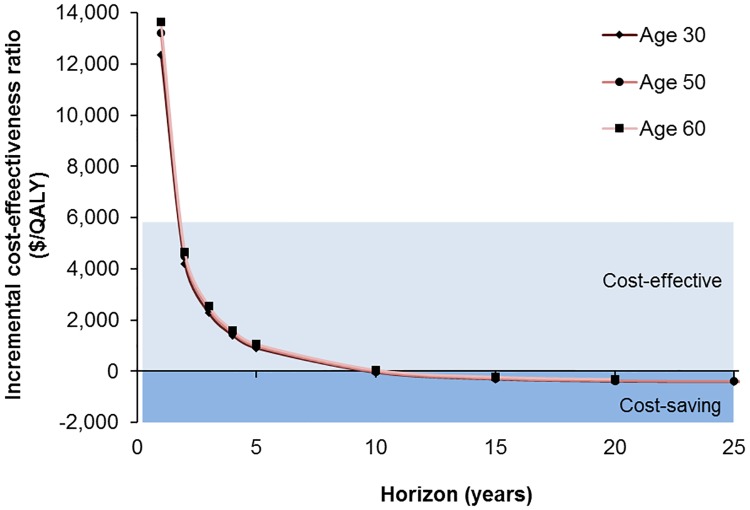
HCV treatment became cost-effective (i.e., ICER < three times India’s per capita annual gross domestic product of $1,942) within 2 years of starting treatment (Fig 3). Furthermore, HCV treatment became cost-saving by 10 years. In other words, by 10 years, the upfront cost of DAAs had been offset by savings from prevention of decompensated cirrhosis and HCC, even if gains in life-years and QALY were ignored. Treatment became cost-saving sooner for patients with advanced disease, i.e. within 4.72 years for those with cirrhosis, and at 11.8 years for those without cirrhosis. We also evaluated the ICER over time for persons treated at different ages (Fig 4); DAAs became cost-effective within 2 years and cost-saving within 10 years, irrespective of the age at treatment.
Fig 3. Cost-effectiveness of directly-acting antiviral drug-based treatment of persons with hepatitis C virus infection at various stages of liver fibrosis in India, depending on modelling time horizon.
Fig 4. Cost-effectiveness of direct-acting antiviral drug-based treatment of persons with hepatitis C virus infection, by age of starting treatment (years) and time horizon.
DAAs became cost-effective within 2 years of initiation of treatment irrespective of patient’s age; and DAAs became cost-saving for patients of age ≤50 around 10 years but never for patients at the age ≥60.
Sensitivity/scenario analyses
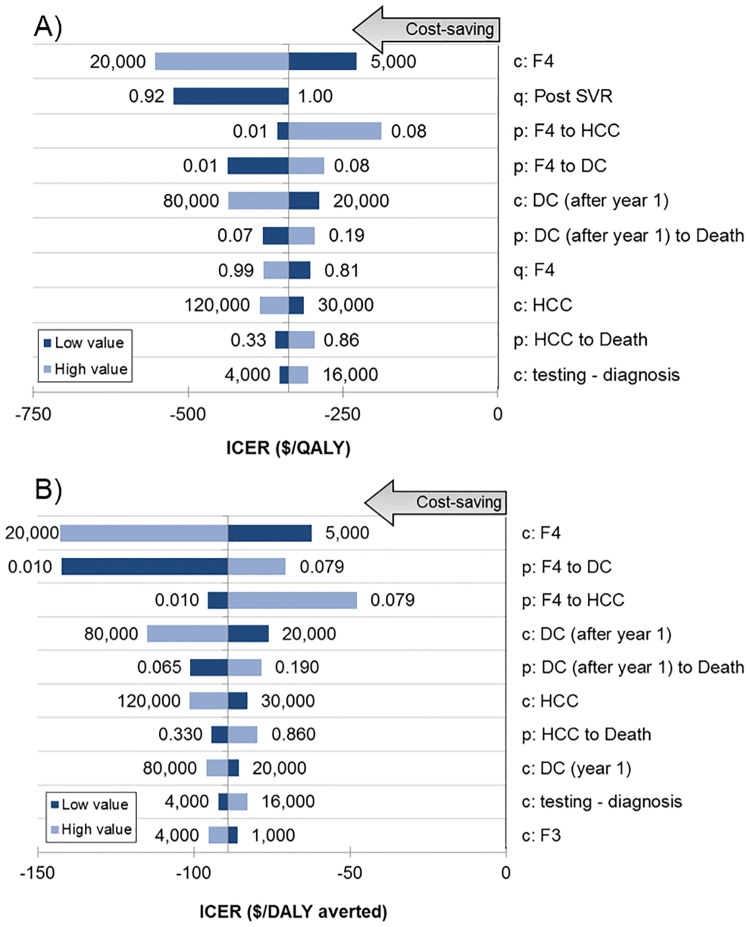
The 10 model parameters to which ICER per QALY was most sensitive are presented in a tornado diagram (Fig 5A). Treatment with DAAs in India always remained cost-saving (ICER/QALY <$0), despite extreme changes in each parameter. The ICER was most sensitive to the costs of management of cirrhosis, post-SVR QoL, and disease progression rate in patients with cirrhosis, cost of HCC management, and cost of HCV diagnosis and testing. Results for ICER per DALY were similar (Fig 5B). In additional scenario analyses on discount rate for costs and QALYs (0% and 5%), age at the time of treatment (20–70 years), drug costs ($100–$900 per 4-week treatment) and mortality rates of liver disease in India compared to developed countries, the DAAs always remained either cost-saving or cost-effective (Table 4). Another scenario analysis showed that DAA treatment remained cost-saving even if rates of progression of fibrosis in HCV infection were different in the Indian population, even though the number of liver events and liver-related deaths did change (Table C in S1 Appendix). A probabilistic sensitivity analysis, which accounted for uncertainty in all model parameters simultaneously, showed DAAs to be cost-saving with a 100% probability (Figure A in S1 Appendix).
Fig 5. Tornado diagram for one-way sensitivity analysis of incremental cost-effectiveness ratio using (A) $ per additional quality-adjusted life-year, and (B) $ per disability-adjusted life-year averted.
Horizontal bars show the variation in incremental cost-effectiveness ratio (ICER; in $/QALY gained or $/DALY averted) with variation in the value of the parameter. In the parameter names, the prefix ‘c’ represents cost of a health-state, ‘q’ the quality-of-life weight and ‘p’ the transition probability from one state to the other. Values of ICER below 0 indicate that the treatment is cost-saving. Abbreviations: QALY = quality-adjusted life-year, DALY = disability-adjusted life-year.
Table 4. Sensitivity analyses of cost-effectiveness results by age at time of treatment, cost of anti-HCV drug therapy and annual discount rate.
| Cost ($) | QALYs | Incremental QALYs | ICER ($/QALY) | |||
|---|---|---|---|---|---|---|
| No treatment | DAA | No treatment | DAA | |||
| Age category | ||||||
| 20 | 2284 | 700 | 16.26 | 21.91 | 5.65 | Cost-saving |
| 25 | 2207 | 694 | 15.78 | 20.86 | 5.08 | Cost-saving |
| 30 | 2108 | 687 | 15.14 | 19.64 | 4.49 | Cost-saving |
| 35 (base case) | 1988 | 679 | 14.42 | 18.31 | 3.89 | Cost-saving |
| 40 | 1847 | 669 | 13.54 | 16.83 | 3.29 | Cost-saving |
| 45 | 1683 | 658 | 12.60 | 15.36 | 2.75 | Cost-saving |
| 50 | 1495 | 646 | 11.47 | 13.73 | 2.27 | Cost-saving |
| 55 | 1291 | 632 | 10.27 | 12.09 | 1.83 | Cost-saving |
| 60 | 1090 | 617 | 8.94 | 10.34 | 1.40 | Cost-saving |
| 65 | 893 | 599 | 7.61 | 8.64 | 1.03 | Cost-saving |
| 70 | 711 | 586 | 6.27 | 7.04 | 0.77 | Cost-saving |
| Drug cost (4 weeks) | ||||||
| $100 (base case) | 1988 | 679 | 14.42 | 18.31 | 3.89 | Cost-saving |
| $300 | 1988 | 1325 | 14.42 | 18.31 | 3.89 | Cost-saving |
| $600 | 1988 | 2295 | 14.42 | 18.31 | 3.89 | 79 |
| $900 | 1988 | 3265 | 14.42 | 18.31 | 3.89 | 329 |
| 0% discount rate | ||||||
| Non-cirrhosis (F0–F3) | 3387 | 597 | 23.61 | 31.94 | 8.33 | Cost-saving |
| Cirrhosis (F4) | 4714 | 2052 | 14.66 | 25.04 | 10.38 | Cost-saving |
| All patients (F0–F4) | 3565 | 792 | 22.41 | 31.02 | 8.61 | Cost-saving |
| 5% discount rate | ||||||
| Non-cirrhosis (F0–F3) | 1275 | 537 | 11.86 | 14.20 | 2.34 | Cost-saving |
| Cirrhosis (F4) | 2572 | 1293 | 8.61 | 12.25 | 3.64 | Cost-saving |
| All patients (F0–F4) | 1449 | 639 | 11.42 | 13.94 | 2.52 | Cost-saving |
| Hazard ratio for liver-related mortality in India compared to our baseline estimate | ||||||
| 1.00 | 1988 | 679 | 14.42 | 18.31 | 3.89 | Cost-saving |
| 1.25 | 1868 | 663 | 14.31 | 18.29 | 3.99 | Cost-saving |
| 1.50 | 1778 | 652 | 14.22 | 18.28 | 4.07 | Cost-saving |
| 1.75 | 1713 | 644 | 14.15 | 18.28 | 4.12 | Cost-saving |
| 2.00 | 1657 | 638 | 14.09 | 18.27 | 4.17 | Cost-saving |
| 2.50 | 1577 | 627 | 14.01 | 18.26 | 4.25 | Cost-saving |
| 3.00 | 1519 | 620 | 13.95 | 18.25 | 4.30 | Cost-saving |
Discussion
Hepatitis C is a major cause of morbidity globally, with estimated 495,000 deaths and loss of 12.6 million DALYs per year [3]. The availability of DAAs has however changed the HCV treatment paradigm, leading to hope of elimination of this infection as a public health threat by the year 2030 [35]. However, the affordability and cost-effectiveness of DAAs, particularly in low-resource settings, have been debated widely. In our analysis, we found that HCV treatment with low-cost DAAs available in India will result in cost-savings because of prevention of decompensated cirrhosis and HCC in future. Furthermore, upfront spending on DAAs will provide a good value for money within as soon as 2 years of initiation of treatment.
Previous studies on cost-effectiveness of DAA-based treatments for HCV have all focused on the situations in developed countries, such as United States and European Union, where the drug costs are high [36]. In these studies, such treatment was found to be cost-effective, i.e. had ICER per QALY of below annual per capita national income, even at high costs prevalent in those countries. However, despite this demonstrated cost-effectiveness, the uptake of HCV treatment in these settings has been lower than expected because of the high cost of treating all infected patients.
By contrast, in several low-income countries, DAAs are available at highly discounted prices. Our cost-effectiveness analysis showed that the treatment of HCV infection with DAAs at the prices prevalent in India was not only cost-effective, but was in fact cost-saving, i.e., the cost of treatment is offset by the savings in future healthcare costs, thus leading to an overall monetary saving while at the same time increasing QALYs. Situations such as this, where pharmacological treatments have been found to be cost-saving, are rare. Thus, HCV treatment with low-priced DAAs is a win-win situation, warranting the use of public money to fund it.
Our results provide a compelling case for India to invest in HCV treatment. Unlike most other pharmacological interventions, HCV treatment with generic DAAs is not merely cost-effective, but also cost-saving. Therefore, investment in HCV treatment paying back for itself over time could be a very rewarding public health investment. For that reason, we estimated the duration in which the upfront cost of DAAs will be offset by the resultant health benefits—a useful parameter for policymakers who prioritize allocation of funds across sectors and diseases. Our analysis found this ‘pay-back’ time to be as short as 10 years, making the public funding of DAA-based treatment of HCV in India quite attractive. In this context, it seems particularly worthwhile to fund the treatment of patients with compensated cirrhosis, since the expenditure would be recouped even faster, i.e. within 5 years.
We recognize that our study had some limitations. First, we did not include patients with HCV genotypes 2, 5 and 6 because their prevalence rate is less than 1% in India. Second, as with most of the previously published models of HCV treatment, we ignored the benefits and costs of re-treatment of patients who failed DAAs. With recent advancements in DAAs, newer compounds that are effective in patients who had not responded to the drugs considered in our analysis. However, these newer drugs, which should be useful for retreatment, are not yet available in India; also, these drugs too are expected to be available at low prices in India. Third, the costs of treatment of HCV sequelae in our study were based on expert opinion than on real data, because of lack of published information. However, our conservative assumptions, if anything, biased the analysis against the DAA-based treatment; further, sensitivity analyses over a wide range of costs showed that our results were robust. Finally, we excluded patients with HIV or hepatitis B virus co-infection, or those at higher risk of HCV reinfection. However, this should not detract from our conclusions since such patients account for only a small proportion of the total HCV-infected pool in the Indian population. On the positive side, our analysis and report follow the principles recommended by a WHO group, aimed at enhancing transparency of economic analyses in the field of viral hepatitis [17]. Of course, such analyses would gain from better cost data on various disease conditions in different settings, an area that needs further work.
Health planners would be interested in knowing whether the treatment is more cost-effective or cost-saving in some particular subgroups, which could then receive priority for treatment. We found that gender and viral genotype did not influence the cost-effectiveness of DAA-based HCV treatment much. However, patient’s disease stage and age at treatment influenced the response, with a much greater cost-effectiveness in those with advanced liver disease. Hence, the governments with limited resources may consider prioritizing treatment of such groups, to maximize the public health impact within their limited resources [37]. Such prioritization is often essential, since ramp-up of public-health treatment programs takes time, and the entire budget is seldom immediately available. Another factor that may guide prioritization is the regional variation in HCV epidemiology within a country; in such cases, modelling for various subnational regions may be helpful.
Though the cost of DAAs has come down, the cost of diagnosis and testing remain high. Thus, with the availability of pan-genotypic DAA-based regimens, it would be important for public-health programs to develop and implement simplified treatment guidelines that obviate the use of pre-treatment HCV genotype testing. Similarly, it would be useful to limit the post-treatment testing to only one occasion, i.e. 12 weeks after completing treatment [37]. Also, in future, use of HCV core antigen test as a cheaper alternative to HCV RNA testing, and reducing the cost of testing through negotiations with service providers, could make HCV treatment with DAAs even more economically attractive. Though our study looked only at persons already diagnosed to have HCV infection, bringing down the testing cost could also help make population-level HCV screening and treatment programs more cost-effective.
The licensing agreements that have made low-cost generic DAAs available in India, allow for sale of these drugs in several other low- and low-middle-income countries at low prices. Hence, our results should generally apply to these other countries too, both for individual case treatment and for public-funded treatment programs.
In conclusion, we found that low-cost generic DAAs available in India can improve patient outcomes and also result in cost-savings in a fairly short time-frame. Therefore, HCV treatment should be a priority for health planners of this country, and other countries where low-cost DAAs are available, not only from the public health viewpoint, but also from the economic perspective.
Supporting information
(DOCX)
Acknowledgments
Authors thanks Yvan Hutin, World Health Organization, for constructive comments that improved the quality of the study. Drs. Seguy, Razia and Hutin, are staff members of the World Health Organization. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions or policies of the World Health Organization.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Blach S, Zeuzem S, Manns M, Altraif I, Duberg A-S, Muljono DH, et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. The Lancet Gastroenterology & Hepatology.(in press). [DOI] [PubMed] [Google Scholar]
- 2.Razavi H, Elkhoury AC, Elbasha E, Estes C, Pasini K, Poynard T, et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology (Baltimore, Md). 2013;57(6):2164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet (London, England). 2016;388(10049):1081–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah N, Pierce T, Kowdley KV. Review of direct-acting antiviral agents for the treatment of chronic hepatitis C. Expert opinion on investigational drugs. 2013;22(9):1107–21. 10.1517/13543784.2013.806482 [DOI] [PubMed] [Google Scholar]
- 5.Iyengar S, Tay-Teo K, Vogler S, Beyer P, Wiktor S, de Joncheere K, et al. Prices, Costs, and Affordability of New Medicines for Hepatitis C in 30 Countries: An Economic Analysis. PLOS Medicine. 2016;13(5):e1002032 10.1371/journal.pmed.1002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raghavan P. Blockbuster Hepatitis C drug now under price control. The Econmic Times, Apr-01-2016(retrieved from: https://goo.gl/jxRtsq, last accessed: Jan-10-2017).
- 7.Westerhout K, Treur M, Mehnert A, Pascoe K, Ladha I, Belsey J. A cost utility analysis of simeprevir used with peginterferon + ribavirin in the management of genotype 1 hepatitis C virus infection, from the perspective of the UK National Health Service. Journal of medical economics. 2015:1–19. [DOI] [PubMed] [Google Scholar]
- 8.Chhatwal J, He T, Lopez-Olivo MA. Systematic Review of Modelling Approaches for the Cost Effectiveness of Hepatitis C Treatment with Direct-Acting Antivirals. PharmacoEconomics. 2016;34(6):551–67. 10.1007/s40273-015-0373-9 [DOI] [PubMed] [Google Scholar]
- 9.Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Annals of internal medicine. 2015;162(6):397–406. 10.7326/M14-1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linas BP, Barter DM, Morgan JR, Pho MT, Leff JA, Schackman BR, et al. The Cost-Effectiveness of Sofosbuvir-Based Regimens for Treatment of Hepatitis C Virus Genotype 2 or 3 Infection. Annals of internal medicine. 2015;162(9):619–29. 10.7326/M14-1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Najafzadeh M, Andersson K, Shrank WH, Krumme AA, Matlin OS, Brennan T, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Annals of internal medicine. 2015;162(6):407–19. 10.7326/M14-1152 [DOI] [PubMed] [Google Scholar]
- 12.Gimeno-Ballester V, Mar J, San Miguel R. Cost-effectiveness analysis of simeprevir with daclatasvir for non-cirrhotic genotype-1b-naive patients plus chronic hepatitis C. Expert review of pharmacoeconomics & outcomes research. 2015:1–10. [DOI] [PubMed] [Google Scholar]
- 13.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. Jama. 2012;308(24):2584–93. 10.1001/jama.2012.144878 [DOI] [PubMed] [Google Scholar]
- 14.Bennett W, Inoue Y, Beck J, Wong J, Pauker S, Davis G. Estimates of the cost-effectiveness of a single course of interferon- 2b in patients with histologically mild chronic hepatitis C. Annals of internal medicine. 1997;127(10):855 [DOI] [PubMed] [Google Scholar]
- 15.Chhatwal J, Ferrante SA, Brass C, El Khoury AC, Burroughs M, Bacon B, et al. Cost-Effectiveness of boceprevir in patients previously treated for chronic hepatitis C genotype 1 Infection in the United States. Value in Health. 2013;16(6):973–86. 10.1016/j.jval.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siebert U, Sroczynski G, Rossol S, Wasem J, Ravens-Sieberer U, Kurth B, et al. Cost effectiveness of peginterferon-2b plus ribavirin versus interferon -2b plus ribavirin for initial treatment of chronic hepatitis C. Gut. 2003;52(3):425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization—Viral Hepatitis Strategic Information and Modelling Reference Group: meeting report. Meeting report | 14–16 June 2016, WHO headquarters, Geneva, Switzerland. http://www.who.int/hepatitis/publications/strategic-information-modelling-meeting/en/ (last accessed: April 14, 2017). Geneva: WHO; 2016.
- 18.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology (Baltimore, Md). 1996;24(2):289–93. [DOI] [PubMed] [Google Scholar]
- 19.American Association for the Study of Liver Diseases and Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C. http://www.hcvguidelines.org/full-report-view Accessed October 22, 2015. [DOI] [PMC free article] [PubMed]
- 20.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. The New England journal of medicine. 2014;370(20):1889–98. 10.1056/NEJMoa1402454 [DOI] [PubMed] [Google Scholar]
- 21.Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology (Baltimore, Md). 2015;61(4):1127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welzel TM, Petersen J, Herzer K, Ferenci P, Gschwantler M, Wedemeyer H, et al. Daclatasvir plus sofosbuvir, with or without ribavirin, achieved high sustained virological response rates in patients with HCV infection and advanced liver disease in a real-world cohort. Gut. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohli A, Kapoor R, Sims Z, Nelson A, Sidharthan S, Lam B, et al. Ledipasvir and sofosbuvir for hepatitis C genotype 4: a proof-of-concept, single-centre, open-label phase 2a cohort study. Lancet Infect Dis. 2015;15(9):1049–54. 10.1016/S1473-3099(15)00157-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardoso AC, Moucari R, Figueiredo-Mendes C, Ripault MP, Giuily N, Castelnau C, et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. Journal of hepatology. 2010;52(5):652–7. 10.1016/j.jhep.2009.12.028 [DOI] [PubMed] [Google Scholar]
- 25.Thein H, Yi Q, Dore G, Krahn M. Estimation of stage specific fibrosis progression rates in chronic hepatitis C virus infection: A meta analysis and meta regression. Hepatology (Baltimore, Md). 2008;48(2):418–31. [DOI] [PubMed] [Google Scholar]
- 26.Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112(2):463–72. [DOI] [PubMed] [Google Scholar]
- 27.Planas R, Ballesté B, Antonio Álvarez M, Rivera M, Montoliu S, Anton Galeras J, et al. Natural history of decompensated hepatitis C virus-related cirrhosis. A study of 200 patients. Journal of hepatology. 2004;40(5):823–30. 10.1016/j.jhep.2004.01.005 [DOI] [PubMed] [Google Scholar]
- 28.Wolfe R, Roys E, Merion R. Trends in Organ Donation and Transplantation in the United States, 1999–2008. American Journal of Transplantation. 2010;10(4p2):961–72. [DOI] [PubMed] [Google Scholar]
- 29.Davis KL, Mitra D, Medjedovic J, Beam C, Rustgi V. Direct economic burden of chronic hepatitis C virus in a United States managed care population. Journal of Clinical Gastroenterology. 2011;45(2):e17 10.1097/MCG.0b013e3181e12c09 [DOI] [PubMed] [Google Scholar]
- 30.McAdam-Marx C, McGarry LJ, Hane CA, Biskupiak J, Deniz B, Brixner DI. All-cause and incremental per patient per year cost associated with chronic hepatitis C virus and associated liver complications in the United States: A managed care perspective. J Manag Care Pharm. 2011;17(7):531–46. 10.18553/jmcp.2011.17.7.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson J, Yao G, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al. A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment. Health Technology Assessment. 2007;11(13):1–202. [DOI] [PubMed] [Google Scholar]
- 32.Chong CAKY, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, et al. Health-state utilities and quality of life in hepatitis C patients. The American journal of gastroenterology. 2003;98(3):630–8. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization, Geneva. "WHO methods and data sources for global burden of disease estimates 2000–2011." http://www.who.int/healthinfo/statistics/GlobalDALYmethods_2000_2011.pdf (last accessed December 22, 2016). 2013. [Google Scholar]
- 34.Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Medical Decision Making. 2006;26(4):391–400. 10.1177/0272989X06290497 [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. 2016. http://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/ (last accessed: January 3, 2017). [Google Scholar]
- 36.Chhatwal J, He T, Hur C, Lopez-Olivo MA. Direct-Acting Antiviral Agents for Patients With Hepatitis C Virus Genotype 1 Infection are Cost Saving. Clin Gastroenterol Hepatol. 2016. [DOI] [PubMed] [Google Scholar]
- 37.WHO guidelines for the screening care and treatment of persons with chronic hepatitis C infection (retrieved from: http://www.who.int/hepatitis/publications/hepatitis-c-guidelines-2016/en/, last accessed: January-09-2017). 2016. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.